Abstract
Background:
Phantom limb pain (PLP) management has been a challenge due to its response heterogeneity and lack of treatment access. This study will evaluate the feasibility of a remotely home-based M1 anodal tDCS combined with motor imagery in phantom limb patients and assess the preliminary efficacy, safety, and predictors of response of this therapy.
Methods:
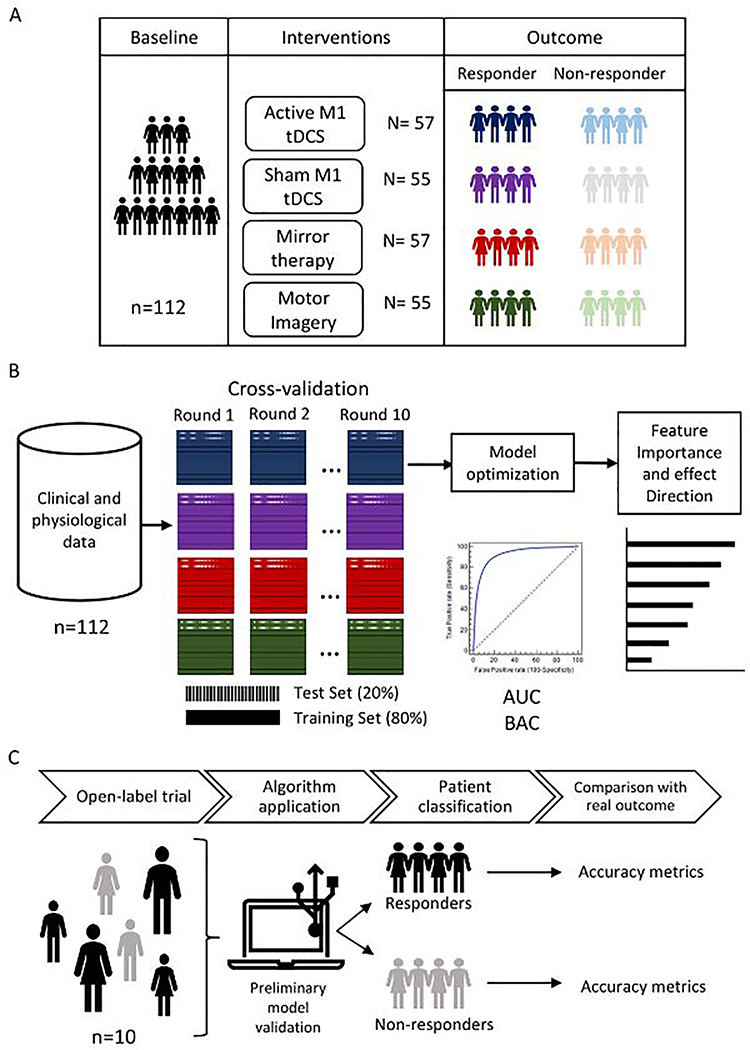
This is a pilot, single-arm, open-label trial in which we will recruit 10 subjects with phantom limb pain. The study will include 20 sessions. All participants will receive active anodal M1 tDCS combined with phantom limb motor imagery training. Our primary outcome will be the acceptability and feasibility of this combined intervention. Moreover, we will assess preliminary clinical (pain intensity) and physiological (motor inhibition tasks and heart rate variability) changes after treatment. Finally, we will implement a supervised statistical learning (SL) model to identify predictors of treatment response (to tDCS and phantom limb motor imagery) in PLP patients. We will also use data from our previous clinical trial (total observations=224 [n=112 x timepoints = 2)) for our statistical learning algorithms. The new prospective data from this open-label study will be used as an independent test dataset.
Discussion:
This protocol proposes to assess the feasibility of a novel, neuromodulatory combined intervention that will allow the design of larger remote clinical trials, thus increasing access to safe and effective treatments for PLP patients. Moreover, this study will allow us to identify possible predictors of pain response and PLP clinical endotypes.
Keywords: phantom limb pain, home-based tDCS, motor imagery, statistical learning, remote trial
1. Introduction
Phantom limb pain (PLP) is a prevalent neuropathic chronic pain condition in individuals who have undergone amputations, characterized by pain in the amputated limb (Flor, 2002; Flor et al., 2006; Nikolajsen & Jensen, 2000). Approximately 50–80% of amputees live with PLP (Limakatso, Bedwell, et al., 2020), and spontaneous pain reduction is not common (Flor, 2002; Nikolajsen & Jensen, 2000). Even two years after amputation, 59% of individuals still report PLP, and only 5–10% report a decrease in intensity (Flor, 2002; Nikolajsen & Jensen, 2000). Thus, PLP remains a significant problem for amputees, deeply impacting their quality of life (Trevelyan et al., 2016).
PLP is difficult to treat with conventional interventions such as pain medication, psychotherapy, and surgery (Erlenwein et al., 2021). The refractory nature of PLP and its resistance to mainstay therapeutic approaches may be explained by the functional and structural reorganization of the brainstem, thalamic nuclei, and somatosensory cortex caused by long-standing limb amputation. For instance, several studies reported a shift of the sensorimotor cortex associated with the phantom limb and an altered connectivity between insula and motor cortex (Birbaumer et al., 1997; Borsook et al., 1998; Flor et al., 1995; Grüsser et al., 2001; Montoya et al., 1998; K. Pacheco-Barrios et al., 2020; Ramachandran et al., 1992). Thus, conventional treatments seem to have limited effects since they do not target the plasticity changes associated with PLP. Given this mechanism, brain stimulation techniques, such as transcranial direct current stimulation (tDCS) and movement representation techniques, have been thought to be possible approaches for improving PLP and reverting maladaptive plasticity (Kevin Pacheco-Barrios, Xianguo Meng, et al., 2020; Thieme et al., 2016).
Bolognini and colleagues have found significant pain relief in amputees with five consecutive sessions of tDCS over the motor cortex in their crossover, double-blind, randomized clinical trial (Bolognini et al., 2015). In addition, a clinical trial from our group has also conveyed significantly effective pain reduction in individuals with PLP with the use of tDCS combined with motor imagery, but slightly smaller effects when combined with mirror therapy (Gunduz et al., 2021). Moreover, Limakatso et al. also found that graded motor imagery had significantly better effects in improving PLP when compared to conventional physical therapy (Limakatso, Madden, et al., 2020).
Despite trials reporting the efficacy and safety of these treatments, access and availability to pain management are increasingly difficult considering the need for physician- or therapist-guided therapies in specialized centers (Saito et al., 2020). Given the potential transportation difficulties of amputees and the current self-isolation and social distancing scenario due to the COVID-19 pandemic (Kevin Pacheco-Barrios, Alejandra Cardenas-Rojas, et al., 2020), there is an increasing demand for more home-based treatments targeted to chronic pain populations (Riggs et al., 2018). Nevertheless, there is a lack of studies evaluating tDCS and motor imagery feasibility as completely remote, home-based therapies for PLP; which is a critical step for clinical trials implementation and clinical translation (Arain et al., 2010).
Moreover, it is thought that treatments for PLP depict limited effectiveness because studies are evaluating therapies on significantly heterogeneous amputee populations (Richardson & Kulkarni, 2017). This suggests that different amputee subgroups may have varying treatment responses to PLP approaches, for instance, based on emotional, cognitive, and sensory-motor profiles (Osumi et al., 2019). Thus, considering the variability of treatment response in the PLP population, there is a need for the identification of possible predictors of response to tDCS and motor representation techniques.
Therefore, we present a protocol of an open-label study to evaluate the acceptability and feasibility of a remotely supervised, home-based, motor cortex anodal tDCS combined with motor imagery in phantom limb patients. Additionally, we will assess its preliminary effectiveness, safety, and physiological markers (indexed by motor inhibition tasks and heart rate variability). Furthermore, we will implement a statistical learning algorithm, based on our previous trial (Gunduz et al., 2021), to identify predictors of response to tDCS and motor imagery and develop PLP endotypes of response. Finally, we will use our open-label study to test the predictive models and PLP endotypes classification.
