Abstract
Background
Mindfulness‐based smoking cessation interventions may aid smoking cessation by teaching individuals to pay attention to, and work mindfully with, negative affective states, cravings, and other symptoms of nicotine withdrawal. Types of mindfulness‐based interventions include mindfulness training, which involves training in meditation; acceptance and commitment therapy (ACT); distress tolerance training; and yoga.
Objectives
To assess the efficacy of mindfulness‐based interventions for smoking cessation among people who smoke, and whether these interventions have an effect on mental health outcomes.
Search methods
We searched the Cochrane Tobacco Addiction Group's specialised register, CENTRAL, MEDLINE, Embase, PsycINFO, and trial registries to 15 April 2021. We also employed an automated search strategy, developed as part of the Human Behaviour Change Project, using Microsoft Academic.
Selection criteria
We included randomised controlled trials (RCTs) and cluster‐RCTs that compared a mindfulness‐based intervention for smoking cessation with another smoking cessation programme or no treatment, and assessed smoking cessation at six months or longer. We excluded studies that solely recruited pregnant women.
Data collection and analysis
We followed standard Cochrane methods. We measured smoking cessation at the longest time point, using the most rigorous definition available, on an intention‐to‐treat basis. We calculated risk ratios (RRs) and 95% confidence intervals (CIs) for smoking cessation for each study, where possible. We grouped eligible studies according to the type of intervention and type of comparator. We carried out meta‐analyses where appropriate, using Mantel‐Haenszel random‐effects models. We summarised mental health outcomes narratively.
Main results
We included 21 studies, with 8186 participants. Most recruited adults from the community, and the majority (15 studies) were conducted in the USA. We judged four of the studies to be at low risk of bias, nine at unclear risk, and eight at high risk. Mindfulness‐based interventions varied considerably in design and content, as did comparators, therefore, we pooled small groups of relatively comparable studies.
We did not detect a clear benefit or harm of mindfulness training interventions on quit rates compared with intensity‐matched smoking cessation treatment (RR 0.99, 95% CI 0.67 to 1.46; I2 = 0%; 3 studies, 542 participants; low‐certainty evidence), less intensive smoking cessation treatment (RR 1.19, 95% CI 0.65 to 2.19; I2 = 60%; 5 studies, 813 participants; very low‐certainty evidence), or no treatment (RR 0.81, 95% CI 0.43 to 1.53; 1 study, 325 participants; low‐certainty evidence). In each comparison, the 95% CI encompassed benefit (i.e. higher quit rates), harm (i.e. lower quit rates) and no difference. In one study of mindfulness‐based relapse prevention, we did not detect a clear benefit or harm of the intervention over no treatment (RR 1.43, 95% CI 0.56 to 3.67; 86 participants; very low‐certainty evidence).
We did not detect a clear benefit or harm of ACT on quit rates compared with less intensive behavioural treatments, including nicotine replacement therapy alone (RR 1.27, 95% CI 0.53 to 3.02; 1 study, 102 participants; low‐certainty evidence), brief advice (RR 1.27, 95% CI 0.59 to 2.75; 1 study, 144 participants; very low‐certainty evidence), or less intensive ACT (RR 1.00, 95% CI 0.50 to 2.01; 1 study, 100 participants; low‐certainty evidence). There was a high level of heterogeneity (I2 = 82%) across studies comparing ACT with intensity‐matched smoking cessation treatments, meaning it was not appropriate to report a pooled result.
We did not detect a clear benefit or harm of distress tolerance training on quit rates compared with intensity‐matched smoking cessation treatment (RR 0.87, 95% CI 0.26 to 2.98; 1 study, 69 participants; low‐certainty evidence) or less intensive smoking cessation treatment (RR 1.63, 95% CI 0.33 to 8.08; 1 study, 49 participants; low‐certainty evidence).
We did not detect a clear benefit or harm of yoga on quit rates compared with intensity‐matched smoking cessation treatment (RR 1.44, 95% CI 0.40 to 5.16; 1 study, 55 participants; very low‐certainty evidence).
Excluding studies at high risk of bias did not substantially alter the results, nor did using complete case data as opposed to using data from all participants randomised.
Nine studies reported on changes in mental health and well‐being, including depression, anxiety, perceived stress, and negative and positive affect. Variation in measures and methodological differences between studies meant we could not meta‐analyse these data. One study found a greater reduction in perceived stress in participants who received a face‐to‐face mindfulness training programme versus an intensity‐matched programme. However, the remaining eight studies found no clinically meaningful differences in mental health and well‐being between participants who received mindfulness‐based treatments and participants who received another treatment or no treatment (very low‐certainty evidence).
Authors' conclusions
We did not detect a clear benefit of mindfulness‐based smoking cessation interventions for increasing smoking quit rates or changing mental health and well‐being. This was the case when compared with intensity‐matched smoking cessation treatment, less intensive smoking cessation treatment, or no treatment. However, the evidence was of low and very low certainty due to risk of bias, inconsistency, and imprecision, meaning future evidence may very likely change our interpretation of the results. Further RCTs of mindfulness‐based interventions for smoking cessation compared with active comparators are needed. There is also a need for more consistent reporting of mental health and well‐being outcomes in studies of mindfulness‐based interventions for smoking cessation.
Plain language summary
Can mindfulness help people to stop smoking?
Key messages
‐ There is currently no clear evidence that mindfulness‐based treatments help people to stop smoking or improve their mental health and well‐being.
‐ However, our confidence in the evidence is low or very low, and further evidence is likely to change our conclusions.
What is mindfulness?
Mindfulness involves focusing attention on your thoughts and feelings and observing them without judgment as they arise and pass away. Mindfulness is believed to help people better control their thoughts and feelings, rather than be controlled by them. Stopping smoking gives rise to distressing urges to smoke and low mood, so mindfulness‐based treatments could improve people's ability to cope with these.
Types of mindfulness‐based therapies include:
‐ mindfulness training (which involves training in mindfulness‐based meditation);
‐ acceptance and commitment therapy (ACT); which doesn't teach meditation but encourages people to embrace their thoughts and feelings rather than fighting them, while making committed behaviour change);
‐ distress tolerance training (which provides parts of the ACT therapy, as well as presenting people who smoke with situations that make them want to smoke. This allows them to practise the skills that they have learnt through ACT);
‐ yoga (which increases awareness of breathing and encourages a connection between mind and body).
What did we want to find out?
We wanted to find out whether mindfulness‐based stop‐smoking programmes work better than other stop‐smoking programmes or no treatment to help people stop smoking.
We wanted to know:
‐ how many people stopped smoking for at least six months;
‐ whether there were changes in people's mental health and well‐being.
What did we do?
We searched for studies that looked at the use of mindfulness to help people stop smoking.
We compared and summarised the results of the studies and rated our confidence in the evidence, based on factors such as study methods and sizes.
What did we find?
We found 21 studies in 8186 young people and adults who smoked.
The studies tested a range of mindfulness‐based treatments, including mindfulness training (8 studies), ACT (8 studies), yoga (3 studies), and distress tolerance training (2 studies). Studies compared these treatments with:
‐ other stop‐smoking treatments that were equally time‐intensive (such as counselling);
‐ other stop‐smoking treatments that were less intensive (such as brief advice);
‐ no treatment.
Most studies took place in the USA (15 studies). Others took place in Hong Kong (2 studies), Brazil (1 study), Ireland (1 study), and Cyprus (1 study). One study did not report the country it took place in.
Main results
We did not find clear evidence that mindfulness helped people to stop smoking. When we grouped studies by the type of mindfulness‐based intervention people received, we found no evidence that people who received mindfulness training, ACT, distress tolerance training, or yoga were more likely to stop than people who received any other stop‐smoking treatments or no support.
Nine studies looked at whether mindfulness‐based stop‐smoking treatments resulted in positive changes in mental health and well‐being, such as reductions in stress or anxiety or improvements in mood. One of these studies found that people who received a mindfulness training programme reported being less stressed than those who received an alternative stop‐smoking treatment. However, the other 8 did not find evidence of a difference in mental health and well‐being between groups.
What are the limitations of the evidence?
Our confidence in the evidence is low to very low as there were problems with the design of studies, findings of studies were very different from one another, and not enough people took part, making it difficult to tell whether mindfulness helps people to stop smoking or was linked to better mental health and well‐being. We need more studies to draw firmer conclusions.
How up to date is this evidence?
We included evidence published to 15 April 2021.
Summary of findings
Background
Description of the condition
Smoking remains a leading cause of preventable death and disease worldwide (WHO 2019). Stopping smoking can result in substantial health gains, even later in life. The sooner a smoker quits, the more they reduce their risk of developing smoking‐related diseases (Doll 2004). The majority of smokers want to quit and many try to quit each year, but quit rates remain low (WHO 2019).
Description of the intervention
In recent decades, mindfulness has increasingly been recognised as an influence on mood and behaviour (Baer 2003; Keng 2011). It has been adopted as an approach for increasing awareness and responding skilfully to mental processes that contribute to emotional distress and maladaptive behaviour (Baer 2003). In current research contexts, mindfulness is typically defined as the psychological process of bringing non‐judgmental attention to experiences occurring in the present moment (Kabat‐Zinn 2013). There are various definitions of mindfulness used in psychological literature. While no consensus has been reached on how to define mindfulness, a two‐component model proposed by Bishop 2004 is often used in research. This operationalises mindfulness as: (i) maintaining attention on the immediate experience, and (ii) maintaining an attitude of openness, curiosity, and acceptance toward this experience, regardless of its valence or desirability.
Mindfulness approaches are not relaxation or mood management techniques, but rather a form of cognitive training to reduce susceptibility to reactive states of mind that might otherwise induce stress or perpetuate psychopathology (Baer 2003). The practice of mindfulness involves focusing attention on the immediate experience of cognitions, emotions, perceptions, and physical sensations and observing them as they arise and pass away. Mindfulness is nondeliberative: it simply involves paying sustained attention to thoughts and feelings without thinking about or evaluating them. A key tenet of mindfulness is that, by noticing thoughts and feelings in a curious and accepting manner, people develop greater tolerance of these phenomena and are able to recognise that they are transient, so they are less likely to respond impulsively to them (Heppner 2015).
There are a range of different treatments based on the principles of mindfulness. Mindfulness‐based stress reduction (MBSR; Kabat‐Zinn 2013) and mindfulness‐based cognitive therapy (MBCT; Segal 2002) use meditation as the primary method of teaching mindfulness. MBSR was developed to treat chronic stress and pain‐related disorders. It uses three techniques: firstly, sitting meditation, which involves mindful attention on the breath and a state of noncritical awareness of cognitions, feelings, and sensations; secondly, Hatha yoga practice, which involves breathing exercises, simple stretches, and postures; and thirdly, body scan, which involves a gradual sweeping of attention through the entire body from feet to head, while employing nonjudgmental awareness of feelings and sensation in each targeted body region (Kabat‐Zinn 2013). MBCT was developed to prevent relapse in depressive disorders. It integrates aspects of cognitive behavioral therapy (CBT) for depression into the MBSR programme (Segal 2002).
Other treatments that incorporate mindfulness include acceptance and commitment therapy (ACT; Hayes 2016), distress tolerance training, dialectical behaviour therapy (DBT; Linehan 2018), and certain types of yoga (Salmon 2009). ACT focuses on increasing people's willingness to experience physical cravings, emotions, and thoughts, and allowing these to come and go while making committed behaviour changes that are guided by their own values (Hayes 2016). Distress tolerance training combines elements drawn from ACT with exposure‐based treatment, allowing ACT skills to be practised within treatment sessions in response to internal triggers (Brown 2008). DBT also has a strong emphasis on acceptance, incorporating strategies to help the patient accept themselves, their current capabilities, and behavioural functioning (Linehan 2018). Yoga is a key component of MBSR (Kabat‐Zinn 2013), and provides an opportunity to practise mindfulness through movement. Forms of yoga that incorporate breathing exercises and directed meditative focus work to still the mind and focus attention (Bock 2012).
How the intervention might work
Mindfulness‐based interventions may aid smoking cessation by teaching individuals to pay attention to, and work mindfully with, negative affective states, cravings, and other symptoms of nicotine withdrawal as they arise, rather than habitually reacting to these unpleasant states by smoking. Proposed mechanisms of action include attention regulation, body awareness, emotion regulation, and change in self‐perspective (Hölzel 2011).
Withdrawal following smoking cessation is acutely associated with heightened levels of stress and negative affect (Shiffman 2004; West 2017). Once withdrawal symptoms have abated, cessation is generally associated with improved mental health (Taylor 2014; Taylor 2021), but early stage acute stress, negative affect, and depression are predictive of relapse (Correa‐Fernández 2012; Glassman 1990; Shiffman 2004; Shiffman 2005). Therefore, interventions that work to reduce these adverse emotional consequences of stopping smoking may enhance quit rates and ultimately prevent relapse. Mindfulness‐based interventions have shown some efficacy in the treatment of psychiatric disorders relating to or involving these negative affective states (Goyal 2014; Marchand 2013).
Further, by teaching smokers to focus their attention on what is happening in the moment, mindfulness‐based interventions bring habitual behaviours into consciousness. This enables people to understand the associative learning process, and focus on affect and craving as central components of positive and negative reinforcement loops (Brewer 2010). By emphasising the transience of affective states and teaching smokers to ‘sit with’ negative affect and craving, mindfulness interventions target and modify learned responses to smoking cues. This may help smokers to quit and may reduce cigarette consumption among those who do not stop smoking completely.
Thus, it has been suggested that mindfulness‐based treatments “may have the relative advantage of teaching a single technique that may lead to the dampening and eventual dismantling of the complex interrelated associative processes of smoking rather than just removing stimuli that might propagate them” (Brewer 2011).
Why it is important to do this review
If found to be effective, mindfulness‐based interventions could add an innovative intervention option to the range of treatments for smoking cessation. A systematic review, including literature to 2016, did not find evidence of a significant impact of mindfulness meditation interventions on abstinence relative to comparator groups (Maglione 2017). However, the evidence identified was of low certainty due to the high levels of heterogeneity and imprecision detected through meta‐analysis. Therefore, there is a need to update this review to include new evidence, in an effort to increase the certainty of the resulting conclusions. In addition, expanding the search to include other interventions that incorporate mindfulness approaches but do not specifically include an element of meditation (e.g. ACT) can add to our understanding of the potential effectiveness of mindfulness for smoking cessation.
The purpose of the present review is to assess the effect of interventions that incorporate mindfulness approaches for smoking cessation, using the robust methodology of Cochrane and the Cochrane Tobacco Addiction Group. This review also represents part of a separate project to evaluate similarities and differences between the standard methodological processes of the Cochrane Tobacco Addiction Group and a novel, machine‐learning approach developed by the Human Behaviour Change Project (Michie 2013).
Objectives
To assess the efficacy of mindfulness‐based interventions for smoking cessation among people who smoke, and whether these interventions have an effect on mental health outcomes.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs) and cluster‐RCTs that measured smoking cessation at least six months from baseline were eligible for this review. We included studies reported as full text, those published as abstract only, and unpublished data, where available. There were no language or date restrictions.
Types of participants
We included current tobacco smokers of any age who were willing to enrol in a smoking cessation study. We excluded studies that only recruited pregnant women, as their particular needs and circumstances warrant their treatment as a separate population, and these are covered in a separate Cochrane Review (Chamberlain 2017).
Types of interventions
We included interventions targeted at tobacco smoking cessation that were either labelled as mindfulness, or involved a mindfulness‐based approach that could be isolated to investigate effectiveness. There were no restrictions on the minimum duration of the intervention. Where a potentially relevant study intervention was not specifically described as being mindfulness‐based, we discussed as a team (of EN, JLB, NL, SJ) whether it was eligible for inclusion. We intentionally adopted an inclusive approach, including interventions that incorporated mindfulness (e.g. ACT or yoga) in addition to those specifically focused on mindfulness meditation (e.g. MBSR or MBCT) to capture the broadest evidence.
Eligible studies had to include at least one of the following comparison (control) interventions:
no smoking cessation treatment;
another smoking cessation intervention, of any length or intensity (including usual care);
another type of mindfulness intervention (e.g. mindfulness of a lower intensity).
Types of outcome measures
Primary outcomes
Smoking abstinence at longest follow‐up
To be eligible for inclusion, studies must have measured abstinence at least six months from the start of the intervention. Following the Cochrane Tobacco Addiction Group's standard methods, we excluded studies that only measured abstinence at less than six‐months' follow‐up.
In studies with more than one measure of abstinence, we preferred the measure with the strictest criteria, in line with the Russell Standard (West 2005). We used prolonged or continuous abstinence in preference to point prevalence abstinence, and preferred biochemically validated abstinence (e.g. using exhaled carbon monoxide or cotinine measures) over self‐report. We favoured biochemically validated point prevalence abstinence over self‐reported continuous or prolonged abstinence.
Mental health and well‐being
This could provide us with information on potential benefits or harms of the mindfulness‐based interventions. Even if comparisons of mindfulness‐based interventions with other smoking cessation interventions do not find a benefit of mindfulness for smoking cessation, improved mental well‐being could be a reason for choosing this treatment over another. We assessed validated measures of the following relevant constructs:
depression;
anxiety;
quality of life;
positive affect;
negative affect;
stress.
We extracted data on these mental health and well‐being outcomes, measured at the longest follow‐up at which abstinence was reported, or as close to this as possible.
Search methods for identification of studies
Electronic searches
We searched the following databases for studies that referred to mindfulness techniques in the title or abstract, or as keywords:
Cochrane Tobacco Addiction Group Specialised Register via the Cochrane Register of Studies (CRS‐Web)
Cochrane Central Register of Controlled Trials (CENTRAL; 2021, issue 3) via CRS‐Web
MEDLINE Ovid (1946 to 15 April 2021)
Embase Ovid (1974 to 15 April 2021)
PsycINFO Ovid (1806 to 15 April 2021)
We searched all databases from inception through to 15 April 2021. At the time of the search, the Register included the results of searches of MEDLINE (via OVID) to update 20210407; Embase (via OVID) to week 202114; PsycINFO (via OVID) to update 20210329. See the Tobacco Addiction Group website for details of the search strategies for these databases. Search strategies are shown in Appendix 1.
By searching CENTRAL and the Cochrane Tobacco Addiction Group’s register, we were able to identify any ongoing studies registered in the World Health Organization's portal (www.who.int/trialsearch) or ClinicalTrials.gov in the USA, and studies reported in Annual Meeting abstracts for the Society for Research on Nicotine and Tobacco (SRNT). We listed in the Characteristics of ongoing studies table any studies that may be candidates for inclusion (i.e. RCTs of smoking cessation interventions using mindfulness‐based approaches with a minimum follow‐up of six months), but for which results are not yet available.
Searching other resources
We checked reference lists of eligible published papers to identify any other relevant papers that may not have been identified by our search, and consulted experts in the field to identify any relevant forthcoming or unpublished research. We contacted the authors of ongoing studies where necessary.
Alongside these manual search strategies, we employed an automated search strategy developed as part of the Human Behaviour Change Project (Michie 2017), using Microsoft Academic. The Human Behaviour Change Project aims to improve upon the human ability to synthesise, interpret and deliver evidence on behaviour change interventions, using Natural Language Processing and Machine Learning technologies to automate the extraction, synthesis, and interpretation of findings from behaviour change intervention evaluation reports. We added any additional studies identified through this method to those found via the manual search, so that we included all relevant evidence. An evaluation comparing these manual and automated methods of study identification will be reported in a separate paper.
Data collection and analysis
Selection of studies
Two review authors (of EN, JLB, NL, SJ), independently checked the titles and abstracts of retrieved studies for relevance, and acquired full study reports of those that may be candidates for inclusion. The review authors resolved any disagreements by mutual consent, or by recourse to a third review author. Two review authors (of EN, JLB, NL, SJ) then independently assessed the full texts for eligibility, resolving any disagreements through discussion and with involvement of a third review author when necessary. We classified as 'exclude' any studies for which we obtained full reports, but that did not meet the inclusion criteria.
Data extraction and management
Two review authors (of EH, EN, JLB, NL, SJ) independently extracted study data and compared their findings. We resolved any disagreements through discussion, involving a third review author where necessary. Where available, we recorded the following information in the Characteristics of included studies table.
Methods: study design, study name (if applicable), study dates, country, number of study centres, study setting, study recruitment procedure
Participants: number (intervention/control), definition of smoker used, specific demographic characteristics (e.g. mean age, age range, gender, ethnicity, socioeconomic status (SES)), mean cigarettes per day, mean Fagerstrom Test for Nicotine Dependence (FTND), relevant inclusion and exclusion criteria
Interventions: description of intervention(s) (details of behavioural support and any pharmacological treatment provided), description of control (details of behavioural support and any pharmacological treatment provided), what comparisons will be constructed between which groups
Outcomes: relevant primary and secondary outcomes measured, time points reported, biochemical validation, definitions of abstinence, mental health measures used, proportion of participants with follow‐up data
Details of any within‐study analyses of moderators of interest: population type; baseline motivation to quit; baseline mental health
Notes: funding for study, and conflicts of interest statements of study authors (extracted verbatim)
Alongside this data extraction of entities that are typically captured in smoking cessation Cochrane Reviews, we also performed data extraction using entities of the Behaviour Change Intervention Ontology, which is being developed as part of the Human Behaviour Change Project (Michie 2017). The ontology consists of granular entities to specify all aspects of behaviour change interventions, such as:
an intervention’s context (including 'setting' (Norris 2020) and 'population');
content (including 'behaviour change techniques'; (Michie 2013)); and
delivery (including 'mode of delivery': how an intervention is provided to participants (Marques 2021); 'source': who delivers interventions (Norris 2021); and 'schedule': how often an intervention is delivered (Michie 2017)).
An evaluation to compare these methods of data extraction will be reported in a separate paper.
Assessment of risk of bias in included studies
Two review authors (of JLB, NL, SJ) independently assessed the risk of bias for each included study. We used RoB 1, following the guidance as set out in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
We assessed the following domains (Higgins 2011):
sequence generation;
allocation concealment,
blinding of outcome assessment;
incomplete outcome data;
selective reporting; and
other sources of bias.
As we were investigating a primarily behavioural intervention, we did not assess the blinding of participants and providers, as it is impossible to blind people to behavioural interventions. This is in accordance with specific guidance from the Cochrane Tobacco Addiction Group.
Each review author recorded information in study reports relevant to each domain and then assessed each domain as either at low, high, or unclear risk of bias. We resolved disagreements by discussion with a third review author. We considered studies to be at high overall risk of bias where we judged at least one domain to be at high risk; at low overall risk of bias where all domains were judged to be at low risk; and at unclear overall risk of bias in all other cases.
Measures of treatment effect
We compared quit rates between intervention and comparator groups for each study. We calculated quit rates on an intention‐to‐treat basis, including all participants originally randomised to a study arm, treating participants lost to follow‐up as relapsed. We calculated a risk ratio (RR) and 95% confidence interval (CI) for each study. We calculated the RR for each study as: (number of participants who reported smoking abstinence in the intervention group/number of participants randomised to the intervention group)/(number of participants who reported smoking abstinence in the control (comparator) group/number of participants randomised to the control (comparator) group).
Due to high levels of variance between studies in interventions and comparators, and in the measurement of mental health and well‐being outcomes, we narratively reported relevant measures of mental health and well‐being.
Unit of analysis issues
The one included cluster‐RCT did not present an analysis adjusting for the clustering effect or report an intracluster correlation coefficient (ICC). Therefore, we used unadjusted data for the primary analysis and performed a sensitivity analysis where we estimated the ICC (0.03), based on the ICC reported in other smoking cessation studies (Fanshawe 2017), and adjusted the analysis on this basis.
In the case of studies with multiple intervention arms, we analysed individual arms separately.
Dealing with missing data
For smoking abstinence, we assumed participants lost to follow‐up to be smoking, as is standard in the field (West 2005). However, we conducted a sensitivity analysis, excluding numbers lost to follow‐up from the denominator.
Assessment of heterogeneity
In order to assess whether it was appropriate to pool studies and conduct meta‐analyses, we assessed the characteristics of included studies to identify any clinical or methodological variance between studies. If we deemed the studies to be homogeneous enough to be combined meaningfully and we could conduct meta‐analyses, we assessed statistical heterogeneity using the I2 statistic (Higgins 2003). We considered an I2 statistic over 50% to indicate moderate to substantial heterogeneity (Deeks 2021). Where the I2 statistic was 80% or more, the direction of individual study effects differed, and heterogeneity was not fully explained by subgroup and sensitivity analyses, we do not report a pooled estimate because it could be misleading. We conducted the subgroup and sensitivity analyses described below to investigate any potential causes of observed heterogeneity.
Assessment of reporting biases
It was not appropriate to assess reporting bias using funnel plots as none of our analyses pooled 10 or more studies.
Data synthesis
We provided a narrative summary of the included studies and, where appropriate, conducted meta‐analyses.
The primary outcome of abstinence provides dichotomous data, therefore, as per the Cochrane Tobacco Addiction Group's standard methods, we combined RRs from individual studies using random‐effects, Mantel‐Haenszel methods, to calculate pooled overall RRs with 95% CIs.
Meaures of our mental health and well‐being outcome typically provided continuous data. Data were too heterogeneous to carry out meta‐analyses, so we tabulated the existing information and summarised narratively.
We also narratively reported the results of any within‐study analyses that have investigated the following moderators of effectiveness at at least six months' follow‐up:
population type;
baseline motivation to quit;
baseline mental health.
Subgroup analysis and investigation of heterogeneity
We carried out subgroup analyses, categorising studies by the type/intensity of control treatment received and mode of intervention delivery. We compared pooled summary statistics across groups and ran statistical tests for subgroup differences.
Sensitivity analysis
For smoking abstinence, we tested the impact of excluding studies deemed to be at overall high risk of bias and compared abstinence rates calculated assuming 'missing equals smoking' with abstinence rates calculated through complete‐case analysis. We also carried out the sensitivity analysis reported above, using an assumed ICC to adjust for potential clustering effects in a cluster‐RCT.
Summary of findings and assessment of the certainty of the evidence
Following standard Cochrane methodology (Schünemann 2021), we created summary of findings tables for smoking abstinence, and mental health and well‐being outcomes, detailing different intervention types in separate tables (mindfulness training; ACT; distress tolerance training; yoga). Also following standard Cochrane methodology (Schünemann 2021), we used the five GRADE considerations (risk of bias, inconsistency, imprecision, indirectness, and publication bias) to assess the certainty of the body of evidence for each outcome, within each comparison, and to draw conclusions about the certainty of evidence within the text of the review.
Results
Description of studies
Results of the search
Our bibliographic database searches and automated search process identified 2900 non‐duplicate records (Figure 1). We screened all records and retrieved the full‐text papers of 166 potentially relevant articles. After screening and checking the full texts, we identified 57 reports relating to 27 studies. Of these, 21 were completed studies (see Characteristics of included studies table) and six were ongoing studies (see Characteristics of ongoing studies table). We were unable to classify one study because the follow‐up period was unclear (see Characteristics of studies awaiting classification table).
1.
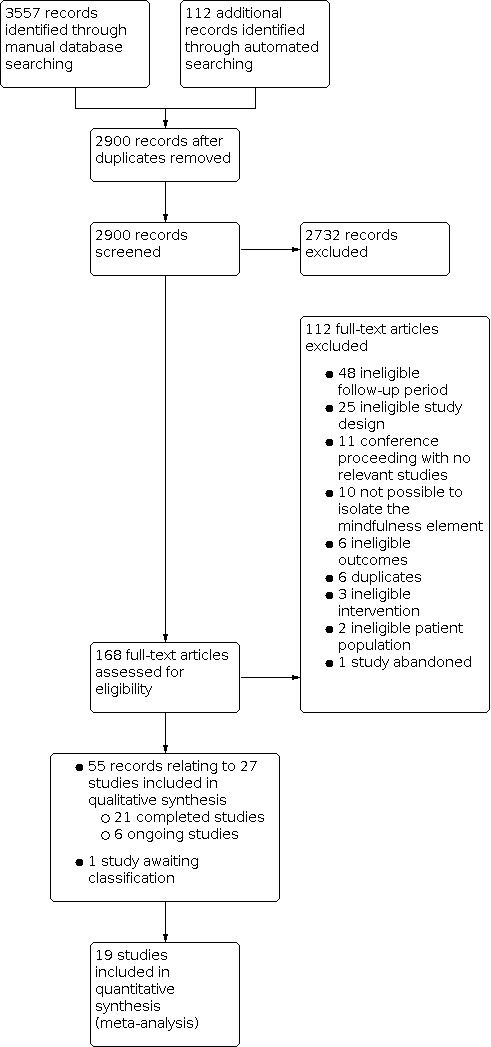
Figure 1: PRISMA flow diagram
Included studies
In total, we included 21 completed studies (Bloom 2020; Bock 2012; Bock 2019; Bricker 2014a; Bricker 2018; Bricker 2020; Brown 2013; Davis 2014a; Davis 2014b; de Souza 2020; Garrison 2020; Gaskins 2015; Gifford 2003; Mak 2020; McClure 2020; O'Connor 2020; Pbert 2020; Savvides 2014; Singh 2014; Vidrine 2016; Weng 2021). Key features of the included studies are summarised below.
Context and participants
Studies were conducted in the USA (15 studies), Hong Kong (2 studies), Brazil (1 study), Ireland (1 study), and Cyprus (1 study). One study (Singh 2014) did not report its location. Participants were recruited from the community (12 studies), online (3 studies), from healthcare centres (2 studies), high schools and universities (2 studies), tobacco treatment services (1 study), and workplaces (1 study). One study was a cluster‐RCT (Pbert 2020), which randomised high schools to different conditions. All other studies were randomised at the individual level.
The total number of participants across studies was 8186. The median sample size was 146 but ranged from 38 to 2637 participants. Two studies deliberately targeted young adults (Pbert 2020; Savvides 2014), two studies low SES smokers (Davis 2014a; Davis 2014b), one study uninsured smokers (Bricker 2014a), one study smokers with a history of early lapse (never able to remain abstinent for more than 72 hours; Brown 2013), and one study adults with mild intellectual disability (Singh 2014). Most studies had similar proportions of men and women or slightly more women than men. The exceptions were Bloom 2020, Bock 2012 and Weng 2021, which targeted only women; Gaskins 2015, which targeted only men; Singh 2014, which recruited 82% men with mild intellectual disability; and Mak 2020, which was conducted in Hong Kong where smoking prevalence among women is low and recruited 71% men.
Studies typically recruited people who smoked at least five cigarettes a day. Although some studies included lighter smokers as well, the average number smoked was over 15 a day in most studies, ranging from five a day in Pbert 2020's sample of high school students to 22 a day in Brown 2013's community sample.
Intervention programmes
Mindfulness training
Eight studies used mindfulness training (which, for the purpose of this review, we define as specific training in mindfulness and mindfulness‐based meditation techniques).
Five studies tested the effectiveness of mindfulness training delivered face‐to‐face. Davis 2014b compared seven weeks of group mindfulness training and meditation practice with an alternative, intensity‐matched, behavioural support programme. Similarly, Vidrine 2016 compared mindfulness‐based addiction treatment (8 x 2‐hour sessions) with an intensity‐matched CBT programme. In the latter study, there was also a second, less intensive comparator arm, in which participants received briefer support intended to represent the intervention a smoker might typically receive if they asked a healthcare provider for help (4 x 5‐ to 10‐minute sessions). All participants received self‐help materials. The other three studies compared mindfulness training with less intensive comparators. Davis 2014a compared an eight‐week mindfulness and meditation training programme with quitline support. Singh 2014 compared mindfulness and meditation training for adults with mild intellectual disability with treatment as usual, which varied between participants and encompassed a range of treatments such as behaviour therapies, nicotine replacement therapy (NRT), and other medications. Weng 2021 provided women in workplaces with self‐help materials and compared the effectiveness of additional mindfulness and meditation training (2 x 2‐hour sessions), with brief advice to follow the advice of the self‐help materials. Provision of pharmacotherapy varied between studies: three studies (Davis 2014a; Davis 2014b; Vidrine 2016), provided participants in both arms with a course of nicotine patches, one study provided no pharmacotherapy (Weng 2021), and one study (Singh 2014), did not specifically provide pharmacotherapy to participants in either arm, although for some comparator arm participants it was part of their usual treatment.
Two studies tested the effectiveness of mindfulness training delivered via smartphone apps. Garrison 2020 was conducted online. It compared a mobile mindfulness training app plus experience sampling (which asked participants to check in 6 times a day for 22 days) with experience sampling only. Pbert 2020 was a cluster‐RCT conducted in high schools. It tested the effectiveness of a mindfulness smartphone app designed for teens against two comparators: firstly, an alternative (non‐mindfulness) smoking cessation app designed for teens and secondly, self‐help materials. Participants in each of the three arms met with the school nurse weekly for four weeks. No pharmacotherapy was provided in either study.
de Souza 2020 was the only study to focus on mindfulness for relapse prevention. All participants received CBT over two phases: a smoking cessation phase (weekly sessions over 4 weeks) and a maintenance phase (6 sessions between weeks 6 and 48). The intervention arm also received eight mindfulness‐based relapse prevention sessions during the maintenance phase. Participants were offered the choice of NRT or bupropion.
Behaviour‐change techniques (BCTs) varied across studies. The most commonly used techniques included body changes (7 studies), problem solving (4 studies), self‐monitoring of behaviour (4 studies), pharmacological support (4 studies), and goal setting (3 studies), with no clear patterning in the number or type of BCTs used across mode of delivery.
Acceptance and commitment therapy (ACT)
Eight studies used ACT.
Three studies tested the effectiveness of ACT delivered exclusively face‐to‐face. McClure 2020 compared a five‐week, group ACT programme with a five‐week, group CBT programme. The two arms were matched for the number and duration of sessions. Participants in both arms were provided with eight weeks of nicotine patches. Gifford 2003 compared a seven‐week programme of individual and group ACT sessions (with no pharmacotherapy) with a lower‐intensity comparator group that received a seven‐week course of nicotine patches. O'Connor 2020 compared six weeks of face‐to‐face, group ACT sessions with six weeks of face‐to‐face, group behavioural support, matched in intensity to the six‐week, face‐to‐face ACT programme. No pharmacotherapy was provided.
One study tested the effectiveness of ACT delivered through a combination of face‐to‐face sessions and a smartphone app. In addition to the face‐to‐face‐only intervention arm, O'Connor 2020 included a second intervention arm in which ACT was delivered via two modalities: the six‐week, face‐to‐face ACT programme and an ACT‐based smartphone app. This combined ACT intervention was compared with a less intensive ACT arm (i.e. 6 weeks of face‐to‐face ACT without the app).
One study tested the effectiveness of ACT delivered exclusively via smartphone app. Bricker 2020 compared an ACT smartphone app with a smoking cessation app based on national clinical practice guidelines. No pharmacotherapy was provided.
One study tested the effectiveness of ACT delivered through a combination of face‐to‐face sessions and telephone calls. Mak 2020 compared ACT delivered in one face‐to‐face session and two follow‐up telephone calls with brief advice (5 minutes). Participants in both arms were also provided with self‐help materials. No pharmacotherapy was provided.
One study tested the effectiveness of ACT delivered exclusively via telephone. Bricker 2014a compared an ACT programme delivered over five telephone calls with standard quitline CBT. The arms were matched for the number and duration of telephone calls. Participants were provided with two weeks of nicotine patches or gum.
Two studies tested the effectiveness of ACT delivered via websites. Bricker 2018 compared an online ACT programme with a national standard online quit programme, with both arms receiving daily messages prompting them to log in. Savvides 2014 compared an avatar‐led, internet‐based ACT programme with a waitlist control. Neither study provided participants with pharmacotherapy.
BCTs varied across studies. The most commonly used techniques included problem solving (6 studies), body changes (5 studies), goal setting (4 studies), and action planning (4 studies), with no clear patterning in the number or type of BCTs used across mode of delivery.
Distress tolerance training
Two studies used distress tolerance training. Distress tolerance training interventions combined elements drawn from ACT with exposure‐based treatment. Exposure included periods of scheduled abstinence prior to sessions and exposure to cues within sessions, allowing ACT skills to be practised within the sessions in response to internal triggers.
Bloom 2020 targeted women who were concerned about post‐cessation weight gain. The intervention was nine weeks of CBT plus distress tolerance training ‐ a face‐to‐face and telephone programme that targeted the fear of anticipated post‐cessation weight gain and facilitated initiation of abstinence, and appetite awareness and mindful eating skills to reduce post‐cessation emotional eating. The comparator was nine weeks of CBT plus smoking health education, which mentioned diet and exercise as strategies for health promotion but did not specifically recommend changing diet or increasing physical activity to prevent post‐cessation weight gain.
Brown 2013 targeted smokers who had previously tried to quit but had never been able to remain abstinent for more than 72 hours. The intervention was eight weeks of face‐to‐face distress tolerance treatment and the comparator was six weeks of standard treatment.
Both studies also provided NRT (8 weeks of nicotine patches) to all participants in the intervention and comparator arms.
BCTs varied across studies: while both used pharmacological support, Brown 2013 used reduce prompts/cues and Bloom 2020 used problem solving, self‐monitoring of behaviour, social support, information about health consequences, and anticipated regret.
Yoga
Three studies used yoga involving a mindfulness‐based approach.
Two studies used Vinyasa yoga (Bock 2012; Gaskins 2015), and one used Iyengar yoga (Bock 2019). In each study, participants in the intervention arm were provided with eight CBT classes and 16 yoga classes over eight weeks. Participants in the comparator arm received CBT and wellness classes over eight weeks. In Bock 2012 and Bock 2019, the comparator was matched to the intervention in terms of the number and duration of wellness classes (16 x 1‐hour classes). However, in Gaskins 2015 the comparator was less intensive than the intervention: the intervention arm received 16 yoga classes over the eight weeks, each lasting 60 to 90 minutes, while the comparator arm received eight brief wellness discussions following the CBT sessions.
None of the studies provided participants with pharmacotherapy, but two studies (Bock 2012; Bock 2019), noted that participants were permitted to use NRT or other medications alongside the programme if they wanted to.
BCTs were similar across studies: all three studies used goal setting, problem solving, and social support. Bock 2012 and Gaskins 2015 also used self‐monitoring of behaviour and body changes, and Gaskins 2015 also used reduce negative emotions.
Outcomes
Smoking abstinence
The included studies provided a range of smoking abstinence outcome measures. Two studies reported the strictest outcome as biochemically verified continuous abstinence (Bock 2019; Davis 2014a), 12 studies defined abstinence as biochemically verified, seven‐day point prevalence (Bloom 2020; Bock 2012; Brown 2013; Davis 2014b; Garrison 2020; Gaskins 2015; Gifford 2003; Mak 2020; O'Connor 2020; Pbert 2020; Vidrine 2016; Weng 2021), one study as biochemically verified, 30‐day point prevalence (McClure 2020), and one study as carbon monoxide less than 10 parts per million (de Souza 2020).
Four additional studies reported self‐reported continuous abstinence (Bricker 2020), self‐reported seven‐day point prevalence (Singh 2014), or self‐reported 30‐day point prevalence (Bricker 2014a; Bricker 2018), without biochemical verification.
Most studies had a maximum follow‐up duration of six months, but six studies collected their final follow‐up data at 12 months (Bricker 2018; Bricker 2020; Gifford 2003; Mak 2020; McClure 2020; Singh 2014). Savvides 2014 reported collecting data on seven‐day and 30‐day point prevalence abstinence at six and 12 months but at the time this report was published data collection was ongoing and the only smoking abstinence outcomes reported are from immediately post‐treatment; we have not been able to find long‐term outcome data reported elsewhere.
Mental health
Ten of the included studies reported collecting data on mental health and well‐being (Bloom 2020; Bock 2012; Brown 2013; Davis 2014a; Davis 2014b; de Souza 2020; Gaskins 2015; Gifford 2003; O'Connor 2020; Vidrine 2016), of which nine analysed and reported on changes in these outcomes. Mental health outcomes included depression, anxiety, perceived stress, and negative and positive affect. The constructs assessed, measures used, and follow‐up durations varied across studies.
Excluded studies
Figure 1 shows the most common reasons for exclusion of studies during full‐text screening, which included: a follow‐up period of less than six months; ineligible study design (not an RCT); conference proceedings with no relevant studies; and an intervention where it was not possible to isolate the effects of the mindfulness element.
In the Characteristics of excluded studies table, we list exclusion reasons for 47 studies. This list is not comprehensive, only containing studies that a reader might plausibly expect to be included.
Risk of bias in included studies
Overall, we judged four of the 21 completed studies to be at low risk of bias, nine studies to be at unclear risk, and the remaining eight studies at high risk of bias.
Details of risk of bias judgments for each domain of each included study can be found in the Characteristics of included studies table. Figure 2 illustrates judgments for each included study.
2.

