Supplemental Digital Content is Available in the Text.
Women are more susceptible to medical cannabis–related adverse effects because of both an inherent sex influence and the unique combinations of cannabis cultivars consumed.
Keywords: Sex, Medical cannabis, Chronic pain, Phytocannabinoids, Adverse effects
Abstract
Studies have shown that women are more susceptible to adverse effects (AEs) from conventional drugs. This study aimed to investigate the differences of medical cannabis (MC)-related AEs between women and men in patients with chronic noncancer pain (CNCP). This is a cross-sectional study of adult patients licensed for MC treatment who were also diagnosed as patients with CNCP by a physician. Data included self-reported questionnaires and comprehensive MC treatment information. Simultaneously, identification and quantification of phytocannabinoids and terpenoids from the MC cultivars were performed. Comparative statistics were used to evaluate differences between men and women. Four hundred twenty-nine patients with CNCP (64% males) reported fully on their MC treatment. Subgrouping by sex demonstrated that the weight-adjusted doses were similar between men and women (0.48 [0.33-0.6] gr for men and 0.47 [0.34-0.66] gr for women). Nonetheless, women reported more than men on MC-related AEs. Further analysis revealed that women consumed different MC cultivar combinations than men, with significantly higher monthly doses of the phytocannabinoids CBD and CBC and significantly lower monthly doses of the phytocannabinoid 373-15c and the terpenoid linalool. Our findings demonstrate sex differences in MC-related AEs among patients with CNCP. Women are more susceptible to MC-related AEs, presumably because of both the inherent sex effect and the consumption of specific phytocannabinoid compositions in the MC cultivar(s). The understanding of these differences may be crucial for planning MC treatments with safer phytocannabinoid and terpenoid compositions and to better inform patients of expected AEs.
1. Introduction
Chronic noncancer pain (CNCP) is the most common qualifying medical condition reported among patients consuming medical cannabis (MC).9 About half of the patients consuming MC are women.12 In a 2016 survey of 1000 patients diagnosed with rheumatoid arthritis, more than half of the respondents who reported current cannabis use were women.24 In addition, in 2 recent multicenter prospective studies of patients with chronic and cancer pain under MC treatment in Israel, women involved were over 40% and over 50% of the samples, respectively.1,3 Moreover, in a cross-sectional study of patients with migraine under MC treatment in Israel, almost 70% were women.5 These demographics are similar for other patient populations who use MC to alleviate symptoms other than pain. For instance, over 50% of patients with cancer who received a license for MC to manage appetite, weakness, nausea, and pain were women.28 Recreational cannabis use rates are consistently higher among men,10 but data from surveys of MC users demonstrate that the difference between sexes is narrowing.
This equal prevalence of use between men and women raises questions regarding sex-dependent effects related to MC. Nevertheless, very few studies evaluated sex-dependent efficacy of MC treatment and even fewer evaluated MC-related adverse effects (AEs).15 Importantly, although CNCP is currently the most researched indication for MC treatment, with over 40 randomized controlled trials, producing many reviews, meta-analyses, and even systemic reviews of systemic reviews on this issue,25 there is still a vast gap in knowledge of sex-related differences.
In general, AEs from conventional drugs are more frequent and severe in women than in men, based on the FDA Adverse Event Reporting System.23 These differences may be due to pharmacokinetic or pharmacodynamic factors, polypharmacy, or differences in reporting patterns. Adding to the complexity of sex differences in MC treatment is the fact that cannabis is not a single plant. There are over a 1000 of different cannabis cultivars or chemovars, each with a unique chemical composition and therefore each with potentially different biological activity. Nonetheless, current regulations do not take into account the sex of patients for monthly dose, cultivars selection, and the MC chemical composition.16
We have recently demonstrated, in a cross-sectional prospective study following up naturalistically on patients with CNCP with a prolonged MC treatment period, that pain intensities and other clinical outcomes remain stable. Although 86% reported on at least 1 AE, the most frequent AEs were gastrointestinal (70%), central nervous system (60%), psychological (45%), ophthalmic (34%), musculoskeletal (31%), and cardiovascular (10%) and were also stable during the follow-up.2 However, we did not investigate whether these considerable rates of AEs could be explained by sex differences. Thus, in the current study, using the same database, we attempted to examine whether there are sex differences in MC-related rates of AEs and whether such differences are associated with the chemical composition of the MC treatment.
2. Methods
2.1. Israeli medical cannabis regulations
Owing to the Israeli Ministry of Health (IMOH) regulations of cannabis use for medical purposes, there are specific indications for which a physician can request a license for a patient. Chronic noncancer pain is a qualifying condition for MC license. Generally, MC application is received by one of the board members of the medical cannabis unit (MCU) that would reply to the physician if his request is approved or declined.16
In Israel, at the time the study was conducted (2015-2019), any physician with any expertise could apply to the IMOH to request an MC license for his or her patients. These physicians decide in collaboration with the patient on the route of administration that is approved by the MCU, either inflorescence for smoking and vaporizing or oil extracts for sublingual use. The monthly dose of MC is decided by the physician, with a starting monthly dose generally indicated as 20 g by the MCU and any increase subjected to its approval. Physicians provide consultation for the selection of a specific MC cultivar or combinations of cultivars. However, the final decision on the MC cultivar or cultivars selected is in the hands of the patient. After approval, the patients' contact details were transferred to 1 of 9 licensed cultivators for treatment instructions, cultivar selection (ie, THC:CBD ratio and sativa or indica dominance), and payment. Every patient goes through a personal trial-and-error process to find the cultivar or the combination of cultivars that best meets his or her therapeutic needs. Titration guidelines of MC treatment (starting dose, doses per day, guidelines for increasing or decreasing of the dose, or maximum dose allowed) are recommended by the physicians but are not enforced. Patients paid a fixed monthly price of about $100 regardless of the amount or the number of cultivars.
Mentionable, no specific information on the prescribing physicians was published by the IMOH at the time the study was conducted. However, Sharon et al. published a generalizable survey in which 95% of 50 Israeli pain specialist physicians applied for MC licenses for their patients.21
2.2. Inclusion criteria
Patients were eligible to participate if they were aged > 18 years, read Hebrew, were diagnosed for CNCP by a physician, and had standing MC license for the treatment of CNCP.
