Supplemental Digital Content is Available in the Text. Persistent visceral pain induced by colitis in rats can be either transferred to controls or counteracted by mean of a faecal microbiota transplant.
Keywords: Microbiota-gut-brain axis, Visceral pain, IBS, IBDs, Faecal microbiota transplantation
Abstract
Recent findings linked gastrointestinal disorders characterized by abdominal pain to gut microbiota composition. The present work aimed to evaluate the power of gut microbiota as a visceral pain modulator and, consequently, the relevance of its manipulation as a therapeutic option in reversing postinflammatory visceral pain persistence. Colitis was induced in mice by intrarectally injecting 2,4-dinitrobenzenesulfonic acid (DNBS). The effect of faecal microbiota transplantation from viscerally hypersensitive DNBS-treated and naive donors was evaluated in control rats after an antibiotic-mediated microbiota depletion. Faecal microbiota transplantation from DNBS donors induced a long-lasting visceral hypersensitivity in control rats. Pain threshold trend correlated with major modifications in the composition of gut microbiota and short chain fatty acids. By contrast, no significant alterations of colon histology, permeability, and monoamines levels were detected. Finally, by manipulating the gut microbiota of DNBS-treated animals, a counteraction of persistent visceral pain was achieved. The present results provide novel insights into the relationship between intestinal microbiota and visceral hypersensitivity, highlighting the therapeutic potential of microbiota-targeted interventions.
1. Introduction
Visceral pain management is a major clinical problem, given the lack of effective and safe drugs.15 The development of a chronic visceral hypersensitivity frequently occurs in patients with a history of intestinal damage. Indeed 20% to 50% of patients affected by inflammatory bowel diseases (IBDs) manifest a chronic abdominal pain also in the remission phase of colitis. On the other hand, an acute gut illness increases the risk of developing the irritable bowel syndrome (IBS) by 7-fold.81 Several mechanisms have been proposed to contribute to the initiation, exacerbation, and persistence of visceral pain. Among them, there is dysbiosis of gut microbiota.32,47,70 The most well-documented line of evidence linking the loss of gastrointestinal microbial homeostasis to the development of chronic visceral hypersensitivity comes from the literature on postinfectious IBS.54 Consistently, the disturbance of the gut microbiota in early life71 as in adulthood affects the local immune response and enhances pain signalling in rodents.45,60,86 The brain, the gut microbiota, and the immune system show reciprocal associations in health and disease.34 The brain, by the autonomic nervous system and the hypothalamic–pituitary–adrenal axis, influences intestinal functions and gut microbial composition.77 On the other hand, microbiota alterations can influence brain structure and function either developmentally or in response to acute perturbations, setting a regulatory loop between the gut and the brain.26,65 Although alterations of the gut bacteria composition or metabolism caused by intestinal inflammation have been correlated to postinflammatory visceral pain persistence,30,33 a definitive cause–effect relationship between dysbiosis and visceral pain persistence as well as the potential of microbiota as pain generator or pain perpetuator has not yet been determined. Microbiota metabolites, namely, short-chain fatty acids (SCFAs), amino acid–derived metabolites, and bile acids, can promote anti-inflammatory and immunoregulatory effects in different cells within the gastrointestinal tract, thereby indirectly affecting visceral sensitivity.57,62 In addition, these products can translocate across the epithelial barrier into the systemic circulation, thus modulating distant organs, such as the brain and spinal cord, by activating or inhibiting specific receptors therein.41,57 The aims of the present work were (1) to evaluate the effect of faecal microbiota transplantation (FMT) from viscerally hypersensitive donors in remission from colitis induced by 2,4-dinitrobezenesulfonic acid (DNBS) on controls visceral sensitivity, (2) to study the mechanisms by which the gut microbiota can directly affect pain perception, and (3) to evaluate the therapeutic efficacy of FMT on persistent postinflammatory visceral pain.
2. Methods
2.1. Animals
For all the experiments described below, 8-week-old male Sprague–Dawley rats (Envigo, Varese, Italy), weighing approximately 220 to 250 g at the beginning of the experimental procedure, were used. Animals were housed in CeSAL (Centro Stabulazione Animali da Laboratorio, University of Florence) and used at least 1 week after their arrival. Before starting the treatments, 4 animals were housed per cage (size 26 × 41 cm). After starting the experimental procedures, animals were individually housed to avoid the damage of the electrodes implanted for electrophysiological measurements. Animals were fed a standard laboratory diet and tap water ad libitum and kept at 23°C ± 1°C with a 12 hours light or dark cycle, light at 7 am. All animal manipulations were performed according to the Directive 2010/63/EU of the European parliament and of the European Union council (September 22, 2010) on the protection of animals used for scientific purposes. The ethical policy of the University of Florence complies with the Guide for the Care and Use of Laboratory Animals of the US National Institutes of Health (NIH Publication No. 85-23, revised 1996; University of Florence assurance number: A5278-01). Formal approval to conduct the described experiments was obtained from the Animal Subjects Review Board of the University of Florence. Experiments involving animals have been reported according to ARRIVE guidelines.66 All efforts were made to minimize animal suffering and to reduce the number of animals used.
2.2. Induction of colitis
Colitis was induced in accordance with the method described previously by Antonioli et al.3 with minor changes. In brief, during a short anaesthesia with isoflurane (2%), 30 mg of DNBS in 0.25 mL of 50% ethanol was administered intrarectally through a polyethylene PE-60 catheter inserted 8 cm proximal to the anus. Control rats received 0.25 mL of saline solution.
2.3. Faecal microbiota transplantation study design
In the first experimental set, rats were randomized into the following treatment groups: control (vehicle, no antibiotic treatment, no FMT; n = 10), antibiotics (abx) + vehicle (antibiotic treatment followed by vehicle administration; n = 10), abx + FMTCTR (antibiotic treatment followed by FMT from controls; n = 10), and abx + FMTDNBS (antibiotic treatment followed by FMT from DNBS treated animals; n = 14). The animals were singly housed and underwent the following antibiotic or antifungal regimen to prepare them to the FMT: Day 0 to 6 rats received a daily oral gavage of amphotericin B (1 mg·kg−1) and metronidazole (100 mg·kg−1) whereas an antibiotic mix (ceftazidime 1 g·L−1, vancomycin 0.5 g·L−1, and neomicin 1 g·L−1) was added to the autoclaved drinking water and changed every 2 days. On day 7, 24 hours after the interruption of the antibiotic treatment, the animals underwent the FMT procedure. FMT was daily performed on days 7 to 11 (set I) and on days 20 to 24 (set II). Behavioural tests were performed at the end of the antibiotic treatment (before starting the FMT), 24 hours and 7 days after each FMT set, and once a week after the last treatment. Effects of the antibiotic or antifungal treatments on the gut microbiota were evaluated by a culture-based analysis for each treatment group. A portion of fresh faecal pellets (50 mg) was dissolved in 1 mL of sterile saline by vigorous vortexing, and the mean viable cell count was then determined by plating 0.1 mL of appropriate dilutions (detection range, 2 × 102 to 16 × 108 CFU/g of faecal pellet) onto (1) MacConkey agar (Oxoid, Cheshire, United Kingdom), selective for isolation of Gram-negative enteric bacteria; (2) CNA-CV Agar (Becton, Dickinson and Company, Franklin Lakes, NJ), selective for isolation of Gram-positive cocci; and (3) Sabouraud glucose agar (Becton, Dickinson and Company), selective for isolation of yeasts. Faecal material collected from different controls (n = 3) or DNBS-treated animals (n = 3) (between 14 and 21 days after the intrarectal injection of the inflammatory agent) have been homogenized into one solution for FMTCTR and FMTDNBS, respectively. Tube containing fecal pellets in sterile saline solution were left on ice for 60 minutes and later homogenized for 2 minutes on ice using a hand-held pellet pestle device with sterile, reuseable pestles. When fully homogenized, the suspended pellets were directly used for the FMT procedure. FMT was performed through oral gavage with a faecal suspension (50 mg·mL−1) in a final volume of 3 mL. The following analyses have been performed on 3 to 6 samples per group collected from the same animals used for the behavioural assessments.
In the second experimental set, rats were randomized into the following treatment groups (n = 5): control (vehicle), DNBS + vehicle (intrarectal injection of DNBS 30 mg followed by vehicle administration), and DNBS + FMTCTR (intrarectal injection of DNBS 30 mg followed by FMT from controls). Colitis was induced in animals by the intrarectal injection of DNBS (30 mg in 0.25 mL EtOH 50%) on day 1. A control group was intrarectally administered with saline solution. DNBS-treated animals did not receive the antibiotic pretreatment before the FMT because it has been reported in the literature that antibiotics can influence the course of the colitis.43,52 Seven days after DNBS injection, the animals were split into 2 groups, respectively, receiving the vehicle or the naive control–derived faecal microbiota suspensions (FMTCTR). Because the resident microbiota represents an obstacle to the engraftment of the new microbiota, FMT was performed for 5 consecutive days/week and the same protocol was repeated for 4 weeks (I-IV set of FMT): on days 7 to 11 (set I), 14 to 18 (set II), 21 to 25 (set III), and 28 to 32 (set IV) after DNBS injection. Behavioural tests were conducted 7 days after DNBS injection (before starting FMT), 3 days after each FMT set, and once a week after the last treatment. For the FMT, faecal material was collected from different control naive animals (n = 3) and homogenized into one tube containing sterile saline solution. Tubes were left on ice for 60 minutes and later homogenized for 2 min on ice using a hand-held pellet pestle device with sterile, reuseable pestles. When fully homogenized, the suspended pellets were directly used for the FMT procedure. FMT was performed by oral gavage with a faecal suspension (100 mg·mL−1) in a final volume of 3 mL.
2.4. Assessment of visceral sensitivity by visceromotor response
The visceromotor response (VMR) to colorectal balloon distension was used as a objective measure of visceral sensitivity in animals. Two electromyographic electrodes were sutured into the external oblique abdominal muscle under deep anaesthesia and exteriorised dorsally.22 Visceromotor response assessment was performed under light anaesthesia (isoflurane 2%) as previously reported.75 A lubricated latex balloon (length: 4.5 cm), assembled to an embolectomy catheter and connected to a syringe filled with water, was used to perform colorectal distension. The balloon was inserted into the colon and positioned 6.5 cm from the anus and was filled with increasing volumes of water (0.5, 1, 2, and 3 mL). The electrodes were relayed to a data acquisition system, and the corresponding electromyographic signal consequent to colorectal stimulation was recorded, amplified and filtered (Animal Bio Amp; ADInstruments, Oxford, United Kingdom), digitised (PowerLab 4/35; ADIinstruments), analysed, and quantified using LabChart 8 (ADInstruments). To quantify the magnitude of the VMR at each distension volume, the area under the curve immediately before the distension (30 seconds) was subtracted from the area under the curve during the balloon distension (30 seconds) and responses were expressed as percentage increase from the baseline. The time elapsed between 2 consecutive distension was 5 minutes. Visceromotor response measurements were performed in 5 animals per group.
2.5. Assessment of visceral sensitivity by abdominal withdrawal reflex
The behavioural responses to colorectal distension (CRD) were assessed in the animals by measuring the abdominal withdrawal reflex (AWR), a semiquantitative score described previously in conscious animals.19 In brief, rats were anesthetized with isoflurane, and a lubricated latex balloon (length: 4.5 cm), attached to polyethylene tubing, assembled to an embolectomy catheter, and connected to a syringe filled with water, was inserted through the anus into the rectum and descending colon of adult rats. The tubing was taped to the tail to hold the balloon in place. Then rats were allowed to recover from the anaesthesia for 30 minutes. AWR measurement consisted of visual observation of animal responses to graded CRD (0.5, 1, 2, and 3 mL) blinded observers who assigned AWR scores: no behavioral response to colorectal distention (0); immobile during colorectal distention and occasional head clinching at stimulus onset (1); mild contraction of the abdominal muscles but absence of abdomen lifting from the platform (2); observed strong contraction of the abdominal muscles and lifting of the abdomen off the platform (3); and arching of the body and lifting of the pelvic structures and scrotum (4). The time elapsed between 2 consecutive distension was 5 minutes. AWR measurements were conducted in 10 to 14 animals per group.
