Abstract
Forty clonally related clinical isolates of Escherichia coli from hospitalized patients were resistant to cefoxitin (MICs, >256 μg/ml) and ceftazidime (MICs, 32 to 256 μg/ml) and were intermediate or resistant to cefotaxime (MICs, 16 to 128 μg/ml) but susceptible to both cefepime (MICs, 0.5 to 2 μg/ml) and imipenem (MICs, 0.125 to 0.25 μg/ml). Resistance to β-lactams was related to high-level production of AmpC β-lactamase and loss of OmpF porin.
Most Escherichia coli strains do not produce clinically relevant levels of the chromosomally encoded AmpC β-lactamase (4). Gene amplification or mutations at either the promoter and/or the attenuator of the structural β-lactamase gene result in AmpC hyperproduction (2, 5, 11, 16, 20). This causes increased resistance to penicillins, cephalosporins, and β-lactam–β-lactamase inhibitor combinations. β-Lactams penetrate into gram-negative bacteria throughout nonspecific porins (17). Two major porins have been described in E. coli: OmpF and OmpC (24). Loss of nonspecific porins in E. coli and other Enterobacteriaceae are related to increased levels of resistance to β-lactams, particularly when combined with production of an efficient β-lactamase(s) (1, 3, 8–10, 13, 21). Zwitterionic cephalosporins (cefepime, cefpirome, etc.) are more active against organisms producing increased levels of inducible chromosomal AmpC β-lactamase than are older oxyimino-cephalosporins (cefotaxime, ceftriaxone, ceftazidime, etc.) (7). We have, however, scarce information on the activity of zwitterionic cephalosporins against E. coli hyperproducing AmpC. In this study, we have evaluated the in vitro activities of imipenem and cephalosporins against clonally related clinical isolates of E. coli hyperproducing chromosomal β-lactamase and the pattern of porin expression in these isolates.
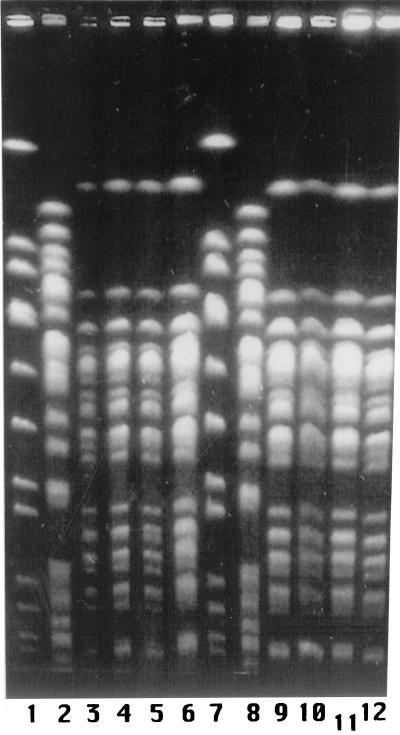
Forty-four cefoxitin-resistant (MICs, >32 μg/ml) isolates of E. coli obtained from clinical samples (January to December 1994) have been studied. Forty isolates were obtained from inpatients, and four were obtained from outpatients. Identification and preliminary susceptibility testing were performed using commercial panels (panels 6P; Pasco). All 40 isolates from inpatients showed similar patterns of resistance to β-lactams, fluoroquinolones, co-trimoxazole, and aminoglycosides. A variety of resistance patterns were observed in isolates from outpatients, none of them being identical to that of isolates from inpatients (data not shown). Genomic DNA from the 44 clinical isolates and from strain ATCC 25922 was separated after digestion with XbaI by pulsed-field gel electrophoresis (PFGE) as previously described (14). All 40 isolates from inpatients presented the same PFGE pattern, in contrast to the 4 isolates from outpatients and strain ATCC 25922, which showed patterns unrelated to each other and to that of isolates from inpatients (Fig. 1). The activities of cefoxitin (Sigma, Madrid, Spain), cefotaxime (Sigma), ceftriaxone (Sigma), ceftazidime (Glaxo, Madrid, Spain), cefepime (Bristol-Myers Squibb, Madrid, Spain), and imipenem (Merck Sharp and Dhome, Madrid, Spain) against all 44 isolates were determined by microdilution (15). MIC ranges and MICs at which 90% of isolates were inhibited, respectively, for the 40 isolates from inpatients were as follows (in micrograms per milliliter): >256 and >256 (cefoxitin); 0.5 to 2 and 2 (cefepime), 16 to 128 and 32 (both cefotaxime and ceftriaxone), 32 to 256 and 128 (ceftazidime), and 0.125 to 0.5 and 0.5 (imipenem). All strains were resistant to cefoxitin and ceftazidime, intermediate or resistant to cefotaxime and ceftriaxone, and susceptible to both cefepime and imipenem (15).