2.3. Instruments
2.3.1. Study questionnaires
Data collection was performed online by secure survey technology Qualtrics (Provo, Utah; version 12018).19 Demographic information included age, sex, body mass index, and tobacco and alcohol consumption habits. Data on pain characteristics included the least, average, and worst weekly pain intensities and pain etiology. Specifically, patients were given the list of pain etiologies with examples for each (eg, “chronic neuropathic pain—such as herniated disc with radiating pain, postherpetic neuralgia, etc.”). Specific information on the pharmaceutical analgesics consumption was also reported. Validated questionnaires included the quality of life (QoL) questionnaire, EuroQol (EQ5),11 and sleep timing section of the Pittsburgh sleep quality index.22 In addition, patients reported on their MC treatment characteristics and related adverse effects, including administration route, cultivator brand, cultivar name, total monthly dose (grams), and monthly dose of each specific cultivar names (grams), as well as adverse effects that patients attributed directly to the MC treatment based on the most frequent AEs in a previously published list of MC related–AEs.4 In the AEs report section, there was an option to report on “other” AEs patients may have experienced, with an open text. After a report of a specific AE, the patients were transferred to a section with questions regarding the frequency (rarely, frequently, or constantly) and severity (mild, moderate, or severe).
2.3.2. Phytocannabinoid profiling of cannabis chemovars
During December 2015 and October 2019, air-dried MC cultivars were obtained from several Israeli MC cultivators. Reagents, analytical standards, and general methodologies for phytocannabinoid and terpenoids extraction and analysis from Cannabis were according to our previously published methods.6,7
For each phytocannabinoid, the concentrations of the acid and its neutral counterpart were summed and reported as the total content. For example, the concentration of total ∆-9-tetrahydrocannabinol (Δ9-THC) was calculated as , with 0.877 being the molar ratio between the 2 compounds that corrects for a change in the mass of (−)-Δ9-trans-tetrahydrocannabinol acid (Δ9-THCA) as a result of decarboxylation. For compounds with no absolute identification, neutral or acid concentrations were used.
For terpenoid analysis, 10 mg of ground cannabis flowers were weighed in a 20 mL amber HS–rounded bottom vial and immediately sealed with a magnetic 32 mm Polytetrafluoroethylene (PTFE) septa cap. Terpenoids were separated using a Trace 1310 gas chromatograph (Thermo Scientific, Bremen, Germany) coupled to a TSQ 8000 Evo triple quadrupole mass spectrometer (Thermo scientific), equipped with a DB-35 MS UI capillary column (30 m × 0.25 mm x 0.25 μm, Agilent Technologies, Palo Alto, CA). A Pal RTC autosampler CTC-Pal (Analytics AG, Zwingen, Switzerland) for automated static headspace injections was used; 1 mL of a sample's gas phase, prepared after 30 minutes agitation of a flower sample with 140°C temperature, was injected in the GC injection port with a split ratio of 1:50. The identification and absolute quantification of terpenoids was performed in MS/MS mode by external calibrations as described by Shapira et al. (2019).20
Because the inflorescences were analyzed in their natural form, monthly consumption of phytocannabinoid doses was calculated using total phytocannabinoid concentrations. This calculation corrects for any differences that may arise in phytocannabinoid profiles as a result of decarboxylation because of mishandling or storage of the MC inflorescences. The median phytocannabinoid concentrations of few separate batches for each cultivar were used in our analyses.
To reduce the variability between analyzed cultivars, only phytocannabinoids and terpenoids with minimum average concentrations of 0.1 g and 400 ppm, respectively, were reported.
2.4. Study procedure
The data for this cross-sectional study were gathered from 2017 through 2019 after approval by the Institutional Ethics Committee of the Technion, Institute of Technology, Haifa, Israel (#011-2016). From an existing database of Israeli patients with preexisting MC license for various indications, we selected patients who agreed electronically to disclose their email address for future studies. Those who reported having a diagnosis of CNCP were sent an email with an explanation on the study design and a link to the online questionnaire. Electronic informed consent was obtained from patients who agreed to participate in the study. No financial compensation was offered to participating patients. While questionnaire data were being collected, the most prominent and most frequently administered cultivars from various approved cultivators in Israel were analyzed for phytocannabinoid content by Electrospray ionization-liquid chromatography (ESI-LC) or mass spectrometry (MS). Importantly, the chemical analyses were performed on the inflorescence of cultivars received from the cultivators and not directly from the patients.
2.5. Statistical analysis
R software (V.1.1.463) with tidyverse29 and atable26 packages were used to analyze differences in outcome measures by the Pearson χ2 test for categorical measures and the Kruskal–Wallis rank-sum test for numeric measures. For the effect size and confidence interval (CI), the Cohen d test was used. The Shapiro–Wilk test of normality demonstrated a nonnormal distribution for all measures; thus, data are presented as median and lower and upper quartiles (interquartile range). Differences were considered significant at the P < 0.05 level. Incidences are presented as number and percentage of patients.
3. Results
3.1. Sample
From 3627 patients with previous MC license for various indications, we selected 429 patients who reported having a diagnosis of CNCP and (1) consumed MC only by the inhalation of inflorescences, as to not compare the effect between different pharmacokinetic routes, and (2) reported fully on their MC treatment, including exact cultivars consumed, and overall and individual cultivars monthly dose. Inhalation of inflorescences was separated to smoking by 324 patients (76%), vaporizing by 86 patients (20%), and alternating both routes by 19 patients (4%). These patients represent the sample that is reported and analyzed in this article.
3.2. Sex subgroups
Sample demographics consisted mainly of men (n = 275, 64%), with a median age of 42 (35-52) years. Subgrouping the sample by sex (Table 1) revealed that women reported on having lower body mass index compared with men (−0.07, 95% CI=−0.28 to 0.13; P < 0.05).
Table 1.
