Abstract
Objective:
To describe sociodemographic and parent psychosocial characteristics associated with patterns of continuous glucose monitor (CGM) use across the first 18 months post-type 1 diabetes (T1D) diagnosis among young children.
Methods:
One hundred fifty-seven parent–child dyads enrolled in a behavioral intervention for parents of young children (1–6 years) newly diagnosed with T1D. Parents reported on baseline sociodemographic characteristics and psychosocial functioning; child CGM use was assessed at five time points during the first 18 months post-diagnosis.
Results:
Most participants (81.8%) used CGM at least once. Four CGM trajectories emerged (always, later/stable, inconsistent, and never). Participants with private insurance were more likely to be in the always, later/stable, or inconsistent groups versus the never group. Youth in the always and later/stable groups had lower mean HbA1c at 18 months than those in the never group.
Conclusions:
Given the health benefits of CGM, further exploration of barriers to CGM use in families with public health insurance is needed. ClinicalTrials.gov identifier: NCT02527525
Keywords: Health insurance, Continuous glucose monitoring, Glycemic outcomes, Pediatrics
Introduction
Continuous glucose monitor (CGM) use among youth is associated with better glycemic control1 as well as parent psychoscial functioning.2 The 2021 American Diabetes Association (ADA) guidelines state that CGM is the preferred method for glucose monitoring in people with type 1 diabetes (T1D).3 Not surprisingly, there has been an increase in CGM use over the last few years, particularly among children ≤6 years.4 Among youth with T1D, glycemic control during the first year post-T1D diagnosis has been found to predict future glycemic control5; thus, the year following diagnosis may be particularly important for the development of skills and behaviors to optimize T1D management. Early CGM initiation at diagnosis has been linked to sustained use and increased wear over time,6 both of which predict better long-term glycemic control. While CGM use enhances glycemic control in youth, little research has focused on the uptake and natural-use patterns of CGM use the year following T1D diagnosis among young children. Few studies have examined whether CGM use patterns soon after diagnosis are associated with later glycemic outcomes among young children.
Both sociodemographic and parent psychosocial functioning factors may have significant impacts on access to and consistent use of diabetes devices, particularly among young children. Several studies have found that children from families with lower education, lower income, a primary language other than English, and single parents have more difficulties accessing and using diabetes devices.7–10 Relatedly, the most commonly cited barriers to CGM use are insurance coverage and device cost.11–13 Furthermore, English-speaking, non-Hispanic, White families report higher rates of device uptake and sustained use than that of families of color.10,14,15
Overall, very little is known about the uptake and patterns of CGM use and glycemic outcomes in young children, particularly those newly diagnosed with T1D. Even less is known about the sociodemographic and parent psychosocial characteristics that relate to CGM use. The aims of the current study are to (1) identify meaningful trajectories of CGM use among young children across 18 months post-T1D diagnosis, and (2) explore whether sociodemographic and parent psychosocial characteristics are associated with CGM trajectories. An exploratory aim was to examine whether CGM use categories are associated with glycemic outcomes at 18 months post-diagnosis.
Methods
Participants
Participants were 157 parent–child dyads (child age M = 4.5 years ±1.7, 91% female, 62% non-Hispanic, White, 76% married) recruited as part of a larger randomized-controlled trial comparing usual care to a behavioral intervention for parents of young children newly diagnosed with T1D. Increasing technology use was not part of the larger study. Parents/legal guardians were eligible if they were ≥21 years old and fluent in English. Families were excluded if the child was also experiencing another major illness (e.g., cancer, cystic fibrosis) or developmental disability (e.g., autism).
Procedures
The Institutional Review Boards at two pediatric academic medical centers, Children's National Hospital and Texas Children's Hospital, approved the study. Recruitment for this study (2016–2019) occurred within 8 weeks of the child's diagnosis (M days post-diagnosis = 29.03 ± 15.39). Families were informed that the research aims were to test a behavioral intervention to support parent's mood and child glycemic control after initial diagnosis. Two hundred seventeen were eligible and consented to participation; 157 completed the baseline psychosocial battery within 8 weeks of T1D diagnosis. Additional details of the behavioral intervention are reported elsewhere.16,17
Measures
Sociodemographic and medical information
Participants self-reported on baseline sociodemographic characteristics. CGM use at baseline, 5, 9, 12, and 18 months post-diagnosis* was based on parent report and corroborated by medical records, and, when available, CGM download data. Medical record reviews were conducted to gather diabetic ketoacidosis (DKA) at diagnosis as well as 18-month A1c. For some of the participants, their 18-month data collection coincided with the onset of the global COVID-19 pandemic and concurrent shift to telehealth diabetes care, limiting in-person A1c data collection.†
Parent psychosocial functioning
The Center for Epidemiological Studies-Depression Scale (CES-D) is a 20-item measure that assesses general depressive symptoms,18 with excellent internal consistency (current sample α = 0.92).18–20 The 7-item Patient Reported Outcomes Measurement Information System-Emotional Distress-Anxiety-Short Form (PROMIS-A) was used to measure parental anxiety (current sample α = 0.93).21 Higher scores on both measures indicate more symptoms (CES-D scores of ≥16 and PROMIS-A scores of ≥24 indicate elevated risk for depression and anxiety, respectively). Table 1 reports details about demographic information and parent psychosocial functioning.