2.6. Profiling of the gut microbiota
Fecal pellets (50 mg) and fecal suspensions (volume equivalent to 50 mg) were processed for the total DNA extraction using the DNeasy PowerLyzer PowerSoil Kit (Qiagen, Hilden, Germany). Next-generation sequencing of 16S ribosomal RNA amplicons (V3-V4 regions) was performed using the Illumina MiSeq platform, according to the Illumina 16S Metagenomic Sequencing Library Preparation protocol (Part # 15044223 Rev. B; URL: http://www.illumina.com/content/dam/illumina-support/documents/documentation/chemistry_documentation/16s/16s-metagenomic-library-prep-guide-15044223-b.pdf), after a 2 × 300 bp paired-end approach. Sequencing results were analyzed using the QIIME 2 suite (Quantitative Insights Into Microbial Ecology).11 In brief, after raw reads denoising (ie, error correction, removal of chimeric and singleton sequences, and joining of denoised paired-end reads), using DADA214 for each sample amplicon sequence variants was inferred. Taxonomic classification of dereplicated amplicon sequence variant was performed using a Naive Bayes classifier trained on the SILVA 16S reference database (release 132) (https://www.arb-silva.de/documentation/release-132/). Microbial diversity was estimated by evaluating alpha diversity (Shannon index) and beta diversity (Bray-Curtis) metrics using specific tools implemented in the QIIME 2 pipeline. The analysis was not performed in a within-subject manner.
The gut microbiota was characterized in a representative number of animals, at different sampling points (day 18, 32, and 46), belonging to the following treatment groups: vehicle + vehicle (n = 3), abx + vehicle (n = 4), abx + FMTCTR (n = 4), and abx + FMTDNBS (n = 4). The first and the last faecal suspension used in the I and II set of either FMTCTR (n = 4) or FMTDNBS (n = 4) were also analysed to determine the composition of the transplanted microbiota.
2.7. Gas chromatography mass spectrometry analysis of short-chain fatty acids
Methanol and tert-butyl methyl ether (Chromasolv grade), sodium bicarbonate and hydrochloric acid (reagent grade), (2H3)Acetic, (2H3)Propionic, (2H7)isobutyric and (2H9)isovaleric (used as internal standards [ISTDs]), acetic acid, propionic acid, butyric acid, isobutyric acid, valeric acid, and isovaleric acid (analytical standards grade) were purchased from Sigma-Aldrich (Milan, Italy). MilliQ water 18 MW was obtained from the Millipore Simplicity system (Milan, Italy). The analysis of SCFAs was performed by an Agilent gas chromatography mass spectrometry system composed with a 5971 single quadrupole mass spectrometer, 5890 gas-chromatograph, and 7673 autosampler.
The SCFAs in the fecal samples were analyzed as free acid form using a SupelcoNukol column, with a 30 m length, 0.25 mm internal diameter, and 0.25 μm of film thickness with the temperatures program as follows: Initial temperature of 40°C was held for 1 minute, then it was increased to 150°C at 30°C/minute, and finally increased to 220°C at 20°C/minute. A 1 μL aliquot of the extracted sample was injected in splitless mode (splitless time 1 minute) at 250°C, whereas the transfer line temperature was 280°C. The carrier flow rate was maintained at 1 mL/minutes. The quantitative determination of SCFAs in each sample was performed by the ratio between the area abundance of the analytes with the area abundance of respective labeled ISTD (isotopic dilution method). The value of this ratio was named the peak area ratio, and it was used as abundance of each analyte in the quantitative evaluation. The ionic signals of SCFAs and the reference ISTDs used for the quantitation of each SCFAs were reported in the supplementary information (available as supplemental digital content at http://links.lww.com/PAIN/B462). Faecal samples were added to sodium bicarbonate 10 mM solution (1:1 w/v). The mixture was sonicated 5 minutes and centrifuged at 5000 rpm for 10 minutes. The supernatants were frozen at −20°C until analysis. The SCFAs were finally extracted as follows: An aliquot of 100 μL of sample solution (corresponding to 0.1 mg of stool sample) was added of 50 μL of ISTDs mixture, 1 mL of tert-butyl methyl ether and 50 μL of 1.0 M HCl solution in 1.5 mL centrifuge tube. Afterwards, each tube was shaken in a vortex apparatus for 2 minutes, centrifuged at 10,000 rpm for 5 minutes, and finally the solvent layer was transferred in autosampler vial and analyzed by the gas chromatography mass spectrometry method. Each sample was prepared and processed, by the method described above in triplicate. The analysis has been performed in 5 to 9 animals per group. Short-chain fatty acids were analysed at each timepoint in the groups receiving the FMT and at day 0, 18, and 46 in the control groups, abx + vehicle, and vehicle + vehicle group. Further details are reported in the supplementary information (available as supplemental digital content at http://links.lww.com/PAIN/B462).
2.8. Histological evaluation of colonic damage
The evaluation of colon damage was performed both macroscopically and histologically, in accordance with the criteria previously reported by Antonioli et al.3 The macroscopic criteria were presence of adhesions between colon and other intra-abdominal organs (0-2), consistency of colonic faecal material (indirect marker of diarrhoea; 0-2), thickening of colonic wall (millimeters), and presence and extension of hyperaemia and macroscopic mucosal damage (0-5). A microscopic analysis was performed on haematoxylin or eosin-stained sections of formalin-fixed full-thickness samples obtained from the distal colon. The microscopic damage was assessed in accordance with the criteria reported previously by Antonioli et al.2 by evaluating the presence of mucosal architecture loss, goblet cell depletion, crypt abscess, cellular infiltration, and tunica muscularis thickening. The analysis has been performed in 4 to 6 animals per group.
2.9. Plasma lipopolysaccharide–binding protein by enzyme-linked immunospecific assay
Plasma lipopolysaccharide–binding protein (LBP) concentrations were determined using the enzyme immunoassay kit (Enzo Life Sciences, Milan, Italy). Rat blood samples were collected in heparinized tubes after decapitation. Samples were centrifuged immediately at 14,000 rpm for 15 minutes, and the resulting plasma was frozen at −80°C until analysis. Lipopolysaccharide-binding protein concentrations were determined using the enzyme immunoassay kit for free mouse and rat LBP as per manufacturers' instruction (Enzo Life Sciences). Sensitivity: range 1 to 50 ng/mL. The analysis has been performed in 3 to 5 animals per group.
2.10. Gene expression analysis by quantitative reverse transcription polymerase chain reaction
Total RNA was extracted from colon samples using the mirVana miRNA Isolation kit (Ambion/Life Technologies, Milan, Italy) according to the manufacturer's recommendations. RNA concentration and quality were determined using a Nanodrop 1000 (Thermo Scientific, Milan, Italy). Quantitative polymerase chain reaction (PCR) was conducted in a LightCycler480 System using PowerUp SYBR Green Master Mix (Applied Biosystems, Milan, Italy) and specific probes designed by Applied Biosystems to rat occludin, ZO-1, tumor necrosis factor (TNF)-α, interleukin (IL)6, IL10, and transforming growth factor (TGF)-β, while using β-Actin as an endogenous control. Experimental samples were run in triplicate with 4 μL (10 ng·μL−1) complementary DNA per reaction. To check for amplicon contamination, each run contained no template controls in triplicate for each probe used. Cycle threshold (Ct) values were recorded. Data were normalised using β-Actin and transformed using the 2−ΔΔCt method. No significant differences were observed in the messenger RNA (mRNA) expression levels of β-actin between groups. The analysis has been performed in 4 to 6 animals per group.
2.11. Statistical analysis
All the experimental procedures were performed by a researcher blind to the treatment. Results were expressed as mean ± SEM. The analysis of variance (ANOVA) was performed by one-way ANOVA with Bonferroni's significant difference procedure used for post-hoc comparisons. P values of less than 0.05 were considered significant. Data were analyzed using the “Origin 9” software (OriginLab, Northampton, MA). Statistical analysis on 16S sequencing data was performed by QIIME2 using nonparametric tests. Differences in relative abundance of bacterial taxa were evaluated using the Kruskal–Wallis test on pairwise or multiple comparisons. The permutational ANOVA (PERMANOVA) test was applied to beta diversity distance matrices generated by QIIME2 to test the significance between samples' clusters observed after principal coordinate analysis (PCoA); significance was determined through 999 permutations. Spearman correlation coefficients between relative abundances of microbial taxa and levels of SCFAs were computed using GraphPad Prism 6 (GraphPad Software, La Jolla, CA).
3. Results
3.1. Faecal microbiota transplantation from dinitrobenzenesulfonic acid donors transfers visceral hypersensitivity
A scheme of the experimental protocol has been reported in Figure 1A. Overtime visceral sensitivity has been assessed by both abdominal withdrawal reflex (AWR) and viscero-motor response (VMR) to colorectal distension (CRD). To limit the effects of the antibiotics on visceral sensitivity, we investigated the minimum treatment duration needed to obtain a sufficient microbiota depletion before proceeding with FMT. The culture-based analyses performed on freshly collected faecal pellets showed no viable bacterial cells after 7 days of treatment. The antibiotic treatment induced visceral hypersensitivity as assessed by AWR (Fig. 1B, day 7), whereas the VMR was unaltered (Fig. 1C, day 7). Antibiotic-induced hypersensitivity was mild on day 12 and completely disappeared on day 18, as assessed, respectively, by AWR (Fig. 1B) and VMR (Fig. 1C). Starting on day 7 and following the scheme reported in Figure 1A, FMT was performed using faeces from control (FMTCTR) or from animals in which a severe visceral pain was induced by the intrarectal injection of DNBS (FMTDNBS).
Figure 1.
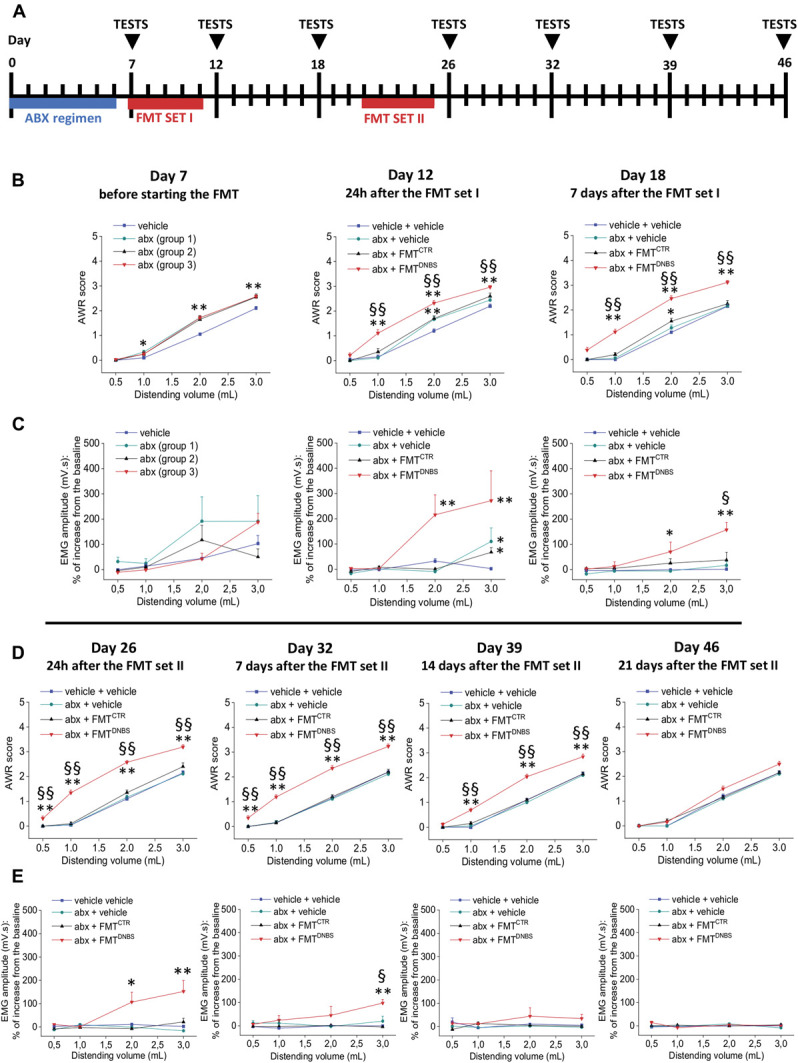
Effect of antibiotic treatment and FMT from DNBS-treated animals on visceral sensitivity of naive recipients. As shown in the scheme (A), rats were treated, with a combination of antibiotics for 7 days; the control group was treated with vehicle. On day 7, the abx-treated animals were divided into 3 groups, FMT from CTR donors, FMT from DNBS donors or vehicle, and were, respectively, administered per os for 5 consecutive days. One week after, the administrations were repeated. Behavioural tests were performed at the end of the antibiotic treatment, 24 hours and 7 days after each cycle of FMT and once week after the last treatment. Visceral sensitivity was assessed in the animals by measuring the AWR (B,D) and the VMR (C,E) to CRD (0.5-3 mL). Each value is the mean ± SEM of 5 rats per group in the VMR test and 10 or 14 (abx + FMTDNBS) rats per group for the AWR test. Statistical analysis was one-way analysis of variance followed by Bonferroni post hoc comparison. *P < 0.05 and **P < 0.01 vs vehicle or vehicle + vehicle–treated animals. §P < 0.05 and §§P < 0.01 vs abx + FMTCTR–treated animals. abx, antibiotics; AWR, abdominal withdrawal reflex; CRD, colorectal distension; CTR, control animals; DNBS, dinitrobenzenesulfonic acid; FMT, faecal microbiota transplantation; VMR, visceromotor response.
