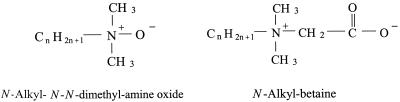Abstract
Alkyl betaines and alkyl dimethylamine oxides have been shown to have pronounced antimicrobial activity when used individually or in combination. Although several studies have been conducted with these compounds in combinations, only equimolar concentrations of the C12/C12 and C16/C14 chain lengths for the betaine and the amine oxide, respectively, have been investigated. This study investigates the antimicrobial activity of a wide range of chain lengths (C8 to C18) for both the betaine and amine oxide and attempts to correlate their micelle-forming capabilities with their biological activity. A broth microdilution method was used to determine the MICs of these compounds singly and in various molar ratio combinations. Activity against both Staphylococcus aureus and Escherichia coli was investigated. Antimicrobial activity was found to increase with increasing chain length for both homologous series up to a point, exhibiting a cutoff effect at chain lengths of approximately 16 for betaine and 14 for amine oxide. Additionally, the C18 oleyl derivative of both compounds exhibited activity in the same range as the peak alkyl compounds. Critical micelle concentrations were correlated with MICs, inferring that micellar activity may contribute to the cutoff effect in biological activity.
As more resistant organisms continue to emerge in society, the identification of additional antimicrobial agents becomes increasingly more important. Compounds such as surfactants are an area to be investigated. Betaines and amine oxides, two types of amphoteric surfactants, have been shown to exhibit antimicrobial activity against a variety of microorganisms (7, 16, 18, 25). Although each of these compounds has shown pronounced activity alone, they have also been used in combination to exhibit a synergistic effect (6).
An equimolar mixture of N-alkyl betaine and N-alkyl-N,N-dimethylamine oxide was patented in 1978 in a compound called C31G (17). With chain lengths ranging from C8 to C18 and buffered in a citrate buffer, C31G was first shown to have pronounced wound healing and deodorizing effects, as well as antimicrobial sensitivity. Further studies showed C31G has exhibited pronounced activity not only against bacteria, but also against yeasts, fungi, sperm, and enveloped viruses (4, 6, 14, 23). Although several studies have been published about this compound in reference to the extent of antimicrobial activity, little work has been conducted with any other chain lengths besides the following two chain-length combinations: (i) C12 betaine-C12 amine oxide and (ii) C16 betaine-C14 amine oxide. Additionally, only an equimolar ratio of the two components has been investigated.
The structures of these two components are shown in Fig. 1. The variation in length of the long hydrocarbon tail is thought to influence the extent of antimicrobial activity. Like most other surfactants, they are believed to be membrane perturbants, disrupting the cell membrane of the microorganism (26). It is believed that interaction with the surface of the microorganism is a function of the polar head groups of the betaine, amine oxide, or mixture of these molecules and that the hydrocarbon tail subsequently becomes integrated with the lipid bilayer of the cell membrane. This integration causes a disruption in the membrane and inevitably causes leakage of the cell contents. The length of the alkyl chain of the surfactants is thought to contribute to the extent of this membrane disruption, because the higher chain lengths may be incorporated into the lipid bilayers of the plasma membrane. The increased hydrophobic effect of these longer chain tails may aid in this disruption (16).
FIG. 1.
Chemical structures of N-alkyl-N,N-dimethylamine oxide and N-alkyl betaine.
In an effort to find an optimal combination of betaine and amine oxide, our study evaluated the extent of antimicrobial activity of a homologous series of betaines, amine oxides, and combinations of these compounds. Being surfactants, their micelle-forming capability is also correlated with their biological activity.
MATERIALS AND METHODS
Betaine and amine oxides.
Compounds ranging in chain length from 8 to 18 carbons for N-alkyl betaine and N-alkyl-N,N-dimethylamine oxide were obtained from two manufacturers, McIntyre Group, Ltd. (University Park, Ill.), and Stepan Co. (Northfield, Ill.). Although not all chain lengths were available, a representative group of samples was acquired. In addition to the alkyl straight chains, oleyl derivatives [C18(o)] of both betaine and amine oxide were also obtained for analysis. Table 1 shows the compounds tested and their manufacturers.