FIG. 1.
PFGE profiles of E. coli clinical isolates hyperproducing chromosomal β-lactamase. Lanes 1, 2, 7, and 8: markers. Lanes 3, 4, 5, 6, 9, 10, 11, and 12: isolates HUS4/94, HUS7/94, HUS23/94, HUS31/94, HUS34/94, HUS36/94, HUS42/94, and HUS47/94, respectively.
Eight isolates from hospitalized patients, including organisms covering the whole range of MICs of ceftazidime and cefotaxime for the 40 isolates from inpatients, were further studied. MICs of cephalosporins and imipenem were determined with Etest strips (AB Biodisk, Solna, Sweden) in the presence of clavulanic acid (2 μg/ml) or BRL 42715 (4 μg/ml). A clinical strain of Klebsiella pneumoniae (HUS57/94) producing SHV-5 β-lactamase (unpublished results) was used as a control in these studies. MICs of both cefoxitin and cefotaxime did not decrease in the presence of clavulanic acid. BRL 42715, however, significantly decreased the MICs of both cefoxitin and cefotaxime (Table 1). Both β-lactamase inhibitors decreased the MIC of cefotaxime, but not of cefoxitin, for K. pneumoniae HUS57/94 (data not shown).
TABLE 1.
MICs of cephalosporins and cephalosporins plus β-lactamase inhibitorsa
| E. coli isolate | MIC (μg/ml) of drug(s) by method
|
pI | β-Lactamase activityb | % β-lactamase inhibition by:
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Microdilution
|
Etest
|
|||||||||||
| CAZ | FOX | CTX | CAZ | FOX | FOX-BRL | CTX | CTX-BRL | CLOXc | CAd | |||
| HUS34/94 | 32 | >256 | 16 | 64 | >256 | 24 | 12 | 0.25 | ≥9 | 470 | 99.6 | 1.9 |
| HUS47/94 | 64 | >256 | 16 | 96 | >256 | 24 | 16 | 0.25 | ≥9 | 215 | 99.1 | 7.6 |
| HUS36/94 | 64 | >256 | 16 | 24 | >256 | 12 | 8 | 0.25 | ≥9 | 218 | 99.0 | 6.3 |
| HUS4/94 | 64 | >256 | 16 | 64 | >256 | 16 | 8 | 0.25 | ≥9 | 346 | 99.1 | 8.1 |
| HUS23/94 | 64 | >256 | 32 | 256e | >256 | 96 | 48 | 0.38 | ≥9 + 5.4f | 2,057 | 32.5 | 31.4 |
| HUS42/94 | 128 | >256 | 32 | 64 | >256 | 64 | 16 | 0.5 | ≥9 | 575 | 99.0 | 0.6 |
| HUS31/94 | 128 | >256 | 32 | 256e | >256 | 24 | 16 | 0.25 | ≥9 | 514 | 99.0 | 1.9 |
| HUS7/94 | 256 | >256 | 128 | 256e | >256 | 8 | 24 | 0.75 | ≥9 | 394 | 87.7 | 2.6 |
Abbreviations for cephalosporins: CAZ, ceftzidime; FOX, cefoxitin; CTX, cefotaxime. Abbreviations for β-lactamase inhibitors: CA, clavulanic acid; BRL, BRL 42715. MICs were determined by microdilution or Etest for eight isolates of AmpC-hyperproducing E. coli
Milliunits of enzyme/milligram of protein.
