Abstract
Traumatic brain injury (TBI) remains a prominent public health concern despite several decades of attempts to develop therapies for the associated neurological and cognitive deficits. Effective models of this condition are imperative for better defining its pathophysiology and testing therapeutics. Human brain organoids are stem cell–derived neural tissues that recapitulate many of the steps of normal neurodevelopment, resulting in the reproduction of a substantial degree of brain architecture. Organoids are highly relevant to clinical conditions because of their human nature and three-dimensional tissue structure, yet they are easier to manipulate and interrogate experimentally than animals. Thus, they have the potential to serve as a novel platform for studying TBI. In this article, we discuss available in vitro models of TBI, active areas of inquiry on brain organoids, and how these two concepts could be merged.
Keywords: Brain organoids, Neurodegeneration, Stem cells, Traumatic brain injury
Upward of 2.87 million patients suffer from traumatic brain injury (TBI) annually in the United States [1]. The resulting neurological and cognitive disabilities significantly impair individual quality of life and constitute an enormous societal burden. Despite decades of intensive efforts, no viable therapies for TBI-associated deficits are currently available. Several basic and translational questions require further examination. Substantial progress has been made in understanding the relevant molecular and cellular pathways in TBI, but the link age to biomechanical forces needs to be explored more fully. The mechanisms underlying post-traumatic neuroinflammation and neurodegeneration are not well understood, nor is the impact of TBI on neural activity and systems-level networks. Optimal repair strategies for TBI-induced damage to neural circuits also remain elusive.
A number of platforms have been developed to model human TBI under controlled conditions and investigate the biological mechanisms that come into play after injury. Most research has been conducted with rodent models, which can reproduce certain biophysical features of TBI and the interplay among various cellular compartments (e.g., vasculature and immune cells). However, the rodent brain is an imperfect match for the human brain because of the former’s lissencephalic nature and low white matter content. Large animal models, such as pigs, mitigate these concerns to some degree but require considerable investments in time and resources. In vitro models provide a number of theoretical advantages, including a more controlled environment and easier access to samples for experimental manipulations and multiple measurements. At the same time, current cell culture–based approaches are likely over-simplified systems that can answer only a subset of questions for TBI. The generalizability of results derived from in vitro cultures of commonly used rodent neurons to human patients is also questionable. Thus, there is certainly room for new TBI models that address the shortcomings of current research platforms. Human brain organoids are a relatively new technology that holds intriguing promise as a novel in vitro model of TBI. Derived from pluripotent stem cell sources, brain organoids recapitulate many steps of normal neurodevelopment and emulate cerebral architecture to a significant degree [2–5]. These entities raise the possibility of an in vitro three-dimensional TBI model that is human in nature and reproducible in a personalized fashion, although caveats do exist. In this article, we review prior in vitro models of TBI, describe current progress in the field of brain organoids, and discuss how these two strands could be merged to create new opportunities to study TBI.
In vitro TBI models
A number of technologies have emerged to study specific aspects of TBI in cell culture systems, which have been reviewed more extensively in studies by Kumaria and Tolias [6] and Morrison et al. [7]. These in vitro platforms can be categorized based on the type of cellular injury induced, which includes static and dynamic mechanical injuries.
Early in vitro models fell primarily in the category of static or quasi-static mechanical injuries. Cellular or axonal transection was performed using various instruments to explore secondary injury mechanisms such as excitotoxicity [8,9]. Alternatively, injury was induced via compression [10] or hydrostatic pressure [11], similar to the forces generated by weight drop and fluid percussion injury models in vivo, respectively. One specific disadvantage of these earlier models was the inability to objectively characterize the biomechanical aspects of the injury, especially with respect to the applied forces and subsequent cellular or tissue deformation. Thus, a certain degree of heterogeneity was inherent to these methods.
Subsequent dynamic mechanical injury models sought, in part, to address this issue. These models also attempted to better model shear deformations of the brain, a major biomechanical contributor to TBI [12]. Controlled fluid shear forces can be applied to neurons using a rotating disk [13] or micropipette [14]. Shear strain also can be induced mechanically via a moving plate attached to a hydrogel embedded with cells [15]. Other investigators have cultured neurons and tissues on flexible membranes that could be deformed in a controlled manner to induce stretch injuries [16,17]. Although the majority of these stretch injuries have been applied to primarily neuronal somata, axon-specific stretch also has been demonstrated [18]. Models of axon stretch injury have revealed downstream axonal pathology after TBI such as proteolytic cleavage of voltage-gated sodium channels [19] and breakdown of the microtubule cytoskeleton [20]. More recently, microfluidic devices have been used as an alternative approach for delivering precise mechanical strains to axons [21].
