Abstract
Purpose:
Neuropathic corneal pain (NCP) is caused by damage or disease of the somatosensory nervous system that innervates the cornea and presents with symptoms of pain or persistent unpleasant sensations, such as burning, dryness, or light sensitivity. This retrospective study aims to assess the efficacy and tolerability of low-dose naltrexone (LDN) in refractory NCP patients.
Methods:
Fifty-nine NCP patients with a centralized component treated with oral LDN 4.5 mg at bedtime for at least four weeks were identified. Thirty out of 59 patients who had a baseline pain score ≥4 on the visual analogue scale had completed the ocular pain assessment survey (OPAS) and presented persistent pain, despite instillation of topical anesthetic drops, were included. Changes in pain scores, comorbidities, side effects, among others, were analyzed. Change in ocular pain scores (scale 0–10) and quality of life (QoL) scores (scale 0–100%) were the main endpoints.
Results:
Mean age (years ± SD) was 45.60 ± 19.30 with a white (80.00%) female (73.33%) predominance. Duration of LDN use was 14.87 ± 11.25 months, and the duration of NCP before treatment was 17.53 ± 17.29 months. Eight patients used LDN as a monotherapy, whereas the remaining used it as an adjunct therapy. LDN resulted in a 49.22% decrease in mean pain score from 6.13 ± 1.93 to 3.23 ± 2.60 (p < 0.001). Mean QoL scores by the OPAS were 5.84 ± 2.57 at the first visit and improved to 3.77 ± 2.91 at the last visit (p = 0.023). Common side effects were vivid dreams, headaches, and stomachache.
Conclusion:
LDN was effective and well-tolerated for NCP treatment.
Keywords: Neuropathic corneal pain, Corneal neuralgia, Low-dose naltrexone
1. Introduction
The cornea is the most densely innervated tissue in the body [1,2], and the corneal nerves, which are part of the somatosensory nervous system, are specialized peripheral sensory neurons [3]. Neuropathic Corneal Pain (NCP) is a condition caused by damage or disease of the somatosensory nervous system that innervates the cornea [4,5], presenting with symptoms of pain or persistent unpleasant sensations, such as severe dryness, burning, foreign body sensation, or light sensitivity, among others. While presence of nerve injury can generate physiological pain responses, thus protecting tissues form acute injuries [6,7], tissue and nerve damage together with persistent inflammation can result in increased nociceptive sensitivity to environmental stimuli, leading to peripheral sensitization. Over time, persistent stimuli may lead to central nervous system (CNS) changes, resulting in central sensitization that contributes to perpetuation of pain signals arising from the CNS [8]. Spontaneous pain, allodynia (pain from typically non-noxious stimuli), and hyperalgesia (pain that are exaggerated in severity and duration), among others, can be indicative of sensitization [6,9]. NCP patients can present with persistent symptoms after the instillation of topical anesthetic drops, such as the proparacaine hydrochloride (in-office test used to suppress stimuli from peripheral nociceptors). The lack of complete resolution with anesthetic drops suggests that a central component of pain/symptoms may be present [4,5,10], which is crucial for treatment guidance [5]. Moreover, NCP patients with the central component of pain may often suffer from concurrent neuropsychiatric co-morbidities, such as anxiety, depression, or migraines among others [11–13].
Low-dose naltrexone (LDN) in doses ranging from 1.5 to 4.5 mg operates through two mechanisms of action, targeting analgesia and neuroinflammation [14,15]. LDN achieves analgesia through transient blockade of opioid receptors, in particular μ− and δ opioid receptors, which in turn result in upregulation of opioid signaling, and thereby increasing the production of endogenous endorphins [15]. In addition, increased endogenous endorphins have been shown to result in a positive impact on quality of life [16,17]. LDN also exerts an anti-inflammatory effect, through specific binding to the Toll-like receptor (TLR)-4, acting as an antagonist, and thereby reducing neuroinflammation and neurotoxicity [14,15,18]. More recent studies have explored the safety and efficacy of off-label use of LDN in chronic pain syndromes, such as fibromyalgia, low back pain, complex regional pain syndromes, and refractory painful diabetic neuropathy [19–21].
Thus, we hypothesize that LDN may be effective in ameliorating pain and discomfort, as well as improving quality of life in NCP patients with a central component of pain, who failed to respond to previous treatments. The purpose of this study was therefore to assess the efficacy and tolerability of LDN in reducing pain and discomfort in NCP patients with a central component of the disease. To our knowledge, this is the first study demonstrating the efficacy of LDN in relieving pain and discomfort in NCP patients.
2. Methods
2.1. Patient selection
This study is a retrospective cohort study, which was conducted at the Cornea Service, New England Eye Center, Department of Ophthalmology, Tufts Medical Center, Tufts University Medical School, Boston, Massachusetts. The Institutional Review Board/Ethics Committee approved the protocol. We ensured compliance with the Health Insurance Portability and Accountability Act and adherence to the tenets of the Declaration of Helsinki. Charts between July 1, 2015 and March 31, 2019 were reviewed. Patients with a diagnosis of ocular pain (ICD-10: H57.1 or ICD-9: 379.91) and whose charts included the keyword “naltrexone” were identified. All patients were required to have been diagnosed by a single cornea specialist (PH), who used the ocular pain ICD codes for diagnosis of NCP. Diagnosis was based on medical history, minimal or absent clinical signs on slit-lamp examination, presence of neuropathic pain or neuropathic like symptoms (burning, stinging, foreign body sensation and photoallodynia), and corneal in vivo confocal microscopic (IVCM) findings [5]. Patients were required to present persistent ocular discomfort/pain after instillation of 0.5% proparacaine hydrochloride (Alcaine; Alcon, Fort Worth, TX). LDN was used off-label as a therapy in these NCP patients, started at 1.5 mg and increased to a final dose of 4.5 mg.