Differences in demographic characteristics between sexes.
| Measure | Males (N = 275, 64%) | Females (N = 154, 36%) | Total (N = 429) | Effect size (CI; P) |
|---|---|---|---|---|
| Median (IQR) | ||||
| Age (y) | 41 (35-52) | 44 (33-53) | 42 (35-52) | −0.01 (−0.21 to 0.19; P = 0.18) |
| Weight (Kg) | 78 (70-90) | 67 (56-78) | 73 (63-85) | −0.73 (−0.92 to 0.55; P < 0.001) |
| BMI | 24 (22-27) | 24 (20-27) | 24 (21-27) | −0.07 (−0.28 to 0.13; P < 0.05) |
| No. of patients (%) | ||||
| Tobacco smoking consumption (yes) | 144 (53) | 78 (51) | 222 (52) | 0.05 (0 to 0.15; P = 0.56) |
| Alcohol consumption (yes) | 100 (37) | 55 (35) | 155 (36) | 0.02 (0 to 0.11; P = 0.92) |
BMI, body mass index; CI, confidence interval; IQR, interquartile range; Kg, kilograms; OR, odds ratio.
3.3. Pain intensity measures
Least, average, and worst pain intensities were similar between sexes, as well as for their pain frequency. However, women were more likely to suffer from headaches (n = 56, 36%) and dysfunctional pain etiologies (n = 70, 45%; mostly fibromyalgia syndrome) than men (n = 55, 20% and n = 43, 16%, respectively). No significant differences between sexes were observed in neuropathic, musculoskeletal, and visceral pain etiologies or in adjuvant analgesic medications consumption, including over-the-counter drugs, nonsteroidal anti-inflammatory drugs, weak and strong opioids, anticonvulsants, antidepressants, and IV analgesic treatments (P > 0.05) (Table 2). The morphine equivalent dose was also similar between the sexes, with median (interquartile range) and mean ± SD of 0 (0-7.5) and 24 ± 102 for men, respectively, and 0 (0-0) and 16 ± 65 for women, respectively. Additional data on QoL and sleep timing differences between the sexes were also examined (Appendix Table 1, available at http://links.lww.com/PAIN/B477), showing that both sleep latency and time in bed were significantly longer for women (0.23, 95% CI = 0.03-0.44; P < 0.01 and 0.37, 95% CI = 0.17-0.57; P < 0.01, respectively). Sleep duration and QoL scores were not significantly different between the sexes.
Table 2.
Differences in pain characteristics between sexes.
| Measure | Males (N = 275, 64%) | Females (N = 154, 36%) | Total (N = 429) | Effect size (CI; P) |
|---|---|---|---|---|
| Median (IQR) | ||||
| Least pain intensity (NPS, 0-10) | 4 (2-5) | 4 (2-6) | 4 (2-6) | 0.17 (−0.03 to 0.38; P = 0.36) |
| Average pain intensity (NPS, 0-10) | 7 (5-8) | 7 (6-8) | 7 (5-8) | 0.18 (−0.01 to 0.38; P = 0.20) |
| Worst pain intensity (NPS, 0-10) | 8 (8-10) | 9 (8-10) | 9 (8-10) | 0.19 (−0.01 to 0.39; P = 0.24) |
| No. of patients (%) | ||||
| Pain frequency | ||||
| Constant | 142 (52) | 85 (55) | 227 (53) | 0.11 (0.02 to 0.21; P = 0.47) |
| Sporadic | 133 (48) | 69 (45) | 202 (47) | |
| Pain etiology* | ||||
| Neuropathic | 205 (75) | 107 (62) | 312 (73) | 0.78 (0.49 to 1.20; P = 0.31) |
| Musculoskeletal | 168 (61) | 88 (57) | 256 (60) | 0.85 (0.56 to 1.30; P = 0.49) |
| Nociplastic | 43 (16) | 70 (45) | 113 (26) | 4.50 (2.80 to 7.30; P < 0.001) |
| Visceral | 33 (12) | 29 (19) | 62 (14) | 0.59 (0.33 to 1.10; P = 0.07) |
| Headaches | 55 (20) | 56 (36) | 111 (26) | 2.30 (1.40 to 3.60; P < 0.001) |
| Any analgesics consumption (yes) | 128 (47) | 70 (45) | 198 (46) | 1.10 (0.69 to 1.6; P = 0.88) |
| OTC analgesics (yes) | 30 (11) | 24 (16) | 54 (13) | 0.67 (0.36 to 1.20; P = 0.22) |
| NSAID analgesics (yes) | 19 (7) | 17 (11) | 36 (8) | 0.60 (0.29 to 1.30; P = 0.20) |
| Weak opioids (yes) | 40 (15) | 21 (14) | 61 (14) | 1.10 (0.59 to 2.00; P = 0.90) |
| Strong opioids (yes) | 57 (21) | 26 (17) | 83 (19) | 1.30 (0.76 to 2.30; P = 0.39) |
| Anticonvulsant adjuvant analgesics (yes) | 26 (10) | 17 (11) | 43 (10) | 1.20 (0.58 to 2.40; P = 0.73) |
| Antidepressant adjuvant analgesics (yes) | 39 (14) | 31 (20) | 70 (16) | 1.50 (0.87 to 2.60; P = 0.15) |
| IV analgesics (yes) | 7 (3) | 9 (6) | 16 (4) | 2.40 (0.77 to 7.60; P = 0.15) |
Weak opioids included buprenorphine hydrochloride, tramadol hydrochloride OD, etc.; strong opioids included fentanyl, methadone, oxycodone hydrochloride, etc.; anticonvulsants included pregabalin, gabapentin, etc.; antidepressants included amitriptyline hydrochloride, duloxetine, etc.; IV, intravenous analgesics included IV ketamine, IV magnesium, and IV lidocaine.
Either as a single pain etiology or in combination with other pain etiologies does not add up to 100%.
CI, confidence interval; IQR, interquartile range; NPS, Numerical Pain Scale; NSAIDs, nonsteroidal anti-inflammatory drugs (including ibuprofen, etoricoxib, etc); OTC, over the counter (including paracetamol and dipyrone).
