Introduction
Until recent years, Candida albicans had fundamentally been linked to cancer as an opportunistic pathogen that takes advantage of an immunosuppressed state [1]. However, there is a growing body of evidence that this dimorphic fungal species may be capable of promoting cancer [2]. C. albicans is a normal commensal of the human body and therefore does not induce damage. However, as an opportunistic pathogen, C. albicans is capable of becoming pathogenic when the host defenses are weakened, causing an array of infections ranging from mucosal to systemic [1]. Oral candidiasis, commonly known as “thrush,” is one of the most common infections of the oral cavity characterized by fungal overgrowth and infiltration of superficial tissues involving the tongue and other oral mucosal sites. Among the spectrum of oral mucosal lesions associated with Candida, chronic hyperplastic candidiasis, also known as candidal leukoplakia, has been associated with the risk of malignant transformation to oral cancer [1,3]. The association between Candida and oral cancer has traditionally been a subject of debate, and many mechanisms of potential interactions between this fungal pathogen and oral carcinogenesis have been described [4]. Mounting evidence has supported a correlation between Candida infection and development of oral epithelial dysplasia [5], a spectrum of histopathological changes that affect the epithelial lining of the oral mucosae displaying increased risk of progression to oral squamous cell carcinoma (OSCC) or oral cancer [3]. In this article, we review prior research directly or indirectly linking Candida and oral cancer (Table 1) and posit that candidiasis may not just be randomly coexisting with oral cancer, but the pathogenetic relationship is also a dominant scenario, including the possibility that C. albicans may initiate or facilitate the development of oral cancer. Further, we describe the main proposed mechanisms by which this yeast species may induce cancer and highlight the need for further future mechanistic studies in oral carcinogenesis models to establish C. albicans as an opportunistic oncogenic pathogen.
Table 1. Overview of select descriptive and mechanistic evidence that directly or indirectly highlight the potential roles of Candida in oral carcinogenesis.
| Year | First author(s) | Type of study | Key experimental approaches | Main findings |
|---|---|---|---|---|
| 1987 | Krogh [13] | Descriptive | Liquid and gas chromatography in yeast isolated from oral premalignancy patients and healthy participants | Elevated Candida albicans strains with nitrosation potential in oral premalignancy |
| 1992 | O’Grady [21] | Mechanistic | 4NQO rat model with Candida coinfection | Candida promotes 4NQO-induced oral carcinogenesis |
| 2002 | McCullough [5] | Descriptive | Oral swish and culture of Candida in oral premalignancy and cancer as well as controls | Increased frequency of oral yeast carriage and colony-forming units in patients with oral epithelial dysplasia and cancer compared with controls |
| 2009 | Dwivedi [22] | Mechanistic | 4NQO mouse model with Candida coinfection | Validation of oral cancer promoting roles of Candida in 4NQO mouse model |
| 2013 | Hebbar [6] | Descriptive | Histology (PAS stain) and oral swish with culture from oral potentially malignant disorders and oral cancer | Presence of Candida hyphae correlates with severity of dysplastic epithelial changes |
| 2015 | Alnuaimi [9] | Descriptive | Isolation of oral yeast and genetic identification with RT-PCR from OSCC versus control patients |
C. albicans is an independent risk factor for oral cancer development Combination with alcohol generates a higher risk C. albicans genotype A predominates in oral cancer |
| 2016 | Alnuaimi [19] | Descriptive | Crystal violet staining/XTT salt reduction assays, agar plate enzyme detection method, and gas chromatography in Candida isolated from OSCC patients and healthy controls | Increased biofilm mass, metabolic activity, high phospholipase, and acetaldehyde production by Candida in oral cancer |
| 2019 | Roy [8] | Descriptive | CHROMagar assay in Candida isolated from oral premalignancy and cancer patients as well as healthy controls | Dysbiosis of mycobiome with emergence of Candida krusei, Candida glabrata, and Candida tropicalis, increasing in patients with dysplastic lesions or OSCC |
| 2019 | Ho [35] | Mechanistic | Diverse comprehensive approaches in cell cultures, murine, and zebrafish models | Virulence factor “candidalysin” activates molecular pathways that have been implicated in carcinogenesis (MAPK pathway and activation of immune responses) in an EGFR-related manner |
| 2021 | Break and Oikonomou [31] | Mechanistic | Comprehensive experimental approaches primarily in mouse models (including Aire-deficient mice), as well as cell cultures, and APECED patients | Hyperactivation of type 1 immune responses leading to epithelial destruction and subsequent Candida superinfection is seen in APECED syndrome, a disease which correlates with uncommon OSCC development |
| 2022 | Vadovic [23] | Mechanistic | Multiple techniques in cell lines, OSCC xenograft mouse model, and 4NQO mouse model | Candida induces increased migration, expression of matrix metalloproteinases, activation of epithelial to mesenchymal transition, and expression of genes implicated in metastatic processes by OSCC cells. The tumor promoting roles of Candida in a 4NQO model were also highlighted |
4NQO, 4-nitroquinoline-1-oxide; APECED, autoimmune polyendocrinopathy–candidiasis–ectodermal dystrophy; EGFR, epidermal growth factor receptor; OSCC, oral squamous cell carcinoma; RT-PCR, real-time PCR.