We utilized the Ocular Pain Assessment Survey (OPAS) in this study [22]. The OPAS is a multi-dimensional survey, that assesses eye pain intensity within the past 24h and 2 weeks, non-eye pain intensity, quality of life assessment, aggravating factors, and associated factors, using numerical rating scales (0–10). To be included in the analysis, patient charts were required to have documented 1) LDN use for at least 4 weeks. 2) completed OPAS questionnaire before treatment with LDN and at the most recent visit; 3) pain level of 4 or higher at the initial visit. Patients were excluded if they had any ocular pathology that might cause nociceptive pain (e.g. active corneal infections, abrasions, recurrent erosion syndrome, angle-closure glaucoma, and anterior uveitis).
2.2. Chart reviews
A detailed chart review was conducted. Demographics, time between visits (i.e. duration of LDN use), concomitant medications, systemic and ocular co-morbidities, ocular surgeries, duration of NCP, side effects, reason for discontinuation, and response to OPAS questions were recorded.
2.3. Statistical analysis
Statistical analysis was performed with SPSS version 20 (IBM SPSS Statistics, Chicago, IL, USA). Distribution of the data was analyzed by Shapiro-Wilk test. Differences between visits were analyzed using paired t-test for normally distributed data and Wilcoxon signed-rank test for non-normally distributed data. Mixed model analyses were performed on OPAS questions with greater than 20 subject responses to test for potential confounders that could have influenced the apparent impact of naltrexone. The mixed model analysis compared initial and last visit responses and included variables of age, sex, race, presence/absence of selected concomitant medications, and presence/absence of selected systemic co-morbidities. The selection of concomitant medications and systemic co-morbidities was based on the need to minimize the impact of outliers on the model statistics. This was accomplished by requiring included concomitant medications and co-morbidities to 1) have at least three subjects in the presence and absence groups for each concomitant medication/co-morbidity and 2) have a significant difference in the change in response (final visit response-initial visit response) between the presence and absence groups. P values less than 0.05 were considered statistically significant.
3. Results
3.1. Demographics
Fifty-nine NCP patients were identified that had been prescribed off-label LDN and 30 patients were included in the final dataset for the efficacy analysis, based on inclusion/exclusion criteria. From the other 29 patients, 9 patients were excluded due to lack of follow-up, 3 patients had baseline pain <4, 12 patients were excluded, as they never used LDN due to cost and lack of insurance coverage, 3 patients stopped LDN due to early side effects, and 2 patients stopped LDN due to lack of improvement. The mean age (years ± SD) was 45.60 ± 19.30 with a white (80.00%) female (73.33%) predominance (Table 1). Systemic comorbidities were a present feature among included NCP patients. Neuropsychiatric and cardiovascular diseases (50.0%), followed by autoimmune diseases (16.66%), were the most frequently observed. When stratified by disease, depression (16.70%) and anxiety (10.00%) were the most common diseases present among neuropsychiatric diseases, while rheumatoid arthritis (13.33%) was the most commonly associated autoimmune disease. Moreover, 22 (73.33%) patients presented with more than one systemic co-morbidity (Table 2). Regarding ocular events, a prior history of dry eye disease (66.70%) was the most prevalent ocular disease followed by the event of ocular surgery (including cataract and refractive surgery – 23.3% recorded among NCP patients included in this study; Table 3). Regarding the use of concomitant topical medications, almost all patients were on topical steroids (86.7%) and autologous serum tears (80.0%). Moreover, most patients were also on other systemic neuromodulatory medications, and the most frequent among them were Nortriptyline (26.6%), Selective Serotonin Reuptake Inhibitors (SSRIs – 26.6%), and Gabapentin (23.3%).
Table 1.
Baseline demographics (N = 30).
| Age, year (SD) | 45.60 (19.30) |
|---|---|
|
| |
| Gender | |
| Male, n (%) | 8 (26.7) |
| Female, n (%) | 22 (73.3) |
| Ethnicity, | |
| Caucasian, n (%) | 24 (80.0) |
| Asian, n (%) | 6 (20.0) |
Table 2.
Systemic co-morbidities of patients with NCP.
| Co-morbidities | Patients n (%) |
|---|---|
|
| |
| Neuro-psychiatric | |
| Depression | 5 (16.7) |
| Anxiety | 3 (10.0) |
| Fibromyalgia | 2 (6.7) |
| Non-migraine Headaches | 2 (6.7) |
| Migraine | 1 (3.3) |
| Multiple Sclerosis | 1 (3.3) |
| Seizures | 1 (3.3) |
| Cardiovascular | |
| Hypertension | 10 (33.3) |
| Tachycardia | 4 (13.3) |
| Hyperlipidemia | 3 (10.0) |
| Autoimmune | |
| Rheumatoid Arthritis | 4 (13.3) |
| Myasthenia Gravis | 1 (3.3) |
| Miscellaneous | |
| Hypothyroidism | 5 (16.7) |
| Asthma | 3 (10.0) |
| Diabetes mellitus | 2 (6.7) |
| Anemia | 1 (3.3) |
| History of Cancer | 1 (3.3) |
Table 3.
Ocular co-morbidities of patients with neuropathic corneal pain.
| Patients, n (%) | |
|---|---|
|
| |
| History of Dry Eye Disease | 20 (66.7) |
| Glaucoma | 2 (6.7) |
| Post-herpetic Keratitis | 1 (3.3) |
| History of Ocular Surgeries | 7 (23.3%) |
| Refractive Surgery (LASIK, PRK) | 1 (3.3) |
| Other ocular surgeries (cataract surgery, pterygium surgery, strabismus surgery) | 6 (20.0) |
(LASIK: laser in-situ keratomileusis, PRK: photo refractive keratectomy).
All surgeries were conducted bilaterally.
A complete list of all systemic and ocular diseases is provided in Tables 2 and 3. A comprehensive list of adjuvant treatments is shown in Supplemental Table 1
3.2. Efficacy
The efficacy results showed that sixteen patients (53.33%) had equal to, or more than 50% improvement in pain, 9 patients (30.00%) had 1–49% improvement, 2 patients (6.67%) did not improve, while 3 (10.00%) patients got worse (Fig. 1). The average percent improvement in pain between the first and last visit was −49.22%. The average ocular pain intensity in the past 24 h was 5.17 ± 1.77 (range 4–10) at the first visit, and 3.85 ± 2.88 (range 0–10) at the last visit (p < 0.011). The average level of pain in the last 2 weeks also decreased from 5.79 ± 0.30 at the first visit to 3.70 ± 0.74 at the last visit (p < 0.01; Fig. 2; Table 4). Similarly, the highest level of pain intensity in the last 24 h decreased from 7.00 ± 1.59 for the initial visit to 4.56 ± 3.10 for the last visit (p = 0.003). A reduction was also observed for the highest level of pain in the last 2 weeks from 6.87 ± 2.07 to 2.42 ± 2.41 for the initial and last visit respectively (p = 0.008).
