Abstract
N-Chlorotaurine, the main representative of long-lived oxidants found in the supernatant of stimulated granulocytes, has been investigated systematically with regard to its antibacterial activity at different physiological concentrations for the first time. N-Chlorotaurine (12.5 to 50 μM) demonstrated a bactericidal effect i.e., a 2 to 4 log10 reduction in viable counts, after incubation at 37°C for 6 to 9 h at pH 7.0, which effect was significantly enhanced in an acidic milieu (at pH 5.0), with a 3 to 4 log10 reduction after 2 to 3 h. Moreover, bacteria were attenuated after being incubated in N-chlorotaurine for a sublethal time, as demonstrated with the mouse peritonitis model. The supernatant of stimulated granulocytes exhibited similar activity. Transmission electron microscopy revealed changes in the bacterial cell membrane and cytoplasmic disintegration with both reacting systems, even in the case of a mere attenuation. The results of this study suggest a significant bactericidal function of N-chlorotaurine and other chloramines during inflammation.
Oxidants are important tools that stimulated human phagocytes use to attack and kill pathogens (26). Upon phagocytosis, neutrophilic and eosinophilic granulocytes and monocytes generate hypochlorite (HOCl) through myeloperoxidase (9, 31, 32). HOCl immediately oxidizes NH groups, and less-reactive, long-lived oxidants, which have been identified as chloramines (R-NHCl compounds), are generated (8, 32, 34).
Chloramine concentrations of 30 to 100 μM have been detected in the supernatants of 0.25 × 107 to 1.0 × 107 stimulated granulocytes. N-Chlorotaurine (NCT) (ClHN—CH2—CH2—SO3−) proved to be the main representative of these compounds and was estimated to reach concentrations of 10 to 50 μM (8, 34). It is the most stable N-chloro amino acid which can be reduced in structure to a β-amino acid (4), and it is thought to maintain oxidation capacity (COX) for many hours during inflammation (8, 23).
Concerning biological functions, it is assumed that the formation of NCT protects human cells from damage by HOCl, which, scavenged by taurine, leads to NCT with very low cytotoxicity (1, 28). On the other hand, NCT inhibits the production of tumor necrosis factor, nitric oxide, and prostaglandins by macrophages, so an immune modulatory function was discussed previously (14, 22).
The bactericidal activities of NCT both at concentrations of 10 to 50 μM and in the supernatant of neutrophils have never been investigated systematically, and controversial results and statements can be found in previous studies. Incubation of Escherichia coli in the supernatant of stimulated granulocytes for 60 to 80 min at pH 7.3 did not lead to a reduction in viable bacterial counts (8), while 200 to 500 μM HOCl in the presence of 10 mM taurine, which equals 200 to 500 μM NCT, killed E. coli after 2 h of incubation at pH 6.6 and 37°C (31). By contrast, there is no doubt about the bactericidal, fungicidal, and virucidal activity of supraphysiological NCT concentrations of 0.55 to 55 mM (15, 16, 18). Moreover, lag of regrowth and attenuation of virulence of Staphylococcus aureus strain Smith diffuse after short, sublethal incubations in 55 mM NCT (i.e., postantibiotic effect [PAE]) have been observed (17). Killing activity of 10 to 200 μM NCT against Schistosoma mansoni and Candida albicans has been reported (33, 36).
In this study we investigated the antibacterial activity of 10 to 50 μM NCT in relation to the totality of chloramines produced by granulocytes at pH 7 and pH 5 with extended incubation times in order to gain more insight into the function of these compounds in the human defense system.
MATERIALS AND METHODS
Reagents and buffers.
Pure NCT as a crystalline sodium salt (Mr = 181.52 g/mol) (15) was dissolved in 0.01 M phosphate (pH 7.0)- or citrate (pH 5.0)-buffered saline. Buffers, sodium chloride, sodium thiosulfate, CaCl2, MgCl2, potassium chloride, and glucose (all reagent grade) were purchased from Merck (Darmstadt, Germany). Dulbecco's buffered saline (pH 7.3) contained 0.133 g of CaCl2, 0.1 g of MgCl2, 0.2 g of KCl, 0.2 g of K2HPO4, 8.0 g of NaCl, 1.15 g of NaH2PO4 and 1.0 g of d-glucose per liter. Phorbol 12-myristate 13-acetate (PMA) and poly-l-lysine hydrobromide (molecular weight, 70,000 to 150,000) were from Sigma (St. Louis, Mo.). Glutaraldehyde and osmium tetroxide were from Agar (Essex, United Kingdom), and ruthenium red was from BDH Chemicals Ltd. (Poole, United Kingdom).
Inactivation of NCT and other chloramines.
A 6% (242 mM) aqueous sodium thiosulfate solution was diluted 250-fold in the test solution to achieve a final concentration of 1 mM.
Bacteria and media.
Bacterial strains (S. aureus Smith diffuse B9 and Streptococcus pyogenes d 68, both slime-producing and highly encapsulated strains [kindly provided by J. Hildebrandt, Sandoz Scientific Center Vienna]; S. aureus ATCC 25923; Staphylococcus epidermidis ATCC 12228; Proteus mirabilis ATCC 14153; E. coli ATCC 11129; and Pseudomonas aeruginosa ATCC 27853) deep frozen for storage were grown overnight on tryptic soy agar (Merck). Colonies from this agar were grown in tryptic soy broth (Merck) at 37°C overnight, centrifuged at 1,800 × g, washed twice in 0.9% NaCl, and diluted in saline to an absorption of 0.08 to 0.01 at 625 nm (ca. 1 × 108 CFU/ml) before use.