TABLE 1.
N-Alkyl betaine and N-alkyl-N,N-dimethylamine oxide derivatives available from manufacturers
| Chain length | Manufacturer
|
|
|---|---|---|
| Betaine | Amine oxide | |
| C8 | McIntyre | McIntyre |
| C10 | McIntyre | |
| C12 | McIntyre | McIntyre or Stepan |
| C14 | Stepan | |
| C16 | McIntyre | Stepan |
| C18 | Stepan | |
| C18 (oleyl) | McIntyre | McIntyre |
Microorganisms.
Escherichia coli (ATCC 25922) and Staphylococcus aureus (ATCC 25923) were obtained from the American Type Culture Collection (ATCC) and used as representative gram-negative and gram-positive organisms. Both microorganisms were maintained in Mueller-Hinton agar and broth, buffered to pH 4.8 for E. coli, and maintained at pH 7.3 for S. aureus. Citric acid buffer (2.5 mM) at pH 5.5 and 7.3 was used as a diluting solution as needed.
Microorganisms were stored in freezer vials at −10°C under conditions recommended by the ATCC. As needed, samples were thawed, warmed to 37°C, and streaked by dilution on agar petri plates for isolation. Inoculated plates were placed in a humidified incubator at 37°C for 24 h, during which time colonies formed. With a sterile loop, colonies were picked and dispersed in broth. After 24 h of incubation, the concentration of microorganisms was adjusted to a turbidity equal to that of a 0.5 McFarland standard, adjusted by diluting the overnight culture to a concentration equivalent to 80% transmittance (625 nm). This standardized suspension has been shown to contain approximately 108 CFU/ml (19).
Antimicrobial evaluation.
A microdilution plate method was used according to National Committee for Clinical Laboratory Standards methods with Mueller-Hinton broth (19). In 96-well dishes, concentrations of N-alkyl betaine and N-alkyl-N,N-dimethylamine oxide ranged from 104 to 1 μM, with the final well column containing no active sample solution. The standardized microorganism suspension was added to each well, and plates were incubated in a 37°C humidified incubator for 24 h before evaluation. The MICs were determined based on visual observation of turbid and nonturbid wells. Samples were run in duplicate.
CMCs.
Selected critical micelle concentrations (CMCs) were determined by using measured surface tension values as a function of concentration. Surface tension measurements at 25°C were determined by the Wilhelmy plate method on a Rosano surface tensiometer. The CMCs were determined by plotting the surface tension against the log of the concentration. The CMC is noted as the sharp change in decreasing surface tension as the concentration of surface active agent is increased.
RESULTS
Betaine.
The antimicrobial activity of a homologous series of N-alkyl betaines was evaluated against S. aureus and E. coli. Table 2 shows the MICs of these compounds. Antimicrobial activity was very poor at lower chain lengths, with MICs of C8 betaine of 2.3 × 104 μM for S. aureus and 1.2 × 104 μM for E. coli. The MICs of the betaine series decreased with increasing chain length, plateauing at the higher chain lengths—around C16 for both microorganisms. The C16 compound exhibited some of the best activity, with MICs of 61 and 120 μM for S. aureus and E. coli, respectively.
TABLE 2.
MICs of a homologous series of N-alkyl betaines for both S. aureus and E. coli
| Compound | MIC (μM)
|
|
|---|---|---|
| S. aureus | E. coli | |
| C8 betaine | 2.3 × 104 | 1.2 × 104 |
| C12 betaine | 150 | 290 |
| C16 betaine | 61 | 120 |
| C18(o) betaine | 110 | 230 |
Amine oxide.
