Abstract
Objective:
Chronic pain is conceptualized as a biopsychosocial phenomenon that involves both physical and emotional processes. The vast majority of research regarding these facets of chronic pain characterizes differences between individuals. In this review, we describe problems with assuming that differences between persons accurately characterize within-person processes. We also provide a systematic review of studies that have examined within-person relationships between pain and affect among individuals with chronic pain.
Method:
Articles published by December 2020 that pertained to within-person assessment of pain and emotion, affect, or mood were identified. Data regarding study design, adherence, and concurrent and prospective relationships among pain and affect variables were extracted and summarized.
Results:
Of 611 abstracts, 55 studies met inclusion criteria. Results suggest that individuals with chronic pain tend to experience increased negative affect and decreased positive affect when experiencing more severe pain (rpooled = .18 and −.19, respectively). However, the size of these effects appeared smaller than between-person associations, and there was evidence of significant variability between individuals. Examination of predictive relationships between pain and affect largely suggested the tendency of symptoms to predict themselves, rather than pain predicting affect or vice versa.
Conclusions:
Consistent with group-level relationships, experiencing more severe pain relative to an individual’s average seems to be associated with more negative affect and less positive affect. However, individuals vary in the size and even direction of these effects. More research is necessary to understand the implications of such variability for the assessment and treatment of chronic pain.
Keywords: Pain, affect, ecological momentary assessment
Pain has been defined as an unpleasant sensory and emotional experience for over 50 years [1]. In the ensuing decades, disentangling relationships between sensory and affective experiences relevant to chronic pain has been a subject of much research [2–4]. For example, group-level research has shown that as many as 52% of patients in pain clinics report concurrent major depression [5]. Risk of death by suicide also appears to be at least twice as high in patients with chronic pain as compared to the general population [6]. Patients with chronic pain who have more severe depressive symptoms are likely to experience worse pain and poorer functioning, including higher risk of opioid misuse and overdose [7–10].
This research suggests that pain and affect are related between individuals. However, physicians and psychologists are often interested in how symptoms fluctuate within individuals over time [11–13]. For example, cognitive-behavioral models posit that pain can lead to negative emotions through maladaptive cognitive and behavioral patterns, and that negative affect can then perpetuate pain [14–17]. Similarly, the dynamic model of affect [18] suggests that positive affect may lessen the relationship between pain and negative affect. These theories are describing dynamic, within-person relationships between pain and affect. Although it is often assumed that between-person differences can be generalized to describe within-person relationships (e.g., if pain and affect are related between persons, then they should be related within individuals over time), this assumption represents an ecological fallacy [19,20]. For example, the between-person relationship between typing speed and errors is negative – faster typists make fewer errors. However, the within-person relationship between typing speed and errors is positive – individuals make more errors when they type faster [21]. In psychological research, within-person associations between negative mood and worry have been shown to be much smaller and more variable than the strong, positive association seen between-persons [20]. Similarly, whereas physical and emotional pain (e.g., painful affect perceived as resulting from emotional rather than physical experiences) were moderately correlated between persons, associations within individuals ranged from nearly zero to strong and positive [22]. Thus, although pain and affect appear to be related between persons, it is critical to understand whether similar relationships are seen within individuals over time, as is suggested by several theories for conceptualizing and treating chronic pain.
We will provide a systematic review of studies in which within-person relationships between pain and affect have been examined using daily diary or ecological momentary assessment (EMA) approaches that allow for sufficient intensive longitudinal data (ILD) to examine within-person relationships over time [23]. Extant reviews of the literature suggest that gathering ILD from patients with chronic pain is highly feasible [24,25]. However, these reviews focused primarily on methodology, rather than the substantive insights gained from ILD. To our knowledge, there have been no reviews of within-person relationships between pain and affect. Furthermore, the field of intensive longitudinal assessment is rapidly developing alongside technological advancements in data collection and analysis, and many recent studies have not yet been reviewed. In the current systematic review, we will provide an overview of the relevant studies, including within-person assessment procedures and the adherence and feasibility associated with these procedures. We will then examine evidence of within-person relationships between pain and affect. Finally, we will discuss these findings with regard to insights for within-person relationships between pain and affect, as well as important future areas of research.
Method
Search Methodology
A literature search was performed following PRISMA guidelines in PsycInfo and PubMed databases and by searching the references of identified articles. Search terms were: [(“diary” OR “ecological momentary assessment” OR “experience sampling”) AND “pain” AND (“mood” OR “depress*” OR “affect” OR “emotion”)]. In PubMed, an additional search term, (NOT “child” OR “adolescent”), was included. The search was performed in July 2020 and updated in December 2020. Papers were excluded that: (1) were not empirical journal articles; (2) utilized a child or adolescent sample; (3) focused on cancer pain; (4) were not available in English; and (5) did not utilize a daily diary, experience sampling, or EMA approach that included assessment of pain and affect, emotions, or mood. Consistent with a prior review [24], studies of chronic pain conditions including general chronic pain, rheumatic diseases (e.g., osteoarthritis, rheumatoid arthritis, fibromyalgia), headache or migraine, back, neck, or shoulder pain, temporomandibular disorder (TMD), irritable bowel syndrome (IBS), and reflex sympathetic dystrophy syndrome (RSDS) or complex regional pain syndrome (CRPS) were eligible for inclusion. Studies that assessed pain but as part of a separate medical condition (e.g., HIV/AIDS, multiple sclerosis, or cancer) were not included, as persistent pain is common but not universal among individuals with these conditions.
Given ongoing debate regarding the nature of emotion [26], we sought to examine any construct related to discrete emotions (e.g., anger, happiness), broader affect domains (e.g., negative and positive affect), or mood states (e.g., depressed mood). As the focus of this review is on the relationship between pain and affect, studies that measured these variables but did not report any relevant analyses (e.g., focused only on the relationship between pain and sleep or treatment outcomes) were also excluded.
Data Extraction and Summary
In order to provide an overview of methodology, we extracted metrics including sample size, assessment modality (e.g., paper, electronic, or phone call), assessment frequency, and adherence from included studies. For any studies that examined between- or within-person correlations between pain and affect variables, we also extracted the correlation coefficients. In order to summarize these statistics across studies, we first composited dependent observations (e.g., multiple relevant results from a single study, multiple studies using overlapping samples) by Z-transforming, averaging, and converting back to r. These independent observations were then meta-analyzed using the metacor() function of the meta package in R version 4.0.3 [27]. Using a random effects model to account for heterogeneity between samples, correlations were Z-transformed and weighted by inverse variance weights before converting back to r, thus accounting for sample size [28–30].
In addition to correlation coefficients, results regarding intraclass correlation coefficients (ICC) and multilevel regression analyses were also extracted. Similar to the procedure for summarizing correlation coefficients, ICC was summarized by z-transforming, averaging, and converting back to r for an estimate of the pooled ICC (ICC+). Given high variability between studies regarding analytic design, multilevel regression results are described rather than statistically combined. Data and code are available at https://osf.io/cdqu3/.
Results
An overview of the search results can be seen in Figure 1. The initial search yielded 611 unique articles, of which 155 were excluded during screening (not an empirical journal article, n = 72; utilized child or adolescent sample, n = 47; focused on cancer pain, n = 28; not available in English, n = 8). Of the remaining 456 full-text articles, 401 were excluded because they did not meet eligibility criteria (did not utilize chronic pain sample, n = 147; did not use daily diary or EMA to assess both pain and mood, n = 225; did not report relevant analyses, n = 29). Thus, 55 articles are included in this review.
Figure 1.