Cloxacillin (250 μM).
Clavulanic acid (2 μM).
Difficult-to-read end-point.
Two β-lactamase bands were detected.
Plasmid DNA was obtained by the alkaline-lysis method (12) and analyzed by electrophoresis in 0.7% agarose gels. All eight isolates contained a plasmid of 5.1 kb; isolate HUS23/94 additionally contained a plasmid of 8.0 kb. Attempts to transfer resistance to cephalosporins from isolates HUS31/94, HUS23/94, HUS36/94, and HUS47/94 to E. coli J53-2 (F− pro met Rifr) by conjugation, transformation (heat shock method), and electroporation, using ampicillin (50 μg/ml), cefoxitin (10 μg/ml), or cefotaxime (10 μg/ml) as selective agents (22), consistently failed. Although strains of E. coli producing AmpC-type β-lactamase from plasmid have been described (6), these results suggest that the most probable cause of β-lactam resistance in our isolates is of chromosomal origin.
Isoelectric point (pI) determinations using crude supernatants were performed by isoelectric focusing using the Phast-System (gel pI range, 3.5 to 9; Pharmacia, Sant Cugat del Vallés, Spain). All eight isolates produced a β-lactamase with a pI of ≥9. Isolate HUS23/94 also produced a β-lactamase with a pI of 5.4, compatible with TEM-1. β-Lactamase activity was determined spectrophotometrically using crude supernatants from sonicated cells. One unit of activity was defined as the amount of enzyme that hydrolyzes 1 μmol of cephaloridine per min at 37°C. Inhibition of β-lactamase was determined after preincubation (10 min at 37°C) of supernatants with cloxacillin (250 μM) or clavulanic acid (2 μM). Cloxacillin and clavulanic acid inhibited 88.7 to 99.6% and 0.6 to 8.1% of the β-lactamase activity, except in isolate HUS23/94. Outer membrane protein profiles from bacteria grown to logarithmic phase in Mueller-Hinton broth or in nutrient broth were determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (12% acrylamide and 6 M urea in the running gel). OmpF porin was absent in the eight isolates grown in either Mueller-Hinton broth or nutrient broth (Fig. 2).
FIG. 2.
Outer membrane protein profiles of E. coli clinical isolates HUS4/94 (lane 2), HUS7/94 (lane 3), HUS23/94 (lane 4), HUS31/94 (lane 5), HUS34/94 (lane 6), HUS36/94 (lane 7), HUS42/94 (lane 8), and HUS47/94 (lane 9). Lane 1: E. coli JF568 (OmpA+ OmpF+ OmpC+).
Hyperproduction of AmpC causing resistance of clinical isolates of E. coli to oxyimino-cephalosporins has been previously documented (2, 4, 16), but the epidemiological relationship of the strains included in those studies has not been reported. In this study we have shown that 40 isolates from inpatients were clonally related but epidemiologically unrelated to other AmpC-hyperproducing strains (data not shown) cultured from outpatients in the same geographical area. These 40 isolates presumably represent a single strain causing a nosocomial outbreak. Data on patients from whom the isolates were obtained are not available for a complete analysis, thus precluding an adequate epidemiological study.
The isolates described in this report were resistant to oxyimino-cephalosporins but susceptible to both cefepime and imipenem, two compounds highly stable to AmpC β-lactamases from other enterobacteria (7). This study suggests that both cefepime and imipenem may represent a therapeutic alternative for infections caused by AmpC-hyperproducing E. coli strains, as suggested for other organisms (23). Loss of both OmpF and OmpC porins in laboratory mutants of E. coli determines an 8- to 16-fold increase in the MICs of cephalosporins (9). Loss of OmpF alone in E. coli mutants expressing increased amounts of AmpC determines a two- to fourfold increase in the MICs of cephalosporins (10). There is scarce information on the number and nature of porins expressed by clinical isolates of E. coli, and the relationship of porin expression, if any, with antimicrobial resistance. Reguera et al. (21) reported that seven out of eight clinical strains of E. coli resistant to amoxicillin-clavulanic acid expressed only OmpC or expressed OmpF with altered electrophoretic mobility. In order to evaluate the role of porins in antimicrobial resistance, it would be necessary to compare Omp profiles and susceptibility data of bacteria cultured in the same medium. OmpF, an important porin for β-lactam penetration (18, 19), is not expressed in high-osmolarity media, such as Mueller-Hinton broth, the most commonly used medium for susceptibility testing. In the isolates herein studied, we have observed that OmpF is not expressed in Mueller-Hinton broth (as would be expected); nor is it expressed in the low-osmolarity medium nutrient broth, suggesting that this porin is actually not expressed by these isolates. This also suggests that resistance due to cooperation between β-lactamase production and altered permeability demonstrated in laboratory mutants of E. coli can also be expressed by clinical isolates.