Table 3 shows the MIC results for the series of homologous N-alkyl-N,N-dimethylamine oxides. Like the betaine series, the amine oxide series followed a similar trend of increased activity with increased chain length. Again exhibiting very poor activity at the low chain lengths, the MICs of the C8 amine oxide were 2.9 × 104 and 3.6 × 104 μM for S. aureus and E. coli, respectively. The activity also increased with chain length, up to approximately C14 to C16, and then tailed off at the higher chain lengths. For the amine oxide series, activity peaked at a chain length of C14 against E. coli, at a MIC of 31 μM, and plateaued at C14 to C16 against S. aureus, at a MIC of 62 μM. In addition, the C18 oleyl compound showed excellent activity against both microorganisms, matching the alkyl chain's peak activity. Being unsaturated in chemical structure, the oleyl compounds do not exhibit the poor solubility problems usually associated with the C18 stearyl compounds.
TABLE 3.
MICs of a homologous series of N-alkyl-N,N-dimethylamine oxides for both S. aureus and E. coli
| Compound | MIC (μM)
|
|
|---|---|---|
| S. aureus | E. coli | |
| C8 amine oxide | 2.9 × 104 | 3.6 × 103 |
| C10 amine oxide | 3.1 × 103 | 340 |
| C12 amine oxide | 340 | 87 |
| C14 amine oxide | 62 | 31 |
| C16 amine oxide | 56 | 56 |
| C18 amine oxide | 102 | 390 |
| C18(o) amine oxide | 51 | 25 |
CMCs.
The CMCs for N-hexadecyl betaine and N-tetradecyl-N,N-dimethylamine oxide were determined by using surface tension measurements as a function of concentration. The remaining CMCs have been collected from the literature and are shown in Table 4. It is well known in the literature that a linear relationship exists between chain length and CMC (22). For a homologous series, the following equation has been used: Log CMC = k1 N + k2, where N is equal to the number of carbon atoms in the alkyl chain and k1 and k2 are constants. A linear relationship was determined for the betaine series in this study, yielding the equation Log CMC = −0.447 N + 4.10 (R2 = 0.995). The amine oxide series also followed a linear trend, yielding the equation Log CMC = −0.438 N + 3.89 (R2 = 0.998). Even with compiled CMCs, determined under different conditions, the linear correlation is still very good.
TABLE 4.
CMCs of N-alkyl betaine and N-alkyl-N,N-dimethylamine oxide as a function of alkyl chain length
| Alkyl chain length | CMC (μM)a
|
|
|---|---|---|
| Betaine | Amine oxide | |
| 8 | 1.86 × 105 (1) | 1.50 × 105 (2) |
| 10 | 1.80 × 104 (1) | 1.5 × 104 (21) |
| 12 | 1.84 × 103 (1) | 1.70 × 103 (10) |
| 14 | 186 (1) | 268 |
| 16 | 20.1 | 24.9 (10) |
| 18 | 4.78 (9) | |
Reference numbers are given in parentheses.
DISCUSSION
Cutoff effect.
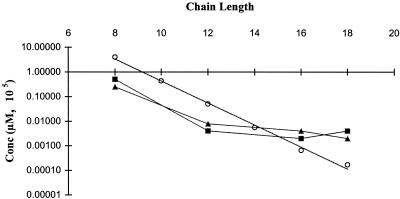
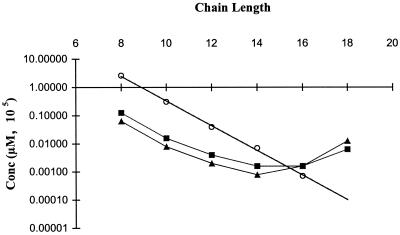
Unlike the log-linear relationship between increasing alkyl chain length and CMC for an entire homologous series, both the betaine and amine oxide series showed this linear relationship with the antimicrobial activity only at the lower chain lengths. Figures 2 and 3 show the relationship of concentration versus alkyl chain length, comparing both the MIC and the CMC. The MICs of both the betaine and the amine oxide series for both microorganisms exhibited a plateauing or parabolic effect with increasing alkyl chain lengths, occurring at chain lengths of approximately 14 to 16. This phenomenon is consistent with current literature regarding biological activity, with numerous studies documenting this type of response with homologous series of long-chain amphiphilic molecules (13, 22, 24).
FIG. 2.
