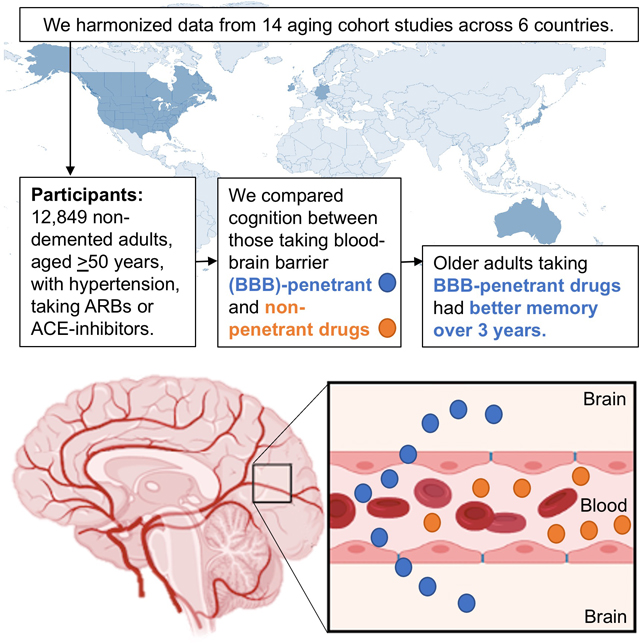
Abstract
Hypertension is an established risk factor for cognitive decline and dementia in older adults, highlighting the potential importance of antihypertensive treatments in prevention efforts. Work surrounding antihypertensive treatments has suggested possible salutary effects on cognition and neuropathology. Several studies have specifically highlighted renin-angiotensin system drugs, including AT1-receptor blockers and angiotensin-converting-enzyme inhibitors, as potentially benefiting cognition in later life. A small number of studies have further suggested renin-angiotensin system drugs that cross the blood-brain barrier may be linked to lower dementia risk compared to their non-penetrant counterparts. The present meta-analysis sought to evaluate the potential cognitive benefits of blood-brain barrier crossing renin-angiotensin system drugs relative to their non-penetrant counterparts. We harmonized longitudinal participant data from 14 cohorts from 6 countries (Australia, Canada, Germany, Ireland, Japan, United States), for a total of 12,849 individuals at baseline, and assessed for blood-brain barrier crossing potential within antihypertensive medications used by cognitively normal participants. We analyzed seven cognitive domains (attention, executive function, language, verbal memory learning, recall, mental status, and processing speed) using analysis of covariance (adjusted for age, sex, and education) and meta-analyses. Older adults taking blood-brain barrier-crossing renin-angiotensin drugs exhibited better memory recall over up to 3 years of follow-up, relative to those taking non-penetrant medications, despite their relatively higher vascular risk burden. Conversely, those taking non-blood-brain barrier-penetrant medications showed better attention over the same follow-up period, although their lower vascular risk burden may partially explain this result. Findings suggest links between blood-brain barrier crossing renin-angiotensin drugs and less memory decline.
Keywords: Hypertension, cognition, blood-brain-barrier, ARBs, ACE-inhibitors, dementia
Graphical Abstract
Introduction
Hypertension is a well-established risk factor for cognitive decline and dementia,1 possibly through its effects on both cerebrovascular disease and Alzheimer’s disease.2,3 Hypertension and vascular risk factors have been linked to deficits in various domains of cognition. Specifically, prior work has found individuals with hypertension exhibit deficits in episodic memory, working memory, executive function,4 attention, and psychomotor speed,5 relative to normotensive persons.
Studies of antihypertensive treatments have reported possible salutary effects on cognition and cerebrovascular disease,6,7 as well as Alzheimer’s disease (AD) neuropathology.8 In comparison to individuals who have never taken any antihypertensive drugs, individuals who have been treated with these medications have been found to be at decreased risk of all dementia, with an 8% risk reduction for every year of use in individuals ≤75 years old.9 In the Systolic Blood Pressure Intervention Trial – Memory and Cognition in Decreased Hypertension (SPRINT MIND) trial, intensive blood pressure lowering to SBP < 120 mm Hg (compared to standard treatment to <140 mm Hg) was linked to 19% reduction in cases of mild cognitive impairment (MCI), which is considered the precursor to dementia.10
In addition to focusing on intensive blood pressure lowering for the prevention of cognitive impairment, some meta-analytic studies have examined the role of specific antihypertensive treatments. A meta-analysis of 19 randomized trials and 11 studies examining the relationships among antihypertensive drug use, cognition, and dementia incidence provided support for the possible cognitive benefits of antihypertensive treatment [effect size 0.05, 95% CI (0.02–0.07)]11. Of the many antihypertensive drug classes available, drugs targeting the renin-angiotensin system (RAS), namely, angiotensin II receptor blockers (ARBs) and angiotensin-converting-enzyme (ACE)-inhibitors, have been highlighted as possibly conferring the greatest benefit.11–15 However, other studies have also shown benefits of calcium channel blockers16–19 and diuretics20 on reducing dementia risk, and it must be acknowledged that results from research linking blood pressure control with RAS drugs to cognitive benefit have been mixed.21–23 It is possible that older trials, which were not originally designed to evaluate cognition, did not include milder forms of cognitive impairment as endpoints. Notably, even the SPRINT MIND trial did not reach statistically significant benefit for dementia, despite showing substantial benefit for MCI. These findings highlight the potential importance of antihypertensive treatment in mild levels of cognitive impairment observed on more sensitive neuropsychological tests.
Pharmacokinetic properties of RAS drugs may also be of importance in modulating potential neurocognitive benefits. Although RAS is classically involved in the maintenance of cardiovascular function and fluid homeostasis in peripheral circulation, the presence of RAS within the central nervous system is also known to operate largely independently of peripheral function.24 Interestingly, RAS in the brain is believed to be involved in functions critical to cognition, including neuronal differentiation, nerve regeneration, and learning and memory.25 Thus, RAS drugs that cross the blood-brain barrier (BBB) may influence cognition through both luminal and abluminal neurovascular effects, including neuronal effects. Consistent with this hypothesis, a small number of studies have found that certain ARBs and ACE-inhibitors with BBB-crossing capability might be linked to lower risk of cognitive decline in older hypertensive adults, when compared to the use of their non-BBB-penetrant counterparts. For example, the Cardiovascular Health Study of 5888 community-dwelling older adults found that the use of BBB-penetrating ACE-inhibitors was associated with 65% less cognitive decline per year of exposure, compared to the use of other antihypertensive drugs.26 Conversely, use of non-BBB-penetrant ACE-inhibitors was associated with a greater risk of incident dementia (adjusted hazard ratio, 1.20; 95% CI, 1.00–1.43 per year of exposure), compared to the use of other antihypertensive drugs.26 Our own research, using data from the prospective Alzheimer’s Disease Neuroimaging Initiative (ADNI) study, found that older adults using BBB-crossing RAS medications had better memory performance over a 3-year-followup, as well as less white matter hyperintensities, compared to users of all other antihypertensive medications.27
Despite these encouraging findings from three studies, larger meta-analytic studies have been hampered by the fact that pharmacokinetic properties are typically not considered in existing studies or routine clinical practice. The present study sought to fill this gap by conducting a large and longitudinal meta-analytic study of existing data recoded to assess the effects of BBB-crossing potential in RAS treatments among hypertensive adults, including data from randomized clinical trials, prospective cohort studies, and retrospective observational studies. We included the latter two types of studies to increase the overall sample size of this observational study. By comparing RAS treatments with versus without BBB-crossing ability, we leveraged existing data to construct a “randomization in nature” design whereby participants were compared based on a pharmacokinetic factor not considered in the selection of treatment. We hypothesized that BBB-crossing potential would be associated with less cognitive dysfunction and attenuated cognitive decline, particularly within the memory domain.
Methods
The authors declare that all supporting data are available within the article, and its online supplementary files.
Search Strategy and Selection Criteria
Guidelines from the Preferred Reporting Systems for Systematic Reviews and Meta-Analyses (PRISMA) were utilized for this study28 and systematic review criteria were documented with the International Prospective Register of Systematic Reviews (PROSPERO) system (registration number: CRD42018086511). The literature search was conducted on January 31, 2018, with no restrictions placed on publication dates.
The initial search stage involved searches of the ALOIS database with the search terms “(hyperten* or blood pressure)” and “Alzheim* or dement* or cognit*”. The ALOIS database contains records of various major healthcare databases, including Medline, Embase, PsycInfo, CINAHL, Literatura Latino Americana em Ciências da Saúde (LILACS), and ongoing trial databases. The databases Web of Science, ProQuest Dissertations and Theses Global, and ProQuest Central were also searched with the same search terms. Original research articles, conference proceedings, and theses were included. Bibliographies of original and review articles were screened for additional references.
Study Selection
We manually reviewed record titles and abstracts using broad inclusion and exclusion criteria. If an article passed a first-level screening, a second-level screening involved a full-text review. If studies contained insufficient information for the calculation of effect sizes, personal communications with authors were made in attempts to obtain data required for this computation. Communications also included requests for further relevant studies, which were screened as above.
Titles and abstracts were screened and assessed according to the inclusion criteria that a study: (1) involves human participants, (2) involves adults aged ≥ 50 with hypertension, (3) assesses the effects of antihypertensive drugs affecting the renin-angiotensin-system: ARBs or ACE-inhibitors, (4) assesses at least one neuropsychological outcome, (5) provides sufficient information in the publication or through contact with the authors to allow for calculation of effect sizes.
Exclusion criteria included (1) studies focused on another condition (e.g. diabetes mellitus), (2) studies in populations with particular diagnoses in which antihypertensive medications were primarily used for other effects other than lowering blood pressure (e.g. studies of vasoactive medications in participants with systolic heart failure for their cardiac remodeling effects), (3) non-pharmacologic interventions for blood pressure control, (4) studies with less than 6-month follow-up, (5) studies for which all medications used were from only one category (all were BBB-crossing or non-BBB-crossing), given that no comparison between the categories could be made. Editorials, correspondence, commentaries, and case reports/series were excluded.
