ABSTRACT
Assessment of safety of COVID-19 vaccines is an ongoing process. This study aims to explore long-term adverse events reported by physicians and dentists who received at least two COVID-19 vaccine doses. A group of physicians and dentists were invited to complete a validated questionnaire that was composed of items on: socio-demographics, medical history, administered vaccines, and long-term adverse events (LTAE). Data of a total of 498 practitioners were included. Age ranged from 22 to 71 years (mean age= 35.75 ± 11.74) with a female majority (N = 348, 69.9%). The most frequently administered vaccines were Pfizer-BioNtech, Sinopharm and AstraZeneca vaccines. A total of 80 (16.0%) participants reported LTAEs which were mainly fatigue, menstrual disturbances, myalgia, arthralgia, dizziness, and headache (N = 32, 15, 8, 6, 4, and 4, respectively). There was no statistically significant association between LTAEs and: age, gender, or medical history (P > .05). The collective symptoms of fatigue, myalgia, arthralgia, dizziness, and headache were significantly associated with Sinopharm vaccine (P = .04). This was further confirmed by general linear multivariate model analysis. Less than 20% of COVID-19 vaccine recipients may complain of LTAEs that are mostly fatigue-related. It seems that factors such as age, gender, and medical status play a negligible role in development of these AEs. On the other hand, Sinopharm vaccine showed the highest significant association with these AEs followed by AstraZeneca vaccine.
KEYWORDS: Long term adverse effects, COVID-19, Dentists, Physicians, Vaccine
1. Introduction
A year has passed since the roll out of Corona virus disease-2019 (COVID-19) vaccines. Several vaccines (utilizing three main platforms) were approved and used globally, namely Pfizer-BioNtech (PB) (RNA-based), AstraZeneca (AZ) (Adenoviral vector-based), and Sinopharm (SF) (inactivated virus). These vaccines have been reported to have efficacy rates ranging from 79% to 95% in reducing risk of moderate to severe illness, hospitalization, and deaths related to COVID-19.1–4
Early reports on these vaccines concluded that their side effects are mostly acute in nature, with a duration ranging from 1 to 10 days.5 Side effects were described as local effects in the injection site and systemic transient manifestations in the form of fatigue, headache, fever, among others. Potential side effects of vaccines, and concerns about their safety profile represent important obstacles against efforts to distribute vaccines among eligible candidates. Public trust in vaccines has been influenced by several political and social factors with concerns over the relatively rapid production, and claims of inadequate testing and monitoring of vaccine safety. Despite efforts of the scientific community to assure the public about the safety and efficacy of vaccines, there has been significant public hesitancy toward COVID-19 vaccination. Moreover, vaccine hesitancy was reported among health-care workers who demonstrated unacceptably low vaccination rates at times.6
To increase vaccination rates several countries mandated vaccination for eligible candidates as soon as vaccines were approved. Recently, with the severe surge of COVID-19 cases and emergence of aggressive virus variants several countries such as the United States, Italy, and France have mandated vaccines starting from July 2021,7 while other countries announced that they will start mandatory vaccination early 2022. Although mandatory vaccine campaigns went relatively smoothly in the developing world, there were sporadic reports of detecting fake vaccine certificates or fake virus tests to evade vaccination.8 In several industrialized countries, on the other hand, resistance by antivaccination movements was encountered, and their sentiments were circulated over social media concerning misinformation that vaccines contain toxic substances, cause illnesses; are ineffective; and that mandating them shall violate personal freedom.9,10
Several reports were available on short-term or acute side effects of vaccines. However, data on long-term or delayed effects of vaccines are still unavailable. It is important that such reports are made available to the public as well as scientific community. More importantly, these data should be provided in a transparent and evidence-based approach. Continuous tracking of COVID-19 vaccine side effects is important and currently some studies are ongoing to track safety of vaccines.11 It is essential at this stage of the COVID-19 pandemic, to maintain the momentum of vaccination campaigns by providing updated data on vaccine safety and adverse events. Data on vaccine safety will also be more valid and reliable if they are provided by trusted groups of healthcare professionals such as physicians and dentists. Further, such groups, having priority for vaccination, have received full vaccination early on after the introduction of those vaccines and some have already received their booster doses as well. This allowed for a relatively sufficient time for monitoring changes in medical condition and to determine if a long-term adverse event (LTAE) has emerged.
Therefore, this study was conducted among a sample of physicians and dentists who had the full vaccination protocol of COVID-19 vaccines to investigate prevalence of self-reported LTAE of vaccines and to explore the potential influence of age, gender, medical history, and vaccine type on development of these events.