FMTDNBS induced, in comparison with FMTCTR, a significant increase of both AWR and VMR to CRD after the set I of FMT (respectively, Figs. 1B and C, days 12-18). Hypersensitivity was consolidated after the set II of FMT (Figs. 1D and E, days 26-32), in particular when evaluated as AWR in awake animals (day 39; Fig. 1D). Three weeks after the interruption of treatments, the effect of FMTDNBS ended, indeed the visceral sensitivity of abx + FMTDNBS animals appeared not different from that registered in controls on day 46 (Figs. 1D and E). FMTCTR did not induce significant effects on pain threshold, showing no differences in comparison with the group abx + vehicle at each time tested.
We observed similar results in preliminary experiments using a longer antibiotic treatment (24 days), although the effect of microbiota transplantation on visceral sensitivity was less clear because of the persistence of antibiotic-induced gut sensitivity alterations (Figure S1, available as supplemental digital content at http://links.lww.com/PAIN/B462).
3.2. Visceral hypersensitivity is accompanied by alteration of the gut microbiota composition
The taxonomic analysis of faecal suspensions, subsequently used for FMT, revealed several differences between control and DNBS-treated animals (Figure S3A, available as supplemental digital content at http://links.lww.com/PAIN/B462). For instance, the phylum Verrucomicrobia showed a diminishing trend in suspensions from DNBS-treated animals, and at lower taxonomic level, the Prevotellaceae family was almost absent in the same suspensions, whereas other families were found to be underrepresented, including Akkermansiaceae and Lactobacillaceae; on the other hand, an expansion of the Bacteroidaceae and Tannerellaceae families was identified (Figure S3A, available as supplemental digital content at http://links.lww.com/PAIN/B462).
Analysis of the alpha diversity in samples collected after the I and II set of FMT (day 18 and 46) showed a significantly greater microbial diversity in samples from the groups of animals receiving abx + FMTCTR and abx + FMTDNBS compared with others (vehicle + vehicle and abx + vehicle; Figure S3B, available as supplemental digital content at http://links.lww.com/PAIN/B462). This observation suggested a stable engraftment of gut microbiota after transplantation, whereas an antibiotic-driven dysbiosis was still persisting in animals of the abx + vehicle group. Such differences were even more marked on day 46, where the II set of FMT was clearly associated with a higher microbial diversity in samples from abx + FMTCTR and abx + FMTDNBS compared with controls. The long-lasting effect of the antibiotic and antifungal treatment was still evident in samples from animals belonging to the abx + vehicle group that displayed an overall lower microbial richness compared with other groups (Figure S3B, available as supplemental digital content at http://links.lww.com/PAIN/B462). On day 32, samples from the abx + FMTDNBS group were characterized by a microbial richness slightly higher, although not significant, than samples from abx + FMTCTR.
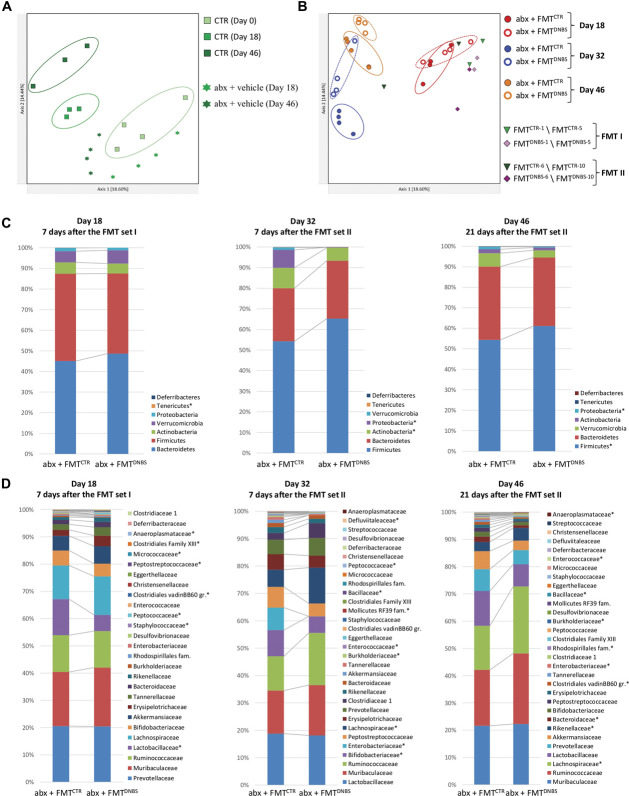
Evaluation of beta diversity through PCoA using the Bray–Curtis metric showed that samples from the abx + vehicle group clustered away from samples of corresponding controls (ie, controls at day 18 and 46), suggesting a different composition of the gut microbiota (Fig. 2A). Defined clusters of samples belonging to the abx + FMTCTR and abx + FMTDNBS groups were identifiable on PCoA plots on day 18 (PERMANOVA, P = 0.03; test statistic, 4.49), day 32 (PERMANOVA, P = 0.04; test statistic, 5.70), and day 46 (PERMANOVA, P = 0.02; test statistic, 3.54), with a more pronounced group separation occurring on day 32 (Fig. 2B). Analysis of beta diversity using the weighted UniFrac metric, which also considers the taxa phylogenetic relatedness in addition to their abundances, revealed a clustering behaviour similar to that previously observed for the abx + FMTCTR and abx + FMTDNBS groups (ie, using the Bray–Curtis metric) (Figs. S4A and B, available as supplemental digital content at http://links.lww.com/PAIN/B462).
Figure 2.
Analysis of beta diversity and taxonomic profiles. Principal coordinate analysis plots based on the Bray–Curtis beta diversity metric showing (A) the clustering pattern (left) among samples from CTR and from antibiotic-treated animals after vehicle administration (abx + vehicle); (B) the clustering pattern (right) among samples from antibiotic-treated animals subjected to FMT from controls (abx + FMTCTR) or from DNBS-treated animals (abx + FMTDNBS). Samples obtained from control (FMTCTR) or DNBS-treated (FMTDNBS) animals and used for the I and II set of FMT are also shown. The percentage of total variance explained is shown for each component. Taxonomic composition at the phylum (C) and family (D) level of samples belonging to the abx + FMTCTR and to the abx + FMTDNBS group; microbial taxa showing a statistically significant difference (Kruskal–Wallis P < 0.05) in their relative abundance between groups are marked with a star (*) in each legend box. abx, antibiotics; CTR, control animals; DNBS, dinitrobenzenesulfonic acid; FMT, faecal microbiota transplantation.
Samples from fecal suspensions obtained from control (FMTCTR) or DNBS-treated (FMTDNBS) animals were found to cluster away from each other (PERMANOVA, P = 0.02; test statistic, 3.81), consistently with their different source; moreover, when grouped by the I or II set of FMT, FMTCTR and FMTDNBS samples clustered away also from their corresponding recipients, although not significantly (Fig. S4B, available as supplemental digital content at http://links.lww.com/PAIN/B462).
Consistently with the sample clustering observed on beta diversity PCoA plots, analysis of the taxonomic composition of samples belonging to the abx + FMTCTR and abx + FMTDNBS groups pointed out to several significant differences at the phylum and family level. In detail, although no major changes in the relative abundance of most represented phyla were detected at day 18, an expansion of the Lactobacillaceae and Staphylococcaceae families was observed in samples from the abx + FMTDNBS and from the abx + FMTCTR groups (Kruskal–Wallis P < 0.043), respectively (Figs. 2C and D). At day 32, marked changes were identified in samples from the abx + FMTDNBS at a phylum level, showing a higher Firmicutes to Bacteroidetes ratio and a contraction of Proteobacteria and Actinobacteria (Kruskal–Wallis P = 0.02); at the family level, the most noticeable change consisted in a large expansion of Lachnospiraceae and a concomitant contraction of Enterobacteriaceae and Burkholderiaceae in samples from the abx + FMTDNBS (Kruskal–Wallis P = 0.02). Additional statistically significant variations (Kruskal–Wallis P < 0.047) have been observed in samples from the abx + FMTDNBS and from the abx + FMTCTR groups at day 32, although occurring at a lower extent (Figs. 2C and D). At day 46, some of the previously observed signatures were still present in samples from the abx + FMTDNBS group, as the higher Firmicutes to Bacteroidetes ratio, an even more pronounced expansion of the Lachnospiraceae family, and a contraction of Enterobacteriaceae and Burkholderiaceae relative abundances (Kruskal–Wallis P < 0.043) (Figs. 2C and D). Interestingly, a peculiar variation trend was observed in the relative abundance of Akkermansiaceae, being overall equally represented in samples from the abx + FMTCTR and abx + FMTDNBS groups at day 18 and 46 and almost disappeared in samples from the abx + FMTDNBS group at day 32 (Fig. 2D). Furthermore, a grouped analysis of the taxonomic profiles of FMTCTR and abx + FMTCTR samples after the I and II set of FMT, as well as of FMTDNBS and abx + FMTDNBS, was performed. Results showed that a close match between the transplanted fecal material and recipient animals was not detectable after both sets of FMT (Fig. S5, available as supplemental digital content at http://links.lww.com/PAIN/B462), thus corroborating the results obtained by the beta diversity analysis on these samples.
3.3. Changes in the levels of faecal short-chain fatty acids are associated with visceral hypersensitivity
Faecal pellets were collected from animals undergoing to FMTs 7 days after the I and the II set of treatment (days 18 and 32) and 3 weeks after the last treatment (day 46). An increase of the total amount of SCFAs was found in the abx + FMTDNBS group in respect to the abx + FMTCTR. This difference was detectable already on day 18, becoming significant on day 32 and extinguished on day 46 (Fig. 3A). Further in-depth analysis revealed that alteration on day 18 were because of an overall increase of fatty acids, whereas on day 32, acetic acid alone was found to be raised, whereas butyric acid significantly decreased (Figs. 3B–D).
Figure 3.
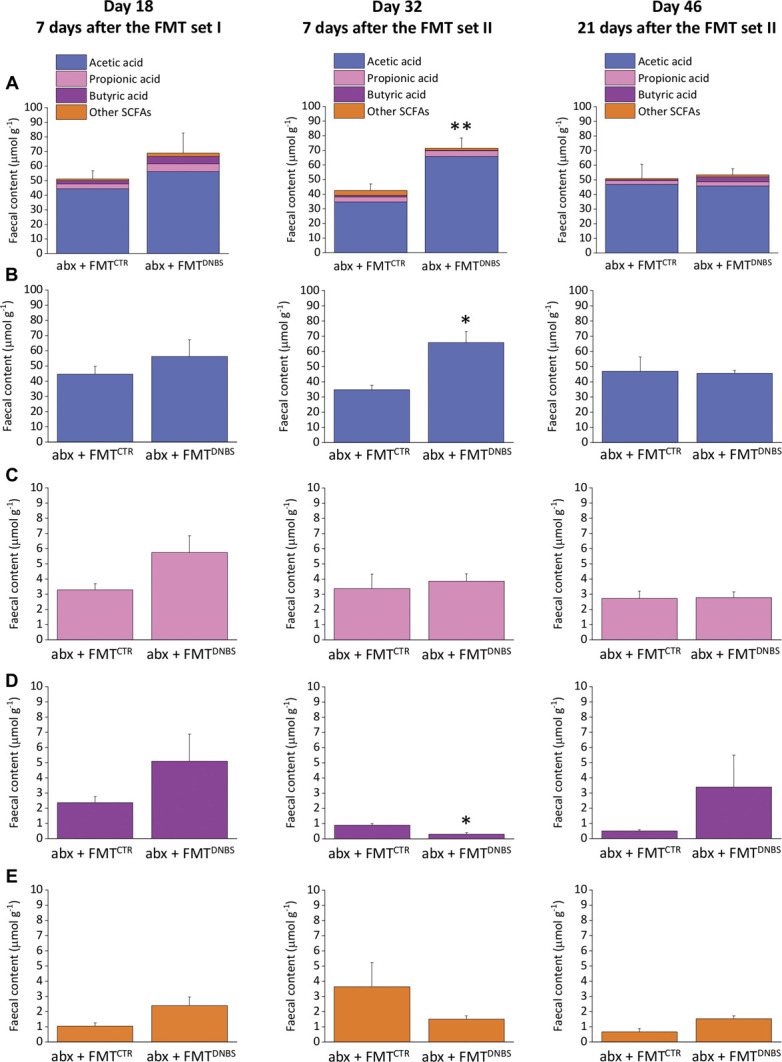
Effect of FMT on faecal SCFAs concentrations. HPLC analysis of (A) total SCFAs, (B) acetic acid, (C) propionic acid, (D) butyric acid, and (E) other fatty acids concentration in faecal pellets. Each value represents the mean ± SEM of 9 (day 18) or 5 (day 32 and 46) animals per group. Statistical analysis was one-way analysis of variance followed by Bonferroni post hoc comparison. **P < 0.01 and *P < 0.05 vs abx + FMTCTR treated animals. abx, antibiotics; CTR, control animals; DNBS, dinitrobenzenesulfonic acid; FMT, faecal microbiota transplantation; SCFA, short-chain fatty acid.
