Giant cell myocarditis (GCM)—a rapidly progressive form of myocarditis—is a rare and potentially fatal diagnosis if not treated immediately. Endomyocardial biopsy is the gold standard for diagnosis. Treatment of GCM involves immunosuppression along with symptomatic management of heart failure, arrhythmias, and the other cardiac sequelae of GCM.1 We describe a case of GCM in a patient without cardiac history or significant comorbid conditions who presented with severe heart failure a few weeks after the BNT162b2 mRNA COVID-19 vaccine (Pfizer-BioNTech).
Case Presentation
A 63-year-old man with a history of psoriasis, diabetes, and tobacco use presented with fever, fatigue, and cough ≈1 week after receiving his second dose of the BNT162b2 vaccine. He received his first dose and experienced self-terminating nausea but otherwise felt fine. During the next 2 weeks, he experienced fever and chills followed by cough, shortness of breath, and a 20-pound unintentional weight loss. He denied any sick contacts during that time period. Due to the severity of symptoms, he presented multiple times to his primary care clinic and urgent care with a negative workup.
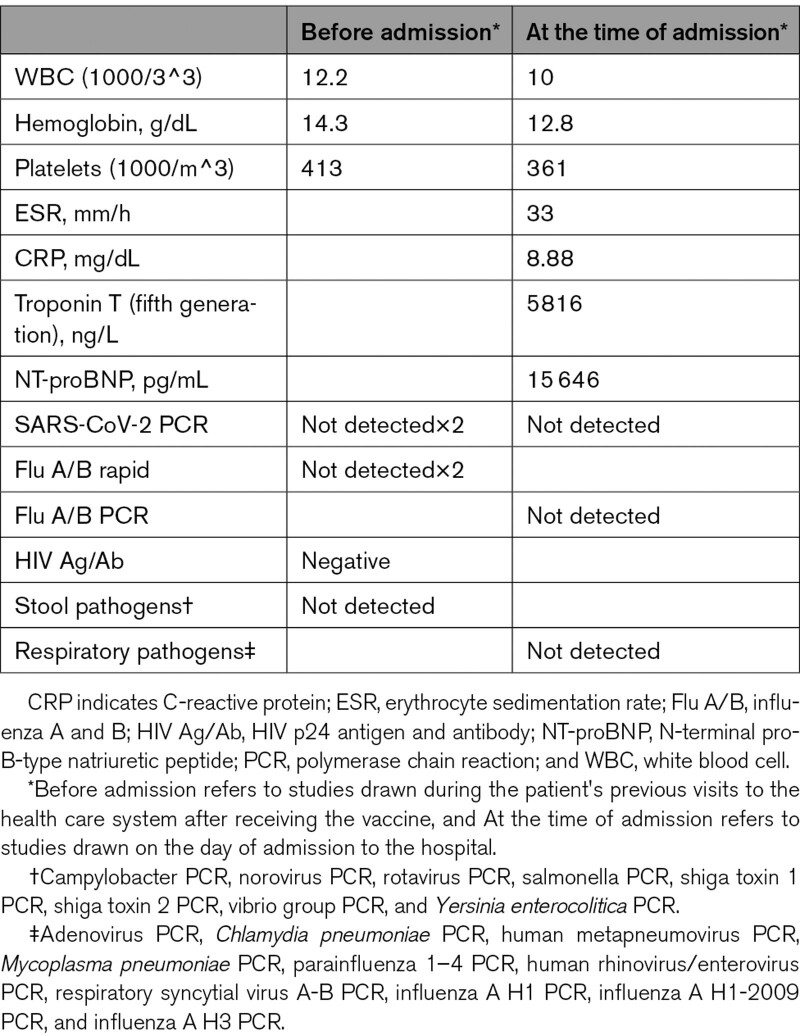
His last visit to urgent care prompted a referral to the emergency department after an ECG showed ST-segment elevation in the setting of clinical heart failure. Studies drawn before admission and in the emergency department are listed in the Table. Echocardiogram revealed an ejection fraction of 35%, biventricular hypertrophy, and apical hypokinesis. Right and left heart catheterization revealed elevated filling pressures, a cardiac index of 1.8 L/min per M^2, and no obstructive coronary artery disease. An intra-aortic balloon pump was placed. Dopamine and dobutamine were initiated. Endomyocardial biopsy was performed revealing lymphocytes, eosinophils, and giant cells, confirming the diagnosis of GCM (Figure 1). Electron microscopy revealed tubuloreticular inclusions (Figure 2). Evaluation for viral inclusion and immunohistochemistry were not performed given the negative workup for infectious etiology of myocarditis. Treatment was started as described in Figure 3. The patient’s condition improved, and he tolerated withdrawal of intra-aortic balloon pump on day 6 and vasopressors on day 8 of admission. Before discharge, cardiac magnetic resonance imaging was performed revealing mildly depressed biventricular systolic function with areas of linear and transmural subepicardial delayed enhancement involving the inferior and septal apical walls compatible with myocarditis. Currently, there is ongoing risk-benefit discussion with the patient regarding discontinuation of immunosuppression.