2. Methods and Analysis
2.1. Participants and Study Design
This is a pilot, single-arm, open-label trial. We will recruit 10 subjects with phantom limb pain (PLP) of any etiology (traumatic, vascular disease, diabetic amputees, or others) and location (upper or lower limb amputees). This protocol is approved by Mass General Brigham Review Board Ethical approval (#2021P000554). All the proceedings and methods of this study are following the Declaration of Helsinki guidelines.
Potential subjects for this research will be identified by inviting physicians or therapists’ referrals, letters, flyers, internet, and newspaper advertisements. All participants will receive active anodal M1 tDCS combined with phantom limb motor imagery training. Subjects and outcome evaluators will not be blinded to the intervention. The study will be done for a total of 5 weeks approximately. All study procedures will be done at the subject’s houses under strict remote supervision by the Neuromodulation Center researchers. Virtual visits will be conducted through Zoom Enterprise provided by Mass General Brigham. The informed consent (eConsent) and all questionnaires will be managed with Electronic Data Capture REDCap (Harris et al., 2009).
The study will consist of 23 visits per participant. During visit 1, we will perform the screening and consent process. In visit 2, we will provide subjects’ training on the procedures to perform during the trial. Then, the baseline assessments will be performed in visit 3. From visit 4 to visit 23, the interventions will be delivered. Finally, during visits 13 and 23, we will perform clinical and physiological assessments at the middle and the end of the protocol, respectively (Figure 1).
Figure 1.
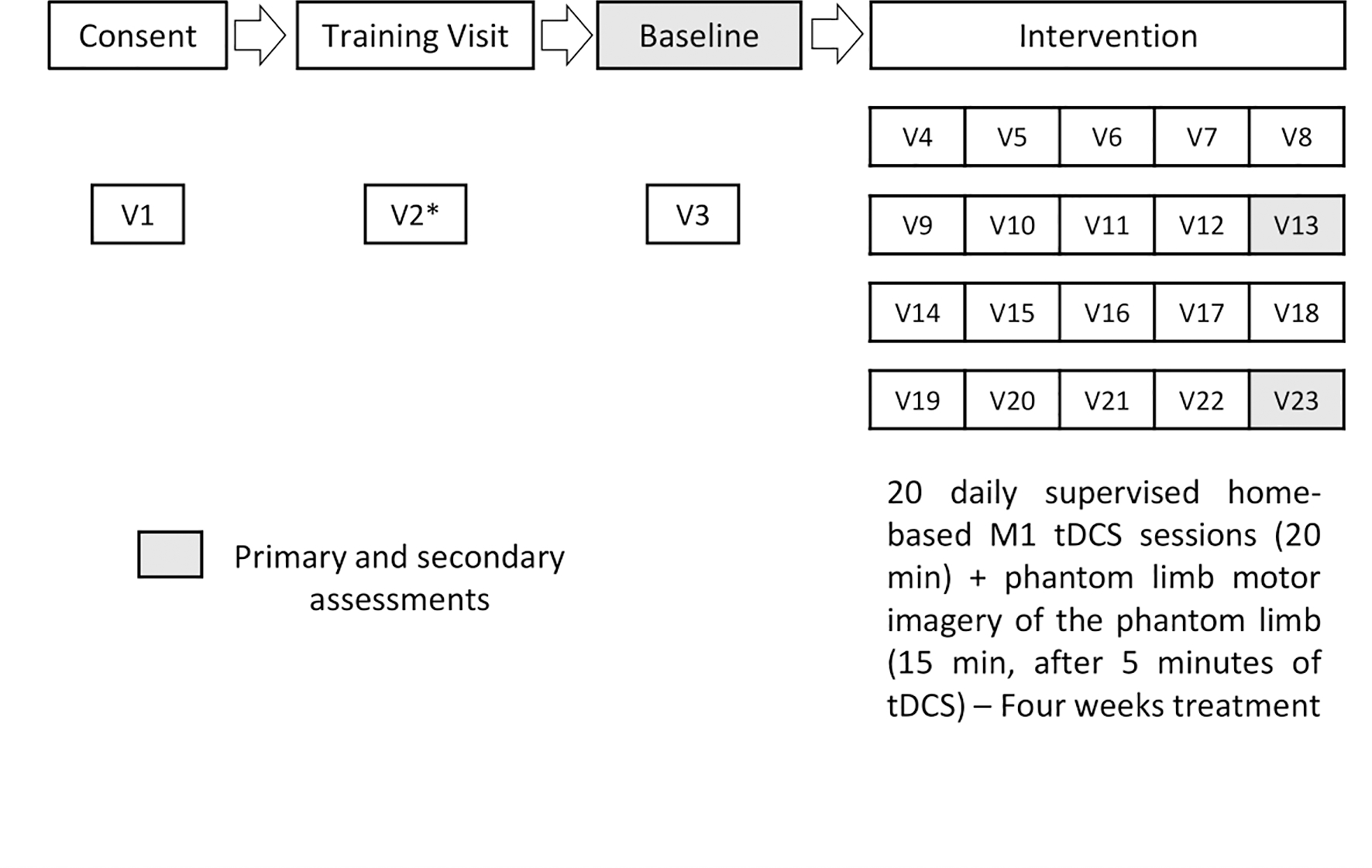
Study timeline (*) Training visits might take more remote visits if necessary.
2.1.1. Inclusion Criteria
Participants will be included in the trial if i) they can provide online informed consent; ii) older than 18 years; iii) have at least 1 month of phantom limb pain (experienced regularly for at least once a week) after the amputation-related wound has completely healed; iv) have an average pain of at least 4 on a numeric rating scale in the previous week (NRS; ranging from 0 to 10); and v) if they are taking any medications, dosages must be stable for at least 2 weeks prior to the enrollment of the study.
2.1.2. Exclusion Criteria
Subjects will be excluded in case of i) pregnancy or trying to become pregnant in the next 2 months, ii); history of alcohol or drug addiction within the past 6 months (self-reported); iii) presence of the following contraindication to transcranial direct current stimulation (ferromagnetic metal in the head [e.g., plates or pins, bullets, shrapnel] or implanted head electronic medical devices [e.g., cochlear implants]); iv) head injury resulting in loss of consciousness for at least 30 min or post-traumatic amnesia greater than 24 hours (as self-reported), with lasting neurological deficits; v) cognitive impairment as assessed by Montreal cognitive assessment; vi) unstable medical conditions (e.g., uncontrolled diabetes, uncompensated cardiac issues, heart failure, or chronic obstructive pulmonary disease); vii) uncontrolled epilepsy, as defined by a previous clinical seizure in the past 3 months in patients with treatment for epilepsy; viii) suffering from severe depression (as defined by a score of >30 in the Beck Depression Inventory); ix) history of unexplained fainting spells or loss of consciousness as self-reported during the last 2 years; or x) local skin infections or inflammatory conditions at the sites required for electrode placement on the head/scalp.
2.2. Interventions
We will provide a combined protocol consisting of home-based M1 tDCS and phantom limb motor imagery to the participants, which will be performed at the same time during each supervised stimulation visit.
