Abstract
Significance:
Immunotherapy promises unprecedented benefits to cancer patients. However, the majority of cancer types, including high-risk neuroblastoma remain immunologically unresponsive. High intensity focused ultrasound (HIFU) is a non-invasive technique that can mechanically fractionate tumors, transforming immunologically “;cold” tumors into responsive “;hot” tumors.
Methods:
We treated <2% of tumor volume in previously unresponsive, large, refractory murine neuroblastoma tumors with mechanical HIFU, and assessed systemic immune response using flow-cytometry, ELISA, and gene sequencing. In addition, we combined this treatment with αCTLA-4 and αPD-L1 to study its effect on the immune response and long-term survival.
Results:
Combining HIFU with αCTLA-4 and αPD-L1 significantly enhances anti-tumor response, improving survival from 0 to 62.5%. HIFU alone causes upregulation of splenic and lymph node NK cells and circulating IL-2, IFN-Ɣ, and DAMPs, whereas immune regulators like CD4+Foxp3+, IL-10, and VEGF-A are significantly reduced. HIFU combined with checkpoint inhibitors induced significant increases in intratumoral CD4+, CD8ɑ+, and CD8α+CD11c+ cells, CD11c+ in regional lymph nodes, and decrease in circulating IL-10 compared to untreated group. We also report significant abscopal effect following unilateral treatment of mice with large, established bilateral tumors using HIFU and checkpoint inhibitors compared to tumors treated with HIFU or checkpoint inhibitors alone (61.1% survival, p<0.0001). This combination treatment significantly also induces CD4+CD44+hiCD62L+low and, CD8α+CD44+hiCD62L+low population and are adoptively transferable imparting immunity, slowing subsequent de novo tumor engraftment.
Conclusion:
Mechanical fractionation of tumors using HIFU can effectively induce immune sensitization in a previously unresponsive murine neuroblastoma model, and promises a novel yet efficacious immuno-adjuvant modality to overcome therapeutic resistance.
INTRODUCTION
Despite the unprecedented potential of cancer immunotherapy, many patients with cancer do not respond to immunotherapy (1,2). Even among those who initially respond, many relapse after some period due to inadequate T-cell recognition resulting from loss of tumor antigen presentation by tumor cells (3,4). Both local and systemic strategies are required to mitigate therapeutic resistance to immunotherapy and transform immunologically “;cold” tumors into responsive “;hot” tumors.
Neuroblastoma is the third most common childhood cancer and arises from the developing sympathetic nerve ganglia in the abdomen, chest or, neck (5,6). Survival for pediatric patients with high-risk neuroblastoma has improved in recent years with the addition of multi-modal therapy including high dose chemotherapy, radiation, autologous stem-cell transplantation, and immunotherapy (7). The costs of therapy associated with acute and late side-effects are high and over 50% of patients still do not survive despite intensive therapy (7). Neuroblastoma cells evade the innate and adaptive immune system by downregulation of human leucocyte antigen (HLA) -class I & II (8,9), and are likely to be ignored by the host T-cell compartment (8,10,11). Various efforts to facilitate immunotherapy-based strategies including engineered T-cells specific to disialoganglioside (GD2), monoclonal antibodies directly targeting GD2, ɣδ T-cells, and vaccine therapies have changed neuroblastoma treatment perspective (12–15). Immune checkpoint inhibitor therapy is a recent advance in cancer therapy for several adult tumors, but similar responses have not been appreciated in pediatric solid tumor malignancies (1,16,17). The lack of therapy effectiveness in pediatric neuroblastoma is due to upregulation of TGF-β and IL-10, and downregulation of ligands that activate receptors expressed on NK and T-cells (8,18). The natural inhibition of hemopoietic stem-cell differentiation, generation of dendritic cells (DCs), T-cell proliferation, and the phenotype of the cellular and humoral immune response to neuroblastoma tumor cells is strikingly similar in human and murine (Neuro2a) hosts (19,20).
Sensitizing and changing the tumor microenvironment is shown to improve the efficacy of checkpoint inhibitor therapy, resulting in systemic tumor regression (21). Minimally invasive treatments such as radiofrequency (RFA) and, cryo-ablation have been used to perform tumor ablation in the clinic that result in an inflammatory response (22–24). High intensity focused ultrasound (HIFU) is a completely noninvasive ablation therapy that is used in the clinic to thermally ablate solid tumors (25,26). Thermal ablation using RFA and HIFU, however, could be unfavorable immunologically due to heat-associated tumor fixation, resulting in poor tumor permeability to immune cells and antigen release deficiency (27,28). In addition to thermal ablation, HIFU can also be used to mechanically fractionate tumors, with minimal thermal effects, referred to as histotripsy (29–31), which may improve anti-tumor immune sensitivity. Together with our collaborators, we have previously characterized this modality of HIFU, boiling histotripsy (BH, which will hereon be referred to as ‘HIFU’), a technique capable of mechanically fractionating tumors with high spatial precision using a clinical HIFU system for ablation (32–34). HIFU-mediated tumor fractionation may cause immunogenic cell death, and create an in situ tumor debris depot within the treated zone, increasing inflammation and, potentially leading to immune sensitization (28,35), which is unlikely to occur in HIFU ablation due to lack of tumor permeability (27).
Herein, we report the role for HIFU in inducing a significant immune response in a previously refractory, large subcutaneous murine neuroblastoma tumor model (Neuro2a)(36). We report that (1) partial mechanical fractionation of tumor using HIFU in combination with checkpoint inhibitors (αCTLA-4 + αPD-L1) significantly prolongs survival in a previously refractory unilateral and bilateral neuroblastoma tumor model; (2) HIFU induces a systemic immune activation of DCs, tumor infiltrating T-cells, proinflammatory cytokine changes, and damage-associated molecular patterns (DAMPs) changes, while downregulating regulatory T-cells, IL-10, TGF-β, and VEGF-A; and (3) HIFU-based tumor mechanical fractionation elicits systemic effector memory that is adoptively transferable.
MATERIALS AND METHODS
Mouse Neuroblastoma Cell Culture & Checkpoint Inhibitor Antibodies
The murine neuroblastoma cell line Neuro2a is derived from an aggressive and metastatic sub-clone of the C1300 neuroblastoma cell line that was cultured from a spontaneous tumor in the spinal cord of A/J mice (ATCC, Manassas, VA). Neuro2a cells were maintained in Dulbecco’s Modified Essential Medium (DMEM) supplemented with 1% penicillin-streptomycin (Invitrogen, Carlsbad, CA) and 10% fetal bovine serum (Gemini Bioproducts, Sacramento, CA). Cells were grown at 37°C under 5% CO2. Anti-mouse checkpoint inhibitors αCTLA-4 (clone 9D9) and αPD-L1 (clone 10F.9G2) were obtained from commercially available source (BioXCell, West Lebanon, NH).