Fig. 1. Percentage change in pain scores between the initial and last visit.
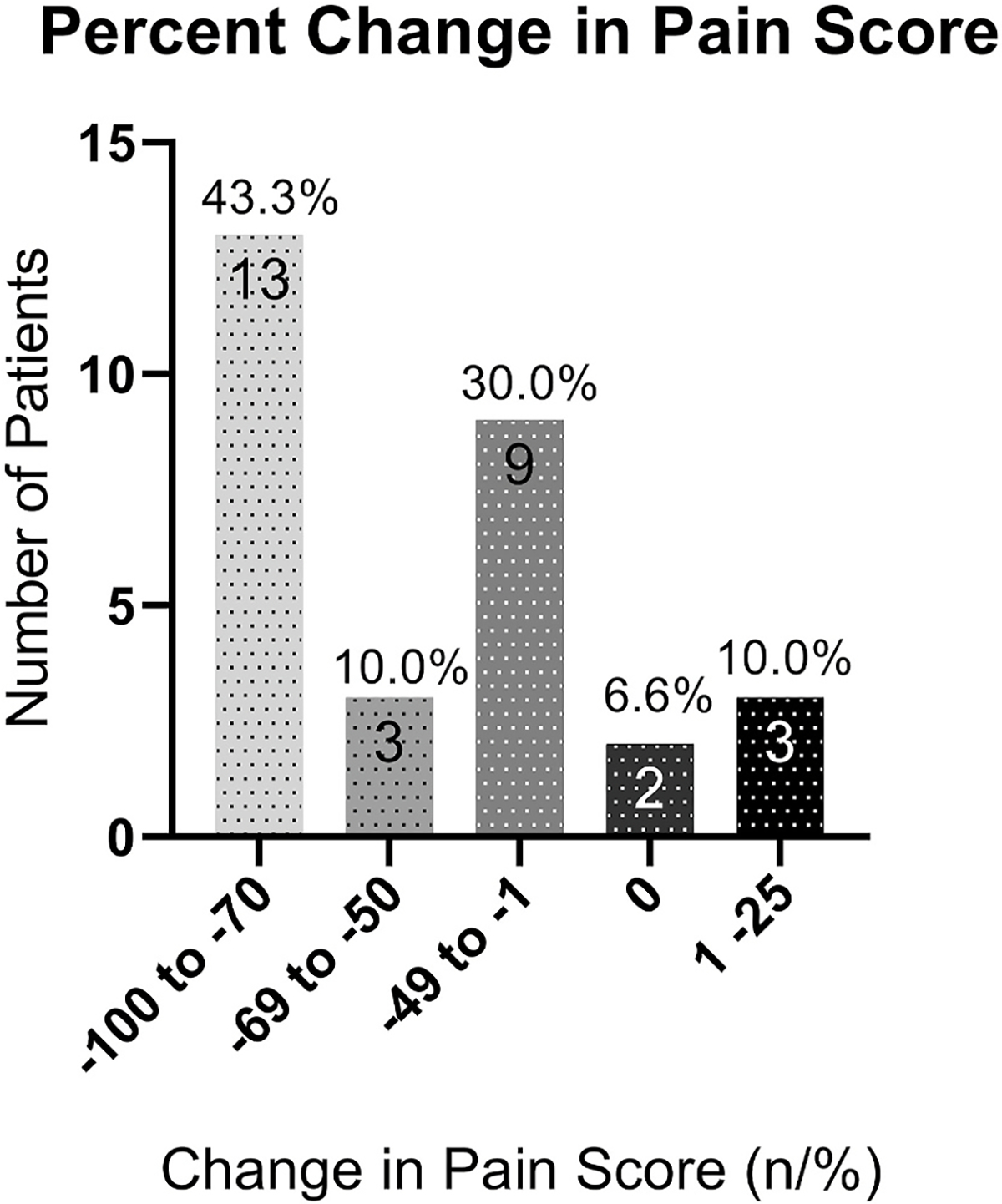
Sixteen patients (53.33%) had equal to or more than 50% improvement, 4 patients (13.33%) had 30–49% improvement, 5 patients (16.67%) had 1–29% improvement, and 5 patients (16.67%) did not improve with use of low dose naltrexone.
Fig. 2.
