Abstract
Background
We estimated COVID-19 vaccine effectiveness against SARS-CoV-2 infection and severe COVID-19 outcomes among individuals with immune-mediated inflammatory diseases in Ontario, Canada.
Methods
In this population-based analysis, we used a test-negative design across four immune-mediated inflammatory disease population-based cohorts, comprising individuals with rheumatoid arthritis, ankylosing spondylitis, psoriasis, and inflammatory bowel disease. We identified all SARS-CoV-2 tests done in these populations between March 1 and Nov 22, 2021 (a period in which there was rapid uptake of vaccines, and the alpha [B.1.1.7] and delta [B.1.617.2] SARS-CoV-2 variants were predominantly circulating in Canada) and separately assessed outcomes of SARS-CoV-2 infection and severe COVID-19 outcomes (hospitalisation due to COVID-19 and death due to COVID-19) for each disease group. We used multivariable logistic regression to estimate the effectiveness of one, two, and three doses of mRNA-based COVID-19 vaccine (BNT162b2 [Pfizer–BioNTech], or mRNA-1273 [Moderna]) among individuals at the time of SARS-CoV-2 testing.
Findings
Between March 1 and Nov 22, 2021, we identified 2127 (5·9%) test-positive cases among 36 145 individuals (26 476 [73·2%] were female and 9669 [26·8%] were male) with rheumatoid arthritis tested, 476 (6·1%) test-positive cases among 7863 individuals (4130 [52·5%] were female and 3733 [47·5%] were male) with ankylosing spondylitis tested, 3089 (6·5%) test-positive cases among 47 199 individuals (26 062 [55·2%] were female and 21 137 [44·8%] were male) with psoriasis tested, and 1702 (5·4%) test-positive cases among 31 311 individuals (17 716 [56·6%] were female and 13 595 [43·4%] were male) with inflammatory bowel disease tested. Adjusted vaccine effectiveness of two doses against infection was 83% (95% CI 80–86) in those with rheumatoid arthritis, 89% (83–93) among those with ankylosing spondylitis, 84% (81–86) among those with psoriasis, and 79% (74–82) among those with inflammatory bowel disease. After two vaccine doses, effectiveness against infection generally peaked 31–60 days after vaccination and waned gradually with each additional month. Vaccine effectiveness against severe outcomes after two doses was 92% (95% CI 88–95) in those with rheumatoid arthritis, 97% (83–99) among those with ankylosing spondylitis, 92% (86–95) among those with psoriasis, and 94% (88–97) among those with inflammatory bowel disease. Vaccine effectiveness after a third dose against infection was similar to or higher than after the second dose (ranging from 76% [47–89] to 96% [72–99]), although due to a paucity of events, estimates could not be calculated for some subgroups for severe outcomes.
Interpretation
Two vaccine doses were found to be highly effective against both SARS-CoV-2 infection and severe COVID-19 outcomes in patients with rheumatoid arthritis, ankylosing spondylitis, psoriasis, and inflammatory bowel disease during the study period. Research is needed to determine the durability of effectiveness of three doses over time, particularly against emerging variants.
Funding
Public Health Agency of Canada
Introduction
Canada's comprehensive universal SARS-CoV-2 testing and surveillance, rapid uptake of COVID-19 vaccines, and population-based linked health datasets have contributed to monitoring of vaccine effectiveness in the general population.1, 2, 3, 4, 5 Less is known about vaccine effectiveness among individuals with immune-mediated inflammatory diseases. Individuals with immune-mediated inflammatory diseases are not only susceptible to severe outcomes associated with SARS-CoV-2,6, 7, 8, 9 but the immunogenicity of mRNA-based COVID-19 vaccines might also be impaired in this patient population.10, 11, 12, 13, 14 Although policy and clinical recommendations on COVID-19 vaccination for people with immune-mediated inflammatory diseases have largely been driven by research on immunogenicity,15, 16 understanding how well these vaccines work in broader immune-mediated inflammatory disease populations remains a priority, especially because they are under-represented in clinical studies. Therefore, we aimed to estimate the vaccine effectiveness of mRNA-based COVID-19 vaccines against SARS-CoV-2 infection and severe COVID-19 outcomes among individuals with a selection of immune-mediated inflammatory diseases in Ontario, Canada, over the period March to November, 2021, during which there was rapid uptake of vaccinations in the province,17 and the predominant circulating SARS-CoV-2 variants were alpha (B.1.1.7; March to June, 2021) and delta (B.1.617.2; June to November, 2021).1
Research in context.
Evidence before this study
We searched PubMed for studies published in English between Jan 1, 2021, and Mar 1, 2022, using different combinations of the terms “SARS-CoV-2”, “COVID-19”, “vaccination”, “vaccine”, “rheumatic diseases”, “rheumatoid arthritis”, “psoriasis”, “inflammatory bowel disease”, “ankylosing spondylitis”, “immune-mediated Inflammatory diseases”, “effectiveness”, and “immunogenicity”. Vaccine effectiveness studies done on the general population have shown that mRNA-based COVID-19 vaccines are highly protective against infections and severe outcomes, such as admission to hospital and death. Studies have shown than the immunogenicity of mRNA-based COVID-19 vaccines might be impaired among individuals with immune-mediated inflammatory diseases, which could translate into reduced vaccine effectiveness. Sparse information exists on the effectiveness of COVID-19 vaccines in patients with immune-mediated inflammatory diseases.
Added value of this study
To our knowledge, this is the largest population-based study of vaccine effectiveness of mRNA-based COVID-19 vaccines in people with immune-mediated inflammatory diseases. Among individuals with rheumatoid arthritis, ankylosing spondylitis, psoriasis, and inflammatory bowel disease tested for SARS-CoV-2 between March 1 and Nov 22, 2021, we estimated high (92–97%) vaccine effectiveness of two doses of mRNA-based COVID-19 vaccines (BNT162b2 or mRNA-1273) against severe COVID-19 outcomes compared with unvaccinated patients. Estimates of vaccine effectiveness against SARS-CoV-2 infection were numerically lower than for severe outcomes. For the outcome of infection with SARS-CoV-2, overall vaccine effectiveness for two doses exceeded 79% across all four patient populations. After the second dose, vaccine effectiveness against infection peaked 31–60 days after vaccination (82–90%) and waned with each additional month, but effectiveness increased again with third doses.
Implications of the available evidence
We found high vaccine effectiveness of mRNA vaccines against severe COVID-19 outcomes among individuals with rheumatoid arthritis, ankylosing spondylitis, psoriasis, and inflammatory bowel disease, which could help individuals with these diseases, who were largely excluded from vaccine trials, make informed decisions about following vaccination recommendations.
Methods
Study design and setting
In this population-based study, we used a common protocol that was used to estimate vaccine effectiveness within the general Ontario population to help facilitate comparisons.1, 2, 3
To assess vaccine effectiveness, we used a test-negative design across four separate population-based cohorts of people with immune-mediated inflammatory diseases. Within each cohort, individuals tested for SARS-CoV-2 served as the nested cohort, whereby vaccine status was compared between SARS-CoV-2 test-positive cases versus test-negative controls.
Our study period was from March 1 to November 22, 2021. The details of the immunisation rollout in Ontario has been previously reported.17 Briefly, due to low vaccine supply, Ontario initially had a slow and low vaccine uptake between December, 2020, and March, 2021, during which time vaccines were prioritised for residents and staff of long-term care and retirement homes, health-care workers, and adults aged 80 years and older. By March, 2021, vaccination of residents was largely prioritised by age (in decreasing age group increments) in rapid succession, resulting in a rapid increase in vaccine uptake of one and two doses among all age groups between March and July, 2021.17 Administration of third doses started in September, 2021.18 Most people with immune-mediated inflammatory diseases in Ontario were given the BNT162b2 (Pfizer–BioNTech) vaccine (>70%) and the mRNA-1273 (Moderna) vaccine (>18%) as part of their initial two doses, and few patients (<10%) received the Covishield vaccine (also known as ChAdOx1 nCoV-19; Oxford–AstraZeneca).17 The pandemic's third wave occurred in the country between March and June, 2021, with a lower incidence of SARS-CoV-2 infections observed between June and November, 2021.19
This study was approved by a privacy impact assessment at ICES (formerly called the Institute for Clinical Evaluative Sciences). The use of the data in this project was authorised under section 45 of Ontario's Personal Health Information Protection Act, which does not require review by a Research Ethics Board. Datasets were linked using unique encoded identifiers and analysed at ICES. ICES is a prescribed entity under Personal Health Information Protection Act. Section 45 of the Personal Health Information Protection Act authorises ICES to collect personal health information, without consent, for the purpose of analysis or compiling statistical information with respect to the management of, evaluation or monitoring of, the allocation of resources to or planning for all or part of the health system.