Various brain tissue surrogates have been utilized in the models described in the earlier passages. By far, the most common are monolayer cultures of primary rodent neurons, although stem cell–derived human neurons are filtering into more recent studies [22]. A significant drawback of these culture models is the lack of cellular microenvironment niches arising from three-dimensional tissue structure. Brain slices, either acute preparations or organotypic cultures, resolve this particular problem. However, results derived from animal brain tissues may not be generalizable to TBI patients, and human brain specimens are not readily available. Engineered neural tissues, such as neurons embedded in hydrogels [23] or seeded on polymer scaffolds [24], can be generated with human cells in large quantities, but thus far they have not reproduced brain architecture to any significant degree. As in vitro TBI models continue to be refined to more accurately replicate human injuries, better substrates also will be needed. Human brain organoids are an intriguing candidate for this purpose.
Properties of current brain organoids
Organoids are simplified in vitro versions of an organ that recapitulate its structural features. Current iterations of organoids are derived from human pluripotent stem cell sources and utilize processes that occur during normal development, such as self-assembly, self-patterning, and self-driven morphogenesis, to generate organ-like architecture [25]. The modern era of human brain organoids effectively began in 2013, when Lancaster et al. [2] reported on whole-brain organoids composed of structural features of multiple brain regions. In the same year, Sasai et al. [3] described the formation of rudimentary neocortex with a multilayered structure in an early version of cortex-specific organoids. A series of subsequent studies refined the structural fidelity of brain organoids, which resulted in improved segregation of cortical layers [4,5]. This literature has been summarized in several excellent reviews by Kelava and Lancaster [26] and Di Lullo and Kriegstein [27] [26,27]. Here, we focus on the properties of brain organoids that have relevance for their use in modeling TBI.
Structural and cellular limitations of brain organoids
Significant excitement has been generated by the advent of human brain organoids because of their ability to emulate cerebral architecture, which is superior to any other in vitro system thus far. Despite this feat, it is important to understand the ways in which organoids fall short in replicating the brain and the implications of these shortcomings on the use of organoids in modeling TBI and other brain disorders. Areas in which brain organoid generation could improve include their structural features, cellular composition, and maturity.
Both whole-brain organoids that consist of multiple cerebral regions [2] and region-specific organoids that reproduce the structure of a single region (e.g., cortex [4,5] and hippocampus [28]) have been reported. Structural differences with the brain exist in both cases. Whole-brain and region-specific organoids are tissues with a generally smooth surface that enlarge to at most 4 mm in size. No gyri naturally occur, although phosphatase and tensin homolog (PTEN) gene deletion induces the expansion of folds in organoids [29]. Especially with cortical organoids, multiple cortical units occur, each with its own central ventricle-like region and surrounding layers of neural progenitors and differentiated neurons. Axon tracts that mimic white matter are not found in organoids but can be coaxed to grow out of organoids using tissue-engineering techniques [30,31]. Recent organoid work has emphasized region-specific organoids (see the following passages). These organoids are, by definition, limited to one brain region, but efforts to fuse different types of organoids as ‘assembloids’ [32,33] or engineering axons between organoids [31] may build systems that can probe interactions among brain regions.
The cellular composition of brain organoids, particularly the region-specific variants, is often incomplete. As an example, cortical organoids are composed of primarily glutamatergic neurons of ventricular/subventricular zone origin. Some interneurons are present [5]. However, the complete complement of interneurons is absent because these organoids have a dorsal rather than a ventral fate and the latter is necessary to generate the ganglionic eminences from which most interneurons arise. This observation highlights the tension that characterizes highly controlled protocols that generate more precise but less complete organoids. Astrocytes [5,34] and oligodendrocytes [35] appear in older organoids following expected timelines in normal neurodevelopment. Other support cells in the brain, such as microglia and endothelial cells derived from the mesoderm, are absent in brain organoids, limiting studies of inflammation, a major component of TBI.