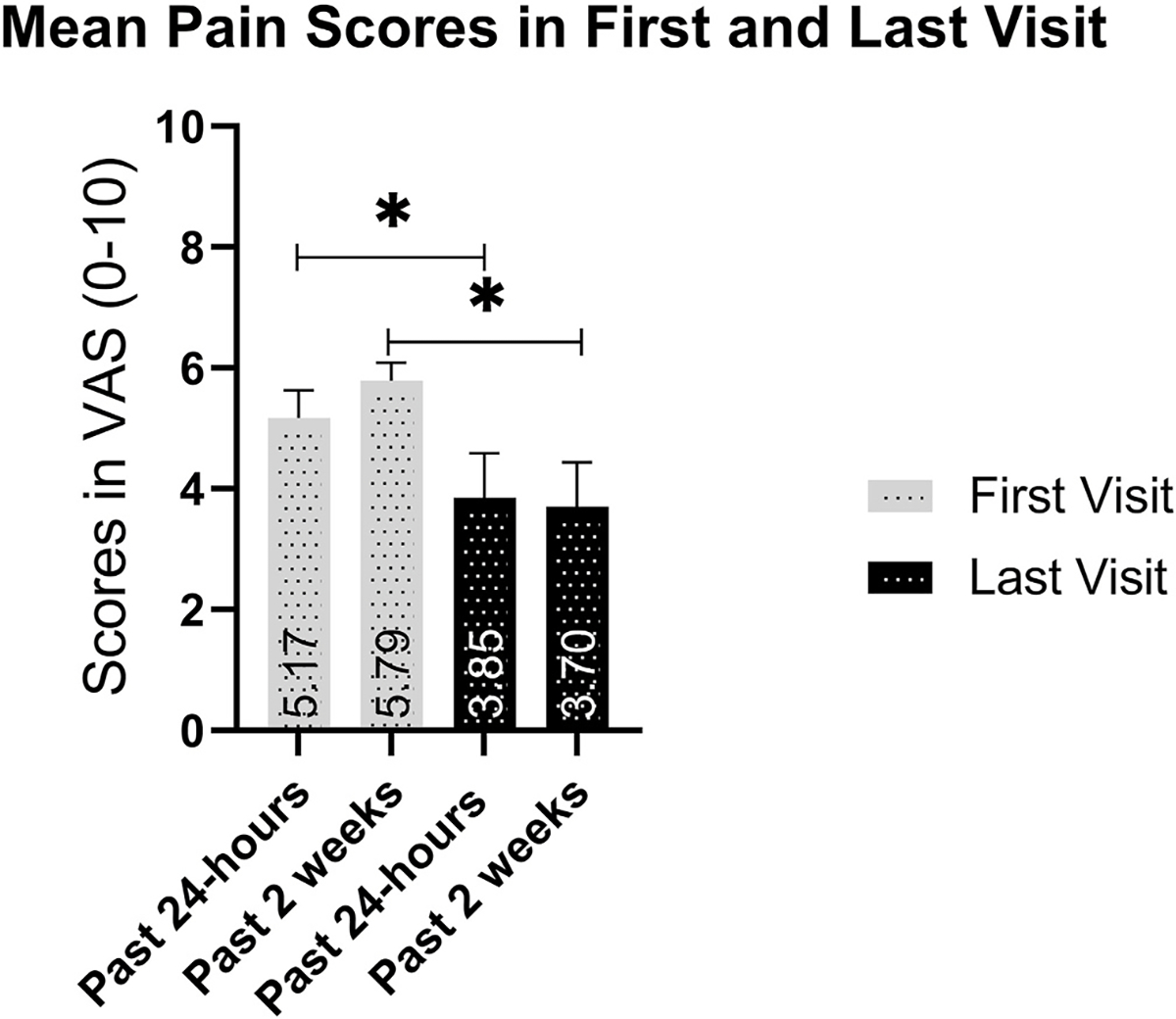
Change in pain scores based on the pain dimension of the ocular pain assessment survey. Pain levels for the past 24 h and past 2 weeks decreased between the initial and last visit with use of low dose naltrexone.
Table 4.
Results from the questions in pain level dimension of the OPAS (h = hours, w = weeks, data in mean ± SD).
| Eye Pain Question | First Visit Score | Last Visit Score | Percent Change in Pain Score | P |
|---|---|---|---|---|
|
| ||||
| Most in 24h | 7.00 ± 0.39 | 4.56 ± 0.77 | −46.21 ± 9.74 | 0.003 |
| Least in 24h | 3.50 ± 0.54 | 2.42 ± 0.60 | −33.70 ± 10.63 | 0.087 |
| Average in 24h | 5.17 ± 0.46 | 3.85 ± 0.74 | −46.83 ± 10.14 | 0.011 |
| Most in 2w | 6.87 ± 0.53 | 4.78 ± 0.80 | −39.30 ± 20.76 | 0.008 |
| Least in 2w | 4.47 ± 0.60 | 2.75 ± 0.69 | −58.03 ± 10.38 | 0.008 |
| Average in 2w | 5.79 ± 0.30 | 3.70 ± 0.74 | −50.14 ± 11.01 | 0.010 |
Light sensitivity (53.3%) and burning (6.7%) were the two most common other symptoms associated to pain and reported in the study, followed by foreign body sensation (3.3%), grittiness (3.3%), and itching (3.3%). Burning and light sensitivity showed improvement from the initial visit (4.39 ± 3.50 and 6.62 ± 3.67), to the last visit (3.78 ± 3.54 and 5.14 ± 4.15, respectively), although there was no significant reduction in either (p = 0.22 and p = 0.16, respectively). The average duration of LDN use was 14.87 ± 11.25 months and duration of NCP symptoms prior to treatment were 17.53 ± 17.29 months, both of which did not correlate with percent change in average ocular pain (Spearman’s rho = −0.163, p = 0.389 and Spearman’s rho = −0.270, p = 0.149 respectively). Mixed model analysis showed that age, sex, and race did not have a significant impact on the results for pain levels. Selected concomitant medications (topical steroids, serum tears, nortriptyline, serotonin-norepinephrine reuptake inhibitors, benzodiazepines, tricyclic antidepressants, carbamazepine, gabapentinoids, opioid agonists, and medically prescribed marijuana) and selected systemic co-morbidities (rheumatoid arthritis and Sjögren’s syndrome) did not have a significant effect on the model (all p > 0.05). This lack of significance provides further evidence that LDN was effective in lowering the pain levels of patients independent of other factors. The off-label use of LDN in NCP patients was effective in reducing pain levels, as well as unpleasant sensations, such as burning and light sensitivity. Moreover, LDN use resulted in improvement of patients’ quality of life as described below.
3.3. Quality of life
Mean QoL scores improved from 5.84 ± 2.57 at the first visit to 3.77 ± 2.91 at the last visit with LDN treatment (p = 0.02). A significant reduction was observed on time spending thinking about pain from the initial visit (7.50 ± 3.06) to the last visit (4.74 ± 3.63), with 35.56% improvement between the visits (p = 0.021). Further, enjoyment of life showed a significant improvement (43.58%) between the visits (from 6.27 ± 3.24 to 3.61 ± 3.42; p = 0.043). All other questions, including reading/using computer, driving watching TV, general activity, mood and sleep, while also showing improvement, did not reach statistical significance. Table 5 and Fig. 3 summarize the results of individual QoL questions.
Table 5.