Study Design
This study was designed to evaluate the role and efficacy of HIFU-based tumor fractionation on treatment of murine neuroblastoma tumors. Experiments were performed on a protocol (IRB# 30499) approved by institutional animal care and use committee (IACUC) at Children’s National Medical Center, Washington, DC. A total of 150 A/J mice with subcutaneous tumors were used in this study (105 unilateral, and 45 bilateral). Mice were assigned randomly to six treatment groups: HIFU + αCTLA-4 + αPD-L1 (N = 16), a combination of αCTLA-4 + αPD-L1 (N = 10), HIFU only (N = 10), combinations of HIFU with only either αCTLA-4 or αPD-L1 (HIFU + αCTLA-4, N = 10); (HIFU + αPD-L1, N =10), and untreated (N = 10). Mice in these groups had large, established unilateral neuroblastoma tumors. In addition, we evaluated HIFU + αCTLA-4 + αPD-L1 (N = 18) in mice with established and large bilateral tumors. Mice in all groups were subcutaneously injected with 1 × 106 neuroblastoma (Neuro2a) cells in the flank, and were randomized once the tumors reached a volume of 1200–1750 mm3. This model was desired over an orthotopic model to reduce variability and to avoid introduction of technical targeting challenges introduced by alternate models. In this and other murine models, >40% mortality by day 100 was previously demonstrated for starting tumor volumes less than 300 mm3 (28,37–39). Combination of αCTLA-4 + αPD-L1 (100 μg/antibody/mouse/time-point) was administered intraperitoneally on ~ days 1, 4 and, 7 after HIFU (Fig. 1A). Mice were euthanized if tumor volumes exceeded 4000 mm3. Mice surviving HIFU alone or combinational treatment were re-challenged with 2x initial tumor burden (2 × 106 cells) >300 days after the first tumor challenge, to test long-term immune memory effect. T-cells were isolated from spleens of surviving mice, and ~8 × 106 cells were adoptively transferred into naïve mice with de novo established (~600–900 mm3 tumor volume) neuroblastoma tumors. Animal caretakers, as well as investigators who analyzed data were blinded to the mice groups.
Figure 1. Adding HIFU to αCTLA-4 + αPD-L1 significantly increases survival in mice.

(A) Experimental timeline for treating mice with large, established unilateral neuroblastoma tumors using combination of HIFU and αCTLA-4 + αPD-L1. Mice were inoculated on day 0, tumors treated with HIFU on day 8, followed by a regimen of αCTLA-4 + αPD-L1. (B) Mice are held using an arm connected to a computer-controlled 3D positioning system, and the lower half of the mice’s body is submerged into a water bath filled with circulating degassed water at ~33°C. Three adjacent, non-overlapping HIFU foci (1.5×1.5×7 mm each) are positioned in the middle of the tumor. (C) H&E –stained histological sections obtained 24-hours after HIFU, demonstrating tumor fractionation and necrosis (red arrow), adjacent to intact tumor (yellow arrowhead). Representative photographs of mice with and without tumors. Red dashed-circle shows typical tumors, measuring ~1200–1750 mm3 that were treated in this study, while yellow dashed-circle shows a completely tumor-free mouse after HIFU + αCTLA-4 + αPD-L1 treatment, with no evidence of iatrogenic effects from HIFU treatment. (D) Kaplan-Meir plots of six groups of mice with unilateral established tumors treated with either individual or combinational approaches. HIFU + αCTLA-4 + αPD-L1 was the only group to show significantly higher survival compared to all other groups, with more than 62% of mice surviving beyond 300 days post- treatment.
Ultrasound-guided HIFU Tumor Fractionation
The HIFU system consists of a transducer that is capable of producing equivalent acoustic pressures to a clinical HIFU transducer (Sonalleve® V2 MR-HIFU system, Profound Medical Inc., Mississauga, ON, Canada) (32). The focal size of our HIFU transducer was 1.5×1.5×7 mm at −6dB level; with a transmit frequency of 1.5 MHz, focal length of 56 mm, and transducer aperture diameter of 75 mm. A commercially available amplifier (1240L, E&I, Rochester, NY) was used to power the HIFU transducer. The HIFU transducer produced a peak positive pressure of 85 MPa, and peak negative pressure of 14 MPa (shock amplitude of 80 MPa), measured in water using a fiber optic probe hydrophone (Rp Acoustics, Leutenbach, Germany). A 13.33 ms-long pulse at 1 Hz pulse repetition frequency, as previously described, was used for in vivo HIFU sonications (32). The HIFU focus was sequentially moved across three adjacent, non-overlapping foci using a computer controlled 3-axis linear-stage (Velmex, Bloomfield, NY) covering ~ 2% tumor volume (acoustically equivalent). The pulsing protocol was applied for 15 seconds/focus (Fig. 1B). Mice were anesthetized using a ketamine-xylazine cocktail (100 mg/kg ketamine and 10 mg/kg xylazine), attached to a custom-built holder, and positioned in the water tank to align the HIFU focus within the tumor. The linear stages were connected to stepper motors (Maxon motors, Fall River, MA) and remotely controlled (Galil motion controller, Rocklin, CA). A diagnostic ultrasound transducer (S12–4, Philips CX50, Philips, Cambridge, MA) was placed co-axially with the HIFU transducer for real-time b-mode image guidance to target the tumor along the axial plane (Figure 1B). This setup enabled a robust and repeatable platform to treat murine tumors. Immediately after HIFU, mice were administered buprenorphine (0.3 mg/ml of buprenorphine diluted to 1:10 with PBS) subcutaneously. Additional doses of buprenorphine were administered at 12, 24, and 48-hours post HIFU treatment, to alleviate any pain caused by tumor fractionation.
Statistical Analysis
All statistical analyses were performed using GraphPad Prism software (GraphPad Software Inc., La Jolla, CA) version 7.0. Sample sizes were determined apriori using a two-sided log rank test with an overall sample size of 30 mice (15 in HIFU + αCTLA-4 + αPD-L1 and 15 in untreated) which achieved a power of 76% at 0.05 significance level to detect a difference of survival proportion. Tumor growth curves are presented as tumor volume over time. Kaplan-Meir plots were populated for all groups post-treatment to demonstrate mice survival. Significance was assessed by log-rank test (Mantel-Cox test). Earlier time points were assessed using Gehan-Breslow-Wilcoxon test and were together presented as percent survival (mean ± standard error). One-way nonparametric ANOVA, followed by Bonferroni correction was used to compare all experimental groups in both ELISA and flow cytometry data. Confidence interval of 95% was used for all tests. Empirical Bayes approach was used to analyze probe-wise microarray gene data and compute t-statistics, F-statistic, and log-odds of differential expression.
RESULTS
HIFU in combination with αCTLA-4 + αPD-L1 cures refractory, unilateral neuroblastoma tumors, leading to significant long-term survival
To assess the role of HIFU mechanical fractionation in survival efficacy, we partially treated large, refractory Neuro2a tumors in mice (Fig. 1C). Treatment using HIFU alone or in combination with single checkpoint inhibitors alone was not effective and had no survival benefit (Fig. 1D, blue, black and, orange lines). Additionally, treatment with both αCTLA-4 + αPD-L1 (without HIFU) resulted in marginal survival (10%, Fig. 1D, red line). In contrast, HIFU combined with αCTLA-4 + αPD-L1 resulted in complete tumor regression at the primary site, with no distant metastases (Fig. 1C) and significantly increased long-term survival (>300 days, p<0.0001) from 0% (untreated, HIFU only, HIFU + αCTLA-4 or HIFU + αPD-L1) to 62.5% (Fig. 1D, green line). Tumor volumes compared 28 days after treatment demonstrate that tumors treated with HIFU + αCTLA-4 + αPD-L1 were significantly smaller when compared to tumors treated with αCTLA-4 + αPD-L1 alone (p = 0.0241) (fig. S1). These results demonstrate that HIFU induces a strong synergistic effect when combined with αCTLA-4 + αPD-L1, and leads to long-term survival in a previously untreatable, refractory neuroblastoma tumor model.