From a broader perspective, current brain organoids are limited in terms of the maturity of their constituent neurons. A combination of genetic [4,5,36], epigenetic [37], and epitranscriptomic data [38] suggest that current brain organoids approximate the human fetal brain up to the second trimester of development. Further maturation is inhibited, at least in part, by the development of a necrotic core as organoid growth outstrips the ability of diffusion to provide adequate nutrient, gas, and waste exchange. This mass transport deficit depletes neural progenitors located centrally within the organoid and impairs the health of differentiated neurons at the periphery. A particularly elegant feature of brain organoids is the appearance of cortical laminae highly reminiscent of in vivo cortex. One consequence of the arrest in organoid development is that this laminar architecture remains in a rudimentary state. Impairment of cortical neuron subtype specification is also associated with metabolic stresses from the in vitro environment [39]. Recent attempts to address the mass transport deficit have involved borrowing techniques from organotypic cultures to decrease organoid thickness [30,40]. Transplantation of organoids into rodent brains is an alternative strategy for advancing organoid maturity by providing access to host vasculature [41] and relieving in vitro stressors [39].
Defining the neural activity of brain organoids
Brain organoids have been investigated primarily from a cellular and molecular perspective with less attention given to characterizing their neural activity. Calcium waves and action potentials induced by current injection were observed in earlier studies [2,4,5], suggesting the potential of brain organoids exhibit neural activity. It was initially reported that spontaneous action potentials occurred in eight-month-old, but not four-month-old, whole-brain organoids [42]. Subsequent studies demonstrated that spontaneous activity occurred at much younger ages in cortical organoids [43], although a more detailed description of the temporal evolution of this activity is needed. There is early evidence of neural network formation in brain organoids. Optical stimulation of light-sensitive cells in whole-brain organoids modulated the activity of distant population of neurons [42], and consistent patterns of neural firing in spatially distinct neurons are observed, suggesting the formation of network-level circuitry. A recent study reported the presence of nested oscillations in brain organoids (i.e., nonoscillatory gamma activity on delta waves) [43], but this computational result may not reflect normal oscillations in the brain. Further study is essential to understand when neural activity appears and how it evolves in brain organoids.
Heterogeneity in organoid generation
Heterogeneity exists in organoid generation protocols. Both batch-to-batch and intrabatch variability has been observed in whole-brain organoids [42]. This variability includes differences in the specific cell types that are present in these organoids. Cortical organoids are more uniform, as shown by the consistent thickness of cortical layers at different time points [5]. Genetic and transcriptomic analyses support the reproducibility of cortical organoids at the level of basic cell identify (i.e., glutamatergic neuron versus GABAergic neuron versus astrocyte), including the generation of consistent organoids from different pluripotent stem cell lines [44,45]. However, concerns persist for even cortical organoids regarding heterogeneity in the identity of cortical areas expressed (e.g., frontal versus occipital cortex) [39] and the number and size of component cortical units.
Modeling TBI with brain organoids
Despite the caveats described in the earlier passages, brain organoids remain a highly promising system for modeling brain disorders, as they are human entities that reproduce a substantial degree of three-dimensional brain architecture [46]. The in vitro nature of brain organoids facilitates experimental manipulations and sampling that would not otherwise be possible in vivo. Brain organoids have already been used to identify pathogenic mechanisms underlying microcephaly caused by Zika virus infection [5,47,48], examine cellular and genetic pathways that are perturbed in schizophrenia [49] and autism spectrum disorder [50], and model the heterogeneity and invasive behavior of glioblastoma multiforme [51–53]. Brain organoids have not been reported for the study of TBI as of yet. Their use to model TBI is a viable option as long as appropriate questions are asked that keep in mind the constraints of the modeling system.
Applications of brain organoids as TBI models
Current brain organoids are roughly equivalent to fetal brains at the second-trimester stage of development. Thus, a TBI model based on brain organoids is most appropriately considered an early fetal TBI model. As with cultures of embryonic rodent neurons, results from an organoid-based TBI model would need to be interpreted with caution, especially if generalizations to adult or even pediatric patients were to be made.
With this qualification in mind, brain organoids could provide an intriguing window into human neuronal physiology after traumatic injury. Applying clinically relevant biomechanical forces to brain organoids provides an opportunity to examine the temporal progression of acute cellular responses to injury occurring within a complex three-dimensional architecture. In particular, directly examining the interplay of multiple cell types may offer important insights into disease pathogenesis. Because organoids contain an incomplete complement of cell types that exist in the brain, additional cellular components, such as microglia or endothelial cells, could be introduced into organoids to assess their specific contribution to postinjury processes. An advantage of organoids over other standard in vitro platforms is their ability to survive on the order of months, which permits direct evaluation of cellular changes over time into the chronic phase of injury. Chronic changes after TBI is a topic of increasing importance as accumulating data suggest that TBI exposure is associated with neurodegenerative pathologies in the months and even years after injury [54,55].
