A new study in Nature Microbiology led by Jillian Banfield and Jennifer Doudna reports a bioinformatic and genetic toolkit for precise gene editing in a bacterial community.
Nearly all microbes on the planet live in communities. Most experimental studies, however, use monoculture to elucidate gene and microbe functions. Modifying the DNA of individual constituents in a microbial community and investigating the phenotypic consequences of these manipulations in a near-native context has thus far been challenging. Most microbes have not been isolated or successfully cultured outside their native environments. Even for those bacteria that can be cultivated, we often lack the robust tools (i.e., replicating plasmids) necessary for gene editing. Thus, there is an unmet need for tools to tackle these numerous challenges and to advance our understanding of the complex phenomena present in native microbial communities.1
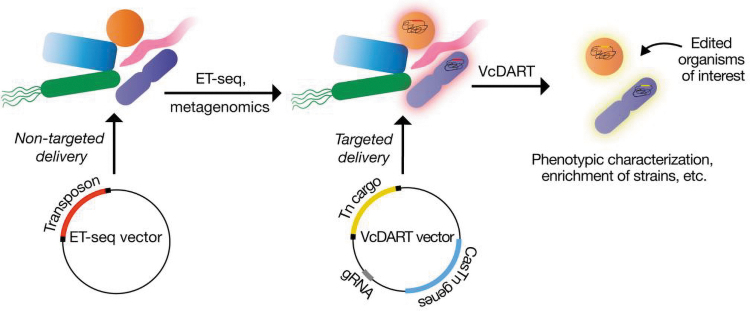
To address these problems, two CRISPR pioneers, Jennifer Doudna and Jillian Banfield, have lead a multidisciplinary team (including The CRISPR Journal's Editor-in-Chief, Rodolphe Barrangou) that reports in Nature Microbiology the development of a bioinformatic and genetic toolkit to identify and manipulate the editable microbes in a community.2 In this workflow, the first step involves prescreening a bacterial community to identify specific bacteria that are amenable to the delivery of nucleic acids and genome editing. This is achieved through a process called environmental transformation sequencing (ET-Seq; Fig. 1).
FIG. 1.
Schematic summary of environmental transformation sequencing and Vibrio cholerae CAST system workflow for organism- and locus-specific genome editing.
In this pipeline, the complex microbial community of interest is first exposed to a randomly integrating mariner transposon delivered via three separate methods: conjugation, electroporation, and natural transformation. Subsequently, in the absence of any selection, the total community DNA is extracted and sequenced to evaluate the location and frequency of genomic integration in each organism. Using a bioinformatic pipeline that normalizes the frequency of the insertion events to an internal standard (added to the microbial sample) and accounts for metagenomic abundance of the individual organisms, the authors were able to measure the species-specific percentage of transposon insertion of each community member quantitatively. ET-Seq is therefore able to evaluate the relative amenability of each bacteria species agnostically within the community to genetic manipulation in a quantitative fashion.
In their study, the authors tested ET-Seq on (1) a synthetic soil community consisting of nine distinct microbes from three separate phyla and (2) an infant gut microbe sample cultured ex vivo. Using the ET-Seq approach, the authors were able to detect genetic insertions reproducibly in both samples, identify microbes within the samples that were amenable to foreign DNA integration, and determine the best method for delivery of the gene editing cargo. Notably, introduction of foreign DNA into multiple microbes comprising the rarer species in the community was not detected. The elegance of the ET-Seq pipeline lies in providing the ability to screen a microbial community rapidly for genetic accessibility without the need for culturing and testing individual strains of the constituent microbes.
Having identified candidate microbes with ET-Seq for gene editing, Rubin et al. engineered a CRISPR-associated Tn7 transposon (CAST) system to deliver targeted gene editing cargo to these bacteria. CAST systems (also called INTEGRATE-insertion of transposable elements by guide RNA–assisted targeting) were first identified from a bioinformatic screen of bacterial and archaeal genomes3 as a naturally occurring fusion of Tn7-like transposons with a CRISPR-Cas systems lacking nuclease activity.3,4 Such a modality combines the programmable RNA-guided targeting of the CRISPR-Cas system with the DNA integration abilities of the Tn7-like transposon. CAST systems have been previously adapted for high-efficiency RNA-guided programmable integration of DNA for microbial gene editing,5,6 including the delivery of transposon cargo with single vector tools via conjugation in a microbial community.7
For their studies, the authors adapted a Vibrio cholerae CAST system6 (called VcDART - DNA-editing-all-in-one RNA-guided CRISPR-Cas Transposase) with negligible off-target insertions. Barcoded transposons enabled the facile tracking using ET-Seq of CRISPR-Cas-guided transposition into the genome of an editable microorganism without the need for selection. The authors generated loss-of-function and gain-of-function mutants in specific targeted organisms within a mock community through locus-specific DNA integration.