Local HIFU treatment alone induces systemic cellular, cytokine, and gene response
Since HIFU was applied locally to fractionate tumors, we first sought to measure any loco-regional and systemic immune responses at 24, 48, and, 72-hours after HIFU alone (Fig. 2A–D).
Figure 2. Systemic cellular, cytokine and genetic signature caused by HIFU mechanical fractionation alone of neuroblastoma tumor.

(A) Box plots presenting cellular changes as percent live cells in the spleen and tumor-draining lymph nodes of mice at 24, 48, and 72-hours following HIFU were compared against cellular changes in untreated mice. Percentage of CD4+Foxp3+, CD11c+, DX5+ (NK cells), CD11bhigh, ratio of CD8+/CD4+Foxp3+, and CD8α+CD11c+ were evaluated using flow cytometry. (B) Representative fluorescent microscopy images of tumor tissues stained with DAPI (blue stain) + PD-L1 (red stain) obtained from untreated mice and from mice at 72-hours post-HIFU. (C) Box plots presenting circulating cytokines in mice at 24, 48, and 72-hours post-HIFU and untreated mice in pg/mL (IL-2, VEGF-A, IL-10, TGF-β1, GM-CSF, IL-6, IFN-Ɣ, and TGF-β2) measured using ELISA from terminal cardiac blood draw. (D) Microarray data from untreated, 24, 48, and 72-hours post-HIFU, represented as log2 change. This was followed-up by qRT-PCR analysis of seven key genes repeated for three different housekeeping genes (GAPDH, ACTB, and GUSB). N ≥ 8 tumors per time-point for each housekeeping gene were used. Asterisks in GAPDH columns represent significance from untreated tumors (p < 0.05), and was significantly high in gene expression for ACTB and GUSB housekeeping genes as well. Significance values for all data analysis were calculated using ANOVA with Bonferroni correction (p< 0.05).
Temporal evolution of cellular response in tumor, lymph nodes, and spleen
Untreated tumors did not have any infiltration of CD4+ and CD8α+ cells, although some resident macrophages (CD68+) were detected (fig. S2). Significant infiltration of both CD4+ and CD8α+ cells was observed in the treated tumor at 24-hours after HIFU alone (fig. S2, p = 0.0001 and 0.033, respectively). This was accompanied by a significant increase in CD68+ cells at 48-hours, compared to untreated tumors (fig. S2, p = 0.0377). Significant increase in NK cells (DX5+) was measured in both spleen and tumor draining lymph nodes at 24-hours post-HIFU (p = 0.0006 and p = 0.0004, respectively, Fig. 2A), with a non-significant increase observed in the contralateral lymph nodes (fig. S3). At this time-point, DC (CD11c+) population also moderately increased in spleen, tumor draining lymph nodes, and contralateral lymph nodes (Fig. 2A & fig. S3), while CD8α+ DCs (CD8α+CD11c+) subset significantly increased at 24-hours post HIFU in both spleen and tumor draining lymph nodes (p = 0.0001 and 0.0002, respectively). At 48-hours post treatment, HIFU also caused a significant reduction in CD4+ subset of regulatory T-cells (CD4+Foxp3+) in the tumor draining lymph nodes (p = 0.0200). Likewise, at 72-hours, CD4+Foxp3+ population was significantly lower in the spleen and tumor draining lymph nodes (p = 3.5e-9 and p = 0.0001, respectively, Fig. 2A). CD4+Foxp3+ population remained unchanged in the contralateral lymph nodes at all time-points after HIFU (fig. S3). Given that a higher cytotoxic to regulatory T-cell ratio (CD8ɑ+/Foxp3+) has been previously shown to signify favorable outcomes in several cancer types (40,41), we measured this cell ratio post-HIFU. CD8ɑ+/Foxp3+ cell ratios were significantly elevated in both spleen and tumor draining lymph nodes at 72-hours post-HIFU treatment (p = 0.0021 and 0.0091, respectively). We observed no significant changes in CD11bhigh in spleen and tumor draining lymph nodes. These results show an early and marked increase in local immune cell infiltration of the tumor after HIFU.
Intra-tumoral expression of PD-L1 post-HIFU
We further investigated the role of HIFU alone in altering the PD-L1 – PD-1 axis by measuring intratumoral PD-L1 expression. Untreated neuroblastoma tumors presented no PD-L1 expression (Fig. 2B). In contrast, there was a significant increase in PD-L1 expression on most tumor cells at 72-hours after HIFU treatment compared to untreated tumor (Fig. 2B, p = 0.0001). This acute increase in PD-L1 expression at 72-hours following HIFU suggests adaptive tumor immune suppression, and loss of T-cell population (fig. S2). Thus anti-PD-L1 treatment is critical for countering this effect and enhancing anti-tumor immune responses (42).
Temporal progression of systemic inflammatory cytokines after HIFU
We then evaluated circulating inflammatory cytokine changes at 24, 48, and 72-hours post-HIFU alone (Fig. 2C). At 24-hours post-HIFU we observed a significant increase in interleukin-2 (IL-2) (p = 0.0148) and granulocyte-macrophage colony-stimulating factor (GM-CSF) (p = 0.0454), plus a significant decrease in vascular endothelial growth factor-A (VEGF-A) (p = 0.0072). At 48-hours post-HIFU interleukin-6 (IL-6) (p = 0.0298) was significantly upregulated, whilst significantly lower concentrations in interleukin-10 (IL-10) (p = 0.0227) were measured. Further, at 72-hours post-HIFU, IL-10 continued to significantly decrease (p = 0.0314), and in contrast interferon-Ɣ (IFN-Ɣ) was significantly higher at 72-hours (p = 1.7e-5). We measured no significant changes in TGF-β1, TGF-β2 (Fig. 2C), TNF-α, IL-12p70, or IL-4 after HIFU treatment (fig. S4).
Gene expression within HIFU-treated tumors
We further characterized intra-tumor genetic patterns 24, 48, and 72-hours following HIFU treatment (Fig. 2D). S100 calcium binding protein A8 (S100a8), S100 calcium binding protein A9 (S100a9), heat shock protein family, member 7 (Hspb7), and lipocalin 2 (Lcn2), were overexpressed significantly at all three time-points, while heat shock protein family A member 1B (Hsp70) and Cd72 expression were significantly overexpressed at 24 and 48-hours post-HIFU, and high mobility group box 1 (Hmgb1) expression did not change post-HIFU.
In summary, our collective evidence from cellular changes, cytokine, and genetic signatures suggest that HIFU treatment of previously refractory neuroblastoma results in early immune cell presence in the tumor, lymph nodes and spleen, converting a non-immunogenic ‘cold’ tumor to an immunogenic ‘hot’ tumor.