2. Materials and Methods
2.1. Participants
Study participants were physicians and dentists registered as group members of specific professional WhatsApp groups. Only practitioners who provided informed consent and who received the full vaccination protocol were eligible for participation.
2.2. Study tool
A questionnaire was designed based on a previously validated and used questionnaire after some modifications5 using Google Forms. It consisted of 35 items distributed in the following sections: first section consisted of 10 questions on demographics (age, gender, height, weight, profession, country of practice, history of chronic disease, hemoglobin level, medications, intake of influenza vaccine); second section on COVID-19 vaccine consisting of 20 questions on type of vaccine for the first, second, and booster dose and associated LTAE. The third section was composed of five questions directed to female practitioners on influence of vaccine on menstrual health. All questions were closed-ended, except questions on weight, height, country of practice and LTAEs which were open ended. The question on LTAEs explained that a LTAE is “a symptom that continued more than one month after vaccine administration” to ensure that the question is clear and to obtain accurate responses. The questionnaire had an introductory paragraph explaining the nature and objectives of the study, and the voluntary and anonymous nature of participation. The study was ethically approved by Taibah University, College of Dentistry Ethics Committee, IRB# TUCDREC/01122021/NDar-Odeh.
2.3. Statistical analysis
Statistical analysis was carried out using Statistical Package for Social Sciences (IBM-SPSS) version 25. Descriptive statistics were obtained in the form of frequencies and percentages describing study sample, vaccines, and LTAEs. Cross tabulation with Chi-square was used to investigate significant associations of various variables with adverse events. General linear model (multivariate analysis of variance- MANOVA) was carried out to investigate significant differences in adverse events between vaccine types with least significant difference (LSD) post-hoc test.
3. Results
3.1. Participants (Demographics)
A total of 505 practitioners participated, however, there were only seven participants who received one of the following vaccines: Moderna, Johnson and Johnson, and Sputnik vaccines. Hence these were excluded leaving a total of 498 participants who comprised the study sample. These were 150 (30.1%) males and 348 (69.9%) females with age range of 22–71 years (mean age = 35.75 ± 11.74 years). Participants were: 266 (53.4%) dentists and 232 (46.6%) physicians. They were practicing in Saudi Arabia (N = 69,13.9%), and Jordan (N = 421,84.5%). However, eight participants (1.6%) practiced outside these two countries.
3.2. Participants (Medical history)
A total of 162 participants (32.5%) indicated presence of systemic illnesses. The most frequently cited diseases were anemia, diabetes mellitus (DM), allergy, thyroid disorders, and hypertension. The frequencies of systemic diseases in the sample are presented in Figure 1. A total of 134 (26.9%) indicated they were taking at least one medication for their illnesses. Data of weight and height indicated that 272 (54.6%) were overweight or obese.
Figure 1.
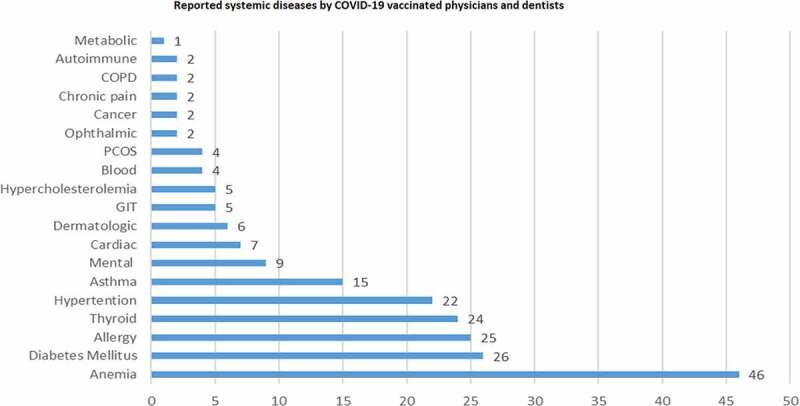
Systemic diseases frequencies reported by COVID-19 vaccinated physicians and dentists. GIT: gastrointestinal disorders; PCOS: polycystic ovarian syndrome; COPD: chronic obstructive pulmonary disease.
3.3. Reported long-term adverse events (LTAE) after vaccine administration
Three vaccines were received by the study sample, as follows: PB vaccine (N = 241, 48.4%), SP vaccine (N = 158, 31.7%), and AZ vaccine (N = 91, 18.3%), AZ (first dose) and PB as the second dose (N = 8, 1.6%). Third dose was received by: 83 (16.7%) (PB); 15 (3.0%) (SP); and 5 (1.0%) (AZ). There were 3 subjects who took Sinopharm first and received one of the other two vaccines as a second dose; however, those were not included due to the small number.