3.4. Medical cannabis treatment measures
The median treatment duration before this study was 2 years ranging from patients treated for more than 1 year to 13 years. Men consumed a higher (40 [30-40] grams) absolute MC dose than women (30 [20-40] grams) (−0.31, 95% CI=−0.51 to 0.11; P < 0.05). Specifically, most men consumed 40 to 100 g MC monthly (n = 141/275, 51%), whereas most women consumed 20 to 30 g MC monthly (n = 99/154, 64%). Although the total MC monthly dose was higher for men, the total weight-adjusted dose was not significantly different between sexes, with 0.48 (0.33-0.6) g/kg/M for men and 0.47 (0.34-0.66) g/kg/M for women (0.11, 95% CI=−0.08 to 0.32; P = 0.44) (Fig. 1). No significant differences were observed between sexes in MC inhalation administration routes, daily frequency of MC consumption, or number of MC cultivars per month (P > 0.05) (Table 3).
Figure 1.
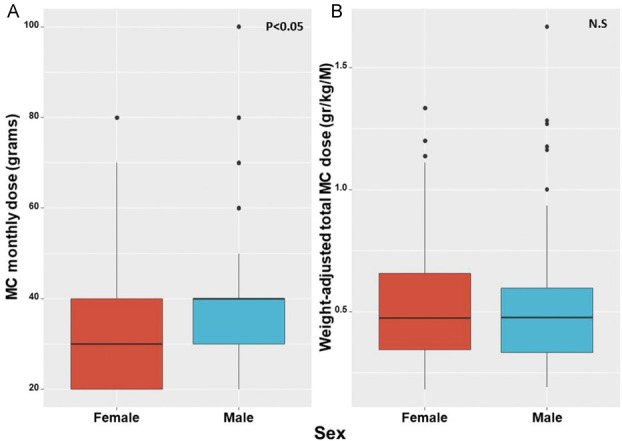
(A) Difference between absolute monthly doses of MC between women and men; (B) Difference between weight-adjusted monthly doses of MC between women and men. gr, grams; kg, kilograms; M, month; MC, medical cannabis; N.S, nonsignificant.
Table 3.
Differences between medical cannabis characteristics and monthly weight-adjusted doses of phytocannabinoids and terpenoids between the sexes.
| Measure | Males (N = 275, 64%) | Females (N = 154, 36%) | Effect size (CI; P) |
|---|---|---|---|
| No. of patients (%) | |||
| MC dose | |||
| 20-30 (gr) | 134 (49) | 99 (64) | 1.90 (1.20 to 2.90; P < 0.005) |
| 40-100 (gr) | 141 (51) | 55 (36) | |
| MC inhalation administration routes | |||
| Smoking | 204 (74) | 120 (78) | 0.04 (0 to 0.14; P = 0.69) |
| Vaporizing | 58 (21) | 28 (18) | |
| Smoking and vaporizing | 13 (5) | 6 (4) | |
| Daily frequency of MC consumption | |||
| 1 | 1 (<1) | 1 (<1) | 0.03 (0 to 0.15; P = 0.95) |
| 2-3 | 72 (26) | 47 (31) | |
| 4-6 | 75 (27) | 50 (32) | |
| >6 | 32 (12) | 18 (12) | |
| Median (IQR) | |||
| No. of MC cultivars per month | 2 (2-3) | 2 (2-3) | −0.18 (−0.37 to 0.02; P = 0.62) |
Percentages are rounded and without decimal points.
CI, confidence interval; IQR, interquartile range; kg, kilograms; ppm, parts per million; mg, milligrams; M, month; MC, medical cannabis.
3.5. Medical cannabis-related adverse effects
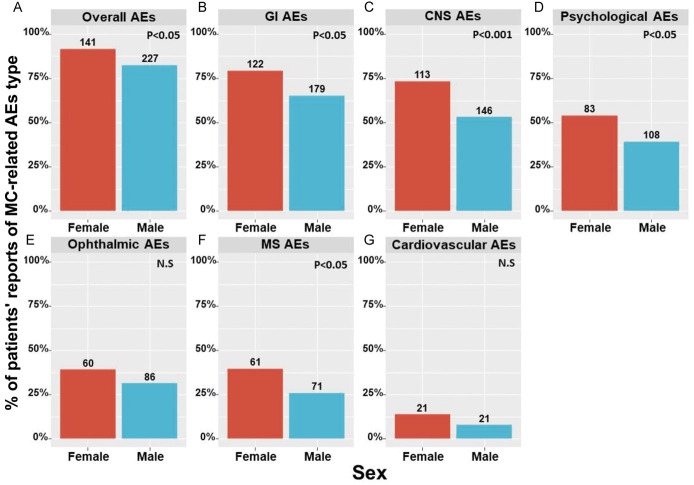
Analyzing the data collected regarding the differences of MC-related AEs between women and men revealed that women reported more than men on such AEs (Fig. 2). Specifically, women reported more than men on central nervous system (73% compared with 53%), gastrointestinal (79% compared with 65%), musculoskeletal (MS) (40% compared with 26%), and psychological AEs (54% compared with 39%). No significant differences were found in cardiovascular and ophthalmic AEs.
Figure 2.
Differences of MC-related AEs between women and men. (A) Overall (B−G) system types as stated. AEs, adverse effects; CNS, central nervous system; GI, gastrointestinal; MS, musculoskeletal; MC, medical cannabis; N.S, nonsignificant; numbers on top of the bars represent the number of patients n.
Particularly, women significantly reported (P < 0.05) more than men on confusion, attention disturbance, dizziness, balance disturbance, fatigue, memory disturbance, coordination disturbance, anxiety, dysphoria, forgetfulness, abdominal pain, nausea, diarrhea, decreased appetite, thirst, joint pain, week limbs, blurred vision, red eyes, and dry eyes AEs. The specific AEs in which statistically significant sex differences were found are displayed in Table 4. All specific AEs, as well as their frequency and severity, are displayed in Appendix Table 2, available at http://links.lww.com/PAIN/B477. Generally, whenever a specific AE was more frequent in women, it was also reported with a higher rank of severity, at significantly higher rates than men.
Table 4.