Figure 2: risk of bias summary
Allocation
Random sequence generation
We judged one study (Singh 2014), to be at high risk of selection bias for sequence generation, because randomisation was via alternate placement in the experimental and control groups. We judged eight studies at low risk of bias (Bock 2019; Bricker 2014a; Bricker 2018; Bricker 2020; Gifford 2003; Mak 2020; O'Connor 2020; Savvides 2014). The risk of bias for the remaining studies was unclear.
Allocation concealment
We judged six studies (Bricker 2014a; Bricker 2018; Bricker 2020; Mak 2020; O'Connor 2020; Weng 2021), to be at low risk of selection bias for allocation concealment, and the remainder to be at unclear risk as there was insufficient information with which to judge.
Blinding
Blinding of participants and personnel (performance bias)
As we were investigating a primarily behavioural intervention, we did not assess the blinding of participants and providers, as it is impossible to blind people to behavioural interventions. This is in accordance with specific guidance from the Cochrane Tobacco Addiction Group.
Blinding of outcome assessment (detection bias)
We rated three studies (Mak 2020; Savvides 2014; Singh 2014), at high risk for detection bias. These studies did not use blinding, they provided different levels of support, and outcomes were self‐reported. This meant we thought there was a high risk of bias being introduced. We judged the remaining studies to be at low risk for detection bias.
Incomplete outcome data
We judged most studies (13 out of 21) to be at low risk of attrition bias. We rated four studies with substantial (> 50%) loss to follow‐up and one study with more than a 20% difference in follow‐up rates between arms at high risk of attrition bias (Davis 2014a; Davis 2014b; de Souza 2020; Gaskins 2015; Mak 2020). The remaining three studies (Bock 2012; Savvides 2014; Singh 2014), did not provide sufficient data on which to judge, and hence we judged them to be at unclear risk.
Selective reporting
Of the 21 studies, we considered 13 to be at low risk of reporting bias, as they reported all prespecified or expected outcomes. We rated two studies (Bock 2012; de Souza 2020), at high risk, as they did not present data as specified in the original protocols. We judged the rest (Bloom 2020; Brown 2013; Gifford 2003; Mak 2020; Savvides 2014; Singh 2014), to be at unclear risk, as we were unable to identify a protocol.
Other potential sources of bias
We judged one study (Savvides 2014), to be at high risk of other bias because it used a waitlist control. This design risks participants in the control arm delaying quitting, knowing that they would be receiving an intervention at a later date. This has the potential to inflate the reported effect of the intervention. We did not find any other studies to be at risk of other bias.
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4
Summary of findings 1. Mindfulness training compared with control for smoking cessation.
| Mindfulness training compared with control for smoking cessation | ||||||
| Patient or population: people who smoke Setting: community; online; tobacco treatment services; high schools; workplaces (USA; Brazil; Hong Kong) Intervention: mindfulness training; mindfulness‐based relapse prevention Comparisons: matched‐intensity smoking cessation treatment; less intensive smoking cessation treatment; no treatment | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with control | Risk with mindfulness training | |||||
| Mindfulness training vs matched‐intensity smoking cessation treatment: smoking cessation (≥ 6‐month follow‐up) | Study population | RR 0.99 (0.67 to 1.46) | 542 (3 RCTs) | ⊕⊕⊝⊝ Lowa,b | ||
| 16 per 100 | 16 per 100 (11 to 24) | |||||
| Mindfulness training vs less intensive smoking cessation treatment: smoking cessation (≥ 6‐month follow‐up) | Study population | RR 1.19 (0.65 to 2.19) | 813 (5 RCTs) | ⊕⊝⊝⊝ Very lowc,d,e | ||
| 11 per 100 | 14 per 100 (7 to 25) | |||||
| Mindfulness training vs no treatment: smoking cessation (≥ 6‐month follow‐up) | Study population | RR 0.81 (0.43 to 1.53) | 325 (1 RCT) | ⊕⊕⊝⊝ Lowf | ||
| 12 per 100 | 10 per 100 (5 to 18) | |||||
| Mindfulness‐based relapse prevention vs no treatment: smoking cessation (≥ 6‐month follow‐up) | Study population | RR 1.43 (0.56 to 3.67) | 86 (1 RCT) | ⊕⊝⊝⊝ Very lowg,h | ||
| 14 per 100 | 20 per 100 (8 to 52) | |||||
| Mental health and well‐being | Studies investigated a range of outcomes: anxiety, depression, negative affect, positive affect, stress. Although 1 study found a statistically significantly greater reduction in perceived stress in people who received mindfulness training compared with those who received a matched‐intensity smoking cessation treatment at 6‐month follow‐up, the other 2 studies found no clinically meaningful between‐group differences in change in mental health and well‐being measures. | 633 (3 RCTs) |
⊕⊝⊝⊝ Very lowi,j | We were unable to meta‐analyse these outcomes and therefore summarised them narratively. | ||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomised controlled trial; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aNot downgraded for risk of bias: we judged one of the three studies to be at high risk of bias, but excluding this study did not change the conclusion. bDowngraded by two levels due to imprecision: the overall number of events was very low (n = 87) and the confidence interval of the effect estimate incorporates clinically relevant potential benefit and harm of the intervention. cDowngraded by one level due to risk of bias: we judged two of the five studies to be at high risk of bias and removing these studies changed the direction of the effect estimate. dDowngraded by one level due to inconsistency: substantial unexplained heterogeneity (I² = 60%). eDowngraded by two levels due to imprecision: the overall number of events was low (n = 101) and the confidence interval of the effect estimate incorporates clinically relevant potential benefit and harm of the intervention. fDowngraded by two levels due to imprecision: the overall number of events was very low (n = 36) and the confidence interval of the effect estimate incorporates clinically relevant potential benefit and harm of the intervention. gDowngraded by two levels due to risk of bias: we judged the sole study to be at high risk of bias. hDowngraded by two levels due to imprecision: the overall number of events was very low (n = 15) and the confidence interval of the effect estimate incorporates clinically relevant potential benefit and harm of the intervention. iDowngraded by one level due to risk of bias: we judged two of the three studies to be at high risk of bias and one of these was the only study to report a meaningful difference in mental health between conditions. jDowngraded by two levels due to inconsistency: mental health and well‐being are measured using a range of different constructs, the interventions include both a standard cessation intervention and an intervention targeted at relapse prevention and the interpretation of results varies across studies.
Summary of findings 2. Acceptance and commitment therapy (ACT) compared with control for smoking cessation.
| Acceptance and commitment therapy (ACT) compared with control for smoking cessation | ||||||
| Patient or population: people who smoke Setting: community; online; primary care; high schools and universities (USA; Cyprus; Hong Kong; Ireland) Intervention: Acceptance and commitment therapy (ACT) Comparisons: matched‐intensity smoking cessation treatment; NRT; brief advice; less intensive ACT | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with control | Risk with ACT | |||||
| ACT vs matched‐intensity smoking cessation treatment: smoking cessation (≥ 6‐month follow‐up) | It was not appropriate to pool data across these studies because there was a high level of heterogeneity (I2 = 82%) and the result may be misleading. | 5723 (5 RCTs) |
⊕⊝⊝⊝ Very lowa,b,c | |||
| ACT vs NRT: smoking cessation (≥ 6‐month follow‐up) | Study population | RR 1.27 (0.53 to 3.02) | 102 (1 RCT) | ⊕⊕⊝⊝ Lowd | ||
| 15 per 100 | 19 per 100 (8 to 45) | |||||
| ACT vs brief advice: smoking cessation (≥ 6‐month follow‐up) | Study population | RR 1.27 (0.59 to 2.75) | 144 (1 RCT) | ⊕⊝⊝⊝ Very lowd,e | ||
| 14 per 100 | 17 per 100 (8 to 37) | |||||
| ACT vs less intensive ACT: smoking cessation (≥ 6‐month follow‐up) | Study population | RR 1.00 (0.50 to 2.01) | 100 (1 RCT) | ⊕⊕⊝⊝ Lowd | ||
| 24 per 100 | 24 per 100 (12 to 48) | |||||
| Mental health and well‐being | One study that compared ACT with NRT found no clinically meaningful difference in negative affect across conditions at all follow‐ups to 12 months. Another study that compared ACT with a matched‐intensity smoking cessation treatment and a less intensive ACT intervention found no clinically meaningful difference in positive mental health across conditions up to 6‐month follow‐up. |
252 (2 RCTs) |
⊕⊝⊝⊝ Very lowf,g | We were unable to meta‐analyse this outcome and therefore summarised narratively. | ||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). ACT: Acceptance and commitment therapy; CI: confidence interval; NRT: nicotine replacement therapy; RCT: randomised controlled trial; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded by two levels due to inconsistency: substantial heterogeneity was detected (I² = 82%). A subgroup analysis grouping by mode of delivery used explained a small amount of this, but substantial heterogeneity remained unexplained. bNot downgraded for indirectness. One study included only smokers without health insurance, but contributed just 17.3% of the weighted effect. cDowngraded by one level due to imprecision: the confidence interval of the effect estimate incorporates clinically relevant potential benefit and harm of the intervention. dDowngraded by two levels due to imprecision: the overall number of events was very low (< 25) and the confidence interval of the effect estimate incorporates clinically relevant potential benefit and harm of the intervention. eDowngraded by two levels due to risk of bias: we judged the sole study to be at high risk of bias. fDowngraded by two levels due to inconsistency: the constructs and measures of mental health used differed across studies, as well as the study comparators. gDowngraded by two levels due to imprecision: two small studies likely lacked sufficient statistical power to detect clinically meaningful effects.
Summary of findings 3. Distress tolerance training compared with control for smoking cessation.
| Distress tolerance training compared with control for smoking cessation | ||||||
| Patient or population: people who smoke Setting: community (USA) Intervention: distress tolerance training Comparisons: matched‐intensity smoking cessation treatment; less intensive smoking cessation treatment | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with control | Risk with distress tolerance training | |||||
| Distress tolerance training vs matched‐intensity smoking cessation treatment: smoking cessation (≥ 6‐month follow‐up) | Study population | RR 0.87 (0.26 to 2.98) | 69 (1 RCT) | ⊕⊕⊝⊝ Lowa | ||
| 14 per 100 | 12 per 100 (4 to 41) | |||||
| Distress tolerance training vs less intensive smoking cessation treatment: smoking cessation (≥ 6‐month follow‐up) | Study population | RR 1.63 (0.33 to 8.08) | 49 (1 RCT) | ⊕⊕⊝⊝ Lowa | ||
| 9 per 100 | 15 per 100 (3 to 73) | |||||
| Mental health and well‐being | One study that compared distress tolerance training with less intensive smoking cessation treatment found no clinically meaningful difference in negative affect at 4 weeks post‐quit. | 49 (1 RCT) | ⊕⊕⊝⊝ Lowb | We were unable to meta‐analyse this outcome and therefore summarised narratively. | ||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomised controlled trial; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded by two levels due to imprecision: the overall number of events was very low (< 10) and the confidence interval of the effect estimate incorporates clinically relevant potential benefit and harm of the intervention. bDowngraded by two levels due to imprecision: the overall number of participants was very low (< 50).
Summary of findings 4. Yoga compared with control for smoking cessation.
| Yoga compared with control for smoking cessation | ||||||
| Patient or population: people who smoke Setting: community (USA) Intervention: yoga Comparison: matched‐intensity smoking cessation treatment; less intensive smoking cessation treatment | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with control | Risk with yoga | |||||
| Yoga vs matched‐intensity smoking cessation treatment: smoking cessation (≥ 6‐month follow‐up) | Study population | RR 1.44 (0.40 to 5.16) | 55 (1 RCT) | ⊕⊝⊝⊝ Very lowa,b | ||
| 13 per 100 | 19 per 100 (5 to 67) | |||||
| Mental health and well‐being | One study compared yoga with matched‐intensity smoking cessation treatment and found no clinically meaningful difference in depression, anxiety, or general well‐being scores between conditions at 8‐week follow‐up after controlling for baseline scores. Another study compared yoga with less intensive smoking cessation treatment and found no clinically meaningful differences in the change in depression or anxiety scores by group up to 6‐month follow‐up. |
93 (2 RCTs) |
⊕⊝⊝⊝ Very lowc,d | We were unable to meta‐analyse this outcome and therefore summarised narratively. | ||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomised controlled trial; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded by two levels due to risk of bias: we judged the sole study to be at high risk of bias. bDowngraded by two levels due to imprecision: the overall number of events was very low (< 10) and the confidence interval of the effect estimate incorporates clinically relevant potential benefit and harm of the intervention. cDowngraded by two levels due to risk of bias: we judged both studies to be at high risk of bias. dDowngraded by two levels due to imprecision: two small studies likely lacked sufficient statistical power to detect clinically meaningful effects
Mindfulness training
Smoking abstinence
Three studies compared an intervention involving mindfulness training with an alternative smoking cessation treatment that was matched for intensity (Davis 2014b; Pbert 2020; Vidrine 2016). Pooled data showed no evidence of a benefit of mindfulness training, with a point estimate very close to the null (RR 0.99, 95% CI 0.67 to 1.46; I2 = 0%; 542 participants; low‐certainty evidence; Analysis 1.1). We judged Davis 2014b at high risk of bias, while the other two studies were at unclear risk. Removing the study at high risk of bias did not substantially change the interpretation of the results (RR 0.82, 95% CI 0.51 to 1.33; I2 = 0%; 2 studies, 407 participants; Analysis 5.1), nor did using complete‐case analysis (RR 1.05, 95% CI 0.69 to 1.59; I2 = 25%; 3 studies, 356 participants; Analysis 6.1). In each of these sensitivity analyses, there was only a minimal impact on the point estimate, with CIs still spanning both benefit (i.e. higher quit rates) and harm (i.e. lower quit rates). A subgroup analysis separating studies by mode of delivery showed no evidence of moderating the effect of mindfulness training interventions in comparison with alternative, matched‐intensity smoking cessation treatment (I2 = 0%). Pbert 2020 was a cluster‐RCT, so we conducted another sensitivity analysis adjusting for any potential clustering effect (assuming an ICC of 0.03); this did not substantially change the overall pooled result (RR 1.01, 95% CI 0.68 to 1.50; I2 = 0%; Analysis 7.1).
1.1. Analysis.