3.4. Long-term adverse events (LTAEs)
A total of 80 participants reported at least one LTAE (16.1%), and these were distributed as follows: one LTAE reported by 71 (14.3%) subjects; two LTAEs reported by 4 subjects (.8%), 3 LTAEs reported by 3 (.6%) subjects and 4 LTAEs reported by 2 (.4%) subjects. This sub-sample had an age range of 23–67 years (mean = 36.6 ± 11.0 years), and most of them were females (N = 59, 73.8%) and healthy (N = 57, 71.3%) with no systemic conditions.
A total of 17 (3.4%) subjects contracted COVID-19 infection after their first vaccination dose. Also 41 (8.2%) contracted the disease after second dose. None of the 17 subjects who indicated infection with COVID-19 after first dose reported symptomatic infection, however, 33 (78.5%) of the 41 subjects who contracted the infection after second dose reported symptomatic infection.
The most frequently reported LTAE among the whole sample was fatigue (N = 32, 6.4%), and the second most frequently reported LTAE among females was menstrual disturbances which were reported by 15 out of 314 menstruating women (N = 15, 4.8%). Adverse events that were reported only once include: anorexia, dry mouth, periodontitis, body malodor, herpes zoster, hypertension, hypothyroidism, low oxygen saturation, pityriasis rosea, taste and smell distortion, thrombosis, bleeding tendency and left arm paresthesia, with the latter 3 LTAEs being reported in recipients of PB and SP vaccines. Adverse events that were reported by more than one participant are presented in Figure 2.
Figure 2.
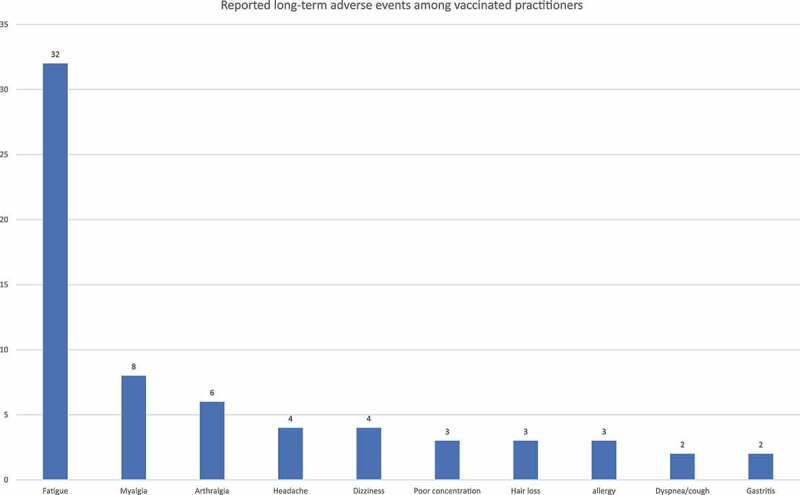
Most frequently reported long-term adverse events among physicians and dentists vaccinated with COVID-19 vaccines.
Cross-tabulation showed that there were no significant association of reported LTAEs with: gender, age groups, history of systemic diseases (or with individual systemic diseases) or types of vaccines (P > .05) (Tables 1–2). Moreover, medication history was not significantly associated with any of reported LTAEs (P > .05).
Table 1.
Cross tabulation of adverse events with age, gender, systemic disease category, and individual systemic diseases
| Long-term adverse events | P-value | |
|---|---|---|
| Gender | ||
| Male (N = 150) | 21 (14.0%) | 0.410 |
| Female (N = 348) | 59 (17.0%) | |
| Age category (years) | ||
| <40 (N = 333) | 52 (15.6%) | 0.699 |
| ≥40 (N = 165) | 28 (17.0%) | |
| Systemic diseases | ||
| Yes (N = 162) | 52 (15.5%) | 0.607 |
| No ( = 336) | 28 (17.3%) | |
| BMI categories | ||
| Normal (N = 226) | 38 (16.8%) | 0.678 |
| Overweight/Obese (N = 272) | 42 (15.4%) | |
| Anemia | ||
| No (452) | 70 (15.5%) | 0.271 |
| Yes (N = 46) | 10 (21.7%) | |
| Diabetes mellitus | ||
| No (472) | 78 (16.5%) | 0.232 |
| Yes (N = 26) | 2 (7.7%) | |
| Allergy | ||
| No (473) | 77 (16.3%) | 0.570 |
| Yes (N = 25) | 3 (12.0%) | |
| Thyroid disorders | ||
| No (474) | 73 (15.4%) | 0.073 |
| Yes (N = 24) | 7 (29.2%) | |
| Hypertension | ||
| No (476) | 76 (16.0%) | 0.782 |
| Yes (N = 22) | 4 (18.2%) | |
| Asthma | ||
| No (483) | 80 (16.6%) | 0.085 |
| Yes (N = 15) | 0 (.0%) |
Table 2.