Correlation of MIC with CMC for a homologous series of N-alkyl betaines. ○, CMC; ■, MIC for S. aureus; ▴, MIC for E. coli.
FIG. 3.
Correlation of MIC with CMC for a homologous series of N-alkyl-N,N-dimethylamine oxides. ○, CMC; ■, MIC for S. aureus; ▴, MIC for E. coli.
Ferguson was one of the first to document this type of effect in 1939 (8) when compiling a combination of studies relating to homologous series of compounds. He referred to this change in biological activity as exhibiting a “cutoff effect” at higher chain lengths.
Several theories have been postulated as to why this cutoff effect occurs. Janoff and Pringle (12, 20) have associated this cutoff with a limit in solubility. They proposed that as the alkyl chain increases, lipid solubility increases at a rate faster than the change in partition coefficient (lipid/aqueous). At these higher chain lengths, partitioning is limited, making the concentration at the site of action insufficient to have a significant effect on the membrane of the cell wall (12, 20). Other accounts attribute this to a decrease in perturbation of the membrane at higher chain lengths, proposing that the longer alkyl chain molecules better mimic molecules in the lipid bilayer, causing less of a disruption in the membrane (7).
Although numerous theories exist, it is likely that in the case of the betaine and amine oxide series, the cutoff in biological activity is due to the micellar action of these compounds. Ross and coworkers first suggested this theory, investigating alkylbenzyldimethylammonium chlorides of various chain lengths (22). As surfactants increase in chain length, their tendency toward micelle formation is greater, noted by the lower CMCs at higher chain lengths. This tendency to form micelles becomes greater than the tendency to move toward the interface (the membrane), and thus the concentration at the site of action becomes decreased. Also, as the size of the diffusing species increases from the size of a monomer to that of micelles, their diffusibility and permeation abilities will decrease, affecting their action on the microbial cell wall.
Comparing the CMC and the MIC in Fig. 2 and 3, the linear CMC line intersects the MIC line at the cutoff point for both the betaine and amine oxide series with both microorganisms investigated. At chain lengths below the cutoff point, the MICs of both compounds are below the CMC line, implying that the monomeric species of these compounds are responsible for an antimicrobial response. Although some micelles may be present at concentrations below the CMC, it is not likely they are significant enough to produce an effect. In the case of the amine oxide series, the MICs for E. coli are up to 2 orders of magnitude lower than the CMC. At these concentrations, it is not likely that the presence of micelles is significant.
At chain lengths above the cutoff point, the MIC is not reached until well above the CMC. Possessing a much lower CMC than the short-chain homologues, fewer monomers will be present at these concentrations, apparently less than are needed to produce a significant biologic effect. Increased overall concentrations are needed to obtain the desired bactericidal effects.
Effects of differences in bacterial strains.
It is also to be noted that both the betaine and amine oxide series show similar trends in activity for both S. aureus and E. coli, with both organisms exhibiting a cutoff effect in approximately the same location. These results are advantageous in a compound selection process, in which only one chain length that exhibits the best broad-spectrum activity can be selected for further development.
Although the similar effect on both the gram-positive and gram-negative organisms is preferred, it is not always the case with different strains of microorganisms. Ferguson noted that the most resistant organisms will often exhibit a cutoff effect much lower in alkyl chain length, while Lien and Hansch more specifically concluded that gram-positive organisms preferred a more lipophilic molecule than the gram-negative one (15). This has been attributed to the cell wall difference between bacterial types and strains. E. coli, a gram-negative rod, exhibits a more complex cell wall than gram-positive organisms such as S. aureus (5). Although both gram-positive and -negative organisms have a similar cytoplasmic membrane inside the outer wall, containing both phospholipids and membrane proteins, the outer walls are very different. Gram-positive bacteria have a very simple cell wall, consisting mainly of a mesh-like structure, while the gram-negative bacterial cell walls contain a layer of peptidoglycan between an outer membrane and the cytoplasmic membrane. This outer membrane contains lipopolysaccharides which are cross-bridged by divalent cations, believed to aid in the stabilization of the outer membrane and also make the membrane more impermeable to lipophilic molecules (3).