Systemic immune effects are sustained after HIFU in combination with αCTLA-4 + αPD-L1 and results in systemic ‘abscopal’ effect and, prolonged survival
Similar to studying immune effects of HIFU only above, we analyzed circulating cytokine and systemic cellular changes in these mice at 24, 48, and 72-hours after the last dose of αCTLA-4 + αPD-L1. We observed significant intratumoral infiltration of helper T-cells (CD4+), cytotoxic T-cells (CD8α+), as well as CD8α+ dendritic cells (CD8ɑ+CD11c+) at 48-hours after the last dose of checkpoint inhibitors (Fig. 3A). In addition, significant CD8ɑ+CD11c+ infiltration into the draining lymph nodes and spleen was measured after the last dose of checkpoint inhibitors (Fig. 3A). CD11c+ infiltration into draining and contralateral lymph nodes, as well as systemic cytokine changes were observed after the last dose of checkpoint inhibitors (Fig. 3A).
Figure 3. HIFU + αCTLA-4 + αPD-L1 treatment of large, established bilateral tumors leads to abscopal effect.
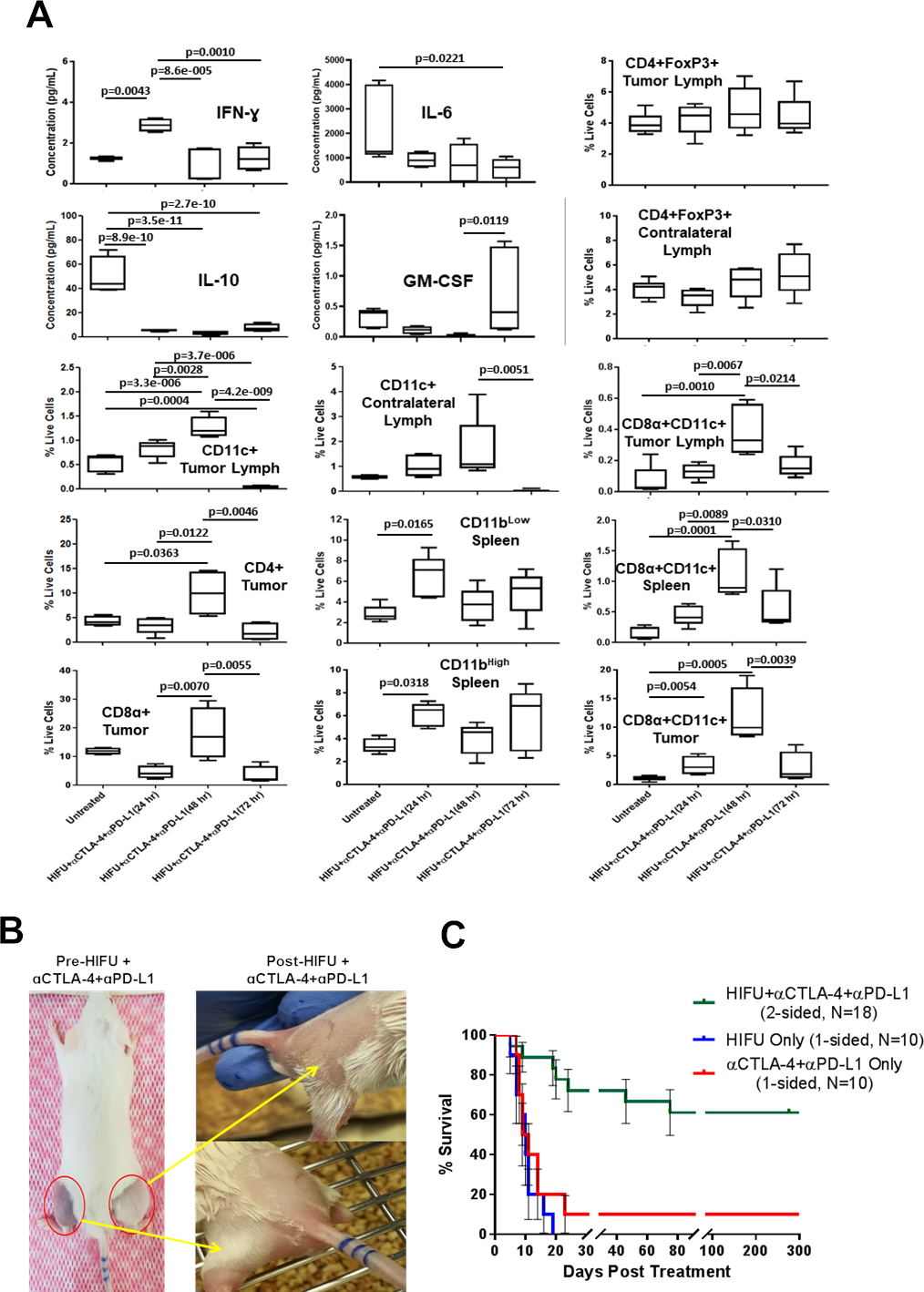
(A) Box plots showing cytokine concentration (pg/mL) and cellular changes (percent live cells), quantified at 24, 48, and 72-hrs after HIFU and the last dose of αCTLA-4 + αPD-L1 and, compared with untreated mice. Significance values for all data analysis were calculated using ANOVA (p< 0.05). (B) Representative photographs of mouse with established and large bilateral neuroblastoma tumors (red circles). After unilateral treatment with HIFU + αCTLA-4 + αPD-L1, both tumors completely regressed, signifying strong abscopal effect. (C) Kaplan-Meir plot showing survival of mice with bilateral neuroblastoma tumors treated with HIFU + αCTLA-4 + αPD-L1 (N = 18). 61.1% of mice with bilateral tumors treated with HIFU + αCTLA-4 + αPD-L1 displayed significantly higher survival compared to mice with treated with HIFU only or αCTLA-4 + αPD-L1 only, beyond day 300.
IFN-Ɣ was significantly elevated at 24-hours after the last dose of αCTLA-4 + αPD-L1 regimem (p = 0.0043). These IFN-Ɣ levels in circulating blood are similar in mice at 72-hours post-HIFU only, suggesting a sustained IFN-Ɣ elevation at day 8 after treating tumors with HIFU. In contrast, mice receiving just αCTLA-4 + αPD-L1 and not supplemented by HIFU did not demonstrate any changes in IFN-Ɣ concentration systemically (fig. S5). In addition, IL-10 significantly reduced by at least 10-fold at 24, 48, and 72-hours after the last dose of αCTLA-4 + αPD-L1 compared to untreated mice (p = 8.9e-10, 3.5e-11, and 2.7e-10, respectively). IL-6 was significantly lower at 72-hours after the last dose (p = 0.0221), while GM-CSF was significantly elevated at 72-hours after the last dose (p = 0.0119). This downward trend in systemic IL-10 concentration is similarly observed in HIFU only treatment of mice with unilateral tumors, suggesting a sustained effect at 10 days after HIFU. Population of dendritic cells (CD11c+) in tumor draining lymph nodes significantly increased at 48-hours compared to untreated mice (p = 3.3e-6), noticeably increasing in contralateral lymph nodes at 48-hours post final dose of αCTLA-4 + αPD-L1, suggesting systemic antigen presenting capability. Also, the frequency of both CD4+ and CD8α+ cells were found to be elevated in tumor at 48-hours after the last dosage of αCTLA-4 + αPD-L1 compared to untreated mice (p = 0.0363), and 24-hours after the last dose of checkpoint inhibitors (p = 0.0070). CD8ɑ+CD11c+ cells were significantly elevated intratumorally, in the spleen and in draining lymph node at 48-hours after the last dose of αCTLA-4 + αPD-L1 (p = 0.0054, 0.0001, and 0.0010, respectively). CD11blow in the spleen was significantly elevated at 24-hours after the last dose of αCTLA-4 + αPD-L1 (p = 0.0165). In addition, CD11bhigh population in the spleen was also significantly elevated at 24-hours post final αCTLA-4 + αPD-L1 dose compared to untreated mice (p = 0.0318). In contrast, mice treated with HIFU only did not show any changes in CD11blow. The CD4+Foxp3+ cell population remained unchanged in both tumor draining and contralateral lymph nodes after the last dose of αCTLA-4 + αPD-L1, although they significantly reduced in mice treated with HIFU alone.