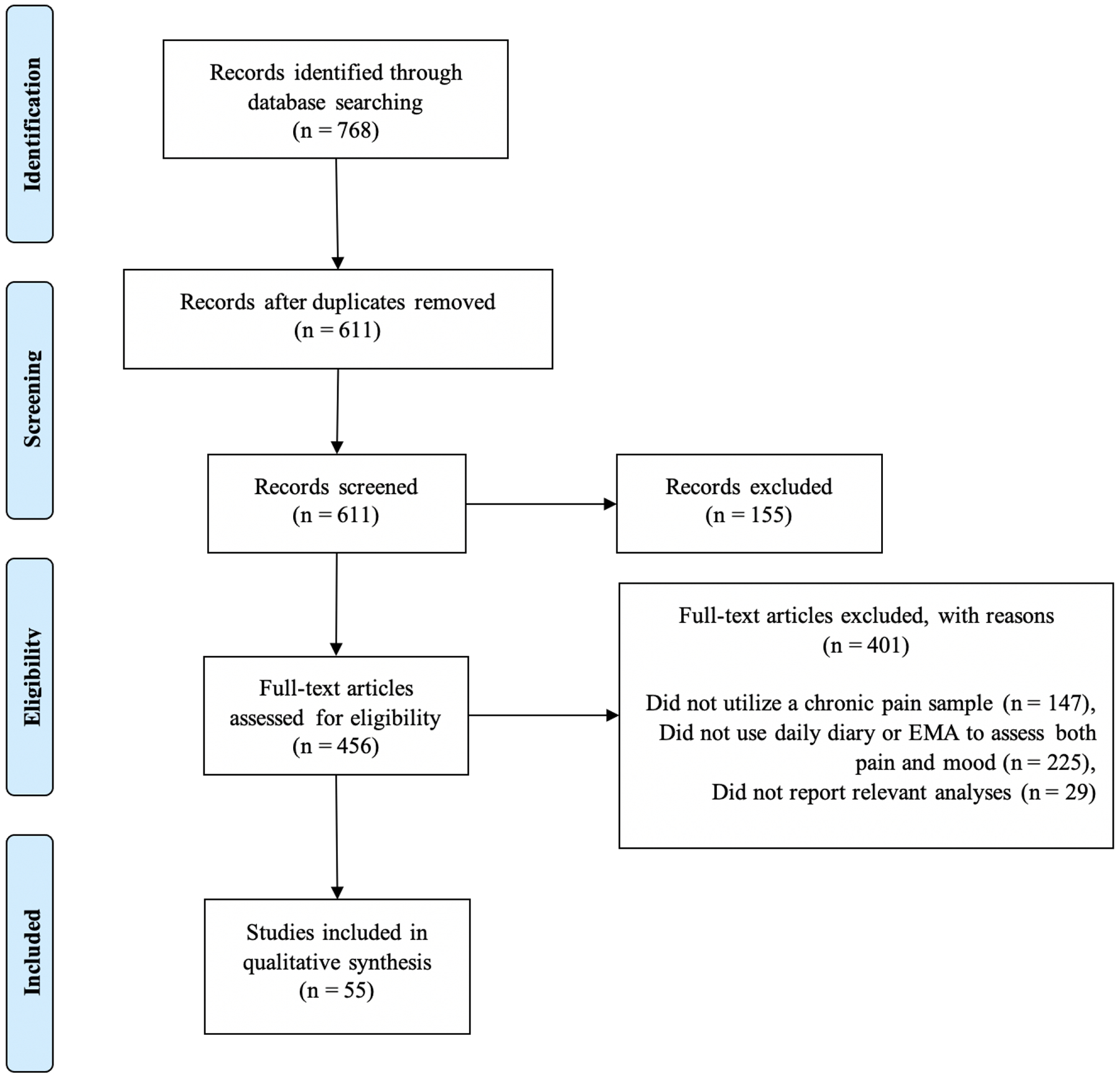
PRISMA diagram of articles included for review
Overview of Included Studies
The 55 included articles reported results from 44 unique samples1 (see Appendix A). The median sample size was 88 participants (SD = 88, range 18 – 356). The most common chronic pain diagnosis was rheumatoid arthritis (n = 10, 23%), followed by general chronic pain (n = 8, 18%), fibromyalgia (n = 5, 11%), and mixed rheumatic diseases (n = 5, 11%). Chronic low back pain, migraine/headache, osteoarthritis, TMD, IBS, and RSDS/CRPS were represented in four or fewer unique samples.
Most of the unique studies (n = 24, 55%) utilized an electronic diary approach. Paper diaries were also common (n = 15, 34%), followed by telephone call procedures (n = 3, 7%). Studies assessed participants for an average of 20 days (SD = 13, range 2 – 56). More than half of studies (n = 25, 58%) assessed participants more than once per day. Taking both length of study and assessments per day into account, participants were tasked with completing between 3 and 168 total assessments (Median = 30, SD = 34). At least two studies found that average levels of pain and affect did not change significantly with repeated sampling [31,32]. In two studies, patients tended to report that the repeated assessments were not burdensome and even useful in understanding their symptoms [33,34].
Consistent with previous reviews [24,25], adherence with repeated assessments appeared good (M = 90%, SD = 8%, range = 73–99%). Notably, at least 19 studies that reported adherence excluded between 1 and 70 participants (M = 15, SD = 20) for reasons including missing, lost, or insufficiently variable data, and several studies reported adherence only for the subset of the sample that was retained. In addition to inflating adherence estimates, this practice may systematically exclude individuals who exhibit more severe symptoms. In one study, higher average negative affect was associated with a greater chance of non-response [35]. Although recent recommendations for ambulatory assessment research suggest the use of adherence thresholds to reduce bias on the estimates caused by data that is not missing at random [36], there are tools to work with this type of missing data [37]. Retaining all participants in the sample will reduce the threat of biased results and increase power, two important considerations in within-person analyses.
Regardless of sampling approach, nearly all studies considered pain to be a unidimensional construct that could be assessed using a single numeric rating scale (e.g., 0–10 or 0–100), whereas many considered affect to have at least two dimensions corresponding to positively and negatively-valanced experiences. Given that disruptions in both dimensions are common among individuals with depression [38,39], both positive and negative affect (henceforth referred to as PA and NA) will be considered in this review.
Evidence of Within-Person Relationships between Pain and Affect
ILD allows for assessment of relationships both within and across individuals. In terms of correlations, between-person relationships are assessed by creating an average of each variable for each individual. A positive between-person correlation suggests that individuals with higher average levels of one variable also tend to have higher average levels of a second variable. Within-person correlations, on the other hand, can be generated by assessing within-person correlations among variables for each individual, and then averaging these estimates across individuals. A positive within-person correlation suggests that, on average, when an individual experiences higher levels of one variable, they also tend to experience higher levels of a second variable2.
Between- and within-person correlations between pain and affect are shown in Table 1. Regarding pain and NA, 12 of 15 studies reported significant positive between-person correlations (rpooled = .32, 95% CI: .22 – .42, p < .001), and 7 of 7 studies reported significant positive within-person correlations (rpooled = .18, 95% CI: .07 – .28, p = .002). Regarding pain and PA, 5 of 9 studies reported significant negative between-person correlations (rpooled = −.24, 95% CI: −.35 – −.12, p < .001), and 4 of 6 studies reported significant negative within-person correlations (rpooled = −.19, 95% CI: −.33 – −.06, p = .007). Thus, most studies indicated that increased pain for an individual was related to increased NA and decreased PA, although within-person effect sizes were smaller than those observed for group-level relationships.
Table 1.
Correlations between pain and affect
| Times per day | Pain with NA | Pain with PA | ||||||
|---|---|---|---|---|---|---|---|---|
| Sample | Study | N | Days | Between | Within | Between | Within | |
| Chronic pain | Kindt et al. [44] | 70 | 14 | 1 | .34** | −.43** | ||
| Mun et al. [109] | 131 | 21 | 3 | .19* | .11** | −.21* | −.18** | |
| Okun et al. [110]✝ | .20* | .17** | −.18* | −.20** | ||||
| Mun et al. [111]✝ | .32** | .08** | ||||||
| Vendrig et al. [84] | 57 | 6 | 8 | −.22* | ||||
| Fragoso et al. [112] | 86 | 5 | 1 | .43** | .29** | |||
| Christian et al. [62] | 85 | 30 | 1 | −.50** | ||||
| Chronic low back pain | Gerhart et al. [113] | 105 | 14 | 5 | .62** | −.16 | ||
| Burns et al. [79] | 22 | 49 | 2 | .57** | ||||
| Grant et al. [47] | 88 | 30 | 2 | .26** | ||||
| Osteoarthritis | Song et al. [114] | 143 | 22 | 1 | .06** | −.01 | ||
| Martire et al. [54]✝ | .08** | −.03 | ||||||
| Nah et al. [115]✝ | .29** | |||||||
| Finan et al. [66] | 151 | 14 | 1 | .40** | −.23** | |||
| Rheumatoid arthritis | Gruszczyńska et al. [64] | 95 | 3 | 1 | .24* | −.18 | ||
| Fifield et al. [51] | 27 | 20 | 1 | −.09 | ||||
| Mixed rheumatic | Hamilton et al. [56] | 49 | 2 | 6 | .06 | −.14 | ||
| Fibromyalgia | Kothari et al. [116] | 220 | 21 | 1 | .24** | −.33** | ||
| Tennen et al. [48] | 71 | 30 | 3 | .20 | −.02 | |||
| Irritable bowel syndrome | Han et al. [59] | 356 | 28 | 1 | .38** | |||
Note. N = reported sample size included in analyses; Days = number of EMA or daily diary assessment days; Times per day = number of EMA or daily diary assessments per day; NA = negative affect; PA = positive affect; Between = between-person correlation; Within = within-person correlation;
Indicates duplicate sample.
p < .05,
p <.01 as reported by the original study. If more than one relevant correlation was reported (e.g., pain with depressed mood and anxious mood, correlations between pain and NA in the morning and evening), then each observation was z-scored before averaging and converting back to r.
Further Examination of Within-Person Relationships using Multilevel Modeling
Correlation analyses of ILD are limited in their ability to perform more advanced functions, including assessing the relative influence of multiple variables or accounting for the effects of time. Multilevel modeling (MLM) is commonly used to answer questions of this nature using ILD [40]. MLM is necessary because of the nested structure of the data (e.g., time points nested within individuals). Considering all data points without nesting within individuals results in biased estimates and spurious results, as an individual’s data points are likely to be more related to one another than they are to another individual’s data [41]. Thus, unnested data violates the assumption of most linear models that residuals be independent [42]. Alternatively, considering only the group-level effects by averaging data points for each individual limits power and ignores within-person variance [43].
Several studies included in the current review reported the proportion of variance in pain and affect that was due to between- versus within-person differences (e.g., intraclass correlation). Across 14 studies, the pooled variance in pain due to between-person differences was 62.14% (range 42–86%), indicating that approximately 40% of the variance in pain was attributed to within-person fluctuations. Similar proportions of variance were found for NA (ICC+ = 61.61%, range 50–73%) and PA (ICC+ = 64.07%, range 50–83%). Thus, across several studies, pain and affect appeared to vary both within and between persons, making it necessary to consider both sources of variance through MLM.
Contemporaneous Relationships.
An overview of studies that utilized MLM to assess within-person relationships between pain and affect is provided in Table 2. Within individuals, pain was positively associated with NA variables across studies of mixed chronic pain [44,45], back pain [46,47], fibromyalgia [48–50], rheumatoid arthritis [51–53], osteoarthritis [54,55], mixed rheumatic disease [56–58], IBS [59], CRPS [60], and migraine [61]. Several studies also found pain to be negatively associated with PA in general chronic pain [44,62], fibromyalgia [48,49,63], rheumatoid arthritis [52,53,64,65], osteoarthritis [55,66], and mixed rheumatic disease [56,58]. Additionally, a higher ratio of PA to NA was associated with less pain in patients with osteoarthritis [55,67].