Cross tabulation of gender, age groups and full vaccine protocol type with reported individual long-term adverse events
| Gender |
Age |
Vaccine |
||||||
|---|---|---|---|---|---|---|---|---|
| Male (n=150) | Female (n=348) | <40 (n=333) | ≥40 (n=165) | PB (n=241) | AZ (n=91) | SP (n=158) | AZ & PB (n=8) | |
| Headache | 2 (1.3%) | 2 (.6%) | 4 (1.2%) | 0 (.0%) | 1 (.4%) | 1 (1.1%) | 2 (1.3%) | 0 (0%) |
| P value | 0.384 | 0.158 | 0.797 | |||||
| Myalgia | 4 (2.7%) | 4 (1.1%) | 7 (2.1%) | 1 (.6%) | 3 (1.2%) | 1 (1.1%) | 4 (2.5%) | 0 (0%) |
| P value | 0.217 | 0.211 | 0.746 | |||||
| Dizziness | 1 (.7%) | 3 (.9%) | 3 (.9%) | 1 (.6%) | 0 (.0%) | 0 (.0%) | 4 (2.5%) | 0 (0%) |
| P value | 0.823 | 0.729 | 0.38 | |||||
| Fatigue | 12 (8.0%) | 20 (5.7%) | 21 (6.3%) | 11 (6.7%) | 11 (4.6%) | 5 (5.5%) | 15 (9.5%) | 1 (12.5%) |
| P value | 0.347 | 0.877 | 0.217 | |||||
| Poor concentration | 1 (.7%) | 2 (.6%) | 3 (.9%) | 0 (.0%) | 1 (.4%) | 2 (2.2%) | 0 (0%) | 0 (0%) |
| P value | 0.903 | 0.221 | 0.159 | |||||
| Arthralgia | 1 (.7%) | 5 (1.4%) | 5 (1.5%) | 1 (.6%) | 2 (.8%) | 1 (1.1%) | 3 (1.9%) | 0 (0%) |
| P value | 0.470 | 0.389 | 0.811 | |||||
| Dyspnea/cough | 0 (.0%) | 2 (.6%) | 1 (.3%) | 1 (.6%) | 2 (.8%) | 0 (0%) | 0 (0%) | 0 (0%) |
| P value | 0.352 | 0.612 | 0544 | |||||
| Hair loss | 1 (.7%) | 2 (.6%) | 2 (.6%) | 1 (.6%) | 3 (1.2%) | 0 (0%) | 0 (0%) | 0 (0%) |
| P value | 0.903 | 0.994 | 0.359 | |||||
| Allergy | 0 (.0%) | 3 (.9%) | 2 (.6%) | 1 (.6%) | 1 (.4%) | 0 (0%) | 2 (1.3%) | 0 (0%) |
| P value | 0.254 | 0.994 | 0.610 | |||||
It was noticed that the most frequently reported symptoms were fatigue and related symptoms (myalgia, arthralgia, headache, and dizziness). Data of participants who had any of these symptoms were compiled and analyzed (N = 47, 9.4%). This group of participants had a mean age of: 35.91 ± 11.26 years, which was not significantly different from mean age of remainder of participants (P = .921). There were no statistically significant differences in the occurrence of fatigue-related symptoms among age groups, genders, date of vaccination or medical history (P > .05) (Table 3.). However, these symptoms were significantly reported more by recipients of SP vaccine (N = 23, 48.9%) (P = .048) (Table 3).
Table 3.