Correlation analyses between the concentration of SCFAs and relative abundances of microbial taxa that were previously found to statistically differ in abx + FMTCTR and abx + FMTDNBS groups revealed that some time-dependant associations were identifiable. In detail, after the I set of FMT at day 18, Peptococcaceae and Clostridiales vadin BB60 group were found to be negatively correlated with butyric acid (Spearman r range: −0.857, −0.821; P < 0.035), whereas the Lactobacillaceae family was positively correlated with acetic acid (Spearman r: +0.850, P: 0.02). After the II set of FMT at day 32, several bacterial families were found to be negatively correlated with acetic acid (Spearman r range: −0.778, −0.881; P < 0.030), including Burkholderiaceae, Enterobacteriaceae, Bifidobacteriaceae, Enterococcaceae, and Mollicutes RF39, as well as 2 phyla including Actinobacteria and Proteobacteria (Spearman r range: −0.928, −0.833; P < 0.015). On day 46, Rikkenellaceae and Anaeroplasmataceae were positively correlated with isobutyric acid (Spearman r range: 0.898, 0.803; P < 0.025) (Fig. S6, available as supplemental digital content at http://links.lww.com/PAIN/B462).
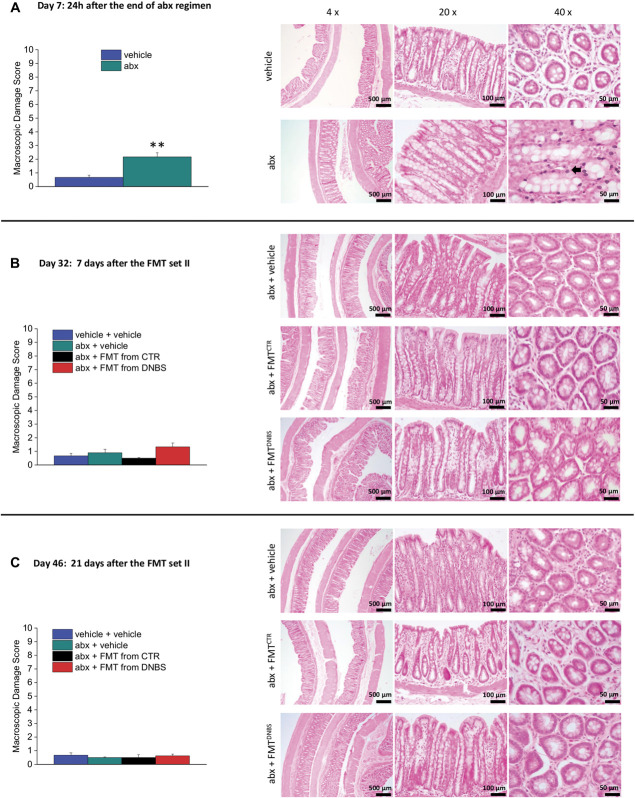
3.4. Faecal microbiota transplantation including the source does not affect colon histology
Colon samples were collected on days 7 (24 hours after the end of antibiotic treatment), 32 (when the effect of FMT on pain was well established) and 46 (when the effect of FMT extinguished). The macroscopic damage score highlights the presence of hyperaemia and a slight thickening of wall after the antibiotic treatment (day 7; Fig. 4A left panel). These results were confirmed by microscopic analysis: An infiltration of neutrophils in mucosa and submucosa was revealed in the animals receiving the antibiotic treatment (day 7; black arrow; Fig. 4A right panel). The tissue alteration disappeared after the interruption of antibiotic treatment. No macroscopic or microscopic differences were observed in the animals receiving FMTs (day 32 and 46; Figs. 4B and C, respectively).
Figure 4.
Histological evaluation of colon damage after the antibiotic regimen and the FMT. Macroscopic damage score (left panel) and representative microphotograph of haematoxylin or eosin-stained colon slices. Each value represents the mean ± SEM of 6 (A - day 7 and B - day 32) or 4 (C - day 46) animals per group. Statistical analysis was one-way analysis of variance followed by Bonferroni post hoc comparison. **P < 0.01 vs vehicle-treated animals. abx, antibiotics; CTR, control animals; DNBS, dinitrobenzenesulfonic acid; FMT, faecal microbiota transplantation.
3.5. Faecal microbiota transplantation does not affect gut permeability factors
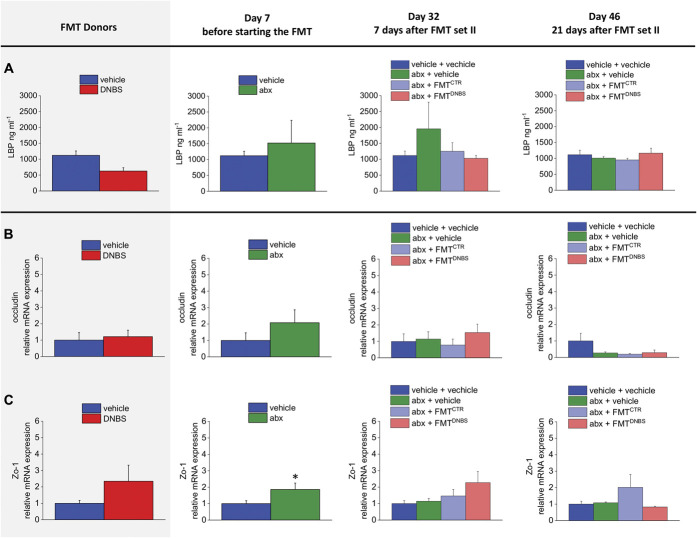
Gut permeability was indirectly evaluated by assessing the plasma levels of LBP (Fig. 5A) and also by measuring the mRNA expression of the tight junctions occludin and Zo-1 in the colon (Figs. 5B and C, respectively).
Figure 5.
Effect of antibiotic regimen and FMT on gut permeability. (A) Elisa assay for LBP in plasma samples. Analysis of occludin (B) and Zo-1(C) gene expression on colon samples by RT-quantitative PCR. The mRNA expression was normalized to β-actin, and fold changes were expressed in comparison with the control group. (A) Each value is the mean ± SEM of 3 or 5 (vehicle + vehicle group) animals per group; (B and C) Each value is the mean ± SEM of 6 (donors, day 7 and day 32) or 4 (day 46) animals per group. Statistical analysis was one-way analysis of variance followed by Bonferroni post hoc comparison. *P < 0.05 and **P < 0.01 vs vehicle-treated animals. abx, antibiotics; CTR, control animals; DNBS, dinitrobenzenesulfonic acid; FMT, faecal microbiota transplantation LBP, lipopolysaccharide-binding protein; mRNA, messenger RNA.
No significant alteration in the plasma levels of LBP was found among the experimental groups; a trend towards increase was observed after the antibiotic treatment (Fig. 5A; day 7).
Real time polymerase chain reaction analysis of colon tight junctions revealed a marginal increase (not statistically significant) of occludin mRNA after the antibiotic treatment (day 7; Fig. 5B). On the contrary, on day 7, after the antibiotic regime, Zo-1 mRNA significantly increased (Fig. 5C). Neither occludin nor Zo-1 was affected by FMTs (Figs. 5B and C, respectively).
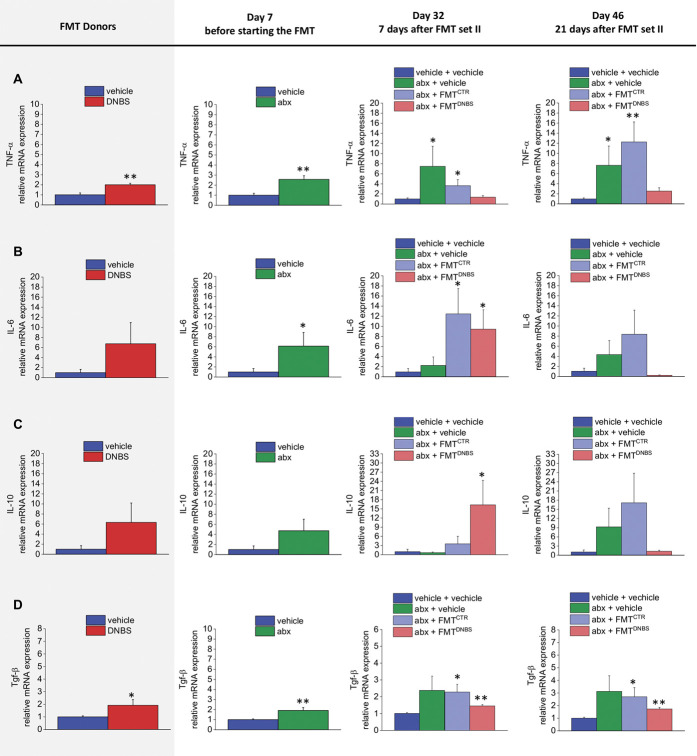
3.6. Faecal microbiota transplantation alters gut cytokines profile, irrespective of the donor
The expression of TNF-α, IL-6, IL-10, and TGF-β mRNA levels was measured in the colon by real time PCR. The antibiotic regimen caused a long-lasting upregulation of both proinflammatory and anti-inflammatory cytokines expression (day 7; Fig. 6). This deregulatory effect of antibiotics was not fixed by FMT (day 32; Fig. 6).
Figure 6.
Effect of antibiotic regimen and FMT on the gut cytokines profile. Analysis of TNF-α (A), IL-6 (B), IL-10 (C), and TGF-β (D) gene expression on colon samples by Real Time PCR. The mRNA expression was normalized to β-actin, and fold changes were expressed in comparison with the control group. Each value is the mean ± SEM of 6 (donors, day 7 and day 32) or 4 (day 46) animals per group. Statistical analysis was one-way analysis of variance followed by Bonferroni post hoc comparison. *P < 0.05 and **P < 0.01 vs vehicle-treated animals. abx, antibiotics; CTR, control animals; DNBS, dinitrobenzenesulfonic acid; FMT, faecal microbiota transplantation; IL, interleukin; mRNA, messenger RNA; TGF, transforming growth factor; TNF, tumor necrosis factor.
On the other hand, the cytokines profile of the FMT recipients did not match the pattern observed in the donors. The expression of the analyzed cytokines (TNF-alpha, IL-6, IL-10, and TGF-β) resulted augmented in DNBS donors in respect to CTR donors (Figs. 6A–D) although only the increase of IL-6 and TGF-β resulted significant (Figs. 6B and D). Despite this, either FMTDNBS or FMTCTR recipient animals showed an overexpression of IL-6 and TGF-β (day 32, Figs. 6B and D, respectively). At the same time, TNF-alpha and IL-10 were respectively upregulated in FMTCTR and FMTDNBS recipients (day 32; Figs. 6A and B, respectively). On day 46, all cytokines were still upregulated in the abx + vehicle group and in the abx + FMTCTR group. By contrast, in the abx + FMTDNBS, all cytokines, with exception of TGF-β (Fig. 6D), returned to the values observed in controls (Figs. 6A–C).