Bactericidal activity of NCT.
Bacteria were diluted 100-fold in buffered NCT solution to 0.2 × 106 to 1.0 × 106 CFU/ml. Immediately subsequent to incubation at 37°C for different lengths of time, aliquots were removed and the NCT was inactivated. Portions (50 μl) of undiluted aliquots as well as of 100-fold dilutions in saline were spread in duplicate onto tryptic soy agar plates with an automatic spiral plater (Don Whitley Scientific Limited, West Yorkshire, United Kingdom), allowing a detection limit of 10 CFU/ml. The plates were incubated at 37°C, and the CFU were counted after 24 and 48 h. Controls without NCT were treated the same way.
Bactericidal activity of supernatant of stimulated granulocytes.
Granulocytes were isolated from heparinized venous blood of healthy adult volunteers by centrifugation through Lymphoprep (Nycomed, Oslo, Norway). Erythrocytes were lysed by treatment with 0.84% NH4Cl at 37°C for 20 min. Granulocytes were suspended in Dulbecco's buffered saline (pH 7.3) to a concentration of 1 × 107 cells/ml and stimulated with 100 ng of PMA per ml for 1 h at 37°C. Subsequently, cells were centrifuged for 10 min at 1,500 × g. The COX in the supernatant was determined as described below (see “stability of long-lived oxidants produced by granulocytes”). Volumes (10 μl) of the bacterial suspension were diluted in 1 ml of supernatant to concentrations of 0.2 × 106 to 1.0 × 106 CFU/ml. After incubation times of 1, 2, 5, and 18 h at 37°C, bacterial counts were performed as described above. A 1-ml portion of each supernatant was inactivated before the addition of bacteria and served as a control. For experiments in acidic solution, the pH was adjusted to 5.0 by the addition of a few microliters of 4 N sulfuric acid per 10 ml of supernatant.
Attenuation of bacteria by NCT and by supernatant of stimulated granulocytes after sublethal incubation time (PAE). (i) Evaluation of PAE in vitro.
Bacteria were diluted 100-fold in buffered NCT solution or the supernatant of stimulated granulocytes to concentrations of 0.5 × 106 to 1.1 × 106 CFU/ml. After a sublethal incubation time of 1 to 3 h at 37°C, oxidants were inactivated. Aliquots were diluted 100-fold in prewarmed tryptic soy broth which was incubated at 37°C. CFU were determined hourly, and the curves of bacterial regrowth were constructed. Buffer solutions without NCT and with inactivated NCT or inactivated supernatant were investigated in parallel as controls.
The duration of the lag of regrowth was calculated using the following equation: lag time = T − C, where T is the time required for the colony count in the test culture to increase one log10 unit above the count at zero time (immediately after 1:1,000 dilution in prewarmed tryptic soy broth) and C is the time required for the same increase in the control culture (3).
(ii) Evaluation of PAE with in vivo model.
The mouse peritonitis model (11) using S. pyogenes d 68 was applied. The animal tests were performed according to the Principles of Animal Care and were approved by the Austrian Federal Government for Science and Research.
Bacteria (0.5 × 105 to 1.0 × 105 CFU/ml) were treated for 30, 60, and 90 min with 50 μM NCT buffer solution and for 5 h with supernatant of stimulated granulocytes at 37°C and pH 7. After inactivation, 0.5-ml volumes of 1:250 to 1:1,000 dilutions in saline were injected intraperitoneally to Swiss mice (6 to 8 weeks old, 24 to 33 g). Control experiments, where chloramines had been inactivated before the addition of pathogens, were performed in parallel. Quantitative cultures were performed from aliquots obtained right before injection to confirm that the CFU counts in samples and controls were equal. Mice were observed for clinical signs of peritonitis, i.e., changes in attitude toward care and refusal of food intake, which were connected with lethal outcome. Blood was obtained from the tail vessels repeatedly, weighed, and diluted with 250 μl of distilled water. Bacterial counts were performed as described above.
Demonstration of antibacterial effect of NCT by electron microscopy.
S. aureus Smith diffuse (1.0 × 108 to 2.0 × 108 CFU/ml), chosen as an encapsulated, highly pathogenic model organism, was incubated in 1 ml of 50 μM NCT solution at pH 7.0 for 30, 60, and 120 min. Control experiments (without NCT) were performed in parallel. Incubation was stopped either by inactivation before centrifugation at 16,000 × g for 5 min or by immediately fixing the pelleted samples.
For transmission electron microscopy, two complementary protocols were employed, ambient-temperature chemical fixation and ultra-rapid cryofixation followed by freeze-substitution. Briefly, chemical fixation was done with glutaraldehyde (2.5% [vol/vol] in 0.1 M sodium cacodylate buffer, pH 7.4, 120 min, 25°C) followed by osmium tetroxide (1% [wt/vol] in double-distilled water, 60 min, 4°C), both supplemented with 0.15% (wt/vol) ruthenium red to improve preservation of cell surface carbohydrates (10, 13). Cryofixation was achieved with slam-freezing. Freeze-substitution was carried out for 8 h at −90°C with anhydrous acetone containing 2% (wt/vol) osmium tetroxide. All samples were embedded in Epon epoxy resin. Thin (80-nm) sections were optionally poststained with uranyl acetate (0.5% [wt/vol]) and lead citrate (30 and 3 min, respectively) and examined with a transmission electron microscope at 60 to 100 kV (Zeiss EM10A [Carl Zeiss, Inc., Oberkochen, Germany] or Jeol 1200EX [JEOL, Ltd., Tokyo, Japan]).
