Abstract
As the most commonly used fluoroquinolone in the United States since 1987, ciprofloxacin has exerted the greatest selective pressure on S. pneumoniae and provides a valuable marker to evaluate the actual and potential emergence of fluoroquinolone resistance in this species. Analysis of susceptibility results obtained with 5,640 strains collected from throughout the United States showed that only 16 (0.3%) of the isolates demonstrated MICs of ≥4 μg/ml. The prevalence of this phenotype was significantly higher (P < 0.05) among penicillin-resistant populations, among isolates from patients >64 years old, and among respiratory isolates. However, >99% of strains had MICs of <4 μg/ml regardless of the risk group examined, and the MIC population distributions were the same for each risk group. These findings demonstrate that the phenotype of a MIC of ≥4 μg/ml remains uncommon after 10 years of ciprofloxacin use; however, these findings are no reason to become complacent with regard to appropriate use of fluoroquinolones and the need to carefully track resistance trends. Equally important is careful analysis of data that result from surveillance in terms of risk factors and other associated trends so that resistance and susceptibility, and their consequences, are neither over- nor underestimated.
The recent targeting of fluoroquinolones for respiratory infections has raised concerns about hastened development and dissemination of resistance among Streptococcus pneumoniae populations (3, 6, 12, 18, 21–24). A decline in the susceptibility of this species to fluoroquinolones would be especially problematic given the rising levels of resistance of pneumococci to several other antimicrobial agents (5, 22). Because ciprofloxacin is the fluoroquinolone with the longest history of clinical use in the United States (since 1987), it likely has exerted the greatest selective pressure on S. pneumoniae and provides a valuable marker to evaluate the actual and potential emergence of fluoroquinolone resistance in this species (2, 20). Although two recent reports suggest that ciprofloxacin resistance among S. pneumoniae strains may be increasing in other countries (4, 7), no such systematic evaluation of ciprofloxacin activity and the factors associated with reduced susceptibility has been done in the United States.
The analysis of current ciprofloxacin activity was done using antimicrobial susceptibility testing results obtained with 5,640 isolates collected from 377 geographically distributed U.S. hospitals during the 1997 to 1998 respiratory season. Susceptibility results were generated using broth microdilution panels (Trek Diagnostics, Inc., Westlake, Ohio) according to the recommended procedures and interpretive criteria of the National Committee for Clinical Laboratory Standards (NCCLS) (15). The relationships between ciprofloxacin activity and the penicillin susceptibility status of isolates, patient age groups, and specimen sources were statistically analyzed using chi-square analysis and, when expected numbers were low, Fisher's exact test (one-sided). Because ciprofloxacin NCCLS interpretive categories do not exist for S. pneumoniae, we used the MIC of ≥4 μg/ml used by others as the criterion for categorizing isolates as having reduced ciprofloxacin susceptibility (4).
(This work was presented in part at the 38th Interscience Conference on Antimicrobial Agents and Chemotherapy, 1998, San Diego, Calif. [M. L. Hickey, C. Thornsberry, D. R. Diakun, S. V. Mani, and D. F. Sahm, Abstr. 38th Conf. Antimicrob. Agents Chemother., abstr. E-20, p. 172, 1998]).
Of the 5,640 isolates tested, 14% were resistant to penicillin and 4% were resistant to ceftriaxone, whereas 33% were resistant to trimethoprim-sulfamethoxazole (SXT) (Table 1). Resistance rates for azithromycin, clarithromycin, and erythromycin were 21, 23, and 24%, respectively. For all β-lactams, macrolides, and SXT, the prevalence of resistant isolates was higher among penicillin-intermediate and -resistant populations.
TABLE 1.