Cross tabulation of vaccine type for the full vaccination protocol, date of vaccination, gender, age groups, systemic disease category, individual systemic diseases, and medication history with occurrence of fatigue and fatigue-related adverse events among vaccinated physicians and dentists
| Sample characteristics | Subjects (N, %) | P value |
|---|---|---|
| Gender | ||
| Males | 17 (36.2%) | 0.342 |
| Females | 30 (63.8%) | |
| Vaccine (protocol) | ||
| PB (n = 241) | 15 (31.9%) | 0.048* |
| AZ (n = 91) | 8 (17.1%) | |
| SP (n = 158) | 23 (48.9%) | |
| AZ-PB (n = 8) | 1 (2.1%) | |
| Age category (years) | ||
| <40 | 33 (9.9%) | 0.609 |
| ≥40 | 14 (8.5%) | |
| Systemic diseases | ||
| Yes | 13 (27.7%) | 0.454 |
| No | 34 (72.3%) | |
| Asthma | ||
| No | 47 (100%) | 0.204 |
| Yes | 0 (.0%) | |
| Anemia | ||
| No | 43 (9.5%) | 0.857 |
| Yes | 4 (8.7%) | |
| Diabetes mellitus | ||
| No | 45 (95.7%) | 0.755 |
| Yes | 2 (4.3%) | |
| Thyroid disorders | ||
| No | 46 (97.9%) | 0.365 |
| Yes | 1 (2.1%) | |
| Autoimmune disease | 0.647 | |
| No | 47 (100%) | |
| Yes | 0 (.0%) | |
| Mental disorders | 0.186 | |
| No | 45 (95.7%) | |
| Yes | 2 (4.3%) | |
| Allergy | ||
| No | 46 (97.9%) | 0.340 |
| Yes | 1 (2.1%) | |
| Cardiac | ||
| No | 46 (97.9%) | 0.659 |
| Yes | 1 (2.1%) | |
| Hypertension | ||
| No | 46 ((97.9%) | 0.422 |
| Yes | 1 2.1%) | |
| BMI categories | 0.411 | |
| Normal | 24 (51.1%) | |
| Overweight/Obese | 23 (48.9%) | |
| Medications use | 0.823 | |
| No | 35 (74.5%) | |
| Yes | 12 (25.5%) |
*: Statistically significant, BMI: Body mass index
General multivariate linear model analysis (MANOVA) was carried out to investigate LTAE across levels of vaccination regimens. Results indicated a Pillaiʻs test value = .08646 and F = . 74,656 that was not significant (P = .920). Canonical correlation (partial Eta) was . 25,376 and partial Eta square (or effect size) was .06439. Only: categorical presence or absence of LTAE, fatigue related AE and dizziness were the only factors to show significant differences at P = .012, .048, and .034, respectively. These were investigated more by post hoc test.
Investigated variables included: fatigue-related AEs (fatigue, myalgia, dizziness, poor concentration, arthralgia), and other AEs including dyspnea, anorexia, menstrual disturbances, gastrointestinal disturbances, and thrombosis. Table 4 shows the only significant differences in LSD (least significant difference) post-hoc test (fatigue, fatigue-related AEs, dizziness, poor concentration), as all nonsignificant findings are not included in the table.
Table 4.
LSD post hoc tests of MANOVA of adverse events of different vaccine protocols showing only significant differences
| |
(I) Vaccine regimen | (J) Vaccine regimen | Mean Difference (I-J) | Std. Error | Sig. | 95% Confidence Interval |
|
|---|---|---|---|---|---|---|---|
| Dependent Variable | Lower Bound | Upper Bound | |||||
| Fatigue-related AEs | PB | SP | −.08* | .030 | .005 | −.14 | −.02 |
| Dizziness | AZ | SP | −.03* | .012 | .031 | −.05 | .00 |
| Fatigue | PB | SP | −.05* | .025 | .050 | −.10 | .00 |
| Poor concentration | AZ | SP | .02* | .010 | .031 | .00 | .04 |
Based on observed means. The error term is Mean Square (Error) = .002.
*The mean difference is significant at the .05 level.
4. Discussion
This study was conducted at a critically challenging stage of COVID-19 pandemic. October and November 2021 were marked by several important events such as expanding emergency use authorization of the PB COVID-19 vaccine to include 5–11-year-old children and expanding eligibility for COVID-19 vaccine boosters to vaccine recipients who are ≥18 years.12 Moreover, during this period the newly emerging variant of SARS-CoV-2, Omicron, was considered by the WHO as a “variant of concern.”13 Health authorities including the CDC and WHO remain, understandably, adamant that vaccines are the effective method for reducing severe disease outcomes including hospitalization and mortality. Public trust in vaccines can be strengthened by reassuring them about the benign nature of vaccine side effects. Whereas the public trust in politicians may sometimes be wavering,14 it appears that a reliable method is to obtain data from vaccinated healthcare workers such as physicians and dentists.5 Scientists believe that there are several factors that point to the safety of COVID-19 vaccines particularly mRNA vaccines such as: rapid degradability and elimination from the body and nature of side effects.15 However, more data are needed to support these beliefs.
Several reports have covered acute side effects of vaccines; however, data on the LTAEs are still scarce. Investigating LTAEs of vaccines is a critical issue that warrants collecting data on several related factors that are eligible confounders in the development of side effects or adverse events in general. This study addressed several factors that may be implicated including systemic diseases, medication intake and BMI. The study results showed that approximately 16% of vaccinated physicians and dentists developed LTAEs after receiving at least two doses of the PB, SP, or AZ vaccines. Several LTAEs were reported in this study with variable frequencies, however, a great majority of these events do not seem to pose any serious risk. Some AEs can be explained such as: thrombosis, paresthesia, and herpes zoster, while others are difficult to explain such as: periodontitis (a chronic inflammatory condition), anorexia or body malodor.