3.7. Transfer of microbiota from healthy controls counteracts postinflammatory visceral pain persistence
Finally, we tested the hypothesis that visceral pain could be suppressed or reduced by FMT from healthy donors. Pain threshold was assessed by evaluating the animal AWR in response to CRD stimulus at different timepoints after DNBS injection and FMT (Fig. 7A). As expected, 7 days after the induction of the damage, the abdominal withdrawal response to CRD was significantly higher in both the groups treated with DNBS (Fig. 7B, day 7). The FMTCTR led to a progressive reduction of visceral hypersensitivity in DNBS-treated animals that became significantly lower after the third transplant cycle (Fig. 7C, day 28) and consolidated after the fourth one (Fig. 7C, day 35). As a result of FMT, DNBS animals displayed a visceral sensitivity significantly lowered up to 17 days after the treatment interruption (Fig. 7D, day 49). On day 56 (Fig. 7D), the behavioural response of these animals to CRD resulted not significantly reduced compared with DNBS + vehicle–treated animals, and no differences were observed between DNBS animals receiving vehicle or FMT on day 63 (Fig. 7D).
Figure 7.
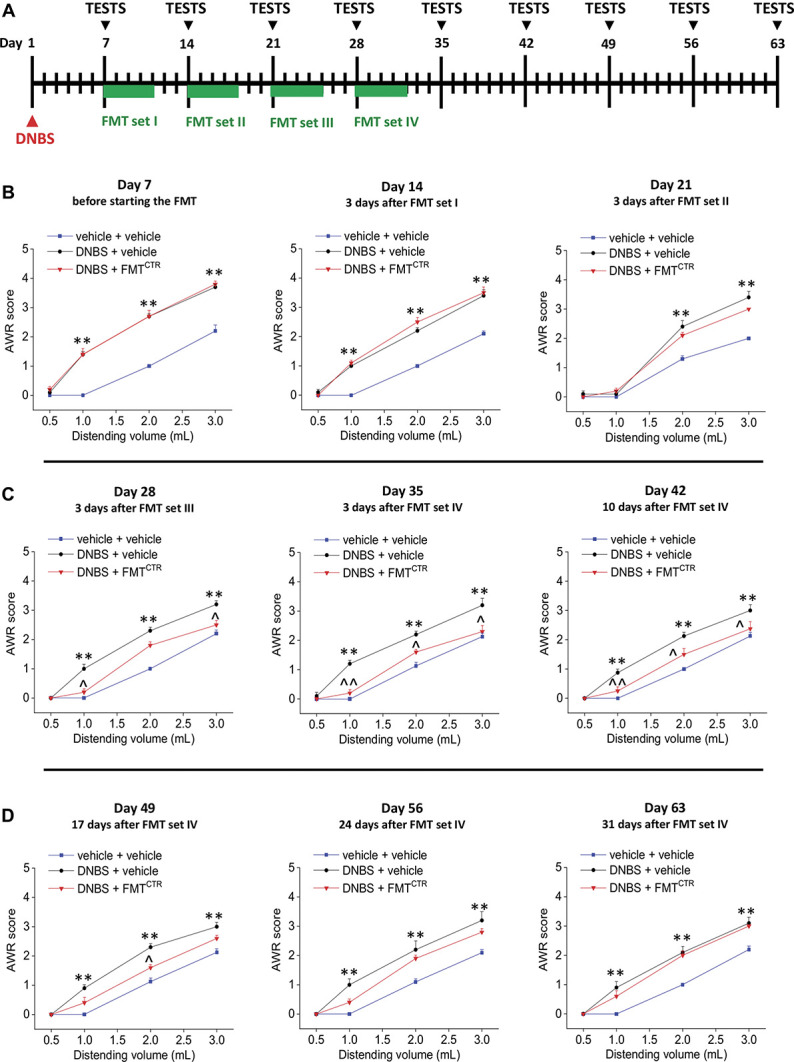
Therapeutic effect of FMT on DNBS-induced postinflammatory visceral pain. As shown in the scheme (A), rats were intrarectally injected with DNBS (30 mg in 0.25 mL EtOH 50%); on day 7, DNBS-injected animals were divided into 2 groups, respectively, administered with the vehicle or the FMT from CTR donors per os for 5 consecutive days. The FMT set was weekly repeated for 4 times. Behavioral tests were performed at the end of the antibiotic treatment, 3 days after each cycle of FMT, and once a week afterwards (B-D). Visceral sensitivity was assessed in the animals by measuring the AWR to CRD (0.5-3 mL). Each value is the mean ± SEM of 5 animals per group. Statistical analysis was one-way analysis of variance followed by Bonferroni post hoc comparison. *P < 0.05 and **P < 0.01 vs vehicle or vehicle + vehicle–treated animals. ^P < 0.05 and ^^P < 0.01 vs DNBS + vehicle–treated animals. abx, antibiotics; AWR, abdominal withdrawal reflex; CRD, colorectal distension; CTR, control animals; DNBS, dinitrobenzenesulfonic acid; FMT, faecal microbiota transplantation.
4. Discussion
The present work highlights the relevance of microbiota in pain signalling from the gut. The FMT from animals affected by visceral pain to healthy rats was sufficient to induce visceral hypersensitivity. On the other hand, the manipulation of gut microbiota by FMT induced a reduction of persistent visceral pain in DNBS animals. A strong association between animals' pain threshold and microbiota composition changes was demonstrated.
Although the gut microbiota seemed to be clearly involved in visceral pain regulation,32,47,70 the evidence collected so far are circumstantial.24,42,90 Moreover, little is known on the evolution of microbiota over time after transplantation from animals affected by a disease, like pain, to naive recipients. The only other evidence about the involvement of microbiota perturbations in visceral pain was obtained in another model of colitis induced by DSS.30 We report the active involvement of microbiota alterations in the persistence of pain after colitis resolution in the animal model induced by DNBS, which differs from the DSS model either for the aetiology, for the localization of the damage, and for the course of the disease, providing different insight into clinical translatability.3,37,38,53,92 Furthermore, the present work demonstrates significant temporal correlations between the composition of bacteria, their products (SCFAs), and pain in the animals receiving FMT rather than in the donors, which represents an original aspect compared with the previous findings. Yet, for the first time, it has been demonstrated that the effect of FMT is long lasting but reversible and that the final balance is strongly affected by the environment where the microbiota is transplanted.
As suggested by the analysis of the beta diversity in samples from FMTDNBS animals, major modifications in the composition and structure of the gut microbiota occurred after the FMT set II (day 32), correlating with a significantly increased response of these animals to CRD. At a later stage (day 46), when the difference in visceral sensitivity perception disappeared, a less pronounced variation in beta diversity was observed.
Evaluation of taxonomic profiles confirmed that marked differences between the animals that underwent the FMTCTR or FMTDNBS were identifiable on day 32 at the phylum and family level for major members of gut microbiota, including protective microbial taxa known to be associated with gastrointestinal health (ie, Akkermansiaceae).17,61 Akkermansia muciniphila is known to mediate a variety of host functions ranging from metabolism to immune regulation.27,28,73 Regarding its role in pain syndromes or functional gastrointestinal disorders, A. muciniphila was found to be significantly reduced in children with IBS78 as well as in animal models of postinflammatory IBS.20 Recently, Guida et al.40 reported that pain related to dysbiosis in vitamin D deficient mice was ameliorated by an anandamide congener, in concomitance with increased levels of Akkermansia, Eubacterium, and Enterobacteriaceae. However, there is little evidence for a pain-alleviating effect mediated by A. muciniphila. One hypothesis is that SCFAs, like propionate and acetate, both endproducts of mucus degradation by Akkermansia, may modulate visceral nociception, as stimulation of the SCFA receptor GPR43 has been shown to decrease visceral pain sensitivity in healthy humans.25,85 The SCFAs have been associated to human gastrointestinal disorders68 and proposed as regulators of visceral sensitivity; since the activation of their receptors, FFAR2/3 regulates leucocyte functions, such as the production of cytokines (eg, TNF-a, IL-2, IL-6, and IL-10), eicosanoids and chemokines (eg, CCL2).88 Nevertheless, the role of SCFAs in visceral and somatic pain perception is still the subject of debate: depending on the context, the effect of SCFAs signalling can be either analgesic or nociceptive.50 Clinical evidence has shown that butyrate, a SCFA found dramatically reduced in the faecal samples of IBS and IBD patients, is highly effective in relieving abdominal pain.4,46 The significant reduction of butyrate in the FMTDNBS group on day 32 is in line with this evidence. Nevertheless, despite the reduction of butyrate on day 32, the total amount of SCFAs increased in these animals both on day 18 and 32 mainly contributed by an overproduction of acetate. Specific bacterial taxa were found to be negatively correlated with the increased acetate levels uniquely on day 32, suggesting the presence of a defined microbiota profile at this stage. Of note, the recently identified bacterial genus Acetatifactor belonging to Lachnospiraceae, one of the most prominent bacterial family being expanded in samples from the FMTDNBS group, and showing strong acetate-producing capabilities,76 resulted overrepresented in FMTDNBS samples (Fig. S4C, available as supplemental digital content at http://links.lww.com/PAIN/B462). However, there are no consistent data in literature reporting a negative impact of acetate on gut physiology. Short-chain fatty acids modulate immune and inflammatory responses through activation of free fatty acid (FFA) receptors type 2 and 3 (FFA2 and FFA3 receptors) and G protein–coupled receptor 109A (GPR109A, known as hydroxyl-carboxylic acid 2, HCAR2) and inhibition of histone deacetylases.56 Recently, HCAR2 was found to regulate neuroinflammation and nervous system plasticity in neuropathic pain10 and multiple sclerosis.18,72 HCAR2 has been found to express in the hypothalamus, a vital integration site during ascending pain transduction.57 Despite all these evidences that suggest the potential mechanistic benefits of SCFA receptors in inflammation and pain transduction, studies directly investigating the role of SCFA receptors in visceral pain are still lacking. By monitoring the time course modifications and evolution of the gut microbiota in this model, emerged that the effects of FMT were long lasting but temporary, demonstrating the presence of a continuous bidirectional influence between the microbiota and the gut, as previously postulated.23,44 FMTDNBS-induced visceral hypersensitivity was evident already 24 hours after the first set of FMT, suggesting the presence of pain mediators in the supernatant obtained from the faeces, as supposed in other studies.13,90 The presence of residual DNBS in the solutions used for the FMT has been excluded by High Performance Liquid Chromatography (HPLC) analysis (Fig. S2, available as supplemental digital content at http://links.lww.com/PAIN/B462). Besides, the presence of pain mediators in the faecal medium cannot be excluded and might explain the acute effect of FMT. By contrast, the long-lasting hyperalgesia observed in these animals is not attributable to the acute stimulation, nor to local intestinal irritation, because colon histological analysis revealed no damage on day 32 (7 days after the last FMT). Although these findings support the idea of an active role of microbiota in pain induction by FMT, it remains to be clarified which factor(s) among the microbiota itself or its metabolic output or some host-derived metabolites contributed the most to this phenomenon. Besides, the apparent longer persistence of visceral hypersensitivity in the AWR test in respect to the VMR test suggested a possible central sensitization to the painful stimulus induced by CRD. Similarly, in the animals treated with DNBS, VMR was significantly decreased after 4 weeks, whereas the augmented AWR persisted up to 13 weeks after DNBS injection.59
A slight increase in gut permeability occurred after the antibiotic treatment, with an increase in the expression of Zo-1, which has been positively correlated with a leaky gut.31 This finding is consistent with the previously demonstrated positive effect of microbiota on gut barrier integrity maintenance.82
The bacterial community participates in maintaining intestinal homeostasis through the “training” of the immune system and inhibiting the growth of pathogens.49 The analysis of gene expression in the gut confirmed a derangement of cytokines as a result of both the antibiotic treatment and the FMT, but no significant correlations were found between the cytokines profiles among the groups and the observed differences in visceral pain. Moreover, the cytokine levels of recipients did not reflect those of the respective DNBS and CTR donors. On day 32, FMTCTR recipients showed increased levels of TNF-α, IL-6, and TGF-β, although their pain threshold was unaltered. At the same time, in FMTDNBS recipients, visceral hypersensitivity was associated to increased levels of IL-10, IL-6, and TGF-β. With respect of FMTCTR, animals receiving FMTDNBS displayed increased levels of IL-10 and a decreased level of TNF-α, despite these differences were not statistically significant. A number of evidence in the literature attests TNF-α contributes to enhance pain,36 whereas IL-10 is mostly reported to counteract the development of pain.55,84 In the gut, both T cells and M2 macrophages can release IL-10 and opioid peptides that can signal to sensitized pain-sensing neurons in the peripheral and central nervous system to reverse or control sensitization and resolve pain.51 Nevertheless, colitis in IL-10−/− mice was not found to be associated with abdominal pain.7 Clinically, the TNFα serum level was significantly higher, and IL-10 was significantly lower in IBS patients compared with healthy controls.21 Yet, mucosal mRNA expression of IL-10 tended to be decreased in IBS patients affected by visceral hypersensitivity.8
Then, we analysed the effect of FMT on tryptophan metabolism (Tables S1 and 2, available as supplemental digital content at http://links.lww.com/PAIN/B462), considering it is differentially affected in intestinal diseases characterized by persistent pain, like IBS and IBDs, and it is directly or indirectly controlled by the microbiota.1 In addition, we measured monoamine in the gut because of their impact on the microbiota–gut–brain axis physiology80 and their critical role in the modulation of pain.5,6,12 A decrease in plasma tryptophan was found in DNBS donors, likely consequent to an increased biosynthesis of serotonin in the gut, as confirmed by the HPLC (Tables S3 and 4, available as supplemental digital content at http://links.lww.com/PAIN/B462). This phenomenon, which is a characteristic consequence of intestinal damages, can directly influence the neuronal firing of visceral afferents.64,69 A plasma tryptophan decrease was also detected in the animals that underwent FMT and correlated to a massive and long-lasting increase of serotonin in the faeces (Table S6, available as supplemental digital content at http://links.lww.com/PAIN/B462), but not in the gut tissue (Table S3, available as supplemental digital content at http://links.lww.com/PAIN/B462). An increase of 5-HT in the gut lumen has been recently demonstrated to selectively modulate the colonization of bacteria species in the gut.35 Nevertheless, no difference was found between the animals receiving the FMT from CTR or from DNBS donors, demonstrating again that 5-HT is not involved in the transfer of pain mediated by FMTDNBS.