Table 1.
Summary of Sociodemographic Characteristics and Related Psychosocial Factors
| Baseline characteristics | M | SD |
|---|---|---|
| Primary caregiver age (years) | 34.9 | 7.0 |
| Child age (years) | 4.5 | 1.6 |
| Hemoglobin A1c (%) | 8.4 | 1.4 |
| N (157) | % | |
| Primary caregiver sex | ||
| Female | 144 | 91.7 |
| Primary caregiver race and ethnicity | ||
| White, non-Hispanic | 97 | 62.2 |
| Black/African American, non-Hispanic | 23 | 14.7 |
| Hispanic/Latinx | 19 | 12.2 |
| Asian/Asian American | 12 | 7.7 |
| Multiracial | 4 | 2.5 |
| American Indian/Alaskan native | 1 | <1% |
| Primary caregiver marital status | ||
| Married | 117 | 76.5 |
| Child sex | ||
| Female | 86 | 54.8 |
| Yearly household income | ||
| <20k | 13 | 9.6 |
| 20–40k | 14 | 10.4 |
| 40–65k | 16 | 11.9 |
| 65–100k | 30 | 22.2 |
| 100k–200k | 43 | 31.9 |
| 200k+ | 19 | 14.1 |
| Insurance type | ||
| Public only | 43 | 27.7 |
| DKA status at diagnosis | 57 | 37.0 |
| Positive family history of T1D | 42 | 26.9 |
| Intervention treatment condition | 115 | 73.2 |
| Psychosocial functioning variables | M | SD |
| Baseline | ||
| CES-D | 16.6 | 11.6 |
| PROMIS-A | 17.9 | 6.2 |
CES-D, Center for Epidemiological Studies-Depression Scale; DKA, diabetic ketoacidosis; PROMIS-A, Patient Reported Outcomes Measurement Information System-Emotional Distress-Anxiety-Short Form; T1D, type 1 diabetes.
Data analyses
Analyses were conducted in SPSS v.27.22 With the exception of child age and 18-month A1c, all variables were dichotomized: CGM use at baseline, 5, 9, 12, and 18 months (1 = yes, 0 = no); insurance (1 = public, 2 = private); marital status (1 = married, 0 = other); parent racial and ethnic background (1 = non-Hispanic, white, 2 = parents of color); CES-D (0 = ≤15, 1 = ≥16); PROMIS-A (0 = ≤23, 1 = ≥24); T1D family history (1 = yes, 0 = no); and DKA at diagnosis (1 = yes, 0 = no). Treatment condition (1 = intervention, 0 = usual care) was examined as a potential covariate.
Omnibus χ2 test of independence and one-way analysis of variance were used to examine differences in demographic and psychosocial characteristics among CGM trajectories. CGM trajectories were developed based on descriptive statistics and prior literature on CGM use over time. Variables with significant differences among the CGM trajectories were entered into a multinomial logistic regression predicting CGM trajectory. Our exploratory aim was examined using the Kruskal–Wallis H test with post hoc comparisons conducted using Mann–Whitney U tests with adjusted p-values for multiple comparisons.
Results
We retained over 97% of our sample from baseline to 18 months post-diagnosis. Overall, 82% of children used CGM at least one time during the 18 months post-diagnosis. Rates of CGM use generally increased over time: at baseline (n = 38; 24.2%), 5 (n = 91; 58.0%), 9 (n = 82; 52.9%), 12 (n = 96; 61.9%), and 18 months (n = 102; 65.8%) post-diagnosis.