The most reported LTAE was fatigue, and its related symptoms of myalgia, arthralgia, dizziness, and headache. These symptoms could have a multifactorial etiology; therefore, statistical analysis was conducted on several possible confounders such as age, gender, and underlying systemic conditions that could predispose to fatigue such as anemia, obesity, and DM among others. All these factors were not found significantly associated with fatigue symptoms. The study also investigated post-vaccination, COVID-19 infection and it was found that, all participants who developed COVID-19 after vaccination whether after first (N = 17) or second dose (N = 42), did not develop long-term manifestations except for five participants who developed either fatigue, menstrual abnormalities, or memory problem. While this confirms that the low incidence of contracting COVID-19 after vaccination is in concordance with the reported efficacy of these vaccines, it also shows that fatigue and related symptoms are most probably not related to COVID-19 infection among this sample. On the other hand, post vaccination COVID-19 infection justifies the maintenance of additional precautionary measures such as distancing and the use of face masks to reduce infection rates.16 The study also analyzed a possible association with vaccine factors such as type, and date of vaccination. The only significant factor was vaccine type as approximately one in two of participants complaining of fatigue and related symptoms have received the full vaccination protocol of SP vaccine. Dizziness was also significantly associated with SP vaccine. When explaining the possible association between a particular vaccine with symptoms of fatigue, caution should be practiced. Fatigue that develops after vaccination is basically triggered by activation of the innate immune system.17 Fatigue, headache, and dizziness have also been reported previously among recipients of human papilloma virus (HPV) vaccines, however, the possible psychological influence of antivaccine media movement in raising suspicions about vaccine safety was highlighted in this case.18 Psychological factors are not only implicated in impairing the immune response to vaccines but are also implicated in the prevalence and severity of vaccine-related side effects.19 Moreover, mild depressive symptoms and chronic stress were identified as factors that prolong inflammatory responses after influenza and pneumococcal pneumonia vaccines in older adults.20,21 Further, some vaccines contain aluminum adjuvant,1 which was blamed for causing myalgia and fatigue following immunization; however, reliable vaccine sources confirm that aluminum salts have been used safely in vaccines since the 1930s,22 and no firm causal relationship between persistent fatigue and aluminum adjuvants was ever established.23 Within the context of COVID-19 vaccines, myalgia and arthralgia persisting for approximately six weeks were noticed among a group of older adults who received AZ vaccine in Korea.24 Headache, on the other hand, may be a delayed presentation associated with thrombotic complications in recipients of adenovirus-based vaccines such as AZ.25 In this study all subjects complaining of headache were younger than 40 years and headache was associated with all three types of vaccines, nonetheless, none complained of thrombosis, which may again highlight the role of immune activation in development of headache and other fatigue related symptoms. Furthermore, among participants who reported thrombosis, bleeding tendency and left arm paresthesia in this study, none received AZ vaccine. Poor concentration, on the other hand, was the only significantly associated AE with AZ vaccine. More research is needed to investigate the association of particular vaccines such as SP vaccine with LTAEs especially that more countries now are authorizing the use of these vaccines.
Finally, this study showed that approximately 5% of females in the reproductive age reported menstrual abnormalities. Menstrual changes have been reported after receiving both mRNA and adenovirus vector covid-19 vaccines; however, menstrual abnormalities were temporary and short-lived, and no adverse effects were noticed concerning fertility.26 The use of other vaccines has also been associated with menstrual changes such as HPV vaccine.27 This is attributed to immune activation which could also affect SARS-CoV-2 infected individuals. A recent study reported that a larger percentage of menstruating women infected with COVID-19 (25%) have also experienced menstrual disruption.28
The study has limitations. It was cross-sectional and was based on self-reported data that could be influenced by participantʻs prior prejudice and misinformation about vaccines. In addition, it was not possible to estimate response rates among individual professional groups of dentists and physicians. Moreover, although the term “long-term adverse events” was clearly defined within this previously validated questionnaire, it was not possible to ensure that all participants have a sufficient understanding of this definition. Nevertheless, it is plausible to say that data provided by participants were accurate and transparent taking into consideration their medical background and relatively long postvaccination tracking period spanning approximately 10 months. Only three vaccines were explored in this study; however, these vaccines are among the first vaccines to be authorized and to be used on a wide scale globally. Tracking of vaccine safety should continue for longer durations of time, and information about other vaccines currently in use, should also be provided.