The decrease of dopamine consequent to the antibiotic treatment and the FMT (Tables S5 and 7, available as supplemental digital content at http://links.lww.com/PAIN/B462) resulted in line to previous data reporting an antibiotic-mediated reduction of this neurotransmitter in the gut, where it plays a role in gastrointestinal motility regulation.58 In accordance, the clearance of gut microbiota by antibiotic cocktail reduced the synthesis of dopamine in intestines and exacerbated liver damage, and the alteration in dopamine levels was restored by the recovery of gut microbiota.83,94 Physiological studies suggest that dopaminergic mechanisms are important in the regulation of gastrointestinal motility,87,89 directly or after its metabolism to noradrenaline.48,63,67 The gut levels of dopamine were reduced also in DNBS donors, which reported alterations in the gastrointestinal motility.2 Further study needs to evaluate the impact of dopamine reduction in the gut of animals receiving the FMT. The antihyperalgesic effect obtained after manipulation of the gut microbiota in DNBS animals contributes further to sustain the role of microbiota in pain signalling. Currently, many discrepancies do exist on the therapeutic potential of FMT.93 So far, it has been considered a good protocol to maintain a low number of FMT and supplied by a single donor.16,91 However, recent clinical studies highlighted the efficacy of a multiple donor and repeated treatment-based therapy in patients affected by ulcerative colitis, chronic intestinal pseudo-obstruction, and IBS.29,39,74 In certain pathological conditions, the interactions between genetic, immunological, and environmental factors might determine a state more resistant to therapeutic microbial manipulation.79 In the preclinical model of colitis-induced visceral hypersensitivity, we obtained a substantial reduction in pain after 4 cycles of FMT, using different donors. It is likely that the “inflammatory” environment wherein the microbiota is transplanted may pose as an obstacle to the engraftment of some microbial taxa compared with others. In this case, the repetition of the FMT could “force” the host to accept the newly introduced microbial communities. These findings suggest the possibility to enhance the therapeutic efficacy of FMT by developing different protocols according to the specific disorder to be treated. Moreover, the FMT outcome is drastically influenced by the microbial diversity of the donor's stool, so the identification of a “super donor” profile is pivotal for therapeutic purposes.91
In conclusion, further evidence about the active contribution of the gut microbiota to visceral pain induction and persistence has been provided by this study, strengthening the role of FMT as a promising therapeutic approach for abdominal pain. However, major knowledge gaps remain. Continued efforts in improving the mechanistic understanding of how visceral sensitivity can be modulated by the gut microbiota are therefore warranted.
Conflict of interest statement
The authors have declared that no conflicts of interest exists.
Appendix A. Supplemental digital content
Supplemental digital content associated with this article can be found online at http://links.lww.com/PAIN/B462 and http://links.lww.com/PAIN/B463.
Acknowledgments
The authors are grateful to Dr G. Clarke (APC Microbiome Ireland & Department of Psychiatry and Neurobehavioural Science, University College Cork, Cork, Ireland) and Dr K. Rea (APC Microbiome Ireland, University College Cork, Cork, Ireland) for their practical and theoretical input in the work. This research was supported by the Italian Ministry of Instruction, University and Research, by the University of Florence, and by the University College Cork.
E. Lucarini, L. Di Cesare Mannelli, C. Nicoletti, V. Di Pilato, and S. M. O'Mahony conceived and designed the study. E. Lucarini, C. Parisio, E. Niccolai, S. Baldi, and G. Bartolucci performed the experiments. L. Micheli and A. Toti analysed the data. E. Lucarini, L. Di Cesare Mannelli, and V. Di Pilato wrote the manuscript. A. Pacini, C. Nicoletti, A. Amedei, and S. M. O'Mahony revised the manuscript. C. Ghelardini, G. M. Rossolini, A. Amedei, and J. F. Cryan supervised the study and provided the experimental resources.
All experiments were performed using rodents in accordance with the International Association for the Study of Pain, the European Union directives, and the National Institutes of Health guidelines on laboratory animal welfare and approved by the Animal Care committee at the University of Florence (Italy).
The data sets generated and analysed during the current study are included as additional files. The 16S rRNA sequence data have been deposited in the NCBI Sequence Read Archive (SRA) database (https://www.ncbi.nlm.nih.gov/bioproject/) under the BioProject accession number PRJNA605112.
Footnotes
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Web site (www.painjournalonline.com).
Contributor Information
Elena Lucarini, Email: elena.lucarini@unifi.it.
Vincenzo Di Pilato, Email: vincenzo.dipilato@unige.it.
Carmen Parisio, Email: carmen.parisio@unifi.it.
Laura Micheli, Email: laura.micheli@unifi.it.
Alessandra Toti, Email: alessandra.toti@unifi.it.
Alessandra Pacini, Email: alessandra.pacini@unifi.it.
Gianluca Bartolucci, Email: gianluca.bartolucci@unifi.it.
Simone Baldi, Email: simone.baldi@unifi.it.
Elena Niccolai, Email: elena.niccolai@unifi.it.
Amedeo Amedei, Email: amedeo.amedei@unifi.it.
Gian Maria Rossolini, Email: gianmaria.rossolini@unifi.it.
Claudio Nicoletti, Email: claudio.nicoletti@unifi.it.
John F. Cryan, Email: J.Cryan@ucc.ie.
Siobhain M. O'Mahony, Email: SOMahony@ucc.ie.
Carla Ghelardini, Email: carla.ghelardini@unifi.it.
References
- [1].Agus A, Planchais J, Sokol H. Gut microbiota regulation of tryptophan metabolism in health and disease. Cell Host Microbe 2018;23:716–24. [DOI] [PubMed] [Google Scholar]
- [2].Antonioli L, Fornai M, Colucci R, Awwad O, Ghisu N, Tuccori M, Del Tacca M, Blandizzi C. Differential recruitment of high affinity A1 and A2A adenosine receptors in the control of colonic neuromuscular function in experimental colitis. Eur J Pharmacol 2011;650:639–49. [DOI] [PubMed] [Google Scholar]
- [3].Antonioli L, Fornai M, Colucci R, Ghisu N, Da Settimo F, Natale G, Kastsiuchenka O, Duranti E, Virdis A, Vassalle C, La Motta C, Mugnaini L, Breschi MC, Blandizzi C, Del Taca M. Inhibition of adenosine deaminase attenuates inflammation in experimental colitis. J Pharmacol Exp Ther 2007;322:435–42. [DOI] [PubMed] [Google Scholar]
- [4].Banasiewicz T, Krokowicz L, Stojcev Z, Kaczmarek BF, Kaczmarek E, Maik J, Marciniak R, Krokowicz P, Walkowiak J, Drews M. Microencapsulated sodium butyrate reduces the frequency of abdominal pain in patients with irritable bowel syndrome. Colorectal Dis 2013;15:204–9. [DOI] [PubMed] [Google Scholar]
- [5].Bandyopadhyaya A, Rajagopalan DR, Rath NP, Herrold A, Rajagopalan R, Napier TC, Tedford CE, Rajagopalan P. The synthesis and receptor binding affinities of DDD-016, a novel, potential, atypical antipsychotic. Medchemcomm 2012;3:580–3. [Google Scholar]
- [6].Bannister K, Dickenson AH. What do monoamines do in pain modulation? Curr Opin Support Palliat Care 2016;10:143–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Basso L, Benamar M, Mas-Orea X, Deraison C, Blanpied C, Cenac N, Saoudi A, Dietrich G. Endogenous control of inflammatory visceral pain by T cell-derived opioids in IL-10-deficient mice. Neurogastroenterol Motil 2020;32:e13743. [DOI] [PubMed] [Google Scholar]
- [8].Bennet SM, Polster A, Törnblom H, Isaksson S, Capronnier S, Tessier A, Le Nevé B, Simrén M, Öhman L. Global cytokine profiles and association with clinical characteristics in patients with irritable bowel syndrome. Am J Gastroenterol 2016;111:1165–76. [DOI] [PubMed] [Google Scholar]
- [9].Blumberg RS, Saubermann LJ, Strober W. Animal models of mucosal inflammation and their relation to human inflammatory bowel disease. Curr Opin Immunol 1999;11:648–56. [DOI] [PubMed] [Google Scholar]
- [10].Boccella S, Guida F, De Logu F, De Gregorio D, Mazzitelli M, Belardo C, Iannotta M, Serra N, Nassini R, de Novellis V, Geppetti P, Maione S, Luongo L. Ketones and pain: unexplored role of hydroxyl carboxylic acid receptor type 2 in the pathophysiology of neuropathic pain. FASEB J 2019;33:1062–73. [DOI] [PubMed] [Google Scholar]
- [11].Bolyen E, Rideout JR, Dillon MR, Bokulich NA, Abnet CC, Al-Ghalith GA, Alexander H, Alm EJ, Arumugam M, Asnicar F, Bai Y, Bisanz JE, Bittinger K, Brejnrod A, Brislawn CJ, Brown CT, Callahan BJ, Caraballo-Rodriguez AM, Chase J, Cope EK, Da Silva R, Diener C, Dorrestein PC, Douglas GM, Durall DM, Duvallet C, Edwardson CF, Ernst M, Estaki M, Fouquier J, Gauglitz JM, Gibbons SM, Gibson DL, Gonzalez A, Gorlick K, Guo J, Hillmann B, Holmes S, Holste H, Huttenhower C, Huttley GA, Janssen S, Jarmusch AK, Jiang L, Kaehler BD, Kang KB, Keefe CR, Keim P, Kelley ST, Knights D, Koester I, Kosciolek T, Kreps J, Langille MGI, Lee J, Ley R, Liu YX, Loftfield E, Lozupone C, Maher M, Marotz C, Martin BD, McDonald D, McIver LJ, Melnik AV, Metcalf JL, Morgan SC, Morton JT, Naimey AT, Navas-Molina JA, Nothias LF, Orchanian SB, Pearson T, Peoples SL, Petras D, Preuss ML, Pruesse E, Rasmussen LB, Rivers A, Robeson MS, II, Rosenthal P, Segata N, Shaffer M, Shiffer A, Sinha R, Song SJ, Spear JR, Swafford AD, Thompson LR, Torres PJ, Trinh P, Tripathi A, Turnbaugh PJ, Ul-Hasan S, van der Hooft JJJ, Vargas F, Vazquez-Baeza Y, Vogtmann E, von Hippel M, Walters W, Wan Y, Wang M, Warren J, Weber KC, Williamson CHD, Willis AD, Xu ZZ, Zaneveld JR, Zhang Y, Zhu Q, Knight R, Caporaso JG. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat Biotechnol 2019;37:852–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Bravo L, Llorca-Torralba M, Berrocoso E, Micó JA. Monoamines as drug targets in chronic pain: focusing on neuropathic pain. Front Neurosci 2019;13:1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Buhner S, Li Q, Vignali S, Barbara G, De Giorgio R, Stanghellini V, Cremon C, Zeller F, Langer R, Daniel H, Michel K, Schemann M. Activation of human enteric neurons by supernatants of colonic biopsy specimens from patients with irritable bowel syndrome. Gastroenterology 2009;137:1425–34. [DOI] [PubMed] [Google Scholar]
- [14].Callahan BJ, McMurdie PJ, Rosen MJ, Han AW, Johnson AJA, Holmes SP. DADA2: high-resolution sample inference from Illumina amplicon data. Nat Methods 2016;13:581–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Camilleri M, Boeckxstaens G. Dietary and pharmacological treatment of abdominal pain in IBS. Gut 2017;66:966–74. [DOI] [PubMed] [Google Scholar]