Oleyl compounds.
In addition to the cutoff peak of the homologous series, the oleyl compounds showed very good antimicrobial activity. The compounds are unsaturated and exhibit a much greater aqueous solubility than that of the other high chain lengths, particularly the C18 stearyl. They likely possess the right lipophilic/hydrophilic balance to allow the molecule to adequately disrupt the cell wall of the microorganism. The CMC of the 9-octadecyl-N,N-dimethylamine oxide has been shown to be 128 μM (11), which is comparable to the CMC of the most antimicrobially active alkyl compounds. Additionally, the CMC of this derivative is greater than the MIC, further supporting the contention that the antimicrobial aspects of these compounds are primarily due to that of the monomer.
Conclusions.
Overall, by comparing the MIC and CMC of the betaine and amine oxide series, this study has provided a better understanding of the relationship between the biological activities of these compounds correlated with their micelle-forming capabilities. It was shown that the majority of compounds provide excellent antimicrobial activity in their monomeric forms, but at chain lengths above the cutoff point, compounds must be in both a micellar form and monomeric form to exhibit a similar antimicrobial effect. Additionally, a range of chain lengths that exhibited some of the best antimicrobial activity was identified for both compounds. For both the betaine and amine oxide, compounds in the range of C14 to C16 were shown to be among the most effective of the alkyl compounds in addition to the unsaturated C18 oleyls.
ACKNOWLEDGMENT
This work was supported in part by the Biosyn Graduate Research Fellowship.
REFERENCES
- 1.Beckett A, Woodward R. Surface-active betaines: N-alkyl-N,N-dimethylglycines and their critical micelle concentrations. J Pharm Pharmacol. 1963;15:422–431. [PubMed] [Google Scholar]
- 2.Benjamin L. Calorimetric studies of the micellization of dimethyl-n-alkylamine oxides. J Phys Chem. 1964;68:3575–3581. [Google Scholar]
- 3.Brooks G, Butel J, Ornston L. Jawetz, Melnick and Adelberg's medical microbiology. 19th ed. Norwalk, Conn: Appleton and Lange; 1991. pp. 15–20. [Google Scholar]
- 4.Calis S, Yulug N, Sumnu M, Ayhan A, Hincal A. A non-antibiotic antimicrobial mixture (C31G): evaluation of the antimicrobial efficiency of C31G on vaginal culture. Boll Chim Farm. 1992;131:335–338. [PubMed] [Google Scholar]
- 5.Campbell N. Biology. 3rd ed. Redwood City, Calif: Benjamin Cummings Publishing; 1993. p. 517. [Google Scholar]
- 6.Corner A-M, Dolan M M, Yankell S L, Malamud D. C31G, a new agent for oral use with potent antimicrobial and antiadherence properties. Antimicrob Agents Chemother. 1988;32:350–353. doi: 10.1128/aac.32.3.350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Devinsky F, Kopecka-Leitmanova A, Sersen F, Balgavy P. Cut-off effect in antimicrobial activity and in membrane perturbation efficiency of the homologous series of N,N-dimethylalkylamine oxides. J Pharm Pharmacol. 1990;42:790–794. doi: 10.1111/j.2042-7158.1990.tb07022.x. [DOI] [PubMed] [Google Scholar]
- 8.Ferguson J. The uses of chemical potentials as indices of toxicity. Proc R Soc Lond B. 1939;127:387–404. [Google Scholar]
- 9.Harrison D, Szule R, Fisch M. Solution behavior of the zwitterionic surfactant octadecyldimethylbetaine. J Phys Chem. 1998;102:6487–6492. [Google Scholar]
- 10.Hoffman H. Correlation between surface and interfacial tensions with micellar structures and properties of surfactant solutions. Prog Colloid Polym Sci. 1990;83:16–28. [Google Scholar]