5. Conclusions
Approximately one in six of COVID-19 vaccine recipients may complain of long-term adverse events. These are mostly fatigue related. The results of this study indicated a wide variability of incidence and nature of LTAE among the three platform-based vaccines. Among these vaccines Pfizer-BioNtech vaccine seems to be the least associated with LTAEs. Future large-scale studies are warranted to investigate the possible role of psychological factors and media effects in determining perceptions toward vaccine adverse effects.
Funding Statement
The author(s) reported there is no funding associated with the work featured in this article.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
Data supporting this study can be obtained upon request from corresponding author.
Institutional review board statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Taibah University, College of Dentistry Ethics Committee, IRB# TUCDREC/01122021/NDar-Odeh.
Informed consent statement
Informed consent was obtained from all subjects participating in the study.
References
- 1.COVID-19 Vaccine (Vero Cell), Inactivated (Sinopharm). [accessed 2021 Dec 3]. file:///C:/Users/PC/Downloads/v.3_21195_Sinopharm-vaccine-explainer-24.pdf.
- 2.Baraniuk C What do we know about China's covid-19 vaccines? BMJ. 2021;373:n912. doi: 10.1136/bmj.n912. [DOI] [PubMed] [Google Scholar]
- 3.Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, Perez JL, Pérez Marc G, Moreira ED, Zerbini C, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383:2603–7. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Voysey M, Clemens S, Madhi SA, Weckx LY, Folegatti PM, Aley PK, Angus B, Baillie VL, Barnabas SL, Bhorat Q, et al. Safety and efficacy of the ChAdox1 nCov-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397:99–111. doi: 10.1016/S0140-6736(20)32661-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abu-Hammad O, Alduraidi H, Abu-Hammad S, Alnazzawi A, Babkair H, Abu-Hammad A, Nourwali I, Qasem F, Dar-Odeh N.. Side effects reported by Jordanian healthcare workers who received COVID-19 vaccines. Vaccines. 2021;9(6):577. doi: 10.3390/vaccines9060577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dzieciolowska S, Hamel D, Gadio S, Dionne M, Gagnon D, Robitaille L, Cook E, Caron I, Talib A, Parkes L, et al. Covid-19 vaccine acceptance, hesitancy, and refusal among Canadian healthcare workers: A multicenter survey. Am J Infect Control. 2021;49(9):1152–57. Epub 2021 Apr 28. doi: 10.1016/j.ajic.2021.04.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Emanuel EJ, Skorton DJ. Mandating COVID-19 vaccination for health care workers. Ann Intern Med. 2021;174(9):1308–10. Epub 2021 Jul 30. doi: 10.7326/M21-3150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mbunge E, Fashoto SG, Akinnuwesi B, Metfula A, Simelane S, Ndumiso N. Ethics for integrating emerging technologies to contain COVID-19 in Zimbabwe. Hum Behav Emerg Technol. 2021;11. doi: 10.1002/hbe2.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Megget K. Even covid-19 can't kill the anti-vaccination movement. BMJ. 2020;369:m2184. doi: 10.1136/bmj.m2184. [DOI] [PubMed] [Google Scholar]
- 10.Tafuri S, Martinelli D, Prato R, Germinario C. Dalla lotta per la libertà alla negazione dellʻevidenza: storia dei movimenti anti-vaccinisti in Europa [from the struggle for freedom to the denial of evidence: history of the anti-vaccination movements in Europe]. Ann Ig. 2011;23:93–99. [PubMed] [Google Scholar]
- 11.Riad A, Schünemann H, Attia S, Peričić TP, Žuljević MF, Jürisson M, Kalda R, Lang K, Morankar S, Yesuf EA, et al. COVID-19 vaccines safety tracking (CoVast): protocol of a multi-center prospective cohort study for active surveillance of COVID-19 vaccines, side effects. Int J Environ Res Public Health. 2021;18(15):7859. doi: 10.3390/ijerph18157859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.U.S. Food & Drug Administration. Comirnaty and Pfizer-BioNTech COVID-19 Vaccine. Washington (DC). https://www.fda.gov/emergency-preparedness-and-response/coronavirus-disease-2019-covid-19/comirnaty-and-pfizer-biontech-covid-19-vaccine.
- 13.World Health Organization. Episode #64 - Why are experts concerned about Omicron? Geneva (Switzerland); [accessed 2021 Nov 30]. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/media-resources/science-in-5/episode-64---why-are-experts-concerned-about-omicron?gclid=Cj0KCQiAu62QBhC7ARIsALXijXSUUyuINe9suSbLc6ekSpdmyE-A-MlMRYFk3ayHwuYGaDzPavgx5iMaAmJsEALw_wcB.