Sex differences in specific medical cannabis-related adverse effects.
| Measure | Males (N = 275, 64%) | Females (N = 154, 36%) | Total (N = 429) | Effect size (CI; P) |
|---|---|---|---|---|
| No. of patients (%) | ||||
| Central nervous system | ||||
| Confusion | 21 (8) | 24 (16) | 45 (10) | 2.20 (1.10 to 4.40; P < 0.05) |
| Impaired attention | 28 (10) | 32 (21) | 60 (14) | 2.30 (1.30 to 4.20; P < 0.005) |
| Dizziness | 12 (4) | 26 (17) | 38 (9) | 4.40 (2.10 to 10.0; P < 0.001) |
| Impaired balance | 13 (5) | 24 (16) | 37 (9) | 3.70 (1.70 to 8.20; P < 0.001) |
| Fatigue | 108 (39) | 88 (57) | 196 (45) | 2.10 (1.40 to 3.10; P < 0.001) |
| Impaired memory | 34 (12) | 45 (29) | 79 (18) | 2.90 (1.70 to 5.00; P < 0.001) |
| Impaired coordination | 6 (2) | 13 (8) | 19 (4) | 4.10 (1.40 to 14.00; P < 0.005) |
| Impaired speech | 10 (4) | 13 (8) | 23 (5) | 2.40 (0.96 to 6.40; P < 0.01) |
| Psychological | ||||
| Anxiety | 19 (7) | 22 (14) | 41 (10) | 2.20 (1.10 to 4.50; P < 0.05) |
| Dysphoria | 39 (14) | 45 (29) | 84 (20) | 2.50 (1.50 to 4.20; P < 0.001) |
| Forgetfulness | 35 (13) | 34 (22) | 69 (16) | 1.90 (1.10 to 3.40; P < 0.05) |
| Gastrointestinal | ||||
| Abdominal pain | 23 (8) | 32 (21) | 55 (13) | 2.90 (1.60 to 5.40; P < 0.001) |
| Nausea | 26 (10) | 28 (18) | 54 (13) | 2.10 (1.10 to 3.90; P < 0.05) |
| Diarrhea | 14 (5) | 17 (11) | 31 (7) | 2.30 (1.00 to 5.20; P < 0.05) |
| Decreased appetite | 28 (10) | 36 (23) | 64 (15) | 2.70 (1.50 to 4.80; P < 0.001) |
| Thirst | 76 (28) | 59 (38) | 135 (31) | 1.60 (1.00 to 2.50; P < 0.05) |
| Musculoskeletal | ||||
| Joint pain | 37 (13) | 39 (25) | 76 (18) | 2.20 (1.30 to 3.70; P < 0.005) |
| Weak limbs | 29 (11) | 29 (19) | 58 (14) | 2.00 (1.10 to 3.60; P < 0.05) |
| Visual | ||||
| Blurred vision | 14 (5) | 19 (12) | 33 (8) | 2.60 (1.20 to 5.80; P < 0.05) |
| Red eyes | 59 (21) | 20 (13) | 79 (18) | 0.55 (0.30 to 0.97; P < 0.05) |
| Dry eyes | 35 (13) | 34 (22) | 69 (16) | 1.90 (1.10 to 3.40; P < 0.05) |
CI, confidence interval; IQR, interquartile range; OR, odds ratio.
3.6. Sex-specific medical cannabis cultivar combinations
The difference in rates of AEs observed between men and women may be attributed to either an inherent difference between the sexes or to differences in the MC they consumed. The variety of cultivars and the option for patients to consume more than 1 cultivar per month created multiple and variable treatment options. Therefore, we calculated from the cultivar(s) the phytocannabinoid and terpenoid monthly doses consumed by each patient.
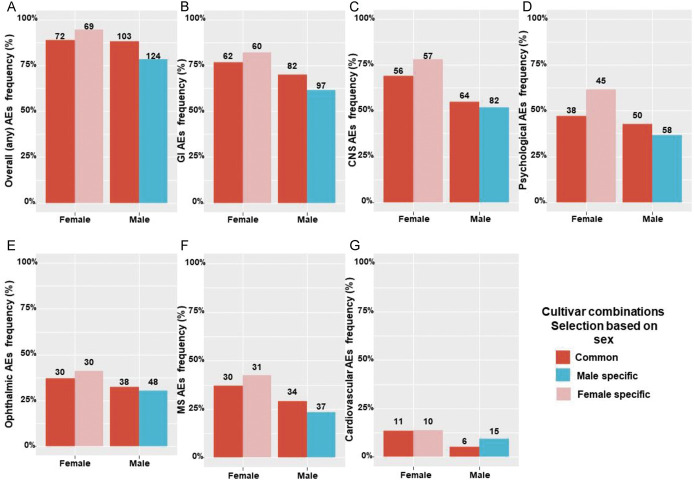
In the entire study sample (n = 429), a total of 41 distinct MC cultivars were consumed, comprising 222 cultivar combinations that were reported by all patients. The patients were divided into 3 groups based on the cultivar combinations they chose. Of these, 111 unique cultivar combinations (50%) were consumed only by men (ie, these combinations were not consumed by patients in the other 2 groups), 70 unique cultivar combinations (32%) were consumed only by women, and 41 unique cultivar combinations (18%) were consumed by both sexes (‘common’). Particular cultivars made up a small percentage of the overall cultivar combinations and therefore are unlikely the underlying reason for the differences in rates of AEs between men and women.
Next, we evaluated the differences in overall and specific rates of AEs between cultivar combinations consumed only by men, only by women, or by both sexes. Although the common cultivar combinations had the same percentage of overall AEs reports (88% both for men and women in this group), the men only or women only groups had different cultivar combinations and reported on more AEs for the woman-specific cultivars (95%) than the man-specific cultivars (78%) (Fig. 3A). We next divided the AEs into specific subtypes (gastrointestinal, central nervous system, psychological, ophthalmic, MS, and cardiovascular AEs) and examined the sex differences per each subtype. Men reported less than women on AEs in the common group as well as the men only cultivar combinations group (Fig. 3B–F). The one exception were cardiovascular AEs, in which men in the sex-specific cultivar combinations group reported on more AEs than men in the common group (Fig. 3G). For women, those in the women only cultivar combinations group reported more on AEs than women in the common group; while for men, those in the men only cultivar combinations group reported less on AEs compared with men in the common group.
Figure 3.