Three types of studies were included: (1) randomized, double-blind trials, in which pharmacological interventions to lower blood pressure were administered for over six months, (2) prospective cohort studies, and (3) retrospective observational studies. Six months was chosen as the cutoff duration for treatment time in RCTs in accordance with other reviews which determined this to be the minimum amount of treatment time required for benefits to be achieved.21 This duration was not applied to exclude prospective cohort studies or retrospective observational studies due to the infrequent recording of treatment duration by studies, as well as inaccurate reporting by participants (e.g. reporting using an ARB in the 1980s, prior to ARBs becoming commercially available in the 1990s).
Participant samples from each study were derived following the inclusion criteria: (a) adults aged ≥50 with hypertension, (b) taking an antihypertensive drug affecting the renin-angiotensin-system: ARBs or ACE-inhibitors, (c) assessed for at least one neuropsychological outcome. We excluded participants with dementia as assessed according to established guidelines at the time of publication, with the exception of the Reasons for Geographic and Racial Differences in Stroke (REGARDS)29 study, which did not assess dementia as a primary endpoint. Details of dementia assessment by study are available in Supplemental Table S1 (please see http://hyper.ahajournals.org). Participants with other comorbidities were not excluded.
Given that multiple publications from individual research groups reported findings from the same participant samples, authors from the most recent publication using the participant sample group were contacted, and the most recent data obtained through these authors were used for analyses. All participating studies had at least one published paper; no authors of unpublished sources (e.g. theses/conference abstracts without corresponding peer-reviewed articles by the same authors) responded to requests for raw medication and neuropsychological data.
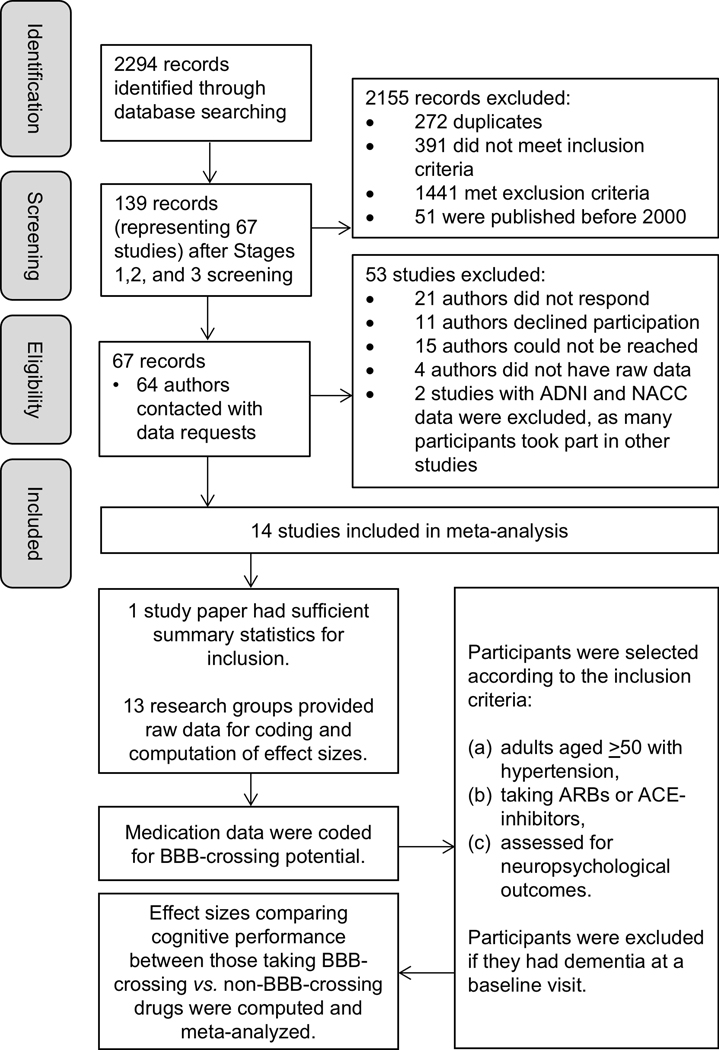
The selection of studies and participants included in the meta-analysis are shown in Figure 1. After full inclusion and exclusion criteria were applied, 67 studies remained. Of these, only three studies had published summary statistics that could be used for the computation of effect sizes for groups of participants taking BBB-crossing drugs and non-BBB-crossing drugs. Thus, all other primary authors and/or study consortiums were contacted, and raw medication and neuropsychological data were requested. Thirteen studies from six countries (Australia, Canada, Germany, Ireland, Japan, and the USA) agreed to participate in our meta-analysis and supplied full raw data. Two research groups provided medication data coded for BBB-crossing potential following set protocol, and the remaining 11 medication datasets were coded by a team of five research assistants. Each dataset was coded twice, and discrepancies were resolved by the first author (JKH). Details of each study are available in Table 1 and Supplemental Table S2 (please see http://hyper.ahajournals.org).
Figure 1:
Flowchart of the selection of studies and participants included in the meta-analysis. ACE = Angiotensin-converting-enzyme, ADNI = Alzheimer’s Disease Neuroimaging Initiative, ARBs = Angiotensin receptor blockers, BBB = blood-brain barrier, NACC = National Alzheimer’s Coordinating Center.
Table 1.
Fourteen studies included in meta-analyses.
| Study abbreviation | Full study name | Citation | Study location | |
|---|---|---|---|---|
| 1 | ACT | Adult Changes in Thought | Kukull et al (2002)48 | USA (WA) |
| 2 | CHCS | Cardiovascular Health Cognition Substudy | Sink et al (2009)26 | USA (NC, MD, CA, PA) |
| 3 | CHAP | Chicago Health and Aging Project | Morris et al (2000)49 | USA (IL) |
| 4 | IIDP | Indianapolis-Ibadan Dementia Project | Liu et al (2013)88 | USA (IN)a |
| 5 | MBS | Maintenance of Balance, Independent Living, Intellect, and Zest in the Elderly Study (MOBILIZE) Boston Study | Leveille (2008)89 | USA (MA) |
| 6 | SKILL | Staying Keen in Later Life | Hudak et al (2013)90 | USA (KY, AL) |
| 7 | REGARDS | Reasons for Geographic and Racial Differences in Stroke | Gillett et al (2015)29 | USA (all continental states) |
| 8 | CAMUI | Combination of Antihypertensive Therapy in the Elderly, Multicenter Investigation | Sato et al (2013)91 | Japan |
| 9 | CARLA | Cardiovascular Disease, Living and Ageing in Halle (CARLA) | Lacruz et al (2016)92 | Germany |
| 10 | CSHA | Canadian Study of Health and Aging | Lindsay et al (2002)46 | Canada |
| 11 | INSPIRED | Investigating Services Provided in the Residential Environment for Dementia | Liu et al (2017)52 | Australia |
| 12 | MSHA | Manitoba Study of Health and Aging | Tyas et al (2001)47 | Canada |
| 13 | TILDA | The Irish Longitudinal Study on Aging | Kenny et al (2011)50 | Ireland |
| 14 | TREND | Tübinger Evaluation of Risk Factors for Early Detection of Neurodegeneration | Heinzel et al (2014)51 | Germany |
The original study had data from Ibadan, Nigeria; however, none of the Nigerian participants took antihypertensive medications. Only participants from Indiana were included in analyses.
Determination of BBB-crossing potential
Determination of BBB-crossing potential was made following previous literature. With regard to ACE-inhibitors, we followed the categories by Sink and colleagues26 who assessed studies of tissue-specific ACE-activity after administration of ACE-inhibitors, as well as tissue-specific imaging of radio-labeled ACE-inhibitors. Captopril, fosinopril, lisinopril, perindopril, ramipril, and trandolapril were classified as BBB-crossing ACE-inhibitors, and benazepril, enalapril, moexipril, and quinapril were classified as non-BBB-crossing ACE-inhibitors. With regard to ARBs, after similar review of the literature, we classified telmisartan30 and candesartan31 as BBB-crossing, and olmestartan, eprosartan, irbesartan, and losartan as non-BBB-crossing. There had to be at least 2 positive autoradiographic studies and not more than 1 negative autoradiographic study for an ARB to be classified as BBB-crossing. Details of our literature review and classification decisions are available in Supplemental Table S3 (please see http://hyper.ahajournals.org).
Data Extraction
Data were extracted from published reports as well as through personal communications with authors. From each study, the following were extracted: (1) study design, (2) objectives, (3) setting, (4) demographic variables (sex, age), (5) comorbidities (burden of comorbidity, number of medications at baseline), (6) baseline cognitive function, (7) subject eligibility and exclusion criteria, (8) number of subjects per group, (9) years of enrollment, (10) duration of follow-up, (11) the study and comparator interventions (i.e., antihypertensive medications used), (12) relevant co-interventions, (13) change in cognitive function, and (14) adverse events (changes in quality of life, all-cause mortality).
The summary statistics required for each study and each outcome for continuous data were (1) means and standard deviations of cognitive measures at baseline and at follow-up, if available, (2) the standard error of the mean change, if follow-up was available, and (3) the number of patients per group at each assessment. A weighted estimate of the effect across studies was computed.
Assessment of quality and publication bias
Given that our meta-analysis only included one randomized trial, the methodological quality of included studies was assessed using an adapted version of the Newcastle-Ottawa Scale (NOS), which was developed to evaluate the quality of non-randomized studies in meta-analyses.32 We selected for studies with at least 6 month follow-up; therefore, we did not assess the adequacy of follow-up duration. Thus, the maximum score that a longitudinal study could receive on the NOS was 8, as opposed to the usual 9. For our purposes, “outcome” was operationalized as cognitive performance, and “exposure” was operationalized as treatment with antihypertensive medication. Studies were assessed for selection (4 points for representativeness of participants, ascertainment of exposure and non-exposure, and demonstration that dementia was absent at the start of the study), comparability (2 points for whether the study controlled for one or more important covariates) and outcome (2 points for whether the study conducted independent, blind assessment or used record linkage, and whether there was a description of subjects lost to follow-up). The maximum score that a cross-sectional study could receive on the NOS was 7, after excluding the criterion on subjects lost to follow-up.