Susceptibility of S. pneumoniae to ciprofloxacin and other antimicrobial agents
| Antimicrobial agent and phenotypea | No. of isolates | MIC (μg/ml)
|
% of isolates that wereb:
|
||||
|---|---|---|---|---|---|---|---|
| Range | 50% | 90% | S | I | R | ||
| Penicillin | |||||||
| All | 5,640 | ≤0.03–>8 | ≤0.03 | 2.0 | 63.9 | 22.5 | 13.7 |
| Pen S | 3,603 | ≤0.03–0.06 | ≤0.03 | 0.06 | 100.0 | 0.0 | 0.0 |
| Pen I | 1,267 | 0.12–1 | 0.5 | 1.0 | 0.0 | 100.0 | 0.0 |
| Pen R | 770 | 2–>8 | 2.0 | 4.0 | 0.0 | 0.0 | 100.0 |
| Amoxicillin-clavulanate | |||||||
| All | 5,640 | ≤0.015–>16 | 0.03 | 1.0 | 81.5 | 9.3 | 9.2 |
| Pen S | 3,603 | ≤0.015–0.5 | ≤0.015 | 0.03 | 100.0 | 0.0 | 0.0 |
| Pen I | 1,267 | ≤0.015–>16 | 0.25 | 1.0 | 76.1 | 19.7 | 4.2 |
| Pen R | 770 | 0.26–>16 | 2.0 | 4.0 | 4.2 | 35.6 | 60.3 |
| Cefuroxime | |||||||
| All | 5,640 | ≤0.12–64 | ≤0.12 | 4.0 | 71.9 | 3.4 | 24.8 |
| Pen S | 3,603 | ≤0.12–1 | ≤0.12 | ≤0.12 | 100.0 | 0.0 | 0.0 |
| Pen I | 1,267 | ≤0.12–16 | 1.0 | 4.0 | 35.5 | 14.8 | 49.7 |
| Pen R | 770 | ≤0.12–64 | 4.0 | 8.0 | 0.3 | 0.1 | 99.6 |
| Ceftriaxone | |||||||
| All | 5,640 | ≤0.015–8 | 0.03 | 1.0 | 84.6 | 11.2 | 4.1 |
| Pen S | 3,603 | ≤0.015–0.5 | 0.03 | 0.06 | 100.0 | 0.0 | 0.0 |
| Pen I | 1,267 | ≤0.015–8 | 0.25 | 1.0 | 84.0 | 13.4 | 2.6 |
| Pen R | 770 | ≤0.015–8 | 1.0 | 2.0 | 13.9 | 60.3 | 25.8 |
| Clarithromycin | |||||||
| All | 5,640 | ≤0.015–>32 | 0.03 | 4.0 | 75.9 | 0.9 | 23.2 |
| Pen S | 3,603 | ≤0.015–>32 | 0.03 | 0.03 | 93.9 | 0.4 | 5.7 |
| Pen I | 1,267 | ≤0.015–>32 | 0.03 | 32 | 54.2 | 2.1 | 43.7 |
| Pen R | 770 | ≤0.015–>32 | 2.0 | >32 | 27.5 | 1.4 | 71.0 |
| Erythromycin | |||||||
| All | 5,640 | ≤0.03–>4 | ≤0.03 | 4.0 | 75.9 | 0.1 | 24.0 |
| Pen S | 3,603 | ≤0.03–>4 | ≤0.03 | 0.06 | 93.9 | 0.0 | 6.1 |
| Pen I | 1,267 | ≤0.03–>4 | 0.06 | >4 | 54.1 | 0.5 | 45.5 |
| Pen R | 770 | ≤0.03–>4 | 4.0 | >4 | 27.5 | 0.1 | 72.3 |
| SXT | |||||||
| All | 5,640 | ≤0.015–>4 | 0.12 | 4.0 | 67.1 | 16.5 | 16.4 |
| Pen S | 3,603 | ≤0.015–>4 | 0.12 | 1.0 | 89.5 | 4.9 | 5.6 |
| Pen I | 1,267 | ≤0.015–>4 | 2.0 | 4.0 | 40.2 | 35.3 | 24.5 |
| Pen R | 770 | 0.06–>4 | 4.0 | >4 | 6.6 | 39.9 | 53.5 |
| Ciprofloxacin | |||||||
| All | 5,640 | ≤0.002–>8 | 0.5 | 1.0 | 99.7 | 0.3 | |
| Pen S | 3,603 | ≤0.002–8 | 0.5 | 1.0 | 99.9 | 0.1 | |
| Pen I | 1,267 | 0.015–>8 | 0.5 | 1.0 | 99.5 | 0.5 | |
| Pen R | 770 | 0.015–>8 | 1.0 | 1.0 | 99.2 | 0.8 | |
Analysis for all isolates, penicillin-susceptible (Pen S) isolates, penicillin-intermediate (Pen I) isolates, and penicillin-resistant (Pen R) isolates.