- [16].Cammarota G, Ianiro G, Tilg H, Rajilic-Stojanovic M, Kump P, Satokari R, Sokol H, Arkkila P, Pintus C, Hart A, Segal J, Aloi M, Masucci L, Molinaro A, Scaldaferri F, Gasbarrini G, Lopez-Sanroman A, Link A, de Groot P, de Vos WM, Hogenauer C, Malfertheiner P, Mattila E, Milosavljevic T, Nieuwdorp M, Sanguinetti M, Simren M, Gasbarrini A. European consensus conference on faecal microbiota transplantation in clinical practice. Gut 2017;66:569–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Cani PD. Human gut microbiome: hopes, threats and promises. Gut 2018;67:1716–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Chen H, Assmann JC, Krenz A, Rahman M, Grimm M, Karsten CM, Köhl J, Offermanns S, Wettschureck N, Schwaninger M. Hydroxycarboxylic acid receptor 2 mediates dimethyl fumarate's protective effect in EAE. J Clin Invest 2014;124:2188–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Chen Y, Lin C, Tang Y, Chen A-Q, Liu C-Y, Lu D-L. ZD 7288, an HCN channel blocker, attenuates chronic visceral pain in irritable bowel syndrome-like rats. World J Gastroenterol 2014;20:2091–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Chen Y, Xiao S, Gong Z, Zhu X, Yang Q, Li Y, Gao S, Dong Y, Shi Z, Wang Y, Weng X, Li Q, Cai W, Qiang W. Wuji Wan formula ameliorates diarrhea and disordered colonic motility in post-inflammation irritable bowel syndrome rats by modulating the gut microbiota. Front Microbiol 2017;8:2307–. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Choghakhori R, Abbasnezhad A, Hasanvand A, Amani R. Inflammatory cytokines and oxidative stress biomarkers in irritable bowel syndrome: association with digestive symptoms and quality of life. Cytokine 2017;93:34–43. [DOI] [PubMed] [Google Scholar]
- [22].Christianson JA, Gebhart GF. Assessment of colon sensitivity by luminal distension in mice. Nat Protoc 2007;2:2624–31. [DOI] [PubMed] [Google Scholar]
- [23].Contijoch EJ, Britton GJ, Yang C, Mogno I, Li Z, Ng R, Llewellyn SR, Hira S, Johnson C, Rabinowitz KM, Barkan R, Dotan I, Hirten RP, Fu SC, Luo Y, Yang N, Luong T, Labrias PR, Lira S, Peter I, Grinspan A, Clemente JC, Kosoy R, Kim-Schulze S, Qin X, Castillo A, Hurley A, Atreja A, Rogers J, Fasihuddin F, Saliaj M, Nolan A, Reyes-Mercedes P, Rodriguez C, Aly S, Santa-Cruz K, Peters L, Suarez-Farinas M, Huang R, Hao K, Zhu J, Zhang B, Losic B, Irizar H, Song WM, Di Narzo A, Wang W, Cohen BL, DiMaio C, Greenwald D, Itzkowitz S, Lucas A, Marion J, Maser E, Ungaro R, Naymagon S, Novak J, Shah B, Ullman T, Rubin P, George J, Legnani P, Telesco SE, Friedman JR, Brodmerkel C, Plevy S, Cho JH, Colombel JF, Schadt EE, Argmann C, Dubinsky M, Kasarskis A, Sands B, Faith JJ. Gut microbiota density influences host physiology and is shaped by host and microbial factors. Elife 2019;8:e40553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Crouzet L, Gaultier E, Del'Homme C, Cartier C, Delmas E, Dapoigny M, Fioramonti J, Bernalier-Donadille A. The hypersensitivity to colonic distension of IBS patients can be transferred to rats through their fecal microbiota. Neurogastroenterol Motil 2013;25:e272–82. [DOI] [PubMed] [Google Scholar]
- [25].Cruz-Aguliar RM, Wantia N, Clavel T, Vehreschild MJ, Buch T, Bajbouj M, Haller D, Busch D, Schmid RM, Stein-Thoeringer CK. An open-labeled study on fecal microbiota transfer in irritable bowel syndrome patients reveals improvement in abdominal pain associated with the relative abundance of Akkermansia muciniphila. Digestion 2019;100:127–38. [DOI] [PubMed] [Google Scholar]
- [26].Cryan JF, Dinan TG. Mind-altering microorganisms: the impact of the gut microbiota on brain and behaviour. Nat Rev Neurosci 2012;13:701–12. [DOI] [PubMed] [Google Scholar]
- [27].Dao MC, Everard A, Aron-Wisnewsky J, Sokolovska N, Prifti E, Verger EO, Kayser BD, Levenez F, Chilloux J, Hoyles L, Dumas ME, Rizkalla SW, Dore J, Cani PD, Clement K. Akkermansia muciniphila and improved metabolic health during a dietary intervention in obesity: relationship with gut microbiome richness and ecology. Gut 2016;65:426–36. [DOI] [PubMed] [Google Scholar]
- [28].Derrien M, Belzer C, de Vos WM. Akkermansia muciniphila and its role in regulating host functions. Microb Pathog 2017;106:171–81. [DOI] [PubMed] [Google Scholar]
- [29].El-Salhy M, Hausken T, Hatlebakk JG. Increasing the dose and/or repeating faecal microbiota transplantation (FMT) increases the response in patients with irritable bowel syndrome (IBS). Nutrients 2019;11:1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Esquerre N, Basso L, Defaye M, Vicentini FA, Cluny N, Bihan D, Hirota SA, Schick A, Jijon HB, Lewis IA, Geuking MB, Sharkey KA, Altier C, Nasser Y. Colitis-induced microbial perturbation promotes postinflammatory visceral hypersensitivity. Cell Mol Gastroenterol Hepatol 2020;10:225–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Fasano A. Intestinal permeability and its regulation by zonulin: diagnostic and therapeutic implications. Clin Gastroenterol Hepatol 2012;10:1096–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Foster JA, Rinaman L, Cryan JF. Stress & the gut-brain axis: regulation by the microbiome. Neurobiol Stress 2017;7:124–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Franzosa EA, Sirota-Madi A, Avila-Pacheco J, Fornelos N, Haiser HJ, Reinker S, Vatanen T, Hall AB, Mallick H, McIver LJ, Sauk JS, Wilson RG, Stevens BW, Scott JM, Pierce K, Deik AA, Bullock K, Imhann F, Porter JA, Zhernakova A, Fu J, Weersma RK, Wijmenga C, Clish CB, Vlamakis H, Huttenhower C, Xavier RJ. Gut microbiome structure and metabolic activity in inflammatory bowel disease. Nat Microbiol 2019;4:293–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Fung TC. The microbiota-immune axis as a central mediator of gut-brain communication. Neurobiol Dis 2020;136:104714. [DOI] [PubMed] [Google Scholar]
- [35].Fung TC, Vuong HE, Luna CDG, Pronovost GN, Aleksandrova AA, Riley NG, Vavilina A, McGinn J, Rendon T, Forrest LR, Hsiao EY. Intestinal serotonin and fluoxetine exposure modulate bacterial colonization in the gut. Nat Microbiol 2019;4:2064–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Gonçalves dos Santos G, Delay L, Yaksh TL, Corr M. Neuraxial cytokines in pain states. Front Immunol 2020;10:3061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Goyal N, Rana A, Ahlawat A, Bijjem KRV, Kumar P. Animal models of inflammatory bowel disease: a review. Inflammopharmacology 2014;22:219–33. [DOI] [PubMed] [Google Scholar]
- [38].Greenwood-Van Meerveld B, Prusator DK, Johnson AC. Animal models of gastrointestinal and liver diseases. Animal models of visceral pain: pathophysiology, translational relevance, and challenges. Am J Physiol Gastrointest Liver Physiol 2015;308:G885–903. [DOI] [PubMed] [Google Scholar]
- [39].Gu L, Ding C, Tian H, Yang B, Zhang X, Hua Y, Zhu Y, Gong J, Zhu W, Li J, Li N. Serial frozen fecal microbiota transplantation in the treatment of chronic intestinal pseudo-obstruction: a preliminary study. J Neurogastroenterol Motil 2017;23:289–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Guida F, Boccella S, Belardo C, Iannotta M, Piscitelli F, De Filippis F, Paino S, Ricciardi F, Siniscalco D, Marabese I, Luongo L, Ercolini D, Di Marzo V, Maione S. Altered gut microbiota and endocannabinoid system tone in vitamin D deficiency-mediated chronic pain. Brain Behav Immun 2020;85:128–41. [DOI] [PubMed] [Google Scholar]
- [41].Guida F, Turco F, Iannotta M, De Gregorio D, Palumbo I, Sarnelli G, Furiano A, Napolitano F, Boccella S, Luongo L, Mazzitelli M, Usiello A, De Filippis F, Iannotti FA, Piscitelli F, Ercolini D, de Novellis V, Di Marzo V, Cuomo R, Maione S. Antibiotic-induced microbiota perturbation causes gut endocannabinoidome changes, hippocampal neuroglial reorganization and depression in mice. Brain Behav Immun 2018;67:230–45. [DOI] [PubMed] [Google Scholar]
- [42].Hadizadeh F, Bonfiglio F, Belheouane M, Vallier M, Sauer S, Bang C, Bujanda L, Andreasson A, Agreus L, Engstrand L, Talley NJ, Rafter J, Baines JF, Walter S, Franke A, D'Amato M. Faecal microbiota composition associates with abdominal pain in the general population. Gut 2018;67:778–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Hernández-Chirlaque C, Aranda CJ, Ocón B, Capitán-Cañadas F, Ortega-González M, Carrero JJ, Suárez MD, Zarzuelo A, Sánchez de Medina F, Martínez-Augustin O. Germ-free and antibiotic-treated mice are highly susceptible to epithelial injury in DSS colitis. J Crohns Colitis 2016;10:1324–35. [DOI] [PubMed] [Google Scholar]
- [44].Hevia A, Bernardo D, Montalvillo E, Al-Hassi HO, Fernandez-Salazar L, Garrote JA, Milani C, Ventura M, Arranz E, Knight SC, Margolles A, Sanchez B. Human colon-derived soluble factors modulate gut microbiota composition. Front Oncol 2015;5:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Hoban AE, Moloney RD, Golubeva AV, McVey Neufeld KA, O'Sullivan O, Patterson E, Stanton C, Dinan TG, Clarke G, Cryan JF. Behavioural and neurochemical consequences of chronic gut microbiota depletion during adulthood in the rat. Neuroscience 2016;339:463–77. [DOI] [PubMed] [Google Scholar]
- [46].Huda-Faujan N, Abdulamir AS, Fatimah AB, Anas OM, Shuhaimi M, Yazid AM, Loong YY. The impact of the level of the intestinal short chain fatty acids in inflammatory bowel disease patients versus healthy subjects. Open Biochem J 2010;4:53–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Hyland NP, Quigley EMM, Brint E. Microbiota-host interactions in irritable bowel syndrome: epithelial barrier, immune regulation and brain-gut interactions. World J Gastroenterol 2014;20:8859–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Iversen L. Biochemistry of biogenic amines. Vol. 3. New York and Philadelphia: Springer Science & Business Media, 2013. [Google Scholar]
- [49].Kamada N, Chen GY, Inohara N, Nunez G. Control of pathogens and pathobionts by the gut microbiota. Nat Immunol 2013;14:685–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Kannampalli P, Shaker R, Sengupta JN. Colonic butyrate- algesic or analgesic? Neurogastroenterol Motil 2011;23:975–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Kavelaars A, Heijnen CJ. T cells as guardians of pain resolution. Trends Mol Med 2021;27:302–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Khan KJ, Ullman TA, Ford AC, Abreu MT, Abadir A, Marshall JK, Talley NJ, Moayyedi P. Antibiotic therapy in inflammatory bowel disease: a systematic review and meta-analysis. Am J Gastroenterol 2011;106:661–73. [DOI] [PubMed] [Google Scholar]