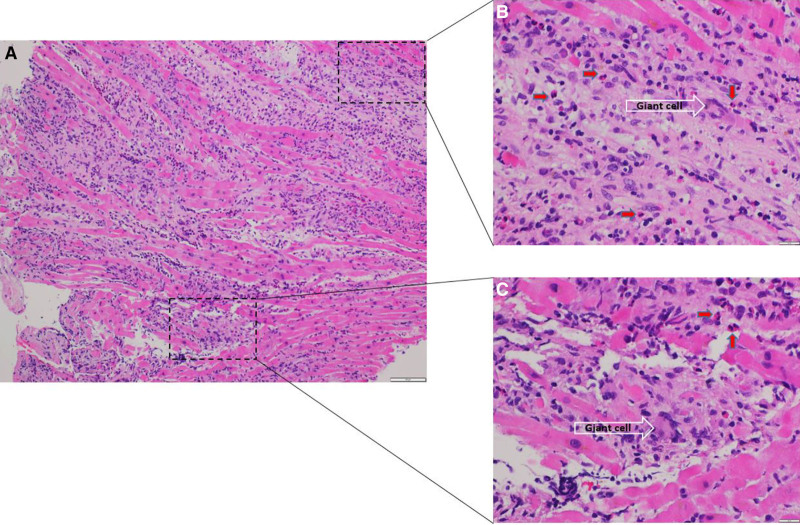
Figure 1.
Heart biopsy with diffuse inflammation and myocyte damage. High magnification showing inflammatory infiltrate including giant cells (white arrow) and eosinophils (red arrows; A, ×10 hematoxylin and eosin stain; B and C, ×40 hematoxylin and eosin stain).
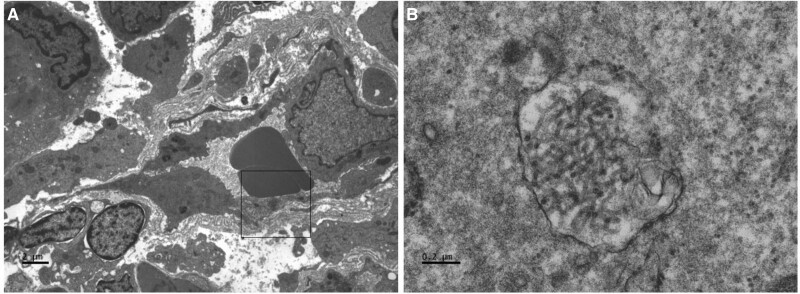
Figure 2.
Electron microscopy showing tubuloreticular inclusions in capillary endothelial cells that have been seen in COVID-19–related collapsing glomerulopathy.2 A, ×4200; (B) ×60 000. Electron microscopy images were obtained by Jesus Macias, senior histotechnologist and electron microscopist at University of California San Diego Health.