Percentages of susceptible (S), intermediate (I), and resistant (R) isolates according to NCCLS breakpoints. NCCLS breakpoints are not available for the fluoroquinolone ciprofloxacin, so a MIC of ≥4 μg/ml was used as the marker for reduced susceptibility.
The prevalence of isolates with reduced susceptibility to ciprofloxacin (MIC ≥4 μg/ml) was 0.3% (16 of 5,640 isolates), and, regardless of the penicillin susceptibility status of the isolates, ciprofloxacin MICs at which 90% of the isolates were inhibited (MIC90s) remained the same (1 μg/ml) (Table 1). The 16 isolates with an MIC of ≥4 μg/ml were obtained from 15 different institutions. The 0.3% prevalence is nearly six times lower than that (1.7%) reported by Chen et al. (4) in their 1998 study of Canadian isolates but is consistent with results of a recently published report in which none of the 1,476 U.S. isolates tested had ciprofloxacin MICs of >4 μg/ml (9). Whether the difference between Canada and the United States in prevalence of strains with MICs of >4 μg/ml results from differences in prescribing behaviors, intrinsic characteristics of the bacterial populations studied, or sampling differences between the two surveillance studies is unknown but merits further investigation. In any case, these differences underscore the need for conducting national and regional surveillance and highlight the importance of avoiding extrapolating one nation's experience to other nations.
Although the prevalence of isolates with reduced susceptibility in this U.S. study was substantially lower than that reported from Canada, similarities with the findings of Chen et al. (4) were noted when results were analyzed using the phenotype of a ciprofloxacin MIC of ≥4 μg/ml to mark reduced susceptibility (Table 2). As with Canadian strains, this phenotype among U.S. strains was statistically associated with penicillin resistance, patient age of >64 years, and respiratory source of the isolate. By patient age group, prevalence ranged from 0 (<15 years) to 0.6% (>64 years). Similarly, a small but significant increase in the prevalence of reduced susceptibility occurred among penicillin-intermediate (0.5%) and penicillin-resistant (0.8%) populations compared with penicillin-susceptible (0.1%) populations. Also, there was a significantly higher prevalence of the phenotype among respiratory isolates (0.5%) than among blood isolates (0.1%). No significant differences in the prevalence of reduced ciprofloxacin susceptibility associated with geographic region or hospital size (i.e., number of beds) were noted (data not shown).
TABLE 2.
Reduced susceptibility to ciprofloxacin (MIC ≥4 μg/ml) and erythromycin resistance among S. pneumoniae isolates according to risk factor
| Risk factor | No. of isolates | No. of isolates (%) withc:
|
|
|---|---|---|---|
| Reduced ciprofloxacin susceptibilitya | Erythromycin resistance | ||
| Patient age (yrs) | |||
| <15 | 1,577 | 0 (0.0) | 474 (30.1)** |
| 15–64b | 2,230 | 5 (0.2) | 462 (20.7) |
| >64 | 1,833 | 11 (0.6)* | 416 (22.7) |
| Penicillin category | |||
| Susceptibleb | 3,603 | 4 (0.1) | 219 (6.1) |
| Intermediate | 1,267 | 6 (0.5)* | 576 (45.5)** |
| Resistant | 770 | 6 (0.8)* | 557 (72.3)** |
| Specimen source | |||
| Blood | 1,641 | 2 (0.1) | 279 (17.0) |
| Respiratory | 2,668 | 12 (0.5)* | 668 (25.1)** |
| Other | 1,331 | 2 (0.2) | 405 (30.4)** |
Ciprofloxacin MIC of ≥4 μg/ml.
Referent group.
∗, difference from reference group is significant (P < 0.05); ∗∗, difference from reference group is highly significant (P < 0.0001).