For scanning electron microscopy, bacteria were rinsed another five times in distilled water, and having been immobilized for 5 min on poly-l-lysine-coated coverslips, they were fixed with glutaraldehyde and osmium tetroxide, without the addition of ruthenium red. After dehydration, the samples were subjected to critical-point drying (CPD 030; BAL-TEC, Balzers, Liechtenstein) and 15-nm gold-palladium sputter-coating (MED 020; BAL-TEC). The specimens were viewed with a Zeiss DSM 982 Gemini field-emission scanning electron microscope.
Stability of long-lived oxidants produced by granulocytes.
The COX of supernatants of stimulated granulocytes was determined by the addition of potassium iodide in molar excess and measurement of the formed triiodide (>N—Cl + 3I− + H+↔I3− + >N—H) at 350 nm which is the maximum wavelength (λmax) of I3− (ɛ = 22,900/mol/cm [34]). Supernatants were stored in the dark at 2, 20, and 37°C. For evaluations of stability, the pH was maintained at 7.3 (Dulbecco's phosphate-buffered saline) or pH 5.0 (obtained by the addition of sulfuric acid).
Statistical analyses.
Student's t test was used for comparison of paired means of two groups of measurements. One-way analysis of variance (Graphpad Software Inc.) and Dunnett's multiple comparison test were applied for evaluation of the significance of lag of regrowth. P values of <0.05 were considered significant.
RESULTS
Bactericidal activity of NCT.
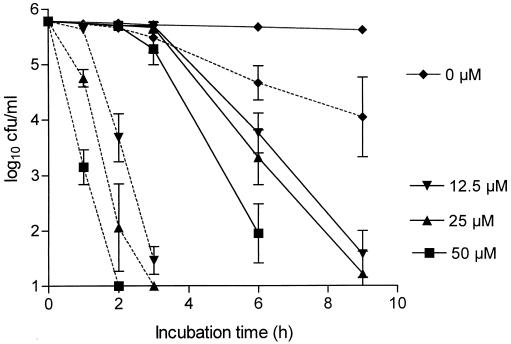
Micromolar concentrations of NCT in buffer solution demonstrated considerable bactericidal activity against all test bacteria, which was increased significantly by an acidic pH. Fig. 1 shows the killing of S. aureus Smith diffuse by 12.5, 25, and 50 μM NCT. In Table 1 the activities of 30 μM NCT at pH 7.0 and 5.0 against other test strains are shown. At pH 7, P. aeruginosa and E. coli were less susceptible than gram-positive cocci and P. mirabilis. This difference disappeared at pH 5.0, where a log10 reduction in CFU of ≥4 was achieved in all strains after an incubation time of 3 to 6 h. S. epidermidis and, to a lesser extent, S. aureus Smith diffuse showed a slow decrease in CFU in acidic milieu in the absence of NCT. Sodium thiosulfate and NCT, which was inactivated by sodium thiosulfate before the addition of bacteria, exerted no bactericidal activity (data not shown).
FIG. 1.
Bactericidal activity of 12.5 to 50 μM NCT against S. aureus Smith diffuse in 0.01 M phosphate-buffered saline at 37°C and pH 7.0 (solid lines) or pH 5.0 (dotted lines). The results are the means ± the standard deviations (SD) of three separate experiments. The differences resulting from both the pH level and the NCT concentration were significant (P < 0.01) except for between 12.5 and 25 μM NCT at pH 7.0.
TABLE 1.
Bactericidal activity of NCTa
| Strain | pH | Log10 reduction in CFU after incubation for:
|
|||||
|---|---|---|---|---|---|---|---|
| 1 h | 2 h | 3 h | 6 h | 9 h | 24 h | ||
| S. aureus ATCC 25923 | 7.0 | NDc | ND | 0.2 (0.5) | 1.9 (0.6) | 2.9 (1.0) | >4.5 (0.3) |
| 5.0 | 0.4 (0.6) | 1.4 (0.4) | 2.3 (0.4) | 3.9 (0.8) | ND | ND | |
| S. epidermidis ATCC 12228 | 7.0 | ND | ND | 0.1 (0.2) | 1.6 (0.3) | 3.0 (0.3) | >4.8 (0.1) |
| 5.0 | 0.9 (0.8) | 2.4 (0.6) | 4.0 (0.6) | >4.8 (0.1) | ND | ND | |
| P. mirabilis ATCC 14153 | 7.0 | ND | ND | 0.6 (0.6) | 2.9 (0.5) | >4.7 (0.1) | >4.7 (0.1) |
| 5.0 | 3.1 (0.4) | 4.5 (0.1) | >4.9 (0.1) | >4.9 (0.1) | ND | ND | |
| E. coli ATCC 11129 | 7.0 | ND | ND | 0.1 (0.3) | 1.1 (1.1) | 2.0 (1.7) | 3.2 (1.5) |
| 5.0 | 2.1 (0.3) | 4.1 (0.5) | >4.8 (0.2) | >4.8 (0.2) | ND | ND | |
| P. aeruginosa ATCC 27853b | 7.0 | ND | ND | 0.3 (0.6) | 0.4 (0.5) | 0.8 (0.6) | 1.7 (0.7) |
| 5.0 | 1.3 (0.6) | 3.3 (0.4) | >5.0 (0.2) | >5.0 (0.2) | ND | ND | |
Bactericidal activity of NCT (30 μM) against 0.2 × 106 to 1.0 × 106 CFU/ml during incubation in buffer solution at 37°C for different periods of time. Values are means (standard deviations) from four different experiments. In control experiments (without NCT), the maximum log10 reduction in CFU was 0.4, except that for S. epidermidis at pH 5.0 it was 0.9 after 3 h and 2.7 after 6 h.