Given the local and systemic immunologic changes with HIFU alone and in combination with αCTLA-4 + αPD-L1, we hypothesize that local unilateral tumor fractionation using HIFU in combination with αCTLA-4 + αPD-L1 induces an abscopal effect in mice with established bilateral tumors (>500 mm3 on each side, >1000 mm3 total tumor burden) (Fig. 3B). We locally treated one side only with HIFU, and followed up with a regimen of systemic αCTLA-4 + αPD-L1 (similar to Fig. 1A). Interestingly, combining αCTLA-4 + αPD-L1 with one-sided HIFU tumor fractionation resulted in complete bilateral tumor regression (Fig. 3B). By day 20, 75% of mice had no tumors, bilaterally (fig. S6). There was a significant improvement in survival (p = 0.0001) compared to mice with unilateral tumors treated with checkpoint inhibitors or HIFU only (Fig. 3C). Overall, 61.1% of mice survived longer than 300 days in this group, with no evidence of local or systemic tumor recurrence. These results provide vital evidence of local treatment of tumor with HIFU in combination with αCTLA-4 + αPD-L1 cause a sustained systemic immune-adjuvant effect and abscopal effect.
HIFU mediated tumor fractionation combined with checkpoint inhibitor using αCTLA-4 + αPD-L1 induces effector memory cells
In view of the fact that HIFU treated tumors continued to regress well beyond the treatment period and the abscopal effect, we re-challenged all surviving mice with twice the number of tumor cells (2 × 106 cells) compared to the initial inoculation (1 × 106 cells). Intriguingly, all mice survived this re-challenge for at least 75 days, without any evidence of tumor formation (Fig. 4A), except for two mice that demonstrated transient tumor formation, which eventually regressed by day 21 (fig. S7A). To determine the cellular mediator of this systemic effector memory response, we measured CD4+CD44+hiCD62L+low and CD8α+CD44+hiCD62L+low cells in the spleen. The percentage of CD4+ and CD8α+ cells expressing effector memory markers in mice that were re-challenged were 3 and 17 folds greater than in naïve mice (p = 0.0001, Fig. 4B & C). To further support the induction of long-term memory response following HIFU and checkpoint inhibitor treatment, we adoptively transferred the isolated T-cells from spleens in mice surviving combination therapy into mice with de novo established tumors (volume: 600–900 mm3). We found tumor growth retardation and prolonged survival in mice that received adoptive T-cells compared to mice that did not receive adoptive T-cells (p = 0.0002) (fig. S7B). Combing HIFU with α-CTLA-4 + α-PD-L1 in previously untreatable and refractory neuroblastoma tumor not only effectively induced tumor regression and systemic abscopal effect, but also induced a long-term immune memory response.
Figure 4. Mice surviving HIFU + αCTLA-4 + αPD-L1 treatment possess significantly high effector memory against neuroblastoma tumors.

(A) Kaplan-Meir plot shows 100% survival in mice re-challenged with 2x the initial tumor inoculation (2 × 106 vs 1 × 106). Mice from both unilateral and bilateral tumor groups were re-challenged and all mice survived. (B) Box plot showing significantly higher effector memory on CD4+ cells in mice treated with HIFU + αCTLA-4 + αPD-L1 compared to naïve mice (C) Box plot presenting significantly higher effector memory on CD8+ cells in mice treated with HIFU + αCTLA-4 + αPD-L1 compared to naïve mice. P-values were determined using unpaired Welch’s t-test.
DISCUSSION
In this study, we demonstrate that partial tumor fractionation using HIFU in combination with αCTLA-4 + αPD-L1 results in an abscopal effect and significantly prolongs survival in previously untreatable, large unilateral and bilateral murine neuroblastoma tumor models. Our results also provide a proof-of-concept for using HIFU to sensitize the systemic immune system, and serving as an adjuvant to checkpoint inhibitor therapy. We also reveal that this systemic anti-tumor effect is mediated by upregulation of dendritic cells, tumor infiltrating T-cells, proinflammatory cytokines, and DAMPs, as well as concurrent downregulation of pro-tumor regulators such as Foxp3, IL-10 and VEGF-A. Furthermore, this combination therapy elicits a systemic effector memory response that results in rejection of tumor re-challenge and can be adoptively transferred.
One of the most important findings in our study is that combining HIFU with αCTLA-4 + αPD-L1 significantly improves long-term survival of mice with large and established otherwise immunologically ‘cold’ neuroblastoma tumors. Tumors in mice were treated when the volume reached 1200–1750 mm3, which is significantly larger than prior reported work in similar or same tumor models (27,28,38,43). The HIFU foci were calculated to cover ~2% of total tumor volume, which may be clinically relevant in treating tumors in patients (44), where complete margin treatment may not be possible. Unlike HIFU-mediated thermal ablation, BH (modality of HIFU used in this study) is capable of mechanically fractionating tissues at high spatial precision and relatively low temperature elevation. In addition, BH’s two-prong approach of debulking tumor tissue via mechanical fractionation while keeping major blood vessels intact (30,45), and simultaneously producing significant sterile inflammation (45), potentially improves efficiency of immune checkpoint inhibitors. The checkpoint inhibitors used in this study are clinically approved for multiple indications and are currently in trials for treating neuroblastoma (NCT02304458) (1,46). Mechanically fractionating <10% of tumor volume with HIFU compared to thermally ablating more than 50% tumor volume, potentially reduces heat convective-associated iatrogenic effects, allows improved blood supply into the tumor and immune sensitization (37). These factors suggest mechanical fractionation using BH may lead to improved immune sensitization against tumor with little or no side effects compared to thermal ablation. This is potentially impactful in clinical translation of this drug-device combination therapy for patients suffering from high-risk or relapsed neuroblastoma, without other effective therapeutic options.