Table 2.
Contemporaneous and lagged relationships between pain and affect
| Lagged | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Sample | Study | N | Lag | Pain*NA | Pain* PA | Pain → NA | Pain → PA | NA → Pain | PA → Pain |
| General CP | Christian et al. [62] | 85 | -- | ✓ | |||||
| Kindt et al. [44] | 70 | -- | ✓ | ✓ | |||||
| CLBP | Grant et al. [47] | 88 | -- | ✓ | |||||
| Burns et al. [46] | 105 | 3h | ✓ | ✗ | |||||
| Finan et al. [66] | 151 | -- | ✗ | ✓ | |||||
| Smith et al. [67] | 120 | -- | ✓ (ratio) | ✓ (ratio) | |||||
| Martire et al. [54] | 145 | 24h | ✓ | ✓ | |||||
| Rivera et al. [55] | 268 | -- | ✓ | ✓ | |||||
| Fifield et al. [51] | 27 | -- | ✓ | ||||||
| Conner et al. [52] | 188 | -- | ✓ | ✓ | |||||
| Gruszczyńska et al. [64] | 95 | -- | ✗ | ✓ | |||||
| Graham-Engeland et al. [65] | 31 | 30m+ | ✗ | ✓ | ✗ | ✗ | |||
| Kwissa-Gajewska et al. [53] | 54 | -- | ✓ | ✓ | |||||
| Hamilton et al. [56] | 49 | -- | ✓ | ✓ | |||||
| Finan et al. [58] | 260 | 24h | ✓ | ✓ | ✗ | ✗ | |||
| Hegarty et al. [57] | 142 | -- | ✓ | ✗ | |||||
| Tennen et al. [48]/Hamilton et al. [49] | 71 | -- | ✓ | ✓ | |||||
| van Middendorp et al. [50] | 333 | -- | ✓ | ||||||
| Finan et al. [117] | 46 | -- | ✓ | ||||||
| IBS | Han et al. [59] | 356 | -- | ✓ | |||||
| CRPS/ RSDS | Feldman et al. [73] | 109 | 24h | ✓ | ✓ | ||||
| Cho et al. [60] | 30 | 24h | ✓ | ✗ | ✗ | ✗ | |||
| Migraine/ headache | Houtveen et al. [71] | 87 | 3.5h+ | ✗ | ✓ | ||||
| Kikuchi et al. [68] | 23 | -- | ✗ | ||||||
| Ciere et al. [61] | 61 | 90m+ | ✓ | ✓ | |||||
Note. ✓ denotes significant finding; ✗ denotes non-significant finding; NA = negative affect; PA = positive affect; CP = chronic pain; CLBP = chronic low back pain; FM = fibromyalgia; RA = rheumatoid arthritis; OA = osteoarthritis; IBS = irritable bowel syndrome; CRPS/RSDS = complex regional pain syndrome/reflex sympathetic dystrophy syndrome; h = hours; m = minutes.
Although the majority of studies included here found that individuals experienced more severe pain when they experienced greater NA and less PA relative to their average emotional state, there was evidence of mixed findings. In four studies, the contemporaneous relationship between NA and pain was not significant [64–66,68]. Additionally, in two studies, the contemporaneous relationship between PA and pain was not significant [57,60]. There are several possible reasons for these mixed findings, including assessing different variables across different time scales. Further, some studies controlled for variables including the opposite affect domain [60,66], depressive symptoms [65], average pain [64], and time [68]. Although many studies that found significant results also controlled for similar variables, it will be important to consider whether contemporaneous relationships between pain and affect are better accounted for by these or other confounds.
Lagged Relationships.
Lagged associations reflect the relationship between one variable assessed at one point in time (t) and another variable assessed at a previous (t−1) or future (t+1) time point. For example, one could examine whether higher than average pain (Paint) is associated with higher-than-average NA at the next assessment point (NAt+1). When examining a lagged association between two variables, it is important to also include the autoregressive effect of a variable on itself. For example, perhaps elevated pain does predict elevated NA, but only because elevated pain was associated with elevated NA at the previous time point, and this elevated NA persisted over time. Thus, pain would not be presumed to cause NA if NA is primarily causing itself. Importantly, several studies have found moderate to strong correlations of pain and affect with themselves, both within and across days [rs = .67 – .97; 52,55,68,73,74].
As can be seen in Table 2, the few studies that assessed within-person relationships between pain and affect over time offered limited support for such effects. When controlling for previous pain levels, affect did not appear to predict pain a few hours later among samples of rheumatoid arthritis or chronic low back pain patients [46,65]. However, in one study of prodromal features of migraine, PA was decreased 0–12 hours before headache onset [71]. With regard to pain predicting affect, there was evidence of greater pain intensity predicting greater NA a few hours later among patients with chronic migraine [61]. In a network analysis of abdominal and mood symptoms in individuals with IBS, there was little evidence of directional effects between the two categories of symptoms [72].
Between days, there was some evidence of affect predicting next-day pain [71,73] and pain predicting next-day affect [54,73]. However, some of these studies did not take into account the autoregressive effects of pain or affect, which limits the ability to draw firm conclusions about causality.
Variability in Within-Person Relationships
The studies reviewed here summarized the average within-person relationships between pain and affect to understand the sample as a whole. However, individuals can vary from one another in these relationships, and such variability may have important clinical implications. Here, we review evidence of between-person variability in within-person symptom dynamics, both with regard to the symptoms themselves (i.e., symptom variance) and relationships among symptoms.
Symptom variance.
ILD offers a unique opportunity to examine the structure of symptoms within individuals. For example, some individuals may experience larger or more frequent fluctuations in pain or affect symptoms. Evidence from the mood disorders literature suggests individuals who experience wider variability in NA also tend to experience worse depressive symptoms and poorer psychological well-being [74–77]. Consistent with these findings, greater within-person variability in NA (as defined by within-person standard deviation) was associated with increased severity of NA among two studies of chronic back pain patients [78,79]. Similarly, greater variability in pain was associated with worse pain severity [79,80]. However, there was mixed evidence of cross-over in these effects. That is, variability in affect was associated with pain intensity or vice versa in some studies [78,81], and not in others [79,80]. When taking temporal relationships into account, instability of NA (i.e., the tendency to experience unusually large and/or frequent changes in affect) was associated with higher pain severity and disability [82,83]. In one study, both mean levels and variability in pain and NA were lower after bright light treatment to improve sleep [79].
The somewhat mixed nature of these findings is unsurprising given that participants were sampled at different rates (e.g., between one and seven times per day). To use Koval’s [76] metaphor, observing the sun once per day versus once per hour would lead to vastly different conclusions about variability. Whereas daily observation would lead to a conclusion of little variability, hourly observation would lead to a conclusion of high variability in the same process. Thus, it will be important in future research to determine the appropriate time scale on which to measure pain and affect based on the research questions at hand.
Relationships between symptoms.
In addition to individual differences in the way that symptoms vary, there is strong evidence of such between-person differences in the within-person relationships of pain and affect symptoms. As shown in Table 1, the within-person correlations between pain and affect appeared smaller than between-person correlations. This is likely because within-person correlations are more accurately described as the average of within-person correlations, wherein relationships are assessed for each individual and averaged across the group. Few studies assessed variation in these relationships. In one study, although most participants had a negative correlation between pain and PA, four participants from the sample exhibited a significant positive correlation [84]. In a large study of women with fibromyalgia, the within-person relationship between anger and pain ranged from −.48 to .70 [50]. Thus, average within-person correlations may not represent the true relationship between pain and affect for many individuals in the group.
Several studies also assessed moderators of within-person relationships using MLM. Results suggest that pain and NA were more strongly related for individuals with greater average NA or depressive symptoms [45,50,53,58]. In one study, pain and NA were also more strongly related for individuals with more severe anxiety symptoms [45]. An exploratory analysis in a sample of rheumatoid arthritis patients suggested that the contemporaneous relationship between pain and affect remained significant only for clinically depressed individuals when compared to the non-clinically depressed subset of the sample [65]. The contemporaneous relationships between pain and affect may also be moderated by depression history, such that individuals with a history of depression exhibit stronger positive relationship between pain and NA [52] and stronger negative relationships between pain and PA [48,52], controlling for current depressive symptoms. Additional moderators of within-person contemporaneous relationships between pain and affect included genotype [85], sleep quality or duration [56], and optimism [53]. Overall, this evidence suggests that within-person relationships between pain and affect are likely to vary between individuals based on a variety of different factors.
Discussion
Numerous studies have suggested that pain and affect are associated between individuals, such that individuals who experience more severe pain also tend to experience more severe NA and depressive symptoms [2–4,86,87]. The results of this review suggest that pain and affect are also associated within individuals over time. Across several studies, individuals tended to experience more NA and less PA when experiencing more severe pain. However, these effects tended to be smaller than between-person associations. There was also evidence of between-person variation in within-person relationships, such that pain and affect may be related more strongly for some individuals than others. Although one might assume from these relationships that pain would predict affect or vice versa within individuals over time, these prospective effects were examined less often, and results were less consistent across studies.