Another attractive avenue for the use brain organoids in studying TBI is high-throughput screening of therapeutics. The viability of this strategy depends on a number of factors, starting with improvements in organoid homogeneity. Miniaturized spinning bioreactors hold the potential for scaling up the generation of brain organoids [5], but novel methods for multiplexing organoid analysis are also needed. Radio frequency identification chips embedded in liver organoids have been used to identify disease phenotypes [56]. Borrowing techniques from tissue matrix arrays may facilitate multiplexed analysis of injured brain organoids as well [57].
Building injury models with brain organoids
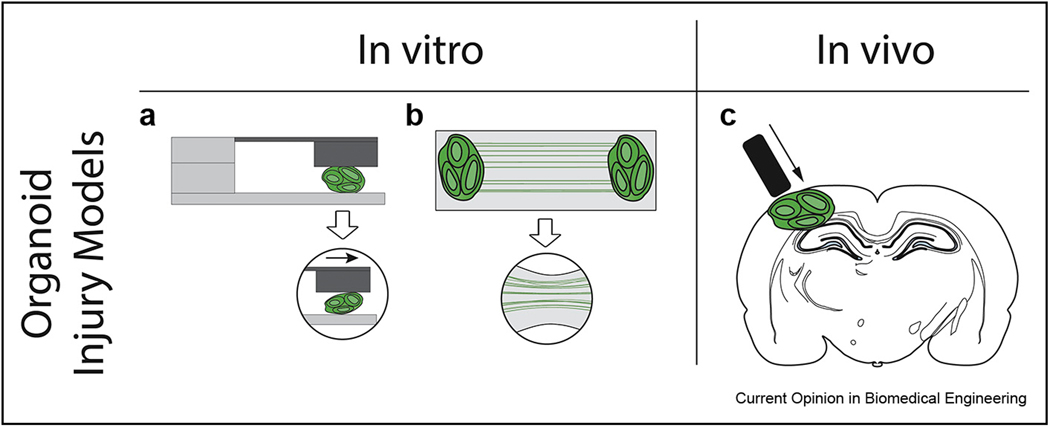
From a practical perspective, developing a TBI model based on brain organoids would rely upon methods that have already been pioneered for other in vitro cultures. Static mechanical injuries would be relatively easy to apply to brain organoids that are fixed in place, but they would suffer from the same problems with other in vitro substrates regarding the inability to quantify applied forces and tissue deformation. Fluid shear forces that have been applied to monolayer cultures [13] may not produce enough injury to cells within a tissue and would need to be augmented for brain organoid injury. Dynamic mechanical injury induced by a moving plate [15] could be readily adapted to brain organoids because it has been applied previously to cells embedded in a hydrogel. It may be that stretch injury could be applied to engineered axons generated from brain organoids (Figure 1a–b) [30,31], although as with fluid shear injuries the amount of force applied would need to be increased to account for differences between axons within tissues as opposed to monolayer cultures. Another possibility for using brain organoids to model TBI would be to take advantage of recent reports of organoid transplantation in rodents [41] and generate brain chimeras amenable to traditional in vivo TBI injuries (Figure 1c).
Figure 1. Examples of injury models based on human brain organoids.
(a) In vitro injury models include direct application of shear to organoid tissue using a moving plate (left) and rapid deformation of axon tracts grown between organoids (right) (b) Brain organoids also could be incorporated into an in vivo injury model. Following integration of transplanted organoids with the host brain, injury is induced using conventional controlled cortical impact or fluid percussion methods. This approach permits experimental manipulation and analysis of injured human brain tissue in the in vivo setting.
Conclusion
Human brain organoids, even whole-brain organoids, are not truly ‘mini-brains,’ and current versions most accurately reflect fetal brain development. However, they recreate brain architecture to a considerable degree, more so than any other in vitro system thus far. Progress is being made on multiple fronts to further improve the fidelity with which organoids reproduce cerebral structure. In parallel, opportunities exist to use brain organoids to complement other models in studying the pathogenesis and treatment of human brain disorders and diseases. Traumatic brain injury is one condition that would benefit from such an approach, especially given the importance of species-specific differences in neural responses to injury and the availability of established in vitro injury models. As work on organoid models of TBI progresses, it will be important to keep in mind the ethical considerations of research involving replica of human brain tissue [58,59]. Combining these considerations with the relevant scientific and bioengineering principles will likely yield a novel and responsible strategy for investigating human TBI.
