Abstract
The 16.5-kbp plasmid pSCFS1 from Staphylococcus sciuri mediated combined resistance to chloramphenicol and florfenicol. The gene responsible for this resistance property, cfr, was cloned and sequenced. The amino acid sequence of the Cfr protein revealed no homology to known acetyltransferases or efflux proteins involved in chloramphenicol and/or florfenicol resistance or to other proteins whose functions are known.
Staphylococcus sciuri is a common inhabitant of the physiological skin flora of most rodents, ungulates, carnivora, and marsupials. Although classified as rarely pathogenic (6), S. sciuri isolates have been obtained occasionally from cases of mastitis in goats (10) and bronchopneumonia in cattle (13). Antimicrobial resistance is common among S. sciuri isolates, and a number of plasmids carrying one or more resistance genes have been identified (11, 13, 14). Resistance to chloramphenicol (CM) in staphylococci has usually been associated with plasmid-borne cat genes (11, 13), whose gene products inactivate CM by diacetylation. CM acetyltransferases, however, are unable to inactivate florfenicol (FF), a fluorinated CM derivative which was licensed in Germany in 1995 as a therapeutic agent to control bacterial respiratory infections in cattle. Genes whose gene products mediate combined resistance to CM and FF by efflux of both drugs have been identified in gram-negative bacteria, such as Salmonella enterica serovar Typhimurium (2) and Photobacterium damselae subsp. piscicida, formerly known as Pasteurella piscicidae (5). In staphylococci and related organisms, FF resistance genes have not been described yet.
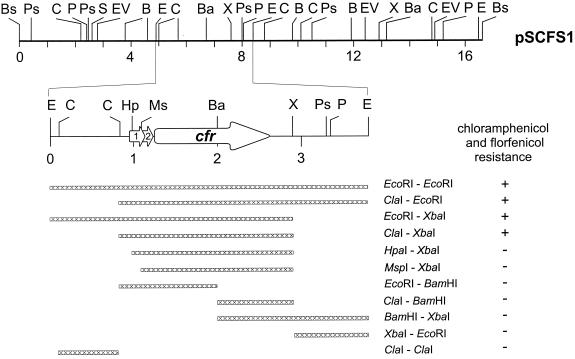
An S. sciuri isolate obtained from the nasal swab of a calf suffering from an infection of the respiratory tract proved to be resistant to tetracycline, erythromycin, kanamycin, CM, and FF. Plasmid analysis revealed the presence of six plasmids in the size range between 1.5 and 16.5 kbp. Experiments involving transformation into protoplasts of Staphylococcus aureus RN4220 (12) and subsequent selection of the transformants on regeneration media containing 20 μg of FF/ml (Essex, Munich, Germany) identified only the 16.5-kbp plasmid, designated pSCFS1, as the mediator of resistance to CM and FF. This plasmid also mediated resistance to erythromycin by an inducibly expressed ermC gene as confirmed by PCR analysis (7). Cloning experiments revealed that the ermC gene was located on a 2.5-kbp PstI fragment of pSCFS1 (data not shown). The original S. sciuri isolate and S. aureus RN4220:pSCFS1 showed FF MICs of 64 μg/ml and CM MICs of 32 μg/ml. Preincubation of these isolates in the presence of either 0.5 μg of FF or 0.5 μg of CM increased the FF MICs to 512 μg/ml and the CM MICs to 64 μg/ml, suggesting that pSCSF1-mediated resistance to FF and CM in both staphylococcal hosts is inducible by FF as well as CM. Plasmid pSCFS1 was mapped (Fig. 1) and subjected to cloning experiments. Restriction fragments of pSCFS1 generated by the enzymes EcoRI and BclI-BamHI were cloned into pBluescript SKII+. The recombinant plasmids were transformed into the recipient strain Escherichia coli HB101 and plated on Luria-Bertani (LB) agar supplemented with 20 μg of FF/ml. Only E. coli HB101 clones which carried a 3.8-kbp EcoRI fragment of pSCFS1 (Fig. 1) grew on these selective plates. Subclones of this EcoRI fragment were produced and tested for their ability to grow on LB agar supplemented with 20 μg of FF/ml (Fig. 1). Subclones which carried a 3-kbp ClaI-EcoRI fragment, a 2.9-kbp EcoRI-XbaI fragment, or a 2-kbp ClaI-XbaI fragment grew on this selective medium and also on LB agar supplemented with 15 μg of CM/ml. The MICs of FF and CM for these subclones were 32 μg/ml; preincubation in the presence of subinhibitory concentrations of FF or CM increased the FF MICs to 64 μg/ml but had no effect on the CM MICs. A lack of increase in CM MICs has also been observed when inducible cat genes from Staphylococcus spp. were expressed in E. coli hosts (15). All subclones generated by BamHI digestion, e.g., those carrying 1.1-kbp ClaI-BamHI and 0.95-kbp BamHI-XbaI fragments (Fig. 1), failed to exhibit resistance to FF and CM.