2.2.1. Home-based Transcranial Direct Current Stimulation (tDCS)
We will use Soterix Medical 1X1 tDCS mini-CT stimulator device (© Soterix Medical Inc.) and FDA-approved home-based tDCS devices used in several clinical trials (Ahn et al., 2019; Pilloni et al., 2020; Riggs et al., 2018; Sivaramakrishnan et al., 2019) with no adverse events (Figure 2). It sends a low-level current from the positive electrode, anode, to the negative electrode, the cathode. During tDCS, low amplitude direct currents are applied via scalp electrodes and penetrate the skull to enter the brain. Direct current will be transferred by a saline-soaked pair of surface sponge electrodes (5×7cm, 35 cm2) and delivered by a specially developed, battery-driven, constant current stimulator. During this trial, subjects will receive 20 daily stimulation sessions with active anodal tDCS over the primary motor cortex (M1), for four weeks and will be allowed to reschedule up to one stimulation visit.
Figure 2.
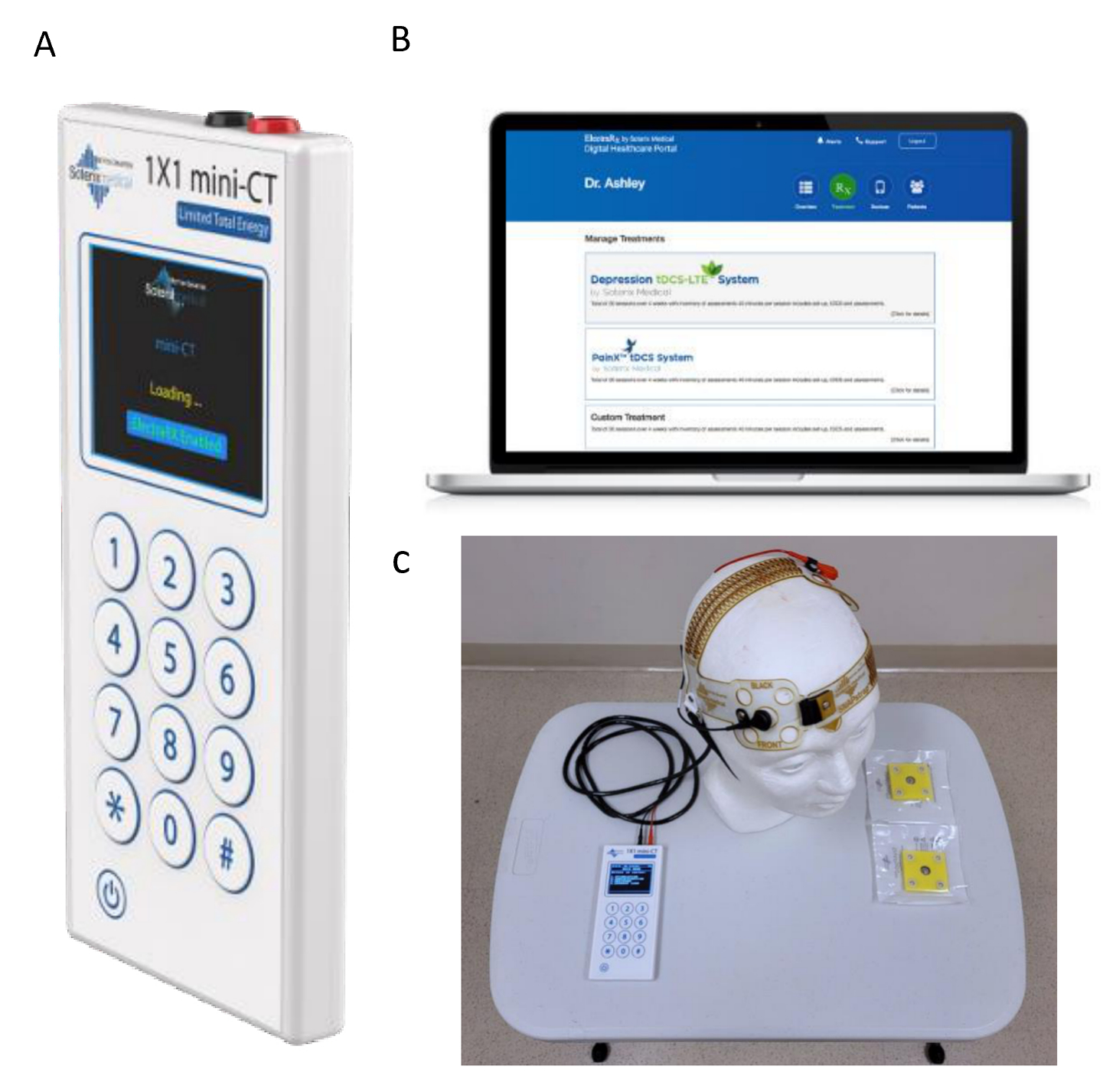
A. Home-based tDCS device (Soterix Inc.) B. Encrypted web platform C. Home-based stimulation kit components in our research center.
An anodal electrode will be placed during each session over M1, contralateral to the most painful amputation side, and the cathode over the contralateral supraorbital area. Two milliamps of tDCS will be applied for 20 minutes (Bolognini et al., 2013; Pinto et al., 2016).
2.2.1.1. Safety strategy
We will use the Soterix ElectraRX software (https://soterixmedical.com/research/remote/electrarx) for remote supervision of the tDCS sessions. This platform gives us unique login information for the research team and the participants. The stimulation protocol is not modifiable in the platform by the subject. It is accessible only by the research team to select an enrolled individual, and assign appropriate stimulation dose (intensity, duration, etc.), assuring the protocol safety. The device contains a skin-contact recognition function. Therefore, the output automatically shows a warning when the contact with the skin is poor and will stop if this is not improved or will not start the stimulation session. Additionally, during the intervention visit, we will connect via Zoom video call. We will have access to remote control of the provided laptop (laptop from the intervention kit used only for study-related activities) for strict supervision during treatment and data collection. A Side Effects Questionnaire for tDCS will be administered upon completion of the stimulation. In addition, we will complete the virtual clinical research visit checklist before any visit to assure safety and privacy. A senior clinician will be available at the time of the visit to answer questions, address unanticipated problems/adverse events or other issues that could be reported during the visit.
2.2.2. Phantom limb motor imagery protocol training
We will perform 15 min of phantom limb motor imagery exercises reported in previous studies (Brunelli et al., 2015; Mallik et al., 2020). The subject will be in a comfortable position, in a quiet environment. In the first part of the training session, the research will lead the subject in a progressive muscle relaxation exercise (McCallie et al., 2006). The subject will be induced to relax through the “body scan technique” for 5 min (Paulson et al., 2013). After that, the subject will be asked to focus on any kinesthetic, kinetic, or exteroceptive sensations from the phantom limb and to find a position that would be comfortable. Finally, for the remaining 10 min, the research will ask the participant to perform “imagined movements” of the phantom limb (toe/ finger, foot/hand, ankle/arm) in sequences. During the training, subjects will be asked to keep their attention focused on the task.
2.2.3. Home-based intervention kit:
As part of the fully online trial approach, we will deliver the intervention and assessment equipment to the participant’s houses after consent and before the training session. This intervention kit will content: ii) Soterix Medical 1X1 tDCS mini-CT stimulator device; ii) Heart rate monitor; iii) Encrypted laptop with camera, headphones, and pre-install software (Soterix ElectraRX software, zoom, team viewer, heart rate monitor software, Go/no-go assessment software).
2.2.4. Training and support for participants
Even though the device is practical to use and provides a safety guarantee, it is convenient to have a training session to address any question and check the compliant and proper usage of the device (Sandran et al., 2019). Therefore, checklist-based training will be conducted by using the device for practice without stimulation. We will provide a video training and manual before the training session. Subjects who have completed all the checklist items will be allowed to use the device for the study after the training session and a final test. This session will include practicing the placement and positioning of the device and preparation materials, starting the stimulator, and troubleshooting common problems. Remote support will be provided via a remote-control program as well as video calling will be used to facilitate the study.