For Aim 1, four meaningful CGM trajectories emerged that were characterized as the following (n = 154): (1) “always” included participants who used CGM at all five assessments (n = 22); (2) “later, stable” included those who initiated CGM at 5, 9, or 12 months post-diagnosis and continued CGM use through the end of the study (n = 53); (3) “inconsistent” included those who initiated CGM at baseline, 5, 9, 12, or 18 months post-diagnosis, yet exhibited an “on/off” pattern of use (n = 51); and (4) “never” included participants who never used CGM (n = 28).
For Aim 2, parent race/ethnicity, child age, parent CES-D/PROMIS-A scores, treatment condition, T1D family history, and DKA at diagnosis did not differ among the four trajectories (p < .05); thus, these were not included in future multivariate analyses. There were group differences by insurance type and marital status (p < .05).
Thus, insurance type and marital status were entered into a multinomial logistic regression with CGM trajectory as the dependent variable (n = 151). Given our interest in understanding correlates of CGM adoption among newly diagnosed families, the “never” group was used as the reference group. Participants with private insurance were more likely than those with only public insurance to be in the “always” (OR = 19.94), “later, stable” (OR = 4.78), and “inconsistent” (OR = 3.75) groups than the “never” group (all p < .05). Marital status was not associated with CGM trajectory in the multivariate model (p > .05).
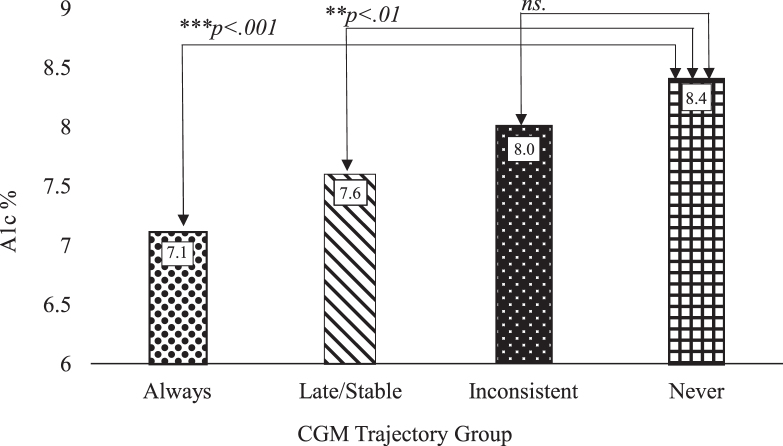
For our exploratory analysis, results indicated significant differences in 18-month A1c based on CGM trajectories (Kruskal–Wallis H = 15.18, p = .002; n = 124). Post hoc analyses with adjusted p-values indicated that those in the “always” (7.1%) and “later, stable” (7.6%) trajectories had lower 18-month HbA1c compared with those in the “never” group (8.4%; p < .003 for both). There were no significant differences in the 18-month A1c between the “inconsistent” and “never” groups (8.0% vs. 8.4%, p = .11) (Fig. 1).
FIG. 1.
Eighteen-month A1c medians by CGM trajectory group.
Discussion
There were sociodemographic disparities among families who never used CGM versus those who used CGM in any pattern during the first 18 months post-diagnosis, with insurance type emerging as the primary predictor of CGM use. Insurance has been cited as a barrier to CGM access, and publicly insured families pursuing CGM often face more obstacles, including providing documentation of at least four blood glucose checks per day and navigating durable medical equipment.13,23–25 As of 2016–2018, there was approximately a threefold difference in CGM use among American children in the lowest versus highest income quintiles, while the difference between those groups among German/Austrian children was negligible.7 Alarmingly, these disparities in CGM use between the poorest and wealthiest quintiles of American children living with T1D for at least one year have worsened over the last decade.7
Our exploratory analyses also indicate that CGM trajectories were associated with 18-month A1c, supporting other data in school-age youth showing that early CGM use (within the first 12 months) post-diagnosis predicts better glycemic outcomes.26 Current results demonstrate that sustained use of CGM predicts better glycemic control compared with those who never used CGM during the 18 months post-diagnosis among young children.
Parent race and ethnicity did not predict CGM trajectory in this study, which conflicts with other findings in the literature. For example, even after Willi et al. controlled for socioeconomic factors among youth in the T1D Exchange (T1DX),27 rates of diabetes technology use were lower among black and Hispanic children compared with white children.27 The T1DX's most recent data showed that among youth living in households with more than $75,000 in annual household income, 31% of Hispanic youth were using CGM compared with 9% of black children.4 Given the relatively small cell sizes in our sample, we were not able to conduct racial and ethnic group comparisons, which may have masked existing disparities.