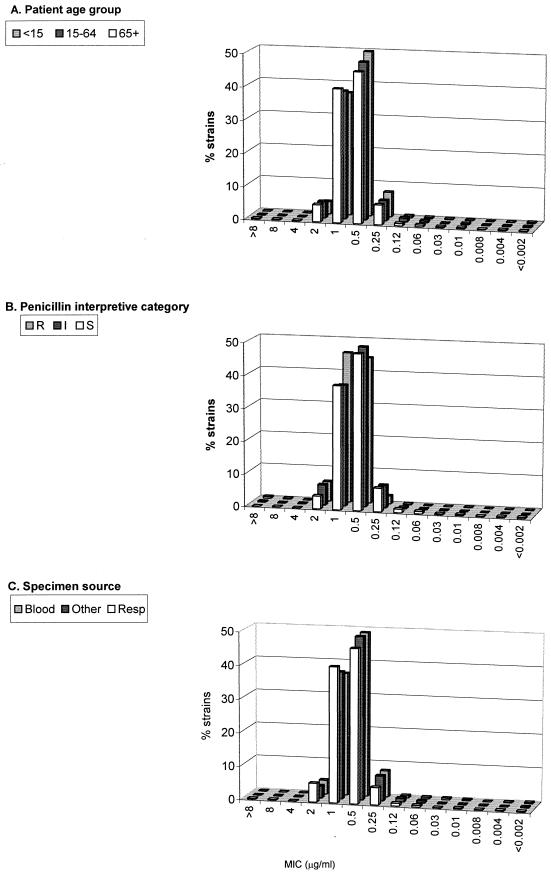
While these statistical associations can be made when analysis is based on the reduced ciprofloxacin susceptibility phenotype of a MIC of ≥4 μg/ml, several points regarding the perspective and context of these findings must be considered. First, even in each of the “high-risk” categories (patient age of >64 years, penicillin resistance, and respiratory isolates), >99% of the isolates had ciprofloxacin MICs of <4 μg/ml (Table 2). Second, in terms of relative resistance, the same analysis for erythromycin showed that correlations between macrolide resistance and patient age, penicillin status of the isolates, and source of isolates were all highly significant (P < 0.0001) and that the percentages of isolates resistant to erythromycin were orders of magnitude higher than the percentages of isolates with the phenotype of a ciprofloxacin MIC of ≥4 μg/ml (Table 2). Finally, tracking ciprofloxacin activity according to MIC distributions rather than by using a phenotypic breakpoint of 4 μg/ml revealed that no discernible population shifts occurred within any of the risk groups of patient age, penicillin susceptibility status, or specimen source (Fig. 1). Further, the MIC distributions (e.g., MIC50 and MIC90 of 0.5 and 1 μg/ml, respectively) were the same for strains tested in this study as those reported in previous studies with strains isolated between 1995 and 1997 (22, 23).
FIG. 1.
Ciprofloxacin MIC distributions for S. pneumoniae according to patient age group, penicillin interpretive category, and specimen source. R, resistant; I, intermediate, S, susceptible; resp, respiratory.
More than 93 million ciprofloxacin prescriptions were written between 1990 and 1998, and the number of annual prescriptions has increased an average of 9% per year since 1990, so that the number of prescriptions written in 1998 (12,897,000) was 73% higher than the number written in 1990 (7,457,000; MS Health, National Prescription Audit Plus). Further, the pharmacokinetic and pharmacodynamic properties of ciprofloxacin are such that, regardless of the route of administration, low levels of the agent (0.04 to 1.6 μg/ml) appear in nasal secretions and saliva (1, 8, 19). Therefore, regardless of site of infection or bacterial species being targeted for eradication by ciprofloxacin use, organisms such as S. pneumoniae that colonize the upper respiratory tract are exposed. This selective pressure, coupled with the ability of pneumococci to acquire resistance to fluoroquinolones through mutations in gyrase and topoisomerase genes and by efflux mechanisms, provides ample opportunity for fluoroquinolone resistance to expand among pneumococcal populations in the United States (10, 11, 13, 14, 16, 17).
With these conditions in mind, the findings of this U.S. study are noteworthy in two respects. First, based on the paucity of strains with MICs of ≥4 μg/ml, ciprofloxacin activity against S. pneumoniae isolates from the United States appears to be relatively stable. Second, regardless of the risk factor examined, >99% of strains had ciprofloxacin MICs of <4 μg/ml, and among 174 isolates for which all three significant risk factors were met (i.e., penicillin-resistant respiratory isolates from patients >64 years old) only three isolates (1.7%) exhibited reduced ciprofloxacin susceptibility. In addition, no shifts in MIC distribution were associated with any of the risk factors studied. Therefore, while statistical relationships between the phenotype of a MIC of ≥4 μg/ml and certain risk factors can be made, the overall significance of this association must be kept in appropriate medical and microbiological perspective.