2.3. Sample size calculation
In this open-label pilot study, we estimated the sample size based on our previous randomized controlled trial results on efficacy effect size (Gunduz et al., 2021) since no previous study on feasibility was conducted. We found an effect size of 1.08 for the combination of anodal M1 tDCS and covered mirror therapy (motor imagery without mirror feedback). Using that estimate, we performed a one-sample two-sided t-test calculation, with an alpha of 5% and power of 80%. In addition, we calculated a sample size of 9, however, we added a 10% due to the potential attrition rate (as reported in previous studies (Brietzke et al., 2020)). Therefore, our final sample size for this pilot study is 10 PLP patients.
2.4. Outcomes
2.3.1. Primary Outcome
Our primary outcome will be the acceptability and feasibility of a remotely supervised home-based M1 anodal tDCS protocol combined with motor imagery in phantom limb patients. We will evaluate the patient’s acceptability to the combined intervention and the delivery modality, using the Acceptability and feasibility measures scale (Table 1), at baseline (Visit 3), and at the end of the intervention (Visit 23). Also, we will evaluate the ability to implement the study as designed, assessing the number of missing sessions and dropouts at the end of the trial (Visit 23). Any participants’ self-reported problems with the intervention and reasons of missing data will be collected as notes in the adverse events form and visit form in REDCap.
Table 1 -.
Assessments
| Tool | Brief description |
|---|---|
| Primary outcome | |
| Acceptability and feasibility measures scale | The scale consists of a self-assessed 5-point Likert format from 1 (Completely disagree) to 5 (Completely agree) assessing subject perceptions about the intervention (Weiner et al., 2017). |
| Secondary outcomes | |
| Demographic data | This survey will age, gender, sex, race, ethnicity, education level, hand dominance, weight, height, and body mass index. |
| Medical history form | History of medical conditions, a list of medications currently using, allergies, history of hospitalizations or visits to an emergency room in the last year, tobacco and alcohol consumption, and emergency and primary care physician contact. |
| Visual Analogue Scale (VAS) for PLP | Subjects will rate their pain from 0 – indicating no pain at all, to 10 – indicating the worst pain felt. This scale is also colored, from green (at 0) to red (at 10), as a visual indicator of pain (Bolognini et al., 2013; Brodie et al., 2007; Moseley, 2006). |
| Visual Analogue Scale (VAS) for Residual limb pain | Any painful sensation in the stump. Subjects will rate their residual limb pain from 0 – indicating no pain at all, to 10 – indicating the worst pain felt. The scale will include colors to help in identifying the correct response (Bolognini et al., 2013). |
| Visual Analog Scale (VAS) for Phantom Limb Sensation | All non-painful sensations in the amputated part of the limb. Subjects will be presented with a scale starting at 0- No phantom limb sensation, to 10 – Full sensation of the amputated limb. The scale will include colors to help in identifying the correct response (Bolognini et al., 2013). |
| Visual Analog Scale (VAS) for Phantom Limb telescoping | Refers to the shrinking and retraction of the phantom towards the residual limb. Subjects will be presented with a scale starting at 0 - indicated that the phantom was enlarged, and 10 meant that the phantom was completely retracted into the stump, the scale will include colors to help in identifying the correct response (Bolognini et al., 2013). |
| Adapted Groningen Questionnaire after Arm Amputation | This questionnaire will be applied to obtain information concerning complaints that may be developed after arm amputation (Kooijman et al., 2000). |
| Adapted Groningen Questionnaire after Leg Amputation | This questionnaire is originally meant to obtain information concerning complaints that may be developed after arm amputation. We adapted the current arm version for lower limb amputation (Kooijman et al., 2000). |
| Prosthesis use questionnaire | This survey will ask for details related to the prosthesis use, including prosthesis use start date, stage of prosthesis training, use duration, usage per day, walking activity with prosthesis per day, use interruption, and prosthesis ownership. |
| Brief Pain Inventory (BPI) – short form | Short self-assessment questionnaire that provides information on various dimensions of pain including how pain developed, the types of pain a patient experiences, time of day pain is experienced, as well as current ways of alleviating pain (Cleeland & Ryan, 1994). It also includes the VAS Pain scale, a simple 10- point scale (0 = “no pain”, 10 = “pain as bad as you can imagine”) (Fregni et al., 2006). |
| Central sensitization inventory | This scale assesses symptoms that are related to central sensitization. It includes two parts: A. 25 common symptoms. B. previous diagnosis of central sensitivity syndromes (CSS) such as fibromyalgia, neck injury, temporomandibular joint disorder or migraine/tension headaches, anxiety, and depression (Neblett, 2018). |
| Pain and medication diary | Subjects will be asked to record the number of phantom limb episodes daily, using a pain diary. They will record the intensity of the strongest episode as well as phantom limb sensation and residual limb pain on a colored visual analog scale included in the diary, where 0 represents no pain at all and 10 represents the highest pain the patient has ever felt. Moreover, subjects will record their current medications and dosages daily in a pain medication diary, until the completion of the study. We will calculate a pain medication index to standardize the assessment (Harden et al., 2005). |
| Beck Depression Inventory | This self-report inventory consists of 21 multiple-choice questions and is a widely used method to classify depression severity. It assesses for the presence of several symptoms related to depression, such as irritability, hopelessness, and decreased cognitive performance. Physical symptoms such as weight loss and fatigue are also included (Santos Portilla et al., 2013; Villamar et al., 2013). |
| Beck Anxiety Inventory | This self-report inventory consists of 21 multiple-choice questions about how the subject has been feeling in the last week, expressed as common symptoms of anxiety (such as numbness and tingling, sweating not due to heat, and fear of the worst happening) (Beck et al., 1988). |
| Pain catastrophizing scale (PCS) | Pain catastrophizing is characterized by the tendency to magnify the threat value of a pain stimulus and to feel helpless in the presence of pain, as well as by a relative inability to prevent or inhibit pain-related thoughts in anticipation of, during, or following a painful event (Quartana et al., 2009). It affects how individuals experience pain, including three main characteristics: rumination, magnification, and helplessness (Vase et al., 2012; Vase et al., 2011). |
| The behavioral inhibition system (BIS)/ behavioral activation system (BAS) Scale | This scale is a 24-item self-report questionnaire designed to measure two motivational systems: the BIS (motivation to avoid aversive outcomes) and BAS (motivation to approach goal-oriented outcomes). Participants respond to each item using a 4-point Likert scale: 1 (very true for me), 2 (somewhat true for me), 3 (somewhat false for me), and 4 (very false for me). The scale has four subscales that were derived via factor analysis. One subscale corresponds to the BIS. Seven items contribute to this score (e.g., “Criticism or scolding hurts me quite a bit”). The remaining three subscales correspond to three components of BAS. BAS Drive measures the motivation to follow one’s goals. Four items contribute to this score. BAS Reward Responsiveness measures the sensitivity to pleasant reinforcers in the environment. Four items contribute to this score. BAS Fun Seeking measures the motivation to find novel rewards spontaneously. Five items contribute to this score (Carver & White, 1994). |
| Expectation for treatment scale (ETS) | The ETS is a well-validated and brief five-item scale for measuring patient expectations, with excellent test-retest properties (Barth et al., 2019). |
| Montreal Cognitive Assessment (MOCA) | This is a sensitive, valid, and reliable 30-point questionnaire that is used extensively in clinical and research settings to measure cognitive impairment. This instrument will be used as a brief screening of cognitive abilities. It will be used as a baseline evaluation (Freitas et al., 2012). |
| Pittsburgh Sleep Quality Index (PSQI) | A self-report measure of the quality and patterns of sleep in adults. It assesses 7 components of sleep quality: subjective sleep quality, sleep latency, sleep duration, habitual sleep efficiency, sleep disturbances, use of sleeping medications, and daytime dysfunction over the last month. Scoring of the answers is based on a Likert scale from “0” (not during the past month) to “3” (3 or more times a week). A total sum of “5” or greater indicates a “poor” sleeper (Buysse et al., 1989). |
| Short version of SF-36 | The short version of the SF-36 health survey is used as a measurement of quality of life. It provides a profile of functional health and well-being scores. It is also used as a psychometrical index of physical and mental health (Aldington et al., 2014; Rahimi et al., 2012; Sinha et al., 2014; Ware Jr et al., 1998). |
| Side Effects Questionnaire for tDCS | At each stimulation session, subjects will complete a questionnaire to evaluate potential adverse effects of tDCS (tingling, burning sensation, headache, neck pain, mood alterations) on a 4-point scale (None, mild, moderate, and severe). If any side effects are reported, the degree of relatedness to the intervention will be assessed on a 5-point scale (Villamar et al., 2013). |
2.3.2. Secondary Outcomes
2.3.2.1. Clinical outcomes
Our secondary outcomes will assess the preliminary effectiveness and safety of M1 anodal tDCS combined with motor imagery in phantom limb patients. Additionally, we will assess predictors of treatment response, based on our previous clinical trial (Gunduz et al., 2021).