Results from the questions in the quality of life dimension of the OPAS (QoL data in mean ± SD).
| Pain affecting: | First Visit Score | Last Visit Score | Percent Change in QoL Score | P |
|---|---|---|---|---|
|
| ||||
| Reading/computer use | 7.60 ± 2.47 | 5.35 ± 3.63 | −37.82 ± 50.97 | 0.059 |
| Driving/watching TV | 7.00 ± 3.01 | 4.98 ± 3.97 | −23.07 ± 66.53 | 0.096 |
| General activity | 4.29 ± 3.02 | 3.08 ± 3.27 | −34.76 ± 65.18 | 0.193 |
| Mood | 6.40 ± 2.95 | 4.13 ± 3.18 | −7.39 ± 109.15 | 0.064 |
| Sleep | 4.00 ± 3.82 | 2.61 ± 2.74 | −31.02 ± 62.36 | 0.169 |
| Enjoying life | 6.27 ± 3.24 | 3.61 ± 3.42 | −43.58 ± 55.47 | 0.043 |
| Time spent thinking about pain | 7.50 ± 3.06 | 4.74 ± 3.63 | −34.56 ± 40.67 | 0.021 |
Fig. 3.
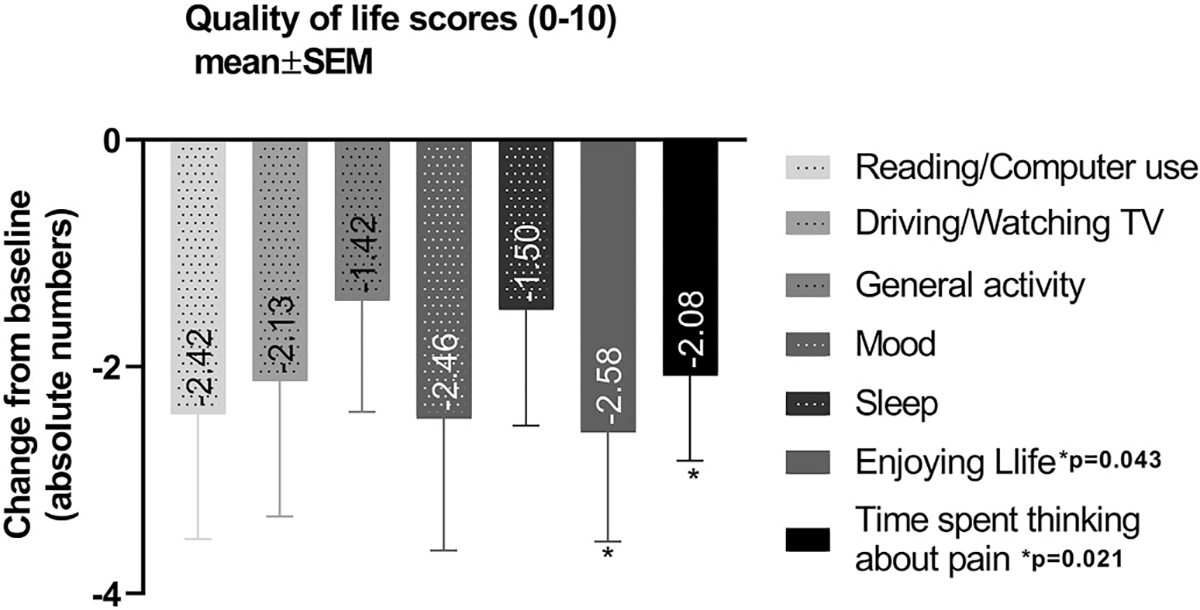
Quality of life score changes with use of low dose naltrexone. A significant reduction was observed on time spending thinking about pain and enjoying life with use of low dose naltrexone.
3.4. Side effects
Three out of the 29 patients not included in the study, presented side effects, and discontinued the treatment before 4 weeks. Six out of 30 included in the efficacy analysis presented side effects while in treatment with LDN for more than 4 weeks. When combined, from a total of 59 LDN prescribed patients, nine (15.25%) presented side effects. Patients that discontinued LDN before 4 weeks and were not included in the efficacy analysis reported headaches (n = 1, 3.4%), nausea (n = 1, 3.4%), increased anxiety (n = 1, 3.4%), sleep disturbances (n = 1, 3.4%) and unwell feeling (n = 1, 3.4%). Patient-reported side effects in the group that was assessed for efficacy analysis included vivid dreams (n = 3, 10.0%) and headaches (n = 2, 6.6%). One patient presented with stomachache that improved after dose reduction from 4.5 mg to 3.0 mg daily. Only one of the patients (3.4%) who used LDN more than 4 weeks and were included in the efficacy analysis discontinued the treatment.
LDN was well-tolerated among most patients. Three patients discontinued LDN before 4 weeks of use, due to nausea, headache, and increased anxiety and sleep disturbances. Stomachache was reported as a minor side effect in one patient and it was resolved with the dose reduction from 4.5 to 3.0 mg/day. All the side effects reported during the period of the study were minors and resolved with time or dose reduction.
4. Discussion
Herein, we demonstrate, for the first time, that NCP patients with the central component of pain, without complete relief of symptoms after topical anesthetic drops, respond to LDN. Interestingly, the improvement in pain and other symptoms of discomfort is noted even in the presence of previously prescribed concomitant systemic medications.
In this study we excluded patients with baseline pain levels below 4. Baseline pain scores below 4 are highly subjective to intraindividual variations. Therefore, they are not considered reliable for the propose of efficacy measurements. Moreover, it is clinically and statistically challenging to demonstrate a significant effect size (risk reduction) of any pain treatment using baseline pain scores below 4. Indeed, most of the articles exploring the efficacy of pain medications use four as baseline pain scores [23–25]. The cutoff value for clinical significance in clinical trials with chronic pain patients is determined as the minimal amount of change in pain that would be valuable and important to patients. Many different approaches to establishing this minimal significant clinical difference have been proposed. Most studies use the cutoff method of 30% to suggests clinical importance. However, to be even more conservative, our group used the cutoff threshold of 50% to achieve clinically relevant results [24,26,27].
There was significant improvement in pain for the past 24 h and for the past two weeks in the current cohort. Due to the novelty of its analgesic and anti-inflammatory property, there are few reports using LDN in chronic pain syndromes. These include one study in refractory painful diabetic neuropathy, where LDN was used as an adjunctive therapy, in which the percentage of pain improved from 90% to 5% after two weeks of use [21]. Two additional reports describing the off-label use of LDN for pain relief in complex regional pain syndrome and chronic low-back pain further reported pain relief in a period up to 90 days [28–30].