In the present study, we found three key mechanistic changes. First, HIFU-mediated mechanical fractionation of tumor tissue elevated concentrations of IFN-Ɣ, while lowering concentrations of IL-10, and sustaining IL-4, TGF-β1, and TGF-β2. These cytokine changes help improve antigen presentation capability (47,48) after HIFU-mediated mechanical fractionation of tumors. Tumor fractionation using HIFU also demonstrates remarkable DAMP changes. Key DAMPs such as S100a8/a9 and HSPs are upregulated post-HIFU, and are of key interest since they are known to bind to pattern recognition receptors (PRR) on the surface of innate immune cells, such as TLRs (49). These genes are also known to activate the S100a8/a9–NK cell axis via the receptor for advanced glycation end products (RAGE) pathway (50), facilitating dendritic cell maturation, and promoting immunogenic cell death (ICD) (51). HIFU tumor fractionation increased HSP27 and HSP70 that may act as a chemoattractant to dendritic cells via the RAGE pathway (as observed in both preclinical and clinical studies) (35,52). We also mechanistically demonstrate the upregulation of CD72 gene, which is linked in inhibition of IFN-Ɣ production by NK cells, although NK cell concentration significantly increased at 24-hrs. CD72 may have inhibited IFN-Ɣ production by NK cells (without interfering with NK cell’s cytotoxic abilities), leading to suppression of IFN-Ɣ until 72-hours post-HIFU (53). We therefore conclude that 24–48 hours post-HIFU, a significant increase in macrophages, CD8α+ DCs, IFN-Ɣ, T-cells, NK cells, and dendritic cells have converted a ‘cold’, non-immunogenic tumor to a ‘hot’, immunogenic tumor, but not adequate to improve overall survival.
Second, when HIFU tumor fractionation is combined with αCTLA-4 + αPD-L1, sustained systemic increase in CD11c+, CD8α+CD11c+, CD4+, and CD8α+ populations, while inhibited IL-10 and CD4+Foxp3+ is observed, leading to abscopal effect and long-term survival in over 61.1% of mice. The presence of significantly high levels of CD11c+ in the draining lymph nodes after tumor fractionation, and downregulating CTLA-4 and PD-L1 suggests a more efficient T-cell priming and activation. Also, CD8α+CD11c+ in the draining lymph nodes directly presents antigens to CD8α+ cells, leading to efficient priming and activation of CD8α+ cells. Combining HIFU tumor-fractionation with αPD-L1, improves T-cell based tumor targeting efficiency. This synergy between HIFU and checkpoint inhibitor therapy causing systemic cellular, cytokine, and DAMP release may have facilitated this effect, leading to significantly improved overall survival.
Third, HIFU in combination with αCTLA-4 + αPD-L1 improves long-term memory responses, and rejected tumor cell re-challenge with a strong adaptive immune response. Nineteen out of twenty-one mice rejected tumor re-challenge, while two presented transient tumor growth (1500–1800 mm3) before completely regressing by day 21 (fig. S7A). Overall, 100% mice initially treated with the HIFU + αCTLA-4 + αPD-L1 combination eventually survived the re-challenge sans further therapy. Since long-term memory markers CD4+CD44+hiCD62L+low and CD8α+CD44+hiCD62L+low were found to be significantly elevated in re-challenged mice compared to untreated mice, suggesting HIFU’s ability to improve T-cell memory . Adoptively transferring T-cells from surviving mice into naïve mice with established neuroblastoma tumor resulted in improved survival compared to mice that did not receive adoptive T-cells. This suggests a role of long-term memory T-cell response in slowing down established tumor growth. Alternatively, the increase in tumor infiltrating lymphocytes in the adoptive transfer group, may have induced upregulation of PD-L1, compounded by the lack of αPD-L1, and may have prevented complete tumor regression and long-term survival.
Some important limitations remain unaddressed. Firstly, it would be valuable to assess the role of priming the immune system with αCTLA-4 prior to HIFU mechanical fractionation, followed by αPD-L1 in evaluating the role of T-cell priming and activation while reducing the potential effects of co-toxicity. Secondly, assessing the role of specific T-cell phenotypes after HIFU + checkpoint inhibitors, which may help support outcomes observed in this study. Finally, it may be interesting to assess the effect of treated tumor volumes in survival outcomes, and establish an ‘exposure-response’ relationship.
In conclusion, partial tumor fractionation of refractory neuroblastoma tumors in mice using HIFU enhances innate and adaptive cellular immunity, converting a ‘cold’ non-immunogenic tumor to a ‘hot’ immunogenic one. Combining this mode of HIFU with αCTLA-4 + αPD-L1 induces potent systemic immunity and cures majority of mice with large, established unilateral and bilateral neuroblastoma tumors. In addition, HIFU treatment of these tumors leads to long-term immune memory. Our group has clinical experience in using HIFU for thermal tumor ablation in patients (54,55) and we have previously demonstrated preclinical feasibility of performing tissue mechanical fractionation on the same clinical MR-HIFU system (32). Thus, combining this technology with checkpoint inhibition is a step closer to clinical translation for patients with previously unresponsive primary neuroblastoma tumor with or without metastatic burden.
Supplementary Material
Translational Relevance.
Immunotherapy promises unprecedented benefits to cancer patients. However, the majority of cancer types, including high-risk neuroblastoma remain immunologically unresponsive. High intensity focused ultrasound (HIFU) is a non-invasive technique that can mechanically fractionate tumors with high spatial precision, potentially transforming immunologically ‘cold’ tumors into responsive ‘hot’ tumors. Herein, we demonstrate that a combination of HIFU mechanical fractionation and checkpoint inhibitors significantly enhance systemic anti-tumor response and survival in previously unresponsive, large refractory murine neuroblastoma tumors. We report a significant abscopal effect was induced following unilateral treatment of large, established bilateral tumors. Furthermore, this immune response is adoptively transferable. Mechanical HIFU opens a favorable time-window for immunotherapy that eventually leads to therapeutic responsiveness in previously immunologically “;cold” tumors. This HIFU approach can be executed on clinical HIFU systems, expediting clinical translation of this novel combination therapy.
Acknowledgements:
The authors thank Karuna Panchapakesan, Dr.Surajit Bhattacharya, Mousumi Basu, Dr.Xiaofang Wu for their assistance in sample preparation and assistance. We also thank Dr.Navid Farr and Dr.Ari Partanen for comments and suggestions regarding HIFU, Dr.Stefan Neirkens and Dr.Luca Gattinoni for help in manuscript development. We also acknowledge the support of the CRI Bioinformatics Unit, a partnership between Children’s Research Institute (CRI), Center for Genetic Medicine Research, Clinical Translational Science Institute at Children’s National (CTSI-CN) and, District of Columbia Intellectual and Developmental Disabilities Research Center (DC-IDDRC).
Funding:
This research was supported in part by Sheikh Zayed Institute for Pediatric Surgical Innovation, Joseph E. Robert, Jr. Center for Surgical Care and Board of Visitors at Children’s National Health System. This work was also supported in part by Center for Interventional Oncology and Intramural Research Program of the National Institutes of Health (NIH), NIH and National Cancer Institute grants ZID# BC011242 & CL040015, and the National Institutes of Health-NCI grant 1U01CA202947-01A1.
Footnotes
Conflict of Interest: The authors declare no conflict of interest.
Data and materials availability: All microarray gene data is publicly made available on gene expression omnibus (GEO ID# GSE137124). Remaining data associated with this study are available in the main text or the supplementary materials.