- 11.Imae T, Araki H, Ikeda S. The absorption spectra and the micelle species of dimethyloleylamine oxide in aqueous solutions. Colloids Surf. 1986;17:221–228. [Google Scholar]
- 12.Janoff A, Pringle M, Miller K. Correlation of general anesthetic potency with solubility in membranes. Biochim Biophys Acta. 1981;649:125–128. doi: 10.1016/0005-2736(81)90017-1. [DOI] [PubMed] [Google Scholar]
- 13.Kanazawa A, Ikeda T, Endo T. Synthesis and antimicrobial activity of dimethyl- and trimethyl-substituted phosphonium salts with alkyl chains of various lengths. Antimicrob Agents Chemother. 1994;38:945–952. doi: 10.1128/aac.38.5.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Krebs F, Miller S, Malamud D, Howett M, Wigdahl B. Inactivation of human immunodeficiency virus type 1 by nonoxynol-9, C31G, or an alkyl sulfate, sodium dodecyl sulfate. Antivir Res. 1999;43:157–173. doi: 10.1016/s0166-3542(99)00044-3. [DOI] [PubMed] [Google Scholar]
- 15.Lien E, Hansch C, Anderson S. Structure activity correlations for antimicrobial agents on gram-positive and gram-negative bacteria. J Med Chem. 1968;11:430–441. doi: 10.1021/jm00309a004. [DOI] [PubMed] [Google Scholar]
- 16.Lindstedt M, Allenmark S, Thompson R A, Edebo L. Antimicrobial activity of betaine esters, quaternary ammonium amphiphiles which spontaneously hydrolyze into nontoxic components. Antimicrob Agents Chemother. 1990;34:1949–1954. doi: 10.1128/aac.34.10.1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Michaels, E. B. August 1978. U.S. patent 4,107,328.
- 18.Mlynarcik D, Cupkova V, Devinsky F, Lacko I. Antimicrobial efficiency of saturated heterocyclic amine oxides. Folia Microbiol. 1978;23:493–495. doi: 10.1007/BF02885581. [DOI] [PubMed] [Google Scholar]
- 19.National Committee for Clinical Laboratory Standards. NCCLS document M7-A2. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. 2nd ed. Villanova, Pa: National Committee for Clinical Laboratory Standards; 1991. [Google Scholar]
- 20.Pringle M, Brown K, Miller K. Can the lipid theories of anesthesia account for the cut off in anesthetic potency in homologous series of alcohols? Mol Pharmacol. 1981;19:49–55. [PubMed] [Google Scholar]
- 21.Rathman J, Christian S. Determination of surfactant activities in micellular solutions of dimethyldodecylamine oxide. Langmuir. 1990;6:391–395. [Google Scholar]
- 22.Ross S, Kwartler C, Bailey J. Colloidal association and biological activity of some related quaternary ammonium salts. J Colloid Sci. 1953;8:385–401. [Google Scholar]
- 23.Thompson K, Malamud D, Storey B. Assessment of the antimicrobial agent C31G as a spermicide: comparison with nonoxynol-9. Contraception. 1996;53:313–318. [PubMed] [Google Scholar]
- 24.Tomlinson E, Brown M, Davis S. Effect of colloidal association on the measured activity of alkylbenzyldimethylammonium chlorides against Pseudomonas aeruginosa. J Med Chem. 1977;20:1277–1282. doi: 10.1021/jm00220a010. [DOI] [PubMed] [Google Scholar]
- 25.Tsubone K, Uchida N, Ito Y. Relation between structure and antimicrobial activity of 2-(N,N,N-trialkylammonio)alkyl hydrogen phosphates. J Pharm Sci. 1991;80:441–444. doi: 10.1002/jps.2600800509. [DOI] [PubMed] [Google Scholar]
- 26.Wyrick P B, Knight S T, Gerbig D G, Jr, Raulston J E, Davis C H, Paul T R, Malamud D. The microbial agent C31G inhibits Chlamydia trachomatis infectivity in vitro. Antimicrob Agents Chemother. 1997;41:1335–1344. doi: 10.1128/aac.41.6.1335. [DOI] [PMC free article] [PubMed] [Google Scholar]