Candida colonization is correlated with oral premalignancy
The earliest studies investigating a potential role for oral yeast in oral carcinogenesis were mostly descriptive, relying on assessing the relative frequencies of fungal species in oral premalignancy and cancer. However, although correlative, these studies have undoubtedly demonstrated increased Candida colonization, as the epithelial lining of the oral mucosa alters from normal to dysplastic epithelium [5,6]. Moreover, Candida recovery from the oral cavity of patients with oral epithelial dysplasia [5] and the presence of hyphae in tissue sections [6] correlated with the severity of dysplastic changes. Further, sequencing techniques revealed alterations in the relative frequencies of the constituents of the oral mycobiome (fungal biome) as a whole [7]. Interestingly, differences in diversity with an abundance of less common non-albicans species have been observed in patients with dysplastic lesions or OSCC [8]. In the context of established oral cancer, C. albicans is considered a risk factor for oral carcinogenesis, while the combination of candidiasis with alcohol drinking generated the highest risk [9]. However, despite the copiousness of incidental evidence, the potential contribution of fungi to oral carcinogenesis remains a debatable subject.
Initiator or facilitator?
Although mechanisms by which bacteria and viruses stimulate cancer development are well investigated, very few studies have explored the role of fungi in this context. Neoplastic processes affecting other organs have been investigated [10], including pancreatic cancer induced by Malassezia genus [11]. However, conversely, the same fungus was shown to correlate with favorable prognosis [12] in oral cancer, highlighting the complex and organ-specific oncogenicity of mycobiome dysbiosis. Within the framework of oral cancer, C. albicans has primarily been studied in the context of being an “initiator” of carcinogenesis. Many possible mechanisms of diverse etiology have been implicated and are summarized in Fig 1. The most widely accepted hypothesis regarding the carcinogenic effect of Candida on the mucosal epithelium is related to the production of carcinogens such as nitrosamine [13] and acetaldehyde (a mutagenic compound that is indisputably carcinogenic) [2,14,15]. Acetaldehyde is the first metabolite of ethanol catabolism in epithelial cells and C. albicans. In the oral cavity, acetaldehyde produces DNA and protein adducts that interfere with normal DNA replication causing point mutations and chromosomal aberrations [16]. Further, acetaldehyde also affects enzymes involved in DNA repair and binds to the essential antioxidant glutathione, indirectly increasing the presence of reactive oxygen species (ROS), which are related to an increase in DNA damage. Mitochondrial damage is also induced by acetaldehyde, increasing ROS production [17,18]. In fact, Candida was shown to display increased metabolic activity and acetaldehyde production in oral cancer compared to healthy controls, reinforcing its potential carcinogenic role [19]. The possible oncogenic effects of Candida strains have been considered to be significantly affected by polymicrobial interactions, and other constituents of the microbiome seem to act antagonistically or synergistically during Candida-related oral carcinogenesis [20].