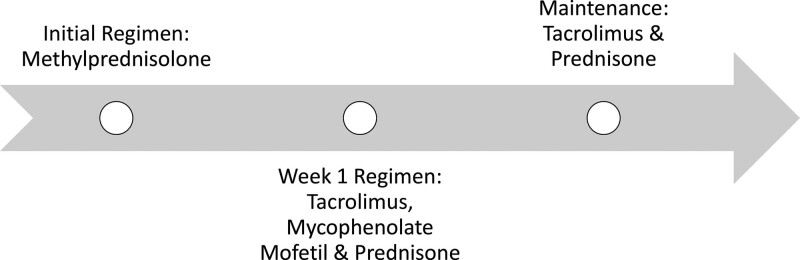
Figure 3.
Immunosuppression regimen for the patient. Initial regimen included methylprednisolone 1 g IV every 24 hours given for 3 doses. Week 1 regimen included tacrolimus with a goal trough of 12 to 15, mycophenolate mofetil 1000 mg BID, and prednisone taper starting at 60 mg daily. Once tacrolimus was at goal, mycophenolate mofetil was discontinued. Tacrolimus trough was stable for the first 4 months, but after developing complications, the goal trough was gradually decreased to 4 to 6. Prednisone was weaned from a starting dose of 60 mg daily to 5 mg daily. He remains on tacrolimus and prednisone at 10 months after diagnosis.
Table.
Studies Drawn Before Admission and in the Emergency Department
Discussion
GCM is a rapidly progressive disease that not only has a high morbidity and mortality rate but can be difficult to diagnose. It typically affects young and middle-aged adults and is believed to be due to T lymphocyte–mediated infiltration and to the myocardium.3 Clinical presentations vary from systolic heart failure, ventricular arrhythmias, and de novo heart block.1,4 Endomyocardial biopsy is the diagnostic gold standard, and histology characteristically shows multinucleated giant cells in the background of a lymphocytic inflammatory infiltrate in the absence of well-formed granulomas.1,4,5
Importantly, there are no specific immunosuppression recommendations for lymphocytic myocarditis that is not associated with a systemic illness. Treatment of GCM is multifactorial, but in contrast to other forms of myocarditis, immunosuppression is considered standard of care. Tapering of immunosuppression should be done with caution, as reports of disease recurrence have been noted to as far as 8 years out from the initial disease presentation.6
The incidence of myocarditis after receiving the BNT162b2 vaccine in the Israeli population has been previously reported to be 2.13 cases per 100 000 vaccinated people in the first 42 days following the first dose of the vaccine.7 While this estimate was determined after adjudication of reported cases of myocarditis by cardiologists using the universally accepted definition of myocarditis by the Centers for Disease Control and Prevention, only one person underwent biopsy. As such, the subtypes of these myocarditis cases remain unknown. Of the 41 reported cases in this case series, only one was similar to our patient’s presentation. Recently, Verma et al8 reported 2 cases of lymphocytic myocarditis within 2 weeks following the COVID-19 vaccine (one received BNT162b2 and the other received mRNA-1273 [Moderna]). Histological assessment in both cases revealed infiltrate predominantly composed of T cells and macrophages, admixed with eosinophils, B cells, and plasma cells. The first patient was successfully treated with immunosuppression, but the second patient died 3 days later.7 Of note, large-vessel giant cell arteritis following the BNT162b2 vaccine has been recently reported. While the clinical presentation is different than GCM, it shares a similar pathophysiology further supporting a possible link between vaccine and GCM.9 Importantly, tubuloreticular inclusions were noted in capillary endothelial cells—a finding seen in COVID-19–related collapsing glomerulopathy further supporting a possible relationship.2 Finally, given the limited data, there are no COVID-19 vaccination guidelines in patients with myocarditis, although the authors suggest avoidance in life-threatening cases.