Comparison 1: Mindfulness training, Outcome 1: Mindfulness training vs matched‐intensity smoking cessation treatment
5.1. Analysis.

Comparison 5: Sensitivity analysis ‐ excluding studies at high risk of bias, Outcome 1: Mindfulness training vs matched‐intensity smoking cessation treatment
6.1. Analysis.

Comparison 6: Sensitivity analysis ‐ complete cases, Outcome 1: Mindfulness training vs matched‐intensity smoking cessation treatment
7.1. Analysis.

Comparison 7: Sensitivity analysis ‐ mindfulness training adjusting for clustering in Pbert 2020, Outcome 1: Mindfulness training vs matched‐intensity smoking cessation treatment
Five studies compared an intervention involving mindfulness training with a less intensive smoking cessation treatment (Davis 2014a; Pbert 2020; Singh 2014; Vidrine 2016; Weng 2021). Pooled data showed no evidence of a benefit of mindfulness training, with the CI spanning both benefit and harm of mindfulness training interventions in comparison with less intensive smoking cessation treatments (RR 1.19, 95% CI 0.65 to 2.19; I2 = 60%; 813 participants; very low‐certainty evidence; Analysis 1.2). We judged Davis 2014a and Singh 2014 at high risk of bias, while the other three studies were at unclear risk. Removing the studies at high risk of bias changed the direction of the effect estimate (from favouring mindfulness training to favouring the comparator) but the CI still spanned both benefit and harm so this did not substantially change the interpretation of the results (RR 0.81, 95% CI 0.50 to 1.33; I2 = 5%; 3 studies, 566 participants; Analysis 5.2). Using complete‐case analysis produced similar results to the main analysis (RR 1.08, 95% CI 0.53 to 2.16; I2 = 62%; 4 studies, 479 participants; Analysis 6.2). Subgroup analyses showed some evidence of moderation by type of comparator (I2 = 68%). While there was no evidence of a benefit of mindfulness training versus less intensive behavioural support (RR 1.31, 95% CI 0.75 to 2.30; I2 = 60%; 2 studies, 453 participants), brief advice (RR 0.47, 95% CI 0.18 to 1.23; 1 study, 213 participants), or self‐help materials (RR 0.75, 95% CI 0.28 to 2.00; 1 study, 96 participants), there was evidence of a benefit of mindfulness training versus mixed treatment (treatment as usual, which varied between participants and encompassed a range of treatments such as behaviour therapies, NRT, and other medications; RR 2.77, 95% CI 1.30 to 5.94; 1 study, 51 participants). However, we judged the latter study at high risk of bias and it had substantial imprecision. Adjusting for clustering in Pbert 2020 (assuming an ICC of 0.03) did not substantially change the overall pooled result (RR 1.22, 95% CI 0.66 to 2.26; I2 = 58%; Analysis 7.2) or the subgroup result for mindfulness training versus self‐help materials (RR 0.80, 95% CI 0.24 to 2.67).
1.2. Analysis.

Comparison 1: Mindfulness training, Outcome 2: Mindfulness training vs less intensive smoking cessation treatment
5.2. Analysis.

Comparison 5: Sensitivity analysis ‐ excluding studies at high risk of bias, Outcome 2: Mindfulness training vs less intensive smoking cessation treatment
6.2. Analysis.

Comparison 6: Sensitivity analysis ‐ complete cases, Outcome 2: Mindfulness training vs less intensive smoking cessation treatment
7.2. Analysis.

Comparison 7: Sensitivity analysis ‐ mindfulness training adjusting for clustering in Pbert 2020, Outcome 2: Mindfulness training vs less intensive smoking cessation treatment
One study compared an intervention involving mindfulness training with no treatment. Garrison 2020 showed no evidence of a benefit of mindfulness training, with the point estimate favouring no treatment over mindfulness training and the CI spanning both benefit and harm of mindfulness training compared with no treatment (RR 0.81, 95% CI 0.43 to 1.53; 325 participants; low‐certainty evidence; Analysis 1.3). We judged this study at unclear risk of bias. Using complete‐case analysis did not substantially change the interpretation of the results (RR 0.77, 95% CI 0.41 to 1.43; 247 participants; Analysis 6.3).
1.3. Analysis.

Comparison 1: Mindfulness training, Outcome 3: Mindfulness training vs no treatment
6.3. Analysis.

Comparison 6: Sensitivity analysis ‐ complete cases, Outcome 3: Mindfulness training vs no treatment
One study compared an intervention involving mindfulness‐based relapse prevention with no treatment. de Souza 2020's point estimate favoured mindfulness‐based relapse prevention over no treatment but there was substantial imprecision, meaning the result could indicate potential harm as well as considerable benefit (RR 1.43, 95% CI 0.56 to 3.67; 86 participants; very low‐certainty evidence; Analysis 1.4). We judged this study at high risk of bias. Using complete‐case analysis did not substantially change the interpretation of the results (RR 1.23, 95% CI 0.72 to 2.10; 20 participants; Analysis 6.4).
1.4. Analysis.

Comparison 1: Mindfulness training, Outcome 4: Mindfulness‐based relapse prevention vs no treatment
6.4. Analysis.

Comparison 6: Sensitivity analysis ‐ complete cases, Outcome 4: Mindfulness‐based relapse prevention vs no treatment
Mental health
Three studies that tested an intervention involving mindfulness training reported on mental health outcomes (Analysis 1.5; very low‐certainty evidence). One study showed evidence of a benefit of mindfulness training on mental health. Davis 2014b (135 participants) analysed perceived stress at six months post‐quit. They observed a statistically significantly greater reduction in perceived stress between baseline and six months in the intervention arm than the intensity‐matched control arm, but this difference was not statistically significant when analysed as intention‐to‐treat.
1.5. Analysis.
Comparison 1: Mindfulness training, Outcome 5: Mental health outcomes
| Mental health outcomes | ||||||
| Study | Depression | Anxiety | Quality of life | Stress | Negative affect | Other |
| Davis 2014a | Not assessed | Not assessed | Not assessed | Not assessed | Depression, Anxiety and Stress Scales (DASS): assessed at 1 month post‐baseline, data only reported for intervention arm | Not assessed |
| Davis 2014b | Not assessed | Not assessed | Not assessed | Perceived Stress Scale (PSS): assessed at 6 months post‐quit. Significantly greater reduction in intervention than control arm (P = 0.03) Intervention arm scored 16.2 at baseline and 13.0 at 6 months post‐quit (−3.2); comparator arm scored 17.5 at baseline and 16.9 at 6 months post‐quit (−0.6). This difference was not significant when analysed as intention to treat | Not assessed | Not assessed |
| de Souza 2020 | Hospital Anxiety and Depression Scale (HAD): assessed at 4 and 12 weeks. No difference across conditions in the change between 4 weeks and 12 weeks (F (3.421) = 17.549; P = 0.074). Intervention arm scored 6.4 at 4 weeks and 6.9 at 12 weeks (+0.5); comparator arm scored 7.6 at 4 weeks and 6.0 at 12 weeks (−1.6) | HAD: assessed at 4 and 12 weeks. No difference across conditions in the change between 4 weeks and 12 weeks (F (0.352) = 1.668; P = 0.558). Intervention arm scored 10.4 at 4 weeks and 8.9 at 12 weeks (−1.4); comparator arm scored 9.5 at 4 weeks and 8.7 at 12 weeks (−0.8) | Not assessed | Not assessed | Positive and Negative Affect Scale (PANAS): assessed at 4 and 12 weeks. No difference across conditions in the change between 4 weeks and 12 weeks (F (0.015) = 0.278; P = 0.903). Intervention arm scored 20.3 at 4 weeks and 19.1 at 12 weeks (−1.2); comparator arm scored 19.2 at 4 weeks and 17.7 at 12 weeks (−1.4) | Positive affect ‐ PANAS: assessed at 4 and 12 weeks. No difference across conditions in the change between 4 weeks and 12 weeks (F (0.904) = 14.395; P = 0.350). Intervention arm scored 25.9 at 4 weeks and 27.2 at 12 weeks (+1.2); comparator arm scored 25.3 at 4 weeks and 24.6 at 12 weeks (−0.7) |
| Vidrine 2016 | Center for Epidemiologic Studies ‐ Depression Scale (CES‐D): assessed 6 times between quit date and 6 months post‐quit. No significant difference in the change between quit date and 6 months post‐quit between intervention arm and either control arm (CBT: β 0.03, 95% CI −0.16 to 0.22, P = 0.762; usual care: β −0.18, 95% CI −0.40 to 0.03, P = 0.099) | Not assessed | Not assessed | 4‐item Perceived Stress Scale ‐ Short Form (PSS‐SF): assessed 6 times between quit date and 6 months post‐quit. No significant difference in the change between quit date and 6 months post‐quit between intervention arm and either control arm (CBT: β 0.09, 95% CI −0.10 to 0.28, P = 0.362; usual care: β −0.16, 95% CI −0.38 to 0.05, P = 0.141) | PANAS: assessed 6 times between quit date and 6 months post‐quit. No significant difference in the change between quit date and 6 months post‐quit between intervention arm and either control arm (CBT: β 0.08, 95% CI ‐0.11 to 0.27, p = 0.403; usual care: β ‐0.19, 95% CI ‐0.40 to 0.03, p = 0.086) | Positive affect ‐ PANAS: assessed 6 times between quit date and 6 months post‐quit. No significant difference in the change between quit date and 6 months post‐quit between intervention arm and either control arm (CBT: β ‐0.09, 95% CI ‐0.29 to 0.10, p = 0.337; usual care: β 0.09, 95% CI ‐0.13 to 0.30, p = 0.444) |
Two studies showed no clear evidence of a benefit of mindfulness training on mental health. de Souza 2020 (86 participants) analysed depression, anxiety, negative affect, and positive affect at 4 and 12 weeks. No statistically significant or clinically meaningful difference between conditions was observed for any outcome at either time point. Vidrine 2016 (412 participants) assessed depression, perceived stress, negative affect, and positive affect at six time points between quit date and six months post‐quit. They analysed changes between quit date and six months and observed no statistically significant or clinically meaningful difference between conditions for any outcome.
In addition, Davis 2014a (196 participants) assessed negative affect at one month post‐baseline, but only reported data for the intervention arm.
Acceptance and commitment therapy (ACT)
Smoking abstinence
Five studies compared an intervention involving ACT with an alternative smoking cessation treatment that was matched for intensity (Bricker 2014a; Bricker 2018; Bricker 2020; McClure 2020; O'Connor 2020). We judged McClure 2020 to be at unclear risk of bias, while the other four studies were at low risk. It was not appropriate to pool data across these five studies because there was a high level of heterogeneity (I2 = 82%; Analysis 2.1), with variation in the direction of effect between studies, and the result may be misleading. Subgroup analyses showed some evidence of moderation by mode of delivery (I2 = 82%), although this didn't account for all variation within subgroups. While there was no evidence of a benefit of ACT delivered face‐to‐face (RR 0.76, 95% CI 0.32 to 1.78; I2 = 68%; 2 studies, 550 participants), by telephone (RR 1.35, 95% CI 0.74 to 2.46; 1 study, 121 participants), or via a website (RR 0.91, 95% CI 0.79 to 1.05; 1 study, 2637 participants), there was evidence of a benefit of ACT delivered via smartphone app (RR 1.77, 95% CI 1.32 to 2.37; 1 study, 2415 participants). Using complete‐case analysis did not substantially change the interpretation of results.
2.1. Analysis.

Comparison 2: Acceptance and commitment therapy (ACT), Outcome 1: ACT vs matched‐intensity smoking cessation treatment
One study compared an intervention involving ACT with NRT. Gifford 2003's point estimate favoured ACT over NRT but there was substantial imprecision, meaning the result could indicate potential harm as well as considerable benefit (RR 1.27, 95% CI 0.53 to 3.02; 102 participants; low‐certainty evidence; Analysis 2.2). We judged this study to be at unclear risk of bias. Using complete‐case analysis increased the point estimate but did not substantially change the interpretation of the results (RR 1.63, 95% CI 0.71 to 3.72; 71 participants; Analysis 6.6).
2.2. Analysis.

Comparison 2: Acceptance and commitment therapy (ACT), Outcome 2: ACT vs NRT
6.6. Analysis.

Comparison 6: Sensitivity analysis ‐ complete cases, Outcome 6: ACT vs NRT
One study compared an intervention involving ACT with brief advice. Mak 2020's point estimate favoured ACT over brief advice but there was substantial imprecision, meaning the result could indicate potential harm as well as considerable benefit (RR 1.27, 95% CI 0.59 to 2.75; 144 participants; very low‐certainty evidence; Analysis 2.3). We judged this study to be at high risk of bias. Using complete‐case analysis reduced the point estimate but did not substantially change the interpretation of the result (RR 1.06, 95% CI 0.54 to 2.11; 66 participants; Analysis 6.7).
2.3. Analysis.

Comparison 2: Acceptance and commitment therapy (ACT), Outcome 3: ACT vs brief advice
6.7. Analysis.

Comparison 6: Sensitivity analysis ‐ complete cases, Outcome 7: ACT vs brief advice
One study compared an intervention involving ACT with less intensive ACT. O'Connor 2020 showed no evidence of a benefit of more intensive ACT, with the point estimate indicating no difference between more and less intensive ACT (RR 1.00, 95% CI 0.50 to 2.01; 100 participants; low‐certainty evidence; Analysis 2.4). We judged this study to be at low risk of bias. Using complete‐case analysis did not substantially change the interpretation of the result (RR 0.94, 95% CI 0.47 to 1.86; 91 participants; Analysis 6.8).
2.4. Analysis.

Comparison 2: Acceptance and commitment therapy (ACT), Outcome 4: ACT vs less intensive ACT
6.8. Analysis.

Comparison 6: Sensitivity analysis ‐ complete cases, Outcome 8: ACT vs less intensive ACT
Within‐study analyses of moderators of interest
Bricker 2018 tested for moderation of the effectiveness of ACT by baseline mental health (depression or anxiety) and commitment to quitting. Quit rates were not found to differ significantly according to these variables.
Gifford 2003 tested the effectiveness of ACT in a subsample of smokers who were highly dependent. While there was no significant difference between the ACT arm and comparator arm in the full sample, ACT was reported to be associated with better long‐term quitting outcomes among nicotine‐dependent participants.
Mental health
Two studies that tested an intervention involving ACT reported on mental health outcomes (Analysis 2.5; very low‐certainty evidence). Both studies showed no evidence of a benefit of ACT on mental health. Gifford 2003 (102 participants) analysed negative affect between conditions at post‐treatment, six months and 12 months and O'Connor 2020 (150 participants) analysed positive mental health at post‐treatment and six months. Neither study observed a statistically significant or clinically meaningful difference between conditions at any time point.
2.5. Analysis.
Comparison 2: Acceptance and commitment therapy (ACT), Outcome 5: Mental health outcomes
| Mental health outcomes | ||||||
| Study | Depression | Anxiety | Quality of life | Stress | Negative affect | Other |
| Gifford 2003 | Not assessed | Not assessed | Not assessed | Not assessed | Profile of Mood States (POMS): assessed at each follow‐up. No difference across conditions at post‐treatment (F (1.83) = 0.688, P = 0.409), 6 months (F (1.82) = 0.438, P = 0.510), or 12 months (F (1.65) = 0.080, P = 0.779) | Not assessed |
| O'Connor 2020 | Not assessed | Not assessed | Not assessed | Not assessed | Not assessed | Positive mental health ‐ Mental Health Continuum ‐ Short Form (MHC‐SF): assessed at post‐treatment and 6 months. No significant difference across conditions at post‐treatment (ACT face‐to‐face vs behavioural support: P = 0.491; ACT face‐to‐face + app vs ACT face‐to‐face: P = 0.865) or 6 months (ACT face‐to‐face vs behavioural support: P = 0.457; ACT face‐to‐face + app vs ACT face‐to‐face: P = 0.990) |
Distress tolerance training interventions
Smoking abstinence
One study compared an intervention involving distress tolerance training with an alternative smoking cessation treatment that was matched for intensity. Bloom 2020 showed no evidence of a benefit of distress tolerance training, with the 95% CI spanning both benefit and harm (RR 0.87, 95% CI 0.26 to 2.98; 69 participants; low‐certainty evidence; Analysis 3.1). We judged this study to be at unclear risk of bias. Using complete‐case analysis did not substantially change interpretation of the result (RR 0.86, 95% CI 0.26 to 2.86; 54 participants; Analysis 6.9).
3.1. Analysis.

Comparison 3: Distress tolerance, Outcome 1: Distress tolerance vs matched‐intensity smoking cessation treatment
6.9. Analysis.

Comparison 6: Sensitivity analysis ‐ complete cases, Outcome 9: Distress tolerance vs matched‐intensity smoking cessation treatment
One study compared a distress tolerance training intervention with a less intensive smoking cessation treatment (Brown 2013). There was substantial imprecision, meaning the result could indicate potential harm as well as considerable benefit (RR 1.63, 95% CI 0.33 to 8.08; 49 participants; low‐certainty evidence; Analysis 3.2). We judged this study to be at unclear risk of bias. Using complete‐case analysis did not substantially change the interpretation of the result (RR 1.68, 95% CI 0.34 to 8.28; 46 participants; Analysis 6.10).
3.2. Analysis.

Comparison 3: Distress tolerance, Outcome 2: Distress tolerance vs less intensive smoking cessation treatment
6.10. Analysis.

Comparison 6: Sensitivity analysis ‐ complete cases, Outcome 10: Distress tolerance vs less intensive smoking cessation treatment
Mental health
One study that tested an intervention involving distress tolerance training reported on a mental health outcome (Analysis 3.3; low‐certainty evidence). Brown 2013 (49 participants) analysed negative affect at four weeks post‐quit and observed no statistically significant or clinically meaningful difference between conditions.
3.3. Analysis.
Comparison 3: Distress tolerance, Outcome 3: Mental health outcomes
| Mental health outcomes | ||||||
| Study | Depression | Anxiety | Quality of life | Stress | Negative affect | Other |
| Bloom 2020 | Patient Health Questionnaire‐9 (PHQ‐9): assessed at each follow‐up, data not reported | Not assessed | Not assessed | Not assessed | Positive and Negative Affect Scale (PANAS): assessed at each follow‐up, data not reported | Not assessed |
| Brown 2013 | Not assessed | Not assessed | Not assessed | Not assessed | Profile of Mood States (POMS): assessed at 4 weeks post‐quit. No difference across conditions. Intervention arm scored 46.2 at baseline and 45.7 at 4 weeks post‐baseline (−0.5); comparator arm scored 46.6 at baseline and 45.5 at 4 weeks post‐baseline (−1.1) | Not assessed |
In addition, Bloom 2020 (69 participants) planned to assess depression and negative affect at each follow‐up (1 month, 3 months, and 6 months post‐treatment), but to our knowledge have not reported these data.
Yoga
Smoking abstinence
One study compared an intervention involving yoga with an alternative smoking cessation treatment that was matched for intensity. Bock 2012's point estimate favoured yoga over alternative smoking cessation treatment but there was substantial imprecision, meaning the result could indicate potential harm as well as considerable benefit (RR 1.44, 95% CI 0.40 to 5.16; 55 participants; very low‐certainty evidence; Analysis 4.1). We judged this study to be at high risk of bias. The number of participants followed up in each arm was unclear so we could not conduct a complete‐case analysis.
4.1. Analysis.