Acknowledgements
This work was supported in part by the Career Development Award IK2-RX002013 from the U.S. Department of Veterans Affairs Rehabilitation Research Development Service to Han-Chiao Isaac Chen.
Footnotes
Disclaimer
The contents of this review do not represent the views of the U.S. Department of Veterans Affairs or the United States Government.
Conflict of interest statement
Nothing declared.
References
Papers of particular interest, published within the period of review, have been highlighted as:
* of special interest
** of outstanding interest
- 1.Prevention CfDCa: Surveillance report of truamatic brain injury-related emergency department visits, hospitalizations, and Deaths–United States. Centers for Disease Control and Prevention, U.S. Department of Heath and Human Services; 2019; 2014. [Google Scholar]
- 2. Lancaster MA, Renner M, Martin CA, Wenzel D, Bicknell LS, Hurles ME, Homfray T, Penninger JM, Jackson AP, Knoblich JA: Cerebral organoids model human brain development and microcephaly. Nature 2013, 501:373–379. * * This study introduced the concept of whole-brain organoids and provided the first demonstration of using organoids to model human brain disorders.
- 3. Kadoshima T, Sakaguchi H, Nakano T, Soen M, Ando S, Eiraku M, Sasai Y: Self-organization of axial polarity, insideout layer pattern, and species-specific progenitor dynamics in human ES cell-derived neocortex. Proc Natl Acad Sci USA 2013, 110:20284–20289. * * Early versions of cortical organoids described in this study showed the possibility of generating rudimentary cortical layers and different areal identities of cortex.
- 4.Pasca AM, Sloan SA, Clarke LE, Tian Y, Makinson CD, Huber N, Kim CH, Park JY, O’Rourke NA, Nguyen KD, et al. : Functional cortical neurons and astrocytes from human pluripotent stem cells in 3D culture. Nat Methods 2015, 12:671–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Qian X, Nguyen HN, Song MM, Hadiono C, Ogden SC, Hammack C, Yao B, Hamersky GR, Jacob F, Zhong C, et al. :Brain-region-specific organoids using mini-bioreactors for modeling ZIKV exposure. Cell 2016, 165:1238–1254. * Miniaturized spinning bioreactors were implemented in this study to improve the reproducibility and structural fidelity of cortical organoids.
- 6.Kumaria A, Tolias CM: In vitro models of neurotrauma. Br J Neurosurg 2008, 22:200–206. [DOI] [PubMed] [Google Scholar]
- 7.Morrison B 3rd, Elkin BS, Dolle JP, Yarmush ML: In vitro models of traumatic brain injury. Annu Rev Biomed Eng 2011, 13: 91–126. [DOI] [PubMed] [Google Scholar]
- 8.Tecoma ES, Monyer H, Goldberg MP, Choi DW: Traumatic neuronal injury in vitro is attenuated by NMDA antagonists. Neuron 1989, 2:1541–1545. [DOI] [PubMed] [Google Scholar]
- 9.Mukhin AG, Ivanova SA, Knoblach SM, Faden AI: New in vitro model of traumatic neuronal injury: evaluation of secondary injury and glutamate receptor-mediated neurotoxicity. J Neurotrauma 1997, 14:651–663. [DOI] [PubMed] [Google Scholar]
- 10.Church AJ, Andrew RD: Spreading depression expands traumatic injury in neocortical brain slices. J Neurotrauma 2005, 22:277–290. [DOI] [PubMed] [Google Scholar]
- 11.Murphy EJ, Horrocks LA: A model for compression trauma: pressure-induced injury in cell cultures. J Neurotrauma 1993, 10:431–444. [DOI] [PubMed] [Google Scholar]
- 12.Gennarelli TA, Thibault LE, Adams JH, Graham DI, Thompson CJ, Marcincin RP: Diffuse axonal injury and traumatic coma in the primate. Ann Neurol 1982, 12:564–574. [DOI] [PubMed] [Google Scholar]
- 13.LaPlaca MC, Thibault LE: Dynamic mechanical deformation of neurons triggers an acute calcium response and cell injury involving the N-methyl-D-aspartate glutamate receptor. J Neurosci Res 1998, 52:220–229. [DOI] [PubMed] [Google Scholar]
- 14.Chung RS, Staal JA, McCormack GH, Dickson TC, Cozens MA, Chuckowree JA, Quilty MC, Vickers JC: Mild axonal stretch injury in vitro induces a progressive series of neurofilament alterations ultimately leading to delayed axotomy. J Neurotrauma 2005, 22:1081–1091. [DOI] [PubMed] [Google Scholar]
- 15. LaPlaca MC, Cullen DK, McLoughlin JJ, Cargill RS 2nd: High rate shear strain of three-dimensional neural cell cultures: a new in vitro traumatic brain injury model. J Biomech 2005, 38: 1093–1105. * Controlled strain was applied to neurons embedded in a three-dimensional hydrogel. This system has direct applicability to an organoid model of TBI.