Conclusion
In this case of GCM following the BNT162b2 vaccine, causality cannot be established definitively, but the time course and absence of an alternative explanation does suggest a relationship. To our knowledge, this is the first biopsy-proven case of GCM associated with the COVID-19 vaccine.
Article Information
Sources of Funding
None.
Disclosures
None.
Footnotes
This manuscript was sent to Ray E. Hershberger, MD, Guest Editor, for review by expert referees, editorial decision, and final disposition.
For Sources of Funding and Disclosures, see page 416.
Contributor Information
Kevin Sung, Email: k4sung@health.ucsd.edu.
Julia McCain, Email: jamccain@health.ucsd.edu.
Kevin R. King, Email: krking@ucsd.edu.
Omonigho Aisagbonhi, Email: oaisagbonhi@health.ucsd.edu.
Eric D. Adler, Email: eradler@ucsd.edu.
References
- 1.Kandolin R, Lehtonen J, Salmenkivi K, Räisänen-Sokolowski A, Lommi J, Kupari M. Diagnosis, treatment, and outcome of giant-cell myocarditis in the era of combined immunosuppression. Circ Heart Fail. 2013;6:15–22. doi: 10.1161/CIRCHEARTFAILURE.112.969261 [DOI] [PubMed] [Google Scholar]
- 2.Gaillard F, Ismael S, Sannier A, Tarhini H, Volpe T, Greze C, Verpont MC, Zouhry I, Rioux C, Lescure FX, et al. Tubuloreticular inclusions in COVID-19-related collapsing glomerulopathy. Kidney Int. 2020;98:241. doi: 10.1016/j.kint.2020.04.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cooper LT, Jr, Berry GJ, Shabetai R. Idiopathic giant-cell myocarditis–natural history and treatment. Multicenter Giant Cell Myocarditis Study Group investigators. N Engl J Med. 1997;336:1860–1866. doi: 10.1056/NEJM199706263362603 [DOI] [PubMed] [Google Scholar]
- 4.Okura Y, Dec GW, Hare JM, Kodama M, Berry GJ, Tazelaar HD, Bailey KR, Cooper LT. A clinical and histopathologic comparison of cardiac sarcoidosis and idiopathic giant cell myocarditis. J Am Coll Cardiol. 2003;41:322–329. doi: 10.1016/s0735-1097(02)02715-8 [DOI] [PubMed] [Google Scholar]
- 5.Ammirati E, Frigerio M, Adler ED, Basso C, Birnie DH, Brambatti M, Friedrich MG, Klingel K, Lehtonen J, Moslehi JJ, et al. Management of acute myocarditis and chronic inflammatory cardiomyopathy: an expert consensus document. Circ Heart Fail. 2020;13:e007405. doi: 10.1161/CIRCHEARTFAILURE.120.007405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maleszewski JJ, Orellana VM, Hodge DO, Kuhl U, Schultheiss HP, Cooper LT. Long-term risk of recurrence, morbidity and mortality in giant cell myocarditis. Am J Cardiol. 2015;115:1733–1738. doi: 10.1016/j.amjcard.2015.03.023 [DOI] [PubMed] [Google Scholar]
- 7.Witberg G, Barda N, Hoss S, Richter I, Wiessman M, Aviv Y, Grinberg T, Auster O, Dagan N, Balicer RD, et al. Myocarditis after Covid-19 vaccination in a large health care organization. N Engl J Med. 2021;385:2132–2139. doi: 10.1056/NEJMoa2110737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Verma AK, Lavine KJ, Lin CY. Myocarditis after Covid-19 mRNA vaccination. N Engl J Med. 2021;385:1332–1334. doi: 10.1056/NEJMc2109975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mejren A, Sørensen C, Gormsen L, Tougaard R, Nielsen B. Large-vessel giant cell arteritis after COVID-19 vaccine. Scand J Rheumatol. 2022;51:154–155. doi: 10.1080/03009742.2021.1961401 [DOI] [PubMed] [Google Scholar]