FIG. 1.
Restriction map of plasmid pSCFS1 from S. sciuri and subcloning strategy for obtaining restriction fragments which mediate (or do not mediate) resistance to CM and FF. Restriction enzyme abbreviations: B, BclI; Ba, BamHI; Bs, BstEII; C, ClaI; E, EcoRI; EV, EcoRV; Hp, HpaI; Ms, MspI; P, PvuII; Ps, PstI; S, SacI; X, XbaI. A distance scale in kilobase pairs is given below each map. Arrows, locations of the cfr reading frames, ORF1 and ORF2, and their directions of transcription.
The sequence of the smallest restriction fragment that conferred resistance to FF and CM, the 2,037-bp ClaI-XbaI fragment, was determined on both strands. Three open reading frames (ORFs) were detected. The BamHI site was located within an ORF for a peptide of 349 amino acids (aa) (positions 570 to 1619). This reading frame, designated cfr (CM and FF resistance) was followed by a pair of inverted repeated sequences of 13 bp, which may represent the transcriptional terminator. The cfr reading frame was preceded by a potential promoter structure (−35: TTTACA, positions 168 to 173; −10: TTACAG, positions 190 to 195; A, position 204) and two overlapping reading frames, ORF1 (positions 237 to 416) and ORF2 (positions 371 to 505), coding for putative peptides of 59 and 44 aa, respectively. The amino acid sequences encoded by both small ORFs did not exhibit significant homology to protein sequences deposited in the databases. Deletion of the cfr gene upstream region as shown in the HpaI-XbaI and the MspI-XbaI subclones (Fig. 1) resulted in sensitivity to FF and CM, suggesting that this region is essential for the expression of combined resistance to FF and CM. Further analysis of the upstream region revealed similarities to the upstream regions of inducible cat genes from Staphylococcus and Bacillus spp. (8, 16). The region between the stop codon of ORF2 and the start codon of cfr comprised a pair of inverted repeated sequences (IR1: positions 515 to 527; IR2: positions 549 to 563) which might be able to form a stable mRNA secondary structure (ΔG = −60.3 kJ/mol). The cfr-associated ribosome binding site was located within the IR2 sequence. Moreover, the terminal part of ORF2 (5′-GTGCAAAAAGAAATTGATTCT-3′) showed considerable homology to previously identified ribosome stall sequences in the reading frames of the regulatory peptides involved in inducible CM resistance (8, 16). A ribosome stalled in the terminal part of ORF2 will overlap the IR1 sequence and abolish mRNA secondary structure formation, thus rendering the cfr-associated ribosome binding site accessible to ribosomes and allowing translation of the cfr transcripts. Assuming that inducible expression of cfr occurs via a translational attenuation-like process (8, 16), deletion of the upstream region which comprises relevant elements for such a regulatory system may explain the loss of resistance to FF and CM.