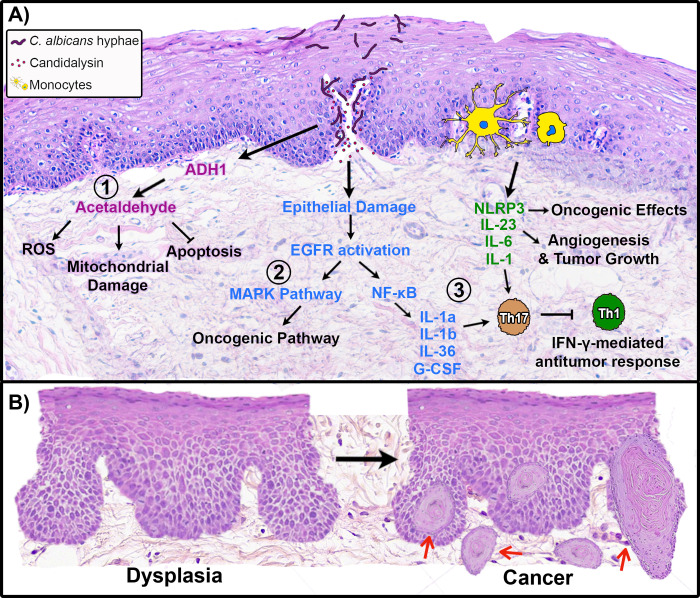
Fig 1. Mechanisms by which Candida albicans may play a role in oral cancer development.
(A) (1) Production of carcinogenic substances. C. albicans, using the enzyme ADH1, is capable of metabolizing alcohol to acetaldehyde, which is carcinogenic. Acetaldehyde binds to proteins and DNA modifying their structure and functionality, resulting in mitochondrial damage, and also reducing antioxidant activity of glutathione leading to increased intracellular levels of ROS. These alterations may produce genome instability linked with inhibition of the apoptotic machinery ultimately leading to tumor development. (2) Activation of oncogenic pathways in epithelial cells by candidalysin. C. albicans secrete candidalysin toxin that damages the epithelial barrier and activates EGFR with downstream up-regulation of the MAPK pathway that has been implicated in various types of cancer. (3) Induction of tumor-promoting immunity. EGFR activation also causes downstream up-regulation of the NFκΒ pathway in epithelial cells resulting in the expression of IL-1a, IL-1b, IL-36, and G-CSF. Myeloid cells including antigen presenting cells and macrophages recognize Candida and secrete tumor promoting cytokines including IL-23, IL-6, and IL-1. Additionally, the NLRP3 inflammasome pathway is activated. Collectively, cytokines secreted by epithelial and myeloid cells result in activation of Th17 (IL-17 secreting) cells. Type 17 immune responses further support cancer progression by antagonizing Th1 (IFNγ secreting) cells. (B) At the oral mucosa, these tumor promoting mechanisms may have the potential of causing cytologic and architectural alterations in the oral epithelium (dysplasia), and their accumulation may lead to the development of OSCC, which is characterized by tumor islands (red arrows) invading the underlying connective tissue. ADH1, alcohol dehydrogenase 1; EGFR, epidermal growth factor receptor; G-CSF, granulocyte colony-stimulating factor; IFNγ, interferon gamma; IL, interleukin; NFκΒ, nuclear factor kappa B; OSCC, oral squamous cell carcinoma; ROS, reactive oxygen species; Th, T helper.
On the other hand, some studies described C. albicans as a promoter or “facilitator” of cancer development, rather than initiators; in one study, oral inoculation with C. albicans or administration of the carcinogen 4NQO [21] failed to cause dysplastic changes in animal models; however, in combination, oral epithelial dysplasia occured, indicating that C. albicans may have promoted dysplastic changes [21–23]. The potential tumor promoting roles of Candida were also confirmed by in vitro studies in oral cancer cells in which C. albicans increased the migration ability, expression of matrix metalloproteinases, secretion of oncometabolites, and expression of metastasis-related genes [23]. An underestimated alternative hypothesis is that oral candidiasis and a dysplastic epithelium are unrelated pathophysiologically or that they display the inverse cause-and-effect relationships, as Candida infections and premalignancy display common predisposing factors, most notably immunosuppression [24,25]. It is also important to consider that defective epithelium with destructed architecture (in the context of oral premalignancy or cancer) may enhance susceptibility to infections [26]. Nevertheless, despite the increasing evidence linking Candida with oral cancer, the largely descriptive nature of prior studies cannot reliably ascertain the underlying pathogenetic mechanisms that implicate Candida or the oral mycobiome in oral carcinogenesis.