The presence of publication bias was assessed by visual inspection of the Begg’s funnel plot and Egger’s regression test. Two studies were excluded from this analysis as they did not have baseline effect sizes; one study did not have baseline cognitive data available in the published report,26 and the other study utilized a six-item cognitive screener, which provided a small range of scores which would not have been meaningful to interpret.29
Statistical Analysis
Outcomes included: (1) neuropsychological performance on measures of mental status, memory, language, executive function, attention, and processing speed, and (2) cognitive change from baseline in all these domains. If multiple tests were used to assess a cognitive domain for any given study, the multiple effect sizes were not averaged. Rather, the most sensitive test for each cognitive domain was selected based on previous literature,33,34 and studies contributed one effect size per cognitive domain. We chose measures that were most harmonious with each other (e.g. Trails Making Test A and Color Trails A, both visual tests of attention) as opposed to measures which tested the same cognitive domain but through different modalities (e.g. Digit Span Forward for Attention, which necessitates auditory/verbal processing) and with less sensitivity for impairment. Tests used for the assessment of each domain are displayed in Table 2.
Table 2.
Neuropsychological measures used for the assessment of various cognitive domains.
| Domain | Test used | k* |
|---|---|---|
| Attention | Trail Making Test A | 3 |
| Color Trails 1 | 1 | |
| Executive Function | Trail Making Test B | 3 |
| Color Trails 2 | 1 | |
| Language | Animal Fluency | 3 |
| Category Fluency | 1 | |
| Memory (Learning) | CERAD Word List Learning | 3 |
| HVLT Immediate Recall | 2 | |
| Word List Learning | 1 | |
| Memory (Recall) | CERAD Word List Recall | 3 |
| HVLT Delayed Recall | 2 | |
| Word List Learning | 1 | |
| Mental Status | Cognitive Abilities Screening Instrument (CASI) | 1 |
| Mini-Mental State Examination (MMSE) | 7 | |
| Modified MMSE | 2 | |
| Cambridge Mental Disorders of the Elderly Examination (CAMDEX) | 1 | |
| Psychogeriatric Assessment-Cognitive Impairment (PAS-Cog) | 1 | |
| Processing Speed | Modified Symbol-Digit Modality Test | 1 |
| Digit Symbol Test | 1 |
k = number of included studies at baseline
This meta-analysis necessitated the combination of data from studies which used similar, but not identical, rating scales for outcome assessment. Effect sizes (Hedge’s g) were calculated using mean differences, analyses of covariance (ANCOVA), or t tests comparing two treated groups, (i) participants taking antihypertensive medications which cross the BBB (the “Crossing group”) and (ii) participants taking antihypertensive medications which do not (the “Non-crossing group”). When ANCOVA was utilized, models were adjusted for age, sex, and educational attainment. Given that pooled studies used different rating scales, the measure of group difference for all outcome measures was the standardized mean difference (SMD) i.e., the absolute mean difference divided by the pooled standard deviation. SMDs can be intuitively interpreted as the groups differing by, for example, one-tenth (g = 0.10) or one (g = 1) standard deviation.
For consistency, effect sizes were calculated by subtracting the scores of the Non-crossing group from the Crossing group. On most tests, a higher score indicates better performance; thus, a positive effect size indicates that the Crossing group performed better on a test than the Non-crossing group, while a negative effect size indicates that the Crossing group performed worse on a test than the Non-crossing group. On timed tests in which greater time taken reflected poorer performance (e.g. Trail Making Test), scores were inverted (i.e. multiplied by −1) to allow for consistent analysis and interpretation of results.
For longitudinal analyses, cognitive change was measured in change scores, computed by subtracting the baseline score from the score at follow-up assessment. SMDs were computed as the absolute mean difference in change scores divided by the pooled standard deviation of change scores. In calculation of change scores, another covariate was added to every model: each individual’s baseline score on the measure being studied, with the exception of the Cardiovascular Health Cognition Substudy (CHCS),26 for which these data were unavailable. A positive effect size indicates that the Crossing group performed better than the Non-crossing group on a test over follow-up, while a negative effect size indicated the reverse. The most amount of data available was at 3 years of follow-up; thus, this cutoff was used for longitudinal analyses. Prior to analyses, participants who had suspected dementia at baseline, as determined using individual study criteria, were dropped. Participants were not dropped for having dementia at follow-up.
Heterogeneity between studies was examined using the I2 and Q statistics, and p < 0.10 was considered to indicate significant heterogeneity.35,36 An I2-statistic above 40% was considered as representing heterogeneity that may be substantial enough to impact the meta-analysis, following Cochrane guidelines37. Maximum likelihood random-effects meta-regression analyses (i.e., moderator analyses) were considered for each cognitive domain to explore possible sources of heterogeneity based on mean age, mean educational attainment, and percentage of male participants.38–40 Meta-regression was only considered when there were ten or more studies contributing to a meta-analysis,37 and only one cognitive domain (mental status) met this cutoff. Given that the amount of heterogeneity in this meta-analysis was inconsequential (0.0%), meta-regression was not conducted.
All results are reported using a random-effects model. The Knapp-Hartung-Sidik-Jonkman (HKSJ) adjustment was used for confidence intervals and the Sidik-Jonkman estimator was used for the tau-squared estimator to weight effect sizes in our meta-analyses, as shown in the forest plots. Analyses were conducted using R version 3.6.2 software41 and the meta,42 dmetar,43 and metafor44 packages as well as SPSS for Mac OS X version 21.0 (SPSS, Armonk, NY: IBM Corp). The Meta-Essentials tool45 was used to conduct Egger’s regression test and produce Begg’s funnel plot.
Data from two studies – the Canadian Study of Health and Aging (CSHA46) and Manitoba Study of Health and Aging (MSHA47) were pooled and analyzed as one. This was due to the small sample sizes that used the medications of interest (MSHA n=15, CSHA n=11), which was likely due to the fact that these drugs were newer and less prescribed in the early 1990s, during data collection. Further, both studies used the same cognitive assessment, and the MSHA was a parallel study to the CSHA, with one-quarter of MSHA participating in the CSHA. Duplicate participants were dropped before analyses.
Results
Clinical/demographic data
After application of full inclusion and exclusion criteria, we included 10 prospective studies, 3 retrospective studies, and 1 randomized trial in our meta-analysis. Demographic characteristics for 12,849 participants with baseline demographic data are shown in Table 3. Differences between the Crossing and Non-crossing groups are shown in Supplemental Table S4 (please see http://hyper.ahajournals.org). Three studies [Adult Changes in Thought (ACT)48, Chicago Health and Aging Project (CHAP)49 and REGARDS29] had higher percentages of male participants than female participants who were taking BBB-crossing drugs vs. non-BBB-crossing drugs (all p’s < 0.02). The Non-crossing group in The Irish Longitudinal Study on Aging (TILDA)50 had higher levels of education than the Crossing group (p = 0.02).
Table 3.
Characteristics of the cohorts at baseline, after exclusion of dementia cases.
| Study | Age, y | Sex, no. (%) | Education, y | Race / ethnicity (%) | APOEe4, no. (%) | ||||
|---|---|---|---|---|---|---|---|---|---|
| Range | Mean (SD) | Female | Male | Range | Mean (SD) | E4 carrier | Missing data | ||
| ACT | 60–90 | 73.6 (6.0) | 861 (55%) | 689 (45%) | 10–20 | 14.8 (2.9) | 88% White, 4% Asian, 3% Black, 5% Others | 343 (22%) | 196 (13%) |
| CAMUI | 63–89 | 74.4 (5.9) | 41 (51%) | 39 (49%) | -- | -- | Japanese | NA | NA |
| CARLA | 60–87 | 73.3 (7.2) | 129 (39%) | 204 (61%) | 9–20 | 14.7 (2.5) | NA | NA | NA |
| CHAP | 64–90+ | 72.9 (5.8) | 458 (64%) | 258 (36%) | 7–20 | 13.2 (2.6) | 53% Black, 47% White | 232 (32%) | 17 (2%) |
| CSHA and MSHA | 65–90 | 79.1 (5.6) | 18 (69%) | 8 (31%) | 3–18 | 10.1 (3.3) | NA | 4 (15%) | 10 (39%) |
| IIDP | 65–89 | 72.4 (6.3) | 135 (68%) | 65 (32%) | 3–16 | 10.1 (2.8) | NA | NA | NA |
| INSPIRED | 66–90+ | 86.1 (7.6) | 82 (79%) | 22 (21%) | 10–16 | 11.9 (2.2) | NA | NA | NA |
| MBS | 69–90+ | 78.4 (5.2) | 110 (55%) | 90 (45%) | 4–18 | 14.6 (2.7) | 82% White, 13% Black, 7% Others | NA | NA |
| SKILL | 62–90+ | 73.7 (6.0) | 99 (55%) | 80 (45%) | 8–20 | 14.0 (2.4) | 87% White, 12% Black, 1% Others | NA | NA |
| TILDA | 50–80+ | 66.5 (8.7) | 472 (47%) | 540 (53%) | 5–18 | 10.6 (4.2) | NA | NA | NA |
| TREND | 50–84 | 66.7 (6.6) | 192 (46%) | 228 (54%) | 9–20 | 14.1 (2.7) | NA | NA | NA |
| REGARDS | 50–90+ | 66.8 (8.5) | 4317 (54%) | 3712 (46%) | 12–16 | 14.1 (1.7) | 55% White, 45% Black | NA | NA |
One study, the Cardiovascular Health Cognition Substudy, did not have baseline demographic data on the sample taking relevant medications. NA = Not available.
Baseline vascular risk factors are displayed in Table 4. The Non-crossing group had a higher proportion of individuals with diabetes than the Crossing group in the ACT study48. In the REGARDS and TILDA studies, the Crossing groups had lower average body mass index and higher proportions of individuals with prior stroke than the Non-crossing groups.
Table 4.