Comparison 4: Yoga, Outcome 1: Yoga vs matched‐intensity smoking cessation treatment
Raw data on the number of quits at six months were not available for two other studies that tested an intervention involving yoga so we could not calculate unadjusted RRs. Bock 2019 reported no significant difference in the odds of smoking abstinence between the intervention arm and matched comparator arm at six‐month follow‐up (P > 0.05). Gaskins 2015 also reported no significant difference in the odds of smoking abstinence between the intervention arm and less intensive comparator arm at six‐month follow‐up (OR 2.38, 95% CI 0.52 to 10.8, P = 0.265). There was substantial imprecision, meaning the result could indicate potential harm as well as considerable benefit.
Mental health
Two studies that tested a yoga intervention reported on mental health outcomes (Analysis 4.2). Both studies showed no evidence of a benefit of yoga on mental health. Bock 2012 (55 participants) analysed depression, anxiety, and general well‐being at eight weeks (post‐treatment) and six months, but only reported data collected at 8 weeks. No statistically significant or clinically meaningful differences between conditions were observed. Gaskins 2015 (38 participants) analysed depression, anxiety, and a composite measure of physical self‐worth, attractiveness, physical strength, and condition, at eight weeks (post‐treatment), three months and six months. No statistically significant or clinically meaningful differences between conditions, nor any significant group by time interactions, were observed.
4.2. Analysis.
Comparison 4: Yoga, Outcome 2: Mental health outcomes
| Mental health outcomes | ||||||
| Study | Depression | Anxiety | Quality of life | Stress | Negative affect | Other |
| Bock 2012 | Center for Epidemiologic Studies ‐ Depression Scale (CES‐D): assessed at 6 months, data only reported for 8‐week follow‐up. No difference across conditions at 8 weeks, controlling for baseline scores (P = 0.86). Intervention arm scored 10.4 at baseline and 9.3 at 8 weeks post‐baseline (−1.1); comparator arm scored 8.8 at baseline and 8.8 at 8 weeks post‐baseline (0) | State‐Trait Anxiety Inventory (STAIT): assessed at 6 months, data only reported for 8‐week follow‐up. No difference across conditions at 8 weeks, controlling for baseline scores (P = 0.09). Intervention arm scored 43.6 at baseline and 39.4 at 8 weeks post‐baseline (−4.2); comparator arm scored 41.6 at baseline and 41.3 at 8 weeks post‐baseline (−0.3) | Not assessed | Not assessed | Not assessed | General wellbeing ‐ 36‐item Short Form Survey (SF‐36): assessed at 6 months, data only reported for 8‐week follow‐up. No difference across conditions at 8 weeks, controlling for baseline scores (P = 0.60). Intervention arm scored 48.8 at baseline and 52.8 at 8 weeks post‐baseline (+4.0); comparator arm scored 52.1 at baseline and 53.0 at 8 weeks post‐baseline (+0.9) |
| Gaskins 2015 | CES‐D: assessed at 8 weeks, 3 months, and 6 months. No significant differences by group or group by time interactions | STAIT: assessed at 8 weeks, 3 months, and 6 months. No significant differences by group or group by time interactions | Not assessed | Not assessed | Not assessed | Physical self‐worth, attractiveness, physical strength, and condition ‐ Physical Self Perception Profile Scale (PSPP): assessed at 8 weeks, 3 months, and 6 months. No significant differences by group or group by time interactions |
Discussion
Summary of main results
The 21 studies in this review did not detect a clear, long‐term benefit of mindfulness‐based smoking cessation interventions (based on mindfulness training, ACT, distress tolerance training, or yoga) when compared with other interventions, or with no intervention, for smoking cessation. This was true when mindfulness‐based interventions were compared with intensity‐matched smoking cessation interventions, less intensive smoking cessation interventions (including less intensive mindfulness), or no treatment. However, one subgroup analysis found a positive effect of an ACT intervention when this was delivered via smartphone application, as opposed to face‐to‐face, through a website, or over the telephone.
Ten studies collected data on mental health and well‐being, of which nine analysed and reported on changes in these outcomes. There was no clear evidence of a positive or negative effect of mindfulness‐based treatments on mental health and well‐being.
Overall completeness and applicability of evidence
The searches conducted for this review were broad, in our attempt to find any study that made any mention of mindfulness‐based approaches. As well as medical databases, we also searched studies registers to identify any ongoing or completed but unpublished registered studies, and supplemented our traditional search strategy with an automated search strategy developed as part of the Human Behaviour Change Project (Michie 2017), using Microsoft Academic. We therefore feel confident in our search approach.
A particular challenge of this review compared with other reviews of smoking cessation treatments was bringing together a diverse evidence base. The studies identified by this review varied widely in their design (e.g. digital versus in person), intervention type (e.g. ACT versus yoga), nature of the comparator, and mental health outcomes assessed, meaning we could not meaningfully pool results in a single meta‐analysis. While we intentionally adopted an inclusive approach to cover a broad range of mindfulness‐based interventions, some of the studies included may have had a looser mindfulness focus (e.g. yoga interventions) than others (e.g. mindfulness training interventions), but our approach to pooling meant that we did not 'dilute' the effects of pure mindfulness interventions. The studies identified in this review were mainly conducted in the USA and all took place in high‐income or higher middle‐income countries. Most studies were carried out in the general population and so may not be applicable to populations with specific requirements or particularly high cigarette dependence.
To be included studies had to assess long‐term abstinence, so most studies were able to contribute cessation data to the relevant comparisons. However, the number of studies and participants contributing to each analysis were low, and further research could strengthen or change findings. In addition, data on mental health outcomes were sparse and varied, meaning we were unable to conduct meta‐analyses for this outcome. We did not assess safety outcomes beyond any adverse effect on mental health and well‐being because the intervention was behavioural and was not considered high risk for adverse events.
Quality of the evidence
Of the 21 studies included in this review, we judged four to be at low risk of bias for all domains, and eight to be at high risk in one or more domains. In many cases, we rated studies at unclear risk of bias because they did not report key information. In these cases, it is impossible to know whether these studies were at any risk of bias or whether the information was simply not reported. To investigate the potential impact on results of studies that we judged to be at high risk of bias, we removed these studies in sensitivity analyses. This did not affect our interpretation of results.
We considered the certainty of the evidence for effectiveness of mindfulness training, ACT, distress tolerance training, and yoga interventions for smoking cessation relative to matched‐intensity smoking cessation treatment, less intensive smoking cessation treatment, or no treatment. We created summary of findings tables and carried out GRADE ratings for each comparison (Table 1; Table 2; Table 3; Table 4).
We judged all comparisons and outcomes to be of low or very low certainty, meaning that the interpretation of effects is likely to change as more studies and information become available. Reasons for downgrading the certainty of evidence included: risk of bias, when all studies pooled were judged at high risk of bias; inconsistency, when there was variance in the characteristics of studies or statistical heterogeneity was high and unexplained; and imprecision, when the absolute number of events was low or confidence intervals were wide and included no difference, or both.
Potential biases in the review process
We took several steps to ensure the review process was robust. We followed standard methods used by the Cochrane Tobacco Addiction Group. Our search strategy included a broad range of databases, including the Cochrane Tobacco Addiction Group's Specialised Register. We followed standard Cochrane practice of requiring two review authors to independently screen studies, extract data, and assess risk of bias. None of the authors of this review were also authors of included studies.
Despite this rigorous approach, it is possible that relevant literature, particularly unpublished or grey literature, may have been missed. We did not evaluate publication bias as there were fewer than 10 studies available for each primary outcome. It is also possible that non‐reporting of information in the published articles may have influenced the risk of bias assessments.
Agreements and disagreements with other studies or reviews
Carim‐Todd 2013 conducted a systematic review of yoga and other mind‐body interventions for smoking cessation. It included 14 studies, of which eight studies were RCTs, one study was a non‐RCT, two studies applied within‐participant controlled designs, and three studies used pre‐post designs. We included just one of these RCTs in our review (Bock 2012); the others did not meet our inclusion criterion of six months' follow‐up. Carim‐Todd 2013 did not meta‐analyse data due to differences in study designs, participants, and outcome measures. The authors reported that all 14 included studies, "observed changes in smoking behavior or in predictors of smoking behavior that could be beneficial for smoking cessation" but more clinical studies with larger sample sizes and carefully monitored interventions were required to draw firm conclusions.
Maglione 2017 conducted a meta‐analysis of mindfulness meditation interventions for smoking cessation. It included 10 RCTs: four mindfulness training studies that we included in the current review (Davis 2014a; Davis 2014b; Singh 2014; Vidrine 2016), and six studies that did not meet our inclusion criterion of six months' follow‐up. Pooled data from six of the 10 studies included by Maglione 2017 showed no significant effect of mindfulness meditation versus comparator interventions (odds ratio 2.52, 95% CI 0.76 to 8.29; I2 = 58%; 936 participants). This is consistent with our results.
Oikonomou 2017 conducted a meta‐analysis of mindfulness training interventions for smoking cessation compared with current behavioural treatments (the Freedom From Smoking programme and smoking quit line). It included four RCTs: two mindfulness training studies we included in the current review (Davis 2014a; Davis 2014b) and two studies that did not meet our inclusion criterion of six months' follow‐up. Pooled data from three of their four included studies showed significantly greater smoking abstinence rates in smokers who received mindfulness treatment compared to control at 17‐ to 24‐week follow‐up (RR 1.88, 95% CI 1.04 to 3.40; I2 = 44%; 419 participants). This differs from our results. The two studies in this analysis that we included in our review showed no benefit of mindfulness training (Davis 2014a; Davis 2014b). We excluded the third from our review because its longest follow‐up was just 17 weeks (Brewer 2011). Brewer 2011 accounts for the difference between the conclusions of Oikonomou 2017's review and our review. Brewer 2011's results indicated a strong benefit of mindfulness training, albeit with high levels of imprecision (RR 4.97, 95% CI 1.52 to 16.22; 88 participants).
Goldberg 2018 conducted a meta‐analysis of mindfulness‐based interventions for psychiatric disorders, including smoking as a form of substance use. It included seven RCTs that focused on smoking cessation, of which four compared a mindfulness‐based intervention with evidence‐based treatment. Three of these studies were included in the current review (Davis 2014a; Davis 2014b; Vidrine 2016), but one did not meet our inclusion criterion of six months' follow‐up. Pooled data from four of the seven included studies showed significantly greater smoking abstinence rates in smokers who received mindfulness treatment compared to control (d 0.42, 95% CI 0.20 to 0.64; I2 = 11%; 587 participants). Similar to Oikonomou 2017, this differs from our results because it included Brewer 2011, which showed a strong benefit of mindfulness on abstinence rates.
Authors' conclusions
Implications for practice.
We did not detect a clear benefit of mindfulness‐based interventions for increasing long‐term smoking quit rates compared with no treatment or alternative smoking cessation treatments that are equally or less intensive. However this evidence is of low or very low certainty, and further evidence is likely to change our conclusions.
We also did not detect a clear benefit of mindfulness‐based interventions for improving mental health and well‐being compared with no treatment or alternative smoking cessation treatments that are equally or less intensive. Again, this evidence is of low or very low certainty, and our conclusions are likely to change with further evidence.
Implications for research.
Further RCTs of mindfulness‐based interventions for smoking cessation are needed, following up participants at six months or longer. Studies with active comparators (i.e. comparing mindfulness‐based interventions to currently used smoking cessation interventions) are likely to be of particular use to decision makers.
Further studies need to be adequately powered to detect potentially small but clinically important differences between mindfulness‐based interventions and active comparators. In order to ensure low risk of bias, they should involve biochemical verification of abstinence along with improved methods of retaining participants to follow‐up points.
There is also a need for more consistent reporting of mental health and well‐being outcomes in studies of mindfulness‐based interventions for smoking cessation. Even if mindfulness is only as successful as other behavioural support in enhancing long‐term quit rates, it may be preferable to some smokers if it improves mental health. Most studies we identified did not report on mental health or well‐being. Those that did assessed a number of different constructs, at different time points, using a variety of measures, meaning we could not meaningfully pool the results. Therefore, it would be useful to develop a consensus on the best ways to measure these outcomes in relevant studies.
History
Protocol first published: Issue 7, 2020
Acknowledgements
This protocol was co‐authored by employees and editors of the Cochrane Tobacco Addiction Group, which receives infrastructure funding from the National Institute for Health Research (NIHR). The views and opinions expressed therein are those of the review authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, National Health Service (NHS) or the Department of Health.
We gratefully acknowledge peer review by Margaret A. Maglione (Adjunct Researcher), RAND Corporation and Dr Ana Howarth (Honorary Research Fellow), St George's, University of London; consumer review by Lee Bromhead; and editorial review by Dr Jamie Hartmann‐Boyce, University of Oxford.
Appendices
Appendix 1. Search strategies
Cochrane Tobacco Addiction Group Specialised Register
MESH DESCRIPTOR Mindfulness EXPLODE ALL
MESH DESCRIPTOR Meditation EXPLODE ALL
MESH DESCRIPTOR Mind‐Body Therapies EXPLODE ALL
MESH DESCRIPTOR Mind‐Body Relations, Metaphysical EXPLODE ALL
MESH DESCRIPTOR Breathing Exercises EXPLODE ALL
((meditat* OR mindful* OR relaxation* mind‐body OR body‐mind)):TI,AB,MH,EMT,KY,XKY
((Samadhi OR Samapatti)):TI,AB,MH,EMT,KY,XKY
((acceptance adj2 commitment)):TI,AB,MH,EMT,KY,XKY
#8 OR #7 OR #6 OR #5 OR #4 OR #3 OR #2 OR #1
CENTRAL
MESH DESCRIPTOR Mindfulness EXPLODE ALL
MESH DESCRIPTOR Meditation EXPLODE ALL
MESH DESCRIPTOR Mind‐Body Therapies EXPLODE ALL
MESH DESCRIPTOR Mind‐Body Relations, Metaphysical EXPLODE ALL
MESH DESCRIPTOR Breathing Exercises EXPLODE ALL
((meditat* OR mindful* OR relaxation* mind‐body OR body‐mind)):TI,AB,MH,EMT,KY,XKY
((Samadhi OR Samapatti)):TI,AB,MH,EMT,KY,XKY
((acceptance adj2 commitment)):TI,AB,MH,EMT,KY,XKY
#8 OR #7 OR #6 OR #5 OR #4 OR #3 OR #2 OR #1
MESH DESCRIPTOR Tobacco Use Disorder EXPLODE ALL NOT SRTAG
MESH DESCRIPTOR Tobacco Use Cessation EXPLODE ALL NOT SRTAG
MESH DESCRIPTOR Tobacco Smoke Pollution EXPLODE ALL NOT SRTAG
MESH DESCRIPTOR Tobacco Use Cessation Products EXPLODE ALL NOT SRTAG
MESH DESCRIPTOR Tobacco, Smokeless EXPLODE ALL NOT SRTAG
(SMOKING* or TOBACCO or TOBACCO‐USE‐DISORDER* or TOBACCO‐SMOKELESS* or TOBACCO‐SMOKE‐POLLUTION* or TOBACCO‐USE‐CESSATION* or NICOTINE*):MH NOT SRTAG
(smoking cessation):MH NOT SRTAG
(SMOKING CESSATION or ANTISMOK*):TI,AB NOT SRTAG
(quit* or smok* or nonsmok* or cigar* or tobacco* or nicotine*):TI NOT SRTAG
MESH DESCRIPTOR Smoking Cessation EXPLODE ALL NOT SRTAG
#10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19
#20 AND #9
MEDLINE/Embase/PsycINFO (in Ovid)
exp Smoking Cessation/
exp "Tobacco Use Disorder"/
(SMOKING* or TOBACCO or "TOBACCO USE DISORDER*" or "TOBACCO USE CESSATION*" or NICOTINE*).mp.
(SMOKING CESSATION or ANTISMOK*).ti,ab.
(quit* or smok* or nonsmok* or cigar* or tobacco* or nicotine*).ti.
smoking cessation.mp.
exp "Tobacco Use Cessation"/
1 or 2 or 3 or 4 or 5 or 6 or 7
exp mindfulness/
exp meditation/
exp "Mind Body Therapies"/
exp "Mind Body Relations, Metaphysical"/
exp Breathing Exercises/
(meditat* or mindful* or "relaxation* mind body" or "body mind").mp.
(Samadhi or Samapatti).mp.
(acceptance adj2 commitment).ti,ab.
9 or 10 or 11 or 12 or 13 or 14 or 15 or 16
8 and 17
Data and analyses
Comparison 1. Mindfulness training.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Mindfulness training vs matched‐intensity smoking cessation treatment | 3 | 542 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.67, 1.46] |
| 1.1.1 Face‐to‐face | 2 | 444 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.64, 1.72] |
| 1.1.2 Smartphone app | 1 | 98 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.29, 2.08] |
| 1.2 Mindfulness training vs less intensive smoking cessation treatment | 5 | 813 | Risk Ratio (M‐H, Random, 95% CI) | 1.19 [0.65, 2.19] |
| 1.2.1 vs. less intensive behavioural support | 2 | 453 | Risk Ratio (M‐H, Random, 95% CI) | 1.31 [0.75, 2.30] |
| 1.2.2 vs. brief advice | 1 | 213 | Risk Ratio (M‐H, Random, 95% CI) | 0.47 [0.18, 1.23] |
| 1.2.3 vs. self‐help materials | 1 | 96 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.28, 2.00] |
| 1.2.4 vs. mixed treatment | 1 | 51 | Risk Ratio (M‐H, Random, 95% CI) | 2.77 [1.30, 5.94] |
| 1.3 Mindfulness training vs no treatment | 1 | 325 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.43, 1.53] |
| 1.4 Mindfulness‐based relapse prevention vs no treatment | 1 | 86 | Risk Ratio (M‐H, Random, 95% CI) | 1.43 [0.56, 3.67] |
| 1.5 Mental health outcomes | 4 | Other data | No numeric data |
Comparison 2. Acceptance and commitment therapy (ACT).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 ACT vs matched‐intensity smoking cessation treatment | 5 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1.1 Face‐to‐face | 2 | 550 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.32, 1.78] |
| 2.1.2 Telephone | 1 | 121 | Risk Ratio (M‐H, Random, 95% CI) | 1.35 [0.74, 2.46] |
| 2.1.3 Website | 1 | 2637 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.79, 1.05] |
| 2.1.4 Smartphone app | 1 | 2415 | Risk Ratio (M‐H, Random, 95% CI) | 1.77 [1.32, 2.37] |
| 2.2 ACT vs NRT | 1 | 102 | Risk Ratio (M‐H, Random, 95% CI) | 1.27 [0.53, 3.02] |
| 2.3 ACT vs brief advice | 1 | 144 | Risk Ratio (M‐H, Random, 95% CI) | 1.27 [0.59, 2.75] |
| 2.4 ACT vs less intensive ACT | 1 | 100 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.50, 2.01] |
| 2.5 Mental health outcomes | 2 | Other data | No numeric data |
Comparison 3. Distress tolerance.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 Distress tolerance vs matched‐intensity smoking cessation treatment | 1 | 69 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.26, 2.98] |
| 3.2 Distress tolerance vs less intensive smoking cessation treatment | 1 | 49 | Risk Ratio (M‐H, Random, 95% CI) | 1.63 [0.33, 8.08] |
| 3.3 Mental health outcomes | 2 | Other data | No numeric data |
Comparison 4. Yoga.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 4.1 Yoga vs matched‐intensity smoking cessation treatment | 1 | 55 | Risk Ratio (M‐H, Random, 95% CI) | 1.44 [0.40, 5.16] |
| 4.2 Mental health outcomes | 2 | Other data | No numeric data |
Comparison 5. Sensitivity analysis ‐ excluding studies at high risk of bias.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 5.1 Mindfulness training vs matched‐intensity smoking cessation treatment | 2 | 407 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.51, 1.33] |
| 5.2 Mindfulness training vs less intensive smoking cessation treatment | 3 | 566 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.50, 1.33] |
| 5.2.1 vs. less intensive behavioural support | 1 | 257 | Risk Ratio (M‐H, Random, 95% CI) | 1.11 [0.57, 2.18] |
| 5.2.2 vs. brief advice | 1 | 213 | Risk Ratio (M‐H, Random, 95% CI) | 0.47 [0.18, 1.23] |
| 5.2.3 vs. self‐help materials | 1 | 96 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.28, 2.00] |
Comparison 6. Sensitivity analysis ‐ complete cases.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 6.1 Mindfulness training vs matched‐intensity smoking cessation treatment | 3 | 356 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.69, 1.59] |
| 6.2 Mindfulness training vs less intensive smoking cessation treatment | 4 | 479 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.53, 2.16] |
| 6.2.1 vs. less intensive behavioural support | 2 | 224 | Risk Ratio (M‐H, Random, 95% CI) | 1.72 [0.62, 4.80] |
| 6.2.2 vs. brief advice | 1 | 165 | Risk Ratio (M‐H, Random, 95% CI) | 0.50 [0.19, 1.29] |
| 6.2.3 vs. self‐help materials | 1 | 90 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.30, 2.08] |
| 6.3 Mindfulness training vs no treatment | 1 | 247 | Risk Ratio (M‐H, Random, 95% CI) | 0.77 [0.41, 1.43] |
| 6.4 Mindfulness‐based relapse prevention vs no treatment | 1 | 20 | Risk Ratio (M‐H, Random, 95% CI) | 1.23 [0.72, 2.10] |
| 6.5 ACT vs matched‐intensity smoking cessation treatment | 5 | 4537 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.70, 1.57] |
| 6.5.1 Face‐to‐face | 2 | 437 | Risk Ratio (M‐H, Random, 95% CI) | 0.71 [0.35, 1.44] |
| 6.5.2 Telephone | 1 | 81 | Risk Ratio (M‐H, Random, 95% CI) | 1.14 [0.66, 1.96] |
| 6.5.3 Website | 1 | 2309 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.81, 1.07] |
| 6.5.4 Smartphone app | 1 | 1710 | Risk Ratio (M‐H, Random, 95% CI) | 1.85 [1.39, 2.47] |
| 6.6 ACT vs NRT | 1 | 71 | Risk Ratio (M‐H, Random, 95% CI) | 1.63 [0.71, 3.72] |
| 6.7 ACT vs brief advice | 1 | 66 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.54, 2.11] |
| 6.8 ACT vs less intensive ACT | 1 | 91 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.47, 1.86] |
| 6.9 Distress tolerance vs matched‐intensity smoking cessation treatment | 1 | 54 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.26, 2.86] |
| 6.10 Distress tolerance vs less intensive smoking cessation treatment | 1 | 46 | Risk Ratio (M‐H, Random, 95% CI) | 1.68 [0.34, 8.28] |
6.5. Analysis.