Data sources and definitions
We collected patient-level data on SARS-CoV-2 testing and COVID-19 vaccination for patients with immune-mediated inflammatory diseases. Ontario residents, comprising approximately 40% of Canada's population of 35 million people, are insured under a single payer health-care system (Ontario's Health Insurance Plan) that covers all medically necessary health services. These contacts for health services are recorded in administrative databases and linked using unique encoded identifiers. We assembled four separate population-based cohorts of individuals aged 16 years or older with rheumatoid arthritis, ankylosing spondylitis, psoriasis, and inflammatory bowel disease using established disease-specific case definitions applied to health administrative data (appendix p 1). These case definitions require multiple health-care contacts for diagnosis codes related to the condition of interest (a minimum of three to five diagnosis codes, often involving a health-care contact with a specialist) and are based on validation studies involving medical chart review.20, 21, 22 These population-based cohorts have been extensively used for previous research, including assessments of SARS-CoV-2 testing and risks of infection, and severe COVID-19 outcomes.6, 17, 23, 24, 25, 26, 27
From within each cohort, we identified all individuals who had real-time RT-PCR tests for SARS-CoV-2 during the study period. Data on SARS-CoV-2 tests (sample collection date and results) were collected from the Ontario Laboratories Information System. The study accrual period ended on Nov 22, 2021, when the first cases of infection with the omicron (B.1.1.529) variant were detected in Ontario (to limit misclassification of variants).
We excluded long-term care residents because they undergo frequent testing, are more frail than other individuals, and have a different threshold for admission to hospital; individuals who received out-of-province vaccinations; and individuals who had received two doses of ChAdOx1 nCoV-19 because effectiveness for that schedule is known to be lower than for mRNA-based vaccines1, 2, 3 and most of the study population received mRNA-based COVID-19 vaccines.17
For vaccine effectiveness against infection, cases were defined as individuals with a laboratory-confirmed positive PCR test for SARS-CoV-2 between March 1 and Nov 22, 2021. The index date was the sample collection date. We identified the first positive test for the individual, and individuals who tested negative were treated as controls. For controls with multiple negative tests, we used the date of a randomly selected negative test as their index date.
Severe outcomes were defined as an admission to hospital (ie, hospitalisation) or death attributed to SARS-CoV-2 infection in test-positive individuals. Severe outcomes were ascertained from an integrated dataset linking Public Health Ontario's Case and Contact Management (CCM) dataset (which contains information on the clinical course of patients with SARS-CoV-2 infection [including hospitalisations and deaths]), the Canadian Institute for Health Information Discharge Abstract Database (as a secondary source to identify hospitalisations and inpatient deaths in individuals with a diagnosis of COVID-19 [ie, an International Classification of Diseases 10th edition code U07.1], and a positive test result within 14 days before or 3 days after admission), and the Ontario Registered Persons Database (as a secondary source for deaths, in which a positive test result must have occurred within 30 days before death or within 7 days post-mortem if COVID-19 was suspected). Infections that occurred during hospital stay for another reason were not considered a severe outcome for the purposes of this analysis. We used the earliest date of sample collection or hospital admission or death as the index date.
At the time of testing, we assessed whether or not individuals had one, two, or three vaccine doses before their testing date. COVID-19 vaccination status, including vaccine product, date of administration, and dose number were determined from COVaxON, a centralised COVID-19 vaccine registry in Ontario.
Statistical analysis
We did analyses separately for each of the four study populations (rheumatoid arthritis, ankylosing spondylitis, psoriasis, and inflammatory bowel disease). To compare characteristics between test-positive cases and test-negative controls and between those vaccinated with at least one dose of mRNA-based vaccine and unvaccinated individuals, we did descriptive analyses and calculated standardised differences (with a standardised difference of >0·10 considered to be a clinically relevant difference).28, 29
We separately estimated overall vaccine effectiveness (for infection and severe outcomes) for one dose of vaccine at least 14 days before testing date, and at least 7 days before testing date for two and three doses, using multivariable logistic regression to compare the odds of vaccination in test-positive cases with the odds of vaccination among test-negative controls, adjusting for covariates that are associated with SARS-CoV-2 infection and vaccination. We estimated vaccine effectiveness as (1 – odds ratio) × 100%. Subsequently, we estimated vaccine effectiveness against infection for each subsequent month after receipt of two doses of vaccine. We also separately estimated adjusted vaccine effectiveness against SARS-CoV-2 infection by vaccine product after one and two doses. We adjusted estimates of vaccine effectiveness for patient age (using age bands as a categorical variable), sex, public health unit region of residence, biweekly period of test (to account for temporal variations in viral activity and regional vaccine roll-out), number of PCR tests for each individual in the 3 months before the start of Ontario's COVID-19 immunisation programme (a proxy for individuals who are at increased risk of SARS-CoV-2 exposure and undergo frequent testing), previous SARS-CoV-2 infection more than 90 days before the index date, presence of any comorbidity that increases the risk of severe COVID-19 (ie, chronic respiratory diseases, chronic heart disease, hypertension, diabetes, chronic kidney disease, other immunosuppressive conditions including receipt of a transplant, other immune disorders, active cancer, advanced liver disease, dementia, frailty, or history of transient ischaemic attack or stroke), a previous influenza vaccination (within the past 2 years, a proxy for health behaviours), and census dissemination area-level quintiles of household income, proportion of people employed as non-health-care-based essential workers (proxy of individuals unable to work from home), average number of people per dwelling, and proportion of self-identified minorities. Full details regarding these covariates are provided in the appendix (pp 2–7).
Because of the length of the study period with changes over time in SARS-CoV-2 infection incidence, PCR testing volumes (ie, higher volumes early on due to higher incidence of infection), and vaccination status (ie, increased vaccination coverage later in the time course) in the underlying population, we did a sensitivity analysis to assess potential bias in patient selection by using the last negative testing episode (rather than a random selection among those with multiple negative tests).
All tests were two-sided and a p value of less than 0·05 was considered to be significant. We do not report estimates of vaccine effectiveness after three doses of vaccine in situations where there were very few individuals with three doses among the test-positive cases, because vaccine effectiveness approximates 100% on the basis of near-zero vaccinated test-positive cases and the 95% CIs were essentially infinite or extremely imprecise.
We did all analyses using SAS (version 9.4; SAS Institute, Cary, NC, USA).
Role of funding source
The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Results
Among 36 145 individuals with rheumatoid arthritis tested for SARS-CoV-2 during the study period, we identified 2127 (5·9%) test-positive cases (appendix p 8). For ankylosing spondylitis, we identified 476 (6·1%) positive cases among 7863 individuals with ankylosing spondylitis tested (appendix p 9). For psoriasis, we identified 3089 (6·5%) positive cases among 47 199 individuals with psoriasis tested (appendix p 10). And for inflammatory bowel disease, we identified 1702 (5·4%) positive cases among 31 311 individuals with inflammatory bowel disease tested (appendix p 11).
Among 36 145 individuals with rheumatoid arthritis, the mean age was 61·2 years (SD 16·5), 20 682 (57·2%) were aged 60 years or older, 26 476 (73·2%) were female, and 9669 (26·8%) were male. Among 7863 individuals with ankylosing spondylitis, the mean age was 52·5 years (SD 15·7), 2615 (33·3%) were aged 60 years or older, 4130 (52·5%) were female, and 3733 (47·5%) were male. Among 47 199 individuals with psoriasis, the mean age was 53·3 years (SD 17·5), 17 954 (38·0%) were aged 60 years or older, 26 062 (55·2%) were female, and 21 137 (44·8%) were male. And among 31 311 individuals with inflammatory bowel disease, the mean age was 50·9 years (SD 17·4), 10 043 (32·1%) were 60 years or older, 17 716 (56·6%) were female, and 13 595 (43·4%) were male. No data were captured on race or ethnicity.