Candida and immune dysfunction during oral carcinogenesis
The transition from normal oral epithelium to dysplasia and ultimately to OSCC (Fig 1B) is a multistep process and is multifactorial in its etiopathogenesis. Owing to this multifactorial process, studies have explored the interplay between tissue inflammation, immunity, and the tumor microenvironment on etiopathogenesis [27]. In the case of Candida the “initiator” scenario, infection of mucosal tissue generates epithelial barrier destruction activating type 17 immune responses [28]. T helper 17 cells, a subset of CD4 T-cells, produce interleukin (IL)-17, which is required for resistance against C. albicans; therefore, Th17 immunity is the dominant response against oral candidiasis. However, other cytokines of the Th17 family, such as IL-23, promote angiogenesis and tumor growth [29]. Moreover, type 17 responses antagonize IL-12 and interferon gamma (IFNγ), both of which are crucial in Th1-type antitumor immune responses [29]. In addition to its direct effect, IL-17 can also favor cancer processes indirectly by recruiting neutrophils. Although these leukocytes are the main effector cells against C. albicans, their presence in tumor tissues also correlates with poor prognosis in some types of cancer [2].
Alternatively, an established epithelial malignancy displaying reduced levels of T-cell inflammation [30] could also possess inadequate Th17 responses, resulting in susceptibility to candidiasis. Another possibility of secondary infection of OSCC as a result of deregulated immune responses is also supported by recent evidence indicating that hyperactivation of IFNγ-induced immunity may cause epithelial destruction with subsequent Candida infection during autoimmune polyendocrinopathy–candidiasis–ectodermal dystrophy syndrome, a disease that also correlates with oral cancer development [31]. The potential of autoimmune hyperactivation of T cells to drive subsequent increase of fungal burden at a malignant mucosal barrier has been previously shown in oncogenesis of the esophagus; however, interestingly in this context, the recruited fungal organisms were shown to futher promote esophageal cancer development [32]. Therefore, the question remains: Does Candida induce epithelial damage facilitating a tumor promoting microenvironment, or does a cancerized field with immune dysfunction drive secondary susceptibility to fungal infections? And regardless, what is the role of host immune responses during these interactions? Perhaps our best understanding of the complex Candida–epithelial barrier–host immunity axis came about with the discovery of candidalysin.
The newest toxin in town: Candidalysin and its oncogenic potential
A cytolytic peptide secreted by C. albicans hyphae capable of disrupting mucosal integrity [33] was recently added to the already impressive list of virulence factors in C. albicans’ armamentarium. Candidalysin, a product of the expression of the ECE1 gene [34], is considered a toxin and was shown to induce epithelial damage, activate the MAPK pathway, and induce secretion of inflammatory cytokines by epithelial cells [33], a process dependent on epidermal growth factor receptor (EGFR) signaling [35]. Additionally, candidalysin was also associated with downstream activation of type 17 immune responses [36], as well as the promotion of the NLRP3 inflammasome [37]. Given that EGFR aberrant expression and activation of the MAPK pathway [38] as well as NLRP3 [39] have been correlated with various epithelial and nonepithelial malignancies, this virulence factor was also associated with tumor-promoting immunity [40,41]. However, despite the significant indications that candidalysin may play a role in initiating or promoting oral carcinogenesis, there is no evidence that Candida strains that overexpress ECE1 predominate during oral cancer. Importantly, these hypotheses have not been confirmed by mechanistic studies in animal models.