Baseline vascular risk factors.
| Study | Taking BBB-crossing drugs | Taking non-BBB-crossing drugs | F or χ 2 value | p | Method of assessment | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Diabetes | Stroke | TIA | BMI | Diabetes | Stroke | TIA | BMI | ||||
| ACT | 16.4% | 3.7% | 8.4% | 27.1 (3.6) | 22.4% | 3.3% | 8.6% | 27.6 (3.5) | D: 5.42 S: 0.08 T: 0.01 B: 3.66 |
D: 0.03 S: 1.00 T: 0.90 B: 0.06 |
Medical record review |
| CAMUI | 19.4% | -- | -- | -- | 25.0% | -- | -- | -- | D: 0.35 | D: 0.60 | Medical record review |
| CHAP | 9.7% | 7.0% | -- | 28.6 (6.2) | 7.9% | 8.9% | -- | 28.5 (5.7) | D: 0.69 S: 0.89 B: 0.17 |
D: 0.43 S: 0.35 B: 0.68 |
Medical record review; participant self-report; clinical examination |
| SKILL | 24.8% | 20.8% | -- | -- | 30.8% | 14.1% | -- | -- | D: 0.80 S: 1.34 |
D: 0.40 S: 0.33 |
Participant self-report |
| REGARDS | 39.1% | 9.7% | 6.0% | 30.4 (6.2) | 38.0% | 8.4% | 5.5% | 31.1 (6.5) | D: 1.08 S: 4.17 T: 1.17 B: 9.58 |
D: 0.30 S: 0.04 T: 0.29 B: 0.01 |
Participant self-report and in-home physical examination |
| MBS | 28.7% | -- | -- | 27.7 (5.3) | 32.6% | -- | -- | 28.5 (5.1) | D: 0.26 B: 0.01 |
D: 0.71 B: 0.97 |
Participant self-report; home or clinical assessment |
| TILDA | 19.8% | 5.0% | 4.7% | 30.2 (4.9) | 16.7% | 1.1% | 2.9% | 30.5 (4.2) | D: 0.92 S: 5.17 T: 1.10 B: 5.83 |
D: 0.40 S: 0.02 T: 0.41 B: 0.02 |
Participant self-report; home or clinical assessment |
D = diabetes, S = stroke, T = TIA, B = Body Mass Index (BMI). BMI was computed as the body mass divided by the square of the body height in kg/m2. BMI results are presented as Mean (Standard Deviation). Significant differences between groups are indicated in boldface type.
Quality assessment and publication bias
Study quality was high across our participating cohorts. As shown in Supplemental Table S2 (please see http://hyper.ahajournals.org), four of eleven longitudinal studies scored 8/8 points on the NOS scale. Five studies scored 7/8, with all losing 1 point for having no mention of blinded assessment, with the exception of CSHA, which lost 1 point for using written self-report medication data, as opposed to ascertainment through records or structured interview. Two studies scored 6/8, losing points for having no mention of blinded assessment as well as using self-report data (MSHA) and being non-representative of the general older adult community [Tübinger Evaluation of Risk Factors for Early Detection of Neurodegeneration (TREND)51]. The TREND study used an enriched sample of individuals who were partially selected for having prodromal risk factors for Alzheimer’s disease and Parkinson’s disease. Two of three cross-sectional studies scored 7/7 points. The remaining study, the Investigating Services Provided in the Residential Environment for Dementia (INSPIRED) study52, was docked one point for being non-representative of the general older adult community, as its participants comprised nursing home residents as opposed to community-dwelling older adults.
Supplemental Figure S1 (please see http://hyper.ahajournals.org) shows Begg’s funnel plot for examination of publication bias for studies with baseline cognitive assessments. The symmetrical distribution of studies on either side of the overall effect line indicates that publication bias was unlikely. The Egger test was not significant for publication bias (p = 0.65).
Heterogeneity
Heterogeneity between studies for each cognitive domain was examined using the I2 and Q statistics. Q statistics are presented in Supplemental Table S5 (please see http://hyper.ahajournals.org), and I2 and associated p-values are presented in individual figures. Results indicated no significant heterogeneity between studies that necessitated meta-regression analyses.
Cross-sectional Results
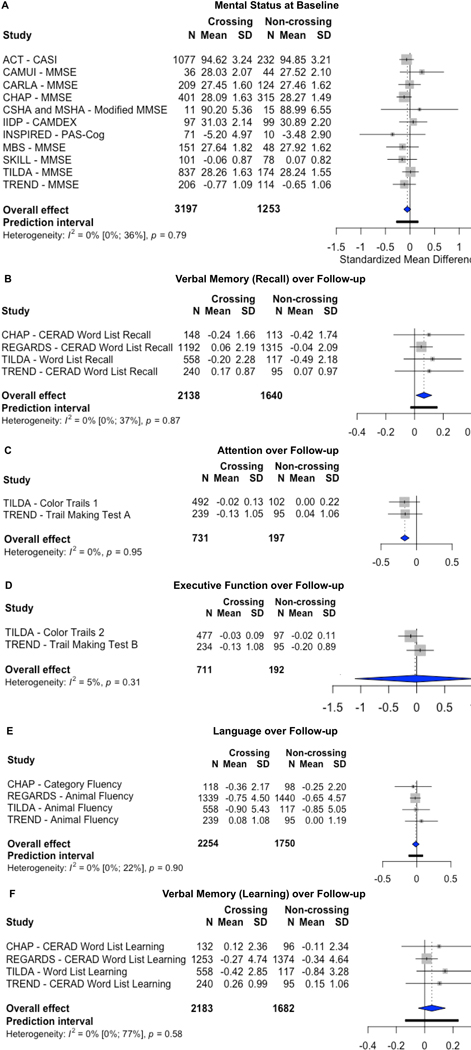
Effect sizes were calculated for mental status (see Figure 2) at baseline assessment as well as the domains of attention, executive function, language, verbal memory (learning), verbal memory (recall), and processing speed (Supplemental Figure S2, please see http://hyper.ahajournals.org). Overall, the effect sizes ranged from a minimum of −0.16 for processing speed, to a maximum of 0.04 for attention. Results indicated no significant difference between the group using non-BBB-crossing drugs and the group using BBB-crossing drugs across any cognitive domains at baseline: attention (g = 0.04, 95% CI [−0.10, 0.18], p = 0.40), executive function (g = 0.04, 95% CI [−0.11, 0.19], p = 0.45), language (g = 0.02, 95% CI [−0.01, 0.06], p = 0.12), mental status (g = −0.05, 95% CI [−0.13, 0.02], p = 0.13), verbal memory (learning), (g = 0.0007, 95% CI [−0.08, 0.08], p = 0.98), verbal memory (recall), (g = −0.04, 95% CI [−0.23, 0.15], p = 0.60), and processing speed (g = −0.16, 95% CI [−2.05, 1.74], p = 0.48).
Figure 2:
Forest plots. (A) There were no significant differences among individuals who took BBB-crossing drugs vs. non-BBB-crossing drugs on measures of mental status at baseline. (B) Individuals who took BBB-crossing drugs outperformed those who took non-BBB-crossing drugs on measures of verbal memory (recall) over time. (C) Individuals who took non-BBB-crossing drugs outperformed those who took BBB-crossing drugs on measures of attention over time. There were no significant differences among individuals who took BBB-crossing drugs vs. non-BBB-crossing drugs on measures of (D) executive function, (E) language, or (F) verbal memory (learning) over 3 years of follow-up.
Longitudinal Results
Effect sizes were calculated for change in scores over time on mental status measures as well as the domains of executive function, language, verbal memory (learning), verbal memory (recall), and attention (see Figure 2). The maximum effect size was 0.07 (95% CI [0.01, 0.12], p = 0.03) for verbal memory (recall), indicating that participants using BBB-crossing drugs exhibited better memory recall ability over 3-year follow-up, relative to those using non-BBB-crossing drugs. The minimum effect size was −0.17 (95% CI [−0.23, −0.10], p = 0.02) for attention, indicating that older adults using non-BBB-crossing drugs displayed better performance on attention measures over time, relative to those using BBB-crossing drugs.
BBB-crossing-potential did not significantly relate to performance over time on mental status (g = 0.02, 95% CI [−0.25, 0.29], p = 0.88, Supplemental Figure S3, please see http://hyper.ahajournals.org) or other cognitive domains: executive function (g = −0.03, 95% CI [−1.10, 1.04], p = 0.80), language (g = −0.01, 95% CI [−0.06, 0.04], p = 0.41), or verbal memory (learning), (g = 0.05, 95% CI [−0.05, 0.15], p = 0.19).
Discussion
The present meta-analysis is the first to evaluate cognitive sequelae of antihypertensive medication BBB-crossing potential in studies of older adults conducted over the past 18 years, with analyses in 13 of 14 studies being previously unpublished. As hypothesized, findings indicate older adults taking BBB-crossing RAS drugs exhibit less decline in memory ability over follow-up, relative to those taking non-BBB-crossing medications. This large meta-analytic result is consistent with our prior observations in a much smaller sample of American and Canadian participants,27 in which older adults taking BBB-crossing drugs showed less memory decline over 3-year follow-up compared to those taking non-BBB-crossing drugs. Our overall effect size was small (g = 0.06); however, this is expected given the cognitively intact nature of our participants. Indeed, it is remarkable that we were able to observe these subtle differences in memory decline in a meta-analytic sample free of dementia and suspected cognitive impairment based on screening exams, and in comparison to individuals taking drugs that differed in BBB-crossing potential but were otherwise quite similar (ARBs and ACEIs).
BBB-crossing potential is not a factor that is considered in prescribing practice. Thus, although there was no formal randomization process, the type of drug that a participant used (BBB-crossing or non-BBB-crossing) was determined randomly, without outside intervention, in our prospective and retrospective studies. The success of this “randomization in nature” design is supported by the fact that we observed no differences in any cognitive domains at baseline between those taking BBB-crossing versus non-crossing RAS drugs, although there were study-specific demographic and clinical differences as discussed below.