Across all four immune-mediated inflammatory disease groups, test-positive cases were more likely to be younger and reside in neighbourhoods with lower income, and were less likely to have had any PCR tests during the 3 months before the start of Ontario's immunisation programme than were test-negative controls (table 1 ; appendix pp 12–14).
Table 1.
Characteristics of individuals with immune-mediated inflammatory diseases, by SARS-CoV-2 test result, at time of testing between March 1 and Nov 22, 2021
|
Rheumatoid arthritis |
Ankylosing spondylitis |
Psoriasis |
Inflammatory bowel disease |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SARS-CoV-2 positive (n=2127) | SARS-CoV-2 negative (n=34 018) | Standardised difference* | SARS-CoV-2 positive (n=476) | SARS-CoV-2 negative (n=7387) | Standardised difference* | SARS-CoV-2 positive (n=3089) | SARS-CoV-2 negative (n=44 110) | Standardised difference* | SARS-CoV-2 positive (n=1702) | SARS-CoV-2 negative (n=29 609) | Standardised difference* | ||
| Age, years | 57·4 (16·1) | 61·4 (16·5) | 0·25 | 50·4 (14·3) | 52·6 (15·8) | 0·15 | 49·4 (16·6) | 53·6 (17·5) | 0·24 | 46·8 (16·8) | 51·1 (17·4) | 0·25 | |
| Sex | |||||||||||||
| Male | 564 (26·5%) | 9105 (26·8%) | 0·01 | 252 (52·9%) | 3481 (47·1%) | 0·12 | 1567 (50·7%) | 19 570 (44·4%) | 0·13 | 860 (50·5%) | 12 735 (43·0%) | 0·15 | |
| Female | 1563 (73·5%) | 24 913 (73·2%) | 0·01 | 224 (47·1%) | 3906 (52·9%) | 0·12 | 1522 (49·3%) | 24 540 (55·6%) | 0·13 | 842 (49·5%) | 16 874 (57·0%) | 0·15 | |
| Number of tests in past 3 months† | |||||||||||||
| 0 | 1776 (83·5%) | 25 520 (75·0%) | 0·21 | 381 (80·0%) | 5467 (74·0%) | 0·14 | 2533 (82·0%) | 32 877 (74·5%) | 0·18 | 1318 (77·4%) | 21 549 (72·8%) | 0·11 | |
| 1 | 250 (11·8%) | 5202 (15·3%) | 0·10 | 70 (14·7%) | 1267 (17·2%) | 0·07 | 416 (13·5%) | 7169 (16·3%) | 0·08 | 274 (16·1%) | 5186 (17·5%) | 0·04 | |
| ≥2 | 101 (4·7%) | 3296 (9·7%) | 0·19 | 25 (5·3%) | 653 (8·8%) | 0·14 | 140 (4·5%) | 4064 (9·2%) | 0·19 | 110 (6·5%) | 2874 (9·7%) | 0·12 | |
| Previous positive test >90 days since testing date‡ | 16 (0·8%) | 639 (1·9%) | 0·10 | 0 | 134 (1·8%) | 0·19 | 15 (0·5%) | 882 (2·0%) | 0·14 | 12 (0·7%) | 471 (1·6%) | 0·08 | |
| Any comorbidity§ | 1473 (69·3%) | 25 165 (74·0%) | 0·10 | 270 (56·7%) | 4569 (61·9%) | 0·10 | 1781 (57·7%) | 27 058 (61·3%) | 0·08 | 938 (55·1%) | 17 714 (59·8%) | 0·10 | |
| Previous influenza vaccination¶ | 862 (40·5%) | 18 373 (54·0%) | 0·27 | 186 (39·1%) | 3460 (46·8%) | 0·16 | 993 (32·1%) | 19 706 (44·7%) | 0·26 | 555 (32·6%) | 13 504 (45·6%) | 0·27 | |
| Neighbourhood income quintile‖ | |||||||||||||
| 1 (lowest) | 526 (24·7%) | 6787 (20·0%) | 0·11 | 98 (20·6%) | 1219 (16·5%) | 0·11 | 708 (22·9%) | 7754 (17·6%) | 0·13 | 301 (17·7%) | 4814 (16·3%) | 0·04 | |
| 2 | 429 (20·2%) | 6625 (19·5%) | 0·02 | 91 (19·1%) | 1323 (17·9%) | 0·03 | 615 (19·9%) | 8389 (19·0%) | 0·02 | 357 (21·0%) | 5575 (18·8%) | 0·05 | |
| 3 | 457 (21·5%) | 6653 (19·6%) | 0·05 | 102 (21·4%) | 1455 (19·7%) | 0·04 | 620 (20·1%) | 8614 (19·5%) | 0·01 | 354 (20·8%) | 5981 (20·2%) | 0·01 | |
| 4 | 386 (18·1%) | 6826 (20·1%) | 0·05 | 94 (19·7%) | 1571 (21·3%) | 0·04 | 619 (20·0%) | 9269 (21·0%) | 0·02 | 356 (20·9%) | 6193 (20·9%) | 0·00 | |
| 5 (highest) | 319 (15·0%) | 7004 (20·6%) | 0·15 | 86 (18·1%) | 1787 (24·2%) | 0·15 | 515 (16·7%) | 9932 (22·5%) | 0·15 | 328 (19·3%) | 6949 (23·5%) | 0·10 | |
Data are mean (SD) or n (%), unless otherwise stated. Proportions might not add up to 100% due to rounding. Additional patient characteristics are provided in the appendix (pp 12–14).
Values of >0·10 are considered to be clinically relevant differences.
In the 3 months before COVID-19 immunisation programme started on Dec 14, 2020.
Previous positive tests by PCR before study period (before March 1, 2021).
Comorbidities include chronic respiratory diseases, chronic heart diseases, hypertension, diabetes, chronic kidney disease, other immunocompromising illness, active cancer, advanced liver disease, dementia, frailty, and history of stroke or transient ischaemic attack (appendix pp 12–14).
Influenza vaccination during 2019–20 or 2020–21 influenza season, or both.
The sum of counts does not equal the column total because of individuals with missing information (<1·0%) for this characteristic.
Among test-positive cases and test-negative controls, 1917 with rheumatoid arthritis, 536 with ankylosing spondylitis, 3168 with psoriasis, and 1906 with inflammatory bowel disease received out-of-province or ChAdOx1 nCoV-19 vaccines and were excluded from the subsequent analyses.
Across all four immune-mediated inflammatory disease groups, at the time of the index test date, unvaccinated patients were generally younger, less likely to have had previous testing, and less likely to have a comorbidity than were patients who had received at least one dose of mRNA-based COVID-19 vaccine (table 2 ; appendix pp 15–17).
Table 2.