Comparison of the Cfr amino acid sequence as deduced from the nucleotide sequence revealed no homology to acetyltransferases or efflux proteins (2, 5, 9) so far known to be associated with resistance to FF and/or CM. However, homology to a number of proteins from a wide variety of bacteria, including Mycobacterium tuberculosis H37RV (accession no. Q10806), Treponema pallidum (accession no. AAC65061), Haemophilus influenzae Rd (accession no. P44665), Pseudomonas aeruginosa PAO1 (accession no. Q51385), E. coli K12 (accession no. P36979), Bacillus subtilis 16 (accession no. CAA74265), the soil bacterium Streptomyces coelicolor A3(2) (accession no. CAA19907), the cyanobacterium Synechocystis sp. strain PCC6803 (accession no. Q55880), and the archaeobacterium Thermotoga maritima MSB8 (accession no. AAD36781) was detected (Fig. 2). The reading frames encoding most of these proteins were identified during whole-genome sequencing of the respective organisms. These proteins have some properties in common: they exhibit similar sizes of 340 to 390 aa, have no known functions, and do not exhibit any specific features such as ATP binding domains which might point to their possible functions. Recently, the terminal 133 aa of a protein from S. aureus (accession no. CAB60749) which shows 53% homology to the Cfr protein have been reported (3). This protein was assumed to be an auxiliary protein which might play a role in the expression of methicillin resistance (3). Analysis of the Cfr protein sequence confirmed the lack of ATP binding domains (1). Use of the TMpred program (http://www.ch.embnet.org/software/TMPRED_form.html) did not result in the detection of any topology typical for transmembrane proteins. This observation suggested that the Cfr protein is unlikely to be secreted or anchored to the membrane (4). Moreover, the negative results of a CM acetyltransferase assay and a bioassay to demonstrate the enzymatic inactivation of FF and CM (12) confirmed that neither the original S. sciuri nor the S. aureus RN4220:pSCFS1 transformant was resistant to FF and CM by enzymatic inactivation of the drugs. Even though the mechanism of Cfr-mediated FF and CM resistance remains to be elucidated, these observations indicate that the cfr gene represents a novel type of transferable CM-FF resistance gene, the product of which confers resistance to both drugs not only in staphylococci but also in E. coli and obviously is not associated with any of the so far known mechanisms of FF and CM resistance.
FIG. 2.
Amino acid alignment of the Cfr protein from S. sciuri with similar proteins from M. tuberculosis H37RV, S. coelicolor A3(2), E. coli K12, H. influenzae Rd, P. aeruginosa PAO1, Synechocystis sp. strain PCC6803, T. maritima MSB8, B. subtilis 16, and T. pallidum produced with the DNAMAN sequence analysis software (Lynnon BioSoft, Vaudreuil, Quebec, Canada). Black boxes, identical amino acids; gray boxes, homologous amino acids which are present in at least 40% of the aligned sequences.
Nucleotide sequence accession number.
The nucleotide sequence of the cfr gene and its adjacent regions has been submitted to the EMBL database and was assigned accession no. AJ249217.
Acknowledgments
C.K. received a scholarship from the Gesellschaft der Freunde der FAL (GdF). This study was supported by a grant from the Deutsche Forschungsgemeinschaft (SCHW 382/6-1).
We thank Georg Wolf for providing the S. sciuri isolate, Keith G. H. Dyke for helpful discussions, and B. Otto for help with sequence analysis.