REFERENCES
- 1.Merchant MS, Wright M, Baird K, Wexler LH, Rodriguez-Galindo C, Bernstein D, et al. Phase I clinical trial of ipilimumab in pediatric patients with advanced solid tumors. Clinical Cancer Research 2016;22(6):1364–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Park JA, Cheung N-KV. Limitations and opportunities for immune checkpoint inhibitors in pediatric malignancies. Cancer treatment reviews 2017;58:22–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sharma P, Hu-Lieskovan S, Wargo JA, Ribas A. Primary, adaptive, and acquired resistance to cancer immunotherapy. Cell 2017;168(4):707–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ribas A, Puzanov I, Dummer R, Schadendorf D, Hamid O, Robert C, et al. Pembrolizumab versus investigator-choice chemotherapy for ipilimumab-refractory melanoma (KEYNOTE-002): a randomised, controlled, phase 2 trial. The lancet oncology 2015;16(8):908–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Irwin MS, Park JR. Neuroblastoma. Pediatric Clinics 2015;62(1):225–56. [DOI] [PubMed] [Google Scholar]
- 6.Ries LAG, Smith MA, Gurney J, Linet M, Tamra T, Young J, et al. Cancer incidence and survival among children and adolescents. United States SEER Program 1975–1995 1999. [Google Scholar]
- 7.Pinto NR, Applebaum MA, Volchenboum SL, Matthay KK, London WB, Ambros PF, et al. Advances in risk classification and treatment strategies for neuroblastoma. Journal of clinical oncology 2015;33(27):3008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Raffaghello L, Prigione I, Airoldi I, Camoriano M, Morandi F, Bocca P, et al. Mechanisms of immune evasion of human neuroblastoma. Cancer letters 2005;228(1–2):155–61. [DOI] [PubMed] [Google Scholar]
- 9.Raffaghello L, Prigione I, Bocca P, Morandi F, Camoriano M, Gambini C, et al. Multiple defects of the antigen-processing machinery components in human neuroblastoma: immunotherapeutic implications. Oncogene 2005;24(29):4634. [DOI] [PubMed] [Google Scholar]
- 10.Contardi E, Palmisano GL, Tazzari PL, Martelli AM, Fala F, Fabbi M, et al. CTLA-4 is constitutively expressed on tumor cells and can trigger apoptosis upon ligand interaction. International journal of cancer 2005;117(4):538–50. [DOI] [PubMed] [Google Scholar]
- 11.Leach DR, Krummel MF, Allison JP. Enhancement of antitumor immunity by CTLA-4 blockade. Science 1996;271(5256):1734–6. [DOI] [PubMed] [Google Scholar]
- 12.Prapa M, Caldrer S, Spano C, Bestagno M, Golinelli G, Grisendi G, et al. A novel anti-GD2/4–1BB chimeric antigen receptor triggers neuroblastoma cell killing. Oncotarget 2015;6(28):24884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zeng Y, Fest S, Kunert R, Katinger H, Pistoia V, Michon J, et al. Anti-neuroblastoma effect of ch14. 18 antibody produced in CHO cells is mediated by NK-cells in mice. Molecular immunology 2005;42(11):1311–9. [DOI] [PubMed] [Google Scholar]
- 14.Marquez-Medina D, Salla-Fortuny J, Salud-Salvia A. Role of gamma-delta T-cells in cancer. Another opening door to immunotherapy. Clinical and Translational Oncology 2012;14(12):891–5. [DOI] [PubMed] [Google Scholar]
- 15.Heczey A, Louis CU, Savoldo B, Dakhova O, Durett A, Grilley B, et al. CAR T cells administered in combination with lymphodepletion and PD-1 inhibition to patients with neuroblastoma. Molecular Therapy 2017;25(9):2214–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nature Reviews Cancer 2012;12(4):252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhu X, McDowell MM, Newman WC, Mason GE, Greene S, Tamber MS. Severe cerebral edema following nivolumab treatment for pediatric glioblastoma: case report. Journal of Neurosurgery: Pediatrics 2017;19(2):249–53. [DOI] [PubMed] [Google Scholar]
- 18.Leen AM, Rooney CM, Foster AE. Improving T cell therapy for cancer. Annu Rev Immunol 2007;25:243–65. [DOI] [PubMed] [Google Scholar]
- 19.Sietsma H, Nijhof W, Dontje B, Vellenga E, Kamps WA, Kok JW. Inhibition of hemopoiesis in vitro by neuroblastoma-derived gangliosides. Cancer research 1998;58(21):4840–4. [PubMed] [Google Scholar]
- 20.Shurin GV, Shurin MR, Bykovskaia S, Shogan J, Lotze MT, Barksdale EM. Neuroblastoma-derived gangliosides inhibit dendritic cell generation and function. Cancer research 2001;61(1):363–9. [PubMed] [Google Scholar]
- 21.Zou W Immunosuppressive networks in the tumour environment and their therapeutic relevance. Nature reviews cancer 2005;5(4):263. [DOI] [PubMed] [Google Scholar]
- 22.Chin JL, Pautler SE, Mouraviev V, Touma N, Moore K, Downey DB. Results of salvage cryoablation of the prostate after radiation: identifying predictors of treatment failure and complications. The Journal of urology 2001;165(6):1937–42. [DOI] [PubMed] [Google Scholar]
- 23.Dromi SA, Walsh MP, Herby S, Traughber B, Xie J, Sharma KV, et al. Radiofrequency ablation induces antigen-presenting cell infiltration and amplification of weak tumor-induced immunity 1. Radiology 2009;251(1):58–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Livraghi T, Solbiati L, Meloni MF, Gazelle GS, Halpern EF, Goldberg SN. Treatment of Focal Liver Tumors with Percutaneous Radio-frequency Ablation: Complications Encountered in a Multicenter Study 1. Radiology 2003;226(2):441–51. [DOI] [PubMed] [Google Scholar]
- 25.Poissonnier L, Chapelon J-Y, Rouviere O, Curiel L, Bouvier R, Martin X, et al. Control of prostate cancer by transrectal HIFU in 227 patients. European urology 2007;51(2):381–7. [DOI] [PubMed] [Google Scholar]
- 26.Chapman A, Ter Haar G. Thermal ablation of uterine fibroids using MR-guided focused ultrasound-a truly non-invasive treatment modality. European radiology 2007;17(10):2505–11. [DOI] [PubMed] [Google Scholar]
- 27.Silvestrini MT, Ingham ES, Mahakian LM, Kheirolomoom A, Liu Y, Fite BZ, et al. Priming is key to effective incorporation of image-guided thermal ablation into immunotherapy protocols. JCI insight 2017;2(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hu Z, Yang XY, Liu Y, Sankin GN, Pua EC, Morse MA, et al. Investigation of HIFU-induced anti-tumor immunity in a murine tumor model. Journal of translational medicine 2007;5(1):34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Eranki A, Farr N, Partanen A, Sharma KV, Rossi CT, Rosenberg AZ, et al. Mechanical fractionation of tissues using microsecond-long HIFU pulses on a clinical MR-HIFU system. International Journal of Hyperthermia 2018:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Khokhlova VA, Fowlkes JB, Roberts WW, Schade GR, Xu Z, Khokhlova TD, et al. Histotripsy methods in mechanical disintegration of tissue: Towards clinical applications. International Journal of Hyperthermia 2015;31(2):145–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vlaisavljevich