Characteristics of individuals with immune-mediated inflammatory diseases, by vaccination status, at time of testing between March 1 and Nov 22, 2021
|
Rheumatoid arthritis |
Ankylosing spondylitis |
Psoriasis |
Inflammatory bowel disease |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Unvaccinated (n=11 238) | At least one dose of mRNA-based vaccine (n=22 990) | Standardised difference* | Unvaccinated (n=2857) | At least one dose of mRNA-based vaccine (n=4470) | Standardised difference* | Unvaccinated (n=17 338) | At least one dose of mRNA-based vaccine (n=26 693) | Standardised difference* | Unvaccinated (n=11 246) | At least one dose of mRNA-based vaccine (n=18 159) | Standardised difference* | ||
| Tested positive for SARS-CoV-2† | 1332 (11·9%) | 720 (3·1%) | 0·34 | 320 (11·2%) | 140 (3·1%) | 0·32 | 2083 (12·0%) | 887 (3·3%) | 0·33 | 1084 (9·6%) | 554 (3·1%) | 0·27 | |
| Age, years | 55·9 (15·9) | 63·9 (16·7) | 0·50 | 48·6 (14·3) | 54·5 (16·6) | 0·38 | 48·1 (16·0) | 56·2 (18·4) | 0·47 | 46·4 (15·6) | 53·1 (18·6) | 0·39 | |
| Sex | |||||||||||||
| Male | 3062 (27·2%) | 5985 (26·0%) | 0·03 | 1421 (49·7%) | 2012 (45·0%) | 0·09 | 8129 (46·9%) | 11 268 (42·2%) | 0·09 | 5169 (46·0%) | 7489 (41·2%) | 0·10 | |
| Female | 8176 (72·8%) | 17 005 (74·0%) | 0·03 | 1436 (50·3%) | 2458 (55·0%) | 0·09 | 9209 (53·1%) | 15 425 (57·8%) | 0·09 | 6077 (54·0%) | 10 670 (58·8%) | 0·10 | |
| Number of tests in past 3 months‡ | |||||||||||||
| 0 | 8752 (77·9%) | 17 028 (74·1%) | 0·09 | 2188 (76·6%) | 3229 (72·2%) | 0·10 | 13 396 (77·3%) | 19 464 (72·9%) | 0·10 | 8419 (74·9%) | 12 953 (71·3%) | 0·08 | |
| 1 | 1726 (15·4%) | 3404 (14·8%) | 0·02 | 492 (17·2%) | 760 (17·0%) | 0·01 | 2820 (16·3%) | 4261 (16·0%) | 0·01 | 2018 (17·9%) | 3132 (17·2%) | 0·02 | |
| ≥2 | 760 (6·8%) | 2558 (11·1%) | 0·15 | 177 (6·2%) | 481 (10·8%) | 0·16 | 1122 (6·5%) | 2968 (11·1%) | 0·16 | 809 (7·2%) | 2074 (11·4%) | 0·15 | |
| Previous positive test >90 days since testing date | 161 (1·4%) | 473 (2·1%) | 0·05 | 35 (1·2%) | 93 (2·1%) | 0·07 | 253 (1·5%) | 599 (2·2%) | 0·06 | 134 (1·2%) | 329 (1·8%) | 0·05 | |
| Any comorbidity§ | 7556 (67·2%) | 17 685 (76·9%) | 0·22 | 1609 (56·3%) | 2896 (64·8%) | 0·17 | 9367 (54·0%) | 17 528 (65·7%) | 0·24 | 6061 (53·9%) | 11 367 (62·6%) | 0·18 | |
| Prior influenza vaccination¶ | 4467 (39·7%) | 13 568 (59·0%) | 0·39 | 1019 (35·7%) | 2310 (51·7%) | 0·33 | 5329 (30·7%) | 13 498 (50·6%) | 0·41 | 3621 (32·2%) | 9287 (51·1%) | 0·39 | |
| Neighbourhood income quintile‖ | |||||||||||||
| 1 (lowest) | 2555 (22·7%) | 4433 (19·3%) | 0·08 | 534 (18·7%) | 715 (16·0%) | 0·07 | 3340 (19·3%) | 4684 (17·5%) | 0·04 | 2036 (18·1%) | 2800 (15·4%) | 0·07 | |
| 2 | 2229 (19·8%) | 4467 (19·4%) | 0·01 | 564 (19·7%) | 775 (17·3%) | 0·06 | 3415 (19·7%) | 5134 (19·2%) | 0·01 | 2217 (19·7%) | 3412 (18·8%) | 0·02 | |
| 3 | 2224 (19·8%) | 4527 (19·7%) | 0·00 | 608 (21·3%) | 851 (19·0%) | 0·06 | 3474 (20·0%) | 5180 (19·4%) | 0·02 | 2317 (20·6%) | 3672 (20·2%) | 0·01 | |
| 4 | 2151 (19·1%) | 4699 (20·4%) | 0·03 | 575 (20·1%) | 982 (22·0%) | 0·05 | 3570 (20·6%) | 5597 (21·0%) | 0·01 | 2345 (20·9%) | 3822 (21·0%) | 0·00 | |
| 5 (highest) | 2033 (18·1%) | 4784 (20·8%) | 0·07 | 562 (19·7%) | 1126 (25·2%) | 0·13 | 3475 (20·0%) | 6008 (22·5%) | 0·06 | 2294 (20·4%) | 4395 (24·2%) | 0·09 | |
Data are n (%) or mean (SD), unless otherwise stated. Proportions might not add up to 100% due to rounding. Additional patient characteristics in the appendix (pp 15–17).
Values of >0·10 are considered to be clinically relevant differences.
Positive test by PCR at index test between March and November, 2021.
In the previous 3 months before COVID-19 immunisation programme started (on Dec 14, 2020).
Comorbidities include chronic respiratory diseases, chronic heart diseases, hypertension, diabetes, chronic kidney disease, other immunocompromising illness, active cancer, advanced liver disease, dementia, frailty, and history of stroke or transient ischaemic attack.
Influenza vaccination during 2019–20 or 2020–21 influenza season, or both.
The sum of counts does not equal the column total because of individuals with missing information (<1·0%) for this characteristic.
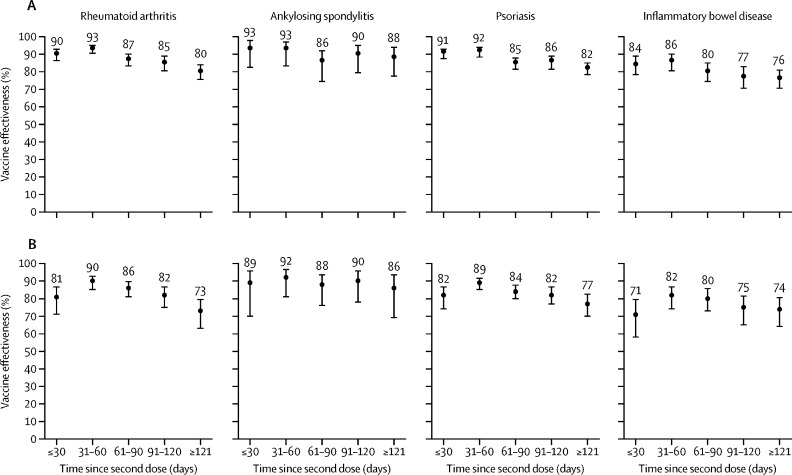
Overall adjusted vaccine effectiveness of two doses of mRNA-based COVID-19 vaccine against SARS-CoV-2 infection was 83% (95% CI 80–86) in patients with rheumatoid arthritis, 89% (83–93) in patients with ankylosing spondylitis, 84% (81–86) in patients with psoriasis, and 79% (74–82) in patients with inflammatory bowel disease (table 3 ). Effectiveness against infection peaked 31–60 days after two doses (adjusted vaccine effectiveness 82–92% across immune-mediated inflammatory disease groups) and overall waned gradually with each additional month (figure ). Estimates of adjusted vaccine effectiveness against infection after three doses of vaccine were near equivalent or higher than those after two doses, although less precise due to fewer patients having received three doses (table 3). Because few test-positive cases had received a third dose of vaccine and due to insufficient follow-up time, we could not assess waning of vaccine effectiveness after third doses over time.
Table 3.