Conclusions and future directions
To date, the exact role that the mycobiome and Candida in particular play in the pathogenesis of oral cancer has been a subject of disagreement. Although numerous studies have provided supporting evidence that Candida may initiate or promote oral epithelial oncogenesis, it is as likely that the increase in Candida colonization in precancerous dysplastic lesions is coincidental, as a result of an altered mucosal barrier that favors the proliferation of these common commensals. Therefore, there is a clear need for comprehensive experimental studies to confidently expound the role of Candida and other fungal species in oral carcinogenesis and provide mechanistic insights to deepen our understanding of the the pathogenesis of oral premalignancy and cancer with regard to its correlation to fungal dysbiosis. Regarding the tumor initiating or promoting effects of Candida, the possible implication of candidalysin in oral tumorigenesis should be validated by in vitro and in vivo approaches. On the other hand, the opposite scenario of passive colonization of oral premalignancy and OSCC by Candida has been minimally studied. Original research investigating whether epithelial changes during tumorigenesis (including altered surface receptor profile or defective intercellular communications) that may create a microenvironment that may facilitate Candida superinfection would also be beneficial to study. Importantly, consideration should be given to the possibility of new individualized therapeutic approaches including antifungal drugs concurrently with antitumor therapies, to minimize the risk of C. albicans and its effect in generating a protumor microenvironment.
Funding Statement
The authors received no specific funding for this work.
References
- 1.Vila T, Sultan AS, Montelongo-Jauregui D, Jabra-Rizk MA. Oral candidiasis: A disease of opportunity. J Fungi (Basel). 2020;6:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ramirez-Garcia A, Rementeria A, Aguirre-Urizar JM, Moragues MD, Antoran A, Pellon A, et al. Candida albicans and cancer: Can this yeast induce cancer development or progression? Crit Rev Microbiol. 2016;42:181–93. doi: 10.3109/1040841X.2014.913004 [DOI] [PubMed] [Google Scholar]
- 3.El-Naggar AK, Chan JK, Grandis JR, Takata T, Slootweg PJ (Eds). WHO classification of head and neck tumours. International Agency for Research on Cancer; 2017 [Google Scholar]
- 4.Di Cosola M, Cazzolla AP, Charitos IA, Ballini A, Inchingolo F, Santacroce L. Candida albicans and Oral Carcinogenesis. A Brief Review. J Fungi (Basel). 2021;7:476. doi: 10.3390/jof7060476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McCullough M, Jaber M, Barrett AW, Bain L, Speight PM, Porter SR. Oral yeast carriage correlates with presence of oral epithelial dysplasia. Oral Oncol. 2002;38:391–3. doi: 10.1016/s1368-8375(01)00079-3 [DOI] [PubMed] [Google Scholar]
- 6.Hebbar PB, Pai A, Sujatha D. Mycological and histological associations of Candida in oral mucosal lesions. J Oral Sci. 2013;55:157–60. doi: 10.2334/josnusd.55.157 [DOI] [PubMed] [Google Scholar]
- 7.Berkovits C, Tóth A, Szenzenstein J, Deák T, Urbán E, Gácser A, et al. Analysis of oral yeast microflora in patients with oral squamous cell carcinoma. Springerplus. 2016;5:1257. doi: 10.1186/s40064-016-2926-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roy SK, Astekar M, Sapra G, Chitlangia RK, Raj N. Evaluation of candidal species among individuals with oral potentially malignant disorders and oral squamous cell carcinoma. J Oral Maxillofac Pathol. 2019;23:302. Erratum in: J Oral Maxillofac Pathol. 2019;23:482. doi: 10.4103/jomfp.JOMFP_111_18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alnuaimi AD, Wiesenfeld D, O’Brien-Simpson NM, Reynolds EC, McCullough MJ. Oral Candida colonization in oral cancer patients and its relationship with traditional risk factors of oral cancer: a matched case-control study. Oral Oncol. 2015;51:139–45. doi: 10.1016/j.oraloncology.2014.11.008 [DOI] [PubMed] [Google Scholar]