In addition to pain, photoallodynia appears to be a critical symptom in NCP as demonstrated by its high frequency in our cohort. Similar to what was observed in this study, photoallodynia or light sensitivity has been described to be a frequent and severe complain among dry eye patient with neuropathic symptoms and has been suggested to be associated with centralized trigeminal pain or ganglionopathy [31,32].
Naltrexone is a nonselective pure opioid antagonist with affinity in particular to μ− and δ opioid receptors. LDN exerts its analgesic effect through a transient opioid receptor blockage, increasing the levels of endogenous endorphins, a phenomenon known as opioid rebound effect [18]. In addition, LDN has an anti-neuroinflammatory effect when it binds to TLR-4 on glial cells throughout the central and peripheral nervous system [15,33]. TLR-4 initiates the cellular signaling pathways in response to pain-induced pro-inflammatory cytokines [14]. The release of cytokines and neurotransmitters, such as interleukin-1, tumor necrosis factor-α, interferon-β, excitatory amino acids, substance P, and nitric oxide, result in neuroinflammation, increased pain sensitivity, cognitive disruption, sleep and mood disorders [14,34,35].
Naltrexone has been approved by the Food and Drug Administration (FDA) for treatment of alcohol dependence or opioid use disorders at doses of at least 50 mg [36]. However, when used at lower doses (1.5–4.5 mg), it has been showed to have other pharmacological properties. The off-label use of LDN was first described in the literature as an adjunct therapy in acquired immune deficiency syndrome (AIDS) patients in the mid-1980s [37]. However, the first reference to LDN studies on chronic pain are from two small placebo-controlled trials that used LDN to reduce pain in patients with fibromyalgia, showing that LDN was superior to placebo in reducing pain associated with fibromyalgia [38, 39]. However, due to the scarcity of larger, controlled, clinical trials on LDN, its use remains off-label for pain. Nevertheless, the promising results on small studies and trials recently published warrant the need for more controlled studies to evaluate the efficacy, safety and drug interactions in larger randomized trials.
In a previous study our group has shown that corneal pain has a significant high negative impact on QoL in patients with NCP, especially with regard to reading, sleep, mood, general activity, relation with other people and enjoying life [13]. In the current cohort we show that LDN promotes a significant improvement in the QoL among NCP patients, reducing the time spent thinking about pain and allowing them to enjoy life. Enhancement of QoL with LDN use has also been described in patients with depression and multiple sclerosis (MS) [40–42]. The mechanisms of action proposed for this benefit has been the link between the μ-opioid receptors and central dopaminergic neurons in the mesencephalon [17]. A single center, double masked, placebo-controlled, crossover study evaluated the efficacy of LDN on self-reported quality of life of patients with MS. As a result the authors concluded that LDN was associated with a significant improvement on mental health quality of life indices [43]. Moreover, LDN has been described as having a role in promoting stress resilience, exercise, social bonding, and emotional well-being, as well as amelioration of psychiatric disorders such as depression [17]. A randomized, double blind, placebo-controlled, counterbalanced, crossover trial assessing daily pain levels in fibromyalgia patients showed that LDN was effective improving general satisfaction with life and improved mood [39].
Although data on side effects linked to LDN is still scarce; the most common side effects described in the literature are vivid dreams and headaches followed by insomnia, nightmares and sleep disturbances that might be addressed by changing the time of taking daily doses usually from bedtime to morning hours, or these disturbances can resolve on their own with prolonged therapy [15,44]. In the current study, LDN was well-tolerated among most of the patients. Three patients discontinued LDN before 4 weeks of use, due to nausea, headache, and increased anxiety and sleep disturbances. Stomachache was reported as a minor side effect in one patient and it was resolved with the dose reduction from 4.5 to 3.0 mg/day. Lie et al. conducted a 12-week prospective study with patients refractory to conventional therapy for inflammatory bowel disease, in which 14.9% of the total patients treated with LDN presented adverse events, such as vivid dreams (8.5%) and headache (2.1%) [44]. Vivid dreams were also reported in the present cohort. Only one patient presented severe headache and discontinued the treatment with LDN. These results are similar to what was observed in other studies where LDN was used as an off -label therapy for chronic pain treatment such as in fibromyalgia and chronic low-back pain [28, 39].
The efficacy of LDN shown in patients with NCP provides a potentially valuable tool in the armamentarium for the treatment of NCP. Previous studies have shown a limited efficacy of gabapentinoids in ameliorating pain in patients with chronic ocular pain. In a retrospective case series of 8 patients receiving oral gabapentinoids as part of their pain regimen, 6 patients showed a partial or complete resolution of their pain/symptoms, while two patients did not improve [45]. Additional studies have shown efficacy in ameliorating pain among NCP patients with the use of self-retained cryopreserved amniotic membrane and autologous serum tears [46,47]. However, patients from these studies had peripheral NCP that resolved with anesthetic drops as opposed to patients in the current study.
All patients included in this study were chronic pain (NCP) patients that have been suffering from moderate to severe chronic pain for months/years and had failed to respond to any previous topical or oral therapy, and as such were refractory. The lack of a standard protocol for treatment, the failure of topical and first-line oral therapy, the severity of the disease, and the devastating impact on patients’ quality of life, justified the off-label use of LDN as an exploratory treatment option. Another aspect that supported the off-label use of LDN in NCP patients, is the good drug tolerability reported in the literature when compared to other neuromodulatory drugs.
Despite the promising results, our study has several limitations. The retrospective nature and the relatively small sample size are the main limitation of this study. However, considering the scarcity of available treatments for NCP patients, the high intensity pain levels, and the extremely negative impact on their quality of life, the current study adds to the clinical available treatment options and is an important contribution to NCP management. Further, given the relatively rare presentation of this disease, larger series are more difficult to conduct. In fact, the current study is the largest to date, reporting the efficacy of any treatment on NCP with the central component of pain. Nevertheless, the current study warrants larger, double-blinded, placebo-controlled, or drug-controlled studies on LDN use among NCP patients in the future.