Estimated vaccine effectiveness of mRNA-based COVID-19 vaccines against SARS-CoV-2 infection and severe outcome, by time between vaccination and testing date
| Test-positive cases | Test-negative controls | Unadjusted vaccine effectiveness (95% CI) | Adjusted vaccine effectiveness (95% CI)* | ||
|---|---|---|---|---|---|
| SARS-CoV-2 infection | |||||
| Rheumatoid arthritis | |||||
| First dose ≥14 days | 311/1801 (17·3%) | 6005/17 393 (34·5%) | 60% (55–65) | 53% (45–59) | |
| Second dose ≥7 days | 244/1734 (14·1%) | 14 330/25 718 (55·7%) | 87% (85–89) | 83% (80–86) | |
| Third dose ≥7 days | 7/1497 (0·5%) | 453/11 841 (3·8%) | 88% (75–94) | 86% (70–94) | |
| Ankylosing spondylitis | |||||
| First dose ≥14 days | 62/421 (14·7%) | 1074/3902 (27·5%) | 55% (40–66) | 49% (30–63) | |
| Second dose ≥7 days | 37/396 (9·3%) | 2876/5704 (50·4%) | 90% (86–93) | 89% (83–93) | |
| Third dose ≥7 days | <6/361 (<1·7%) | 89/2917 (3·1%) | NR | 82% (20–96) | |
| Psoriasis | |||||
| First dose ≥14 days | 334/2655 (12·6%) | 6548/23 586 (27·8%) | 63% (58–67) | 55% (48–60) | |
| Second dose ≥7 days | 314/2635 (11·9%) | 17 230/34 268 (50·3%) | 87% (85–88) | 84% (81–86) | |
| Third dose ≥7 days | <6/2322 (<0·3%) | 245/17 283 (1·4%) | NR | 96% (72–99) | |
| Inflammatory bowel disease | |||||
| First dose ≥14 days | 202/1400 (14·4%) | 4570/15 907 (28·7%) | 58% (51–64) | 49% (40–57) | |
| Second dose ≥7 days | 231/1429 (16·2%) | 11 560/22 897 (50·5%) | 81% (78–84) | 79% (74–82) | |
| Third dose ≥7 days | 7/1205 (0·6%) | 300/11 637 (2·6%) | 78% (53–90) | 76% (47–89) | |
| Severe outcomes | |||||
| Rheumatoid arthritis | |||||
| First dose ≥14 days | 53/305 (17·4%) | 6005/17 393 (34·5%) | 60% (46–70) | 74% (63–81) | |
| Second dose ≥7 days | 35/287 (12·2%) | 14 330/25 718 (55·7%) | 89% (84–92) | 92% (88–95) | |
| Third dose ≥7 days | <6/254 (<2·4%) | 453/11 841 (3·8%) | NR | 88% (48–97) | |
| Ankylosing spondylitis | |||||
| First dose ≥14 days | 6/46 (13·0%) | 1074/3902 (27·5%) | 61% (7–83) | 76% (35–91) | |
| Second dose ≥7 days | <6/42 (<14·3%) | 2876/5704 (50·4%) | NR | 97% (83–99) | |
| Third dose ≥7 days | <6/41 (<14·6%) | 89/2917 (3·1%) | NR | NR† | |
| Psoriasis | |||||
| First dose ≥14 days | 37/269 (13·8%) | 6548/23 586 (27·8%) | 59% (41–71) | 72% (59–82) | |
| Second dose ≥7 days | 25/257 (9·7%) | 17 230/34 268 (50·3%) | 89% (84–93) | 92% (86–95) | |
| Third dose ≥7 days | <6/232 (<2·6%) | 245/17 283 (1·4%) | NR | NR† | |
| Inflammatory bowel disease | |||||
| First dose ≥14 days | 30/173 (17·3%) | 4570/15 907 (28·7%) | 48% (23–65) | 65% (44–78) | |
| Second dose ≥7 days | 14/157 (8·9%) | 11 560/22 897 (50·5%) | 90% (83–94) | 94% (88–97) | |
| Third dose ≥7 days | <6/145 (<4·1%) | 300/11 637 (2·6%) | NR | NR† | |
Data are n/N (%), where n is number vaccinated, and N is total cases, unless otherwise stated. Exact patient numbers and unadjusted vaccine estimates cannot be provided for groups containing fewer than six patients to maintain patient anonymity. Vaccine effectiveness is (1 – odds ratio) × 100%. Severe outcome is defined as hospitalisation or death attributed to SARS-CoV-2 infection. NR=not reportable.
Adjusted for age, sex, region, biweekly period of test, number of previous SARS-CoV-2 tests, past SARS-CoV-2 infection, presence of any comorbidity, previous receipt of influenza vaccine, and area-level sociodemographic variables.
Not reported due to extremely imprecise 95% confidence intervals due to near zero exposures among test-positive cases.
Figure.
Unadjusted (A) and adjusted* (B) vaccine effectiveness against SARS-CoV-2 infection by time since second dose for those tested for SARS-CoV-2 between March 1 and Nov 22, 2021
Datapoints are vaccine effectiveness, with whiskers showing 95% CIs. *Adjusted for age, sex, region, biweekly period of test, number of previous SARS-CoV-2 tests, past SARS-CoV-2 infection, presence of any comorbidity, previous receipt of influenza vaccine, and area-level sociodemographic variables.
Across all four immune-mediated inflammatory disease groups, adjusted vaccine effectiveness estimates against SARS-CoV-2 infection were higher for mRNA-1273 than for BNT162b2 after one and two doses (appendix p 18). Estimates for vaccine effectiveness after three doses could not be precisely determined and so are not presented here.
For rheumatoid arthritis, among 2127 test-positive cases, 352 (16·5%) had a severe outcome including 16 (0·8%) deaths attributed to COVID-19. For ankylosing spondylitis, among 476 test-positive cases, 50 (10·5%) had a severe outcome. For psoriasis, among 3089 test-positive cases, 298 (9·6%) had a severe outcome, of whom 13 (0·4%) died. For inflammatory bowel disease, among 1702 test-positive cases, 196 (11·5%) had a severe outcome. Fewer than six patients died in each group of ankylosing spondylitis and inflammatory bowel disease; therefore, we cannot report the exact numbers due to privacy protection regulations.
Adjusted vaccine effectiveness of two doses against severe outcome was high across all four immune-mediated inflammatory disease groups: 92% (95% CI 88–95) in patients with rheumatoid arthritis, 97% (83–99) in patients with ankylosing spondylitis, 92% (86–95) in patients with psoriasis, and 94% (88–97) in patients with inflammatory bowel disease (table 3). Among test-positive patients who had a severe outcome, there were too few patients who had received three doses of vaccine to precisely estimate vaccine effectiveness for patients with ankylosing spondylitis, psoriasis, and inflammatory bowel disease.
The assessment of potential selection bias through the sensitivity analysis did not change study findings (data not shown).
Discussion
Over a 9 month period in 2021, we assessed the initial vaccine effectiveness of mRNA-based COVID-19 vaccines in patients with rheumatoid arthritis, ankylosing spondylitis, psoriasis, and inflammatory bowel disease. We found high (92–97%) adjusted vaccine effectiveness of two doses of an mRNA-based COVID-19 vaccines (BNT162b2 or mRNA-1273) against severe COVID-19 outcomes compared with unvaccinated patients. Although vaccine effectiveness estimates against infection were lower than for severe outcomes, COVID-19 vaccines still offered very good protection against infection during the study period. For second doses, adjusted vaccine effectiveness against infection peaked at 31–60 days after vaccination (82–92%) and waned with each additional month but rebounded for those who received three doses. Because administration of third doses only commenced on Sept 14, 2021, in Ontario, Canada, and our study accrual period was only up to Nov 22, 2021, we were unable to assess waning of third dose effectiveness in this study.
We found high vaccine effectiveness against severe outcomes in individuals with immune-mediated inflammatory diseases that are similar to those found in the larger general population in Ontario.1, 2, 3 However, vaccine effectiveness against infection among people with immune-mediated inflammatory diseases was slightly lower than that estimated for the general population, which was estimated to be above 90% for symptomatic infection shortly after two doses of vaccine (and rebound to >90% after a third dose) during a similar study time frame.1, 3 We were unable to estimate vaccine effectiveness for symptomatic infection, which might also explain differences in estimates of vaccine effectiveness compared with the general population. Possibly the increased age, altered immune response, high burden of comorbidities, and use of immunosuppressant therapy in people with immune-mediated inflammatory diseases reduces vaccine effectiveness against infection.6, 9, 30 People with immune-mediated inflammatory diseases might also be more likely to get tested for SARS-CoV-2 infection than people in the general population because they know they are more clinically vulnerable, leading to higher detection of infections than in the general population.
Estimates of COVID-19 vaccine effectiveness against infection and seroconversion rates are generally lower in people with immune-mediated inflammatory diseases than in the general population.11, 12, 31, 32, 33, 34 Two systematic reviews reported that immunocompromised groups, including people with immune-mediated inflammatory diseases, had lower seroconversion and antibody titres after first and second doses of COVID-19 vaccines than did immunocompetent controls.11, 35 Studies have also found that additional doses of vaccine and temporary discontinuation of immunosuppressant therapy might improve immunogenicity.10, 13, 14 Thus, the reduced immune response to COVID-19 vaccines that has been observed in clinical studies is probably translates to slightly lower vaccine effectiveness in the larger population of people with immune-mediated inflammatory diseases, as we found in our study. We also found that adjusted vaccine effectiveness estimates against SARS-CoV-2 infection were generally higher for mRNA-1273 than for BNT162b2, for both one and two doses; a finding that has been signalled in other studies1, 36 and is potentially a result in differences in the mRNA content and higher dose of mRNA-1273 than BNT162b2.