From Group-Level Data to Individual-Level Relationships
Consistent with observations of other psychological and somatic phenomena [20,22], within-person correlations between pain and affect were often weaker than between-person associations. There are several potential reasons for this disconnect. First, it is possible that pain and affect are only weakly associated for the majority of individuals. Importantly, the interpretation of this association is quite different from the interpretation of the moderate to strong between-person correlations between pain and affect. Whereas between-person correlations signify that individuals who experience more pain also tend to experience more NA and less PA, within-person correlations signify that when an individual is experiencing more severe pain, they tend to concurrently experience more NA and less PA. If, for example, an individual’s affect levels tend to fluctuate, but their pain remains consistently severe, the association between the two would not be strong. However, even a weak association between pain and affect may provide the opportunity to noticeably relieve pain levels by reducing NA or increasing PA through psychological intervention such as cognitive behavioral therapy. Given limited within-person research, the clinical implications of smaller effect sizes seen in analyses using ILD are largely unknown.
It is also possible that within-person effects appear smaller due to variability between individuals. Summarizing within-person effects requires averaging across all individuals in the group. Thus, although one effect is reported, there may be individuals for whom the relationship is stronger or weaker. In the only two studies to have reported the range of within-person correlations between pain and affect, participants varied in both the strength and directions of these relationships [50,84]. Similarly, several studies reported significant moderators of within-person relationships between pain and affect, such that these effects were stronger for individuals with greater symptomatology [45,50,53,58,65]. Weak average or fixed within-person effects may therefore be due to high levels of between-person variability.
This disconnect between group- and individual-level results is perhaps unsurprising, as it is commonly accepted that no two individuals are exactly alike. Clinicians often face this problem, known as the therapist’s dilemma, as they are tasked with treating a single individual, whereas the vast majority of clinical research is conducted at the group level [88,89]. If variation from the group is minor and rare, then using group-level information to characterize an individual is a relatively safe assumption. However, if such variation is major and frequent, then relying on group-level information may result in failed treatments, ongoing impairment, and billions of dollars in wasted healthcare costs. In the case of chronic pain and emotional symptoms, we believe that we do not yet know the extent of this problem, as very few studies reported the degree to which individuals varied from one another in regard to within-person relationships between pain and affect.
Many researchers have advocated for purely idiographic (i.e., individual-level) analysis as a means of characterizing the full extent of between-person differences in within-person relationships [11,19–21,90]. In contrast to the studies reviewed here, which focused on characterizing groups of patients on average, idiographic research focuses on what is true for each individual. Several analytic approaches have been applied to ILD to generate idiographic, or personalized, models of within-person symptom dynamics [12,89]. These approaches differ from more traditional MLM approaches in that they estimate person-specific effects. Although traditional multilevel models can estimate individual differences in effects, these estimates can be biased by a range of factors including variability, skew, and centering [12,91,92]. Thus, idiographic or multilevel approaches that prioritize within-person variation may be necessary to understand person-specific relationships between pain and emotions.
Methodological Challenges and Limitations of Existing Research
In addition to the level of analysis, the results of this review suggest several methodological challenges in the assessment of within-person relationships between pain and emotion. Many of these concerns pertain broadly to ILD collection. However, we will consider them here in the context of the current review. First, regarding sampling, a large proportion of studies assessed individuals only once per day, although sampling frequency ranged from once to 10 times per day. This aspect of intensive longitudinal studies is often chosen arbitrarily or with an emphasis on decreasing participant burden. However, it has important implications for study results and interpretations. To return to Koval’s metaphor [76], observing the sun’s position in the sky once per day at 4pm would lead to a conclusion of very little change. Yet, observable change is occurring on a faster timescale. As shown in a simulation study, symptom dynamics (e.g., on the order of seconds) cannot be uncovered when sampling frequency is insufficient (e.g., surveys on the order of hours) [93]. Thus, understanding the rate at which pain and emotions fluctuate is a critical step in identifying within-person symptom dynamics. Fortunately, there is evidence that more frequent sampling is not associated with lower adherence [94]. Therefore, it may be possible to survey individuals several times per day and use empirical methods (e.g., time-varying effect models) to identify the appropriate lag lengths [95,96].
Regarding adherence, studies reviewed here suggested reason for optimism about the level of burden incurred by ILD collection among patients with chronic pain. Importantly, the good adherence observed across studies is likely skewed by the recommendation that participants be excluded for non- adherence based on a pre-defined cutoff [36]. In addition to inflating adherence estimates, this practice eliminates the data of individuals who may vary systematically from the sufficiently compliant subset of the sample, especially regarding symptom severity [37]. In future studies, techniques for estimating and handling missing data should be considered to reduce the potentially detrimental effects of this bias.
Future Directions
The results of this review suggest several important directions for future research. First, it will be critical to characterize the extent to which within-person relationships between pain and affect vary between individuals. Fully idiographic or multi-level approaches that produce accurate person-specific estimates can be applied to ILD to characterize this degree of difference. Several methods have recently been developed or applied to ILD, including vector autoregression [97] and dynamic structural equation modeling [11,98]. Notably, many of these methods require large amounts of data per person (e.g., ideally 100 time points or more). They also require careful consideration of time scale and other methodological challenges that cut across EMA research. However, the results of this and prior reviews suggest that longer or more intensive assessment is feasible in chronic pain samples [24,25].
In addition to providing person-specific estimates, many of these methods have the capability to incorporate data beyond self-report. Pain and affect are inherently subjective experiences. However, physiological and behavioral data may provide additional insight into when and why pain and NA are exacerbated. Many smartphone applications and wearable technologies are now available to capture variables including pulse, blood pressure, location, movement, and sleep quality [99,100]. These objective variables can be combined with subjective assessment of pain and affect to test hypotheses and deliver just-in-time adaptive interventions. Research that combines EMA with laboratory assessment may also be useful, as at least one study found different relationships between pain and affect in naturalistic versus laboratory settings [66].
Idiographic and multilevel methods may also be useful in disentangling the fundamental relationships between pain and affect. Pain and emotion share several conceptual, functional, and neurobiological commonalities [4]. For the purposes of this review, we considered pain and affective experiences to be two separate constructs. This was reflected in the literature, as pain was commonly assessed using a single-item numeric rating scale (e.g., “How intense is your pain on a scale of 0–100?”), and emotions, affect, or mood were assessed using specific terms (e.g., sadness, happiness, anger) that were often composited into negative and positive affect scores. However, pain is defined as a physical and emotional experience characterized by sensory and affective dimensions [101,102]. Thus, it is possible that pain and affect are not completely distinct constructs, but different dimensions of the same subjective experience. Dynamic factor models can be used to assess the degree to which pain and affect are distinct versus overlapping constructs, as well as whether this structure varies between individuals [98,103]. Such understanding of the conceptual and functional overlaps between affect associated with pain and affect associated with other emotional experiences may have interesting implications for the conceptualization and treatment of co-occurring pain and mood symptoms.
Finally, idiographic and multi-level models that characterize within-person relationships between pain and affect may be helpful in identifying mechanisms involved in the successful treatment of co-occurring pain and emotional symptoms. EMA approaches have previously been integrated into chronic pain treatment protocols to assess outcomes [79,104–106]. However, these studies focused on the impact of treatment on mean levels of symptoms, rather than changes in relationships between symptoms. For example, previous EMA research suggests that individuals are more prone to use opioids and at higher dosages when experiencing greater NA [107]. Idiographic and multilevel models could be used to examine whether this pattern of misuse changes with treatment aimed at reducing opioid misuse. Idiographic and multilevel models could also be used to help identify individuals at high risk for opioid misuse prior to initiation of opioid therapy and lead to the suggestion of alternative treatments for chronic pain, including psychotherapy [108]. Amid a nation-wide opioid epidemic, idiographic and multilevel models could help researchers and clinicians mitigate risk of opioid misuse, addiction, and overdose.
Limitations of the Current Review
Many articles reviewed here had relatively small samples that may not be fully representative of patients with chronic pain, especially regarding race and socioeconomic status. Additionally, it is possible that relevant articles were not identified. Although new applications of daily diary and EMA data are suggested (e.g., idiographic and multilevel analytic methods that allow for the interpretation of person-specific estimates), these methods are relatively new and have not been widely applied in the field. Thus, it remains unclear to what extent it will be feasible to collect the necessary data, as well as whether the data will satisfy necessary assumptions. Furthermore, although it appears based on variability in within-person relationships that traditional methods of analysis have limitations, there are also benefits to these approaches. Namely, between-person approaches can be useful in identifying risk and protective factors for individuals on average, which may in some cases be sufficient for the research and clinical questions at hand. Finally, there is currently limited guidance for meta-analysis of EMA studies, especially given the multiple sources of power (e.g., number of participants and number of time points). Further guidance and open data sharing practices can help aid in the development of this rapidly growing area of research.
Conclusions
Chronic pain is considered a biopsychosocial phenomenon, in which the perception of pain is highly influenced by emotional experiences in addition to many other psychological, social, and biological factors [2]. However, relationships involving pain and affect have largely been examined between individuals. A growing body of evidence suggests that what is true across individuals on average may not accurately characterize within-person relationships over time [19,20]. The results of this review suggest that it is feasible and acceptable to gather the ILD necessary to examine within-person relationships between pain and affect among individuals with chronic pain. Furthermore, although pain appears to be associated with increased NA and decreased PA for individuals on average, the strength and even direction of these relationships may vary considerably across individuals. Thus, it is critical to further examine within-person relationships between pain and affect using methods that can accurately estimate variability in person-specific effects. Such idiographic and multilevel research has the potential to provide better understanding of the complex relationships between physical and psychological symptoms, and ultimately facilitate more targeted treatment for individuals with chronic pain.
Acknowledgments
Madelyn R. Frumkin and Thomas L. Rodebaugh were supported by the National Institutes of Health [5R01DC017451-02].