In a proof-of-concept experiment, the authors used VcDART to deliver antibiotic resistance genes or lactose-assimilation genes (lacY and lacZ) to a specific member (Pseudomonas simiae) of a synthetic soil community. By culturing the synthetic soil community containing this P. simiae mutant on media with antibiotics or with lactose as the sole carbon source, the authors could significantly enrich the relative abundance of P. simiae within the community. The VcDART methodology was also successfully applied for strain-specific enrichment of specific Escherichia coli strains identified by ET-Seq within an infant gut microbiota sample. Within this community, two candidate strains of E. coli were modified by VcDART-mediated integration of antibiotic markers at unique loci, followed by enrichment via antibiotic selection. Consequently, the modified E. coli became the dominant strains within the community, facilitating the successful assembly of their genomes—a feat that was not possible from the pre-edit community.
As with all new techniques, the establishment of new capabilities also brings new challenges. The current detection limits of ET-Seq may make its applicability to low-abundance organisms in communities challenging. The precision of the various editing events is dependent on the CRISPR-Cas transposase, but the accuracy of the VcDART system is a strength. Nevertheless, ET-Seq combined with VcDART, even in its current form, makes possible several key advances in furthering our understanding of microbial communities and allowing for rational engineering of them.
The most immediate application of this workflow would be the enrichment of specific members from a microbial community for metagenomic applications through targeted integration of selection factors. This could be used to enrich specific organisms present at low abundance in a diverse community or simply to edit microbes that have no plasmid tools. The ability to make targeted edits to a specific strain without significantly disrupting other unedited members will be particularly useful. The organism-specific targeting methodology opens up the possibility for systematically characterizing complex interactions between microbiome members (such as quorum sensing networks) in a near-native setting.
Overall, ET-seq combined with VcDART offers a range of new capabilities, greatly improving the accessibility of potentially underappreciated microbes in their native contexts.
Author Disclosure Statement
J.B.-D. is a scientific advisory board member of SNIPR Biome and Excision Biotherapeutics and a scientific advisory board member and co-founder of Acrigen Biosciences. The Bondy-Denomy lab receives research support from Felix Biotechnology. The remaining authors have no disclosures.
Funding Information
CRISPR-Cas studies in the Bondy-Denomy lab (J.B.-D). are supported by the University of California, San Francisco, Program for Breakthrough Biomedical Research funded in part by the Sandler Foundation, the National Institutes of Health (R01GM127489), and the Innovative Genomics Institute.
References
- 1. Sheth RU, Cabral V, Chen SP, et al. Manipulating bacterial communities by in situ microbiome engineering. Trends Genet 2016;32:189–200. DOI: 10.1016/j.tig.2016.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rubin BE, Diamond S, Cress BF, et al. Species- and site-specific genome editing in complex bacterial communities. Nat Microbiol 2022;7:34–47. DOI: 10.1038/s41564-021-01014-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Peters JE, Makarova KS, Shmakov S, et al. Recruitment of CRISPR-Cas systems by Tn7-like transposons. Proc Natl Acad Sci U S A 2017;114:E7358–E7366. DOI: 10.1073/pnas.1709035114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Faure G, Shmakov SA, Yan WX, et al. CRISPR-Cas in mobile genetic elements: counter-defence and beyond. Nat Rev Microbiol 2019;17:513–525. DOI: 10.1038/s41579-019-0204-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Strecker J, Ladha A, Gardner Z, et al. RNA-guided DNA insertion with CRISPR-associated transposases. Science 2019;365:48–53. DOI: 10.1126/science.aax9181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Klompe SE, Vo PLH, Halpin-Healy TS, et al. Transposon-encoded CRISPR-Cas systems direct RNA-guided DNA integration. Nature 2019;571:219–225. DOI: 10.1038/s41586-019-1323-z. [DOI] [PubMed] [Google Scholar]
- 7. Vo PLH, Ronda C, Klompe SE, et al. CRISPR RNA-guided integrases for high-efficiency, multiplexed bacterial genome engineering. Nat Biotechnol 2021;39:480–489. DOI: 10.1038/s41587-020-00745-y. [DOI] [PMC free article] [PubMed] [Google Scholar]