For this organism, the mean log10 reduction in CFU after 72 h of incubation was 2.9 (standard deviation, 0.6).
ND, not determined.
Bactericidal activity of supernatant of stimulated granulocytes.
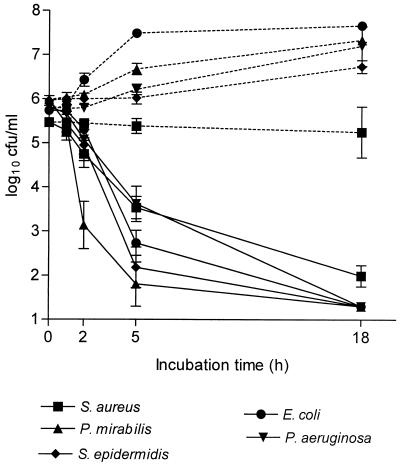
A concentration of 1 × 107 granulocytes/ml produced an oxidant concentration of 41.5 ± 13.0 μM (value is mean ± standard deviation, n = 22). At pH 7.3, a slow bactericidal action against S. pyogenes d 68 (1.6 and 3.0 log10 reduction after 18 and 42 h of incubation, respectively) was observed and a bacteriostatic activity against the other test bacteria was found. At pH 5.0, however, the bactericidal activity of supernatants, containing oxidant concentrations of 27, 34, 57, and 61 μM, against all strains was significant (Fig. 2). Treatment of supernatants with sodium thiosulfate (controls) resulted in loss of antibacterial activity at both pH values.
FIG. 2.
Bactericidal activity of the supernatant of stimulated granulocytes at pH 5.0 and 37°C. For control experiments, inactivation by the addition of thiosulfate was carried out prior to addition of bacteria. The results are the means ± the standard errors of the means (SEM) of 3 to 4 separate experiments. All differences between control (dotted lines) and test samples (solid lines) were significant (P < 0.01).
In three additional experiments with supernatants containing oxidant concentrations of 27, 31, and 35 μM, P. aeruginosa and E. coli were hardly killed by supernatant after 5 to 18 h at 37°C and pH 5.0. The COX decreased about two-thirds, with the oxidant concentration reaching 10 μM within 5 h (for stability of chloramines, see below), a level obviously below a bactericidal concentration. Indeed, when these bacteria were washed after 5 h of incubation and reincubated in a portion of the same supernatant stored at 2°C in the meantime, the log10 reduction of viable counts exceeded 3 after 18 h. This indicates a dependency of the killing time on the concentration of oxidants.
Postantibiotic effect of NCT and supernatant of granulocytes.
In vitro, lag of regrowth was detected in all test strains which had been incubated in supernatant or 30 μM NCT for a sublethal incubation time (Table 2). Similar to the bactericidal activity, the PAE was promoted by acidic pH.
TABLE 2.
PAE of bacteria
| Organism | Lag of regrowth (h) after incubation ina:
|
|||
|---|---|---|---|---|
| NCT
|
Sup
|
|||
| pH 7.0 | pH 5.0 | pH 7.3 | pH 5.0 | |
| S. aureus | 0.9–1.1 | 1.0–1.2 | 0.8–1.4 | 0.3–0.9 |
| S. epidermidis | 0.7–0.9 | 1.3–1.3 | 0.6–1.3 | 0.4–1.0 |
| P. mirabilis | 2.1–3.2 | 1.3–1.4 | 0 | 0.7–1.4 |
| E. coli | 0.8–1.1 | 0.3–0.5 | 0–0.3 | 0.8–1.5 |
| P. aeruginosa | 0.3–0.3 | 0.4–1.3 | 0–0.6 | 0.4–1.0 |
| S. pyogenes | 1.5–3.0 | 2.9–3.0 | 1.4–3.2 | 3.0–3.7 |
| S. aureus Smith diffuse | 0.8–2.0 | 0.5–1.4 | 0.6–1.2 | 0.9–1.4 |
Bacteria were incubated at 37°C in 30 μM NCT at pH 7.0 for 3 h or pH 5.0 for 1 h or in the supernatant (Sup) of stimulated granulocytes at pH 7.3 for 3 h or pH 5.0 for 1 h. Values are ranges from two to three independent experiments.