We will evaluate the analgesic effects of the combined treatment measured by changes in PLP (indexed by a Visual Analog Scale) measured at the end of the intervention (4 weeks, visit 23). Additionally, we will assess changes in residual limb pain, phantom limb sensations, pain interference, central sensitization inventory, pain catastrophizing, quality of life, depression, and anxiety. See Table 1 for the description of all tests that we will perform in this trial.
2.3.2.2. Physiological outcomes
We will assess physiological variables such as heart rate variability and motor inhibition tasks (Go/no-go and stop-signal reaction tasks). The motor inhibition tasks will be programmed in the Gorilla Experiment Builder (www.gorilla.sc) (Anwyl-Irvine et al., 2018). The order of the tasks will be counterbalanced among subjects.
Heart rate variability (HRV) recording.
We will monitor the heart rate variability using a POLAR H10 heart rate sensor device (https://www.polar.com/us-en/products/accessories/h10_heart_rate_sensor), during the visits 3, 13 and 23 (Gilgen-Ammann et al., 2019). The recordings will be performed using a portable ECG sensor on a chest band. Participants will be comfortably seated in a chair. The recording will be for 5 min minutes as recommended in previous trials (Brunoni et al., 2013; Nikolin et al., 2017). After removing ectopic beats, HRV will be analyzed offline.
The HRV measurements will be (i) total power ≈≤0.4 Hz, (ii) very low frequencies ≤0.04 Hz (VLF), (iii) low frequencies 0.04–0.15 Hz (LF), (iv) high frequencies 0.15–0.4 Hz (HF), and (v) LF/HF ratio, as we performed in previous studies from our group (Morales-Quezada et al., 2015). In addition, given that the HRV signal also has random fluctuations and fractal structures (Costa et al., 2017; Goldberger et al., 2002) instead of being only regular quasi-periodic oscillations), we will also employ a non-linear method to calculate the short-term detrended fluctuation exponent (DFA) and sample entropy.
Go/No-go task
The GNG task was adapted from a previous study (Ahn et al., 2019). The participants will be instructed to seat approximately 70 cm from the monitor and to keep their hands over the space bar. The task comprises one practice block with 25 trials and two experimental blocks with 192 trials each. The practice block will include a feedback at the end of each trial. Each block contains three types of trial, namely no-go trial, frequent go trial, and infrequent go trial (see Appendix - Figure 1A). The frequent go trials represent 75% of the trials in each experimental block (i.e., 144 trials), whilst the no-go and the infrequent go trials correspond to 12.5% of the trials each (i.e., 24 trials each). Each experimental block started with 10 frequent go trials to allow familiarization with the task. Every trial starts with a fixation cross for 750 ms followed by a geometric shape for 750 ms. The geometric shape determines the type of the trial that is the action that the participant should perform. The square instructs the participant to withdraw any motor action (i.e., no-go trial), while the circle and the triangle are the instruction to press the space bar as fast as possible with the index finger (i.e., go trials). Regarding go trials, the circle is the representation of the frequent go trials and the triangle is the infrequent go-trials.
The accuracy and the response time (RT) in each type of trial will be recorded. Additionally, in case of lack of response during the 750 ms, a no-go response will be considered. A no-go response in a go trial will be considered an omission error, whilst a go response (i.e., press the space bar) in a no-go trial will be considered a commission error. Additionally, considering the number of omission and commission errors, it will be calculated the d-prime (d’ = Z Hits – Z False Alarms). The d’ derives from signal detection theory and addresses the ability to distinguish targets and non-targets (Hinton et al., 2018). In addition, the RT will be only analyzed in trials where participants executed a go response, but responses with less than 150 ms will be excluded from the data analysis (Chikazoe et al., 2009). Therefore, the data analysis will comprise the omission errors, commission errors, d’, and the RTs for each type of trial.
Stop-Signal Reaction Time task
The SSRTT followed the latest guidelines about the appropriate configuration of the task (Aron et al., 2019). The task will start with a practice block with 24 trials and will be followed by four experimental blocks with 64 trials each. In the practice block, it will be provided feedback at the end of the trial. Each experimental block includes 48 go trials (i.e., 75% of total trials) and 64 stop trials (i.e., 25% of total trials). At the beginning of the block, the first 10 trials will be “go” trials. Each trial starts with a fixation cross for 1000 ms followed by an arrow that can be oriented to the right or the left. The orientation of the arrow determines the response, namely, if the arrow is pointed to the right, the subject should press the “J” button, whilst if it is pointed to the left, the subject should press the “F” button.
Moreover, in stop trials, it will be displayed a stop signal (i.e., circle red frame around the arrow) after a variable delay, warning subjects to inhibit their response (see Appendix - Figure 1B). The delay of the stop signal will be adjusted according to the participant’s performance following a staircase-tracking algorithm (Band & Van Boxtel, 1999). The goal of the tracking procedure is to ensure a p(response|stop-signal) of 0.5. Therefore, the initial stop-signal delay (SSD) at the beginning of each block was 250 ms and it was constantly updated accordingly. For instance, after a correct inhibition (i.e., the subject did not press any button in a stop trial), the SSD increased 25 ms, while after an incorrect inhibition, the SSD decreased 25 ms. The minimum SSD possible will be 100 ms and the maximum 400 ms. Hence, the staircase-tracking algorithm will allow the adjustment of the difficulty in inhibiting a motor action in the next stop trial. For a successful tracking procedure, the participants will be instructed to respond as accurately and fast as possible according to the arrow in the screen and not wait for the stop signal.
The accuracy and RT of go trials will be analyzed after excluding trials next to stop trial and trials next to an incorrect go trial. Furthermore, mean RT will only comprise correct go trials and without outliers (i.e., 2 SDs above the mean RT). Regarding stop trials, the data analysis will include the p(respond|stop-signal), SSD, and the stop-signal reaction time (SSRT). The SSD will be the most frequent interval for each subject, while the SSRT will be estimated using the integration method. This method implies ranking the RTs and selecting the nth RT (n = number of RTs × p(respond|stop-signal)). At last, SSRT is estimated by the subtraction of SSD from the nth RT and averaging by each SSD from each subject. The SSRT indicates the minimum time necessary to inhibit an already initiated response.
2.5. Statistical Analysis
We will report the feasibility and acceptability questionnaire using descriptive statistics (absolute and relative measurements) as our primary outcome. Based on a recent systematic review on tDCS (Fregni et al., 2021), we will defined as a feasible home-based tDCS intervention if at least 80% of the stimulation visits are performed and the attrition rate is less than 30%. Additionally, participant’s self-reported problems with the intervention and reasons for missing data will be used to further describe the intervention’s feasibility. Among the secondary outcomes, the primary analysis will be PLP indexed by VAS. PLP will be analyzed using changes in pain after treatment (post-pre). To analyze these data, we will use a paired non-parametric model. Additionally, we will assess the secondary outcomes using non-parametric approaches. We will consider two-sided p-value and 95% confidence intervals (CIs). Additional statistical models for secondary outcomes will be developed in an exploratory manner. Therefore, we will not correct p values for multiple comparisons. Analyses will be conducted using standard statistical software such as STATA and R (Team, 2013).