Difference of overall (A), gastrointestinal (B), central nervous system (C), psychological (D), opthalmic (E), musculoskeletal (F), and cardiovascular (G) adverse events rates of sex common vs sex-specific medical cannabis cultivar combinations. AEs, adverse effects; CNS, central nervous system; GI, gastrointestinal; MS, musculoskeletal; numbers on top of the bars represent the number of patients n.
Weight-adjusted monthly doses of the most prominent phytocannabinoids and terpenoids consumed only by men, only by women, or by both sexes are presented in Table 5. Mentionable, women in the sex-specific cultivar combinations group consumed significantly higher monthly doses of the phytocannabinoids cannabidiol and cannabichromene, while consuming significantly lower monthly doses of the phytocannabinoid 373-15c and the terpenoid linalool. Men in the sex-specific cultivar combinations group consumed significantly higher monthly doses of the phytocannabinoid CBN and the terpenoid β-myrcene, while consuming significantly lower monthly doses of the phytocannabinoid 331-18b.
Table 5.
Differences between the monthly weight-adjusted doses of phytocannabinoids and terpenoids between sexes in the 3 cultivar combinations groups.
| Measure | Male sex–specific cultivar combinations (N = 158) | Female sex–specific cultivar combinations (N = 73) | Common cultivar combinations (N = 198) | (χ2)*/Kruskal–Wallis rank†(P) |
|---|---|---|---|---|
| No. of patients (%) | ||||
| Sex | ||||
| Male | 158 (100) | 0 | 117 (59) | 220.99* (<0.001) |
| Female | 0 | 73 (100) | 81 (41) | |
| Phytocannabinoids | Median (IQR) mg/kg/M | |||
| ∆9-trans-tetrahydrocannabinol (∆9-THC) | 65 (40-92) | 57 (37-83) | 67 (46-94) | 5.01† (0.08) |
| ∆9-THC-C4 | 0.27 (0.16-0.38) | 0.24 (0.13-0.40) | 0.27 (0.19-0.41) | 4.59† (0.10) |
| ∆9-Tetrahydrocannabivarin (THCV) | 0.63 (0.41-0.98) | 0.55 (0.40-0.85) | 0.70 (0.46-1.00) | 4.70† (0.09) |
| Cannabidiol (CBD) | 0.30 (0.18-0.51) | 0.38 (0.20-12.00) | 0.27 (0.18-0.39) | 11.10† (<0.005) |
| Cannabigerol (CBG) | 2.00 (1.30-2.90) | 2.00 (1.20-2.90) | 2.00 (1.40-3.00) | 0.54† (0.76) |
| Cannabinol (CBN) | 0.31 (0.17-0.48) | 0.26 (0.14-0.41) | 0.24 (0.16-0.36) | 6.06† (<0.05) |
| Cannabichromene (CBC) | 0.77 (0.55-0.97) | 0.90 (0.58-1.40) | 0.64 (0.46-0.96) | 14.66† (<0.001) |
| 331-18b | 0.36 (0.23-0.56) | 0.45 (0.27-0.63) | 0.45 (0.28-0.61) | 8.93† (<0.05) |
| 373-15c | 0.17 (0-0.57) | 0.08 (0-0.24) | 0.34 (0-0.63) | 12.09† (<0.005) |
| Missing N (for phytocannabinoids) | 4 | 4 | 8 | |
| Terpenoids | Median (IQR) ppm/kg/M | |||
| α-pinene | 362 (225-616) | 337 (244-475) | 270 (167-486) | 5.3† (0.07) |
| β-pinene | 258 (158-527) | 286 (178-388) | 226 (138-373) | 1.33† (0.51) |
| Ledene | 408 (205-653) | 413 (158-555) | 396 (214-680) | 0.94† (0.62) |
| Limonene | 795 (512-1600) | 908 (461-1200) | 705 (424-1200) | 2.56† (0.28) |
| Linalool | 820 (468-1300) | 639 (360-1000) | 988 (583-1500) | 8.62† (<0.05) |
| Trans β-farnesene | 364 (245-494) | 382 (257-525) | 293 (212-467) | 4.38† (0.11) |
| α-fenchol | 647 (414-1100) | 561 (381-871) | 598 (396-1000) | 0.69† (0.71) |
| α-humulene | 682 (386-984) | 540 (391-727) | 596 (402-999) | 1.32† (0.52) |
| α-terpineol | 510 (273-804) | 401 (303-617) | 435 (22-748) | 1.49† (0.47) |
| β-caryophyllene | 2100 (1300-3300) | 1700 (1000-2500) | 2100 (1500-3300) | 3.50† (0.17) |
| β-myrcene | 1200 (742-2100) | 1100 (754-1500) | 692 (420-1900) | 11.31† (<0.005) |
| Missing N (for terpenoids) | 63 | 40 | 63 |
Pearson Chi-squared test.
Kruskal–Wallis rank-sum test.
IQR, interquartile range; percentages are rounded and without decimal points; kg, kilograms; mg, milligrams; M, month; ppm, parts per million.
4. Discussion
In this study, we evaluated the sex differences in MC-related AEs of patients with CNCP. To overcome the complexity of multiple cultivars in MC treatment, we calculated patients' monthly dose consumption of specific MC chemovar constituents. We found that although the weight-adjusted monthly dose of MC was similar among the sexes, and pain intensities were also similar, women reported on higher rates of MC-related AEs.