5. Conclusion
In summary, we demonstrate that NCP patients with the central component of pain benefit of using LDN. Patients demonstrate significant improvement of pain and symptoms of discomfort. Moreover, use of LDN resulted in significant improvement of quality of life. Taking into account the limited number of therapeutic options and the positive efficacy and tolerability profile of LDN, this medication could be considered as an alternative off-label therapeutic modality for the treatment of patients with refractory NCP.
Supplementary Material
Financial support
NIH R61-NS113341 (PH), Massachusetts Lions Eye Research Fund Inc. (PH), Lions Club International Foundation (PH), Bettingen Foundation (PH), Tufts Medical Center Institutional support (PH), Research to Prevent Blindness Challange Grant to the Department of Ophthalmology.
Acronyms and abbreviations
- NCP
neuropathic corneal pain
- LDN
low-dose naltrexone
- QoL
quality of life
- CNS
central nervous system
- CRPS
complex regional pain syndrome
- TLR-4
toll-like receptor 4
- NF-KB
nuclear factor-kBB
- JNK
c-Jun-N-terminal kinase
- ERK
extracellular signal-related kinase
- BNDF
brain-derived neurotrophic factor
- OGF
opioid growth factor
- OGFr
opioid growth factor receptor
- Met5
Metenkephalin
- MS
multiple sclerosis
- IVCM
in vivo confocal microscopy
- OPAS
ocular pain assessment survey
- FDA
food and drug administration
Footnotes
Declaration of competing interest
G Dieckmann: None; M. C. Ozmen: None; S. M. Cox: None; R. C. Engert: None; P. Hamrah: grants and personal fees from Novartis, Shire, Ocunova, and Coopervision, outside the submitted work. In addition, P. Hamrah has a patent System for detecting micro-neuromas and methods of use therefore pending.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jtos.2020.12.003.
References
- [1].Muller LJ, Marfurt CF, Kruse F, Tervo TM. Corneal nerves: structure, contents and function. Exp Eye Res 2003;76:521–42. [DOI] [PubMed] [Google Scholar]
- [2].Belmonte C, Aracil A, Acosta MC, Luna C, Gallar J. Nerves and sensations from the eye surface. Ocul Surf 2004;2:248–53. [DOI] [PubMed] [Google Scholar]
- [3].Belmonte C, Nichols JJ, Cox SM, Brock JA, Begley CG, Bereiter DA, et al. TFOS DEWS II pain and sensation report. Ocul Surf 2017;15:404–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Goyal S, Hamrah P. Understanding neuropathic corneal pain–gaps and current therapeutic approaches. Semin Ophthalmol 2016;31:59–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Dieckmann G, Goyal S, Hamrah P. Neuropathic corneal pain: approaches for management. Ophthalmology 2017;124:S34–s47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Belmonte C, Acosta MC, Merayo-Lloves J, Gallar J. What causes eye pain? Curr Ophthalmol Rep 2015;3:111–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Basbaum AI, Bautista DM, Scherrer G, Julius D. Cellular and molecular mechanisms of pain. Cell 2009;139:267–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Borsook D, Youssef AM, Barakat N, Sieberg CB, Elman I. Subliminal (latent) processing of pain and its evolution to conscious awareness. Neurosci Biobehav Rev 2018;88:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Kobayashi S Organization of neural systems for aversive information processing: pain, error, and punishment. Front Neurosci 2012;6:136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Crane AM, Feuer W, Felix ER, Levitt RC, McClellan AL, Sarantopoulos KD, et al. Evidence of central sensitisation in those with dry eye symptoms and neuropathic-like ocular pain complaints: incomplete response to topical anaesthesia and generalised heightened sensitivity to evoked pain. Br J Ophthalmol 2017;101: 1238–43. [DOI] [PubMed] [Google Scholar]
- [11].Dieckmann G, Koseoglu DN, Jamali A, Lopez MJ, Moein HR, Hamrah P. Demographics and risk factors of patients with neuropathic corneal pain. Poster presented at: American Academy of Ophthalmology; November 11–14, 2017; New Orleans, LA PO058. [Google Scholar]
- [12].Chang VS, Rose TP, Karp CL, Levitt RC, Sarantopoulos C, Galor A. Neuropathic-like ocular pain and nonocular comorbidities correlate with dry eye symptoms. Eye Contact Lens 2018;44(Suppl 2):S307–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Lopez MJ, Jamali A, Dieckmann G, Koseoglu D, Ramesh N, Khaksari B, et al. Corneal pain has a negative impact on the quality of life of patients with neuropathic corneal pain. Invest Ophthalmol Vis Sci 2018;59:138. [Google Scholar]
- [14].Toljan K, Vrooman B. Low-dose naltrexone (LDN)-Review of therapeutic utilization. Med Sci 2018;6:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Younger J, Parkitny L, McLain D. The use of low-dose naltrexone (LDN) as a novel anti-inflammatory treatment for chronic pain. Clin Rheumatol 2014;33:451–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Lutz PE, Kieffer BL. Opioid receptors: distinct roles in mood disorders. Trends Neurosci 2013;36:195–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Brown N, Panksepp J. Low-dose naltrexone for disease prevention and quality of life. Med Hypotheses 2009;72:333–7. [DOI] [PubMed] [Google Scholar]
- [18].Trofimovitch D, Baumrucker SJ. Pharmacology update: low-dose naltrexone as a possible nonopioid modality for some chronic, nonmalignant pain syndromes. Am J Hosp Palliat Care 2019;36:907–12. [DOI] [PubMed] [Google Scholar]
- [19].Patten DK, Schultz BG, Berlau DJ. The safety and efficacy of low-dose naltrexone in the management of chronic pain and inflammation in multiple sclerosis, fibromyalgia, crohn’s disease, and other chronic pain disorders. Pharmacotherapy 2018;38:382–9. [DOI] [PubMed] [Google Scholar]