Statistical Learning predictive model
Besides, we will develop a supervised statistical learning (SL) model to predict treatment response (≥50% of reduction of baseline PLP VAS) after tDCS and motor imagery intervention. We will use clinical and neurophysiological data from our previous trial (total observations=224 [n=112 x timepoints = 2) (Gunduz et al., 2021). An SL approach of feature engineering, data pre-processing, and model optimization will be used to create the most accurate predictive model. The SL approach will be optimized for small datasets to avoid overfitted models (Doan et al., 2021; Pruksawan et al., 2019). A principal component analysis algorithm will be used to obtain the most differentiable features, which will be used as features to train multiple classifiers (e.g., support vector machine and multilayer perceptron). We will divide the data for training (80%) and testing (20%), then 10-fold cross-validation with five repeats will be used to minimize over-fitting (Hastie et al., 2001). Finally, we will perform feature importance (Altmann et al., 2010) and accumulated local effect (Apley & Zhu, 2020) algorithms to identify the most important prediction and the direction of their effects, respectively. The classification accuracy will be assessed by balance accuracy (BAC), sensitivity, specificity, and area under the curve (AUC). We will use this dataset for testing. We will use the Phyton programing language to perform the modeling. The statistical learning analysis pipeline is shown in Figure 3.
Figure 3.
Statistical learning analysis pipeline A. Summary of our previous trial (1) B. Models will be trained to classify responders and non-responders at the study endpoint. We will optimize the models and measure the performance using the area under the curve (AUC) and balance accuracy (BAC) C. We will use the open-label trial data to validate the model comparing the prediction to the real outcome.
3. Discussion
This study evaluates the feasibility of a remotely supervised home-based M1 anodal tDCS combined with motor imagery in phantom limb patients and assesses the preliminary effectiveness, safety, and predictors of response of this therapy. This study will improve the development of non-pharmacological treatments for PLP and chronic pain and optimize the use of neuromodulation in chronic pain.
In this context, motor imagery seems to be the optimal behavioral intervention to activate the sensorimotor cortex, as shown by several studies (Bowering et al., 2013; Herrador Colmenero et al., 2018; Volz et al., 2015), and thus an optimal combination to enhance the effects of M1 tDCS. Besides, optimizing the tDCS protocol and implementing a fully online trial approach for non-invasive brain stimulation can help design future clinical trials in the field to enhance the subject’s recruitment, maximize the adherence to the intervention, and facilitate performing well-powered trials.
Furthermore, the development of a home-based and low-cost intervention in this condition will improve the accessibility to pain management, decreasing geographical and economic health care inequalities in pain medicine(Kempner, 2018). Also, given the burden of in-patient treatment and the current COVID-19 pandemic scenario, more pressing needs for remote therapies have emerged to enhance patient adherence and ensure good quality of care in the home environment (Castelo-Branco & Fregni, 2020; Eccleston et al., 2020).
Moreover, it has been reported a 20% chance of having persistent opioid use after amputation (Steen et al., 2020). The adverse events related to opioid intake include dependency, tolerability, somnolence, constipation, nausea, hyperalgesia, dry mouth, and urinary retention (Ricardo Buenaventura et al., 2008). Hence, our intervention may be a good option to reduce the use of opioids on this population as a non-pharmacologic, safe, and inexpensive alternative. Additionally, it can reduce the misuse of non-steroidal anti-inflammatory drugs, leading to renal, hematological, gastrointestinal, and cardiovascular side effects (Vonkeman & van de Laar, 2010).
The feasibility of home-based tDCS has been evaluated for different conditions, including stress, mild cognitive impairment, minimally conscious state, and other chronic pain conditions such as fibromyalgia (Ahn et al., 2019; Martens et al., 2018; Park et al., 2019; Sivaramakrishnan et al., 2019). Evaluating the feasibility and safety for individuals with PLP is essential given the uniqueness of this condition and the need for more accessible and safe treatments for this condition. PLP has been demonstrated as a difficult condition to treat (Aternali & Katz, 2019), and transportation difficulties are usually an extra barrier confronted by this population, resulting in a lack of access to physical therapy and pain management. Therefore, a home-based treatment method would increase treatment accessibility for this population.
Part of the absence of effective therapies for PLP can be associated with the lack of full comprehension regarding the central mechanisms that trigger phantom limb pain. To further understand these mechanisms, it is important to determine and understand possible predictors of treatment response in this condition. Some studies have tried to evaluate the characteristics related to pain severity (Münger et al., 2020) and the characteristics related to the pain response (Osumi et al., 2019). Noteworthy, one of the aims of this study will be to optimize the pain treatment in PLP using a statistical learning algorithm to select and characterize pain responders based on their clinical and physiological characteristics. This innovative approach will allow a better resource allocation since we could identify the subjects that are more likely to respond to this combined neuromodulation treatment based on their clinical endotypes.
Given that this will be an open-label trial, we cannot extrapolate results regarding the efficacy of home-based tDCS for PLP. Nonetheless, the primary aim of this trial will be to assess the feasibility of administering this intervention in a home-based scenario to provide information for further, larger trials.
3.1. Conclusion
This protocol proposes to test the acceptability and feasibility of a novel, combined intervention of home-based tDCS and motor imagery techniques to allow greater access to safe and effective treatments for individuals with phantom limb pain. Given the lack of effective treatments for this condition and the challenges of the in-person treatment regarding adherence and displacement, having a fully remote treatment option will enhance adherence and accessibility to pain management. Confirming the feasibility of these two treatments in a fully remote setting will bestow the framework for a larger trial to assess the efficacy of these interventions for PLP. Moreover, this protocol will allow us to identify possible predictors of pain response in the amputee population with PLP, providing further understanding of the central mechanisms of this condition and the identification of PLP clinical endotypes.
Supplementary Material
7. Acknowledgments
We acknowledge Alba Navarro-Flores, MD, Universidad Nacional Federico Villarreal, for her help on the figures design. She was not compensated for her contributions.
6. Funding
This study was supported by an NIH RO1 grant (1R01HD082302-01A1) and the Spaulding Research Institute (Spaulding Research Accelerator Program). They have no control over any aspect of the research study.