The apparent advantage in memory among participants taking BBB-crossing drugs versus non-crossing drugs was observed on longitudinal analysis of memory decline among cognitively intact older adults, suggesting that any potential memory benefit could have implications for future risk of memory impairment. Thus, our findings have clear implications for efforts to identify older adults at risk for future memory decline and to preserve memory ability in those who are still cognitively healthy. Alzheimer’s disease is the most common cause of dementia and is characterized by early decline in memory recall.53 Memory deficits are also observed in vascular cognitive impairment,54 the second most common cause of dementia. In longitudinal studies, hypertension has been linked to Alzheimer’s and vascular pathologies, including amyloid-beta (Aβ) plaques (in the neocortex and hippocampus)55 and neurofibrillary tangles,8,55–57 in addition to smaller brain volumes and increased white matter hyperintensity (WMH) volume.27,58
Whether the use of BBB-crossing RAS drugs is linked to attenuation of Alzheimer’s and/or cerebrovascular pathologies awaits future biomarker and neuropathological studies. There is a body of evidence that various components of brain RAS are altered in AD; for a review, please see Kehoe (2018).59 In addition to postmortem studies which have found increased ACE levels in AD brains compared to control brains60,61, ACE has been found to be associated with earlier age of AD onset62 and more adverse levels of CSF amyloid.63 Additionally, angiotensin-converting-enzyme-2 (ACE2), a crucial RAS component which counter-regulates the hypertensive actions of the classic pathway, is reduced in AD compared with controls, and associated with increasing Aβ and tau pathology.64 Recent exciting work showed that enhancement of ACE2 activity lowered hippocampal Aβ and restored cognition in mouse models of AD, as well as prevented cognitive decline in young mice who received the enhancement chronically.65 Further, in an experimental study of mice with an ACE coding variant found in AD (R1279Q), hippocampal neurodegeneration was completely rescued with brain-penetrant drugs. The authors stressed that brain-penetrant ARBs and ACE-inhibitors may be protective against AD or neurodegeneration more generally, as their effects of reducing neuroinflammation and reactive oxygen species (ROS) are protective against dopaminergic neuron death.66
It is noteworthy that we were able to replicate our previously reported findings of benefit in memory recall, especially given that our sample contained a substantial proportion of individuals with increased vascular risk. We noted elevated vascular risk factors in the REGARDS study,29 which had proportionally more participants with diabetes as well as higher average BMI relative to other studies in our meta-analysis. This study contributed the highest-weighted effect size to the finding of better memory ability in older adults taking BBB-crossing drugs relative to those taking non-BBB-crossing drugs. Furthermore, the BBB-crossing group within this study also had a significantly higher proportion of participants with stroke compared to the non-BBB-crossing group. These findings could suggest a greater link between the use of BBB-crossing RAS drugs and memory decline in older adults with greater vascular risk burden. Future studies evaluating the role of vascular risk factor and cerebrovascular disease burden in the use of BBB-crossing RAS drugs are warranted.
Despite findings supporting our hypothesis regarding BBB-crossing RAS drugs and memory decline, our overall findings were mixed, as we unexpectedly found that older adults taking non-BBB-crossing drugs displayed better attention over follow-up, compared to the BBB-crossing group. Several factors may explain this unexpected finding. In contrast to memory recall ability, attentional processes may be impacted by spurious factors unrelated to neuropathological processes, including test engagement, stress and depression. Decline in attention ability has been linked to cerebrovascular disease impacting frontal-subcortical networks, as well as stroke in general.67,68 As noted above, studies contributing to the BBB-crossing group exhibited greater vascular risk burden and stroke50 than the non-crossing group, potentially accounting for greater attention deficiencies in this group. It is important to note that several BBB-crossing medications (i.e., perindopril69, ramipril70, candesartan71) have been found to benefit stroke patients or those with high vascular disease, in terms of reducing risk of stroke (compared to placebo)69,70 as well as major vascular events (e.g. cerebral hemorrhage, myocardial infarction, death related to cardiovascular causes, and non-fatal stroke). First indications for these drugs may have been for stroke prevention or improving stroke outcomes, although we could not assess for these indications across all studies. Thus, it remains unclear whether the BBB-crossing group may have been pre-disposed to stroke compared to the non-crossing group. We also noted a slightly lower average level of educational attainment in the BBB-crossing group. These differences make it all the more remarkable that the BBB-crossing group displayed better memory ability over time despite these cognitive disadvantages in terms of vascular risk, stroke, education and attention ability. Future studies should examine the role of vascular risk factor burden in modifying the relationship between cognitive function and the use of specific antihypertensive medications with BBB-crossing versus non-crossing properties.
Three studies in our meta-analysis had a significantly higher proportion of male vs. female participants who were prescribed a BBB-crossing drug. Prior work has shown that compared to other classes of antihypertensive medications, ARBs and ACE-inhibitors are slightly more protective against the onset of AD in men [OR = 0.931 (CI: 0.895–0.969)], but not women [OR = 0.985 (CI: 0.963–1.007)].13 Estrogen in women is hypothesized to be protective against both hypertension and AD, as it prevents the production and vasoconstrictive effects of Ang II, and may potentially facilitate amyloid degradation.72 While we controlled for sex in our meta-analyses, subgroup analyses of men vs. women in order to identify those who might benefit the most from specific medications (i.e. BBB-crossing vs. non-BBB-crossing ARBs and ACE-inhibitors) is another important area of future study.
Independent of systemic vascular benefits, the ARBs and ACE-inhibitors that cross the BBB may affect the brain and exert additional salutary effects that include increasing cerebral blood flow and protecting against inflammation and vascular oxidative stress.73 In animal models, a BBB-crossing ARB (candesartan) has been found to have anti-inflammatory effects,74 and a BBB-crossing ACE-inhibitor (zofenopril) has been demonstrated to dose-dependently decrease intracellular reactive oxygen species and superoxide formation.75 Given their neuroprotective effects of lowering inflammation and oxidative stress, BBB-penetrant ARBs and ACE-inhibitors may work to potentially reduce cerebrovascular dysfunction overall, particularly in the context of cerebrovascular disease.
Our finding further implicates memory consolidation mechanisms that are underpinned by medial temporal and diencephalic circuitry.76 As we have reviewed previously,77 animal studies examining the cognitive effects of the protective ACE2-Ang(1–7)-Mas axis within the RAS show that Mas activity may enhance memory by modulating function of the hippocampus, the structure with the highest Mas receptor density.78 BBB-penetrant drugs are hypothesized to promote this ACE2-Ang(1–7)-Mas axis, which has been found in human brains.78,79 In support of memory benefits, Ang-(1–7) improves the performance of mice and rats on spatial working memory tasks.80,81 Thus, one area of future study may be whether the use of BBB-penetrant drugs aids memory consolidation through promotion of the salutary effects of Mas and Ang-(1–7) on brain memory centers.
To our knowledge, there have only been three studies that have examined the associations between BBB-crossing potential of antihypertensive drugs and cognition. These studies indicated that use of the BBB-crossing drugs was linked to less cognitive decline26,82 and preserved memory27 over time, compared to use of the non-BBB-crossing drugs. With the exception of these three studies, none of the many empirical studies examining hypertension and cognition have considered the pharmacokinetic properties of these drugs. This is because BBB-crossing potential is not a consideration made in prescribing practices. Future studies evaluating antihypertensive medications in the context of cognitive decline should consider pharmacokinetic drug properties in addition to drug class and mechanism of action.
Additional strengths of our meta-analysis relative to prior studies include exhaustive search of the literature leading to very large sample size (N=12,849 at baseline), examination of sensitive neuropsychological tests across multiple domains rather than brief screening measures of global cognition, use of raw cognitive data (as opposed to published summary statistics), and examination of the same covariates (age, sex, and educational attainment), and longitudinal analyses that control for individual variation based on each individual’s baseline score. These strengths produced adjusted estimates of effects together with standard errors, which give the least biased and most precise estimates of the effect sizes. We also took a systematic and empirical approach to classification decisions regarding which ARBs were BBB-crossing or non-BBB-crossing. Previous work has been less clear on the rationale used in the classification of BBB-crossing potential.
Limitations of our study include the possibility of publication bias, which is inherent in any meta-analysis, but which we attempted to overcome by sourcing for unpublished studies (e.g. dissertations, conference abstracts). We also faced the inevitable inclusion of underpowered studies, which is not uncommon in meta-analyses: 66% of Cochrane meta-analyses themselves are underpowered.83 Nevertheless, we highlight that the majority of the data in our analyses were unpublished findings, as we were examining novel associations within established datasets. Additionally, we do not have data on the dose, potency, or distribution of these drugs throughout the body (e.g. whether they are homogenously distributed or concentrated in certain tissues). We also caution that while ACE-inhibitors are described as a drug “class”, this classification is due to their biological function (i.e., ACE inhibition) rather than chemical structure, which is the more frequent practice. Future work may consider sub-analyses by structure, as done by Solfrizzi et al (2013)84, who provide a review of this issue.
We were also unable to control for differences in race/ethnicity and vascular risk among our participants, as these data were inconsistently recorded, and because vascular contributions to dementia were not always a focus of the original studies. Additionally, we could not assess whether a participant’s antihypertensive medication regimen changed between baseline and follow-up, as this was infrequently re-assessed in the original studies. Further, in a number of our analyses, one study contributed more weight than all other studies combined. Nevertheless, with regard to the Attention analyses, the effect sizes of the two contributing studies were comparable (−0.17 and −0.16). With regard to the Memory (Recall) analyses, the effect sizes of the studies contributing the least weight (0.10, 0.10, 0.13) were twice that of the REGARDS study (0.05, which contributed 70% of weight). The fact that our overall effect and finding of better recall over time was heavily weighted by the smallest effect size among our studies is remarkable.
Perspectives
Hypertension occurs decades prior to the onset of AD symptoms85 and exerts its effects on hemodynamics and atherosclerotic mechanisms over many years. Hence, it may have the most impact on cognition early on in the disease course,86 and the degree and mechanism of blood pressure control may be long-term determinants of cognitive function in those with hypertension. Given that current pharmaceutical treatments for dementia have only had modest effects on symptom improvement, modifying risk factors such as hypertension represents a promising line of work toward dementia prevention.87 New drug development necessitates enormous investments of time and money, making the repurposing of existing medications a key research goal. Our meta-analysis found that in a large, international, and cognitively intact sample, the use of BBB-crossing ARBs and ACE-inhibitors was linked to better memory recall over 3 years of follow-up compared to their non-BBB-crossing counterparts. This finding has clear implications for individuals who remain symptom-free, but who may experience later cognitive benefit from simply changing their antihypertensive regimen.
Supplementary Material
Novelty and Significance
1. What is New?
Prior research has highlighted possible benefits of ARBs and ACE-inhibitors but few studies have examined their ability to cross the blood-brain-barrier.
2. What is Relevant?
Hypertension occurs decades prior to cognitive decline with age, chronically impacting brain health.
The type of antihypertensive medication and its ability to cross the blood-brain-barrier may influence later cognitive function.
3. Summary
BBB-crossing potential of ARBs and ACE-inhibitors is associated with better verbal memory over 3-years of follow-up.