Comparison 6: Sensitivity analysis ‐ complete cases, Outcome 5: ACT vs matched‐intensity smoking cessation treatment
Comparison 7. Sensitivity analysis ‐ mindfulness training adjusting for clustering in Pbert 2020.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 7.1 Mindfulness training vs matched‐intensity smoking cessation treatment | 3 | 501 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.68, 1.50] |
| 7.1.1 Face‐to‐face | 2 | 444 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.64, 1.72] |
| 7.1.2 Smartphone app | 1 | 57 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.25, 2.77] |
| 7.2 Mindfulness training vs less intensive smoking cessation treatment | 5 | 773 | Risk Ratio (M‐H, Random, 95% CI) | 1.22 [0.66, 2.26] |
| 7.2.1 vs. less intensive behavioural support | 2 | 453 | Risk Ratio (M‐H, Random, 95% CI) | 1.31 [0.75, 2.30] |
| 7.2.2 vs. brief advice | 1 | 213 | Risk Ratio (M‐H, Random, 95% CI) | 0.47 [0.18, 1.23] |
| 7.2.3 vs. self‐help materials | 1 | 56 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.24, 2.67] |
| 7.2.4 vs. mixed treatment | 1 | 51 | Risk Ratio (M‐H, Random, 95% CI) | 2.77 [1.30, 5.94] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Bloom 2020.
| Study characteristics | ||
| Methods |
Study design: RCT Location: USA Setting: community Recruitment: paper and electronic advertisements Study dates: not reported |
|
| Participants | N = 69 Specialist population?: women motivated to quit smoking and concerned about post‐cessation weight gain Definition of smoker used: ≥ 5 combustible cpd for at least the past year Participant characteristics: 100% female; average age: 50 years; 74% white; 42% SES; average cpd: 16; nicotine dependence: average FTND 4.8 |
|
| Interventions | All participants received CBT, including preparation for quit date, reinforcement, and support for quitting, discussion of past and ongoing quit experiences, initiation of self‐monitoring, identification of triggers and high‐risk situations, development of coping strategies for triggers, obtaining social support, instruction in how to use nicotine patches, and relapse prevention. Recommendations for how to minimise weight gain were brief, de‐emphasised, and consistent with standard CBT (e.g. take a walk to cope with craving instead of smoking, eat low‐calorie snacks) Comparator CBT + smoking health education. Diet and exercise were mentioned as strategies for health promotion and prevention of disease but no specific recommendations for how to change diet or increase physical activity Mode of delivery: face‐to‐face (individual and group), telephone Intensity: 9 sessions (1 x 60 min, 8 x 90 min) and 1 phone call (20 min) over 9 weeks Pharmacotherapy: 8 weeks of nicotine patches from quit date, adjusted to individual cpd Type of therapist/provider: each session was co‐led by 2 trained group leaders, including a physician, doctoral‐level psychologists, and clinical psychology doctoral students BCTs: 1.2 Problem solving; 2.3 Self‐monitoring of behaviour; 3.1 Social support; 5.1 Information about health consequences; 11.1 Pharmacological support Intervention CBT + distress tolerance for weight concern. Based on ACT and included: psychoeducation about the relationship between smoking and weight; distress tolerance skills; discussion of weight concern as a barrier to successful initiation of abstinence; and values‐oriented living skills targeting reduction of emotional eating after quitting Mode of delivery: face‐to‐face (individual and group), telephone Intensity: 9 sessions (1 x 60 min, 8 x 90 min) and 1 phone call (20 min) over 9 weeks Pharmacotherapy: 8 weeks of nicotine patches from quit date, adjusted to individual cpd Type of therapist/provider: each session was co‐led by 2 trained group leaders, including a physician, doctoral‐level psychologists, and clinical psychology doctoral students BCTs: 1.2 Problem solving; 2.3 Self‐monitoring of behaviour; 3.1 Social support; 5.1 Information about health consequences; 5.5 Anticipated regret; 11.1 Pharmacological support |
|
| Outcomes |
Definition of abstinence: 7‐day point prevalence Longest follow‐up: 6 months Biochemical verification: CO ≤ 6 ppm Other relevant outcomes reported: negative affect |
|
| Notes |
Relevant comparisons: distress tolerance for weight concern + CBT + NRT vs health education + CBT + NRT Funding source: National Institute on Drug Abuse (grant number K23DA035288) Author conflicts of interest: “RAB has equity ownership in Health behaviour Solutions, Inc., which is developing products for tobacco cessation that are not related to this study. The terms of this arrangement have been reviewed and approved by the University of Texas at Austin in accordance with its policy on objectivity in research. The other authors have no interests to declare.” |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stated randomised but method not specified |
| Allocation concealment (selection bias) | Unclear risk | Concealment not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Abstinence biochemically verified |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | At 6‐month follow‐up 21.2% (7/33) were lost to follow‐up in the intervention group and 22.2% (8/36) in the control group |
| Selective reporting (reporting bias) | Unclear risk | No protocol available |
Bock 2012.
| Study characteristics | ||
| Methods |
Study design: RCT Location: USA Setting: community Recruitment: advertisements in local newspapers, websites and television, flyers posted at physicians’ offices and stores Study dates: 2007‐10 |
|
| Participants | N = 55 Specialist population?: women Definition of smoker used: ≥ 5 cpd Participant characteristics: 100% female; average age: 4 years; 82% white; 35% college graduate; average cpd: 16; nicotine dependence: average FTND 5.0 |
|
| Interventions | All participants were provided with an 8‐week group CBT programme for smoking cessation, including quit day in week 2, self‐monitoring, stimulus control, coping with high‐risk situations, and stress management for smoking cessation. The programme also focused on topics of concern to women when quitting, including healthy eating, weight management, and balancing multiple roles and multiple demands. Comparator Group CBT + wellness classes Mode of delivery: face‐to‐face (group) Intensity: 8 CBT sessions (x 1 h) and 16 wellness classes (x 1 h) over 8 weeks Pharmacotherapy: none provided, but participants were permitted to use NRT or other smoking cessation medications in conjunction with the programme Type of therapist/provider: wellness: PhD psychologist; CBT: psychologist with > 10 years' experience in conducting smoking cessation groups BCTs: 1.1 Goal setting (behaviour); 1.2 Problem solving; 2.3 Self‐monitoring of behaviour; 3.1 Social support (unspecified) Intervention: Group CBT + Vinyasa yoga Mode of delivery: face‐to‐face (group) Intensity: 8 CBT sessions (x 1 h) and 16 yoga classes (x 1 h) over 8 weeks Pharmacotherapy: none provided, but participants were permitted to use NRT or other smoking cessation medications in conjunction with the programme Type of therapist/provider
BCTs: 1.1 Goal setting (behaviour); 1.2 Problem solving; 2.3 Self‐monitoring of behaviour; 3.1 Social support (unspecified); 12.6 Body changes |
|
| Outcomes |
Definition of abstinence: 7‐day point prevalence Longest follow‐up: 6 months Biochemical verification: salivary cotinine < 15 ng/mL Other relevant outcomes reported: depression, anxiety, general well‐being |
|
| Notes |
Relevant comparisons: Vinyasa yoga + CBT vs wellness classes + CBT Funding source: National Center for Complementary and Alternative Medicine (AT003669) Author conflicts of interest: “No competing financial interests exist for any of the authors.” |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stated randomised but method not specified |
| Allocation concealment (selection bias) | Unclear risk | Concealment not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Abstinence biochemically validated |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Attrition not reported |
| Selective reporting (reporting bias) | High risk | Mental health outcomes not reported in paper as specified in protocol |
Bock 2019.
| Study characteristics | ||
| Methods |
Study design: RCT Location: USA Setting: community Recruitment: advertisements on local radio stations and websites, flyers and brochures at physician’s offices and stores Study dates: not reported |
|
| Participants | N = 227 Specialist population?: no Definition of smoker used: ≥ 5 cpd Participant characteristics: 56% female; average age: 46 years; 86% white; 28% high school education or less; average cpd: 17; nicotine dependence: average FTND 4.9 |
|
| Interventions | All participants were provided with an 8‐week group CBT programme for smoking cessation, including planning for a targeted quit day (week 4), handling smoking triggers, coping with cravings, and managing withdrawal. Comparator Group CBT + wellness classes Mode of delivery: face‐to‐face (group) Intensity: 8 CBT sessions (x 1 h) and 16 wellness classes (x 1 h) over 8 weeks Pharmacotherapy: none provided, but participants were permitted to use NRT or other smoking cessation medications in conjunction with the programme Type of therapist/provider
BCTs: 1.1 Goal setting (behaviour), 1.2 Problem solving, 3.1 Social support, 12.6 Body changes Intervention Group CBT + Iyengar yoga Mode of delivery: face‐to‐face (group) Intensity: 8 CBT sessions (x 1 h) and 16 yoga classes (x 1 h) over 8 weeks Pharmacotherapy: none provided, but participants were permitted to use NRT or other smoking cessation medications in conjunction with the programme Type of therapist/provider
BCTs: 1.1 Goal setting (behaviour), 1.2 Problem solving; 3.1 Social support (unspecified) |
|
| Outcomes |
Definition of abstinence: continuous (based on 7‐day point prevalence at end of treatment and follow‐up) Longest follow‐up: 6 months Biochemical verification: CO < 10 ppm or salivary cotinine < 15 mg/mL Other relevant outcomes reported: none |
|
| Notes |
Relevant comparisons: Iyengar yoga + CBT vs wellness classes + CBT Funding source: National Institutes of Health, National Center for Complementary and Integrative Health (R01 AT006948) Author conflicts of interest: “The authors have no competing financial interests to declare.” |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote [protocol]: “The randomization scheme generated by the study statistician uses a permuted block randomization procedure, with small, random sized blocks. Randomization is stratified based on gender and level of nicotine dependence.” |
| Allocation concealment (selection bias) | Unclear risk | Concealment not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Abstinence biochemically verified |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | At 6‐month follow‐up 6.2% (7/113) were lost to follow‐up in the intervention group and 4.4% (5/114) in the control group |
| Selective reporting (reporting bias) | Low risk | Prespecified outcomes reported |
Bricker 2014a.
| Study characteristics | ||
| Methods |
Study design: RCT Location: USA Setting: community Recruitment: uninsured callers to the South Carolina State Quitline Study dates: 2012‐13 |
|
| Participants | N = 121 Specialist population?: uninsured Definition of smoker used: ≥ 10 cpd for at least the past 12 months Participant characteristics: 69% female; average age: 39 years; 73% white; 55% high school education or less; nicotine dependence: 71% HSI score ≥ 4; 39% positive depression screen |
|
| Interventions |
Comparator: standard CBT‐based counselling intervention offered through the South Carolina State Quitline Mode of delivery: telephone Intensity: 5 calls (1 x 30 min, 4 x 15 min) Pharmacotherapy: 2 weeks of nicotine patch or gum (participant’s choice) Type of therapist/provider: bachelor's‐ or master's‐level providers with ≥ 3 years of general counselling experience and > 100 h of training BCTs: 1.2 Problem solving, 1.4 Action planning, 3.1 Social support (unspecified), 11.1: Pharmacological support, 12.4 Distraction Intervention: ACT programme
Mode of delivery: telephone Intensity: 5 calls (1 x 30 min, 4 x 15 min) Pharmacotherapy: 2 weeks of nicotine patch or gum (participant’s choice) Type of therapist/provider: bachelor's‐ or master's‐level providers with ≥ 3 years of general counselling experience and > 100 h of training BCTs: 1.4 Action planning, 3.1 Social support (unspecified), 11.1: Pharmacological support |
|
| Outcomes |
Definition of abstinence: 30‐day point prevalence Longest follow‐up: 6 months Biochemical verification: none Other relevant outcomes reported: none |
|
| Notes |
Relevant comparisons: ACT (telephone) + NRT vs CBT (quitline) + NRT Funding source: National Institute on Drug Abuse (R21DA030646) Author conflicts of interest: “In 2011 Dr. Heffner served as a consultant for Pfizer.” |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised by automated algorithm |
| Allocation concealment (selection bias) | Low risk | Quote: “Randomized study arm assignments were computer generated and concealed from participants after eligibility was determined and consent for participation was obtained. Neither research staff nor participants had access to upcoming randomized study arm assignments” |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Abstinence self‐reported but no face‐to‐face contact so no difference in intensity; differential report unlikely |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | At 6‐month follow‐up 27.1% (16/59) were lost to follow‐up in the intervention group and 38.7% (24/62) in the control group |
| Selective reporting (reporting bias) | Low risk | Prespecified outcomes reported |
Bricker 2018.
| Study characteristics | ||
| Methods |
Study design: RCT Location: USA Setting: online Recruitment: targeted Facebook adverts, an online survey panel, search engine results, friends/family referral, Google ads, and earned media Study dates: 2014‐19 |
|
| Participants | N = 2637 Specialist population?: no Definition of smoker used: ≥ 5 cpd for the last year Participant characteristics: 79% female; average age: 46 years; 73% white; 28% high school education or less; nicotine dependence: average FTND 5.6; 56% current depressive symptoms; 34% current anxiety symptoms; 48% current panic disorder symptoms; 53% current PTSD symptoms; 30% current social anxiety symptoms |
|
| Interventions |
Comparator: Smokefree online quit programme available for 12 months Intensity: up to 4 daily messages (via text or email) designed to encourage logging in for 28 days after randomisation, unless they opted out Mode of delivery: website Pharmacotherapy: none Type of therapist/provider: N/A BCTs: 1.2 Problem solving, 1.4 Action planning, 5.1 Information about health consequences Intervention: WebQuit online ACT programme with attached online forum available for 12 months Intensity: up to 4 daily messages (via text or email) designed to encourage logging in for 28 days after randomisation, unless they opted out Mode of delivery: website Pharmacotherapy: none Type of therapist/provider: N/A BCTs: 1.4 Action planning, 2.3 Self‐monitoring of behaviour |
|
| Outcomes |
Definition of abstinence: 30‐day point prevalence Longest follow‐up: 12 months Biochemical verification: none Other relevant outcomes reported: none |
|
| Notes |
Relevant comparisons: ACT online programme (WebQuit) vs Smokefree online programme Funding source: National Cancer Institute (R01 CA166646; R01CA192849); National Institute on Drug Abuse (R01 DA038411) Author conflicts of interest: “In July 2016, Dr. Jonathan Bricker was a consultant to Glaxo Smith Kline, the makers of a nicotine replacement therapy. He now serves on the Scientific Advisory Board of Chrono Therapeutics, the makers of a nicotine replacement therapy device. Other authors have no declarations.” |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “Using randomly permuted block randomization, stratified by daily smoking frequency (≤20 vs. ≥21), education (≤ high school vs. ≥ some college), and gender (male vs. female).” |
| Allocation concealment (selection bias) | Low risk | Quote: “Random assignments were concealed from participants until after study eligibility, consent, and baseline data was obtained. Neither research staff nor study participants had access to upcoming randomized study arm assignments.” |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Abstinence self‐reported but no face‐to‐face contact so no difference in intensity; differential report unlikely |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | At 12‐month follow‐up 13.5% (178/1319) were lost to follow‐up in the intervention group and 11.4% (150/1318) in the control group |
| Selective reporting (reporting bias) | Low risk | Prespecified outcomes reported |
Bricker 2020.
| Study characteristics | ||
| Methods |
Study design: RCT Location: USA Setting: online Recruitment: nationally via Facebook ads, a survey sampling company, search engine results, and referral from friends and family Study dates: 2017‐19 |
|
| Participants | N = 2415 Specialist population?: no Definition of smoker used: ≥ 5 cpd for at least the past year Participant characteristics: 70% female; average age: 38 years; 69% white; 41% high school education or less; nicotine dependence: average FTND 5.9; 48.5% positive depression screen |
|
| Interventions |
Comparator: QuitGuide smartphone app including content on motivations to quit, preparing to quit (including quit plan, triggers, social support, and advice on pharmacotherapy), avoiding cravings, and remaining abstinent Mode of delivery: smartphone app Pharmacotherapy: none BCTs: 1.1 Goal setting (behaviour), 1.2 Problem solving, 3.1 Social support (unspecified), 5.1 Information about health consequences, 8.2 Behaviour substitution Intervention: iCanQuit smartphone app; ACT programme teaching skills for coping with urges, staying motivated and preventing relapse, and including advice on pharmacotherapy Mode of delivery: smartphone app Pharmacotherapy: none BCTs: 1.1 Goal setting (behaviour), 1.2 Problem solving, 2.3 Self‐monitoring of behaviour, 4.1 Instruction on how to perform behaviour (where to and what NRTs) |
|
| Outcomes |
Definition of abstinence: prolonged Longest follow‐up: 12 months Biochemical verification: none Other relevant outcomes reported: none |
|
| Notes |
Relevant comparisons: ACT app (iCanQuit) vs QuitGuide app Funding source: National Cancer Institute (R01CA192849) Author conflicts of interest: “Dr Bricker reported receiving grants from the National Cancer Institute during the conduct of the study; serving on the scientific advisory board for and receiving personal fees from Chrono Therapeutics outside the submitted work; and reported that the Fred Hutchinson Cancer Research Center has applied for a US patent that pertains to the content of the iCanQuit application. 2Morrow, Inc, a Kirkland, Washington–based software company, has licensed this technology from the Fred Hutchinson Cancer Research Center. Dr Bricker had no personal financial relationships with this patent application, the licensing agreement, or 2Morrow, Inc. Ms Mull reported receiving grants from the National Institutes of Health/National Cancer Institute during the conduct of the study. Dr Heffner reported receiving nonfinancial support from Pfizer outside the submitted work. None of the authors has a financial relationship with the iCanQuit application and thus will not receive any compensation when it becomes publicly available. No other disclosures were reported.” |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “The random allocation sequence was generated by a database manager and implemented automatically by the study website.” |
| Allocation concealment (selection bias) | Low risk | Quote: “Random assignments were concealed from participants throughout the trial. The random allocation sequence was generated by a database manager and implemented automatically by the study website. Neither research staff nor study participants had access to upcoming randomized study group assignments.” |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Abstinence self‐reported but no face‐to‐face contact so no difference in intensity; differential report unlikely |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | At 12‐month follow‐up 30.9% (375/1214) were lost to follow‐up in the intervention group and 27.5% (330/1201) in the control group |
| Selective reporting (reporting bias) | Low risk | Prespecified outcomes reported |
Brown 2013.
| Study characteristics | ||
| Methods |
Study design: RCT Location: USA Setting: unclear, recruitment from community Recruitment: newspaper and radio advertisements targeting smokers who had “previous difficulty quitting for even short periods of time” Study dates: not reported |
|
| Participants | N = 49 Specialist population?: smokers with a history of early lapse (< 72 h post‐quit) Definition of smoker used: ≥ 15 cpd for at least the past 3 years Participant characteristics: 49% female; average age: 48 years; 90% white; 33% high school education or less; average cpd: 22; nicotine dependence: average FTND 6.3; 29% one or more depressive episodes |
|
| Interventions |
Comparator: standard treatment covering self‐monitoring, identifying triggers, developing self‐management strategies for coping with triggers, and relapse prevention skills Mode of delivery: face‐to‐face (group), telephone Intensity: 6 face‐to‐face group sessions (x 90 min) and 1 phone call (20 min) over 6 weeks Pharmacotherapy: 8 weeks of nicotine patches from quit date (4 weeks x 21 mg, 2 x 14 mg, 2 x 7 mg) Type of therapist/provider: doctoral‐level psychologists or trainees (trained by senior investigators) BCTs: 1.2 Problem solving, 2.3 Self‐monitoring of behaviour, 8.2 Behaviour substitution; 11.1 Pharmacological support Intervention: distress tolerance treatment. This included exercises aimed at increasing participants’ tolerance of distress while maintaining a focus on the valued life goals associated with quitting smoking Mode of delivery: face‐to‐face (individual and group) Intensity: 6 individual sessions (x 50 min) and 9 group sessions (x 2 h) over 8 weeks Pharmacotherapy: 8 weeks of nicotine patches from quit date (4 weeks x 21 mg, 2 x 14 mg, 2 x 7 mg) Type of therapist/provider: doctoral‐level psychologists or trainees (trained by senior investigators) BCTs: 7.3 Reduce prompts/cues: 'nicotine fading', 11.1 pharmacological support |
|
| Outcomes |
Definition of abstinence: 7‐day point prevalence Longest follow‐up: 6 months Biochemical verification: CO ≤ 5 ppm and cotinine ≤ 10 ng/mL Other relevant outcomes reported: negative affect |
|
| Notes |
Relevant comparisons: distress tolerance training + NRT vs standard treatment + NRT Funding source: National Institute on Drug Abuse (DA017332) Author conflicts of interest: “None declared” |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not reported |
| Allocation concealment (selection bias) | Unclear risk | Concealment not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Abstinence biochemically verified |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | At 6‐month follow‐up 7.4% (2/27) were lost to follow‐up in the intervention group and 4.8% (1/21) in the control group |
| Selective reporting (reporting bias) | Unclear risk | No protocol available |
Davis 2014a.
| Study characteristics | ||
| Methods |
Study design: RCT Location: USA Setting: community Recruitment: advertisements on television, newspaper, and flyers, with ad placement in low‐SES neighbourhoods Study dates: 2010‐13 |
|
| Participants | N = 196 Specialist population?: moderately low SES Definition of smoker used: ≥ 5 cpd Participant characteristics: 50% female; average age: 42 years; 77% white; 49% high school education or less; average cpd: 16; average number of years smoked: 22 |
|
| Interventions |
Comparator: smoking cessation counselling through the Wisconsin Tobacco Quit Line Mode of delivery: telephone Intensity: encouraged to make repeated calls to the Quit Line, one visit to study centre to collect nicotine patches Pharmacotherapy: 4 weeks of nicotine patches (21 mg) from quit date Type of therapist/provider: not reported BCTs: 1.1 Goal setting (behaviour), 11.1 Pharmacological support Intervention: Mindfulness Training for Smokers (MTS) Mode of delivery: face‐to‐face (group), video Intensity: 10 sessions (total of 32 h) over 8 weeks Pharmacotherapy: 4 weeks of nicotine patches (21 mg) from quit date Type of therapist/provider: instructors with no formal addiction training, but with successful completion of the 2‐day MTS Instructor Training Course BCTs: 1.2 Problem solving, 3.1 Social support, 4.1 Instruction on how to perform behaviour, 11.1 Pharmacological support, 11.2 Reducing negative emotions, 12.6 Body changes |
|
| Outcomes |
Definition of abstinence: continuous Longest follow‐up: 6 months Biochemical verification: CO < 7 ppm Other relevant outcomes reported: none |
|
| Notes |
Relevant comparisons: mindfulness training + NRT vs quit line counselling + NRT Funding source: National Institute on Drug Abuse (K23DA022471) Author conflicts of interest: Not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient detail to make a judgment Quote: “Consented individuals were asked to complete baseline assessment visit (carbon monoxide (CO) breath testing and self‐report measures) and then undergo randomization via random draws to either the Control Group (Quit Line + 4 weeks Nicotine Replacement Therapy (NRT)) or MTS (MTS + 4 weeks NRT)” |
| Allocation concealment (selection bias) | Unclear risk | Concealment not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Abstinence biochemically verified |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Attrition was high in both groups: 78.1% (82/105) in the intervention group and 64.8% (59/91) in the control group |
| Selective reporting (reporting bias) | Low risk | Prespecified abstinence outcomes were reported. However, the depression, anxiety and stress outcome (DASS) was not reported for both arms |
Davis 2014b.
| Study characteristics | ||
| Methods |
Study design: RCT Location: USA Setting: community Recruitment: advertisements placed on television, newspaper, and flyers (targeted in low‐SES areas) Study dates: 2011‐12 |
|
| Participants | N = 135 Specialist population?: people living in low‐SES areas Definition of smoker used: ≥ 5 cpd, uses no other tobacco products Participant characteristics: 47% female; average age: 45 years; 88% white; 35% high school education or less; average cpd: 18; nicotine dependence: average FTND 4.8 |
|
| Interventions |
Comparator: American Lung Association's Freedom from Smoking (FFS) programme, enhanced to match the Mindfulness Training for Smokers (MTS) intervention more closely (FFS‐E) Mode of delivery: face‐to‐face (group), video, audio recording Intensity: 24 h over 7 weeks Pharmacotherapy: 2 weeks of nicotine patches from quit date Type of therapist/provider: master's degree in psychology, no specialised training or certification in addiction therapy (FFS instructors are typically laypeople), provided with a 2‐day FFS‐E teacher‐training course BCTs: 1.1 Goal setting (behaviour): set a quit plan, 11.1 Pharmacological support, 12.6 Body changes Intervention: Mindfulness Training for Smokers (MTS) Mode of delivery: face‐to‐face (group), video, audio recording Intensity: 7 classes (x 2.5 h) and a quit day retreat (6.5 h) over 7 weeks (total 24 h) Pharmacotherapy: 2 weeks of nicotine patches from quit date Type of therapist/provider: master's degree in psychology (except for one who had a PhD in Sociology), no specialised training or certification in addiction therapy, provided with a 2‐day MTS teacher‐training course BCTs: 1.2 Problem solving, 3.1 Social support (unspecified), 4.1 Instruction on how to perform behaviour, 11.1 Pharmacological support, 11.2 Reducing negative emotions, 12.6 Body changes |
|
| Outcomes |
Definition of abstinence: 7‐day point prevalence Longest follow‐up: 6 months Biochemical verification: CO < 7 ppm Other relevant outcomes reported: stress |
|
| Notes |
Relevant comparisons: mindfulness training + NRT vs Freedom From Smoking programme + NRT Funding source: National Institute on Drug Abuse (K23 DA022471) Author conflicts of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not reported |
| Allocation concealment (selection bias) | Unclear risk | Concealment not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Abstinence biochemically verified |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Attrition was high in both groups: 57.4% (39/68) in the intervention group and 55.2% (37/67) in the control group |
| Selective reporting (reporting bias) | Low risk | Prespecified outcomes reported |
de Souza 2020.
| Study characteristics | ||
| Methods |
Study design: RCT Location: Brazil Setting: outpatient public health tobacco treatment service Recruitment: by phone from a waiting list of an outpatient public health tobacco treatment service Study dates: 2012‐16 |
|
| Participants | N = 86 Specialist population?: no Definition of smoker used: ≥ 10 cpd Participant characteristics: 80% female; average age: 50 years; 85% high school education or less; 33.7% average cpd ≥ 20 |
|
| Interventions |
Comparator: standard treatment (CBT + maintenance sessions) Mode of delivery: unclear Intensity:
Pharmacotherapy: choice of NRT or bupropion Type of therapist/provider: physician with experience treating smoking and with training in the standard treatment approach BCTs: 5.1 Information about health consequences, 11.1 Pharmacological support Intervention: standard treatment (CBT + maintenance sessions) + mindfulness‐based relapse prevention (MBRP) Mode of delivery: group sessions, audio CD Intensity:
Pharmacotherapy: choice of NRT or bupropion Type of therapist/provider: certified MBRP instructor who had received MBRP training; physician with experience treating smoking and with training in the standard treatment approach BCTs: 1.2 Problem solving, 2.3 Self‐monitoring (behaviour), 5.1 Information about health consequences, 11.1 Pharmacological support, 12.6 Body changes |
|
| Outcomes |
Definition of abstinence: CO < 10 ppm Longest follow‐up: 6 months Biochemical verification: CO < 10 ppm Other relevant outcomes reported: depression, anxiety, positive and negative affect |
|
| Notes |
Relevant comparisons: mindfulness‐based relapse prevention + CBT + NRT/bupropion vs CBT + NRT/bupropion Funding source: Fundação de Amparo à Pesquisa do Estado de São Paulo (2013/02316‐5), Conselho Nacional de Desenvolvimento Científico e Tecnológico (870470/1997‐3), Fundação de Amparo à Pesquisa do Estado de Minas Gerais (APQ‐04279‐10) and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (552452/2011) Author conflicts of interest: “None declared.” |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not reported |
| Allocation concealment (selection bias) | Unclear risk | Concealment not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Abstinence biochemically verified |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Attrition was high in both groups: at 24‐week follow‐up 75.0% (33/44) were lost to follow‐up in the intervention group and 78.6% (33/42) in the control group |
| Selective reporting (reporting bias) | High risk | Some prespecified outcomes not reported (dependence, maintenance of abstinence at 12 months, change in smoking urges) |
Garrison 2020.
| Study characteristics | ||
| Methods |
Study design: RCT Location: USA Setting: online Recruitment: online ads on Google, Reddit and smokefree.gov website, social media posts, word of mouth, and blog posts Study dates: 2014‐16 |
|
| Participants | N = 325 Specialist population?: no Definition of smoker used: ≥ 5 cpd, ≤ 3 months past‐year abstinence Participant characteristics: 72% female; average age: 41 years; 81% white; 16% high school education or less; average cpd: 16 |
|
| Interventions |
Comparator: experience sampling (ES) only Mode of delivery: smartphone app Intensity: 22 days of check‐ins 6 x a day Pharmacotherapy: none BCTs: 2.3 Self‐monitoring of behaviour Intervention: mobile mindfulness training with experience sampling (MMT‐ES) Mode of delivery: smartphone app Intensity: 22 days of training modules (5–15 min/d) Pharmacotherapy: none BCTs: 2.3 Self‐monitoring of behaviour, 2.4 Self‐monitoring of outcome(s) of behaviour, 12.6 Body changes: body scan |
|
| Outcomes |
Definition of abstinence: 7‐day point prevalence Longest follow‐up: 6 months Biochemical verification: CO < 10 ppm verified by video by a blinded researcher Other relevant outcomes reported: none |
|
| Notes |
Relevant comparisons: mobile mindfulness training app + experience sampling vs experience sampling Funding source: American Heart Association [14CRP18200010] and National Institute on Drug Abuse grant [K12DA00167] Author conflicts of interest: “Judson A. Brewer and Prasanta Pal own stock in Claritas Mindsciences, the company that developed the apps used in this study. All other authors declare that they have no competing interests.” |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not reported |
| Allocation concealment (selection bias) | Unclear risk | Concealment not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Abstinence biochemically verified |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | At 6‐month follow‐up 21.7% (31/143) were lost to follow‐up in the intervention group and 25.4% (47/185) in the control group |
| Selective reporting (reporting bias) | Low risk | Prespecified abstinence outcomes were reported. However, 4‐item Perceived Stress Scale (PSS) was not reported |
Gaskins 2015.
| Study characteristics | ||
| Methods |
Study design: RCT Location: USA Setting: community Recruitment: advertisements on radio and local newspapers, flyers in local papers and posted at local venues (e.g. pharmacies, supermarkets), and brief descriptions of the study listed in guides published by local yoga studios Study dates: 2009‐11 |
|
| Participants | N = 38 Specialist population?: men Definition of smoker used: ≥ 5 cpd for the past year Participant characteristics: 0% female; average age: 40 years; 95% white; 11% less than high school education; 13% household income < USD 10,000; average cpd: 19; nicotine dependence: average FTND 4.8 |
|
| Interventions |
Comparator: CBT + wellness classes Mode of delivery: face‐to‐face (individual), written materials, video Intensity: 8 CBT sessions (x 30 min) each followed by a brief wellness discussion over 8 weeks Pharmacotherapy: none Type of therapist/provider: CBT: doctoral‐level counsellor; wellness: smoking counsellor BCTs: 1.1 Goal setting (behaviour), 1.2 Problem solving, 2.3 Self monitoring of behaviour, 3.1 Social support, 11.2 Reduce negative emotions Intervention: CBT + Vinyasa yoga Mode of delivery: face‐to‐face (individual CBT, group yoga) Intensity: 16 Vinyasa yoga classes (x 60–90 min) and 8 CBT sessions (x 30 min) over 8 weeks Pharmacotherapy: none Type of therapist/provider: CBT: doctoral‐level counsellor; yoga: certified yoga instructors BCTs: 1.1 Goal setting (behaviour), 1.2 Problem solving, 2.3 Self‐monitoring of behaviour, 3.1 Social support (unspecified), 11.2 Reduce negative emotions, 12.6 Body changes (yoga and relaxation) |
|
| Outcomes |
Definition of abstinence: 7‐day point prevalence Longest follow‐up: 6 months Biochemical verification: CO < 8 ppm Other relevant outcomes reported: depression, anxiety |
|
| Notes |
Relevant comparisons: Vinyasa yoga + CBT vs wellness classes + CBT Funding source: National Institutes of Health, National Center for Complementary, and Alternative Medicine (R21AT003669) Author conflicts of interest: “Ronnesia B. Gaskins, Ernestine Jennings, Herpreet Thind, Joseph Fava, Santina Horowitz, Ryan Lantini, Bruce M. Becker, and Beth C. Bock declare that they have no conflict of interest.” |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not reported |
| Allocation concealment (selection bias) | Unclear risk | Concealment not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Abstinence biochemically verified |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Attrition was different across groups, with high attrition in the yoga group: 56.5% (13/23) in the intervention group vs. 26.7% (4/15) in the control group |
| Selective reporting (reporting bias) | Low risk | Prespecified outcomes reported |
Gifford 2003.
| Study characteristics | ||
| Methods |
Study design: RCT Location: USA Setting: community Recruitment: through newspaper and radio advertisements and flyers Study dates: not reported |
|
| Participants | N = 102 Specialist population?: no Definition of smoker used: self‐identified nicotine dependence, ≥ 10 cpd for the last 12 months Participant characteristics: 59% female; average age: 43 years; 77% white; 4% some high school or less; average cpd: 21; nicotine dependence: average FTND 5.4 |
|
| Interventions |
Comparator: nicotine replacement therapy Mode of delivery: face‐to‐face Intensity: 1‐h session plus weekly return visits to receive new patches for following week Pharmacotherapy: 7 weeks of nicotine patches (22 mg/d for 4 weeks, followed by patches of 11 mg/d for 3 weeks) Type of therapist/provider: psychiatrist with extensive training in the medical management of smoking cessation, including NRT, or psychiatry resident under supervision of psychiatrist BCTs: 4.1 Instruction on how to perform behaviour, 11.1 Pharmacological support Intervention: ACT Mode of delivery: face‐to‐face (individual and group), telephone call if missed face‐to‐face individual session Intensity: 7 individual sessions and 7 group sessions over 7 weeks (duration of sessions not reported) Pharmacotherapy: none Type of therapist/provider: ACT therapist BCTs: 1.1 Goal setting (behaviour), 1.2 Problem solving, 1.9: Commitment, 7.3 Reduce prompts and cues, 8.7 Graded tasks, 12.6 Body changes |
|
| Outcomes |
Definition of abstinence: 7‐day point prevalence Longest follow‐up: 12 months Biochemical verification: CO < 11 ppm Other relevant outcomes reported: negative affect |
|
| Notes |
Relevant comparisons: ACT vs NRT Funding source: National Institutes of Health, National Cancer Institute (CA84813) Author conflicts of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “If participants were accepted into the study, they were randomly assigned to treatment condition using a random numbers generator” |
| Allocation concealment (selection bias) | Unclear risk | Concealment not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Abstinence biochemically verified |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | At 12‐month follow‐up 38.3% (18/47) were lost to follow‐up in the intervention group and 23.6% (13/55) in the control group |
| Selective reporting (reporting bias) | Unclear risk | No protocol available |
Mak 2020.
| Study characteristics | ||
| Methods |
Study design: RCT Location: Hong Kong Setting: primary care Recruitment: from 6 primary healthcare centres Study dates: 2012‐15 |
|
| Participants | N = 144 Specialist population?: no Definition of smoker used: ≥ 1 cpd in the past 30 days Participant characteristics: 29% female; average age: 46 years; 22% primary education or less; 18% unemployed; 16% household income ≤ HKD 9999; 17% average cpd > 20; 38% high nicotine dependence |
|
| Interventions |
Comparator: brief advice + self‐help materials Mode of delivery: face‐to‐face, written materials Intensity: brief 5 minute talk Pharmacotherapy: none Type of therapist/provider: not reported BCTs: 1.2 Problem solving, 4.1 Instruction on how to perform the behaviour, 5.1 Information about health consequences Intervention: ACT + self‐help materials Mode of delivery: face‐to‐face, telephone, written materials Intensity: 1 face‐to‐face ACT session and 2 telephone follow‐up sessions (each 15‐20 min) Pharmacotherapy: none Type of therapist/provider: experienced health counsellor trained in the principles of ACT applied in smoking cessation BCTs: 1.2 Problem solving, 4.1 instruction on how to perform the behaviour, 5.3 Information about social and environmental consequences, 12.6 Body changes |
|
| Outcomes |
Definition of abstinence: 7‐day point prevalence Longest follow‐up: 12 months Biochemical verification: CO < 6 ppm was measured but validated quit rates are not reported Other relevant outcomes reported: none |
|
| Notes |
Relevant comparisons: ACT + self‐help vs brief advice + self‐help Funding source: Health and Medical Research Fund of the Food and Health Bureau of the Hong Kong SAR Government (10111861) Author conflicts of interest: “The authors declare that they have no competing interests.” |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “The process of randomization was based on computer‐generated, block randomization with random block sizes, which were placed in sealed opaque envelopes." |
| Allocation concealment (selection bias) | Low risk | Quote: “The process of randomization was based on computer‐generated, block randomization with random block sizes, which were placed in sealed opaque envelopes." |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Although abstinence was biochemically verified, only unverified quit rates are reported and we were unable to obtain verified rates from the study authors. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Attrition was high: at 12‐month follow‐up 50.0% (35/70) were lost to follow‐up in the intervention group and 58.1% (43/74) in the control group |
| Selective reporting (reporting bias) | Unclear risk | Abstinence was defined as prespecified, although 12 month follow‐up was not prespecified. 6 month rates are not reported so unclear whether the reporting of 12‐month rates is an example of selective reporting |
McClure 2020.
| Study characteristics | ||
| Methods |
Study design: RCT Location: USA Setting: healthcare clinics Recruitment: potential participants were identified via automated medical records Study dates: 2011‐15 |
|
| Participants | N = 450 Specialist population?: no Definition of smoker used: ≥ 10 cpd Participant characteristics: 53% female; average age: 51 years; 83% white; 22% high school education or less; nicotine dependence: average FTND 4.9; 30% current depression; 12% current anxiety |
|
| Interventions |
Comparator: CBT, including motivational content (the risks of smoking and benefits of quitting, understanding one’s personal reasons for quitting), behavioural exercises (tracking one’s smoking), and psychoeducational content (how to use the nicotine patch, how to set a quit date) Mode of delivery: face‐to‐face (group) Intensity: 5 sessions (x 90 min) over 5 weeks Pharmacotherapy: 8 weeks of nicotine patches, adjusted to individual cpd Type of therapist/provider: master’s‐level counsellor with training in CBT, supervised by licensed clinical psychologists with expertise in CBT BCTs: 1.1 Goal setting (behaviour), 1.2 Problem solving, 1.4 Action planning (action plan review), 1.9 Commitment, 3.1 Social support (unspecified), 11.1 Pharmacological support, 12.6 Body changes Intervention: ACT Mode of delivery: face‐to‐face (group) Intensity: 5 sessions (x 90 min) over 5 weeks Pharmacotherapy: 8 weeks of nicotine patches, adjusted to individual cpd Type of therapist/provider: master’s‐level counsellor with training in ACT, supervised by licensed clinical psychologists with expertise in ACT BCTs: 1.1 Goal setting (behaviour), 1.2 Problem solving, 1.4 Action planning (action plan review), 1.9 Commitment, 3.1 Social support (unspecified), 11.1 Pharmacological support, 12.6 Body changes |
|
| Outcomes |
Definition of abstinence: 30‐day point prevalence Longest follow‐up: 12 months Biochemical verification: salivary cotinine < 15 ng/mL Other relevant outcomes reported: none |
|
| Notes |
Relevant comparisons: ACT + NRT vs CBT + NRT Funding source: National Cancer Institute (R01CA151251) Author conflicts of interest: “In July 2016, JB was a consultant to GlaxoSmithKline, the makers of a nicotine‐replacement therapy. He now serves on the Scientific Advisory Board of Chrono Therapeutics, the makers of a nicotine‐replacement therapy device. JLH has also received support from Pfizer. No other authors have conflicts to disclose.” |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not reported |
| Allocation concealment (selection bias) | Unclear risk | Concealment not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Abstinence biochemically verified |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | At 12‐month follow‐up 22.8% (51/224) were lost to follow‐up in the intervention group and 22.1% (50/226) in the control group |
| Selective reporting (reporting bias) | Low risk | Prespecified outcomes reported |
O'Connor 2020.
| Study characteristics | ||
| Methods |
Study design: RCT Location: Ireland Setting: community Recruitment: from the community by self‐selection Study dates: 2016‐18 |
|
| Participants | N = 150 Specialist population?: no Definition of smoker used: ≥ 10 cpd for the past 12 months Participant characteristics: 53% female; average age: 36 years; average years in education: 16.7; 75% employed; average cpd: 17; nicotine dependence: average FTND 4.7 |
|
| Interventions |
Comparator: behavioural support programme based on motivational interviewing Mode of delivery: face‐to‐face (group) Intensity: 6 sessions (x 90 min) over 6 weeks Pharmacotherapy: none Type of therapist/provider: a doctoral‐level and a bachelor’s‐level staff member who had undergone training in smoking cessation from the National Centre for Smoking Cessation and Training (NCSCT) BCTs: 1.2 Problem solving, 1.4 Action planning, 5.1 Information about health consequences Intervention 1: ACT Mode of delivery: face‐to‐face (group) Intensity: 6 sessions (x 90 min) over 6 weeks Pharmacotherapy: none Type of therapist/provider: psychology doctoral student with training in ACT and a doctoral‐level peer‐reviewed ACT trainer BCTs: 1.4 Action planning, 12.6 Body changes Intervention 2: ACT + SmartQuit smartphone app Mode of delivery: face‐to‐face (group), smartphone app Intensity: 6 sessions (x 90 min) over 6 weeks Pharmacotherapy: none Type of therapist/provider: psychology doctoral student with training in ACT and a doctoral‐level peer‐reviewed ACT trainer BCTs: 1.2 Problem solving, 1.4 Action planning, 12.6 Body changes |
|
| Outcomes |
Definition of abstinence: 7‐day point prevalence Longest follow‐up: 6 months Biochemical verification: CO < 10 ppm Other relevant outcomes reported: positive mental health |
|
| Notes |
Relevant comparisons:
Funding source: Irish Research Council Government of Ireland Postgraduate Scholarship Author conflicts of interest: “The authors declare that there are no conflicts of interest.” |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “the allocation sequence was generated with random block sizes of 3, 6 and 9 by a researcher with no clinical involvement in the trial using an online randomization tool” |
| Allocation concealment (selection bias) | Low risk | Quote: “allocation sequence was concealed from the researcher (MOC) enrolling participants in sequentially numbered, opaque, sealed envelopes” |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Abstinence biochemically verified |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | At 6‐month follow‐up 12.0% (6/50) were lost to follow‐up in the combined ACT + app group, 6.0% (3/50) in the ACT group, and 18.0% (9/50) in the control group |
| Selective reporting (reporting bias) | Low risk | Prespecified outcomes reported |
Pbert 2020.
| Study characteristics | ||
| Methods |
Study design: cluster‐RCT Location: USA Setting: high schools Recruitment: parents of all 9‐12 grade students were sent letters allowing them to opt out of their adolescent participating in the study Study dates: 2014‐16 |
|
| Participants | N = 146 (across 9 schools) Specialist population?: high school students Definition of smoker used: average of ≥ 5 cpd for the past 7 days Participant characteristics: 56% female; average age: 17 years; 90% white; 69% in reduced or free lunch programme; average cpd: 5; 23% high level of nicotine dependence; 43% elevated depressive symptoms |
|
| Interventions | All participants had weekly visits with the school nurse for 4 weeks, plus written self‐help materials or 1 of 2 smartphone apps Comparator 1: written self‐help materials (pamphlets) Mode of delivery: written materials, face‐to‐face Intensity: weekly visits with the school nurse over 4 weeks Pharmacotherapy: none Type of therapist/provider: school nurse BCTs: 1.2 Problem solving, 4.1 Instruction on how to perform behaviour Comparator 2: National Cancer Institute QuitSTART App, a free smartphone app developed as a smoking cessation resource for teens Mode of delivery: smartphone app, face‐to‐face Intensity: app intensity unclear, weekly visits with the school nurse over 4 weeks Pharmacotherapy: none Type of therapist/provider: school nurse, smartphone app BCTs: 1.1 Goal setting (behaviour), 1.2 Problem solving, 2.3 Self‐monitoring of behaviour, 15.4 Self‐talk Intervention: craving to quit (C2Q) app adapted for teens, based on core elements of mindfulness training for smoking Mode of delivery: smartphone app, face‐to‐face Intensity: 22 training modules plus 4 bonus modules (5‐15 min/module), weekly visits with the school nurse over 4 weeks Pharmacotherapy: none Type of therapist/provider: school nurse, smartphone app BCTs: 1.1 Goal setting (behaviour), 1.2 Problem solving, 2.3 Self‐monitoring of behaviour |
|
| Outcomes |
Definition of abstinence: 7‐day point prevalence Longest follow‐up: 6 months Biochemical verification: salivary cotinine < 11.4 ng/mL Other relevant outcomes reported: none |
|
| Notes |
Relevant comparisons:
Funding source: National Institute on Drug Abuse (R34 DA037886) Author conflicts of interest: "JB owns stock in Claritas MindSciences, the company that developed the Craving to Quit app. The other authors declare that they have no competing interest." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not reported |
| Allocation concealment (selection bias) | Unclear risk | Concealment not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Abstinence biochemically verified |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | At 6‐month follow‐up 8.3% (4/48) were lost to follow‐up in the intervention (C2Q) group, 2.0% (1/50) in the QuitSTART group, and 4.2% (2/48) in the materials group |
| Selective reporting (reporting bias) | Low risk | Prespecified outcomes reported |
Savvides 2014.
| Study characteristics | ||
| Methods |
Study design: RCT Location: Cyprus Setting: high schools and universities Recruitment: from the cafeterias and psychology classes of the universities and through the Ministry of Education and head teachers of the schools Study dates: not reported |
|
| Participants | N = 165 Specialist population?: young adults Definition of smoker used: ≥ 1 cpd Participant characteristics: 65% female; average age: 22 years; 32% weekly allowance/income < EUR 50; average cpd: 9; average FTND score: 3.1 |
|
| Interventions |
Comparator: waitlist Intensity: none Pharmacotherapy: none Intervention: avatar‐led, internet‐based, ACT Mode of delivery: online Intensity: 6 sessions (x 25 min) spaced out over a minimum of 3 days between each session Pharmacotherapy: none BCTs: 1.1 Goal setting (behaviour), 1.2 Problem solving, 1.5 Review behavioural goals, 1.9 Commitment, 10.3 Non‐specific reward, 11.2 Reducing negative emotions, 12.3 Avoidance/Reducing exposure to cues for the behaviour, 12.6 Body changes, 13.4 Valued self‐identity |
|
| Outcomes |
Definition of abstinence: 30‐day point prevalence Longest follow‐up: 12 months Biochemical verification: none Other relevant outcomes reported: none |
|
| Notes |
Notes: quitting outcomes were collected but not reported Funding source: not reported Author conflicts of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Group assignment was done using an online random number generator" |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Group assignment was done using an online random number generator" However, it is unclear if this was sufficient to conceal allocation. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Biochemical validation not used and unequal levels of contact between study arms |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Attrition at 12‐month follow‐up not reported |
| Selective reporting (reporting bias) | Unclear risk | No protocol available |
| Other bias | High risk | Waitlist control ‐ participants in the control arm may have delayed quitting, knowing that they would be receiving an intervention at a later date. This has the potential to inflate the reported effect of the intervention. |
Singh 2014.
| Study characteristics | ||
| Methods |
Study design: RCT Location: not reported Setting: community Recruitment: by referrals from families, supported living and group home supervisors, and primary care physicians Study dates: not reported |
|
| Participants | N = 51 Specialist population?: adults with mild intellectual disability Definition of smoker used: unclear Participant characteristics: 18% female; average age: 34 years; average cpd: 12; average years smoking: 16 |
|
| Interventions |
Comparator: treatment as usual, including motivational therapies, behaviour therapies, NRTs, non‐nicotine medicines, or a combination of the above Mode of delivery: not reported Intensity: unclear, varied across participants Pharmacotherapy: none specifically provided, although for some usual care included NRT Type of therapist/provider: community therapists BCTs: 2.3 Self‐monitoring (behaviour), 11.1 Pharmacological support Intervention: training in the use of mindfulness and meditation techniques Mode of delivery: face‐to‐face (individual or small groups) or via Skype Intensity: 2 training sessions (1 x 1 h, 1 x 45 min), 10 supervised practice sessions (x 30 min over 5 days), weekly contact with trainer Pharmacotherapy: none Type of therapist/provider: trainer with a 35‐year history of service provision to people with intellectual and developmental disabilities and long‐standing personal meditation practice, clinical expertise, and experience in mindful service delivery to individuals at all levels of intellectual functioning BCTs: 1.9 Commitment, 2.3 Self‐monitoring of behaviour, 12.4 Distraction, 12.6 Body changes |
|
| Outcomes |
Definition of abstinence: 7‐day point prevalence Longest follow‐up: 12 months Biochemical verification: none Other relevant outcomes reported: none |
|
| Notes |
Relevant comparisons: mindfulness and meditation training vs treatment as usual Funding source: not reported Author conflicts of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Randomisation was via alternate placement in the experimental and control groups |
| Allocation concealment (selection bias) | Unclear risk | Concealment not reported |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Abstinence self‐reported, differing levels of face‐to‐face contact between arms |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Only reported attrition during treatment |
| Selective reporting (reporting bias) | Unclear risk | No protocol available |
Vidrine 2016.
| Study characteristics | ||
| Methods |
Study design: RCT Location: USA Setting: community Recruitment: local print media Study dates: 2007‐10 |
|
| Participants | N = 412 Specialist population?: no Definition of smoker used: average of ≥ 5 cpd for the past year, expired air CO ≥ 8 ppm Participant characteristics: 55% female; average age: 49 years; 42% white; 9% less than high school education; 58% household income < USD 30,000/year; average cpd: 20; nicotine dependence: 39% first cigarette within 5 min of waking; 17% history of depression |
|
| Interventions |
Comparator 1: usual care (intended to be equivalent to the intervention a smoker might receive when asking a healthcare provider for help) + self‐help materials Mode of delivery: face‐to‐face (individual), written materials Intensity: 4 sessions (x 5‐10 min) Pharmacotherapy: 6 weeks of nicotine patches, adjusted to individual cpd Type of therapist/provider: master's‐level therapists BCTs: 1.1 Goal setting (behaviour), 1.2 Problem solving, 3.1 Social support (unspecified), 4.1 Instruction on how to perform behaviour: self‐help, 11.1 Pharmacological support Comparator 2: CBT using a problem‐solving/coping skills training approach based on relapse prevention theory and national guidelines + self‐help materials Mode of delivery: face‐to‐face (group), written materials Intensity: 8 sessions (x 2 h) Pharmacotherapy: 6 weeks of nicotine patches, adjusted to individual cpd Type of therapist/provider: master's‐level therapists BCTs: 1.1 Goal setting (behaviour), 1.2 Problem solving, 4.1 Instruction on how to perform behaviour: self‐help, 5.1 Information about health consequences, 9.2 Pros and cons, 11.1 Pharmacological support Intervention: mindfulness‐based addiction treatment (MBAT), which integrates mindfulness‐based stress reduction (MBSR) with a cognitive behavioural/relapse prevention theory‐based approach + self‐help materials Mode of delivery: face‐to‐face (group), written materials Intensity: 8 sessions (x 2 h) Pharmacotherapy: 6 weeks of nicotine patches, adjusted to individual cpd Type of therapist/provider: master's‐level therapists skilled in delivering MBSR BCTs: 1.1 Goal setting (behaviour), 1.2 Problem solving, 3.1 Social support (unspecified), 4.1 Instruction on how to perform behaviour: self‐help, 11.1 Pharmacological support, 11.2 Reduce negative emotions, 12.6 Body changes |
|
| Outcomes |
Definition of abstinence: 7‐day point prevalence Longest follow‐up: 6 months post‐quit day Biochemical verification: CO < 6 ppm. or salivary cotinine < 20 ng/mL if did not attend follow‐up in person Other relevant outcomes reported: depression, stress, positive and negative affect |
|
| Notes |
Relevant comparisons:
Funding source: National Institute on Drug Abuse, Centers for Disease Control and Prevention, National Cancer Institute, National Center for Complementary and Integrative Health, and Oklahoma Tobacco Settlement Endowment Trust Author conflicts of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not reported |
| Allocation concealment (selection bias) | Unclear risk | Concealment not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Abstinence biochemically verified |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | At 6‐month follow‐up 33.1% (51/154) were lost to follow‐up in the mindfulness‐based addiction treatment group, 34.8% (54/155) in the CBT group and 35.9% (37/103) in the usual care group |
| Selective reporting (reporting bias) | Low risk | Prespecified outcomes reported |
Weng 2021.
| Study characteristics | ||
| Methods |
Study design: RCT Location: Hong Kong Setting: workplace Recruitment: from companies in “women‐related industries such as … beauty and retail sectors” or individually through outreaching recruitment sessions Study dates: 2015‐16 |
|
| Participants | N = 213 Specialist population?: women Definition of smoker used: ≥ 1 cpd in the past 3 months Participant characteristics: 100% female; average age: 34 years; 90% secondary education or below; 72% monthly income ≤ HKD 20,000; average cpd: 11; nicotine dependence: 19% FTND ≥ 6 |
|