The higher rates of reported AEs in women may be due to physiological differences. An in vivo study in a rat model showed that the metabolism of the major phytocannabinoid ∆9-THC is different between the sexes; when given the same ∆9-THC dose, female rats produced higher levels of the active metabolite 11-hydroxy-THC in the brain and demonstrated lower pain sensitivity than male rats.27 Another preclinical study has shown that gonadal sex hormones affect cannabinoid concentrations in several brain areas.8 Previous studies have shown that the drug plasma concentrations could be affected by sex, in additional to other factors such as dose, administration route, frequency of consumption, drug–drug interaction, age, body weight, pathophysiology status, and smoking.17,18 In the current study, we controlled for some of these factors, for example, by adjusting the dose to weight or by dividing the sample according to the administration route, but still, they cannot be excluded completely. It is important to note that women reported higher rates of MC-related AEs, although there was no difference in pain intensities between men and women. Contradictory to this, a study among recreational cannabis users found increased antinociception among healthy human men compared with women.13 A possible explanation from previous studies on recreational use is that women are more susceptible to the adverse effects of ∆9-THC at a low dose.14
Another possible explanation for women reporting on higher rates of MC-related AEs is the unique MC cultivar combinations they consumed. Using a more in-depth perspective, we demonstrated that for most types of AEs, for the group of cultivars consumed by both men and women, the rate of AEs reports was either higher among women or similar. This suggested an inherent role of the sex itself on the AEs report. However, we discovered that some cultivar combinations were consumed only by a specific sex. We found that in these groups, women in the women only cultivar combination group reported on higher rates of AEs compared with the women in the common cultivar group, whereas men in the men only cultivar combinations group mostly reported on lower rates of AEs than the men in the common cultivar group. This finding suggests a role for the phytocannabinoid and terpenoid compositions as possible contributors for the differences in MC-related AEs, in addition to inherent sex differences.
Using our laboratory's unique analytical ability that allows for the quantification of phytocannabinoids and terpenoids,7,20 we were able to evaluate all the weight-adjusted monthly doses of the compounds for each patient. We found that different weight-adjusted monthly doses of phytocannabinoids and terpenoids were consumed by the women only or men only cultivar combinations groups. We demonstrated that Δ9-THC doses were not significantly different between the groups. Women in the sex-specific cultivar combinations group consumed significantly higher monthly doses of the phytocannabinoids CBD and CBC and lower monthly doses of 373-15c and the terpenoid linalool, whereas men in the sex-specific cultivar combinations group consumed significantly higher monthly doses of the phytocannabinoid CBN and the terpenoid β-myrcene and lower monthly doses of the phytocannabinoid 331-18b. The different weight-adjusted monthly doses of these specific phytocannabinoids and terpenoids may be responsible to the higher rates of AEs reported by women. How these compounds contribute to the prevalence of AEs remains to be investigated.
The differences found between men and women in the consumed cultivar combinations can arise from either the individual choice of the patients or by the recommendations from physicians and cultivators that supplied them with the treatment. In the case of individual choice, it is possible that factors affected by the phytocannabinoid and terpenoid composition, such as the taste and smell of the MC treatment or other positive effects that were not assessed here, contributed to the choice of specific cultivars, leading men and women to consume different cultivar combinations.
4.1. Limitations
The current study has a few limitations. First, self-report bias could have occurred. However, the questionnaire was anonymous and validated. Second, because of our study design, we did not have access to patient's data before MC treatment initiation, making it impossible to make causal conclusions. Third, because we investigated patients in the sample that consumed MC by inflorescence inhalation only, findings cannot be generalized to other consumption routes. Fourth, because report on AEs was performed after 1-month treatment for the entire month, a recall bias may have occurred. In addition, the specific characteristics of the prescribing physicians are unknown, and as such, selection bias could not be controlled for. Finally, because there was no direct contact with the patients and the MC cultivars for analysis were collected from the cultivators by matching name and batch, there is some measure of extrapolation when associating the patients' AEs reports.
4.2. Conclusions
Although CNCP is one of the most frequent indications for MC treatment, there is no knowledge regarding the response of specific subgroups of the patient population with CNCP to MC treatment. The current study results demonstrate that women are more susceptible to MC-related AEs, presumably because of both an inherent sex effect and to the consumption of specific phytocannabinoid and terpenoid compositions in the MC cultivar(s). These results may shed light on the differential effects of MC between sexes. Physicians and future clinical studies should address sex differences to establish safer MC treatments and better prepare patients for the expected prevalence of MC-related AEs.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Appendix A. Supplemental digital content
Supplemental digital content associated with this article can be found online at http://links.lww.com/PAIN/B477.
Acknowledgments
All authors discussed the results and commented on the article. The study was partially funded by the Evelyn Gruss Lipper Charitable Foundation. This sponsor had no role or influence on the study or on this submission. The authors have no conflicts of interest to declare. The data that support the findings of this study are available from the corresponding author on reasonable request. Some data may not be made available because of privacy or ethical restrictions.
Footnotes
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Web site (www.painjournalonline.com).
Contributor Information
Gil M. Lewitus, Email: lgil@technion.ac.il.
Yelena Vysotski, Email: lalatrololo@gmail.com.
Paula Berman, Email: bermansh@gmail.com.
Anna Shapira, Email: annasha@technion.ac.il.
Shiri Procaccia, Email: shiri.procaccia@gmail.com.
David Meiri, Email: dmeiri@technion.ac.il.