- 16.Ellis EF, McKinney JS, Willoughby KA, Liang S, Povlishock JT: A new model for rapid stretch-induced injury of cells in culture: characterization of the model using astrocytes. J Neurotrauma 1995, 12:325–339. [DOI] [PubMed] [Google Scholar]
- 17.Morrison B 3rd, Cater HL, Benham CD, Sundstrom LE: An in vitro model of traumatic brain injury utilising two-dimensional stretch of organotypic hippocampal slice cultures. J Neurosci Methods 2006, 150:192–201. [DOI] [PubMed] [Google Scholar]
- 18.Smith DH, Wolf JA, Lusardi TA, Lee VM, Meaney DF: High tolerance and delayed elastic response of cultured axons to dynamic stretch injury. J Neurosci 1999, 19:4263–4269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Iwata A, Stys PK, Wolf JA, Chen XH, Taylor AG, Meaney DF, Smith DH: Traumatic axonal injury induces proteolytic cleavage of the voltage-gated sodium channels modulated by tetrodotoxin and protease inhibitors. J Neurosci 2004, 24: 4605–4613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tang-Schomer MD, Patel AR, Baas PW, Smith DH: Mechanical breaking of microtubules in axons during dynamic stretch injury underlies delayed elasticity, microtubule disassembly, and axon degeneration. Faseb J 2010, 24:1401–1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dolle JP, Morrison B 3rd, Schloss RS, Yarmush ML: An organotypic uniaxial strain model using microfluidics. Lab Chip 2013, 13:432–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dolle JP, Jaye A, Anderson SA, Ahmadzadeh H, Shenoy VB, Smith DH: Newfound sex differences in axonal structure underlie differential outcomes from in vitro traumatic axonal injury. Exp Neurol 2018, 300:121–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cullen DK, Stabenfeldt SE, Simon CM, Tate CC, LaPlaca MC: In vitro neural injury model for optimization of tissue-engineered constructs. J Neurosci Res 2007, 85:3642–3651. [DOI] [PubMed] [Google Scholar]
- 24.Tang-Schomer MD, White JD, Tien LW, Schmitt LI, Valentin TM, Graziano DJ, Hopkins AM, Omenetto FG, Haydon PG, Kaplan DL: Bioengineered functional brain-like cortical tissue. Proc Natl Acad Sci U S A 2014, 111:13811–13816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sasai Y: Next-generation regenerative medicine: organogenesis from stem cells in 3D culture. Cell Stem Cell 2013, 12: 520–530. [DOI] [PubMed] [Google Scholar]
- 26.Kelava I, Lancaster MA: Dishing out mini-brains: current progress and future prospects in brain organoid research. Dev Biol 2016, 420:199–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Di Lullo E, Kriegstein AR: The use of brain organoids to investigate neural development and disease. Nat Rev Neurosci 2017, 18:573–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sakaguchi H, Kadoshima T, Soen M, Narii N, Ishida Y, Ohgushi M, Takahashi J, Eiraku M, Sasai Y: Generation of functional hippocampal neurons from self-organizing human embryonic stem cell-derived dorsomedial telencephalic tissue. Nat Commun 2015, 6:8896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li Y, Muffat J, Omer A, Bosch I, Lancaster MA, Sur M, Gehrke L, Knoblich JA, Jaenisch R: Induction of expansion and folding in human cerebral organoids. Cell Stem Cell 2017, 20:385–396 e383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Giandomenico SL, Mierau SB, Gibbons GM, Wenger LMD, Masullo L, Sit T, Sutcliffe M, Boulanger J, Tripodi M, Derivery E, et al. : Cerebral organoids at the air-liquid interface generate diverse nerve tracts with functional output. Nat Neurosci 2019, 22:669–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Cullen DK, Gordian-Velez WJ, Struzyna LA, Jgamadze D, Lim J, Wofford KL, Browne KD, Chen HI: Bundled three-dimensional human axon tracts derived from brain organoids. iScience 2019, 21:57–67. * This study demonstrated the possibility of engineering axon tracts from human brain organoids to achieve a pre-specified architecture.