The level of resistance to cephalosporins varied in different (but clonally related) isolates. It does not seem probable that variations in β-lactamase activity accounts for the differences in susceptibility to cefotaxime and ceftazidime. This is suggested by both the poor correlation between enzyme activity and MICs (even if strain HUS23/94 is not considered) and by the differences of MICs of cefoxitin and cefotaxime in the presence of BRL 42715. It is possible that other mechanisms (penicillin-binding protein expression, active efflux, etc.) contribute to the observed phenotypes of resistance. New studies are in progress to evaluate these possibilities.
Acknowledgments
We gratefully acknowledge the assistance of Janet Dawson in the preparation of the manuscript.
This work was supported in part by grants from Consolidation of Research Groups, Consejería de Educación, Junta de Andalucía, to L.M.-M., A.P. and E.J.P. and the Comisión Interministerial de Ciencia y Tecnología, Ministerio de Educación, Spain (grant PB96-0197) to V.J.B.
REFERENCES
- 1.Bradford P A, Urban C, Mariano N, Projan S J, Rahal J J, Bush K. Imipenem resistance in Klebsiella pneumoniae is associated with the combination of ACT-1, a plasmid-mediated AmpC β-lactamase, and the loss of an outer membrane protein. Antimicrob Agents Chemother. 1997;41:563–569. doi: 10.1128/aac.41.3.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Caroff N, Espaze E, Berard I, Richet H, Reynaud A. Mutations in the ampC promoter of Escherichia coli isolates resistant to oxyiminocephalosporins without extended spectrum beta-lactamase production. FEMS Microbiol Lett. 1999;173:459–465. doi: 10.1111/j.1574-6968.1999.tb13539.x. [DOI] [PubMed] [Google Scholar]
- 3.Chow J W, Shlaes D M. Imipenem resistance associated with the loss of a 40 kDa outer membrane protein in Enterobacter aerogenes. J Antimicrob Chemother. 1991;28:499–504. doi: 10.1093/jac/28.4.499. [DOI] [PubMed] [Google Scholar]
- 4.Cooksey R, Swenson J, Clark N, Gay E, Thornsberry C. Patterns and mechanisms of β-lactamase resistance among isolates of Escherichia coli from hospitals in the United States. Antimicrob Agents Chemother. 1990;34:739–745. doi: 10.1128/aac.34.5.739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Edlund T, Grundstrom T, Normark S. Isolation and characterization of DNA repetitions carrying the chromosomal β-lactamase gene of Escherichia coli K12. Mol Gen Genet. 1979;173:115–125. doi: 10.1007/BF00330301. [DOI] [PubMed] [Google Scholar]
- 6.Fosberry A P, Payne D J, Lawlor E J, Hodgson J E. Cloning and sequence analysis of blaBIL-1, a plasmid-mediated class C β-lactamase gene in Escherichia coli BS. Antimicrob Agents Chemother. 1994;38:1182–1185. doi: 10.1128/aac.38.5.1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fung-Tomc J C. Fourth-generation cephalosporins. Clin Microbiol Newsl. 1997;19:129–136. [Google Scholar]
- 8.Hiraoka M, Okamoto R, Inoue M, Mitsuhashi S. Effects of β-lactamases and omp mutation on susceptibility to β-lactam antibiotics in Escherichia coli. Antimicrob Agents Chemother. 1989;33:382–386. doi: 10.1128/aac.33.3.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jacoby G A, Sutton L. β-Lactamases and β-lactam resistance in Escherichia coli. Antimicrob Agents Chemother. 1985;28:703–705. doi: 10.1128/aac.28.5.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jacoby G A, Carreras I. Activities of β-lactam antibiotics against Escherichia coli strains producing extended-spectrum β-lactamases. Antimicrob Agents Chemother. 1990;34:858–862. doi: 10.1128/aac.34.5.858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jaurin B, Grundström T, Edlund T, Normark S. The E. coli β-lactamase attenuator mediates growth-dependent regulation. Nature. 1981;290:221–225. doi: 10.1038/290221a0. [DOI] [PubMed] [Google Scholar]