By linking a centralised vaccine registry with laboratory and health administrative data, we created large cohorts to study vaccine effectiveness, overcoming the sample size challenges faced by clinical studies. We used a test-negative study design to enable us to do comparative assessments with the general population estimates of vaccine effectiveness from previous Ontario reports that used a similar protocol.1, 2, 3 The test-negative design in traditional case-control studies is purported to reduce selection bias associated with differential health care-seeking behaviour between cases and controls, and reduce misclassification of cases,37, 38, 39 resulting in comparable estimates with those of case-control and cohort studies (although all might underestimate true vaccine effectiveness) and randomised controlled trials.38, 40 However, bias might still occur due to unmeasured differences between vaccinated and unvaccinated patients, and testing patterns might also differ between vaccinated and unvaccinated patients.
The preferred approach in test-negative designs is to sample patients who present for testing with symptomatic disease, otherwise including patients with asymptomatic disease can create a downward bias resulting in underestimation of vaccine effectiveness. Unfortunately, information on symptoms (at the time of testing) was only available on a subset of patients and so we were unable to estimate vaccine effectiveness against symptomatic infection. This approach might have resulted in lower estimates of vaccine effectiveness for the outcome of infection than for the outcome of symptomatic infection.
Ontario's centralised vaccine registry minimised misclassification of vaccination status, and during our study period there were no changes in PCR testing eligibility. Even with the increased use of rapid antigen tests (which were not captured in our datasets), all individuals who tested positive with rapid antigen tests were advised to obtain PCR tests for confirmation throughout our study period. The proportion of SARS-CoV-2 infections that are not confirmed by PCR is unclear; however, our estimates of vaccine effectiveness against severe outcomes are not biased by this uncertainty because, for this measure, all individuals would have received a PCR test upon hospital admission.
Another potential limitation of our study is misclassification error using administrative data to identify people with immune-mediated inflammatory diseases. Our immune-mediated inflammatory disease case definitions were previously validated by medical chart reviews, yielding high specificity (approximately 99%) and positive predictive values (rheumatoid arthritis: 78%, inflammatory bowel disease: 71–81%).20, 22, 41 Misclassified patients (false positives) usually have a similar immune-mediated inflammatory disease diagnosis.20, 21 Because of the population-based nature of our data, the patients with immune-mediated inflammatory diseases in our study encompass a wide spectrum of disease states that are likely to be highly generalisable to real-world populations across different settings; however, we were not able to assess phenotype, disease activity, or severity. Heterogeneity in vaccine effectiveness might exist across different risk groups, such as those receiving pharmacological therapy.9, 30, 42, 43, 44, 45, 46, 47, 48 We did not assess the effects or control for immunosuppressant therapies because prescription drug data were limited to a subset of patients who qualify for the publicly funded drug programme in the province (primarily those aged ≥65 years). Therefore, despite controlling for potential confounders, residual confounding might have affected our results.
Finally, we restricted our study period to predate the omicron SARS-CoV-2 variant for several methodological reasons. The omicron variant has shown differences in disease severity for both vaccinated and unvaccinated individuals within Canada49 and other countries,50, 51 which could confound our results. Moreover, because of the high vaccine coverage in our population with immune-mediated inflammatory diseases by the time omicron was circulating17 and strict public health measures among unvaccinated individuals, estimating vaccine effectiveness for only the initial part of the omicron wave could lead to downward bias. Although the dominant circulating variants of SARS-CoV-2 are changing over time, we postulated a priori that any differences observed (comparing estimates of vaccine effectiveness within Ontario's population with immune-mediated inflammatory diseases within Ontario's general population1, 2, 3) will probably hold true for omicron and future variants. Early general population-based analyses (up to Dec 26, 2021) from Ontario reported that vaccine effectiveness against infection with the omicron variant has been lower than that observed against infection with the delta variant, but still remains high against severe outcomes for omicron.3 Unfortunately, universal PCR testing in Ontario is no longer available, restricting our ability to replicate our analyses in Ontario's population with immune-mediated inflammatory diseases for the outcome of infection with the omicron variant.
In summary, between March and November, 2021, we estimated high vaccine effectiveness of mRNA-based COVID-19 vaccines against severe outcomes and infection among individuals with immune-mediated inflammatory diseases. These findings are crucial to help these individuals, who were excluded from vaccine trials, make informed decisions about following vaccine recommendations and to inform future vaccine strategies. Future research is needed to understand how long effectiveness of three doses of these vaccines remains durable, particularly against emerging variants.
For more on ICES requirements for data access see www.ices.on.ca/DAS
Data sharing
The study dataset is held securely in coded form at ICES. Although legal data sharing agreements between ICES and data providers (eg, health-care organisations and governments) prohibit ICES from making the dataset publicly available, access might be granted to those who meet prespecified criteria for confidential access, available at www.ices.on.ca/DAS or via email (das@ices.on.ca). The full dataset creation plan and underlying analytical code are available from the authors on request, understanding that the computer programs might rely on coding templates or macros that are unique to ICES and are therefore either inaccessible or require modification.
Declaration of interests
GGK has received honoraria for speaking or consultancy from AbbVie, Janssen, Pfizer, and Takeda; has received research support from Janssen, AbbVie, GlaxoSmithKline, Merck, Ferring, and Shire; has been a consultant for Gilead; and shares ownership of two patents (Treatment of inflammatory disorders, autoimmune disease, and PBC, UTI Limited Partnership, assignee. patent WO2019046959A1. PCT/CA2018/051098. Sept 7, 2018). EIB has acted as a legal consultant for Hoffman La-Roche and Peabody & Arnold, and consultant for McKesson Canada for matters unrelated to a medication used to treat inflammatory bowel disease or COVID-19 (ie, unrelated to the submitted work). LE reports grants from AbbVie, Novartis, UCB, Pfizer, and Eli Lilly. All other authors declare no competing interests.
Acknowledgments
Acknowledgments
This project was supported by funding from the Public Health Agency of Canada, through the Vaccine Surveillance Reference group and the COVID-19 Immunity Task Force. The views expressed here do not necessarily represent the views of the Public Health Agency of Canada. The study was supported by ICES (formerly known as the Institute for Clinical Evaluative Sciences), which is funded by the Ontario Ministry of Health (MOH) and the Ministry of Long-Term Care (MLTC). Parts of this material are based on data and information compiled and provided by the MOH, the Canadian Institute for Health Information, Cancer Care Ontario, Ontario Health, and Public Health Ontario (case-level data from CCM and COVID-19 laboratory data). We thank the staff of Ontario's public health units who are responsible for COVID-19 case and contact management and data collection. We are grateful to the Ontario residents without whom this research would be impossible. The opinions, results, and conclusions reported in this Article are those of the authors and are independent of the data sources; no endorsement is intended or should be inferred. JW receives support from the Arthritis Society Stars Career Development Award. JCK is supported by Clinician-Scientist Award from the University of Toronto Department of Family and Community Medicine. LE is Canada research chair (tier 2) in equity in care of rheumatic disorders. DL is the Mary Pack Chair in Rheumatology Research from the University of British Columbia and The Arthritis Society. EIB holds the Northbridge Financial Corporation Chair in Inflammatory Bowel Disease, a joint Hospital-University Chair between University of Toronto, The Hospital for Sick Children, and the SickKids Foundation. JAA-Z is the BC Lupus Society Research Scholar and the Walter and Marilyn Booth Research Scholar. SB is a James McGill Professor of Medicine.
Contributors
JW contributed to study design, data acquisition, statistical analysis, interpretation of data, manuscript writing, and is responsible for the overall content as guarantor. JCK contributed to study design, statistical analysis, interpretation of data, and manuscript editing. SC contributed to data acquisition, data preparation, statistical analysis, and manuscript editing. LE, EIB, GGK, CH, JAA-Z, and DL contributed to study design, interpretation of data, and manuscript editing. HC contributed to study design, data preparation, interpretation of data, and manuscript editing. SB contributed to study design, data acquisition, statistical analysis, interpretation of data, and manuscript editing. All authors had final responsibility for the decision to submit for publication. JW, SC, HC, and SB accessed and verified the underlying study data.