Because pneumococci have the capability of developing fluoroquinolone resistance, the current status of ciprofloxacin is no reason to become complacent with regard to appropriate use of fluoroquinolones and the need to carefully track resistance trends by both categorical and MIC distributions. This is an especially important caution given the ongoing development and release of fluoroquinolones for respiratory infections. Equally important is careful analysis of data that result from surveillance in terms of risk factors and other associated trends so that resistance and susceptibility, and their consequences, are neither over- nor underestimated.
Acknowledgments
This study was supported by Bayer Corp. (West Haven, Conn.).
We thank Geriann Piazza for editing the manuscript.
REFERENCES
- 1.Adler D, Maier H. Gyrase inhibitor ciprofloxacin in human parotid saliva. J Clin Chem Clin Biochem. 1989;27:232–233. [PubMed] [Google Scholar]
- 2.Arcieri G, Griffith E, Gruenwaldt G, Heyd A, O'Brien B, Screen P, Becker N, August R. A survey of clinical experience with ciprofloxacin, a new quinolone antimicrobial. J Clin Pharmacol. 1988;28:179–189. doi: 10.1002/j.1552-4604.1988.tb05741.x. [DOI] [PubMed] [Google Scholar]
- 3.Brueggemann A B, Kugler K C, Doern G V. In vitro activity of BAY 12-8039, a novel 8-methoxyquinolone, compared to activities of six fluoroquinolones against Streptococcus pneumoniae, Haemophilus pneumoniae, and Moraxella catarrhalis. Antimicrob Agents Chemother. 1997;41:1594–1597. doi: 10.1128/aac.41.7.1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen D K, McGeer A, De Azavedo J C, Low D E. Decreased susceptibility of Streptococcus pneumoniae to fluoroquinolones in Canada. N Engl J Med. 1999;341:233–239. doi: 10.1056/NEJM199907223410403. [DOI] [PubMed] [Google Scholar]
- 5.Doern G V, Pfaller M A, Kugler K, Freeman J, Jones R N. Prevalence of antimicrobial resistance among respiratory tract isolates of Streptococcus pneumoniae in North America: 1997 results from the SENTRY Antimicrobial Surveillance Program. Clin Infect Dis. 1998;27:764–770. doi: 10.1086/514953. [DOI] [PubMed] [Google Scholar]
- 6.Felmingham D, Robbins M J, Tesfaslasie Y, Harding I, Shrimpton S, Gruneberg R N. Antimicrobial susceptibility of community-acquired lower respiratory tract bacterial pathogens isolated in the UK during the 1995–1996 cold season. J Antimicrob Chemother. 1998;41:411–415. doi: 10.1093/jac/41.3.411. [DOI] [PubMed] [Google Scholar]
- 7.Goldsmith C E, Moore J E, Murphy P G, Ambler J E. Increased incidence of ciprofloxacin resistance in penicillin-resistant pneumococci in Northern Ireland. J Antimicrob Chemother. 1998;41:420–421. doi: 10.1093/jac/41.3.420. [DOI] [PubMed] [Google Scholar]
- 8.Gonzalez M A, Uribe F, Moisen S D, Fuster A P, Selen A, Welling P G, Painter B. Multiple-dose pharmacokinetics and safety of ciprofloxacin in normal volunteers. Antimicrob Agents Chemother. 1984;26:741–744. doi: 10.1128/aac.26.5.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jacobs M R, Bajaksouzian S, Zilles A, Lin G, Pankuch G A, Appelbaum P C. Susceptibilities of Streptococcus pneumoniae and Haemophilus influenzae to 10 oral antimicrobial agents based on pharmacodynamic parameters: 1997 U.S. Surveillance Study. Antimicrob Agents Chemother. 1999;43:1901–1908. doi: 10.1128/aac.43.8.1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Janoir C, Zeller V, Kitzis M D, Moreau N J, Gutmann L. High-level fluoroquinolone resistance in Streptococcus pneumoniae requires mutations in parC and gyrA. Antimicrob Agents Chemother. 1996;40:2760–2764. doi: 10.1128/aac.40.12.2760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jorgensen J H, Weigel L M, Ferraro M J, Swenson J M, Tenover F C. Activities of newer fluoroquinolones against Streptococcus pneumoniae clinical isolates including those with mutations in the gyrA, parC, and parE loci. Antimicrob Agents Chemother. 1999;43:329–334. doi: 10.1128/aac.43.2.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Klugman K P, Gootz T D. In vitro and in vivo activity of trovafloxacin against Streptococcus pneumoniae. J Antimicrob Chemother. 1997;39(Suppl. B):51–55. doi: 10.1093/jac/39.suppl_2.51. [DOI] [PubMed] [Google Scholar]