- 10.Vallianou N, Kounatidis D, Christodoulatos GS, Panagopoulos F, Karampela I, Dalamaga M. Mycobiome and Cancer: What Is the Evidence? Cancers (Basel). 2021. Jun 24;13(13):3149. doi: 10.3390/cancers13133149 ; PMCID: PMC8269322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aykut B, Pushalkar S, Chen R, Li Q, Abengozar R, Kim JI, et al. The fungal mycobiome promotes pancreatic oncogenesis via activation of MBL. Nature. 2019;574:264–7. doi: 10.1038/s41586-019-1608-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mohamed N, Litlekalsøy J, Ahmed IA, Martinsen EMH, Furriol J, Javier-Lopez R, et al. Analysis of Salivary Mycobiome in a Cohort of Oral Squamous Cell Carcinoma Patients From Sudan Identifies Higher Salivary Carriage of Malassezia as an Independent and Favorable Predictor of Overall Survival. Front Cell Infect Microbiol. 2021;11:673465. doi: 10.3389/fcimb.2021.673465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Krogh P, Hald B, Holmstrup P. Possible mycological etiology of oral mucosal cancer: catalytic potential of infecting Candida albicans and other yeasts in production of N-nitrosobenzylmethylamine. Carcinogenesis. 1987;8:1543–8. doi: 10.1093/carcin/8.10.1543 [DOI] [PubMed] [Google Scholar]
- 14.Mohd Bakri M, Mohd Hussaini H, Rachel Holmes A, David Cannon R, Mary RA. Revisiting the association between candidal infection and carcinoma, particularly oral squamous cell carcinoma. J Oral Microbiol. 2010;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nieminen MT, Uittamo J, Salaspuro M, Rautemaa R. Acetaldehyde production from ethanol and glucose by non-Candida albicans yeasts in vitro. Oral Oncol. 2009;45:e245–8. doi: 10.1016/j.oraloncology.2009.08.002 [DOI] [PubMed] [Google Scholar]
- 16.Seitz HK, Stickel F. Molecular mechanisms of alcohol-mediated carcinogenesis. Nat Rev Cancer. 2007;7:599–612. doi: 10.1038/nrc2191 [DOI] [PubMed] [Google Scholar]
- 17.Manzo-Avalos S, Saavedra-Molina A. Cellular and mitochondrial effects of alcohol consumption. Int J Environ Res Public Health. 2010;7:4281–304. doi: 10.3390/ijerph7124281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Seitz HK, Homann N. The role of acetaldehyde in alcohol-associated cancer of the gastrointestinal tract. Novartis Found Symp. 2007;285:110–9; discussion 119–4, 198–199. doi: 10.1002/9780470511848.ch8 [DOI] [PubMed] [Google Scholar]
- 19.Alnuaimi AD, Ramdzan AN, Wiesenfeld D, O’Brien-Simpson NM, Kolev SD, Reynolds EC, et al. Candida virulence and ethanol-derived acetaldehyde production in oral cancer and non-cancer subjects. Oral Dis. 2016;22:805–14. doi: 10.1111/odi.12565 [DOI] [PubMed] [Google Scholar]
- 20.Arzmi MH, Dashper S, McCullough M. Polymicrobial interactions of Candida albicans and its role in oral carcinogenesis. J Oral Pathol Med. 2019;48:546–51. doi: 10.1111/jop.12905 [DOI] [PubMed] [Google Scholar]
- 21.O’Grady JF, Reade PC. Candida albicans as a promoter of oral mucosal neoplasia. Carcinogenesis. 1992;13:783–6. doi: 10.1093/carcin/13.5.783 [DOI] [PubMed] [Google Scholar]
- 22.Dwivedi PP, Mallya S, Dongari-Bagtzoglou A. A novel immunocompetent murine model for Candida albicans-promoted oral epithelial dysplasia. Med Mycol. 2009;47:157–67. doi: 10.1080/13693780802165797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vadovics M, Ho J, Igaz N, Alföldi R, Rakk D, Veres É, et al. Candida albicans Enhances the Progression of Oral Squamous Cell Carcinoma In Vitro and In Vivo. mBio. 2022;13:e03144–21. doi: 10.1128/mBio.03144-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mortaz E, Tabarsi P, Mansouri D, Khosravi A, Garssen J, Velayati A, et al. Cancers related to immunodeficiencies: Update and perspectives. Front Immunol. 2016;7:365. doi: 10.3389/fimmu.2016.00365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Romani L. Immunity to fungal infections. Nat Rev Immunol. 2011;11:275–88. doi: 10.1038/nri2939 [DOI] [PubMed] [Google Scholar]