Supplementary Material
References
- 1.Nasreen S, Chung H, He S, et al. Effectiveness of COVID-19 vaccines against symptomatic SARS-CoV-2 infection and severe outcomes with variants of concern in Ontario. Nat Microbiol. 2022;7:379–385. doi: 10.1038/s41564-021-01053-0. [DOI] [PubMed] [Google Scholar]
- 2.Chung H, He S, Nasreen S, et al. Effectiveness of BNT162b2 and mRNA-1273 COVID-19 vaccines against symptomatic SARS-CoV-2 infection and severe COVID-19 outcomes in Ontario, Canada: test negative design study. BMJ. 2021;374 doi: 10.1136/bmj.n1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buchan SA, Chung H, Brown KA, et al. Effectiveness of COVID-19 vaccines against omicron or delta symptomatic infection and severe outcomes. medRxiv. 2022 doi: 10.1101/2021.12.30.21268565. published online on Jan 28. (preprint). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bok K, Sitar S, Graham BS, Mascola JR. Accelerated COVID-19 vaccine development: milestones, lessons, and prospects. Immunity. 2021;54:1636–1651. doi: 10.1016/j.immuni.2021.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zheng C, Shao W, Chen X, Zhang B, Wang G, Zhang W. Real-world effectiveness of COVID-19 vaccines: a literature review and meta-analysis. Int J Infect Dis. 2022;114:252–260. doi: 10.1016/j.ijid.2021.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eder L, Croxford R, Drucker AM, et al. COVID-19 hospitalizations, intensive care unit stays, ventilation and death among patients with immune-mediated inflammatory diseases compared to controls. J Rheumatol. 2022 doi: 10.3899/jrheum.211012. publsihed online Feb 1. [DOI] [PubMed] [Google Scholar]
- 7.Fagni F, Simon D, Tascilar K, et al. COVID-19 and immune-mediated inflammatory diseases: effect of disease and treatment on COVID-19 outcomes and vaccine responses. Lancet Rheumatol. 2021;3:e724–e736. doi: 10.1016/S2665-9913(21)00247-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Akiyama S, Hamdeh S, Micic D, Sakuraba A. Prevalence and clinical outcomes of COVID-19 in patients with autoimmune diseases: a systematic review and meta-analysis. Ann Rheum Dis. 2021;80:384–391. doi: 10.1136/annrheumdis-2020-218946. [DOI] [PubMed] [Google Scholar]
- 9.Sparks JA, Wallace ZS, Seet AM, et al. Associations of baseline use of biologic or targeted synthetic DMARDs with COVID-19 severity in rheumatoid arthritis: results from the COVID-19 Global Rheumatology Alliance physician registry. Ann Rheum Dis. 2021;80:1137–1146. doi: 10.1136/annrheumdis-2021-220418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Furer V, Eviatar T, Zisman D, et al. Immunogenicity and safety of the BNT162b2 mRNA COVID-19 vaccine in adult patients with autoimmune inflammatory rheumatic diseases and in the general population: a multicentre study. Ann Rheum Dis. 2021;80:1330–1338. doi: 10.1136/annrheumdis-2021-220647. [DOI] [PubMed] [Google Scholar]
- 11.Di Fusco M, Lin J, Vaghela S, et al. COVID-19 vaccine effectiveness among immunocompromised populations: a targeted literature review of real-world studies. Expert Rev Vaccines. 2022 doi: 10.1080/14760584.2022.2035222. published online Feb 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Whitaker HJ, Tsang RS, Byford R, et al. Pfizer-BioNTech and Oxford AstraZeneca COVID-19 vaccine effectiveness and immune response among individuals in clinical risk groups. J Infect. 2022 doi: 10.1016/j.jinf.2021.12.044. published online Jan 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schmiedeberg K, Vuilleumier N, Pagano S, et al. Efficacy and tolerability of a third dose of an mRNA anti-SARS-CoV-2 vaccine in patients with rheumatoid arthritis with absent or minimal serological response to two previous doses. Lancet Rheumatol. 2022;4:e11–e13. doi: 10.1016/S2665-9913(21)00328-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rubbert-Roth A, Vuilleumier N, Ludewig B, Schmiedeberg K, Haller C, von Kempis J. Anti-SARS-CoV-2 mRNA vaccine in patients with rheumatoid arthritis. Lancet Rheumatol. 2021;3:e470–e472. doi: 10.1016/S2665-9913(21)00186-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Curtis JR, Johnson SR, Anthony DD, et al. American College of Rheumatology guidance for COVID-19 vaccination in patients with rheumatic and musculoskeletal diseases: version 3. Arthritis Rheumatol. 2021;73:e60–e75. doi: 10.1002/art.41928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hazlewood GS, Pardo JP, Barnabe C, et al. Canadian Rheumatology Association recommendation for the use of COVID-19 vaccination for patients with autoimmune rheumatic diseases. J Rheumatol. 2021;48:1330–1339. doi: 10.3899/jrheum.210288. [DOI] [PubMed] [Google Scholar]
- 17.Widdifield J, Eder L, Chen S, et al. COVID-19 vaccination uptake among individuals with immune-mediated inflammatory diseases in Ontario, Canada, between December 2020 and October 2021: a population-based analysis. J Rheumatol. 2022 doi: 10.3899/jrheum.211148. published online Jan 15. [DOI] [PubMed] [Google Scholar]
- 18.Kuenzig ME, Widdifield J, Bernatsky S, Kaplan GG, Benchimol EI. Uptake of third doses of SARS-CoV-2 vaccines among people with inflammatory bowel disease in Ontario, Canada. Lancet Gastroenterol Hepatol. 2022;7:288–289. doi: 10.1016/S2468-1253(22)00054-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.PublicHealthOntario Ontario COVID-19 data tool. February, 2022. https://www.publichealthontario.ca/en/data-and-analysis/infectious-disease/covid-19-data-surveillance/covid-19-data-tool?tab=trends
- 20.Eder L, Widdifield J, Rosen CF, et al. Identifying and characterizing psoriasis and psoriatic arthritis patients in Ontario administrative data: a population-based study From 1991 to 2015. J Rheumatol. 2020;47:1644–1651. doi: 10.3899/jrheum.190659. [DOI] [PubMed] [Google Scholar]
- 21.Widdifield J, Bernatsky S, Paterson JM, et al. Accuracy of Canadian health administrative databases in identifying patients with rheumatoid arthritis: a validation study using the medical records of rheumatologists. Arthritis Care Res (Hoboken) 2013;65:1582–1591. doi: 10.1002/acr.22031. [DOI] [PubMed] [Google Scholar]
- 22.Benchimol EI, Guttmann A, Mack DR, et al. Validation of international algorithms to identify adults with inflammatory bowel disease in health administrative data from Ontario, Canada. J Clin Epidemiol. 2014;67:887–896. doi: 10.1016/j.jclinepi.2014.02.019. [DOI] [PubMed] [Google Scholar]
- 23.Benchimol EI, Manuel DG, Guttmann A, et al. Changing age demographics of inflammatory bowel disease in Ontario, Canada: a population-based cohort study of epidemiology trends. Inflamm Bowel Dis. 2014;20:1761–1769. doi: 10.1097/MIB.0000000000000103. [DOI] [PubMed] [Google Scholar]
- 24.Haroon NN, Paterson JM, Li P, Inman RD, Haroon N. Patients with ankylosing spondylitis have increased cardiovascular and cerebrovascular mortality: a population-based study. Ann Intern Med. 2015;163:409–416. doi: 10.7326/M14-2470. [DOI] [PubMed] [Google Scholar]
- 25.Eder L, Widdifield J, Rosen CF, et al. Trends in the prevalence and incidence of psoriasis and psoriatic arthritis in ontario, canada: a population-based study. Arthritis Care Res (Hoboken) 2019;71:1084–1091. doi: 10.1002/acr.23743. [DOI] [PubMed] [Google Scholar]
- 26.Widdifield J, Bernatsky S, Bombardier C, Paterson M. Rheumatoid arthritis surveillance in Ontario: monitoring the burden, quality of care and patient outcomes through linkage of administrative health data. Healthc Q. 2015;18:7–10. doi: 10.12927/hcq.2015.24439. [DOI] [PubMed] [Google Scholar]