| Interventions | All participants were given a 50‐page self‐help smoking cessation booklet (about 9000 words) tailored to women smokers in workplaces and offered an optional 1‐h health talk (on the hazards of smoking and benefits and methods of quitting) Comparator: brief advice to follow the booklet’s advice Mode of delivery: face‐to‐face (individual), written materials Intensity: 1 session (presumably brief) + optional 1‐h health talk Pharmacotherapy: none Type of therapist/provider: trained counsellors BCTs: 4.1 Instruction on how to perform the behaviour, 5.1 Information about health consequences Intervention: brief mindfulness training Mode of delivery: face‐to‐face (group), written materials Intensity: 2 sessions (x 2 h) over 2 weeks + optional 1‐h health talk Pharmacotherapy: none Type of therapist/provider: certified clinical therapist BCTs: 4.1 Instruction on how to perform behaviour (self‐help guide), 5.1 Information about health consequences, 11.2 Reducing negative emotions, 12.6 Body changes |
|
| Outcomes |
Definition of abstinence: 7‐day point prevalence Longest follow‐up: 6 months Biochemical verification: CO < 4 ppm and salivary cotinine < 10 ng/mL Other relevant outcomes reported: none |
|
| Notes |
Relevant comparisons: brief mindfulness training + self‐help materials vs brief advice + self‐help materials Funding source: Lok Sin Tong Benevolent Society Kowloon Author conflicts of interest: “The authors declared that there is no conflict of interest.” |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not reported |
| Allocation concealment (selection bias) | Low risk | Quote: “The allocation sequence was generated by a researcher who was not involved in participant recruitment.” |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Abstinence biochemically verified |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | At 6‐month follow‐up 24.6% (28/114) were lost to follow‐up in the intervention group and 20.2% (20/99) in the control group |
| Selective reporting (reporting bias) | Low risk | Prespecified outcomes reported |
ACT: acceptance and commitment therapy; BCT: behaviour change techniques; CBT: cognitive behavioural therapy; cpd: cigarettes per day; FTND: Fagerström Test for Nicotine Dependence (Heatherton 1991); HSI: Heaviness of Smoking Index (Heatherton 1989); NRT: nicotine replacement therapy; ppm: parts per million; PTSD: post‐traumatic stress disorder; RCT: randomised controlled trial; SES: socioeconomic status
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Aggarwal 2017 | Ineligible follow‐up period |
| Arcari 1997 | Ineligible follow‐up period |
| Arora 2013 | Not possible to isolate the mindfulness element |
| Baruffi 2014 | Ineligible patient population |
| Bloom 2017 | Ineligible study design |
| Bowen 2009 | Ineligible follow‐up period |
| Brewer 2011 | Ineligible follow‐up period |
| Bricker 2014b | Ineligible follow‐up period |
| Chirikos 2004 | Ineligible patient population |
| Cropley 2007 | Ineligible follow‐up period |
| Davis 2013 | Ineligible follow‐up period |
| Davoudi 2017 | Ineligible follow‐up period |
| Elibero 2011 | Ineligible follow‐up period |
| Elwafi 2013 | Ineligible follow‐up period |
| Gifford 2011 | Not possible to isolate the mindfulness element |
| Heffner 2013 | Ineligible follow‐up period |
| Hemenway 2021 | Ineligible follow‐up period |
| Hernandez‐Lopez 2009 | Ineligible study design |
| Janes 2019 | Ineligible follow‐up period |
| Jang 2019 | Ineligible intervention |
| Jones 2015 | Ineligible follow‐up period |
| Jones 2017 | Not possible to isolate the mindfulness element |
| Karelka 2020 | Ineligible follow‐up period |
| Kochupillai 2005 | Ineligible study design |
| Luberto 2016 | Ineligible follow‐up period |
| Luk 2019 | Not possible to isolate the mindfulness element |
| Luo 2018 | Ineligible follow‐up period |
| Minami 2018 | Ineligible follow‐up period |
| Mineyama 2019 | Ineligible study design |
| Mujcic 2018 | Not possible to isolate the mindfulness element |
| NCT01314378 | Ineligible follow‐up period |
| NCT04038255 | Ineligible follow‐up period |
| Otto 2020 | Ineligible follow‐up period |
| Pakhale 2014 | Ineligible study design |
| Rogojanski 2011a | Ineligible follow‐up period |
| Rogojanski 2011b | Ineligible follow‐up period |
| Ruscio 2016 | Ineligible follow‐up period |
| Sarkar 2017 | Not possible to isolate the mindfulness element |
| Schuman‐Olivier 2014 | Ineligible follow‐up period |
| Shahab 2013 | Ineligible follow‐up period |
| Sharma 2013 | Ineligible study design |
| Sidhu 2016 | Ineligible study design |
| Spears 2019 | Ineligible follow‐up period |
| Sussman 2004 | Not possible to isolate the mindfulness element |
| Tang 2013 | Ineligible outcomes |
| Ussher 2009 | Ineligible follow‐up period |
| Zeng 2016 | Ineligible study design |
Characteristics of studies awaiting classification [ordered by study ID]
Pumariega 2020.
| Methods |
Study design: RCT Location: Brazil Setting: Psychosocial Care Center for Alcohol and Drugs Recruitment: from smokers seeking treatment at a substance centre Study dates: 2017 |
| Participants | N = 52 Specialist population?: smokers seeking treatment Definition of smoker used: unclear Participant characteristics: 83% female; average age: 49 years; 74% white; 69% lower middle socioeconomic class; 43% high nicotine dependence |
| Interventions |
Comparator: usual care based on CBT Intervention: mindfulness‐based relapse prevention |
| Outcomes |
Definition of abstinence: unclear Longest follow‐up: unclear Biochemical verification: CO Other relevant outcomes reported: depression, anxiety |
| Notes | Follow‐up period unclear |
CBT: cognitive behavioural therapy; RCT: randomised controlled trial
Characteristics of ongoing studies [ordered by study ID]
CTRI/2020/01/022692.
| Study name | Yoga as a complementary intervention for tobacco cessation: a PROBE trial |
| Methods |
Study design: RCT Location: India Setting: Centre of Integrative Medicine and Research, All India Institute of Medical Sciences Recruitment: not reported Study dates: 2020‐23 (estimated) |
| Participants | Target N = 200 Specialist population?: no Definition of smoker used: ≥ 5 cpd for at least the past year |
| Interventions |
Comparator: exercise + wellness programme Mode of delivery: face‐to‐face, video, audio recordings and written materials Intensity: 4 supervised sessions in the first week followed by 1 weekly session for 7 weeks Pharmacotherapy: Type of therapist/provider: institutionally certified yoga therapist + study wellness counsellor or other healthcare professional (e.g. psychologist) Intervention: yoga + wellness programme Mode of delivery: face‐to‐face, video and telephone Intensity: 4 supervised sessions in the first week followed by weekly sessions for 7 weeks Pharmacotherapy: none Type of therapist/provider: institutionally certified yoga therapist + study wellness counsellor or other healthcare professional (e.g. psychologist) BCTs: 4.1 Instruction on how to perform behaviour, 5.1 Information about health consequences, 8.6 Generalisation of a target behaviour, 12.6 Body changes |
| Outcomes |
Definition of abstinence: unclear Longest follow‐up: 6 months Biochemical verification: salivary cotinine and CO (threshold not reported) Other relevant outcomes: depression, anxiety, quality of life |
| Starting date | 2020 |
| Contact information | Gautam Sharma, All India Institute of Medical Sciences (AIIMS) |
| Notes |
Contacted PI for update: no update to report Funding source: Centre for Integrative Medicine and Research (CIMR) Institute Name: All India Institute of Medical Sciences (AIIMS), New Delhi Author conflicts of interest: not reported |
NCT01098955.
| Study name | Smoking cessation treatment for head & neck cancer patients |
| Methods |
Study design: RCT Location: USA Setting: cancer centre Recruitment: from patients planning to or currently undergoing treatment, or in follow‐up care Study dates: 2010‐2021 (estimated) |
| Participants |
Specialist population?: current or history of head and neck, lung, breast, gastrointestinal, or genitourinary cancer Definition of smoker used: ≥ 1 cpd |
| Interventions |
Comparator: motivational and behavioural counselling Mode of delivery: not reported Intensity: 6 counselling sessions (x 1 h) delivered over a 5‐week period Pharmacotherapy: varenicline (2 mg daily for 12 weeks) Type of therapist/provider: not reported BCTs: 3.1 Social support (unspecified), 11.1 Pharmacological support Intervention: ACT Mode of delivery: not reported Intensity: 6 counselling sessions (x 1 h) delivered over a 5‐week period Pharmacotherapy: varenicline (2 mg daily for 12 weeks) Type of therapist/provider: not reported BCTs: 1.9 Commitment, 3.1 Social support (unspecified): counselling, 11.1 Pharmacological support |
| Outcomes |
Definition of abstinence: unclear Longest follow‐up: 26 weeks after target quit date Biochemical verification: unclear Other relevant outcomes: none |
| Starting date | 2010 |
| Contact information | Jan Blalock, PhD M.D. Anderson Cancer Center |
| Notes |
Contacted PI for update: no update to report Funding source: not reported Author conflicts of interest: not reported |
NCT01982110.
| Study name | A mindfulness based application for smoking cessation (MBSC) |
| Methods |
Study design: RCT Location: USA Setting: community Recruitment: not reported Study dates: 2013‐18 |
| Participants | N = 5 Specialist population?: no Definition of smoker used: ≥ 5 cpd |
| Interventions |
Comparator: behavioural smoking cessation (NCI Quit Pal app) Mode of delivery: smartphone app Intensity: not reported Pharmacotherapy: none Type of therapist/provider: n/a BCTs: unclear Intervention: mindfulness‐based therapy (Craving to Quit app) Mode of delivery: smartphone app Intensity: not reported Pharmacotherapy: none Type of therapist/provider: n/a BCTs: 12.6 Body changes |
| Outcomes |
Definition of abstinence: unclear Longest follow‐up: 6 months Biochemical verification: CO (threshold not reported) Other relevant outcomes: none |
| Starting date | 2013 |
| Contact information | Jennifer K Penberthy, PhD University of Virginia |
| Notes |
Contacted PI for update: no response Funding source: not reported Author conflicts of interest: not reported |
NCT02037360.
| Study name | Mobile mindfulness training for smoking cessation |
| Methods |
Study design: RCT Location: USA Setting: not reported Recruitment: not reported Study dates: 2015‐17 |
| Participants | N = 1251 Specialist population?: no Definition of smoker used: ≥ 5 cpd with < 3 months' abstinence in the past year |
| Interventions |
Comparator: standard smartphone app to support smokers to quit Mode of delivery: smartphone app Intensity: not reported Pharmacotherapy: none Type of therapist/provider: n/a BCTs: 1.1 Goal setting (behaviour), 1.3 Goal setting (outcome), 2.3 Self‐monitoring (behaviour), 3.1 Social support (unspecified) Intervention: smartphone app that provides training in mindfulness for smoking cessation Mode of delivery: smartphone app Intensity: 22 modules (x 10‐15 minutes each) + 5 bonus modules available upon completion of earlier modules Pharmacotherapy: none Type of therapist/provider: n/a BCTs: 1.1 Goal setting (behaviour), 1.2 Problem solving, 1.3 Goal setting (outcome), 2.3 Self‐monitoring (behaviour), 12.6 Body changes |
| Outcomes |
Definition of abstinence: 7‐day point prevalence Longest follow‐up: 6 months Biochemical verification: not reported Other relevant outcomes: none |
| Starting date | 2015 |
| Contact information | Judson Brewer, MD PhD University of Massachusetts, Worcester |
| Notes |
Contacted PI for update: data were never analysed Funding source: not reported Author conflicts of interest: not reported |
NCT02421991.
| Study name | Telephone‐delivered interventions for smoking cessation (TALK) |
| Methods |
Study design: RCT Location: USA Setting: not reported Recruitment: not reported Study dates: 2015‐19 |
| Participants | N = 1275 Specialist population?: no Definition of smoker used: ≥ 10 cpd for at least the past 12 months |
| Interventions |
Comparator: CBT Mode of delivery: telephone Intensity: 5 weekly sessions Pharmacotherapy: NRT Type of therapist/provider: not reported BCTs: 3.1 Social support Intervention: Acceptance and Commitment Therapy (ACT) Mode of delivery: telephone Intensity: 5 weekly sessions Pharmacotherapy: NRT Type of therapist/provider: not reported BCTs: 3.1 Social support (note: full details of therapy not provided) |
| Outcomes |
Definition of abstinence: 30‐day point prevalence Longest follow‐up: 12 months Biochemical verification: not reported Other relevant outcomes: none |
| Starting date | 2015 |
| Contact information | Jonathan B Bricker, Ph.D. Fred Hutchinson Cancer Research Center |
| Notes |
Contacted PI for update: no update to report Funding source: not reported Author conflicts of interest: not reported |
NCT03253445.
| Study name | Individual acceptance and commitment therapy (ACT) for smoking cessation for schizophrenic patients |
| Methods |
Study design: RCT Location: Hong Kong Setting: not reported Recruitment: not reported Study dates: 2014‐18 (estimated) |
| Participants | N = 160 (target) Specialist population?: diagnosed schizophrenia Definition of smoker used: ≥ 1 cpd |
| Interventions | All participants are given a brief educational talk on encouraging quitting smoking (about 5 min) and a self‐help leaflet on smoking cessation Comparator: ACT + brief advice + self‐help materials Mode of delivery: face‐to‐face, written materials Intensity: 10 sessions (x 20‐30 min) Pharmacotherapy: none Type of therapist/provider: n/a BCTs: 1.9 Commitment, 4.1 Instruction on how to perform the behaviour Intervention: social support + brief advice + self‐help materials Mode of delivery: face‐to‐face, written materials Intensity: 10 sessions (x 5 min) Pharmacotherapy: none Type of therapist/provider: n/a BCTs: 3.1 Social support (unspecified), 4.1 Instruction on how to perform the behaviour |
| Outcomes |
Definition of abstinence: 7‐day point prevalence Longest follow‐up: 6 months Biochemical verification: CO (threshold not reported), urinary cotinine (threshold not reported) Other relevant outcomes: none |
| Starting date | 2014 |
| Contact information | Dr. Yim Wah Mak, Assistant Professor, The Hong Kong Polytechnic University |
| Notes |
Contacted PI for update: no response Funding source: not reported Author conflicts of interest: not reported |
ACT: acceptance and commitment therapy; BCT: behaviour change techniques; CBT: cognitive behavioural therapy; cpd: cigarettes per day; n/a: not applicable; NRT: nicotine replacement therapy; PI: principal investigator; RCT: randomised controlled trial
Differences between protocol and review
We specified in our protocol that we would include measures of depression, anxiety, quality of life, and stress as indicators of mental health and well‐being. We also included data on positive and negative affect as several of our included studies had reported on these outcomes.
We originally proposed that in the case of cluster‐RCTs, we would extract a direct estimate of the required effect from an analysis that properly accounted for the cluster design, and where such data were unavailable, we would perform an approximately correct analysis if we could extract the intracluster correlation coefficient (ICC). However, the one cluster‐RCT we identified as suitable for inclusion did not present an analysis adjusting for the clustering effect or report an ICC. Therefore, we used unadjusted data for the primary analysis and performed a sensitivity analysis where we estimated the ICC (0.03), based on the ICC reported in other smoking cessation studies (Fanshawe 2017), and adjusted the analysis on this basis.
We originally planned to conduct subgroup analyses categorising studies by (broad) intervention type, the type/intensity of control treatment received, population type, baseline motivation to quit, baseline mental health, number of intervention behaviour change techniques, mode of intervention delivery, and type of therapist. Given the studies covered a diverse set of interventions, we did not consider it appropriate to pool all studies in a single meta‐analysis, so we stratified our pooled analyses by intervention type. It was then possible to conduct subgroup analyses by the type and intensity of control treatment received and mode of intervention delivery.
Contributions of authors
SJ wrote the protocol with input from all review authors. JLB carried out the manual searches and JT the automated searches. SJ, JLB, NL and EN screened studies and extracted data. SJ conducted the analyses and wrote the review with input from all review authors.
Sources of support
Internal sources
-
Nuffield Department of Primary Care Health Sciences, University of Oxford, UK
Editorial base for the Cochrane Tobacco Addiction Group
External sources
-
Cancer Research UK, UK
SJ's salary was paid by a grant funded by Cancer Research UK (C1417/A22962)
-
University College London, UK
JB's salary was paid by University College London
-
National Institute for Health Research, UK
Infrastructure funding for the Cochrane Tobacco Addiction Group
Declarations of interest
All review authors declare no financial links with tobacco companies or e‐cigarette manufacturers or their representatives.
SJ: none reported
JB has undertaken research and consultancy for manufacturers of smoking cessation medications (Pfizer and Johnson & Johnson).
EN: none reported
JLB: none reported
EH: none reported
NL: none reported
New
References
References to studies included in this review
Bloom 2020 {published data only}
- Bloom EL, Ramsey SE, Abrantes AM, Hunt L, Wing RR, Kahler CW, et al.A pilot randomized controlled trial of distress tolerance treatment for weight concern in smoking cessation among women. Nicotine & Tobacco Research 2020;22(9):1578-86. [DOI: 10.1093/ntr/ntaa026] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bock 2012 {published data only}
- Bock BC, Fava JL, Gaskins R, Morrow KM, Williams DM, Jennings E, et al.Yoga as a complementary treatment for smoking cessation in women. Journal of Women's Health 2012;21(2):240-8. [DOI: 10.1089/jwh.2011.2963] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bock BC, Morrow KM, Becker BM, Williams DM, Tremont G, Gaskins RB, et al.Yoga as a complementary treatment for smoking cessation: rationale, study design and participant characteristics of the Quitting-in-Balance study. BMC Complementary and Alternative Medicine 2010;10:14. [DOI: 10.1186/1472-6882-10-14] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT00492310.Yoga for women attempting smoking cessation. https://clinicaltrials.gov/ct2/show/NCT00492310 first received 27 June 2007. [URL: https://clinicaltrials.gov/ct2/show/NCT00492310]
Bock 2019 {published data only}
- Bock BC, Dunsiger SI, Rosen RK, Thind H, Jennings E, Fava JL, et al.Yoga as a complementary therapy for smoking cessation: results from BreathEasy, a randomized clinical trial. Nicotine & Tobacco Research 2019;21(11):1517-23. [DOI: 10.1093/ntr/nty212] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bock BC, Rosen RK, Fava JL, Gaskins RB, Jennings E, Thind H, et al.Testing the efficacy of yoga as a complementary therapy for smoking cessation: design and methods of the BreathEasy trial. Contemporary Clinical Trials 2014;38(2):321-32. [DOI: 10.1016/j.cct.2014.06.003] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bock BC, Thind H, Dunsiger S, Fava JL, Jennings E, Becker BM, et al.Who enrolls in a quit smoking program with yoga therapy? American Journal of Health Behavior 2017;41(6):740-9. [DOI: 10.5993/AJHB.41.6.8] [PMID: ] [DOI] [PubMed] [Google Scholar]
Bricker 2014a {published data only}
- Bricker JB, Bush T, Zbikowski SM, Mercer LD, Heffner JL.Randomized trial of telephone-delivered acceptance and commitment therapy versus cognitive behavioral therapy for smoking cessation: a pilot study. Nicotine & Tobacco Research 2014;16(11):1446-54. [DOI: 10.1093/ntr/ntu102] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heffner JL, Mull K, Mercer L, Vilardaga R, Bricker J.Effects of two telephone-delivered smoking cessation interventions on hazardous drinking rates: ACT vs. CBT. Alcoholism: Clinical and Experimental Research 2014;38:137A. [Google Scholar]
- Vilardaga R, Heffner JL, Mercer LD, Bricker JB.Do counselor techniques predict quitting during smoking cessation treatment? A component analysis of telephone-delivered Acceptance and Commitment Therapy. Behaviour Research and Therapy 2014;61:89-95. [PMID: 10.1016/j.brat.2014.07.008] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bricker 2018 {published data only}
- Bricker JB, Mull KE, McClure JB, Watson NL, Heffner JL.Improving quit rates of web-delivered interventions for smoking cessation: full scale randomized trial of WebQuit.org versus Smokefree.gov. Addiction (Abingdon, England) 2018;113(5):914-23. [DOI: 10.1111/add.14127] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bricker JB, Sridharan V, Zhu Y, Mull KE, Heffner JL, Watson NL, et al.Trajectories of 12-month usage patterns for two smoking cessation websites: exploring how users engage over time. Journal of Medical Internet Research 2018;20(4):e10143. [DOI: 10.2196/10143] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heffner JL, Mull KE, Watson NL, McClure JB, Bricker JB.Smokers with bipolar disorder, other affective disorders, and no mental health conditions: Comparison of baseline characteristics and success at quitting in a large 12-month behavioral intervention randomized trial. Drug and Alcohol Dependence 2018;193:35-41. [DOI: 10.1016/j.drugalcdep.2018.08.034] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perski O, Watson NL, Mull KE, Bricker JB.Identifying content-based engagement patterns in a smoking cessation website and associations with user characteristics and cessation outcomes: a sequence and cluster analysis. Nicotine & Tobacco Research 2021;23(7):1103-12. [DOI: 10.1093/ntr/ntab008] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bricker 2020 {published data only}
- Bricker JB, Watson NL, Mull KE, Sullivan BM, Heffner JL.Efficacy of smartphone applications for smoking cessation: a randomized clinical trial. JAMA Internal Medicine 2020;180(11):1472-80. [DOI: 10.1001/jamainternmed.2020.4055] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Brown 2013 {published data only}
- Brown RA, Palm Reed KM, Bloom EL, Minami H, Strong DR, Lejuez CW, et al.Development and preliminary randomized controlled trial of a distress tolerance treatment for smokers with a history of early lapse. Nicotine & Tobacco Research 2013;15(12):2005-15. [DOI: 10.1093/ntr/ntt093] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Davis 2014a {published data only}
- Davis J, Goldberg S, Anderson M, Baker T.Randomized trial comparing mindfulness training for smokers to standard of care smoking cessation therapy. In: SRNT Annual Meeting Abstracts. 2012:6. [URL: https://cdn.ymaws.com/srnt.site-ym.com/resource/resmgr/Conferences/Past_Annual_Meetings/2012_Annual_Meeting_Abstract.pdf]
- Davis JM, Goldberg S, Anderson MC, Manley A, Smith S, Baker T.Randomized trial on mindfulness training for smokers targeted to a disadvantaged population. Substance Use & Misuse 2014;49(5):571-85. [DOI: 10.3109/10826084.2013.770025] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg SB, Davis JM, Hoyt WT.The role of therapeutic alliance in mindfulness interventions. Journal of Clinical Psychology 2013;69(9):936-50. [DOI: 10.1002/jclp.21973] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg SB, Del Re AC, Hoyt WT, Davis JM.The secret ingredient in mindfulness interventions? A case for practice quality over quantity. Journal of Counseling Psychology 2014;61(3):491-7. [DOI: 10.1037/cou0000032] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg SB, Manley AR, Smith SS, Greeson JM, Russell E, Van Uum S, et al.Hair cortisol as a biomarker of stress in mindfulness training for smokers. Journal of Alternative and Complementary Medicine 2014;20(8):630-4. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT01093599.Trial on the effectiveness of Mindfulness Training for Smokers (MTS). https://clinicaltrials.gov/ct2/show/NCT01093599 first received 16 February 2015. [URL: https://clinicaltrials.gov/ct2/show/NCT01093599]
Davis 2014b {published data only}
- Davis JM, Manley AR, Goldberg SB, Smith SS, Jorenby DE.Randomized trial comparing mindfulness training for smokers to a matched control. Journal of Substance Abuse Treatment 2014;47(3):213-21. [DOI: 10.1016/j.jsat.2014.04.005] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg SB, Manley AR, Smith SS, Greeson JM, Russell E, Van Uum S, et al.Hair cortisol as a biomarker of stress in mindfulness training for smokers. Journal of Alternative and Complementary Medicine 2014;20(8):630-4. [DOI: 10.1089/acm.2014.0080] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
de Souza 2020 {published data only}
- Souza IC, Kozasa EH, Bowen S, Richter KP, Sartes LM, Colugnati FA, et al.Effectiveness of mindfulness-based relapse prevention program as an adjunct to the standard treatment for smoking: a pragmatic design pilot study. Nicotine & Tobacco Research 2020;22(9):1605-13. [DOI: 10.1093/ntr/ntaa057] [PMID: ] [DOI] [PubMed] [Google Scholar]
Garrison 2020 {published data only}
- Garrison KA, Pal P, O'Malley SS, Pittman BP, Gueorguieva R, Rojiani R, et al.Craving to quit: a randomized controlled trial of smartphone app–based mindfulness training for smoking cessation. Nicotine & Tobacco Research 2020;22(3):324-31. [DOI: 10.1093/ntr/nty126] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrison KA, Pal P, Rojiani R, Dallery J, O'Malley SS, Brewer JA.A randomized controlled trial of smartphone-based mindfulness training for smoking cessation: a study protocol. BMC Psychiatry 2015;15:83. [DOI: 10.1186/s12888-015-0468-z] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roos CR, Brewer JA, O'Malley SS, Garrison KA.Baseline craving strength as a prognostic marker of benefit from smartphone app-based mindfulness training for smoking cessation. Mindfulness 2019;10:2165-71. [DOI: ] [Google Scholar]
Gaskins 2015 {published data only}
- Gaskins RB, Jennings EG, Thind H, Fava JL, Horowitz S, Lantini R, et al.Recruitment and initial interest of men in yoga for smoking cessation: QuitStrong, a randomized control pilot study. Translational Behavioral Medicine 2015;5(2):177-88. [DOI: 10.1007/s13142-014-0295-7] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gifford 2003 {published data only}
- Gifford ER.Acceptance -based treatment of regulatory internal stimuli in nicotine-dependent smokers: a controlled comparison with transdermal nicotine replacement [Dissertation]. ProQuest Dissertations Publishing, 2003. [ISBN: 978-0-496-38626-0] [URL: https://www.proquest.com/dissertations-theses/acceptance-based-treatment-regulatory-internal/docview/305311847/se-2?accountid=14511] [Google Scholar]
- Gifford EV, Kohlenberg BS, Hayes SC, Antonuccio DO, Piasecki MM, Rasmussen-Hall ML, et al.Acceptance-based treatment for smoking cessation. Behavior Therapy 2004;35(4):689-705. [DOI: 10.1016/S0005-7894(04)80015-7] [DOI] [Google Scholar]
Mak 2020 {published data only}
- Mak YW, Leung DY, Loke AY.Effectiveness of an individual acceptance and commitment therapy for smoking cessation, delivered face-to-face and by telephone to adults recruited in primary health care settings: a randomized controlled trial. BMC Public Health 2020;20:1719. [DOI: 10.1186/s12889-020-09820-0] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mak YW, Loke AY.The acceptance and commitment therapy for smoking cessation in the primary health care setting: a study protocol. BMC Public Health 2015;15:105. [DOI: 10.1186/s12889-015-1485-z] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT01652508.Acceptance and commitment therapy for smoking cessation in the primary care setting. https://clinicaltrials.gov/ct2/show/NCT01652508 first received 30 July 2012. [URL: https://clinicaltrials.gov/ct2/show/NCT01652508]
McClure 2020 {published data only}
- McClure JB, Bricker J, Mull K, Heffner JL.Comparative effectiveness of group-delivered acceptance and commitment therapy versus cognitive behavioral therapy for smoking cessation: a randomized controlled trial. Nicotine & Tobacco Research 2020;22(3):354-62. [DOI: 10.1093/ntr/nty268] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT01533974.PATH partnering to achieve tobacco-free health (PATH). https://clinicaltrials.gov/ct2/show/NCT01533974 first received 16 February 2012. [URL: https://clinicaltrials.gov/ct2/show/NCT01533974]
O'Connor 2020 {published data only}
- NCT02901171.The contribution of a smartphone application to acceptance and commitment therapy group treatment for smoking cessation. clinicaltrials.gov/ct2/show/NCT02901171 first received 15 September 2016. [URL: https://clinicaltrials.gov/ct2/show/NCT02901171]
- O'Connor M, Whelan R, Bricker J, McHugh L.Randomized controlled trial of a smartphone application as an adjunct to acceptance and commitment therapy for smoking cessation. Behavior Therapy 2020;51(1):162-77. [DOI: 10.1016/j.beth.2019.06.003] [PMID: ] [DOI] [PubMed] [Google Scholar]
Pbert 2020 {published data only}
- NCT02218281.Developing a smartphone app With mindfulness training for teen smoking cessation. https://clinicaltrials.gov/ct2/show/NCT02218281 first received 18 August 2014. [URL: https://clinicaltrials.gov/ct2/show/NCT02218281]
- Pbert L, Druker S, Crawford S, Frisard C, Trivedi M, Osganian SK, et al.Feasibility of a smartphone app with mindfulness training for adolescent smoking cessation: Craving to Quit (C2Q)-Teen. Mindfulness 2020;11(3):720-33. [DOI: 10.1007/s12671-019-01273-w] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Savvides 2014 {published data only}