- 26.Moutsopoulos NM, Konkel JE. Tissue-Specific Immunity at the oral mucosal barrier. Trends Immunol. 2018;39:276–87. doi: 10.1016/j.it.2017.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Georgaki M, Theofilou VI, Pettas E, Stoufi E, Younis RH, Kolokotronis A, et al. Understanding the complex pathogenesis of oral cancer. A comprehensive review. Oral Surg Oral Med Oral Pathol. Oral Radiol. 2021;132:566–79. doi: 10.1016/j.oooo.2021.04.004 [DOI] [PubMed] [Google Scholar]
- 28.Gaffen SL, Moutsopoulos NM. Regulation of host-microbe interactions at oral mucosal barriers by type 17 immunity. Sci Immunol. 2020;5:eaau4594. doi: 10.1126/sciimmunol.aau4594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Langowski JL, Zhang X, Wu L, Mattson JD, Chen T, Smith K, et al. IL-23 promotes tumour incidence and growth. Nature. 2006;442:461–5. doi: 10.1038/nature04808 [DOI] [PubMed] [Google Scholar]
- 30.Gajewski TF, Schreiber H, Fu YX. Innate and adaptive immune cells in the tumor microenvironment. Nat Immunol. 2013;14:1014–22. doi: 10.1038/ni.2703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Break TJ, Oikonomou V, Dutzan N, Desai JV, Swidergall M, Freiwald T, et al. Aberrant type 1 immunity drives susceptibility to mucosal fungal infections. Science. 2021;371:eaay5731. doi: 10.1126/science.aay5731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhu F, Willette-Brown J, Song NY, Lomada D, Song Y, Xue L, et al. Autoreactive T Cells and Chronic Fungal Infection Drive Esophageal Carcinogenesis. Cell Host Microbe. 2017. Apr 12;21(4):478–493.e7. doi: 10.1016/j.chom.2017.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moyes DL, Wilson D, Richardson JP, Mogavero S, Tang SX, Wernecke J, et al. Candidalysin is a fungal peptide toxin critical for mucosal infection. Nature. 2016;532:64–8. doi: 10.1038/nature17625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Birse CE, Irwin MY, Fonzi WA, Sypherd PS. Cloning and characterization of ECE1, a gene expressed in association with cell elongation of the dimorphic pathogen Candida albicans. Infect Immun. 1993;61:3648–55. doi: 10.1128/iai.61.9.3648-3655.1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ho J, Yang X, Nikou SA, Kichik N, Donkin A, Ponde NO, et al. Candidalysin activates innate epithelial immune responses via epidermal growth factor receptor. Nat Commun. 2019;10:2297. doi: 10.1038/s41467-019-09915-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Verma AH, Richardson JP, Zhou C, Coleman BM, Moyes DL, Ho J, et al. Oral epithelial cells orchestrate innate type 17 responses to Candida albicans through the virulence factor candidalysin. Sci Immunol. 2017;2:eaam8834. doi: 10.1126/sciimmunol.aam8834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kasper L, König A, Koenig PA, Gresnigt MS, Westman J, Drummond RA, et al. The fungal peptide toxin candidalysin activates the NLRP3 inflammasome and causes cytolysis in mononuclear phagocytes. Nat Commun. 2018;9:4260. doi: 10.1038/s41467-018-06607-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dhillon AS, Hagan S, Rath O, Kolch W. MAP kinase signalling pathways in cancer. Oncogene. 2007;26:3279–90. doi: 10.1038/sj.onc.1210421 [DOI] [PubMed] [Google Scholar]
- 39.Wang H, Luo Q, Feng X, Zhang R, Li J, Chen F. NLRP3 promotes tumor growth and metastasis in human oral squamous cell carcinoma. BMC Cancer. 2018;18:500. doi: 10.1186/s12885-018-4403-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ho J, Camilli G, Griffiths JS, Richardson JP, Kichik N, Naglik JR. Candida albicans and candidalysin in inflammatory disorders and cancer. Immunology. 2021;162:11–6. doi: 10.1111/imm.13255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Engku Nasrullah Satiman EAF, Ahmad H, Ramzi AB, Abdul Wahab R, Kaderi MA, Wan Harun WHA, et al. The role of Candida albicans candidalysin ECE1 gene in oral carcinogenesis. J Oral Pathol Med. 2020;49:835–41. doi: 10.1111/jop.13014 [DOI] [PubMed] [Google Scholar]