- 27.Eder L, Croxford R, Drucker AM, et al. Understanding COVID-19 risk in patients with immune mediated inflammatory diseases: a population-based analysis of SARS-CoV-2 testing. Arthritis Care Res (Hoboken) 2021 doi: 10.1002/acr.24781. https://dooi.org/10.1002/acr.24781 published online Sept 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Austin PC. Using the standardized difference to compare the prevalence of a binary variable between two groups in observational research. Commun Stat Simul Comput. 2009;38:1228–1234. [Google Scholar]
- 29.Mamdani M, Sykora K, Li P, et al. Reader's guide to critical appraisal of cohort studies: 2. Assessing potential for confounding. BMJ. 2005;330:960–962. doi: 10.1136/bmj.330.7497.960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gianfrancesco M, Hyrich KL, Al-Adely S, et al. Characteristics associated with hospitalisation for COVID-19 in people with rheumatic disease: data from the COVID-19 Global Rheumatology Alliance physician-reported registry. Ann Rheum Dis. 2020;79:859–866. doi: 10.1136/annrheumdis-2020-217871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Khan N, Mahmud N. Effectiveness of SARS-CoV-2 vaccination in a veterans affairs cohort of patients with inflammatory bowel disease with diverse exposure to immunosuppressive medications. Gastroenterology. 2021;161:827–836. doi: 10.1053/j.gastro.2021.05.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yelin I, Katz R, Herzel E, et al. Associations of the BNT162b2 COVID-19 vaccine effectiveness with patient age and comorbidities. medRxiv. 2021 doi: 10.1101/2021.03.16.21253686. Published online May 24. (preprint). [DOI] [Google Scholar]
- 33.Ben-Tov A, Banon T, Chodick G, Kariv R, Assa A, Gazit S. BNT162b2 messenger RNA COVID-19 vaccine effectiveness in patients with inflammatory bowel disease: preliminary real-world data during mass vaccination campaign. Gastroenterology. 2021;161:1715–1717. doi: 10.1053/j.gastro.2021.06.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hadi YB, Thakkar S, Shah-Khan SM, Hutson W, Sarwari A, Singh S. COVID-19 vaccination is safe and effective in patients with inflammatory bowel disease: analysis of a large multi-institutional research network in the United States. Gastroenterology. 2021;161:1336–1339. doi: 10.1053/j.gastro.2021.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee ARYB, Wong SY, Chai LYA, et al. Efficacy of COVID-19 vaccines in immunocompromised patients: systematic review and meta-analysis. BMJ. 2022;376 doi: 10.1136/bmj-2021-068632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dickerman BA, Gerlovin H, Madenci AL, et al. Comparative effectiveness of BNT162b2 and mRNA-1273 vaccines in U.S. Veterans. N Engl J Med. 2022;386:105–115. doi: 10.1056/NEJMoa2115463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sullivan SG, Tchetgen Tchetgen EJ, Cowling BJ. Theoretical basis of the test-negative study design for assessment of influenza vaccine effectiveness. Am J Epidemiol. 2016;184:345–353. doi: 10.1093/aje/kww064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.De Serres G, Skowronski DM, Wu XW, Ambrose CS. The test-negative design: validity, accuracy and precision of vaccine efficacy estimates compared to the gold standard of randomised placebo-controlled clinical trials. Euro Surveill. 2013;18 doi: 10.2807/1560-7917.es2013.18.37.20585. [DOI] [PubMed] [Google Scholar]
- 39.Dean NE, Hogan JW, Schnitzer ME. COVID-19 vaccine effectiveness and the test-negative design. N Engl J Med. 2021;385:1431–1433. doi: 10.1056/NEJMe2113151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Orenstein EW, De Serres G, Haber MJ, et al. Methodologic issues regarding the use of three observational study designs to assess influenza vaccine effectiveness. Int J Epidemiol. 2007;36:623–631. doi: 10.1093/ije/dym021. [DOI] [PubMed] [Google Scholar]
- 41.Widdifield J, Bombardier C, Bernatsky S, et al. An administrative data validation study of the accuracy of algorithms for identifying rheumatoid arthritis: the influence of the reference standard on algorithm performance. BMC Musculoskelet Disord. 2014;15:216. doi: 10.1186/1471-2474-15-216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Geisen UM, Berner DK, Tran F, et al. Immunogenicity and safety of anti-SARS-CoV-2 mRNA vaccines in patients with chronic inflammatory conditions and immunosuppressive therapy in a monocentric cohort. Ann Rheum Dis. 2021;80:1306–1311. doi: 10.1136/annrheumdis-2021-220272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Haberman RH, Herati R, Simon D, et al. Methotrexate hampers immunogenicity to BNT162b2 mRNA COVID-19 vaccine in immune-mediated inflammatory disease. Ann Rheum Dis. 2021;80:1339–1344. doi: 10.1136/annrheumdis-2021-220597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bugatti S, De Stefano L, Balduzzi S, et al. Methotrexate and glucocorticoids, but not anticytokine therapy, impair the immunogenicity of a single dose of the BNT162b2 mRNA COVID-19 vaccine in patients with chronic inflammatory arthritis. Ann Rheum Dis. 2021;80:1635–1638. doi: 10.1136/annrheumdis-2021-220862. [DOI] [PubMed] [Google Scholar]
- 45.Simon D, Tascilar K, Fagni F, et al. SARS-CoV-2 vaccination responses in untreated, conventionally treated and anticytokine-treated patients with immune-mediated inflammatory diseases. Ann Rheum Dis. 2021;80:1312–1316. doi: 10.1136/annrheumdis-2021-220461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Spiera R, Jinich S, Jannat-Khah D. Rituximab, but not other antirheumatic therapies, is associated with impaired serological response to SARS- CoV-2 vaccination in patients with rheumatic diseases. Ann Rheum Dis. 2021;80:1357–1359. doi: 10.1136/annrheumdis-2021-220604. [DOI] [PubMed] [Google Scholar]
- 47.Shen C, Risk M, Schiopu E, et al. Efficacy of COVID-19 vaccines in patients taking immunosuppressants. Ann Rheum Dis. 2022 doi: 10.1136/annrheumdis-2021-222045. published online Feb 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mahil SK, Bechman K, Raharja A, et al. The effect of methotrexate and targeted immunosuppression on humoral and cellular immune responses to the COVID-19 vaccine BNT162b2: a cohort study. Lancet Rheumatol. 2021;3:e627–e637. doi: 10.1016/S2665-9913(21)00212-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ulloa AC, Buchan SA, Daneman N, Brown KA. Early estimates of SARS-CoV-2 omicron variant severity based on a matched cohort study, Ontario, Canada. medRxiv. 2022 doi: 10.1101/2021.12.24.21268382. published online Jan 2. (preprint). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Veneti L, Bøås H, Bråthen Kristoffersen A, et al. Reduced risk of hospitalisation among reported COVID-19 cases infected with the SARS-CoV-2 omicron BA.1 variant compared with the delta variant, Norway, December 2021 to January 2022. Euro Surveill. 2022;27 doi: 10.2807/1560-7917.ES.2022.27.4.2200077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bager P, Wohlfahrt J, Bhatt S, et al. Reduced risk of hospitalisation associated with infection with SARS-CoV-2 omicron relative to delta: a Danish cohort study. SSRN. 2022 https://papers.ssrn.com/sol3/papers.cfm?abstract_id=4008930 published online Jan 14. (preprint). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The study dataset is held securely in coded form at ICES. Although legal data sharing agreements between ICES and data providers (eg, health-care organisations and governments) prohibit ICES from making the dataset publicly available, access might be granted to those who meet prespecified criteria for confidential access, available at www.ices.on.ca/DAS or via email (das@ices.on.ca). The full dataset creation plan and underlying analytical code are available from the authors on request, understanding that the computer programs might rely on coding templates or macros that are unique to ICES and are therefore either inaccessible or require modification.