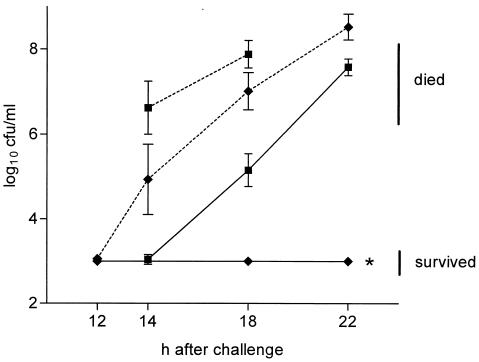
In vivo, Swiss mice challenged intraperitoneally with S. pyogenes d 68 preincubated in supernatant for a sublethal incubation time of 5 h survived or developed a retarded septicemia in blood, depending on the number of CFU injected (Fig. 3). Similarly, six of seven mice challenged with S. pyogenes pretreated with 50 μM NCT for 1.5 h survived, while all seven control animals died (60 to 140 CFU injected per mouse in both groups). Incubation times of 30 and 60 min were too short to induce a significant PAE in vivo.
FIG. 3.
In vivo PAE of S. pyogenes d 68 attenuated by incubation in supernatant of stimulated granulocytes for 5 h at pH 7.0. The mice were injected intraperitoneally with 200 to 324 CFU (n = 5) (■) or 14 to 76 CFU (n = 10) (⧫) of bacteria pretreated with oxidizing and inactivated supernatant, respectively. CFU in tail blood were counted, and the results are the means ± the SEM of 5 to 10 mice. All differences between control (dotted lines) and test samples (solid lines) were significant (P < 0.01). ∗, detection limit (no bacterial growth).
Electron microscopy of bacteria attenuated by NCT.
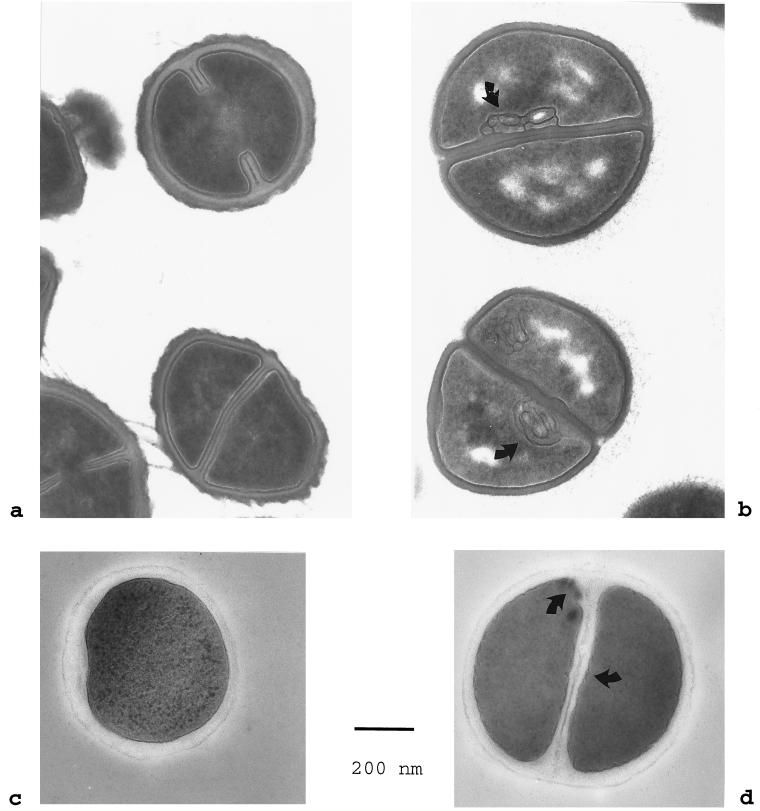
Scanning electron microscopy of attenuated S. aureus Smith diffuse did not reveal any effects on the bacterial surface. However, distinct ultrastructural changes were found by transmission electron microscopy performed with thin sections. After sublethal incubation in 50 μM NCT for 60 min, chemically fixed cells regularly displayed mesosome-like structures. By contrast, the controls, as well as samples incubated for 30 min only, were devoid of membrane infoldings. After 120 min, the cytoplasm also showed segregation patterns (Fig. 4a and b). When S. aureus was regrown in tryptic soy broth, differences between the samples and the controls were no longer observable. Cryofixed, freeze-substituted samples generally displayed similar but less-prominent changes in the bacterial cell membrane and the cytoplasm (Fig. 4c and d).
FIG. 4.
Transmission electron microscopy of S. aureus Smith diffuse. (a and c) Morphology of control bacteria incubated in phosphate-buffered saline for 120 min as seen after chemical fixation and after cryofixation and freeze-substitution, respectively; (b) prominent mesosome-like membrane infoldings (arrows) and segregation of the cytoplasm in chemically fixed specimens after 120 min of incubation in 50 μM NCT solution (pH 7.0); (d) undulations and slight infoldings of the bacterial cell membrane (arrows) after 120 min of incubation in NCT as seen in cryofixed, freeze-substituted samples. Bar = 200 nm. Magnification, ×62,500.
Stability of long-lived oxidants produced by granulocytes.
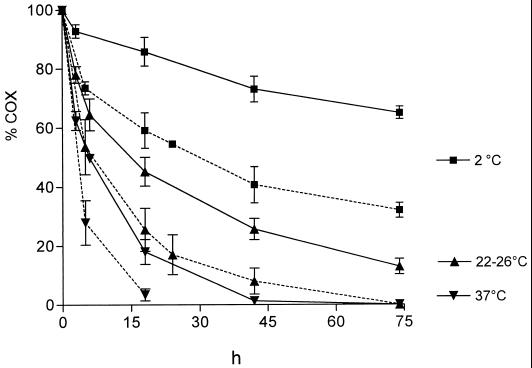
Time courses of COX at different conditions are shown in Fig. 5. As expected, stability increased at lower temperatures. The decrease in the COX was hastened by acidification. In any case, oxidative activity was detectable for at least 18 h.