References
- [1].Aviram J, Lewitus GM, Vysotski Y, Uribayev A, Procaccia S, Cohen I, Leibovici A, Abo-Amna M, Akria L, Goncharov D, Mativ N, Kauffman A, Shai A, Hazan O, Bar-Sela G, Meiri D. Short-term medical cannabis treatment regimens produced beneficial effects among palliative cancer patients. Pharmaceuticals 2020;13:435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Aviram J, Lewitus GM, Vysotski Y, Yellin B, Berman P, Shapira A, Meiri D. Prolonged medical cannabis treatment is associated with quality of life improvement and reduction of analgesic medication consumption in chronic pain patients. Front Pharmacol 2021;12:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Aviram J, Pud D, Gershoni T, Schiff‐Keren B, Ogintz M, Vulfsons S, Yashar T, Haim‐Moshe A, Brill S, Amital H, Goor‐Aryeh I, Robinson D, Green L, Segal R, Fogelman Y, Tsvieli O, Yellin B, Vysotski Y, Morag O, Tashlykov V, Sheinfeld R, Goor R, Meiri D, Eisenberg E. Medical cannabis treatment for chronic pain: outcomes and prediction of response. Eur J Pain 2021;25:359–74. [DOI] [PubMed] [Google Scholar]
- [4].Aviram J, Samuelly-Leichtag G. Efficacy of cannabis-based medicines for pain management: a systematic review and meta-analysis of randomized controlled trials. Pain Physician 2017;20:E755–96. [PubMed] [Google Scholar]
- [5].Aviram J, Vysotski Y, Berman P, Lewitus GM, Eisenberg E, Meiri D. Migraine frequency decrease following prolonged medical cannabis Treatment : a cross—sectional study. Brain Sci 2020;10:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Baram L, Peled E, Berman P, Yellin B, Besser E, Benami M, Louria-Hayon I, Lewitus GM, Meiri D. The heterogeneity and complexity of Cannabis extracts as antitumor agents. Oncotarget 2019;10:4091–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Berman P, Futoran K, Lewitus GM, Mukha D, Benami M, Shlomi T, Meiri D. A new ESI-LC/MS approach for comprehensive metabolic profiling of phytocannabinoids in Cannabis. Sci Rep 2018;8:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Bisogno T, Wenger T, Manzanares J, Milone A, Berrendero F, Marzo VDi, Ramos JA, Ferna JJ. Sex steroid influence on cannabinoid CB 1 receptor mRNA and endocannabinoid levels in the anterior pituitary gland. Biochem Biophys Res Commun 2000;270:260–6. [DOI] [PubMed] [Google Scholar]
- [9].Boehnke KF, Gangopadhyay S, Clauw DJ, Haffajee RL. Qualifying conditions of medical cannabis license holders in the United States. Health Aff (Millwood) 2019;38:295–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Brabete AC, Greaves L, Hemsing N, Stinson J. Sex-and gender-based analysis in cannabis treatment outcomes: a systematic review. Int J Environ Res Public Health 2020;17:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Brooks R, Group E. EuroQol: the current state of play. Health Policy (New York) 1996;37:53–72. [DOI] [PubMed] [Google Scholar]
- [12].Cooper ZD, Craft RM. Sex-dependent effects of cannabis and cannabinoids: a translational perspective. Neuropsychopharmacology 2018;43:34–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Cooper ZD, Haney M. Sex-dependent effects of cannabis-induced analgesia. Drug Alcohol Depend 2016;167:112–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Fogel JS, Kelly TH, Westgate PM, Lile JA. Pharmacology, Biochemistry and Behavior Sex differences in the subjective effects of oral Δ 9 -THC in cannabis users. Pharmacol Biochem Behav 2017;152:44–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Haroutounian S, Meidan R, Davidson E. The effect of medicinal cannabis on pain and quality of life outcomes in chronic pain: a prospective open-label study. Clin J Pain 2016;32:1036–43. [DOI] [PubMed] [Google Scholar]
- [16].Landshaft Y, Albo B, Mechoulam R, Afek A. The Updated Green Book (May 2019): The Official Guide to Clinical Care in Medical Cannabis. Available at: https://www.health.gov.il/hozer/CN_106_2019.pdf 2019. Available: https://www.health.gov.il/hozer/mmk154_2016.pdf. Accessed June 8, 2020. [Google Scholar]
- [17].Luscombe D. Factors influencing plasma drug concentrations. J Int Med Res 1977;51:82–97. [PubMed] [Google Scholar]
- [18].Pan SD, Zhu LL, Chen M, Xia P, Zhou Q. Weight-based dosing in medication use: what should we know? Patient Prefer Adherence 2016;10:549–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Qualtrics LLC. Qualtrics (Version 12018). Provo, UT. Available at: http//www.qualtrics.com. Accessed August 9, 2021. [Google Scholar]
- [20].Shapira A, Berman P, Futoran K, Guberman O, Meiri D. Tandem mass spectrometric quantification of 93 terpenoids in Cannabis using static headspace (SHS) injections. Anal Chem 2019;91:11425–32. [DOI] [PubMed] [Google Scholar]
- [21].Sharon H, Goldway N, Goor-Aryeh I, Eisenberg E, Brill S. Personal experience and attitudes of pain medicine specialists in Israel regarding the medical use of cannabis for chronic pain. J Pain Res 2018;11:1411–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Shochat T, Tzischinsky O, Oksenberg A, Peled R. Validation of the Pittsburgh sleep quality index Hebrew translation (PSQI-H) in a sleep clinic sample. Isr Med Assoc J 2007;9:853–6. [PubMed] [Google Scholar]
- [23].Soldin OP, Chung SH, Mattison DR, Bandiera SM. Sex differences in drug disposition. J Biomed Biotechnol 2011:187103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Ste-Marie PA, Shir Y, Rampakakis E, Sampalis JS, Karellis A, Cohen M, Starr M, Ware MA, Fitzcharles M-A. Survey of herbal cannabis (marijuana) use in rheumatology clinic attenders with a rheumatologist confirmed diagnosis. PAIN 2016;157:2792–7. [DOI] [PubMed] [Google Scholar]
- [25].Stockings E, Campbell G, Hall WD, Nielsen S, Zagic D, Rahman R, Murnion B, Farrell M, Weier M, Degenhardt L. Cannabis and cannabinoids for the treatment of people with chronic non-cancer pain conditions: a systematic review and meta-analysis of controlled and observational studies. PAIN 2018;159:1932–54. [DOI] [PubMed] [Google Scholar]
- [26].Ströbel A, Haynes A. R package atable: Create Tables for Reporting Clinical Trials. Version 0.1.5. Comprehensive R Archive Network (CRAN), 2019. Available at: https://cran.r-project.org/web/packages/atable/atable.pdf. Accessed December 1, 2019. [Google Scholar]
- [27].Tseng AH, Harding JW, Craft RM. Pharmacokinetic factors in sex differences in -tetrahydrocannabinol-induced behavioral effects in rats. Behav Brain Res 2004;154:77–83. [DOI] [PubMed] [Google Scholar]
- [28].Waissengrin B, Urban D, Leshem Y, Garty M, Wolf I. Patterns of Use of medical cannabis among Israeli cancer patients: a single institution experience. J Pain Symptom Manage 2015;49:223–30. [DOI] [PubMed] [Google Scholar]
- [29].Wickham H. Tidyverse: Easily install and load ’Tidyverse" packages. Version 1.3.0. Compr R Arch Netw, 2019. Available: https://cran.r-project.org/web/packages/tidyverse/tidyverse.pdf. Accessed August 9, 2021. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental digital content associated with this article can be found online at http://links.lww.com/PAIN/B477.