Footnotes
4 Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
8 References
- Ahn H, Sorkpor S, Miao H, Zhong C, Jorge R, Park L, . . . Cho RY (2019). Home-based self-administered transcranial direct current stimulation in older adults with knee osteoarthritis pain: An open-label study. Journal of Clinical Neuroscience, 66, 61–65. [DOI] [PubMed] [Google Scholar]
- Altmann A, Toloşi L, Sander O, & Lengauer T (2010). Permutation importance: a corrected feature importance measure. Bioinformatics, 26(10), 1340–1347. [DOI] [PubMed] [Google Scholar]
- Anwyl-Irvine A, Massonnié J, Flitton A, Kirkham N, & Evershed J (2018). Gorillas in our Midst: Gorilla. sc, a new web-based Experiment Builder. bioRxiv, 438242. [Google Scholar]
- Apley DW, & Zhu J (2020). Visualizing the effects of predictor variables in black box supervised learning models. Journal of the Royal Statistical Society: Series B (Statistical Methodology), 82(4), 1059–1086. [Google Scholar]
- Arain M, Campbell MJ, Cooper CL, & Lancaster GA (2010). What is a pilot or feasibility study? A review of current practice and editorial policy. BMC medical research methodology, 10(1), 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aron AR, Band GPH, Beste C, Bissett PG, Brockett AT, Brown JW, . . . Colzato LS (2019). A consensus guide to capturing the ability to inhibit actions and impulsive behaviors in the stop-signal task. [DOI] [PMC free article] [PubMed]
- Aternali A, & Katz J (2019). Recent advances in understanding and managing phantom limb pain. F1000Research, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Band GPH, & Van Boxtel GJM (1999). Inhibitory motor control in stop paradigms: review and reinterpretation of neural mechanisms. Acta psychologica, 101(2–3), 179–211. [DOI] [PubMed] [Google Scholar]
- Birbaumer N, Lutzenberger W, Montoya P, Larbig W, Unertl K, Töpfner S, . . . Flor H (1997). Effects of regional anesthesia on phantom limb pain are mirrored in changes in cortical reorganization. Journal of Neuroscience, 17(14), 5503–5508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolognini N, Olgiati E, Maravita A, Ferraro F, & Fregni F (2013). Motor and parietal cortex stimulation for phantom limb pain and sensations. PAIN®, 154(8), 1274–1280. [DOI] [PubMed] [Google Scholar]
- Bolognini N, Spandri V, Ferraro F, Salmaggi A, Molinari ACL, Fregni F, & Maravita A (2015). Immediate and sustained effects of 5-day transcranial direct current stimulation of the motor cortex in phantom limb pain. The journal of Pain, 16(7), 657–665. [DOI] [PubMed] [Google Scholar]
- Borsook D, Becerra L, Fishman S, Edwards A, Jennings CL, Stojanovic M, . . . Breiter H (1998). Acute plasticity in the human somatosensory cortex following amputation. Neuroreport, 9(6), 1013–1017. [DOI] [PubMed] [Google Scholar]
- Bowering KJ, O’Connell NE, Tabor A, Catley MJ, Leake HB, Moseley GL, & Stanton TR (2013). The effects of graded motor imagery and its components on chronic pain: a systematic review and meta-analysis. The journal of Pain, 14(1), 3–13. [DOI] [PubMed] [Google Scholar]
- Brietzke AP, Zortea M, Carvalho F, Sanches PRS, Danton P Jr, da Silva Torres IL, . . . Caumo W (2020). Large treatment effect with extended home-based transcranial direct current stimulation over dorsolateral prefrontal cortex in fibromyalgia: a proof of concept sham-randomized clinical study. The journal of Pain, 21(1–2), 212–224. [DOI] [PubMed] [Google Scholar]
- Brunelli S, Morone G, Iosa M, Ciotti C, De Giorgi R, Foti C, & Traballesi M (2015). Efficacy of progressive muscle relaxation, mental imagery, and phantom exercise training on phantom limb: a randomized controlled trial. Archives of physical medicine and rehabilitation, 96(2), 181–187. [DOI] [PubMed] [Google Scholar]
- Brunoni AR, Kemp AH, Dantas EM, Goulart AC, Nunes MA, Boggio PS, . . . Benseñor IM (2013). Heart rate variability is a trait marker of major depressive disorder: evidence from the sertraline vs. electric current therapy to treat depression clinical study. International Journal of Neuropsychopharmacology, 16(9), 1937–1949. [DOI] [PubMed] [Google Scholar]
- Castelo-Branco L, & Fregni F (2020). Home-based transcranial direct current stimulation (tDCS) to prevent and treat symptoms related to stress: a potential tool to remediate the behavioral consequences of the COVID-19 isolation measures? Frontiers in Integrative Neuroscience, 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chikazoe J, Jimura K, Asari T, Yamashita K. i., Morimoto H, Hirose S, . . . Konishi S (2009). Functional dissociation in right inferior frontal cortex during performance of go/no-go task. Cerebral cortex, 19(1), 146–152. [DOI] [PubMed] [Google Scholar]
- Costa MD, Davis RB, & Goldberger AL (2017). Heart rate fragmentation: a new approach to the analysis of cardiac interbeat interval dynamics. Frontiers in physiology, 8, 255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doan QH, Le T, & Thai D-K (2021). Optimization strategies of neural networks for impact damage classification of RC panels in a small dataset. Applied Soft Computing, 102, 107100. [Google Scholar]
- Eccleston C, Blyth FM, Dear BF, Fisher EA, Keefe FJ, Lynch ME, . . . de C Williams AC (2020). Managing patients with chronic pain during the COVID-19 outbreak: considerations for the rapid introduction of remotely supported (eHealth) pain management services. Pain, 161(5), 889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erlenwein J, Diers M, Ernst J, Schulz F, & Petzke F (2021). Clinical updates on phantom limb pain. Pain Reports, 6(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flor H (2002). Phantom-limb pain: characteristics, causes, and treatment. The Lancet Neurology, 1(3), 182–189. [DOI] [PubMed] [Google Scholar]
- Flor H, Elbert T, Knecht S, Wienbruch C, Pantev C, Birbaumers N, . . . Taub E (1995). Phantom-limb pain as a perceptual correlate of cortical reorganization following arm amputation. Nature, 375(6531), 482–484. [DOI] [PubMed] [Google Scholar]
- Flor H, Nikolajsen L, & Jensen TS (2006). Phantom limb pain: a case of maladaptive CNS plasticity? Nature Reviews Neuroscience, 7(11), 873–881. [DOI] [PubMed] [Google Scholar]
- Fregni F, El-Hagrassy MM, Pacheco-Barrios K, Carvalho S, Leite J, Simis M, . . . Venkatasubramanian G (2021). Evidence-Based Guidelines and Secondary Meta-Analysis for the Use of Transcranial Direct Current Stimulation in Neurological and Psychiatric Disorders. International Journal of Neuropsychopharmacology, 24(4), 256–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilgen-Ammann R, Schweizer T, & Wyss T (2019). RR interval signal quality of a heart rate monitor and an ECG Holter at rest and during exercise. European journal of applied physiology, 119(7), 1525–1532. [DOI] [PubMed] [Google Scholar]
- Goldberger AL, Amaral LA, Hausdorff JM, Ivanov PC, Peng C-K, & Stanley HE (2002). Fractal dynamics in physiology: alterations with disease and aging. Proceedings of the national academy of sciences, 99(suppl 1), 2466–2472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grüsser SM, Winter C, Mühlnickel W, Denke C, Karl A, Villringer K, & Flor H (2001). The relationship of perceptual phenomena and cortical reorganization in upper extremity amputees. Neuroscience, 102(2), 263–272. [DOI] [PubMed] [Google Scholar]
- Gunduz ME, Pacheco-Barrios K, Bonin Pinto C, Duarte D, Vélez FGS, Gianlorenco ACL, . . . Fregni F (2021). Effects of Combined and Alone Transcranial Motor Cortex Stimulation and Mirror Therapy in Phantom Limb Pain: A Randomized Factorial Trial. Neurorehabilitation and Neural Repair, 15459683211017509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, & Conde JG (2009). Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. Journal of biomedical informatics, 42(2), 377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hastie T, Tibshirani R, & Friedman J (2001). The elements of statistical learning. Springer series in statistics. In: . Springer. [Google Scholar]
- Herrador Colmenero L, Perez Marmol JM, Martí-García C, Querol Zaldivar M. d. l. Á., Tapia Haro RM, Castro Sánchez AM, & Aguilar-Ferrándiz ME (2018). Effectiveness of mirror therapy, motor imagery, and virtual feedback on phantom limb pain following amputation: A systematic review. Prosthetics and orthotics international, 42(3), 288–298. [DOI] [PubMed] [Google Scholar]