FIG. 5.
Stability of long-lived oxidants in the supernatant of stimulated granulocytes (1 × 107/ml) at pH 7.3 (solid lines) and pH 5.0 (dotted lines) and at different temperatures. The results are the means ± the SD of several experiments (n = 5 for pH 7.3 and n = 3 to 5 for pH 5.0). The differences resulting from both temperature and pH were significant (P < 0.01).
DISCUSSION
In this study, the bactericidal activities of pure NCT at concentrations produced by granulocytes and of the supernatant of stimulated granulocytes have been demonstrated for the first time. Similar to previous findings obtained using 0.1 to 55.0 mM NCT (15–18), killing times not only were dependent on the NCT concentration but also decreased markedly by lowering the pH. The same pH dependence, too, was observed with the supernatant of stimulated granulocytes. This phenomenon, which has not yet been cleared up, may be connected with a higher reactivity of NCT which manifests itself in an increase of the redox potential at a lower pH (W. Gottardi, unpublished data).
The supernatant of leukocytes proved to be less bactericidal than pure NCT with the same initial oxidant concentration. This may have been the result of a more rapid decrease of COX due to chlorine consumption in the supernatant during the long incubation times needed for killing of bacteria. Subsequent to incubation in the presence of 106 staphylococci/ml, for 18 h at 37°C and pH 7, 80% of the oxidants were left in NCT solution while in supernatants, only 20% were left after the same period (Fig. 5). The reason might be the lower stability of other chloramines like N-chloro α-amino acids (27) prevailing along with NCT in the natural system. Some of them, e.g., monochloramine (NH2Cl) and the N-chloro derivatives of glycine, α-alanine and β-alanine, have demonstrated higher bactericidal activity than NCT in vitro (16, 31), while N-chloro albumin is less effective (16). The net antimicrobial effect of these long-lived oxidants in the supernatant is determined by the singular effects of the different compounds and their individual concentrations.
HOCl played no role in our supernatant experiments because it is consumed immediately in the presence of the organic compounds (23). The same is true for oxygen radicals, and hydrogen peroxide is known to decrease below bactericidal levels after a 1-h stimulation of cells (23, 30). The removal of bacterial killing by addition of thiosulfate, however, indicates the responsibility of oxidants for the antibacterial effects and rules out significant participation of other toxic compounds of the supernatant. Therefore, only long-lived oxidants, i.e., chloramines, come into question in analyses of bactericidal effect.
Considering the role of NCT and other chloramines in the destruction of pathogens in vivo, intracellular as well as extracellular sites have to be taken into account. Within phagosomes of neutrophils, it has been estimated that 10 to 60% of the HOCl produced by myeloperoxidase is used to form chloramines, and NCT is a major part of these compounds (29). On the other hand, most of the taurine (50 mM) was found to reside in the cytosolic compartment of neutrophils, and the granular concentration which is available for direct reaction with HOCl is about 100 μM (7). The exact granular concentration of NCT after the oxidative burst is unknown, but micromolar levels are plausible. The majority of phagocytosed bacteria is killed within a few minutes, but further reduction in the numbers of CFU occurs as time passes (21). This prolonged killing may be attributed, at least in part, to chloramines, whereas HOCl will be scavenged in the early phase of attack. The pH in phagosomes of neutrophils increases initially to 7.5 after the oxidative burst and decreases to 5.5 to 6.5 within 60 min thereafter (2, 24). This acidic pH will enhance the activity of NCT and chloramines against internalized bacteria.
As to extracellular domains, further equilibration by transhalogenation (NCT + R—NH2↔taurine + R—NHCl) as well as consumption of active chlorine by reaction with thiols will take place. Nevertheless, a few features support the possibility that microbicidal levels are achieved at local extracellular interstitial sites of inflammation. Because of their high stability (Fig. 5) (29), chloramines are thought to provide COX for some hours at inflammatory sites (23), and in inflammation samples enriched with NCT 10 to 55% of the initial COX remained for at least 2 h (16). There is some evidence that the intercellular microenvironment of granulocytes is protected from scavengers of oxidants (28). Chloramines are produced by continuously arriving granulocytes and monocytes, which may compensate for the loss of COX. In bronchial exudates of patients suffering from cystic fibrosis, chloramine concentrations of 118 ± 25 μM have been detected, indicating a high level of long-lived oxidants present extracellularly in vivo (35). It has been shown that bacterial inflammatory processes are predominantly accompanied by acidic pH (12, 25) which supports killing by these agents.
A considerable feature of the action of chloramines is the PAE at physiological concentrations, demonstrated for the first time in this study. Lag of bacterial regrowth connected with a mitigated course of infection takes place long before killing starts. The extent of PAE seems to correlate with bacterial virulence, since it was most pronounced in S. pyogenes d 68, which is highly pathogenic for mice. Therefore, the rapid attenuation of bacteria mediated by chloramines may be considered the first step in inactivation of invading pathogens.