Appendix A. Overview of included studies
| Disease | Citation | N | Assessment type | Days | Times per day | Adherence |
|---|---|---|---|---|---|---|
| General Chronic Pain | Marceau et al. (2007) | 36 | Combined | 28 | 1 | |
| Suso-Ribera et al. (2018) | 38 | Electronic | 30 | 2 | 75.7% | |
| Vendrig et al. (1997) | 57 | Paper | 6 | 8 | 88.3% | |
| Kindt et al. (2016) Rost et al. (2016)* |
70 | Electronic | 14 | 1 | 96.4% | |
| Christian et al. (2015) | 85 | Electronic | 30 | 1 | 77.7% | |
| Fragoso et al. (2018) | 86 | Electronic | 5 | 1 | ||
| Schneider et al. (2012) | 106 | Electronic | 28 | 1 | 98.3% | |
| Mun et al. (2016) Okun et al. (2016)* Mun et al. (2018)* |
131 | Phone call | 21 | 3 | 89.5% | |
| Chronic Low Back Pain | Burns et al. (2020) | 22 | Electronic | 49 | 2 | 76.6% |
| Grant et al. (2002) | 88 | Paper | 30 | 2 | ||
| Burns et al. (2015) Gerhart et al. (2017)* Gerhart et al. (2018)* |
105 | Electronic | 14 | 5 | 87.1% | |
| Osteoarthritis | Smith et al. (2016) | 120 | Phone call | 7 | 4 | 80.8% |
| Song et al. (2015) Nah et al. (2020)* Martire et al. (2018)* |
143 | Electronic | 22 | 3 | 73.0% | |
| Finan et al. (2013) | 151 | Electronic | 14 | 1 | 83.0% | |
| Rivera et al. (2020) | 268 | Phone call | 7 | 4 | 78.0% | |
| Rheumatoid Arthritis | Cruise et al. (1996) | 18 | Paper | 7 | 7 | 94.0% |
| Fifield et al. (2004) | 27 | Paper | 20 | 3 | 99.0% | |
| Graham-Engeland et al. (2016) | 31 | Electronic | 7 | 5 | ||
| Stone et al. (1997) | 34 | Paper | 7 | 7 | 83.0% | |
| Kwissa-Gajewska et al. (2018) | 54 | Paper | 7 | 1 | 98.2% | |
| Connelly et al. (2007) | 94 | Paper | 30 | 1 | 96% | |
| Gruszczyska et al. (2015) | 95 | Paper | 3 | 1 | ||
| Finan et al. (2010) | 170 | Paper | 29 | 1 | 82.6% | |
| Conner et al. (2006) | 188 | Paper | 30 | 1 | 97.7% | |
| Mun et al. (2017) | 231 | Paper | 30 | 1 | 97.0% | |
| Mixed Rheumatic Diseases | Hamilton et al. (2007) | 49 | Paper | 2 | 6 | |
| Schneider et al. (2018) | 100 | Electronic | 14 | 8 | 86.0% | |
| Hegarty et al. (2015) Hegarty et al. (2016)* |
142 | Paper | 7 | 4 | 97.4% | |
| Mak et al. (2020)a | 290 | Electronic | 7–31 | 3–9 | ||
| Finan et al. (2009) | 260 | Electronic | 30 | 1 | 92.5% | |
| Fibromyalgia | Finan et al. (2010)* | |||||
| Hardy et al. (2011) | 27 | Electronic | 5 | 2 | 98.2% | |
| Rost et al. (2020) | 46 | Electronic | 14 | 1 | 93.8% | |
| Tennen et al. (2006) Hamilton et al. (2008)* |
71 | Electronic | 30 | 3 | 98.3% | |
| Kothari et al. (2015) | 220 | Electronic | 21 | 4 | 84.8% | |
| Van Middendorp et al. (2010) | 333 | Paper | 28 | 1 | 89.0% | |
| Irritable Bowel Syndrome | Han et al. (2019) | 356 | Unknown | 28 | 1 | |
| Drukker et al. (2020) | 24 | Electronic | 7 | 10 | ||
| Temporomandibular Disorder | Aaron et al. (2004) | 62 | Electronic | 56 | 3 | 85.0% |
| Aaron et al. (2005)* | ||||||
| RSDS/CRPS | Cho et al. (2013) | 30 | Electronic | 10 | 1 | 99.9% |
| Feldman et al. (1999) | 109 | Paper | 28 | 1 | 91.0% | |
| Migraine/Headache | Kikuchi et al. (2015) | 23 | Electronic | 7 | 6 | 97.0% |
| Ciere et al. (2019) | 61 | Electronic | 7 | 9 | 89.0% | |
| Houtveen et al. (2013) | 87 | Electronic | 21 | 4 | 89.5% | |
| Turner et al. (2019) | 95 | Electronic | 49 | 2 | 90.7% |
Note. RSDS/CRPS = reflex sympathetic dystrophy syndrome or complex regional pain syndrome; N = reported sample size included in analyses. If the sample was mixed (i.e., chronic pain and healthy controls), only the sample size of individuals with chronic pain is reported; Assessment type = method of delivering EMA or daily diary assessments; Days = number of EMA or daily diary assessment days; Times per day = number of EMA or daily diary assessments per day;
indicates duplicate sample. If adherence estimates were not provided by the authors, adherence was estimated by dividing the reported number of completed assessments over the total possible assessments. Some studies did not use all assessments in analysis. The number of assessments associated with adherence is reported.
Mak and Schneider (2020) is a meta-analysis of three separate samples that used different sampling frameworks.
Footnotes
Declarations of interest: none.
Duplicate samples were identified by cross-referencing papers by diagnosis, authors, and sample size. It is possible that additional duplicates exist, given that few studies acknowledged this explicitly.
We aimed to exclude papers that did not use clustering for within-person analysis. However, few authors directly reported their method, making it possible that some such results are included.
References
- [1].Merskey H, Bogduk N, International Association for the Study of Pain Classification of chronic pain: Descriptions of chronic pain syndromes and definitions of pain terms, Pain. 3 (1986) S1–S226. [PubMed] [Google Scholar]
- [2].Gatchel RJ, Peng YB, Peters ML, Fuchs PN, Turk DC, The biopsychosocial approach to chronic pain: scientific advances and future directions, Psychol. Bull 133 (2007) 581–624. doi: 10.1037/0033-2909.133.4.581. [DOI] [PubMed] [Google Scholar]
- [3].Lumley MA, Cohen JL, Borszcz GS, Cano A, Radcliffe AM, Porter LS, Schubiner H, Keefe FJ, Pain and emotion: a biopsychosocial review of recent research, J. Clin. Psychol 67 (2011) 942–968. doi: 10.1002/jclp.20816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Gilam G, Gross JJ, Wager TD, Keefe FJ, Mackey SC, What is the relationship between pain and emotion? Bridging constructs and communities, Neuron. 107 (2020) 17–21. doi: 10.1016/j.neuron.2020.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Bair MJ, Robinson RL, Katon W, Kroenke K, Depression and pain comorbidity, Arch. Intern. Med 163 (2003) 2433. doi: 10.1001/archinte.163.20.2433. [DOI] [PubMed] [Google Scholar]
- [6].Tang NK, Crane C, Suicidality in chronic pain: A review of the prevalence, risk factors and psychological links, Psychol. Med 36 (2006) 575. doi: 10.1017/S0033291705006859. [DOI] [PubMed] [Google Scholar]
- [7].Bair MJ, Wu J, Damush TM, Sutherland JM, Kroenke K, Association of depression and anxiety alone and in combination with chronic musculoskeletal pain in primary care patients., Psychosom. Med 70 (2008) 890–7. doi: 10.1097/PSY.0b013e318185c510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Hooten WM, Shi Y, Gazelka HM, Warner DO, The effects of depression and smoking on pain severity and opioid use in patients with chronic pain., Pain. 152 (2011) 223–9. doi: 10.1016/j.pain.2010.10.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Grattan A, Sullivan MD, Saunders KW, Campbell CI, von Korff MR, Depression and prescription opioid misuse among chronic opioid therapy recipients with no history of substance abuse, Ann. Fam. Med 10 (2012) 304–311. doi: 10.1370/afm.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Dunn KM, Saunders KW, Rutter CM, Banta-Green CJ, Merrill JO, Sullivan MD, Weisner CM, Silverberg MJ, Campbell CI, Psaty BM, Von Korff M, Opioid prescriptions for chronic pain and overdose: A cohort study, Ann. Intern. Med 152 (2010) 85–92. doi: 10.7326/0003-4819-152-2-201001190-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Piccirillo ML, Rodebaugh TL, Foundations of idiographic methods in psychology and applications for psychotherapy, Clin. Psychol. Rev 71 (2019) 90–100. doi: 10.1016/j.cpr.2019.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Wright AGC, Woods WC, Personalized models of psychopathology, Annu. Rev. Clin. Psychol 16 (2020) 49–74. doi: 10.1146/annurev-clinpsy-102419-125032. [DOI] [PubMed] [Google Scholar]
- [13].DeRubeis RJ, The history, current status, and possible future of precision mental health, Behav. Res. Ther 123 (2019). doi: 10.1016/j.brat.2019.103506. [DOI] [PubMed] [Google Scholar]
- [14].Jensen MP, Romano JM, Turner JA, Good AB, Wald LH, Patient beliefs predict patient functioning: Further support for a cognitive-behavioural model of chronic pain, Pain. 81 (1999) 95–104. doi: 10.1016/S0304-3959(99)00005-6. [DOI] [PubMed] [Google Scholar]
- [15].Turner JA, Holtzman S, Mancl L, Mediators, moderators, and predictors of therapeutic change in cognitive-behavioral therapy for chronic pain, Pain. 127 (2007) 276–286. doi: 10.1016/j.pain.2006.09.005. [DOI] [PubMed] [Google Scholar]
- [16].Keefe FJ, Dunsmore J, Burnett R, Behavioral and Cognitive-Behavioral Approaches to Chronic Pain: Recent Advances and Future Directions, 1992. https://psycnet.apa.org/record/1993-02754-001 (accessed October 19, 2020). [DOI] [PubMed]