- 12.Kado C I, Liu S T. Rapid procedure for detection and isolation of large and small plasmids. J Bacteriol. 1981;145:1365–1373. doi: 10.1128/jb.145.3.1365-1373.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Martínez-Martínez L, Pascual A, Hernández-Allés S, Suárez A I, Tran J, Benedí V J, Jacoby G A. Role of β-lactamases and porins in the activity of carbapenems and cephalosporins against Klebsiella pneumoniae. Antimicrob Agents Chemother. 1999;43:1657–1661. doi: 10.1128/aac.43.7.1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maslow J N, Slutsky A M, Arbeit R D. Application of pulsed-field gel electrophoresis to molecular epidemiology. In: Persing D H, Smith T F, Tenover F C, White T J, editors. Diagnostic molecular microbiology. Washington, D.C.: American Society for Microbiology; 1993. pp. 563–572. [Google Scholar]
- 15.National Committee for Clinical Laboratory Standards. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, fourth ed. Approved standard M7-A4. Wayne, Pa: National Committee for Clinical Laboratory Standards; 1997. [Google Scholar]
- 16.Nelson E C, Elisha B G. Molecular basis of AmpC hyperproduction in clinical isolates of Escherichia coli. Antimicrob Agents Chemother. 1999;43:957–959. doi: 10.1128/aac.43.4.957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nikaido H. Outer membrane barrier as a mechanism of antimicrobial resistance. Antimicrob Agents Chemother. 1989;33:1831–1836. doi: 10.1128/aac.33.11.1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nikaido H, Liu W, Rosenberg E Y. Outer membrane permeability and beta-lactamase stability of dipolar ionic cephalosporins containing methoxyimino substituents. Antimicrob Agents Chemother. 1990;34:337–342. doi: 10.1128/aac.34.2.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nikaido H, Rosenberg E Y, Foulds J. Porin chanels in Escherichia coli: studies with β-lactams in intact cells. J Bacteriol. 1983;153:232–240. doi: 10.1128/jb.153.1.232-240.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Olsson O, Bergstrom S, Normark S. Identification of a novel AmpC β-lactamase promoter in a clinical isolate of Escherichia coli. EMBO J. 1982;1:1411–1416. doi: 10.1002/j.1460-2075.1982.tb01331.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reguera J A, Baquero F, Pérez-Díaz J C, Martínez J L. Factors determining resistance to β-lactams combined with β-lactamase inhibitors in Escherichia coli. J Antimicrob Chemother. 1991;27:569–575. doi: 10.1093/jac/27.5.569. [DOI] [PubMed] [Google Scholar]
- 22.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. pp. 1.74–1.84. [Google Scholar]
- 23.Sanders W E, Tenney J H, Kessler R E. Efficacy of cefepime in the treatment of infections due to multiply resistant Enterobacter species. Clin Infect Dis. 1996;23:454–461. doi: 10.1093/clinids/23.3.454. [DOI] [PubMed] [Google Scholar]
- 24.Schmitges C J, Henning U. The major proteins of the E. coli outer cell-envelope membrane. Heterogeneity of protein 1. Eur J Biochem. 1976;63:47–52. doi: 10.1111/j.1432-1033.1976.tb10205.x. [DOI] [PubMed] [Google Scholar]