Although past research has shown that CGM use and parent psychosocial functioning are positively associated,2 parent psychosocial functioning did not predict CGM uptake nor sustained use in this study. During the challenging new-onset period, it is possible that sociodemographic factors are more directly related to CGM access and use than parent psychosocial factors. Whether parent psychosocial characteristics are more strongly related to CGM use among those with longer T1D durations remains an area of future scientific inquiry.
This study has several strengths, including five CGM assessment points during the first 18 months post-diagnosis with high retention (97%) as well as more racial/ethnic and socioeconomic diversity than many other T1D studies. However, findings should be considered in light of its limitations. Although we used multiple data sources to confirm CGM use at each assessment, the study did not measure CGM wear time, which limits interpretation of the degree of CGM use. This study was conducted over a three-year period during which CGM technology usability and access improved significantly. Increasing usability of CGMs over time is a potentially confounding factor due to changes such as nonadjunctive dosing indications, factory calibrated CGMs, and improved accuracy (i.e., lower mean absolute relative difference), which may have influenced families' decisions to begin or maintain CGM use. The CGM technology available to families also changed over the course of the study (i.e., only DEXCOM G4 technology was available to families at the beginning of the study period, but DEXCOM G5 and G6 technology becoming available throughout the study period). While there were no significant differences in CGM trajectory based on CGM type used, it is possible that differences in available technology alone could have influenced the timing at which some families chose to start using CGM. Lastly given the relatively small numbers within each racial and ethnic category, we were not able to examine differences among the various racial and ethnic categories, and we did not measure associated factors that may have impacted CGM access or other outcomes, such as racism/discrimination, acculturation, and language.28
Future studies should examine mechanisms that may explain the association between insurance type and CGM use among larger sample sizes, with special consideration to reasons families are not presented with the option to begin technology use soon after diagnosis and barriers inherent in the CGM initiation process (e.g., navigating durable medical equiptment).9,14,27 Continued research exploring characteristics of families who sustain or cease device use over time informs clinical care, highlighting the importance of increasing access to and education around diabetes technology across demographic groups to ultimately improve health outcomes.
Author Disclosure Statement
B.E.M. has research support from Tandem and Dexcom. No other authors have competing financial interests.
Funding Information
This research was supported by grant R01DK102561 from the National Institute of Diabetes and Digestive and Kidney Diseases (last author PI).
In the current study, 5, 9, 12, and 18 months post-diagnosis correspond approximately to 3, 6, 9, and 15 months postrandomization in the trial.16
Participants from whom we do (n = 125) and do not (n = 32) have 18-month A1c data did not differ by key sociodemographics, such as parent race/ethnicity, insurance type, or marital status (p > .05).
References
- 1. DeSalvo DJ, Miller KM, Hermann JM, et al. ; T1D Exchange and DPV Registries: Continuous glucose monitoring and glycemic control among youth with type 1 diabetes: international comparison from the T1D Exchange and DPV Initiative. Pediatr Diab 2018;19:1271–1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Burckhardt MA, Roberts A, Smith GJ, et al. : The use of continuous glucose monitoring with remote monitoring improves psychosocial measures in parents of children with type 1 diabetes: a randomized crossover trial. Diabetes Care 2018;41:2641–2643. [DOI] [PubMed] [Google Scholar]
- 3. American Diabetes Association: 7. Diabetes technology: standards of Medical Care in Diabetes—2020. Diabetes Care 2020;43(Supplement 1):S77–S88. [DOI] [PubMed] [Google Scholar]
- 4. Foster NC, Beck RW, Miller KM, et al. : State of type 1 diabetes management and outcomes from the T1D Exchange in 2016–2018. Diabetes Technol Ther 2019;21:66–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Shalitin S, Phillip M: Which factors predict glycemic control in children diagnosed with type 1 diabetes before 6.5 years of age?. Acta Diabetol 2012;49:355–362. [DOI] [PubMed] [Google Scholar]
- 6. Prahalad P, Addala A, Scheinker D, et al. : CGM initiation soon after type 1 diabetes diagnosis results in sustained CGM use and wear time. Diabetes Care 2020;43:e3–e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Blackman SM, Raghinaru D, Adi S, et al. : Insulin pump use in young children in the T1D Exchange clinic registry is associated with lower hemoglobin A1c levels than injection therapy. Pediatr Diabetes 2014;15:564–572. [DOI] [PubMed] [Google Scholar]