- [17].Rudy TE, Kerns RD, Turk DC, Chronic pain and depression: toward a cognitive-behavioral mediation model, 1988. https://www.sciencedirect.com/science/article/pii/0304395988902205 (accessed October 19, 2020). [DOI] [PubMed]
- [18].Zautra A, Smith B, Affleck G, Tennen H, Examinations of chronic pain and affect relationships: Applications of a dynamic model of affect., J. Consult. Clin. Psychol 69 (2001) 786–795. doi: 10.1037/0022-006X.69.5.786. [DOI] [PubMed] [Google Scholar]
- [19].Molenaar PCM, A manifesto on psychology as idiographic science: Bringing the person back into scientific psychology, this time forever, Meas. Interdiscip. Res. Perspect 2 (2004) 201–218. doi: 10.1207/s15366359mea0204_1. [DOI] [Google Scholar]
- [20].Fisher AJ, Medaglia JD, Jeronimus BF, Lack of group-to-individual generalizability is a threat to human subjects research, Proc. Natl. Acad. Sci 115 (2018) E6106–E6115. doi: 10.1073/pnas.1711978115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Hamaker EL, Why researchers should think “within-person”: A paradigmatic rationale, in: Mehl M, Conner T (Eds.), Handb. Res. Methods Stud. Dly. Life, The Guilford Press, New York, 2012: pp. 43–61. [Google Scholar]
- [22].Frumkin MR, Haroutounian S, Rodebaugh TL, Examining emotional pain among individuals with chronic physical pain: Nomothetic and idiographic approaches, J. Psychosom. Res 136 (2020) 110172. doi: 10.1016/j.jpsychores.2020.110172. [DOI] [PubMed] [Google Scholar]
- [23].Ariens S, Ceulemans E, Adolf JK, Time series analysis of intensive longitudinal data in psychosomatic research: A methodological overview, J. Psychosom. Res 137 (2020) 110191. doi: 10.1016/j.jpsychores.2020.110191. [DOI] [PubMed] [Google Scholar]
- [24].May M, Junghaenel DU, Ono M, Stone AA, Schneider S, Ecological Momentary Assessment methodology in chronic pain research: A systematic review, J. Pain 19 (2018) 699–716. doi: 10.1016/j.jpain.2018.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Morren M, Dulmen S, Ouwerkerk J, Bensing J, Compliance with momentary pain measurement using electronic diaries: A systematic review, Eur. J. Pain 13 (2009) 354–365. doi: 10.1016/j.ejpain.2008.05.010. [DOI] [PubMed] [Google Scholar]
- [26].Shackman AJ, Wager TD, The emotional brain: Fundamental questions and strategies for future research, Neurosci. Lett 693 (2019) 68–74. doi: 10.1016/j.neulet.2018.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Schwarzer G, meta: An R package for meta-analysis, R News. 7 (2007) 40–45. [Google Scholar]
- [28].Hedges L, Olkin I, Statistical methods for meta-analysis, Academic Press, London, 1985. [Google Scholar]
- [29].Lipsey MW, Wilson DB, Practical meta-analysis, SAGE Publications Inc., 2001. [Google Scholar]
- [30].Field AP, Gillett R, How to do a meta-analysis, Br. J. Math. Stat. Psychol 63 (2010) 665–694. doi: 10.1348/000711010X502733. [DOI] [PubMed] [Google Scholar]
- [31].Cruise CE, Broderick J, Porter L, Kaell A, Stone AA, Reactive effects of diary self-assessment in chronic pain patients, Pain. 67 (1996) 253–258. doi: 10.1016/0304-3959(96)03125-9. [DOI] [PubMed] [Google Scholar]
- [32].Aaron LA, Turner JA, Mancl L, Brister H, Sawchuk CN, Electronic diary assessment of pain-related variables: Is reactivity a problem?, J. Pain 6 (2005) 107–115. doi: 10.1016/j.jpain.2004.11.003. [DOI] [PubMed] [Google Scholar]
- [33].Marceau LD, Link C, Jamison RN, Carolan S, Electronic Diaries as a Tool to Improve Pain Management: Is There Any Evidence?, Pain Med. 8 (2007) S101–S109. doi: 10.1111/j.1526-4637.2007.00374.x. [DOI] [PubMed] [Google Scholar]
- [34].Suso-Ribera C, Castilla D, Zaragozá I, Ribera-Canudas MV, Botella C, García-Palacios A, Validity, reliability, feasibility, and usefulness of pain monitor, Clin. J. Pain 34 (2018) 900–908. doi: 10.1097/AJP.0000000000000618. [DOI] [PubMed] [Google Scholar]
- [35].Aaron LA, Mancl L, Turner JA, Sawchuk CN, Klein KM, Reasons for missing interviews in the daily electronic assessment of pain, mood, and stress, Pain. 109 (2004) 389–398. doi: 10.1016/j.pain.2004.02.014. [DOI] [PubMed] [Google Scholar]
- [36].Trull TJ, Ebner-Priemer UW, Ambulatory assessment in psychopathology research: A review of recommended reporting guidelines and current practices, J. Abnorm. Psychol 129 (2020) 56–63. doi: 10.1037/abn0000473. [DOI] [PubMed] [Google Scholar]
- [37].Ji L, Chow S-M, Schermerhorn AC, Jacobson NC, Cummings EM, Handling missing data in the modeling of intensive longitudinal sata, Struct. Equ. Model. A Multidiscip. J 25 (2018) 715–736. doi: 10.1080/10705511.2017.1417046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Watson D, Naragon-Gainey K, On the specificity of positive emotional dysfunction in psychopathology: Evidence from the mood and anxiety disorders and schizophrenia/schizotypy, Clin. Psychol. Rev 30 (2010) 839–848. doi: 10.1016/j.cpr.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Watson D, Clark LA, Carey G, Positive and negative affectivity and their relation to anxiety and depressive disorders., J. Abnorm. Psychol 97 (1988) 346–353. doi: 10.1037/0021-843X.97.3.346. [DOI] [PubMed] [Google Scholar]
- [40].Schafer T, Models for intensive longitudinal data, Oxford University Press, 2006. [Google Scholar]
- [41].Hox J, Multilevel Modeling: When and Why, in: Balderjahn(Ed.), Classif. Data Anal. Data Highw, Springer-Verlag, Berlin, 1998: pp. 147–154. doi: 10.1007/978-3-642-72087-1_17. [DOI] [Google Scholar]
- [42].Hoffman L, Rovine MJ, Multilevel models for the experimental psychologist: Foundations and illustrative examples, Behav. Res. Methods 39 (2007) 101–117. doi: 10.3758/BF03192848. [DOI] [PubMed] [Google Scholar]
- [43].Krull JL, MacKinnon DP, Multilevel modeling of individual and group level mediated effects, Multivariate Behav. Res 36 (2001) 249–277. doi: 10.1207/S15327906MBR3602_06. [DOI] [PubMed] [Google Scholar]
- [44].Kindt S, Vansteenkiste M, Loeys T, Goubert L, Helping motivation and well-being of chronic pain couples: a daily diary study., Pain. 157 (2016) 1551–1562. doi: 10.1097/j.pain.0000000000000550. [DOI] [PubMed] [Google Scholar]
- [45].Mak HW, Schneider S, Individual differences in momentary pain-affect coupling and their associations with mental health in patients with chronic pain, J. Psychosom. Res 138 (2020) 110227. doi: 10.1016/j.jpsychores.2020.110227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Burns JW, Gerhart JI, Post KM, Smith DA, Porter LS, Schuster E, Buvanendran A, Fras AM, Keefe FJ, The communal coping model of pain catastrophizing in daily life: A within-couples daily diary study, J. Pain 16 (2015) 1163–1175. doi: 10.1016/j.jpain.2015.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Grant LD, Long BC, Willms JD, Women’s adaptation to chronic back pain: Daily appraisals and coping strategies, personal characteristics and perceived spousal responses, J. Health Psychol 7 (2002) 545–563. doi: 10.1177/1359105302007005675. [DOI] [PubMed] [Google Scholar]
- [48].Tennen H, Affleck G, Zautra A, Depression history and coping with chronic pain: A daily process analysis., Heal. Psychol 25 (2006) 370–379. doi: 10.1037/0278-6133.25.3.370. [DOI] [PubMed] [Google Scholar]
- [49].Hamilton NA, Affleck G, Tennen H, Karlson C, Luxton D, Preacher KJ, Templin JL, Fibromyalgia: The role of sleep in affect and in negative event reactivity and recovery., Heal. Psychol 27 (2008) 490–497. doi: 10.1037/0278-6133.27.4.490. [DOI] [PubMed] [Google Scholar]
- [50].van Middendorp H, Lumley MA, Moerbeek M, Jacobs JWG, Bijlsma JWJ, Geenen R, Effects of anger and anger regulation styles on pain in daily life of women with fibromyalgia: A diary study, Eur. J. Pain 14 (2010) 176–182. doi: 10.1016/j.ejpain.2009.03.007. [DOI] [PubMed] [Google Scholar]
- [51].Fifield J, McQuillan J, Armeli S, Tennen H, Reisine S, Affleck G, Chronic strain, daily work stress and pain among workers with rheumatoid arthritis: Does job stress make a bad day worse?, Work Stress. 18 (2004) 275–291. doi: 10.1080/02678370412331324996. [DOI] [Google Scholar]
- [52].Conner TS, Tennen H, Zautra AJ, Affleck G, Armeli S, Fifield J, Coping with rheumatoid arthritis pain in daily life: Within-person analyses reveal hidden vulnerability for the formerly depressed, Pain. 126 (2006) 198–209. doi: 10.1016/j.pain.2006.06.033. [DOI] [PubMed] [Google Scholar]