- 32. Birey F, Andersen J, Makinson CD, Islam S, Wei W, Huber N, Fan HC, Metzler KRC, Panagiotakos G, Thom N, et al. : Assembly of functionally integrated human forebrain spheroids. Nature 2017, 545:54–59. * The concept of organoid “assembloids,” the fusion of the different organoid types to generate a more complex system, was developed in this study.
- 33.Xiang Y, Tanaka Y, Cakir B, Patterson B, Kim KY, Sun P, Kang YJ, Zhong M, Liu X, Patra P, et al. : hESC-derived thalamic organoids form reciprocal projections when fused with cortical organoids. Cell Stem Cell 2019, 24:487–497 e487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sloan SA, Darmanis S, Huber N, Khan TA, Birey F, Caneda C, Reimer R, Quake SR, Barres BA, Pasca SP: Human astrocyte maturation captured in 3D cerebral cortical spheroids derived from pluripotent stem cells. Neuron 2017, 95:779–790 e776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Marton RM, Miura Y, Sloan SA, Li Q, Revah O, Levy RJ, Huguenard JR, Pasca SP: Differentiation and maturation of oligodendrocytes in human three-dimensional neural cultures. Nat Neurosci 2019, 22:484–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Camp JG, Badsha F, Florio M, Kanton S, Gerber T, WilschBrauninger M, Lewitus E, Sykes A, Hevers W, Lancaster M, et al. : Human cerebral organoids recapitulate gene expression programs of fetal neocortex development. Proc Natl Acad Sci U S A 2015, 112:15672–15677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Luo C, Lancaster MA, Castanon R, Nery JR, Knoblich JA, Ecker JR: Cerebral organoids recapitulate epigenomic signatures of the human fetal brain. Cell Rep 2016, 17: 3369–3384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yoon KJ, Ringeling FR, Vissers C, Jacob F, Pokrass M, Jimenez-Cyrus D, Su Y, Kim NS, Zhu Y, Zheng L, et al. : Temporal control of mammalian cortical neurogenesis by m(6)A methylation. Cell 2017, 171:877–889 e817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bhaduri A, Andrews MG, Mancia Leon W, Jung D, Shin D, Allen D, Jung D, Schmunk G, Haeussler M, Salma J, et al. : Cell stress in cortical organoids impairs molecular subtype specification. Nature 2020. [DOI] [PMC free article] [PubMed]
- 40.Qian X, Su Y, Adam CD, Deutschmann AU, Pather SR, Goldberg EM, Su K, Li S, Lu L, Jacob F, et al. : Sliced human cortical organoids for modeling distinct cortical layer formation. Cell Stem Cell 2020:766–781. e769. [DOI] [PMC free article] [PubMed]
- 41. Mansour AA, Goncalves JT, Bloyd CW, Li H, Fernandes S, Quang D, Johnston S, Parylak SL, Jin X, Gage FH: An in vivo model of functional and vascularized human brain organoids. Nat Biotechnol 2018, 36:432–441. * This study is the first detailed description of human brain organoids transplanted into rodent hosts, providing initial evidence of their anatomic and functional integration with the host brain.