- 8. Commissariat PV, Boyle CT, Miller KM, et al. : Insulin pump use in young children with type 1 diabetes: sociodemographic factors and parent-reported barriers. Diabetes Technol Ther 2017;19:363–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wong JC, Foster NC, Maahs DM, et al. : Real-time continuous glucose monitoring among participants in the T1D Exchange clinic registry. Diabetes Care 2014;37:2702–2709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Telo GH, Volkening LK, Butler DA, Laffel LM: Salient characteristics of youth with type 1 diabetes initiating continuous glucose monitoring. Diabetes Technol Ther 2015;17:373–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Addala A, Auzanneau M, Miller K, et al. : A decade of disparities in diabetes technology use and HbA1c in pediatric type 1 diabetes: a transatlantic comparison. Diabetes Care 2021;44:133–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tanenbaum ML, Hanes SJ, Miller KM, et al. : Diabetes device use in adults with type 1 diabetes: barriers to uptake and potential intervention targets. Diabetes Care 2017;40:181–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wong JJ, Barley RC, Hanes S, et al. : Parental perspectives: identifying profiles of parental attitudes and barriers related to diabetes device use. Diabetes Technol Ther 2020;22:674–680. [DOI] [PubMed] [Google Scholar]
- 14. Sheikh K, Bartz SK, Lyons SK, DeSalvo DJ: Diabetes device use and glycemic control among youth with type 1 diabetes: a single-center, cross-sectional study. J Diabetes Res 2018;2018:5162162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lai CW, Lipman TH, Willi SM, Hawkes CP: Racial and ethnic disparities in rates of continuous glucose monitor initiation and continued use in children with type 1 diabetes. Diabetes Care. 2021;44:255–257. [DOI] [PubMed] [Google Scholar]
- 16. Hilliard ME, Tully C, Monaghan M, et al. : Design and development of a stepped-care behavioral intervention to support parents of young children newly diagnosed with type 1 diabetes. Contemp Clin Trials 2017;62:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tully C, Clary L, Monaghan M, et al. : Implementation and preliminary feasibility of an individualized, supportive approach to behavioral care for parents of young children newly diagnosed with type 1 diabetes. Cogn Behav Pract 2021;28:293–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Radloff LS: The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Meas 1977;1:385–401. [Google Scholar]
- 19. Bartlett SJ, Kolodner K, Butz AM, et al. : Maternal depressive symptoms and emergency department use among inner-city children with asthma. Arch Pediatr Adolesc Med 2001;155:347–353. [DOI] [PubMed] [Google Scholar]
- 20. Friis R, Nanjundappa G: Diabetes, depression and employment status. Soc Sci Med 1986;23:471–475. [DOI] [PubMed] [Google Scholar]
- 21. Pilkonis PA, Choi SW, Reise SP, et al. ; PROMIS Cooperative Group. Item banks for measuring emotional distress from the Patient-Reported Outcomes Measurement Information System (PROMIS®): depression, anxiety, and anger. Assessment 2011;18:263–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. IBM Corp: Released 2020. IBM SPSS Statistics for Windows, Version 27.0. Armonk, NY: IBM Corp, 2020. [Google Scholar]
- 23. Anderson JE, Gavin JR, Kruger DF: Current eligibility requirements for CGM coverage are harmful, costly, and unjustified. Diabetes Technol Ther 2020;22:169–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Markowitz JT, Pratt K, Aggarwal J, et al. : Psychosocial correlates of continuous glucose monitoring use in youth and adults with type 1 diabetes and parents of youth. Diabetes Technol Ther 2012;14:523–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Telgener PC, Lowe S: A look at the current reimbursement environment for continuous glucose monitoring (CGM): understanding the fundamentals. J Diab Sci Technol 2008;2:681–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Patton SR, Noser AE, Youngkin EM, et al. : Early initiation of diabetes devices relates to improved glycemic control in children with recent-onset type 1 diabetes mellitus. Diabetes Technol Ther 2019;21:379–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Willi SM, Miller KM, DiMeglio LA, et al. ; T1D Exchange Clinic Network. Racial-ethnic disparities in management and outcomes among children with type 1 diabetes. Pediatrics 2015;135:424–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Peek ME, Odoms-Young A, Quinn MT, et al. : Racism in healthcare: its relationship to shared decision-making and health disparities: A response to Bradby. Soc Sci Med (1982) 2010;71:13. [DOI] [PMC free article] [PubMed] [Google Scholar]