This assumption is supported by the early occurrence of mesosomes and cytoplasmic disintegration of S. aureus Smith diffuse during sublethal exposure to NCT, as revealed by electron microscopy. Although mesosomes are artifactual structures resulting from chemical fixation, they are only inducible under certain physiological conditions (5, 6). Their constant occurrence after 60 min of incubation with sublethal NCT concentrations provides strong indirect evidence for alterations related to the bacterial cell membrane.
The results of this study provide support for NCT and other chloramines being powerful tools of granulocytes to impair pathogens. Thus, in addition to (i) detoxification of hypochlorite, (ii) providing COX to create more microbicidal chloramines like NH2Cl, and (iii) inducing immune modulatory effects, potent antimicrobial activity is probably a major function of NCT in the human defense system. The conceived use of NCT-Na as a natural disinfectant in human medicine, which is supported by positive results of clinical studies concerning tolerability in curing conjunctivitis (19, 20), gets additional encouragement in view of the bactericidal endogenous operation.
ACKNOWLEDGMENTS
This study was supported by the Austrian Science Fund (FWF) (grant no. P12298-MED) and by the Legerlotz Foundation. The spiral plater was financed by the Jubiläumsfonds of the Austrian National Bank (project no. 6801/1).
We acknowledge M. P. Dierich, head of the Institute of Hygiene and Social Medicine, and I. Jenewein for continuous support. We are grateful to Marieluise Kunc and Karin Gutleben (University of Innsbruck) as well as Arja Strandell and Marko Kolari (University of Helsinki) for excellent technical assistance in isolation of granulocytes, bacterial culture, and preparation of samples for electron microscopy.
REFERENCES
- 1.Cantin A M. Taurine modulation of hypochlorous acid-induced lung epithelial cell injury in vitro. Role of anion transport. J Clin Investig. 1994;93:606–614. doi: 10.1172/JCI117013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cech P, Lehrer R I. Phagolysosomal pH of human neutrophils. Blood. 1984;63:88–95. [PubMed] [Google Scholar]
- 3.Craig W A, Gudmundsson S. Postantibiotic effect. In: Lorian V, editor. Antibiotics in laboratory medicine. Baltimore, Md: Williams & Wilkins; 1996. pp. 296–329. [Google Scholar]
- 4.Cunningham C, Tipton K F, Dixon H B. Conversion of taurine into N-chlorotaurine (taurine chloramine) and sulphoacetaldehyde in response to oxidative stress. Biochem J. 1998;330:939–945. doi: 10.1042/bj3300939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dubochet J, McDowall A W, Menge B, Schmid E N, Lickfeld K G. Electron microscopy of frozen-hydrated bacteria. J Bacteriol. 1983;155:381–390. doi: 10.1128/jb.155.1.381-390.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ebersold H R, Cordier J L, Luthy P. Bacterial mesosomes: method dependent artifacts. Arch Microbiol. 1981;130:19–22. doi: 10.1007/BF00527066. [DOI] [PubMed] [Google Scholar]
- 7.Green T R, Fellman J H, Eicher A L, Pratt K L. Antioxidant role and subcellular location of hypotaurine and taurine in human neutrophils. Biochim Biophys Acta. 1991;1073:91–97. doi: 10.1016/0304-4165(91)90187-l. [DOI] [PubMed] [Google Scholar]
- 8.Grisham M B, Jefferson M M, Melton D F, Thomas E L. Chlorination of endogenous amines by isolated neutrophils. J Biol Chem. 1984;259:10404–10413. [PubMed] [Google Scholar]
- 9.Harrison J E, Schultz J. Studies on the chlorinating activity of myeloperoxidase. J Biol Chem. 1976;251:1371–1374. [PubMed] [Google Scholar]
- 10.Hayat M A. Fixation for electron microscopy. New York, N.Y: Academic Press; 1981. [Google Scholar]
- 11.Hunt G A, Moses A J. Acute infection of mice with Smith strain of Staphylococcus aureus. Science. 1958;128:1574–1575. doi: 10.1126/science.128.3338.1574. [DOI] [PubMed] [Google Scholar]
- 12.Light R W. Parapneumonic effusions and empyema. Clin Chest Med. 1985;6:55–62. [PubMed] [Google Scholar]
- 13.Luft J H. Ruthenium red and violet. I. Chemistry, purification, methods of use for electron microscopy and mechanisms of action. Anat Rec. 1971;171:347–368. doi: 10.1002/ar.1091710302. [DOI] [PubMed] [Google Scholar]
- 14.Marcinkiewicz J. Neutrophil chloramines: missing links between innate and acquired immunity. Immunol Today. 1997;18:577–580. doi: 10.1016/s0167-5699(97)01161-4. [DOI] [PubMed] [Google Scholar]
- 15.Nagl M, Gottardi W. In vitro experiments on the bactericidal action of N-chlorotaurine. Hyg Med. 1992;17:431–439. [Google Scholar]
- 16.Nagl M, Gottardi W. Enhancement of the bactericidal efficacy of N-chlorotaurine by inflammation samples and selected N-H compounds. Hyg Med. 1996;21:597–605. [Google Scholar]