- 42.Quadrato G, Nguyen T, Macosko EZ, Sherwood JL, Min Yang S, Berger DR, Maria N, Scholvin J, Goldman M, Kinney JP, et al. : Cell diversity and network dynamics in photosensitive human brain organoids. Nature 2017, 545:48–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Trujillo CA, Gao R, Negraes PD, Gu J, Buchanan J, Preissl S, Wang A, Wu W, Haddad GG, Chaim IA, et al. : Complex oscillatory waves emerging from cortical organoids model early human brain network development. Cell Stem Cell 2019. [DOI] [PMC free article] [PubMed]
- 44.Yoon SJ, Elahi LS, Pasca AM, Marton RM, Gordon A, Revah O, Miura Y, Walczak EM, Holdgate GM, Fan HC, et al. : Reliability of humancorticalorganoidgeneration. Nat Methods 2019, 16:75–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Velasco S, Kedaigle AJ, Simmons SK, Nash A, Rocha M, Quadrato G, Paulsen B, Nguyen L, Adiconis X, Regev A, et al. : Individual brain organoids reproducibly form cell diversity of the human cerebral cortex. Nature 2019, 570:523–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen HI, Song H, Ming GL: Applications of human brain organoids to clinical problems. Dev Dynam 2018. [DOI] [PMC free article] [PubMed]
- 47.Cugola FR, Fernandes IR, Russo FB, Freitas BC, Dias JL, Guimaraes KP, Benazzato C, Almeida N, Pignatari GC, Romero S, et al. : The Brazilian Zika virus strain causes birth defects in experimental models. Nature 2016, 534:267–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Garcez PP, Loiola EC, Madeiro da Costa R, Higa LM, Trindade P, Delvecchio R, Nascimento JM, Brindeiro R, Tanuri A, Rehen SK: Zika virus impairs growth in human neurospheres and brain organoids. Science 2016, 352:816–818. [DOI] [PubMed] [Google Scholar]
- 49.Ye F, Kang E, Yu C, Qian X, Jacob F, Yu C, Mao M, Poon RYC, Kim J, Song H, et al. : DISC1 regulates neurogenesis via modulating kinetochore attachment of ndel1/nde1 during mitosis. Neuron 2017, 96:1041–1054. e1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mariani J, Coppola G, Zhang P, Abyzov A, Provini L, Tomasini L, Amenduni M, Szekely A, Palejev D, Wilson M, et al. : FOXG1Dependent dysregulation of GABA/glutamate neuron differentiation in autism spectrum disorders. Cell 2015, 162: 375–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hubert CG, Rivera M, Spangler LC, Wu Q, Mack SC, Prager BC, Couce M, McLendon RE, Sloan AE, Rich JN: A three-dimensional organoid culture system derived from human glioblastomas recapitulates the hypoxic gradients and cancer stem cell heterogeneity of tumors found in vivo. Cancer Res 2016, 76:2465–2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jacob F, Salinas RD, Zhang DY, Nguyen PTT, Schnoll JG, Wong SZH, Thokala R, Sheikh S, Saxena D, Prokop S, et al. : A patient-derived glioblastoma organoid model and biobank recapitulates inter- and intra-tumoral heterogeneity. Cell 2020, 180:188–204 e122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bhaduri A, Di Lullo E, Jung D, Muller S, Crouch EE, Espinosa CS, Ozawa T, Alvarado B, Spatazza J, Cadwell CR, et al. : Outer radial glia-like cancer stem cells contribute to heterogeneity of glioblastoma. Cell Stem Cell 2020, 26: 48–63 e46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Johnson VE, Stewart JE, Begbie FD, Trojanowski JQ, Smith DH, Stewart W: Inflammation and white matter degeneration persist for years after a single traumatic brain injury. Brain 2013, 136:28–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.McKee AC, Cantu RC, Nowinski CJ, Hedley-Whyte ET, Gavett BE, Budson AE, Santini VE, Lee HS, Kubilus CA, Stern RA: Chronic traumatic encephalopathy in athletes: progressive tauopathy after repetitive head injury. J Neuropathol Exp Neurol 2009, 68:709–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kimura M, Asuma M, Zhang R, Thompson W, Mayhew CN, Takebe T: Digitalized human organoid for wireless phenotyping. iScience 2018. [DOI] [PMC free article] [PubMed]
- 57.Beachley VZ, Wolf MT, Sadtler K, Manda SS, Jacobs H, Blatchley MR, Bader JS, Pandey A, Pardoll D, Elisseeff JH: Tissue matrix arrays for high-throughput screening and systems analysis of cell function. Nat Methods 2015, 12:1197–1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Farahany NA, Greely HT, Hyman S, Koch C, Grady C, Pasca SP, Sestan N, Arlotta P, Bernat JL, Ting J, et al. : The ethics of experimenting with human brain tissue. Nature 2018, 556:429–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chen HI, Wolf JA, Blue R, Song MM, Moreno JD, Ming GL, Song H: Transplantation of human brain organoids: revisiting the science and ethics of brain chimeras. Cell Stem Cell 2019, 25:462–472. [DOI] [PMC free article] [PubMed] [Google Scholar]