REFERENCES
- 1.Allignet J, Loncle V, El Solh N. Sequence of a staphylococcal plasmid gene, vga, encoding a putative ATP-binding protein involved in resistance to virginiamycin A-like antibiotics. Gene. 1992;117:45–51. doi: 10.1016/0378-1119(92)90488-b. [DOI] [PubMed] [Google Scholar]
- 2.Arcangioli M A, Leroy-Setrin S, Martel J L, Chaslus-Dancla E. A new chloramphenicol and florfenicol resistance gene linked to an integron structure in Salmonella typhimurium DT104. FEMS Microbiol Lett. 1999;174:327–332. doi: 10.1111/j.1574-6968.1999.tb13586.x. [DOI] [PubMed] [Google Scholar]
- 3.De Lencastre H, Wu S W, Pinho M G, Ludovice A M, Filipe S, Gardete S, Sobral R, Gill S, Chung M, Tomasz A. Antibiotic resistance as a stress response: complete sequencing of a large number of chromosomal loci in Staphylococcus aureus strain COL that impact on the expression of resistance to methicillin. Microb Drug Resist. 1999;5:163–175. doi: 10.1089/mdr.1999.5.163. [DOI] [PubMed] [Google Scholar]
- 4.Foulger D, Errington J. A 28 kbp segment from the spoVM region of Bacillus subtilis 168 genome. Microbiology. 1998;144:801–805. doi: 10.1099/00221287-144-3-801. [DOI] [PubMed] [Google Scholar]
- 5.Kim E, Aoki T. Sequence analysis of the florfenicol resistance gene encoded in the transferable R plasmid from a fish pathogen, Pasteurella piscicida. Microbiol Immunol. 1996;40:665–669. doi: 10.1111/j.1348-0421.1996.tb01125.x. [DOI] [PubMed] [Google Scholar]
- 6.Kloos W E, Schleifer K H. Genus IV. Staphylococcus. In: Sneath P H A, Mair N S, Sharpe M E, Holt J G, editors. Bergey's manual of systematic bacteriology. Vol. 2. Baltimore, Md: Williams & Wilkins; 1986. pp. 1013–1035. [Google Scholar]
- 7.Lodder G, Werckenthin C, Schwarz S, Dyke K G H. Molecular analysis of naturally occurring ermC-encoding plasmids in staphylococci isolated from animals with and without previous contact with macrolide/lincosamide antibiotics. FEMS Immunol Med Microbiol. 1997;18:7–15. doi: 10.1111/j.1574-695X.1997.tb01022.x. [DOI] [PubMed] [Google Scholar]
- 8.Lovett P S. Translational attenuation as the regulator of inducible cat genes. J Bacteriol. 1990;172:1–6. doi: 10.1128/jb.172.1.1-6.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Murray I A, Shaw W V. O-Acetyltransferases for chloramphenicol and other natural products. Antimicrob Agents Chemother. 1997;41:1–6. doi: 10.1128/aac.41.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Poutrel B. Udder infection of goats by coagulase-negative staphylococci. Vet Microbiol. 1984;9:131–137. doi: 10.1016/0378-1135(84)90028-2. [DOI] [PubMed] [Google Scholar]
- 11.Schwarz S, Cardoso M, Blobel H. Detection of a novel chloramphenicol resistance plasmid from “equine” Staphylococcus sciuri. J Vet Med B. 1990;37:674–679. doi: 10.1111/j.1439-0450.1990.tb01113.x. [DOI] [PubMed] [Google Scholar]
- 12.Schwarz S, Cardoso M, Blobel H. Plasmid-mediated chloramphenicol resistance in Staphylococcus hyicus. J Gen Microbiol. 1990;135:3329–3336. doi: 10.1099/00221287-135-12-3329. [DOI] [PubMed] [Google Scholar]
- 13.Schwarz S, Grölz-Krug S. A chloramphenicol/streptomycin-resistance plasmid from a clinical strain of Staphylococcus sciuri and its structural relationships to other staphylococcal resistance plasmids. FEMS Microbiol Lett. 1991;82:319–322. doi: 10.1016/0378-1097(91)90281-e. [DOI] [PubMed] [Google Scholar]
- 14.Schwarz S, Noble W C. Tetracycline resistance genes in staphylococci from the skin of pigs. J Appl Bacteriol. 1994;76:320–326. doi: 10.1111/j.1365-2672.1994.tb01635.x. [DOI] [PubMed] [Google Scholar]
- 15.Schwarz S, Spies U, Cardoso M. Cloning and sequence analysis of a plasmid-encoded chloramphenicol acetyltransferase gene from Staphylococcus intermedius. J Gen Microbiol. 1991;137:977–981. doi: 10.1099/00221287-137-4-977. [DOI] [PubMed] [Google Scholar]
- 16.Stokes H W, Hall R M. Sequence analysis of the inducible chloramphenicol resistance determinant in the Tn1696 integron suggests regulation by translational attenuation. Plasmid. 1991;26:10–19. doi: 10.1016/0147-619x(91)90032-r. [DOI] [PubMed] [Google Scholar]