- 17.Nagl M, Hengster P, Semenitz E, Gottardi W. The postantibiotic effect of N-chlorotaurine on Staphylococcus aureus. Application in the mouse peritonitis model. J Antimicrob Chemother. 1999;43:805–809. doi: 10.1093/jac/43.6.805. [DOI] [PubMed] [Google Scholar]
- 18.Nagl M, Larcher C, Gottardi W. Activity of N-chlorotaurine against herpes simplex- and adenoviruses. Antiviral Res. 1998;38:25–30. doi: 10.1016/s0166-3542(98)00005-9. [DOI] [PubMed] [Google Scholar]
- 19.Nagl M, Miller B, Daxecker F, Ulmer H, Gottardi W. Tolerance of N-chlorotaurine, an endogenous antimicrobial agent, in the rabbit and human eye—a phase I clinical study. J Ocul Pharmacol Ther. 1998;14:283–290. doi: 10.1089/jop.1998.14.283. [DOI] [PubMed] [Google Scholar]
- 20.Nagl M, Teuchner B, Pöttinger E, Ulmer H, Gottardi W. Tolerance of N-chlorotaurine, a new antimicrobial agent, in infectious conjunctivitis—a phase II pilot study. Ophthalmologica. 2000;214:111–114. doi: 10.1159/000027477. [DOI] [PubMed] [Google Scholar]
- 21.Nielsen S L, Black F T, Storgaard M, Obel N. Evaluation of a method for measurement of intracellular killing of Staphylococcus aureus in human neutrophil granulocytes. APMIS. 1995;103:460–468. [PubMed] [Google Scholar]
- 22.Park E, Quinn M R, Wright C E, Schuller Levis G. Taurine chloramine inhibits the synthesis of nitric oxide and the release of tumor necrosis factor in activated RAW 264.7 cells. J Leukoc Biol. 1993;54:119–124. doi: 10.1002/jlb.54.2.119. [DOI] [PubMed] [Google Scholar]
- 23.Passo S A, Weiss S J. Oxidative mechanisms utilized by human neutrophils to destroy Escherichia coli. Blood. 1984;63:1361–1368. [PubMed] [Google Scholar]
- 24.Segal A W, Geisow M, Garcia R, Harper A, Miller R. The respiratory burst of phagocytic cells is associated with a rise in vacuolar pH. Nature. 1981;290:406–409. doi: 10.1038/290406a0. [DOI] [PubMed] [Google Scholar]
- 25.Simmen H P, Blaser J. Analysis of pH and pO2 in abscesses, peritoneal fluid, and drainage fluid in the presence or absence of bacterial infection during and after abdominal surgery. Am J Surg. 1993;166:24–27. doi: 10.1016/s0002-9610(05)80576-8. [DOI] [PubMed] [Google Scholar]
- 26.Smith J A. Neutrophils, host defense, and inflammation: a double-edged sword. J Leukoc Biol. 1994;56:672–686. doi: 10.1002/jlb.56.6.672. [DOI] [PubMed] [Google Scholar]
- 27.Stelmaszynska T, Zgliczynski J M. Myeloperoxidase of human neutrophilic granulocytes as chlorinating enzyme. Eur J Biochem. 1974;45:305–312. doi: 10.1111/j.1432-1033.1974.tb03555.x. [DOI] [PubMed] [Google Scholar]
- 28.Tatsumi T, Fliss H. Hypochlorous acid and chloramines increase endothelial permeability: possible involvement of cellular zinc. Am J Physiol. 1994;267:H1597–H1607. doi: 10.1152/ajpheart.1994.267.4.H1597. [DOI] [PubMed] [Google Scholar]
- 29.Test S T, Lampert M B, Ossanna P J, Thoene J G, Weiss S J. Generation of nitrogen-chlorine oxidants by human phagocytes. J Clin Investig. 1984;74:1341–1349. doi: 10.1172/JCI111544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Test S T, Weiss S J. Quantitative and temporal characterization of the extracellular H2O2 pool generated by human neutrophils. J Biol Chem. 1984;259:399–405. [PubMed] [Google Scholar]
- 31.Thomas E L. Myeloperoxidase, hydrogen peroxide, chloride antimicrobial system: effect of exogenous amines on the antibacterial action against Escherichia coli. Infect Immun. 1979;25:110–116. doi: 10.1128/iai.25.1.110-116.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thomas E L, Grisham M B, Jefferson M M. Cytotoxicity of chloramines. Methods Enzymol. 1986;132:585–593. doi: 10.1016/s0076-6879(86)32043-3. [DOI] [PubMed] [Google Scholar]
- 33.Wagner D K, Collins Lech C, Sohnle P G. Inhibition of neutrophil killing of Candida albicans pseudohyphae by substances which quench hypochlorous acid and chloramines. Infect Immun. 1986;51:731–735. doi: 10.1128/iai.51.3.731-735.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weiss S J, Klein R, Slivka A, Wei M. Chlorination of taurine by human neutrophils. J Clin Investig. 1982;70:598–607. doi: 10.1172/JCI110652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Witko Sarsat V, Delacourt C, Rabier D, Bardet J, Nguyen A T, Descamps Latscha B. Neutrophil-derived long-lived oxidants in cystic fibrosis sputum. Am J Respir Crit Care Med. 1995;152:1910–1916. doi: 10.1164/ajrccm.152.6.8520754. [DOI] [PubMed] [Google Scholar]
- 36.Yazdanbakhsh M, Eckmann C M, Roos D. Killing of schistosomula by taurine chloramine and taurine bromamine. Am J Trop Med Hyg. 1987;37:106–110. doi: 10.4269/ajtmh.1987.37.106. [DOI] [PubMed] [Google Scholar]