- Savvides SN.Evaluating an acceptance and commitment therapy internet-based intervention for smoking cessation in young adults [Dissertation]. University of Cyprus, Faculty of Social Sciences and Education, 2014. [URL: https://gnosis.library.ucy.ac.cy/handle/7/39519] [Google Scholar]
Singh 2014 {published data only}
- Singh NN, Lancioni GE, Myers RE, Karazsia BT, Winton AS, Singh J.A randomized controlled trial of a mindfulness-based smoking cessation program for individuals with mild intellectual disability. International Journal of Mental Health and Addiction 2014;12:153-68. [DOI: 10.1007/s11469-013-9471-0] [DOI] [Google Scholar]
Vidrine 2016 {published data only}
- NCT00297479.Group therapy for nicotine dependence: mindfulness and smoking. https://clinicaltrials.gov/ct2/show/NCT00297479 first received 28 February 2006. [URL: https://clinicaltrials.gov/ct2/show/NCT00297479]
- Ruscio AC, Uniformed Services University Of The Health Sciences Bethesda United States.Mindfulness and tobacco dependence in cigarette smokers: mediating mechanisms. Defense Technical Information Center, available from apps.dtic.mil/sti/citations/AD1013176 2012:92.0. [URL: https://apps.dtic.mil/sti/citations/AD1013176]
- Spears CA, Hedeker D, Li L, Wu C, Anderson NK, Houchins SC, et al.Mechanisms underlying mindfulness-based addiction treatment versus cognitive behavioral therapy and usual care for smoking cessation. Journal of Consulting and Clinical Psychology 2018;85(11):1029-40. [DOI: 10.1037/ccp0000229] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidrine JI, Businelle MS, Reitzel LR, Cao Y, Cinciripini PM, Marcus MT, et al.Coping mediates the association of mindfulness with psychological stress, affect, and depression among smokers preparing to quit. Mindfulness 2014;6(3):433-43. [DOI: 10.1007/s12671-014-0276-4] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidrine JI, Spears CA, Heppner WL, Reitzel LR, Marcus MT, Cinciripini PM, et al.Efficacy of mindfulness based addiction treatment (MBAT) for smoking cessation and lapse. Journal of Consulting and Clinical Psychology 2016;84(9):824-38. [DOI: 10.1037/ccp0000117] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Weng 2021 {published data only}
- NCT02497339.Mindfulness training for smoking cessation in women in workplaces. clinicaltrials.gov/ct2/show/NCT02497339 first received 14 July 2015. [URL: https://clinicaltrials.gov/ct2/show/NCT02497339]
- Weng X, Luk TT, Lau OS, Suen YN, Lee JJ, Li WH, et al.Brief mindfulness training for smoking cessation in Chinese women in workplaces: a pilot randomized controlled trial. Addictive Behaviors 2021;113:106677. [DOI: 10.1016/j.addbeh.2020.106677] [PMID: ] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Aggarwal 2017 {published data only}
- Aggarwal A, Kumar K.Yoga as a powerful tool for smoking cessation-convincing results of a community based randomised controlled trial. Journal of Thoracic Oncology 2017;12(S1):S1486-S1487. [Google Scholar]
Arcari 1997 {published data only}
- Arcari PM.Efficacy of a workplace smoking cessation program: Mindfulness meditation vs cognitive-behavioral interventions [Dissertation]. Dissertation Abstracts International: Section B: The Sciences and Engineering, 1997. [Google Scholar]
Arora 2013 {published data only}
- Arora M, Reddy KS, Sarkar BK, Shahab L, West R, Ahluwalia JS.Cluster randomised trial of a brief tobacco cessation intervention for low income communities, India. Respiratory Medicine 2013;107:S5. [Google Scholar]
Baruffi 2014 {published data only}
- Baruffi M, Spatola CA, Manzoni GM, Castelnuovo G, Molinari E, Malfatto G, et al.The ACTonHEART study: rationale and design of a randomized controlled clinical trial comparing a brief intervention based on acceptance and commitment therapy to usual secondary prevention care of coronary heart disease. Health and Quality of Life Outcomes 2014;12(1):22. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bloom 2017 {published data only}
- Bloom EL, Wing RR, Kahler CW, Thompson JK, Meltzer S, Hecht J, et al.Distress tolerance treatment for weight concern in smoking cessation among women: the WE QUIT pilot study. Behavior Modification 2017;41(4):468-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bowen 2009 {published data only}
- Bowen S, Marlatt A.Surfing the urge: brief mindfulness-based intervention for college student smokers. Psychology of Addictive Behaviors 2009;23(4):666-71. [DOI: 10.1037/a0017127] [PMID: ] [DOI] [PubMed] [Google Scholar]
Brewer 2011 {published data only}
- Brewer JA, Mallik S, Babuscio TA, Nich C, Johnson HE, Deleone CM, et al.Mindfulness training for smoking cessation: results from a randomized controlled trial. Drug and Alcohol Dependence 2011;119(1):72-80. [DOI: 10.1016/j.drugalcdep.2011.05.027] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bricker 2014b {published data only}
- Bricker JB, Mull KE, Kientz JA, Vilardaga R, Mercer LD, Akioka KJ, et al.Randomized, controlled pilot trial of a smartphone app for smoking cessation using acceptance and commitment therapy. Drug and Alcohol Dependence 2014;143:87-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chirikos 2004 {published data only}
- Chirikos TN, Herzog TA, Meade CD, Webb MS, Brandon TH.Cost-effectiveness analysis of a complementary health intervention: the case of smoking relapse prevention. International Journal of Technology Assessment in Health Care 2004;20(4):475-80. [DOI] [PubMed] [Google Scholar]
Cropley 2007 {published data only}
- Cropley M, Ussher M, Charitou E.Acute effects of a guided relaxation routine (body scan) on tobacco withdrawal symptoms and cravings in abstinent smokers. Addiction 2007;102(6):989-93. [DOI: 10.1111/j.1360-0443.2007.01832.x] [PMID: ] [DOI] [PubMed] [Google Scholar]
Davis 2013 {published data only}
- Davis JM, Mills DM, Stankevitz KA, Manley AR, Majeskie MR, Smith SS.Pilot randomized trial on mindfulness training for smokers in young adult binge drinkers. BMC Complementary and Alternative Medicine 2013;13:215. [DOI] [PMC free article] [PubMed] [Google Scholar]
Davoudi 2017 {published data only}
- Davoudi M, Omidi A, Sehat M, Sepehrmanesh Z.The effects of acceptance and commitment therapy on man smokers' comorbid depression and anxiety symptoms and smoking cessation: a randomized controlled trial. Addiction and Health 2017;9(3):129-38. [PMC free article] [PubMed] [Google Scholar]
Elibero 2011 {published data only}
- Elibero A, Van Rensburg KJ, Drobes DJ.Acute effects of aerobic exercise and Hatha yoga on craving to smoke. Nicotine & Tobacco Research 2011;13(11):1140-8. [DOI: 10.1093/ntr/ntr163] [PMID: ] [DOI] [PubMed] [Google Scholar]
Elwafi 2013 {published data only}
- Elwafi HM, Witkiewitz K, Mallik S, Thornhill TA, Brewer JA.Mindfulness training for smoking cessation: moderation of the relationship between craving and cigarette use. Drug and Alcohol Dependence 2013;130:222-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gifford 2011 {published data only}
- Gifford EV, Kohlenberg BS, Hayes SC, Pierson HM, Piasecki MP, Antonuccio DO, et al.Does acceptance and relationship focused behavior therapy contribute to bupropion outcomes? A randomized controlled trial of functional analytic psychotherapy and acceptance and commitment therapy for smoking cessation. Behavior Therapy 2011;42(4):700-15. [DOI] [PubMed] [Google Scholar]
Heffner 2013 {published data only}
- Heffner JL, Wyszynski CM, Comstock B, Mercer LD, Bricker J.Overcoming recruitment challenges of web-based interventions for tobacco use: the case of web-based acceptance and commitment therapy for smoking cessation. Addictive Behaviors 2013;38(10):2473-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hemenway 2021 {published data only}
- Hemenway M, Witkiewitz K, Unrod M, Brandon KO, Brandom TH, Wetter DW, et al.Development of a mindfulness-based treatment for smoking cessation and the modification of alcohol use: a protocol for a randomized controlled trial and pilot study findings. Contemporary Clinical Trials 2021;100:106218. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hernandez‐Lopez 2009 {published data only}
- Hernandez-Lopez M, Luciano MC, Bricker JB, Roales-Nieto JG, Montesinos F.Acceptance and commitment therapy for smoking cessation: a preliminary study of its effectiveness in comparison with cognitive behavioral therapy. Psychology of Addictive Behaviors 2009;23(4):723-30. [DOI] [PubMed] [Google Scholar]
Janes 2019 {published data only}
- Janes AC, Datko M, Roy A, Barton B, Druker S, Neal C, et al.Quitting starts in the brain: a randomized controlled trial of app-based mindfulness shows decreases in neural responses to smoking cues that predict reductions in smoking. Neuropsychopharmacology 2019;44(9):1631-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jang 2019 {published data only}
- Jan S, Lee JA, Jang BH, Shin YC, Ko SG, Park S.Clinical effectiveness of traditional and complementary medicine interventions in combination with nicotine replacement therapy on smoking cessation: a randomized controlled pilot trial. Journal of Alternative and Complementary Medicine 2019;25(5):526-34. [DOI] [PubMed] [Google Scholar]
Jones 2015 {published data only}
- Jones HA, Heffner JL, Mercer L, Wyszynski CM, Vilardaga R, Bricker JB.Web-based acceptance and commitment therapy smoking cessation treatment for smokers with depressive symptoms. Journal of Dual Diagnosis 2015;11(1):56-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jones 2017 {published data only}
- Jones C, Bennett B.Smoking cessation using group auricular acupuncture and mindfulness. Journal of Spinal Cord Medicine 2017;40(5):623-4. [Google Scholar]
Karelka 2020 {published data only}
- Karelka M, Savvides SN, Gloster A.An avatar-led intervention promotes smoking cessation in young adults: a pilot randomized clinical trial. Annals of Behavioral Medicine 2020;54(10):747-60. [DOI] [PubMed] [Google Scholar]
Kochupillai 2005 {published data only}
- Kochupillai V, Kumar P, Singh D, Aggarwal D, Bhardwaj N, Bhutani M, et al.Effect of rhythmic breathing (Sudarshan Kriya and Pranayam) on immune functions and tobacco addiction. Annals of the New York Academy of Sciences 2005;1056:242-52. [DOI] [PubMed] [Google Scholar]
Luberto 2016 {published data only}
- Luberto C, McLeish A.The effects of a brief mindfulness exercise on state mindfulness, smoking, and affective outcomes among adult smokers. Journal of Alternative and Complementary Medicine (New York, N.Y.) 2016;22(6):A82-A83. [Google Scholar]
Luk 2019 {published data only}
- Luk TT, Li WHC, Cheung DY, Wong SW, Kwont AC, Lai VW, et al.Chat-based instant messaging support combined with brief smoking cessation interventions for Chinese community smokers in Hong Kong: rationale and study protocol for a pragmatic, cluster-randomized controlled trial. Contemporary Clinical Trials 2019;77:70-5. [DOI] [PubMed] [Google Scholar]
Luo 2018 {published data only}
- Luo M, Gardiner P, D'Amico S, Charlot M, Lasser KE, Kathuria H.Feasibility, acceptability, and effectiveness of a mindfulness-based smoking cessation program for cancer patients. Global Advances in Health and Medicine 2018;7:170. [Google Scholar]
Minami 2018 {published data only}
- Minami H, Brinkman HR, Nahvi S, Arnsten JH, Rivera-Mindt M, Wetter DW, et al.Rationale, design and pilot feasibility results of a smartphone-assisted, mindfulness-based intervention for smokers with mood disorders: Project mSMART MIND. Contemporary Clinical Trials 2018;66:36-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mineyama 2019 {published data only}
- Mineyama Y, Hyder Ferry L, Arechiga A, Dos Santos H, Berk L.The effect of a tobacco dependence treatment program with stress management through mindfulness technique training in US veterans. Advances in Mind-body Medicine 2019;33(2):12-17. [PubMed] [Google Scholar]
Mujcic 2018 {published data only}
- Mujcic A, Blankers M, Boon B, Engels R, Van Laar M.Internet-based self-help smoking cessation and alcohol moderation interventions for cancer survivors: a study protocol of two RCTs. BMC Cancer 2018;18(1):364. [DOI] [PMC free article] [PubMed] [Google Scholar]
NCT01314378 {published data only}
- NCT01314378.Effects of intensive behavioral training program on impulsivity and inhibitory control in smokers (IBTP). clinicaltrials.gov/ct2/show/NCT0131437 first received 14 March 2011. [URL: https://clinicaltrials.gov/ct2/show/NCT01314378]
NCT04038255 {published data only}
- NCT04038255.Mindfulness based smoking cessation among cancer survivors. clinicaltrials.gov/ct2/show/nct04038 first received 30 July 2019. [URL: https://clinicaltrials.gov/ct2/show/nct04038255]
Otto 2020 {published data only}
- Otto MW, Zvolensky MJ, Rosenfield D, Hoyt DL, Witkiewitz K, McKee SA, et al.A randomized controlled trial protocol for engaging distress tolerance and working memory to aid smoking cessation in low socioeconomic status (SES) adults. Health Psychology 2020;39(9):815-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pakhale 2014 {published data only}
- Pakhale S, Armstrong MA, Garde A, Reid B, Aitken D, Mullen KA, et al.A pilot implementation of Buddhist mindfulness training, combined with the Ottawa model for smoking cessation in an out-patient respirology clinic setting. American Journal of Respiratory and Critical Care Medicine 2014;189:A2796. [Google Scholar]
Rogojanski 2011a {published data only}
- Rogojanski J, Vettese LC, Antony MM.Coping with cigarette cravings: comparison of suppression versus mindfulness-based strategies. Mindfulness 2011;2(1):14-26. [DOI: ] [Google Scholar]
Rogojanski 2011b {published data only}
- Rogojanski J, Vettese LC, Antony MM.Role of sensitivity to anxiety symptoms in responsiveness to mindfulness versus suppression strategies for coping with smoking cravings. Journal of Clinical Psychology 2011;67(4):439-45. [DOI: 10.1002/jclp.20774] [PMID: ] [DOI] [PubMed] [Google Scholar]
Ruscio 2016 {published data only}
- Ruscio AC, Muench C, Brede E, Waters AJ.Effect of brief mindfulness practice on self-reported affect, craving, and smoking: a pilot randomized controlled trial using ecological momentary assessment. Nicotine & Tobacco Research 2016;18(1):64-73. [DOI] [PubMed] [Google Scholar]
Sarkar 2017 {published data only}
- Sarkar BK, West R, Arora M, Ahluwalia JS, Reddy KS, Shahab L.Effectiveness of a brief community outreach tobacco cessation intervention in India: a cluster-randomised controlled trial (the BABEX Trial). Thorax 2017;72(2):167-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schuman‐Olivier 2014 {published data only}
- Schuman-Olivier Z, Hoeppner BB, Evins AE, Brewer JA.Finding the right match: mindfulness training may potentiate the therapeutic effect of nonjudgment of inner experience on smoking cessation. Substance Use and Misuse 2014;49(5):586-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
Shahab 2013 {published data only}
- Shahab L, Sarkar BY, West R.The acute effects of yogic breathing exercises on craving and withdrawal symptoms in abstaining smokers. Psychopharmacology 2013;225(4):875-82. [DOI: 10.1007/s00213-012-2876-9] [PMID: ] [DOI] [PubMed] [Google Scholar]
Sharma 2013 {published data only}
- Sharma MP, Sharma MK.Mindfulness, an integrated approach for cessation of smoking in workplace. International Journal of Yoga 2013;6(1):80. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sidhu 2016 {published data only}
- Sidhu AK, Sussman S, Tewari A, Bassi S, Arora M.Project EX-India: a classroom-based tobacco use prevention and cessation intervention program. Addictive Behaviors 2016;53:53-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Spears 2019 {published data only}
- Spears CA, Abroms LC, Glass CR, Hedeker D, Eriksen MP, Cottrell-Daniels C, et al.Mindfulness- based smoking cessation enhanced with mobile technology (iquit mindfully): pilot randomized controlled trial. JMIR mHealth and uHealth 2019;7(6):e13059. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sussman 2004 {published data only}
- Sussman S, McCuller WJ, Zheng H, Pfingston YM, Miyano J, Dent CW.Project EX: a program of empirical research on adolescent tobacco use cessation. Tobacco Induced Diseases 2004;2(3):119-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tang 2013 {published data only}
- Tang YY, Tang R, Posner MI.Brief meditation training induces smoking reduction. Proceedings of the National Academy of Sciences of the United States of America 2013;110(34):13971-5. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ussher 2009 {published data only}
- Ussher M, Cropley M, Playle S, Mohidin R, West R.Effect of isometric exercise and body scanning on cigarette cravings and withdrawal symptoms. Addiction 2009;104(7):1251-7. [DOI: 10.1111/j.1360-0443.2009.02605.x] [PMID: ] [DOI] [PubMed] [Google Scholar]
Zeng 2016 {published data only}
- Zeng EY, Heffner JL, Copeland WK, Mull KE, Bricker JB.Get with the program: adherence to a smartphone app for smoking cessation. Addictive Behaviors 2016;63:120-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies awaiting assessment
Pumariega 2020 {published data only}
- Pumariega YN, Calheiros PR, Cardenas RN, Farias ED, Torres CD.Relapse prevention based on mindfulness in the treatment of tobacco dependence - Brazil [Prevenção de recaídas baseada em mindfulness no tratamento do tabagismo – Brasil]. CES Psicología 2020;13(2):129-43. [DOI: ] [Google Scholar]
References to ongoing studies
CTRI/2020/01/022692 {published data only}CTRI/2020/01/022692
- CTRI/2020/01/022692.Yoga as a complementary intervention for tobacco cessation: a PROBE trial. www.ctri.nic.in/Clinicaltrials/pdf_generate.php?trialid=33207&EncHid=&modid=&compid=%27,%2733207det%27 first received 10 January 2020. [URL: http://www.ctri.nic.in/Clinicaltrials/pdf_generate.php?trialid=33207&EncHid=&modid=&compid=%27,%2733207det%27]
NCT01098955 {published data only}
- NCT01098955.Smoking cessation treatment for head & neck cancer patients. clinicaltrials.gov/ct2/show/NCT01098955 first received 5 April 2010. [https://clinicaltrials.gov/ct2/show/NCT01098955]
NCT01982110 {published data only}
- NCT01982110.A mindfulness based application for smoking cessation (MBSC). clinicaltrials.gov/ct2/show/NCT01982110 first received 13 November 2013. [URL: https://clinicaltrials.gov/ct2/show/NCT01982110]
NCT02037360 {published data only}
- NCT02037360.Mobile mindfulness training for smoking cessation. clinicaltrials.gov/ct2/show/NCT02037360 first received 15 January 2014. [URL: https://clinicaltrials.gov/ct2/show/NCT02037360]
NCT02421991 {published data only}
- NCT02421991.Telephone-delivered interventions for smoking cessation (TALK). clinicaltrials.gov/ct2/show/NCT02421991 first received 21 April 2015.
NCT03253445 {published data only}
- NCT03253445.Individual Acceptance and Commitment Therapy (ACT) for smoking cessation for schizophrenic patients. clinicaltrials.gov/ct2/show/NCT03253445 first received 17 August 2017.
Additional references
Baer 2003
- Baer RA.Mindfulness training as a clinical intervention: a conceptual and empirical review. Clinical Psychology: Science and Practice 2003;10(2):125-43. [DOI: 10.1093/clipsy.bpg015] [DOI] [Google Scholar]
Bishop 2004
- Bishop SR, Lau M, Shapiro S, Carlson L, Anderson ND, Carmody J, et al.Mindfulness: a proposed operational definition. Clinical Psychology: Science and Practice 2004;11(3):230-41. [DOI: 10.1093/clipsy.bph077] [DOI] [Google Scholar]
Brewer 2010
- Brewer JA, Bowen S, Smith JT, Marlatt GA, Potenza MN.Mindfulness-based treatments for co-occurring depression and substance use disorders: what can we learn from the brain? Addiction 2010;105(10):1698-706. [DOI: 10.1111/j.1360-0443.2009.02890.x] [DOI] [PMC free article] [PubMed] [Google Scholar]
Brown 2008
- Brown RA, Palm KM, Strong DR, Lejuez CW, Kahler CW, Zvolensky MJ, et al.Distress tolerance treatment for early-lapse smokers: rationale, program description, and preliminary findings. Behavior Modification 2008;32(3):302-32. [DOI: ] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Carim‐Todd 2013
- Carim-Todd L, Mitchell SH, Oken BS.Mind–body practices: an alternative, drug-free treatment for smoking cessation? A systematic review of the literature. Drug and Alcohol Dependence 2013;132(3):399-410. [DOI: ] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Chamberlain 2017
- Chamberlain C, O’Mara-Eves A, Porter J, Coleman T, Perlen SM, Thomas J, et al.Psychosocial interventions for supporting women to stop smoking in pregnancy. Cochrane Database of Systematic Reviews 2017, Issue 2. Art. No: CD001055. [DOI: 10.1002/14651858.CD001055.pub5] [DOI] [PMC free article] [PubMed] [Google Scholar]
Correa‐Fernández 2012
- Correa-Fernández V, Ji L, Castro Y, Heppner WL, Vidrine JI, Costello TJ, et al.Mediators of the association of major depressive syndrome and anxiety syndrome with postpartum smoking relapse. Journal of Consulting and Clinical Psychology 2012;80(4):636-48. [DOI: 10.1037/a0027532] [DOI] [PMC free article] [PubMed] [Google Scholar]
Deeks 2021
- Deeks JJ, Higgins JP, Altman DG editor(s).Chapter 10: Analysing data and undertaking meta-analyses. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane, 2021. Available from training.cochrane.org/handbook/archive/v6.2/chapter-10.
Doll 2004
- Doll R, Peto R, Boreham J, Sutherland I.Mortality in relation to smoking: 50 years’ observations on male British doctors. BMJ 2004;328(7455):1519-27. [DOI: 10.1136/bmj.38142.554479.AE] [DOI] [PMC free article] [PubMed] [Google Scholar]
Fanshawe 2017
- Fanshawe TR, Halliwell W, Lindson N, Aveyard P, Livingstone-Banks J, Hartmann-Boyce J.Tobacco cessation interventions for young people.. Cochrane Database of Systematic Reviews 2017, Issue 11. Art. No: CD003289. [DOI: 10.1002/14651858.CD003289.pub6] [DOI] [PMC free article] [PubMed] [Google Scholar]
Glassman 1990
- Glassman AH, Helzer JE, Covey LS, Cottler LB, Stetner F, Tipp JE, et al.Smoking, smoking cessation, and major depression. Journal of the American Medical Association 1990;264(12):1546-9. [DOI: 10.1001/jama.1990.03450120058029] [DOI] [PubMed] [Google Scholar]
Goldberg 2018
- Goldberg SB, Tucker RP, Greene PA, Davidson RJ, Wampold BE, Kearney DJ, et al.Mindfulness-based interventions for psychiatric disorders: a systematic review and meta-analysis. Clinical Psychology Review 2018;59:52-60. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Goyal 2014
- Goyal M, Singh S, Sibinga EM, Gould NF, Rowland-Seymour A, Sharma R, et al.Meditation programs for psychological stress and well-being: a systematic review and meta-analysis. JAMA Internal Medicine 2014;174(3):357-68. [DOI: 10.1001/jamainternmed.2013.13018] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hayes 2016
- Hayes SC, Strosahl KD, Wilson KG.Acceptance and Commitment Therapy. 2nd edition. New York: Guilford Press, 2016. [Google Scholar]
Heatherton 1989
- Heatherton TF, Kozlowski LT, Frecker RC, Rickert W, Robinson J.Measuring the heaviness of smoking: using self-reported time to the first cigarette of the day and number of cigarettes smoked per day. British Journal of Addiction 1989;84(7):791-9. [DOI: 10.1111/j.1360-0443.1989.tb03059.x] [DOI] [PubMed] [Google Scholar]
Heatherton 1991
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO.The Fagerström Test for Nicotine Dependence: a revision of the Fagerstrom Tolerance Questionnaire. British Journal of Addiction 1991;86(9):1119-27. [DOI: ] [DOI] [PubMed] [Google Scholar]
Heppner 2015
- Heppner WL, Spears CA, Vidrine JI, Wetter DW.Mindfulness and emotion regulation. In: Ostafin BD, Robinson MD, Meier BP, editors(s). Handbook of Mindfulness and Self-Regulation. New York: Springer, 2015:107-20. [DOI: 10.1007/978-1-4939-2263-5_9] [DOI] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG.Measuring inconsistency in meta-analyses. BMJ 2003;327:557-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Altman DG, Sterne JA editor(s).Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. training.cochrane.org/handbook/archive/v5.1/.
Hölzel 2011
- Hölzel BK, Lazar SW, Gard T, Schuman-Olivier Z, Vago DR, Ott U.How does mindfulness meditation work? Proposing mechanisms of action from a conceptual and neural perspective. Perspectives on Psychological Science 2011;6(6):537-59. [DOI: 10.1177/1745691611419671] [DOI] [PubMed] [Google Scholar]
Kabat‐Zinn 2013
- Kabat-Zinn J.Full Catastrophe Living, revised edition: How to Cope With Stress, Pain and Illness Using Mindfulness Meditation. London: Hachette UK, 2013. [Google Scholar]
Keng 2011
- Keng S-L, Smoski MJ, Robins CJ.Effects of mindfulness on psychological health: a review of empirical studies. Clinical Psychology Review 2011;31(6):1041-56. [DOI: 10.1016/j.cpr.2011.04.006] [DOI] [PMC free article] [PubMed] [Google Scholar]
Linehan 2018
- Linehan MM.Cognitive-behavioral Treatment of Borderline Personality Disorder. New York: Guilford Publications, 2018. [Google Scholar]
Maglione 2017
- Maglione MA, Ruelaz Maher A, Ewing B, Colaiaco B, Newberry S, Kandrack R, et al.Efficacy of mindfulness meditation for smoking cessation: a systematic review and meta-analysis. Addictive Behaviors 2017;69:27-34. [DOI: 10.1016/j.addbeh.2017.01.022] [DOI] [PubMed] [Google Scholar]
Marchand 2013
- Marchand WR.Mindfulness meditation practices as adjunctive treatments for psychiatric disorders. Psychiatric Clinics of North America 2013;36(1):141-52. [DOI: 10.1016/j.psc.2013.01.002] [DOI] [PubMed] [Google Scholar]
Marques 2021
- Marques MM, Carey RN, Norris E, Evans F, Finnerty AN, Hastings J, et al.Delivering behaviour change interventions: development of a mode of delivery ontology. Wellcome Open Research 2021;5:125. [DOI: 10.12688/wellcomeopenres.15906.2] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Michie 2013
- Michie S, Richardson M, Johnston M, Abraham C, Francis J, Hardeman W, et al.The behavior change technique taxonomy (v1) of 93 hierarchically clustered techniques: building an international consensus for the reporting of behavior change interventions. Annals of Behavioral Medicine 2013;46(1):81-95. [DOI: 10.1007/s12160-013-9486-6] [DOI] [PubMed] [Google Scholar]
Michie 2017
- Michie S, Thomas J, Johnston M, Aonghusa PM, Shawe-Taylor J, Kelly MP, et al.The Human Behaviour-Change Project: harnessing the power of artificial intelligence and machine learning for evidence synthesis and interpretation. Implementation Science 2017;12(1):121. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Norris 2020
- Norris E, Marques MM, Finnerty AN, Wright AJ, West R, Hastings J, et al.Development of an Intervention Setting Ontology for behaviour change: specifying where interventions take place. Wellcome Open Research 2020;5:124. [DOI: 10.12688/wellcomeopenres.15904.1] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Norris 2021
- Norris E, Wright AJ, Hastings J, West R, Boyt N, Michie S.Specifying who delivers behaviour change interventions: development of an Intervention Source Ontology. Wellcome Open Research 2021;6:77. [DOI: 10.12688/wellcomeopenres.16682.1] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Oikonomou 2017
- Oikonomou MT, Arvanitis M, Sokolove RL.Mindfulness training for smoking cessation: a meta-analysis of randomized-controlled trials. Journal of Health Psychology 2017;22(14):1841-50. [DOI: 10.1177/1359105316637667] [PMID: ] [DOI] [PubMed] [Google Scholar]
Salmon 2009
- Salmon P, Lush E, Jablonski M, Sephton SE.Yoga and mindfulness: clinical aspects of an ancient mind/body practice. Cognitive and Behavioral Practice 2009;16(1):59-72. [DOI: ] [Google Scholar]
Schünemann 2021
- Schünemann HJ, Higgins JP, Vist GE, Glasziou P, Akl EA, Skoetz N, et al.Chapter 14: Completing ‘Summary of findings’ tables and grading the certainty of the evidence. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 202a). Cochrane, 2021. Available from training.cochrane.org/handbook/archive/v6.2/chapter-14.
Segal 2002
- Segal ZV, Williams JM, Teasdale JD.Mindfulness-based Cognitive Therapy for Depression: A New Approach to Preventing Relapse. New York: Guilford Press, 2002. [Google Scholar]
Shiffman 2004
- Shiffman S, West R, Gilbert D, SRNT Work Group on the Assessment of Craving and Withdrawal in Clinical Trials.Recommendation for the assessment of tobacco craving and withdrawal in smoking cessation trials. Nicotine & Tobacco Research 2004;6(4):559-614. [DOI: 10.1080/14622200410001734067] [DOI] [PubMed] [Google Scholar]
Shiffman 2005
- Shiffman S.Dynamic influences on smoking relapse process. Journal of Personality 2005;73(6):1715-48. [DOI: 10.1111/j.0022-3506.2005.00364.x] [DOI] [PubMed] [Google Scholar]
Taylor 2014
- Taylor G, McNeill A, Girling A, Farley A, Lindson-Hawley N, Aveyard P.Change in mental health after smoking cessation: systematic review and meta-analysis. BMJ 2014;348:g1151. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Taylor 2021
- Taylor GM, Lindson N, Farley A, Leinberger-Jabari A, Sawyer K, te Water Naude R, et al.Smoking cessation for improving mental health. Cochrane Database of Systematic Reviews 2021, Issue 3. Art. No: CD013522. [DOI: 10.1002/14651858.CD013522.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
West 2005
- West R, Hajek P, Stead L, Stapleton J.Outcome criteria in smoking cessation trials: proposal for a common standard. Addiction 2005;100(3):299-303. [DOI: 10.1111/j.1360-0443.2004.00995.x] [DOI] [PubMed] [Google Scholar]
West 2017
- West R.Tobacco smoking: health impact, prevalence, correlates and interventions. Psychology & Health 2017;32(8):1018-36. [DOI: 10.1080/08870446.2017.1325890] [DOI] [PMC free article] [PubMed] [Google Scholar]
WHO 2019
- World Health Organization.Tobacco. www.who.int/news-room/fact-sheets/detail/tobacco (accessed 20 May 2020).