- 13.Martinez J L, Alonsa A, Gomez-Gomez J M, Baquero F. Quinolone resistance by mutations in chromosomal gyrase genes. Just the tip of the iceberg? J Antimicrob Chemother. 1998;42:683–688. doi: 10.1093/jac/42.6.683. [DOI] [PubMed] [Google Scholar]
- 14.Munoz R, De La Campa A G. ParC subunit of DNA topoisomerase IV of Streptococcus pneumoniae is a primary target of fluoroquinolones and cooperates with DNA gyrase A subunit in forming resistance phenotype. Antimicrob Agents Chemother. 1996;40:2252–2257. doi: 10.1128/aac.40.10.2252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.National Committee for Clinical Laboratory Standards. Performance standards for antimicrobial susceptibility testing, 9th informational supplement. Approved standard M100-S9. Wayne, Pa: National Committee for Clinical Laboratory Standards; 1999. [Google Scholar]
- 16.Pan X-S, Fisher L M. Cloning and characterization of the parC and parE genes of Streptococcus pneumoniae encoding DNA topoisomerase IV: role in fluoroquinolone resistance. J Bacteriol. 1996;178:4060–4069. doi: 10.1128/jb.178.14.4060-4069.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pan X-S, Fisher L M. DNA gyrase and topoisomerase IV are dual targets of clinafloxacin action in Streptococcus pneumoniae. Antimicrob Agents Chemother. 1998;42:2810–2816. doi: 10.1128/aac.42.11.2810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pankuch G A, Jacobs M R, Appelbaum P C. Activity of CP99,219 compared with DU-6859a, ciprofloxacin, ofloxacin, levofloxacin, lomefloxacin, tosufloxacin, sparfloxacin and grepafloxacin against penicillin-susceptible and -resistant pneumococci. J Antimicrob Chemother. 1995;35:230–232. doi: 10.1093/jac/35.1.230. [DOI] [PubMed] [Google Scholar]
- 19.Piercy E A, Bawdon R E, Mackowiak P A. Penetration of ciprofloxacin into saliva and nasal secretions and effect of the drug on the oropharyngeal flora of ill subjects. Antimicrob Agents Chemother. 1989;33:1645–1646. doi: 10.1128/aac.33.9.1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thomson C J. The global epidemiology of resistance to ciprofloxacin and the changing nature of antibiotic resistance: a 10 year perspective. J Antimicrob Chemother. 1999;43(Suppl. A):31–40. doi: 10.1093/jac/43.suppl_1.31. [DOI] [PubMed] [Google Scholar]
- 21.Thornsberry C, Jones M E, Hickey M L, Mauriz Y, Kahn J, Sahm D F. Resistance surveillance of Streptococcus pneumoniae, Haemophilus influenzae and Moraxella catarrhalis isolated in the United States, 1997–1998. J Antimicrob Chemother. 1999;44:749–759. doi: 10.1093/jac/44.6.749. [DOI] [PubMed] [Google Scholar]
- 22.Thornsberry C, Ogilvie P T, Holley H P, Jr, Sahm D F. In vitro activity of grepafloxacin and 25 other antimicrobial agents against Streptococcus pneumoniae: correlation with penicillin resistance. Clin Ther. 1998;20:1179–1190. doi: 10.1016/s0149-2918(98)80113-6. [DOI] [PubMed] [Google Scholar]
- 23.Thornsberry C, Ogilvie P T, Holley H P, Jr, Sahm D F. Survey of susceptibilities of Streptococcus pneumoniae, Haemophilus influenzae, and Moraxella catarrhalis isolates to 26 antimicrobial agents: a prospective U.S. study. Antimicrob Agents Chemother. 1999;43:2612–2623. doi: 10.1128/aac.43.11.2612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Visalli M A, Jacobs M R, Appelbaum P C. Anti-pneumococcal activity of BAY 12-8039, a new quinolone, compared with activities of three other quinolones and four oral β-lactams. Antimicrob Agents Chemother. 1997;41:2786–2789. doi: 10.1128/aac.41.12.2786. [DOI] [PMC free article] [PubMed] [Google Scholar]