- Hinton KE, Lahey BB, Villalta-Gil V, Boyd BD, Yvernault BC, Werts KB, . . . Landman BA (2018). Right fronto-subcortical white matter microstructure predicts cognitive control ability on the Go/No-go task in a community sample. Frontiers in human neuroscience, 12, 127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempner J (2018). Towards a Socially-just neuroethics of inequalities in pain treatment. In Developments in Neuroethics and Bioethics (Vol. 1, pp. 105–125). Elsevier. [Google Scholar]
- Limakatso K, Bedwell GJ, Madden VJ, & Parker R (2020). The prevalence and risk factors for phantom limb pain in people with amputations: A systematic review and meta-analysis. PloS one, 15(10), e0240431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limakatso K, Madden VJ, Manie S, & Parker R (2020). The effectiveness of graded motor imagery for reducing phantom limb pain in amputees: a randomised controlled trial. Physiotherapy, 109, 65–74. [DOI] [PubMed] [Google Scholar]
- Mallik AK, Pandey SK, Srivastava A, Kumar S, & Kumar A (2020). Comparison of Relative Benefits of Mirror Therapy and Mental Imagery in Phantom Limb Pain in Amputee Patients at a Tertiary Care Center. Archives of Rehabilitation Research and Clinical Translation, 2(4), 100081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martens G, Lejeune N, O’Brien AT, Fregni F, Martial C, Wannez S, . . . Thibaut A (2018). Randomized controlled trial of home-based 4-week tDCS in chronic minimally conscious state. Brain stimulation, 11(5), 982–990. [DOI] [PubMed] [Google Scholar]
- McCallie MS, Blum CM, & Hood CJ (2006). Progressive muscle relaxation. Journal of human behavior in the social environment, 13(3), 51–66. [Google Scholar]
- Montoya P, Ritter K, Huse E, Larbig W, Braun C, Töpfner S, . . . Birbaumer N (1998). The cortical somatotopic map and phantom phenomena in subjects with congenital limb atrophy and traumatic amputees with phantom limb pain. European Journal of Neuroscience, 10(3), 1095–1102. [DOI] [PubMed] [Google Scholar]
- Morales-Quezada L, Cosmo C, Carvalho S, Leite J, Castillo-Saavedra L, Rozisky JR, & Fregni F (2015). Cognitive effects and autonomic responses to transcranial pulsed current stimulation. Experimental brain research, 233(3), 701–709. [DOI] [PubMed] [Google Scholar]
- Münger M, Pinto CB, Pacheco-Barrios K, Duarte D, Enes Gunduz M, Simis M, . . . Fregni F (2020). Protective and risk factors for phantom limb pain and residual limb pain severity. Pain Practice, 20(6), 578–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolajsen L, & Jensen TS (2000). Phantom limb pain. Current review of pain, 4(2), 166–170. [DOI] [PubMed] [Google Scholar]
- Nikolin S, Boonstra TW, Loo CK, & Martin D (2017). Combined effect of prefrontal transcranial direct current stimulation and a working memory task on heart rate variability. PloS one, 12(8), e0181833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osumi M, Inomata K, Inoue Y, Otake Y, Morioka S, & Sumitani M (2019). Characteristics of phantom limb pain alleviated with virtual reality rehabilitation. Pain Medicine, 20(5), 1038–1046. [DOI] [PubMed] [Google Scholar]
- Pacheco-Barrios K, Cardenas-Rojas A, Giannoni-Luza S, & Fregni F (2020). COVID-19 pandemic and Farr’s law: A global comparison and prediction of outbreak acceleration and deceleration rates. PloS one, 15(9), e0239175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacheco-Barrios K, Meng X, & Fregni F (2020). Neuromodulation techniques in phantom limb pain: A systematic review and meta-analysis. Pain Medicine, 21(10), 2310–2322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacheco-Barrios K, Pinto CB, Velez FGS, Duarte D, Gunduz ME, Simis M, . . . Guidetti M (2020). Structural and functional motor cortex asymmetry in unilateral lower limb amputation with phantom limb pain. Clinical Neurophysiology, 131(10), 2375–2382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J, Oh Y, Chung K, Kim KJ, Kim CO, & Park JY (2019). Effect of home-based transcranial direct current stimulation (tDCS) on cognitive function in patients with mild cognitive impairment: a study protocol for a randomized, double-blind, cross-over study. Trials, 20(1), 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulson S, Davidson R, Jha A, & Kabat-Zinn J (2013). Becoming conscious: the science of mindfulness. Ann NY Acad Sci, 1303(1), 87–104. [DOI] [PubMed] [Google Scholar]
- Pilloni G, Choi C, Shaw MT, Coghe G, Krupp L, Moffat M, . . . Charvet L (2020). Walking in multiple sclerosis improves with tDCS: a randomized, double-blind, sham-controlled study. Annals of Clinical and Translational Neurology, 7(11), 2310–2319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto CB, Velez FGS, Bolognini N, Crandell D, Merabet LB, & Fregni F (2016). Optimizing rehabilitation for phantom limb pain using mirror therapy and transcranial direct current stimulation: A randomized, double–blind clinical trial study protocol. JMIR research protocols, 5(3), e5645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruksawan S, Lambard G, Samitsu S, Sodeyama K, & Naito M (2019). Prediction and optimization of epoxy adhesive strength from a small dataset through active learning. Science and technology of advanced materials, 20(1), 1010–1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramachandran VS, Rogers-Ramachandran D, Stewart M, & Pons TP (1992). Perceptual correlates of massive cortical reorganization. SCIENCE-NEW YORK THEN WASHINGTON-, 258, 1159–1159. [DOI] [PubMed] [Google Scholar]
- Ricardo Buenaventura M, Rajive Adlaka M, & Nalini Sehgal M (2008). Opioid complications and side effects. Pain physician, 11, S105–S120. [PubMed] [Google Scholar]
- Richardson C, & Kulkarni J (2017). A review of the management of phantom limb pain: challenges and solutions. Journal of pain research, 10, 1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riggs A, Patel V, Paneri B, Portenoy RK, Bikson M, & Knotkova H (2018). At-home transcranial direct current stimulation (tDCS) with telehealth support for symptom control in chronically-ill patients with multiple symptoms. Frontiers in behavioral neuroscience, 12, 93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito K, Okada A, Matsumura Y, & Rekimoto J (2020, 2020). Remote Treatment System of Phantom Limb Pain by Displaying Body Movement in Shared VR Space.
- Sandran N, Hillier S, & Hordacre B (2019). Strategies to implement and monitor in-home transcranial electrical stimulation in neurological and psychiatric patient populations: a systematic review. Journal of neuroengineering and rehabilitation, 16(1), 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivaramakrishnan A, Datta A, Bikson M, & Madhavan S (2019). Remotely supervised transcranial direct current stimulation: a feasibility study for amyotrophic lateral sclerosis. NeuroRehabilitation, 45(3), 369–378. [DOI] [PubMed] [Google Scholar]
- Steen T, Lirk PB, & Sigurdsson MI (2020). The demographics of persistent opioid consumption following limb amputation. Acta Anaesthesiologica Scandinavica, 64(3), 361–367. [DOI] [PubMed] [Google Scholar]
- Team RC (2013). R: A language and environment for statistical computing.
- Thieme H, Morkisch N, Rietz C, Dohle C, & Borgetto B (2016). The efficacy of movement representation techniques for treatment of limb pain—a systematic review and meta-analysis. The journal of Pain, 17(2), 167–180. [DOI] [PubMed] [Google Scholar]
- Trevelyan EG, Turner WA, & Robinson N (2016). Perceptions of phantom limb pain in lower limb amputees and its effect on quality of life: a qualitative study. British journal of pain, 10(2), 70–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volz MS, Suarez-Contreras V, Portilla ALS, & Fregni F (2015). Mental imagery-induced attention modulates pain perception and cortical excitability. BMC neuroscience, 16(1), 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vonkeman HE, & van de Laar MAFJ (2010, 2010). Nonsteroidal anti-inflammatory drugs: adverse effects and their prevention. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.