- [53].Kwissa-Gajewska Z, Gruszczyńska E, Relationship between daily pain and affect in women with rheumatoid arthritis: lower optimism as a vulnerability factor., J. Behav. Med 41 (2018) 12–21. doi: 10.1007/s10865-017-9874-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Martire LM, Hemphill RC, Zhaoyang R, Stephens MAP, Franks MM, Stanford AM, Daily Marital Tension and Symptom Severity in Older Adults With Diabetes or Osteoarthritis., Ann. Behav. Med 52 (2018) 842–853. doi: 10.1093/abm/kax062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Rivera NV, Parmelee PA, Smith DM, The impact of social interactions and pain on daily positive and negative affect in adults with osteoarthritis of the knee, Aging Ment. Health 24 (2020) 8–14. doi: 10.1080/13607863.2018.1506744. [DOI] [PubMed] [Google Scholar]
- [56].Hamilton NA, Catley D, Karlson C, Sleep and the affective response to stress and pain., Heal. Psychol 26 (2007) 288–295. doi: 10.1037/0278-6133.26.3.288. [DOI] [PubMed] [Google Scholar]
- [57].Hegarty RSM, Conner TS, Stebbings S, Treharne GJ, Feel the fatigue and be active anyway: Physical activity on high-fatigue days protects adults with arthritis from decrements in same-day positive mood, Arthritis Care Res. (Hoboken) 67 (2015) 1230–1236. doi: 10.1002/acr.22582. [DOI] [PubMed] [Google Scholar]
- [58].Finan PH, Zautra AJ, Davis MC, Daily affect relations in fibromyalgia patients reveal positive affective disturbance, Psychosom. Med 71 (2009) 474–482. doi: 10.1097/PSY.0b013e31819e0a8b. [DOI] [PubMed] [Google Scholar]
- [59].Han CJ, Jarrett ME, Heitkemper MM, Relationships between abdominal pain and fatigue with psychological distress as a mediator in women with Irritable Bowel Syndrome, Gastroenterol. Nurs 43 (2019) 28–39. doi: 10.1097/SGA.0000000000000383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Cho S, McCracken LM, Heiby EM, Moon D-E, Lee J-H, Pain acceptance-based coping in complex regional pain syndrome Type I: daily relations with pain intensity, activity, and mood., J. Behav. Med 36 (2013) 531–538. doi: 10.1007/s10865-012-9448-7. [DOI] [PubMed] [Google Scholar]
- [61].Ciere Y, Snippe E, Padberg M, Jacobs B, Visser A, Sanderman R, Fleer J, The role of state and trait positive affect and mindfulness in affective reactivity to pain in chronic migraine., Heal. Psychol 38 (2019) 94–102. doi: 10.1037/hea0000692. [DOI] [PubMed] [Google Scholar]
- [62].Christian MS, Eisenkraft N, Kapadia C, Dynamic associations among somatic complaints, human energy, and discretionary behaviors: Experiences with pain fluctuations at work, Adm. Sci. Q 60 (2015) 66–102. doi: 10.1177/0001839214553655. [DOI] [Google Scholar]
- [63].Finan PH, Hessler EE, Amazeen PG, Butner J, Zautra AJ, Tennen H, Oscillations in daily pain prediction accuracy, Nonlinear Dynamics. Psychol. Life Sci 14 (2010) 27–46. [PMC free article] [PubMed] [Google Scholar]
- [64].Gruszczyńska E, Knoll N, Meaning-focused coping, pain, and affect: a diary study of hospitalized women with rheumatoid arthritis, Qual. Life Res 24 (2015) 2873–2883. doi: 10.1007/s11136-015-1031-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Graham-Engeland JE, Zawadzki MJ, Slavish DC, Smyth JM, Depressive symptoms and momentary mood predict momentary pain among rheumatoid arthritis patients, Ann. Behav. Med 50 (2016) 12–23. doi: 10.1007/s12160-015-9723-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Finan PH, Quartana PJ, Smith MT, Positive and negative affect dimensions in chronic knee osteoarthritis: effects on clinical and laboratory pain., Psychosom. Med 75 (2013) 463–470. doi: 10.1097/PSY.0b013e31828ef1d6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Smith DM, Parmelee PA, Within-day variability of fatigue and pain smong African Americans and non-Hispanic whites with osteoarthritis of the knee, Arthritis Care Res. (Hoboken) 68 (2016) 115–122. doi: 10.1002/acr.22690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Kikuchi H, Yoshiuchi K, Ando T, Yamamoto Y, Influence of psychological factors on acute exacerbation of tension-type headache: Investigation by ecological momentary assessment, J. Psychosom. Res 79 (2015) 239–242. doi: 10.1016/j.jpsychores.2015.06.008. [DOI] [PubMed] [Google Scholar]
- [69].Hardy JK, Crofford LJ, Segerstrom SC, Goal conflict, distress, and pain in women with fibromyalgia: a daily diary study., J. Psychosom. Res 70 (2011) 534–540. doi: 10.1016/j.jpsychores.2010.10.013. [DOI] [PubMed] [Google Scholar]
- [70].Mun CJ, Thummala K, Davis MC, Karoly P, Tennen H, Zautra AJ, Predictors and social consequences of daily pain expectancy among adults with chronic pain, Pain. 158 (2017) 1224–1233. doi: 10.1097/j.pain.0000000000000903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Houtveen JH, Sorbi MJ, Prodromal functioning of migraine patients relative to their interictal state--an ecological momentary assessment study., PLoS One. 8 (2013) e72827. doi: 10.1371/journal.pone.0072827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Drukker M, Peters JCH, Vork L, Mujagic Z, Rutten BPF, van Os J, Masclee AAM, Kruimel JW, Leue C, Network approach of mood and functional gastrointestinal symptom dynamics in relation to childhood trauma in patients with irritable bowel syndrome and comorbid panic disorder, J. Psychosom. Res 139 (2020) 110261. doi: 10.1016/j.jpsychores.2020.110261. [DOI] [PubMed] [Google Scholar]
- [73].Feldman SI, Downey G, Schaffer-Neitz R, Pain, negative mood, and perceived support in chronic pain patients: A daily diary study of people with reflex sympathetic dystrophy syndrome., J. Consult. Clin. Psychol 67 (1999) 776–785. doi: 10.1037//0022-006x.67.5.776. [DOI] [PubMed] [Google Scholar]
- [74].Schoevers RA, Van Borkulo CD, Lamers F, Servaas MN, Bastiaansen JA, Beekman ATF, Van Hemert AM, Smit JH, Penninx BWJH, Riese H, Affect fluctuations examined with ecological momentary assessment in patients with current or remitted depression and anxiety disorders, Psychol. Med (2020) 1–10. doi: 10.1017/S0033291720000689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Bos EH, Jonge P, Cox RFA, Affective variability in depression: Revisiting the inertia–instability paradox, Br. J. Psychol 110 (2019) 814–827. doi: 10.1111/bjop.12372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Koval P, Pe ML, Meers K, Kuppens P, Affect dynamics in relation to depressive symptoms: Variable, unstable or inert?, Emotion. 13 (2013) 1132–1141. doi: 10.1037/a0033579. [DOI] [PubMed] [Google Scholar]
- [77].Houben M, Van Den Noortgate W, Kuppens P, The relation between short-term emotion dynamics and psychological well-being: A meta-analysis., Psychol. Bull 141 (2015) 901–930. doi: 10.1037/a0038822. [DOI] [PubMed] [Google Scholar]
- [78].Gerhart JI, Burns JW, Bruehl S, Smith DA, Post KM, Porter LS, Schuster E, Buvanendran A, Fras AM, Keefe FJ, Variability in negative emotions among individuals with chronic low back pain, Pain. 159 (2018) 342–350. doi: 10.1097/j.pain.0000000000001102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Burns JW, Gerhart J, Rizvydeen M, Kimura M, Burgess HJ, Morning bright light treatment for chronic low back pain: Potential impact on the volatility of pain, mood, function, and sleep, Pain Med. 21 (2020) 1153–1161. doi: 10.1093/pm/pnz235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Stone AA, Broderick JE, Porter LS, Kaell AT, The experience of rheumatoid arthritis pain and fatigue: Examining momentary reports and correlates over one week, Arthritis Care Res. (Hoboken) 10 (1997) 185–193. doi: 10.1002/art.1790100306. [DOI] [PubMed] [Google Scholar]
- [81].Schneider S, Junghaenel DU, Keefe FJ, Schwartz JE, Stone AA, Broderick JE, Individual differences in the day-to-day variability of pain, fatigue, and well-being in patients with rheumatic disease: associations with psychological variables., Pain. 153 (2012) 813–822. doi: 10.1016/j.pain.2012.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Rost S, Van Ryckeghem DML, Koval P, Sütterlin S, Vögele C, Crombez G, Affective instability in patients with chronic pain: A diary approach, Pain. 157 (2016) 1783–1790. doi: 10.1097/j.pain.0000000000000582. [DOI] [PubMed] [Google Scholar]
- [83].Rost S, Crombez G, Sütterlin S, Vögele C, Veirman E, Van Ryckeghem DML, Altered regulation of negative affect in patients with fibromyalgia: A diary study, Eur. J. Pain (2020) 1–11. doi: 10.1002/ejp.1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Vendrig AA, Lousberg R, Within-person relationships among pain intensity, mood and physical activity in chronic pain: a naturalistic approach., Pain. 73 (1997) 71–76. doi: 10.1016/s0304-3959(97)00075-4. [DOI] [PubMed] [Google Scholar]