- [53].Kiesler P, Fuss IJ, Strober W. Experimental models of inflammatory bowel diseases. Cell Mol Gastroenterol Hepatol 2015;1:154–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Klem F, Wadhwa A, Prokop LJ, Sundt WJ, Farrugia G, Camilleri M, Singh S, Grover M. Prevalence, risk factors, and outcomes of irritable bowel syndrome after infectious enteritis: a systematic review and meta-analysis. Gastroenterology 2017;152:1042–54.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Laumet G, Bavencoffe A, Edralin JD, Huo XJ, Walters ET, Dantzer R, Heijnen CJ, Kavelaars A. Interleukin-10 resolves pain hypersensitivity induced by cisplatin by reversing sensory neuron hyperexcitability. PAIN 2020;161:2344–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Li M, van Esch BCAM, Wagenaar GTM, Garssen J, Folkerts G, Henricks PAJ. Pro- and anti-inflammatory effects of short chain fatty acids on immune and endothelial cells. Eur J Pharmacol 2018;831:52–9. [DOI] [PubMed] [Google Scholar]
- [57].Li S, Hua D, Wang Q, Yang L, Wang X, Luo A, Yang C. The role of bacteria and its derived metabolites in chronic pain and depression: recent findings and research progress. Int J Neuropsychopharmacol 2020;23:26–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Li ZS, Schmauss C, Cuenca A, Ratcliffe E, Gershon MD. Physiological modulation of intestinal motility by enteric dopaminergic neurons and the D2 receptor: analysis of dopamine receptor expression, location, development, and function in wild-type and knock-out mice. J Neurosci 2006;26:2798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Lucarini E, Parisio C, Branca JJV, Segnani C, Ippolito C, Pellegrini C, Antonioli L, Fornai M, Micheli L, Pacini A, Bernardini N, Blandizzi C, Ghelardini C, Di Cesare Mannelli L. Deepening the mechanisms of visceral pain persistence: an evaluation of the gut-spinal cord relationship. Cells 2020;9:1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Luczynski P, Tramullas M, Viola M, Shanahan F, Clarke G, O'Mahony S, Dinan TG, Cryan JF. Microbiota regulates visceral pain in the mouse. Elife 2017;6:e25887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Macchione IG, Lopetuso LR, Ianiro G, Napoli M, Gibiino G, Rizzatti G, Petito V, Gasbarrini A, Scaldaferri F. Akkermansia muciniphila: key player in metabolic and gastrointestinal disorders. Eur Rev Med Pharmacol Sci 2019;23:8075–83. [DOI] [PubMed] [Google Scholar]
- [62].Macia L, Tan J, Vieira AT, Leach K, Stanley D, Luong S, Maruya M, Ian McKenzie C, Hijikata A, Wong C, Binge L, Thorburn AN, Chevalier N, Ang C, Marino E, Robert R, Offermanns S, Teixeira MM, Moore RJ, Flavell RA, Fagarasan S, Mackay CR. Metabolite-sensing receptors GPR43 and GPR109A facilitate dietary fibre-induced gut homeostasis through regulation of the inflammasome. Nat Commun 2015;6:6734. [DOI] [PubMed] [Google Scholar]
- [63].Martinucci I, Blandizzi C, de Bortoli N, Bellini M, Antonioli L, Tuccori M, Fornai M, Marchi S, Colucci R. Genetics and pharmacogenetics of aminergic transmitter pathways in functional gastrointestinal disorders. Pharmacogenomics 2015;16:523–39. [DOI] [PubMed] [Google Scholar]
- [64].Mawe GM, Hoffman JM. Serotonin signalling in the gut—functions, dysfunctions and therapeutic targets. Nat Rev Gastroenterol Hepatol 2013;10:473–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Mayer EA, Tillisch K, Gupta A. Gut/brain axis and the microbiota. J Clin Invest 2015;125:926–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].McGrath JC, Lilley E. Implementing guidelines on reporting research using animals (ARRIVE etc.): new requirements for publication in BJP. Br J Pharmacol 2015;172:3189–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Mittal R, Debs LH, Patel AP, Nguyen D, Patel K, O'Connor G, Grati M, Mittal J, Yan D, Eshraghi AA, Deo SK, Daunert S, Liu XZ. Neurotransmitters: the critical modulators regulating gut-brain Axis. J Cell Physiol 2017;232:2359–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Niccolai E, Baldi S, Ricci F, Russo E, Nannini G, Menicatti M, Poli G, Taddei A, Bartolucci G, Calabrò AS, Stingo FC, Amedei A. Evaluation and comparison of short chain fatty acids composition in gut diseases. World J Gastroenterol 2019;25:5543–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Nikolaus S, Schulte B, Al-Massad N, Thieme F, Schulte DM, Bethge J, Rehman A, Tran F, Aden K, Hasler R, Moll N, Schutze G, Schwarz MJ, Waetzig GH, Rosenstiel P, Krawczak M, Szymczak S, Schreiber S. Increased tryptophan metabolism is associated with activity of inflammatory bowel diseases. Gastroenterology 2017;153:1504–16.e2. [DOI] [PubMed] [Google Scholar]
- [70].O'Mahony SM, Clarke G, Dinan TG, Cryan JF. Early-life adversity and brain development: is the microbiome a missing piece of the puzzle? Neuroscience 2017;342:37–54. [DOI] [PubMed] [Google Scholar]
- [71].O'Mahony SM, Felice VD, Nally K, Savignac HM, Claesson MJ, Scully P, Woznicki J, Hyland NP, Shanahan F, Quigley EM, Marchesi JR, O'Toole PW, Dinan TG, Cryan JF. Disturbance of the gut microbiota in early-life selectively affects visceral pain in adulthood without impacting cognitive or anxiety-related behaviors in male rats. Neuroscience 2014;277:885–901. [DOI] [PubMed] [Google Scholar]
- [72].Offermanns S, Schwaninger M. Nutritional or pharmacological activation of HCA(2) ameliorates neuroinflammation. Trends Mol Med 2015;21:245–55. [DOI] [PubMed] [Google Scholar]
- [73].Ouyang J, Lin J, Isnard S, Fombuena B, Peng X, Marette A, Routy B, Messaoudene M, Chen Y, Routy JP. The bacterium Akkermansia muciniphila: a sentinel for gut permeability and its relevance to HIV-related inflammation. Front Immunol 2020;11:645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Paramsothy S, Kamm MA, Kaakoush NO, Walsh AJ, van den Bogaerde J, Samuel D, Leong RWL, Connor S, Ng W, Paramsothy R, Xuan W, Lin E, Mitchell HM, Borody TJ. Multidonor intensive faecal microbiota transplantation for active ulcerative colitis: a randomised placebo-controlled trial. Lancet 2017;389:1218–28. [DOI] [PubMed] [Google Scholar]
- [75].Parisio C, Lucarini E, Micheli L, Toti A, Di Cesare Mannelli L, Antonini G, Panizzi E, Maidecchi A, Giovagnoni E, Lucci J. Researching new therapeutic approaches for abdominal visceral pain treatment: preclinical effects of an assembled system of molecules of vegetal origin. Nutrients 2020;12:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Pfeiffer N, Desmarchelier C, Blaut M, Daniel H, Haller D, Clavel T. Acetatifactor muris gen. nov., sp. nov., a novel bacterium isolated from the intestine of an obese mouse. Arch Microbiol 2012;194:901–7. [DOI] [PubMed] [Google Scholar]
- [77].Rhee SH, Pothoulakis C, Mayer EA. Principles and clinical implications of the brain–gut–enteric microbiota axis. Nat Rev Gastroenterol Hepatol 2009;6:306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Rigsbee L, Agans R, Shankar V, Kenche H, Khamis HJ, Michail S, Paliy O. Quantitative profiling of gut microbiota of children with diarrhea-predominant irritable bowel syndrome. Am J Gastroenterol 2012;107:1740–51. [DOI] [PubMed] [Google Scholar]
- [79].Sartor RB. Genetics and environmental interactions shape the intestinal microbiome to promote inflammatory bowel disease versus mucosal homeostasis. Gastroenterology 2010;139:1816–19. [DOI] [PubMed] [Google Scholar]
- [80].Skolnick SD, Greig NH. Microbes and monoamines: potential neuropsychiatric consequences of dysbiosis. Trends Neurosci 2019;42:151–63. [DOI] [PubMed] [Google Scholar]
- [81].Stern EK, Brenner DM. Gut microbiota-based therapies for irritable bowel syndrome. Clin Transl Gastroenterol 2018;9:e134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Stevens BR, Goel R, Seungbum K, Richards EM, Holbert RC, Pepine CJ, Raizada MK. Increased human intestinal barrier permeability plasma biomarkers zonulin and FABP2 correlated with plasma LPS and altered gut microbiome in anxiety or depression. Gut 2018;67:1555–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Strandwitz P. Neurotransmitter modulation by the gut microbiota. Brain Res 2018;1693:128–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Vanderwall AG, Milligan ED. Cytokines in pain: harnessing endogenous anti-inflammatory signaling for improved pain management. Front Immunol 2019;10:3009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Vanhoutvin SA, Troost FJ, Kilkens TO, Lindsey PJ, Hamer HM, Jonkers DM, Venema K, Brummer RJ. The effects of butyrate enemas on visceral perception in healthy volunteers. Neurogastroenterol Motil 2009;21:952-e976. [DOI] [PubMed] [Google Scholar]
- [86].Verdú EF, Bercik P, Verma-Gandhu M, Huang XX, Blennerhassett P, Jackson W, Mao Y, Wang L, Rochat F, Collins SM. Specific probiotic therapy attenuates antibiotic induced visceral hypersensitivity in mice. Gut 2006;55:182–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Vieira-Coelho MA, Soares-da-Silva P. Dopamine formation, from its immediate precursor 3,4-dihydroxyphenylalanine, along the rat digestive tract. Fundam Clin Pharmacol 1993;7:235–43. [DOI] [PubMed] [Google Scholar]
- [88].Vinolo MA, Rodrigues HG, Nachbar RT, Curi R. Regulation of inflammation by short chain fatty acids. Nutrients 2011;3:858–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Walker JK, Gainetdinov RR, Mangel AW, Caron MG, Shetzline MA. Mice lacking the dopamine transporter display altered regulation of distal colonic motility. Am J Physiol Gastrointest Liver Physiol 2000;279:G311–8. [DOI] [PubMed] [Google Scholar]
- [90].Wang P, Du C, Chen F-X, Li C-Q, Yu Y-B, Han T, Akhtar S, Zuo X-L, Tan X-D, Li Y-Q. BDNF contributes to IBS-like colonic hypersensitivity via activating the enteroglia-nerve unit. Sci Rep 2016;6:20320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Wilson BC, Vatanen T, Cutfield WS, O'Sullivan JM. The super-donor phenomenon in fecal microbiota transplantation. Front Cell Infect Microbiol 2019;9:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Wirtz S, Popp V, Kindermann M, Gerlach K, Weigmann B, Fichtner-Feigl S, Neurath MF. Chemically induced mouse models of acute and chronic intestinal inflammation. Nat Protoc 2017;12:1295–309. [DOI] [PubMed] [Google Scholar]
- [93].Xu D, Chen VL, Steiner CA, Berinstein JA, Eswaran S, Waljee AK, Higgins PDR, Owyang C. Efficacy of fecal microbiota transplantation in irritable bowel syndrome: a systematic review and meta-analysis. Am J Gastroenterol 2019;114:1043–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Xue R, Zhang H, Pan J, Du Z, Zhou W, Zhang Z, Tian Z, Zhou R, Bai L. Peripheral dopamine controlled by gut microbes inhibits invariant natural killer T cell-mediated hepatitis. Front Immunol 2018;9:2398. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental digital content associated with this article can be found online at http://links.lww.com/PAIN/B462 and http://links.lww.com/PAIN/B463.