- [20].Younger J, Parkitny L, McLain D. The use of low-dose naltrexone (LDN) as a novel anti-inflammatory treatment for chronic pain. Clin Rheumatol 2014;33:451–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Hota D, Srinivasan A, Dutta P, Bhansali A, Chakrabarti A. Off-label, low-dose naltrexone for refractory painful diabetic neuropathy. Pain Med 2016;17:790–1. [DOI] [PubMed] [Google Scholar]
- [22].Qazi Y, Hurwitz S, Khan S, Jurkunas UV, Dana R, Hamrah P. Validity and reliability of a novel ocular pain assessment survey (OPAS) in quantifying and monitoring corneal and ocular surface pain. Ophthalmology 2016;123:1458–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Olsen MF, Bjerre E, Hansen MD, Tendal B, Hilden J, Hróbjartsson A. Minimum clinically important differences in chronic pain vary considerably by baseline pain and methodological factors: systematic review of empirical studies. J Clin Epidemiol 2018;101:87–106.e2. [DOI] [PubMed] [Google Scholar]
- [24].Farrar JT, Young JP Jr, LaMoreaux L, Werth JL, Poole RM. Clinical importance of changes in chronic pain intensity measured on an 11-point numerical pain rating scale. Pain 2001;94:149–58. [DOI] [PubMed] [Google Scholar]
- [25].Farrar JT, Young JP Jr, LaMoreaux L, Werth JL, Poole RM. Clinical importance of changes in chronic pain intensity measured on an 11-point numerical pain rating scale. Pain 2001;94:149–58. [DOI] [PubMed] [Google Scholar]
- [26].Younger J, McCue R, Mackey S. Pain outcomes: a brief review of instruments and techniques. Curr Pain Headache Rep 2009;13:39–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Olsen MF, Bjerre E, Hansen MD, Tendal B, Hilden J, Hróbjartsson A. Minimum clinically important differences in chronic pain vary considerably by baseline pain and methodological factors: systematic review of empirical studies. J Clin Epidemiol 2018;101:87–106. e2. [DOI] [PubMed] [Google Scholar]
- [28].Ghai B, Bansal D, Hota D, Shah CS. Off-label, low-dose naltrexone for refractory chronic low back pain. Pain Med 2014;15:883–4. [DOI] [PubMed] [Google Scholar]
- [29].Sturn KM, Collin M. Low-dose naltrexone: a New therapy option for complex regional pain syndrome type I patients. Int J Pharm Compd 2016;20:197–201. [PubMed] [Google Scholar]
- [30].Weinstock LB, Cottel J, Aldridge L, Egeberg A. Low-dose naltrexone therapy for psoriasis. Int J Pharm Compd 2020;24:94–6. [PubMed] [Google Scholar]
- [31].Galor A, Levitt RC, Felix ER, Sarantopoulos CD. What can photophobia tell us about dry eye? Expet Rev Ophthalmol 2016;11:321–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Rosenthal P, Borsook D. Ocular neuropathic pain. Br J Ophthalmol 2016;100: 128–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Bsibsi M, Ravid R, Gveric D, van Noort JM. Broad expression of Toll-like receptors in the human central nervous system. J Neuropathol Exp Neurol 2002;61:1013–21. [DOI] [PubMed] [Google Scholar]
- [34].Cant R, Dalgleish AG, Allen RL. Naltrexone inhibits IL-6 and TNFalpha production in human immune cell subsets following stimulation with ligands for intracellular toll-like receptors. Front Immunol 2017;8:809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Ji RR, Nackley A, Huh Y, Terrando N, Maixner W. Neuroinflammation and central sensitization in chronic and widespread pain. Anesthesiology 2018;129:343–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Sudakin D Naltrexone: not just for opioids anymore. J Med Toxicol 2016;12:71–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Bihari B Efficacy of low dose naltrexone as an immune stabilizing agent for the treatment of HIV/AIDS. AIDS Patient Care 1995;9:3. [PubMed] [Google Scholar]
- [38].Younger J, Mackey S. Fibromyalgia symptoms are reduced by low-dose naltrexone: a pilot study. Pain Med 2009;10:663–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Younger J, Noor N, McCue R, Mackey S. Low-dose naltrexone for the treatment of fibromyalgia: findings of a small, randomized, double-blind, placebo-controlled, counterbalanced, crossover trial assessing daily pain levels. Arthritis Rheum 2013; 65:529–38. [DOI] [PubMed] [Google Scholar]
- [40].Brown N, Panksepp J. Low-dose naltrexone for disease prevention and quality of life. Med Hypotheses 2009;72:333–7. [DOI] [PubMed] [Google Scholar]
- [41].Sharafaddinzadeh N, Moghtaderi A, Kashipazha D, Majdinasab N, Shalbafan B. The effect of low-dose naltrexone on quality of life of patients with multiple sclerosis: a randomized placebo-controlled trial. Mult Scler 2010;16:964–9. [DOI] [PubMed] [Google Scholar]
- [42].Cree BAC, Kornyeyeva E, Goodin DS. Pilot trial of low-dose naltrexone and quality of life in multiple sclerosis. Ann Neurol 2010;68:145–50. [DOI] [PubMed] [Google Scholar]
- [43].Cree BA, Kornyeyeva E, Goodin DS. Pilot trial of low-dose naltrexone and quality of life in multiple sclerosis. Ann Neurol 2010;68:145–50. [DOI] [PubMed] [Google Scholar]
- [44].Lie MRKL, van der Giessen J, Fuhler GM, A de Lima, Peppelenbosch MP, van der Ent C, et al. Low dose Naltrexone for induction of remission in inflammatory bowel disease patients. J Transl Med 2018;16:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Small LR, Galor A, Felix ER, Horn DB, Levitt RC, Sarantopoulos CD. Oral gabapentinoids and nerve blocks for the treatment of chronic ocular pain. Eye Contact Lens 2019;46(3):174–81. 10.1097/ICL.0000000000000630. [DOI] [PubMed] [Google Scholar]
- [46].Morkin MI, Hamrah P. Efficacy of self-retained cryopreserved amniotic membrane for treatment of neuropathic corneal pain. Ocul Surf 2018;16:132–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Aggarwal S, Kheirkhah A, Cavalcanti BM, Cruzat A, Colon C, Brown E, et al. Autologous serum tears for treatment of photoallodynia in patients with corneal neuropathy: efficacy and evaluation with in vivo confocal microscopy. Ocul Surf 2015;13:250–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