- [85].Finan PH, Zautra AJ, Davis MC, Lemery-Chalfant K, Covault J, Tennen H, COMT moderates the relation of daily maladaptive coping and pain in fibromyalgia., Pain. 152 (2011) 300–307. doi: 10.1016/j.pain.2010.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Linton SJ, A review of psychological risk factors in back and neck pain, Spine (Phila. Pa. 1976) 25 (2000) 1148–1156. [DOI] [PubMed] [Google Scholar]
- [87].Goesling J, Clauw DJ, Hassett AL, Pain and depression: An integrative review of neurobiological and psychological factors, Curr. Psychiatry Rep 15 (2013) 421. doi: 10.1007/s11920-013-0421-0. [DOI] [PubMed] [Google Scholar]
- [88].Levine F, Sandeen E, Murphy C, The therapist’s dilemma: Using nomothetic information to answer idiographic questions, Psychother. Theory, Res. Pract. Train 29 (1992) 410–415. doi: 10.1037/h0088544. [DOI] [Google Scholar]
- [89].Piccirillo ML, Beck ED, Rodebaugh TL, A clinician’s primer for idiographic research: Considerations and recommendations, Behav. Ther 50 (2019) 938–951. doi: 10.1016/j.beth.2019.02.002. [DOI] [PubMed] [Google Scholar]
- [90].Barlow DH, Nock MK, Why can’t we be more idiographic in our research?, Perspect. Psychol. Sci 4 (2009) 19–21. doi: 10.1111/j.1745-6924.2009.01088.x. [DOI] [PubMed] [Google Scholar]
- [91].Nickell S, Biases in dynamic models with fixed effects, Econometrica. 49 (1981) 1417. doi: 10.2307/1911408. [DOI] [Google Scholar]
- [92].Hamaker EL, Grasman RPPP, To center or not to center? Investigating inertia with a multilevel autoregressive model, Front. Psychol 5 (2015) 1492. doi: 10.3389/fpsyg.2014.01492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Haslbeck JMB, Ryan O, Recovering within-person dynamics from psychological time series, PsyArXiv. (2020). doi: 10.31234/OSF.IO/DYMHW. [DOI] [PubMed] [Google Scholar]
- [94].Eisele G, Vachon H, Lafit G, Kuppens P, Houben M, Myin-Germeys I, Viechtbauer W, The effects of sampling frequency and questionnaire length on perceived burden, compliance, and careless responding in experience sampling data in a student population, Assessment. (2020) 107319112095710. doi: 10.1177/1073191120957102. [DOI] [PubMed] [Google Scholar]
- [95].Jacobson NC, Chow SM, Newman MG, The Differential Time-Varying Effect Model (DTVEM): A tool for diagnosing and modeling time lags in intensive longitudinal data, Behav. Res. Methods 51 (2019) 295–315. doi: 10.3758/s13428-018-1101-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Tan X, Shiyko MP, Li R, Li Y, Dierker L, A time-varying effect model for intensive longitudinal data, Psychol. Methods 17 (2012) 61–77. doi: 10.1037/a0025814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Bringmann LF, Ferrer E, Hamaker EL, Borsboom D, Tuerlinckx F, Modeling nonstationary emotion dynamics in dyads using a time-varying vector-autoregressive model, Multivariate Behav. Res 3171 (2018) 1–22. doi: 10.1080/00273171.2018.1439722. [DOI] [PubMed] [Google Scholar]
- [98].Asparouhov T, Hamaker EL, Muthén B, Dynamic structural equation models, Struct. Equ. Model 25 (2018) 359–388. doi: 10.1080/10705511.2017.1406803. [DOI] [Google Scholar]
- [99].Ebner-Priemer UW, Trull TJ, Ambulatory assessment: An innovative and promising approach for clinical psychology, Eur. Psychol 14 (2009) 109–119. doi: 10.1027/1016-9040.14.2.109. [DOI] [Google Scholar]
- [100].Cornet VP, Holden RJ, Systematic review of smartphone-based passive sensing for health and wellbeing, J. Biomed. Inform 77 (2018) 120–132. doi: 10.1016/j.jbi.2017.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Treede R-D, Rief W, Barke A, Aziz Q, Bennett MI, Benoliel R, Cohen M, Evers S, Finnerup NB, First MB, Giamberardino MA, Kaasa S, Kosek E, Lavandʼhomme P, Nicholas M, Perrot S, Scholz J, Schug S, Smith BH, Svensson P, Vlaeyen JWS, Wang S-J, A classification of chronic pain for ICD-11., Pain. 156 (2015) 1003–7. doi: 10.1097/j.pain.0000000000000160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Price DD, Psychological and neural mechanisms of the affective dimension of pain., Science (80-.) 288 (2000) 1769–1772. doi: 10.1126/science.288.5472.1769. [DOI] [PubMed] [Google Scholar]
- [103].Molenaar PCM, Nesselroade JR, The recoverability of P-technique factor analysis, Multivariate Behav. Res 44 (2009) 130–141. doi: 10.1080/00273170802620204. [DOI] [PubMed] [Google Scholar]
- [104].Garland EL, Hanley AW, Kline A, Cooperman NA, Mindfulness-Oriented Recovery Enhancement reduces opioid craving among individuals with opioid use disorder and chronic pain in medication assisted treatment: Ecological momentary assessments from a stage 1 randomized controlled trial, Drug Alcohol Depend. 203 (2019) 61–65. doi: 10.1016/j.drugalcdep.2019.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Garland EL, Bryan CJ, Finan PH, Thomas EA, Priddy SE, Riquino MR, Howard MO, Pain, hedonic regulation, and opioid misuse: Modulation of momentary experience by Mindfulness-Oriented Recovery Enhancement in opioid-treated chronic pain patients, Drug Alcohol Depend. 173 (2017) S65–S72. doi: 10.1016/j.drugalcdep.2016.07.033. [DOI] [PubMed] [Google Scholar]
- [106].Litt MD, Shafer DM, Ibanez CR, Kreutzer DL, Tawfik-Yonkers Z, Momentary pain and coping in temporomandibular disorder pain: exploring mechanisms of cognitive behavioral treatment for chronic pain., Pain. 145 (2009) 160–168. doi: 10.1016/j.pain.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Carpenter RW, Lane SP, Bruehl S, Trull TJ, Concurrent and lagged associations of prescription opioid use with pain and negative affect in the daily lives of chronic pain patients., J. Consult. Clin. Psychol 87 (2019) 872–886. doi: 10.1037/ccp0000402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].de C. Williams AC, Eccleston C, Morley S, Psychological therapies for the management of chronic pain (excluding headache) in adults., Cochrane Database Syst. Rev 11 (2012) CD007407. doi: 10.1002/14651858.CD007407.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Mun CJ, Karoly P, Okun MA, Kim H, Tennen H, Affect, work-goal schemas, and work-goal striving among adults with chronic pain: a multilevel structural equation analysis, J. Behav. Med 39 (2016) 288–299. doi: 10.1007/s10865-015-9696-4. [DOI] [PubMed] [Google Scholar]
- [110].Okun M, Karoly P, Mun CJ, Kim H, Pain-contingent interruption and resumption of work goals: A within-day diary analysis, J. Pain 17 (2016) 65–75. doi: 10.1016/j.jpain.2015.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Mun CJ, Karoly P, Okun MA, Does working memory moderate the within-person associations between pain intensity and negative affect and pain’s interference with work goal pursuit?, Clin. J. Pain 34 (2018) 566–576. doi: 10.1097/AJP.0000000000000569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Fragoso ZL, McGonagle AK, Chronic pain in the workplace: A diary study of pain interference at work and worker strain, Stress Heal. 34 (2018) 416–424. doi: 10.1002/smi.2801. [DOI] [PubMed] [Google Scholar]
- [113].Gerhart JI, Burns JW, Post KM, Smith DA, Porter LS, Burgess HJ, Schuster E, Buvanendran A, Fras AM, Keefe FJ, Relationships between sleep quality and pain-related factors for people with chronic low back pain: Tests of reciprocal and time of day effects, Ann. Behav. Med 51 (2017) 365–375. doi: 10.1007/s12160-016-9860-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Song S, Graham-Engeland JE, Mogle J, Martire LM, The effects of daily mood and couple interactions on the sleep quality of older adults with chronic pain., J. Behav. Med 38 (2015) 944–955. doi: 10.1007/s10865-015-9651-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Nah S, Martire LM, Zhaoyang R, Perceived patient pain and spousal caregivers’ negative affect: The moderating role of spouse confidence in patients’ pain management, J. Aging Health (2020) 089826432091963. doi: 10.1177/0898264320919631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Kothari DJ, Davis MC, Yeung EW, Tennen HA, Positive affect and pain: Mediators of the within-day relation linking sleep quality to activity interference in fibromyalgia, Pain. 156 (2015) 540–546. doi: 10.1097/01.j.pain.0000460324.18138.0a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Finan PH, Zautra AJ, Davis MC, Lemery-Chalfant K, Covault J, Tennen H, Genetic influences on the dynamics of pain and affect in fibromyalgia., Heal. Psychol 29 (2010) 134–142. doi: 10.1037/a0018647. [DOI] [PMC free article] [PubMed] [Google Scholar]