E, Maxwell A, Warnez M, Johnsen E, Cain C, Xu Z. Histotripsy-induced cavitation cloud initiation thresholds in tissues of different mechanical properties. Ultrasonics, Ferroelectrics, and Frequency Control, IEEE Transactions on 2014;61(2):341–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Eranki A, Farr N, Partanen A, Sharma KV, Chen H, Rossi CT, et al. Boiling histotripsy lesion characterization on a clinical magnetic resonance imaging-guided high intensity focused ultrasound system. PloS one 2017;12(3):e0173867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Canney MS, Khokhlova VA, Bessonova OV, Bailey MR, Crum LA. Shock-induced heating and millisecond boiling in gels and tissue due to high intensity focused ultrasound. Ultrasound in medicine & biology 2010;36(2):250–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Eranki A, Mikhail AS, Negussie AH, Katti PS, Wood BJ, Partanen A. Tissue-mimicking thermochromic phantom for characterization of HIFU devices and applications. International Journal of Hyperthermia 2019;36(1):518–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.van den Bijgaart RJ, Eikelenboom DC, Hoogenboom M, Fütterer JJ, den Brok MH, Adema GJ. Thermal and mechanical high-intensity focused ultrasound: perspectives on tumor ablation, immune effects and combination strategies. Cancer Immunology, Immunotherapy 2017;66(2):247–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Johnson BD, Yan X, Schauer DW, Orentas RJ. Dual expression of CD80 and CD86 produces a tumor vaccine superior to single expression of either molecule. Cellular immunology 2003;222(1):15–26. [DOI] [PubMed] [Google Scholar]
- 37.Cano-Mejia J, Burga RA, Sweeney EE, Fisher JP, Bollard CM, Sandler AD, et al. Prussian blue nanoparticle-based photothermal therapy combined with checkpoint inhibition for photothermal immunotherapy of neuroblastoma. Nanomedicine: Nanotechnology, Biology and Medicine 2017;13(2):771–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Srinivasan P, Wu X, Basu M, Rossi C, Sandler AD. PD-L1 checkpoint inhibition and anti-CTLA-4 whole tumor cell vaccination counter adaptive immune resistance: A mouse neuroblastoma model that mimics human disease. PLoS medicine 2018;15(1):e1002497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chakrabarti L, Morgan C, Sandler AD. Combination of Id2 knockdown whole tumor cells and checkpoint blockade: a potent vaccine strategy in a mouse neuroblastoma model. PLoS One 2015;10(6):e0129237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shen Z, Zhou S, Wang Y, Li R-l, Zhong C, Liang C, et al. Higher intratumoral infiltrated Foxp3+ Treg numbers and Foxp3+/CD8+ ratio are associated with adverse prognosis in resectable gastric cancer. Journal of cancer research and clinical oncology 2010;136(10):1585–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yao X, Ahmadzadeh M, Lu Y-C, Liewehr DJ, Dudley ME, Liu F, et al. Levels of peripheral CD4+ FoxP3+ regulatory T cells are negatively associated with clinical response to adoptive immunotherapy of human cancer. Blood 2012:blood-2011–10-386482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mühlbauer M, Fleck M, Schütz C, Weiss T, Froh M, Blank C, et al. PD-L1 is induced in hepatocytes by viral infection and by interferon-α and-γ and mediates T cell apoptosis. Journal of hepatology 2006;45(4):520–8. [DOI] [PubMed] [Google Scholar]
- 43.Lina Chakrabarti CM, Anthony D. Sandler. Combination of Id2 knockdown whole tumor cells and checkpoint blockade: a potent vaccine strategy in a mouse neuroblastoma model. PLoS One 2015;10(6):e0129237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bass R, Fleshner N, Finelli A, Barkin J, Zhang L, Klotz L. Oncologic and functional outcome of partial gland ablation with HIFU for localized prostate cancer. The Journal of urology 2018. [DOI] [PubMed] [Google Scholar]
- 45.Schade GR, Wang Y-N, D’Andrea S, Hwang JH, Liles WC, Khokhlova TD. Boiling Histotripsy Ablation of Renal Cell Carcinoma in the Eker Rat Promotes a Systemic Inflammatory Response. Ultrasound in medicine & biology 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gong J, Chehrazi-Raffle A, Reddi S, Salgia R. Development of PD-1 and PD-L1 inhibitors as a form of cancer immunotherapy: a comprehensive review of registration trials and future considerations. Journal for immunotherapy of cancer 2018;6(1):8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.D’andrea A, Aste-Amezaga M, Valiante NM, Ma X, Kubin M, Trinchieri G. Interleukin 10 (IL-10) inhibits human lymphocyte interferon gamma-production by suppressing natural killer cell stimulatory factor/IL-12 synthesis in accessory cells. Journal of Experimental Medicine 1993;178(3):1041–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Strobl H, Knapp W. TGF-β1 regulation of dendritic cells. Microbes and Infection 1999;1(15):1283–90. [DOI] [PubMed] [Google Scholar]
- 49.Wheeler DS, Chase MA, Senft AP, Poynter SE, Wong HR, Page K. Extracellular Hsp72, an endogenous DAMP, is released by virally infected airway epithelial cells and activates neutrophils via Toll-like receptor (TLR)-4. Respiratory research 2009;10(1):31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Narumi K, Miyakawa R, Ueda R, Hashimoto H, Yamamoto Y, Yoshida T, et al. Proinflammatory proteins S100A8/S100A9 activate NK cells via interaction with RAGE. The Journal of Immunology 2015:1402301. [DOI] [PubMed] [Google Scholar]
- 51.Re F, Strominger JL. Toll-like receptor 2 (TLR2) and TLR4 differentially activate human dendritic cells. Journal of Biological Chemistry 2001;276(40):37692–9. [DOI] [PubMed] [Google Scholar]
- 52.Hundt W, O’Connell-Rodwell CE, Bednarski MD, Steinbach S, Guccione S. In vitro effect of focused ultrasound or thermal stress on HSP70 expression and cell viability in three tumor cell lines. Academic radiology 2007;14(7):859–70. [DOI] [PubMed] [Google Scholar]
- 53.Alcón VL, Luther C, Balce D, Takei F. B-cell co-receptor CD72 is expressed on NK cells and inhibits IFN-γ production but not cytotoxicity. European journal of immunology 2009;39(3):826–32. [DOI] [PubMed] [Google Scholar]
- 54.Yarmolenko PS, Eranki A, Partanen A, Celik H, Kim A, Oetgen M, et al. Technical aspects of osteoid osteoma ablation in children using MR-guided high intensity focussed ultrasound. International Journal of Hyperthermia 2018;34(1):49–58. [DOI] [PubMed] [Google Scholar]
- 55.Sharma KV, Yarmolenko PS, Celik H, Eranki A, Partanen A, Smitthimedhin A, et al. Comparison of noninvasive high-intensity focused ultrasound with radiofrequency ablation of osteoid osteoma. The Journal of pediatrics 2017;190:222–8. e1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