Acknowledgements
Thank you to all the Principal Investigators, authors, statisticians and workgroups of the various studies who responded with kindness and generosity to the request for data for this meta-analytic project and who facilitated the data sharing efforts: Eric Larson, Paul Crane, Walter Kukull, Bill Lee, KatieRose Richmire (ACT); Nobuyuki Sato, Naoyuki Hasebe, Yasuaki Saijo (CAMUI); Lamiaa Hassan, Maria Elena Lacruz, Johannes Haerting, Karin Halina Greiser (CARLA); Denis Evans, Kumar Bharat Rajan, Martha Clare Morris, Todd Beck, Bo Yu (CHAP); Danielle Laurin, Pierre-Hugues Carmichael, Joan Lindsay (CSHA); Hugh Hendrie, Sujuan Gao, Katie Lane (Indianapolis-Ibadan Dementia Project); Enwu Liu (INSPIRED); Lewis Lipsitz (MBS); Phil St. John, Suzanne Tyas (MSHA); Jennifer Manly, Virginia Howard, Suzanne Judd, Mary Cushman, Margaret Stewart, Ya Yuan (REGARDS); Elizabeth Hudak, Jerri Edwards, Karlene K. Ball (SKILL); Frank Moriarty, Orna Donoghue (TILDA); Sebastian Heinzel, and Daniela Berg (TREND). Thank you to all the participants who took part in the 14 studies included in our meta-analysis: from Australia, the INSPIRED study; from Canada, the CSHA and MSHA studies; from Germany, the CARLA and TREND studies; from Ireland, the TILDA study; from Japan, the CAMUI study; and from the USA, the ACT, CHCS, CHAP, Indianapolis-Ibadan Dementia Project, MBS, SKILL, and REGARDS studies. Thank you to the VaSC Lab Research Assistants (Samantha Lask, Delilah Leibowitz, Hannah Pavlov, Aye Waddy, Katherine Weir), who coded thousands of rows of medication data. The Graphical Abstract was created with BioRender.com.
Researchers interested in using TILDA data may access the data for free from the following sites: Irish Social Science Data Archive (ISSDA) at University College Dublin http://www.ucd.ie/issda/data/tilda/; Interuniversity Consortium for Political and Social Research (ICPSR) at the University of Michigan http://www.icpsr.umich.edu/icpsrweb/ICPSR/studies/34315
Sources of Funding
Dr. Nation is supported by the National Institute on Aging (NIA), National Institutes of Health, Grants R01AG064228, R01AG060049, P50AG016573, and P01AG052350, as well as by the Alzheimer’s Association Grant No. AARG-17-532905. We gratefully acknowledge the funding bodies which supported the following projects: ACT: Grants U01 AG006781 (Dr. Larson), AG16976 (Dr. Kukull), and AG05136 from the National Institute on Aging, National Institutes of Health; CAMUI: The Waksman Foundation of Japan Inc.; CARLA: The CARLA study was funded by a grant from the German Research Foundation (DFG) as part of the Collaborative Research Center 598 ‘Heart Failure in the Elderly - Cellular Mechanisms and Therapy’ at the Medical Faculty of the Martin Luther University Halle-Wittenberg, by a grant from the Wilhelm-Roux Programme of the Martin Luther University Halle-Wittenberg, by the Ministry of Education and Cultural Affairs of Saxony-Anhalt, and by the Federal Employment Office; CHAP: Grants AG-11101 and AG-10161 from the National Institute on Aging; CSHA: The core study was funded by the Seniors’ Independence Research Program through the National Health Research and Development Program (NHRDP) of Health Canada (project no. 6606-3954-MC (S)). Additional funding was provided by Pfizer Canada Incorporated through the Medical Research Council/Pharmaceutical Manufacturers Association of Canada Health Activity Program, NHRDP (project no. 6603-1417-302 (R)), Bayer Incorporated, and the British Columbia Health Research Foundation (project no. 38 (93-2) and no. 34 (96-1)); Indianapolis-Ibadan Dementia Project: The project is supported by NIH grants R01 AG09956, P30 AG10133, R01 AG019181 and R24 MH080827; INSPIRED: This study was funded by the National Health and Medical Research Council (NHMRC) Partnership Centre on Dealing with Cognitive and Related Functional Decline in Older People (CDPC) (Grant No. GNT9100000); MBS: This research was supported by the National Institute on Aging: Research Nursing Home Program Project Grant P01AG004390; MSHA: This study was supported by a cooperative agreement [grant number U01 NS041588] from the National Institute of Neurological Disorders and Stroke (NINDS) and [grant number R01 HL080477] and [grant number T32 HL07594-24] from the National Heart, Lung, and Blood Institute (NHLBI). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NINDS, the NHLBI, or the National Institutes of Health; SKILL: The SKILL study was supported in part by the National Institutes of Health/National Institute on Aging grant 5 R37 AG05739-16, Improvement of Visual Processing in Older Adults, Karlene K. Ball, principal investigator. Jerri D. Edwards was supported as a Co-Investigator of this study; TILDA: TILDA is funded by the Department of Health with support from the Health Research Board; Science Foundation Ireland; The Atlantic Philanthropies; and Irish Life plc.; TREND: This study was supported by intramural funding as well as by the German Center for Neurodegenerative Diseases (DZNE); REGARDS: This research project is supported by cooperative agreement U01 NS041588 co-funded by the National Institute of Neurological Disorders and Stroke (NINDS) and the National Institute on Aging (NIA), National Institutes of Health, Department of Health and Human Service. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NINDS or the NIA. Representatives of the NINDS were involved in the review of the manuscript but were not directly involved in the collection, management, analysis or interpretation of the data. The authors thank the other investigators, the staff, and the participants of the REGARDS study for their valuable contributions. A full list of participating REGARDS investigators and institutions can be found at http://www.uab.edu/soph/regardsstudy/
Footnotes
Disclosures
None.
References
- 1.Duron E, Hanon O. Hypertension, cognitive decline and dementia. Arch Cardiovasc Dis. 2008;101(3):181–189. doi: 10.1016/S1875-2136(08)71801-1 [DOI] [PubMed] [Google Scholar]
- 2.de la Torre JC. Alzheimer Disease as a Vascular Disorder. Stroke. 2002;33(4):1152–1162. doi: 10.1161/01.STR.0000014421.15948.67 [DOI] [PubMed] [Google Scholar]
- 3.Zlokovic B V. Neurovascular pathways to neurodegeneration in Alzheimer’s disease and other disorders. Nat Rev Neurosci. 2011;12(12):723–738. doi: 10.1038/nrn3114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Saxby BK, Harrington F, McKeith IG, Wesnes K, Ford GA. Effects of Hypertension on Attention, Memory, and Executive Function in Older Adults. Heal Psychol. 2003;22(6):587–591. doi: 10.1037/0278-6133.22.6.587 [DOI] [PubMed] [Google Scholar]
- 5.Waldstein SR. The relation of hypertension to cognitive function. Curr Dir Psychol Sci. 2003;12(1):9–13. doi: 10.1111/1467-8721.01212 [DOI] [Google Scholar]
- 6.Nasrallah IM, Pajewski NM, Auchus AP, et al. Association of intensive vs standard blood pressure control with cerebral white matter lesions. JAMA - J Am Med Assoc. 2019;322(6):524–534. doi: 10.1001/jama.2019.10551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kettani FZ, Dragomir A, Côté R, et al. Impact of a better adherence to antihypertensive agents on cerebrovascular disease for primary prevention. Stroke. 2009;40(1):213–220. doi: 10.1161/STROKEAHA.108.522193 [DOI] [PubMed] [Google Scholar]
- 8.Hoffman LB, Schmeidler J, Lesser GT, et al. Less Alzheimer disease neuropathology in medicated hypertensive than nonhypertensive persons. Neurology. 2009;72(20):1720–1726. doi: 10.1212/01.wnl.0000345881.82856.d5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haag MDM, Hofman A, Koudstaal PJ, Breteler MMB, Strieker BHC. Duration of antihypertensive drug use and risk of dementia: A prospective cohort study. Neurology. 2009;72(20):1727–1734. doi: 10.1212/01.wnl.0000345062.86148.3f [DOI] [PubMed] [Google Scholar]
- 10.Kjeldsen SE, Narkiewicz K, Burnier M, Oparil S. Intensive blood pressure lowering prevents mild cognitive impairment and possible dementia and slows development of white matter lesions in brain: the SPRINT Memory and Cognition IN Decreased Hypertension (SPRINT MIND) study. Blood Press. 2018;27(5):247–248. doi: 10.1080/08037051.2018.1507621 [DOI] [PubMed] [Google Scholar]
- 11.Levi Marpillat N, MacQuin-Mavier I, Tropeano AI, Bachoud-Levi AC, Maison P. Antihypertensive classes, cognitive decline and incidence of dementia: A network meta-analysis. J Hypertens. 2013;31(6):1073–1082. doi: 10.1097/HJH.0b013e3283603f53 [DOI] [PubMed] [Google Scholar]
- 12.Davies NM, Kehoe PG, Ben-Shlomo Y, Martin RM. Associations of anti-hypertensive treatments with Alzheimer’s disease, vascular dementia, and other dementias. J Alzheimer’s Dis. 2011;26(4):699–708. doi: 10.3233/JAD-2011-110347 [DOI] [PubMed] [Google Scholar]
- 13.Barthold D, Joyce G, Wharton W, Kehoe P, Zissimopoulos J. The association of multiple anti-hypertensive medication classes with Alzheimer’s disease incidence across sex, race, and ethnicity. PLoS One. 2018;13(11):1–18. doi: 10.1371/journal.pone.0206705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ye R, Hu Y, Yao A, et al. Impact of renin - angiotensin system-targeting antihypertensive drugs on treatment of Alzheimer’s disease: A meta-analysis. Int J Clin Pract. 2015;69(6):674–681. doi: 10.1111/ijcp.12626 [DOI] [PubMed] [Google Scholar]
- 15.Hajjar I, Okafor M, McDaniel D, et al. Effects of Candesartan vs Lisinopril on Neurocognitive Function in Older Adults With Executive Mild Cognitive Impairment: A Randomized Clinical Trial. JAMA Netw open. 2020;3(8):e2012252. doi: 10.1001/jamanetworkopen.2020.12252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Trompet S, Westendorp RGJ, Kamper AM, de Craen AJM. Use of calcium antagonists and cognitive decline in old age. The Leiden 85-plus study. Neurobiol Aging. 2008;29(2):306–308. doi: 10.1016/j.neurobiolaging.2006.10.006 [DOI] [PubMed] [Google Scholar]
- 17.Hussain S, Singh A, Rahman SO, Habib A, Najmi AK. Calcium channel blocker use reduces incident dementia risk in elderly hypertensive patients: A meta-analysis of prospective studies. Neurosci Lett. 2018;671(September 2017):120–127. doi: 10.1016/j.neulet.2018.02.027 [DOI] [PubMed] [Google Scholar]
- 18.Lovell MA, Abner E, Kryscio R, Xu L, Fister SX, Lynn BC. Calcium Channel Blockers, Progression to Dementia, and Effects on Amyloid Beta Peptide Production. Oxid Med Cell Longev. 2015;2015. doi: 10.1155/2015/787805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hwang D, Kim S, Choi H, et al. Calcium-channel blockers and dementia risk in older adults – National health insurance service – Senior cohort (2002–2013). Circ J. 2016;80(11):2336–2342. doi: 10.1253/circj.CJ-16-0692 [DOI] [PubMed] [Google Scholar]
- 20.Tully PJ, Hanon O, Cosh S, Tzourio C. Diuretic antihypertensive drugs and incident dementia risk. J Hypertens. 2016;34(6):1027–1035. doi: 10.1097/HJH.0000000000000868 [DOI] [PubMed] [Google Scholar]
- 21.McGuinness B, Todd S, Passmore P, Bullock R. Blood pressure lowering in patients without prior cerebrovascular disease for prevention of cognitive impairment and dementia. Cochrane Database Syst Rev. 2009;(4). doi: 10.1002/14651858.CD004034.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fournier A, Oprisiu-Fournier R, Serot JM, et al. Prevention of dementia by antihypertensive drugs: How AT1-receptor-blockers and dihydropyridines better prevent dementia in hypertensive patients than thiazides and ACE-inhibitors. Expert Rev Neurother. 2009;9(9):1413–1431. doi: 10.1586/ern.09.89 [DOI] [PubMed] [Google Scholar]
- 23.Anderson C, Teo K, Gao P, et al. Renin-angiotensin system blockade and cognitive function in patients at high risk of cardiovascular disease: Analysis of data from the ONTARGET and TRANSCEND studies. Lancet Neurol. 2011;10(1):43–53. doi: 10.1016/S1474-4422(10)70250-7 [DOI] [PubMed] [Google Scholar]
- 24.Wright JW, Harding JW. Brain angiotensin receptor subtypes in the control of physiological and behavioral responses. Neurosci Biobehav Rev. 1994;18(1):21–53. doi: 10.1016/0149-7634(94)90034-5 [DOI] [PubMed] [Google Scholar]
- 25.Llorens-Cortes C, Mendelsohn FAO. Organisation and functional role of the brain angiotensin system. JRAAS - J Renin-Angiotensin-Aldosterone Syst. 2002;3(SUPPL. 1):39–48. doi: 10.3317/jraas.2002.029 [DOI] [PubMed] [Google Scholar]
- 26.Sink KM, Leng X, Williamson J, et al. Angiotensin-converting enzyme inhibitors and cognitive decline in older adults with hypertension: Results from the cardiovascular health study. Arch Intern Med. 2009;169(13):1195–1202. doi: 10.1001/archinternmed.2009.175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ho JK, Nation DA. Memory is preserved in older adults taking AT1 receptor blockers. Alzheimer’s Res Ther. 2017;9(1):1–14. doi: 10.1186/s13195-017-0255-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009;6(7). doi: 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gillett SR, Thacker EL, Letter AJ, et al. Correlates of Incident Cognitive Impairment in the REasons for Geographic and Racial Differences in Stroke (REGARDS) Study. Clin Neuropsychol. 2015;29(4):466–486. doi: 10.1080/13854046.2015.1042524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Unger T. Inhibiting renin-angiotensin in the brain: The possible therapeutic implications. Blood Press Suppl. 2001;10(1):12–16. doi: 10.1080/080370501750066453 [DOI] [PubMed] [Google Scholar]
- 31.Unger T. Inhibiting angiotensin receptors in the brain: possible therapeutic implications. Curr Med Res Opin. 2003;19(5):449–451. doi: 10.1185/030079903125001974 [DOI] [PubMed] [Google Scholar]
- 32.Wells GA, Shea B, O’Connel D et al. The Newcastle-Ottawa scale (NOS) for assessing the quailty of nonrandomised studies in meta-analyses. http://wwwohrica/programs/clinical_epidemiology/oxfordhtm 2009. Feb 1. Published online 2009:2009.
- 33.Lezak MD, Howieson DB, Bigler ED, Tranel D. Neuropsychological Assessment. 5th ed. Oxford University Press; 2012. [Google Scholar]
- 34.Strauss E, Sherman EM, Spreen O. A Compendium of Neuropsychological Tests: Administration, Norms, and Commentary. American Chemical Society; 2006. [Google Scholar]
- 35.Higgins JPT, Thomas J, Chandler J, et al. , eds. Cochrane Handbook for Systematic Reviews of Interventions. Wiley; 2019. doi: 10.1002/9781119536604 [DOI] [Google Scholar]
- 36.Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. doi: 10.1136/bmj.327.7414.557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Deeks JJ, Higgins JP, Altman DG. Cochrane Handbook for Systematic Reviews of Interventions. 2nd ed. (Higgins JP, Thomas J, Chandler J, et al. , eds.). John Wiley & Sons; 2019. [Google Scholar]
- 38.Thompson SG, Higgins JPT. How should meta-regression analyses be undertaken and interpreted? Stat Med. 2002;21(11):1559–1573. doi: 10.1002/sim.1187 [DOI] [PubMed] [Google Scholar]
- 39.van Houwelingen HC, Arends LR, Stijnen T. Advanced methods in meta-analysis: multivariate approach and meta-regression. Stat Med. 2002;21(4):589–624. doi: 10.1002/sim.1040 [DOI] [PubMed] [Google Scholar]
- 40.Viechtbauer W. MiMa: An S-Plus/R function to fit meta-analytic mixed-, random-, and fixed-effects models. Published 2006. Accessed December 17, 2019. www.wvbauer.com
- 41.Team RC. R: A language and environment for statistical computing. Published online 2014. [Google Scholar]
- 42.Balduzzi S, Rücker G, Schwarzer G. How to perform a meta-analysis with R: a practical tutorial. Evid Based Ment Heal. 2019;22(4):153–160. doi: 10.1136/ebmental-2019-300117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Harrer M, Cuijpers P, Furukawa T, Ebert DD. Dmetar: Companion R package for the guide “Doing meta-analysis in R”. R package version 0.0.9000. http://dmetar.protectlab.org [Google Scholar]
- 44.Viechtbauer W. Conducting Meta-Analyses in R with the metafor Package. J Stat Softw. 2010;36(3). doi: 10.18637/jss.v036.i03 [DOI] [Google Scholar]
- 45.Suurmond R, van Rhee H, Hak T . Introduction, comparison, and validation of Meta-Essentials : A free and simple tool for meta-analysis. Res Synth Methods. 2017;8(4):537–553. doi: 10.1002/jrsm.1260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lindsay J, Laurin D, Verreault R, et al. Risk factors for Alzheimer’s disease: A prospective analysis from the Canadian Study of Health and Aging. Am J Epidemiol. 2002;156(5):445–453. doi: 10.1093/aje/kwf074 [DOI] [PubMed] [Google Scholar]
- 47.Tyas SL, Manfreda J, Strain LA, Montgomery PR. Risk factors for Alzheimer’s disease: A population-based, longitudinal study in Manitoba, Canada. Int J Epidemiol. 2001;30(3):590–597. doi: 10.1093/ije/30.3.590 [DOI] [PubMed] [Google Scholar]
- 48.Kukull WA, Higdon R, Bowen JD, et al. Dementia and Alzheimer disease incidence: A prospective cohort study. Arch Neurol. 2002;59(11):1737–1746. doi: 10.1001/archneur.59.11.1737 [DOI] [PubMed] [Google Scholar]
- 49.Morris MC, Scherr PA, Hebert LE, et al. Association between blood pressure and cognitive function in a biracial community population of older persons. Neuroepidemiology. 2002;21(3):123–130. doi: 10.1159/000054809 [DOI] [PubMed] [Google Scholar]
- 50.Kenny RA, Whelan BJ, Cronin H, et al. The Design of the Irish Longitudinal Study on Ageing.; 2010. [Google Scholar]
- 51.Heinzel S, Liepelt-Scarfone I, Roeben B, et al. A neurodegenerative vascular burden index and the impact on cognition. Front Aging Neurosci. 2014;6(JUL):1–9. doi: 10.3389/fnagi.2014.00161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liu E, Dyer SM, O’Donnell LK, et al. Association of cardiovascular system medications with cognitive function and dementia in older adults living in nursing homes in Australia. J Geriatr Cardiol. 2017;14(6):407–415. doi: 10.11909/j.issn.1671-5411.2017.06.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Grober E, Kawas C. Learning and retention in preclinical and early Alzheimer’s disease. Psychol Aging. 1997;12(1):183–188. doi: 10.1037/0882-7974.12.1.183 [DOI] [PubMed] [Google Scholar]
- 54.Stephens S, Kenny RA, Rowan E, et al. Neuropsychological characteristics of mild vascular cognitive impairment and dementia after stroke. Int J Geriatr Psychiatry. 2004;19(11):1053–1057. doi: 10.1002/gps.1209 [DOI] [PubMed] [Google Scholar]
- 55.Petrovitch H, White LR, Izmirilian G, et al. Midlife blood pressure and neuritic plaques, neurofibrillary tangles, and brain weight at death: the HAAS. Neurobiol Aging. 2000;21(1):57–62. doi: 10.1016/S0197-4580(00)00106-8 [DOI] [PubMed] [Google Scholar]
- 56.Arvanitakis Z, Capuano AW, Lamar M, et al. Late-life blood pressure association with cerebrovascular and Alzheimer disease pathology. Neurology. 2018;91(6):e517–e525. doi: 10.1212/WNL.0000000000005951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hajjar I, Brown L, Mack WJ, Chui H. Impact of angiotensin receptor blockers on Alzheimer disease neuropathology in a large brain autopsy series. Arch Neurol. 2012;69(12):1632–1638. doi: 10.1001/archneurol.2012.1010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lane CA, Barnes J, Nicholas JM, et al. Associations between blood pressure across adulthood and late-life brain structure and pathology in the neuroscience substudy of the 1946 British birth cohort (Insight 46): an epidemiological study. Lancet Neurol. 2019;18(10):942–952. doi: 10.1016/S1474-4422(19)30228-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kehoe PG. The coming of age of the angiotensin hypothesis in Alzheimer’s disease: Progress toward disease prevention and treatment? J Alzheimer’s Dis. 2018;62(3):1443–1466. doi: 10.3233/JAD-171119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Arregui A, Perry EK, Rossor M, Tomlinson BE. Angiotensin Converting Enzyme in Alzheimer’s Disease: Increased Activity in Caudate Nucleus and Cortical Areas. J Neurochem. 1982;38(5):1490–1492. doi: 10.1111/j.1471-4159.1982.tb07930.x [DOI] [PubMed] [Google Scholar]
- 61.Barnes NM, Cheng CHK, Costall B, Naylor RJ, Williams TJ, Wischik CM. Angiofensin converting enzyme density is increased in temporal cortex from patients with Alzheimer’s disease. Eur J Pharmacol. 1991;200(2–3):289–292. doi: 10.1016/0014-2999(91)90584-D [DOI] [PubMed] [Google Scholar]
- 62.Kehoe PG, Katzov H, Andreasen N, et al. Common variants of ACE contribute to variable age-at-onset of Alzheimer’s disease. Hum Genet. 2004;114(5):478–483. doi: 10.1007/s00439-004-1093-y [DOI] [PubMed] [Google Scholar]
- 63.Kehoe PG, Katzov H, Feuk L, et al. Haplotypes extending across ACE are associated with Alzheimer’s disease. Hum Mol Genet. 2003;12(8):859–867. doi: 10.1093/hmg/ddg094 [DOI] [PubMed] [Google Scholar]
- 64.Kehoe PG, Wong S, Al Mulhim N, Palmer LE, Miners JS. Angiotensin-converting enzyme 2 is reduced in Alzheimer’s disease in association with increasing amyloid-β and tau pathology. Alzheimer’s Res Ther. 2016;8(1):1–10. doi: 10.1186/s13195-016-0217-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Evans CE, Miners JS, Piva G, et al. ACE2 activation protects against cognitive decline and reduces amyloid pathology in the Tg2576 mouse model of Alzheimer’s disease. Acta Neuropathol. 2020;139(3):485–502. doi: 10.1007/s00401-019-02098-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cuddy LK, Prokopenko D, Cunningham EP, et al. Aβ-accelerated neurodegeneration caused by Alzheimer’s-associated ACE variant R1279Q is rescued by angiotensin system inhibition in mice. Sci Transl Med. 2020;12(563). doi: 10.1126/scitranslmed.aaz2541 [DOI] [PubMed] [Google Scholar]
- 67.Pugh KG, Lipsitz LA. The microvascular frontal-subcortical syndrome of aging. Neurobiol Aging. 2002;23(3):421–431. doi: 10.1016/S0197-4580(01)00319-0 [DOI] [PubMed] [Google Scholar]
- 68.Lincoln N, Majid M, Weyman N. Cognitive rehabilitation for attention deficits following stroke. In: Lincoln N, ed. Cochrane Database of Systematic Reviews. John Wiley & Sons, Ltd; 2000. doi: 10.1002/14651858.CD002842 [DOI] [PubMed] [Google Scholar]
- 69.MacMahon S, Neal B, Tzourio C, et al. Randomised trial of a perindopril-based blood-pressure-lowering regimen among 6105 individuals with previous stroke or transient ischaemic attack. Lancet. 2001;358(9287):1033–1041. doi: 10.1016/S0140-6736(01)06178-5 [DOI] [PubMed] [Google Scholar]
- 70.England TN. Effects of an Angiotensin-Converting–Enzyme Inhibitor, Ramipril, on Cardiovascular Events in High-Risk Patients. N Engl J Med. 2000;342(3):145–153. doi: 10.1056/NEJM200001203420301 [DOI] [PubMed] [Google Scholar]
- 71.Lithell H, Hansson L, Skoog I, Elmfeldt D. The Study on Cognition and Prognosis in the Elderly ( SCOPE ): principal results of a randomized double-blind intervention trial. J Hypertens. 2003;21(5):875–886. doi: 10.1097/01.hjh.0000059028.82022.89 [DOI] [PubMed] [Google Scholar]
- 72.O’Hagan TS, Wharton W, Kehoe PG. Interactions between oestrogen and the renin angiotensin system - Potential mechanisms for gender differences in Alzheimer’s disease. Am J Neurodegener Dis. 2012;1(3):266–279. [PMC free article] [PubMed] [Google Scholar]
- 73.Zhou J, Pavel J, Macova M, et al. AT1 receptor blockade regulates the local angiotensin II system in cerebral microvessels from spontaneously hypertensive rats. Stroke. 2006;37(5):1271–1276. doi: 10.1161/01.STR.0000217404.64352.d7 [DOI] [PubMed] [Google Scholar]
- 74.Benicky J, Sánchez-Lemus E, Honda M, et al. Angiotensin II AT1 receptor blockade ameliorates brain inflammation. Neuropsychopharmacology. 2011;36(4):857–870. doi: 10.1038/npp.2010.225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cominacini L, Pasini AF, Garbin U, et al. Zofenopril inhibits the expression of adhesion molecules on endothelial cells by reducing reactive oxygen species. Am J Hypertens. 2002;15(10 I):891–895. doi: 10.1016/S0895-7061(02)02995-3 [DOI] [PubMed] [Google Scholar]
- 76.Zola-Morgan S, Squire L. The primate hippocampal formation: evidence for a time-limited role in memory storage. Science (80- ). 1990;250(4978):288–290. doi: 10.1126/science.2218534 [DOI] [PubMed] [Google Scholar]
- 77.Ho JK, Nation DA. Cognitive benefits of angiotensin IV and angiotensin-(1–7): A systematic review of experimental studies. Neurosci Biobehav Rev. 2018;92(January):209–225. doi: 10.1016/j.neubiorev.2018.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Metzger R, Bader M, Ludwig T, Berberich C, Bunnemann B, Ganten D. Expression of the mouse and rat mas proto-oncogene in the brain and peripheral tissues. FEBS Lett. 1995;357(1):27–32. doi: 10.1016/0014-5793(94)01292-9 [DOI] [PubMed] [Google Scholar]
- 79.Xia H, Lazartigues E. Angiotensin-converting enzyme 2 in the brain: Properties and future directions. J Neurochem. 2008;107(6):1482–1494. doi: 10.1111/j.1471-4159.2008.05723.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Uekawa K, Hasegawa Y, Senju S, et al. Intracerebroventricular Infusion of Angiotensin-(1–7) Ameliorates Cognitive Impairment and Memory Dysfunction in a Mouse Model of Alzheimer’s Disease. J Alzheimer’s Dis. 2016;53(1):127–133. doi: 10.3233/JAD-150642 [DOI] [PubMed] [Google Scholar]
- 81.Xie W, Zhu D, Ji L, Tian M, Xu C, Shi J. Angiotensin-(1–7) improves cognitive function in rats with chronic cerebral hypoperfusion. Brain Res. 2014;1573(264):44–53. doi: 10.1016/j.brainres.2014.05.019 [DOI] [PubMed] [Google Scholar]
- 82.Wharton W, Goldstein FC, Zhao L, Steenland K, Levey AI, Hajjar I. Modulation of renin-angiotensin system may slow conversion from mild cognitive impairment to Alzheimer’s disease. J Am Geriatr Soc. 2015;63(9):1749–1756. doi: 10.1111/jgs.13627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Turner RM, Bird SM, Higgins JPT. The Impact of Study Size on Meta-analyses: Examination of Underpowered Studies in Cochrane Reviews. Gluud LL, ed. PLoS One. 2013;8(3):e59202. doi: 10.1371/journal.pone.0059202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Solfrizzi V, Scafato E, Frisardi V, et al. Angiotensin-converting enzyme inhibitors and incidence of mild cognitive impairment. the Italian Longitudinal Study on Aging. Age (Omaha). 2013;35(2):441–453. doi: 10.1007/s11357-011-9360-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kelley BJ, Petersen RC. Alzheimer’s Disease and Mild Cognitive Impairment. Neurol Clin. 2007;25(3):577–609. doi: 10.1016/j.ncl.2007.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bellew KM, Pigeon JG, Stang PE, Fleischman W, Gardner RM, Baker WW. Hypertension and the rate of cognitive decline in patients with dementia of the Alzheimer type. Alzheimer Dis Assoc Disord. 2004;18(4):208–213. [PubMed] [Google Scholar]
- 87.Middleton LE, Yaffe K. Promising strategies for the prevention of dementia. Arch Neurol. 2009;66(10):1210–1215. doi: 10.1001/archneurol.2009.201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Liu H, Gao S, Hall KS, et al. Optimal Blood Pressure for Cognitive Function: Findings from an Elderly African-American Cohort Study. J Am Geriatr Soc. 2013;61(6):875–881. doi: 10.1111/jgs.12259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Leveille SG, Kiel DP, Jones RN, et al. The MOBILIZE Boston Study: Design and methods of a prospective cohort study of novel risk factors for falls in an older population. BMC Geriatr. 2008;8:1–14. doi: 10.1186/1471-2318-8-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hudak EM, Edwards JD, Athilingam P, McEvoy CL. A Comparison of Cognitive and Everyday Functional Performance Among Older Adults With and Without Hypertension. Clin Gerontol. 2013;36(2):113–131. doi: 10.1080/07317115.2012.749322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sato N, Saijo Y, Sasagawa Y, et al. Combination of antihypertensive therapy in the elderly, multicenter investigation (CAMUI) trial: Results after 1 year. J Hypertens. 2013;31(6):1245–1255. doi: 10.1097/HJH.0b013e32835fdf60 [DOI] [PubMed] [Google Scholar]
- 92.Lacruz ME, Tiller D, Kluttig A, et al. Association of late-life changes in blood pressure and cognitive status. J Geriatr Cardiol. 2016;13(1):37–43. doi: 10.11909/j.issn.1671-5411.2016.01.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.