- 14.Mendoza RU, Dayrit MM, Alfonso CR, Ong MMA. Public trust and the COVID-19 vaccination campaign: lessons from the Philippines as it emerges from the Dengvaxia controversy. Int J Health Plann Manage. 2021;36(6):2048–55. Epub 2021 Aug 19. doi: 10.1002/hpm.3297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Centers for Disease Control and Prevention, National Center for Immunization and Respiratory Diseases. Possible Side Effects After Getting a COVID-19 Vaccine. Washington (DC). [accessed 2021 Nov 30]. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/expect/after.html.
- 16.Elsayed S, Abu-Hammad O, Alolayan A, Althagafi N, Ayed Y, Eldeen YS, Dar-Odeh N. Getting to know SARS-CoV-2: towards a better understanding of the factors influencing transmission. Pesqui Bras Odontopediatria Clin Integr. 2020;20:1–7. [Google Scholar]
- 17.Sampath V, Rabinowitz G, Shah M, Jain S, Diamant Z, Jesenak M, Rabin R, Vieths S, Agache I, Akdis M, et al. Vaccines and allergic reactions: the past, the current COVID-19 pandemic, and future perspectives. Allergy. 2021;76(6):1640–60. Epub 2021 Jun 4. doi: 10.1111/all.14840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ward D, Thorsen NM, Frisch M, Valentiner-Branth P, Mølbak K, Hviid A. A cluster analysis of serious adverse event reports after human papillomavirus (HPV) vaccination in Danish girls and young women, September 2009 to August 2017. Euro Surveill. 2019;24(19):1800380. doi: 10.2807/1560-7917.ES.2019.24.19.1800380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Madison AA, Shrout MR, Renna ME, Kiecolt-Glaser JK. Psychological and behavioral predictors of vaccine efficacy: considerations for COVID-19. Perspect Psychol Sci. 2021;16(2):191–203. Epub 2021 Jan 27. doi: 10.1177/1745691621989243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Glaser R, Robles TF, Sheridan J, Malarkey WB, Kiecolt-Glaser JK. Mild depressive symptoms are associated with amplified and prolonged inflammatory responses after influenza virus vaccination in older adults. Arch Gen Psychiatry. 2003;60(10):1009–14. doi: 10.1001/archpsyc.60.10.1009. [DOI] [PubMed] [Google Scholar]
- 21.Glaser R, Sheridan J, Malarkey WB, MacCallum RC, Kiecolt-Glaser JK. Chronic stress modulates the immune response to a pneumococcal pneumonia vaccine. Psychosom Med. 2000;62(6):804–07. doi: 10.1097/00006842-200011000-00010. [DOI] [PubMed] [Google Scholar]
- 22.Centers for Disease Control and Prevention, National Center for Immunization and Respiratory Diseases. Adjuvants and Vaccines. Washington (DC). [accessed 2021 Dec 4]. https://www.cdc.gov/vaccinesafety/concerns/adjuvants.html.
- 23.Löffler P. Review: vaccine Myth-Buster - cleaning up with prejudices and dangerous misinformation. Front Immunol. 2021;12:663280. doi: 10.3389/fimmu.2021.663280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hyun H, Song JY, Seong H, Yoon JG, Noh JY, Cheong HJ, Kim WJ. Polyarthralgia and Myalgia Syndrome after ChAdox1 nCOV-19 vaccination. J Korean Med Sci. 2021;36(34):e245. doi: 10.3346/jkms.2021.36.e245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.García-Azorín D, Do TP, Gantenbein AR, Hansen JM, Souza MN, Obermann M, Pohl H, Schankin CJ, Schytz HW, Sinclair A, et al. Delayed headache after COVID-19 vaccination: a red flag for vaccine induced cerebral venous thrombosis. J Headache Pain. 2021;22:108. doi: 10.1186/s10194-021-01324-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Male V. Menstrual changes after covid-19 vaccination. BMJ. 2021;374:n2211. doi: 10.1136/bmj.n2211. [DOI] [PubMed] [Google Scholar]
- 27.Suzuki S, Hosono A. No association between HPV vaccine and reported post-vaccination symptoms in Japanese young women: results of the Nagoya study. Papillomavirus Res. 2018. ;5:96–103. Epub 2018 Feb 23. doi: 10.1016/j.pvr.2018.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li K, Chen G, Hou H, Liao Q, Chen J, Bai H, Lee S, Wang C, Li H, Cheng L, et al. Analysis of sex hormones and menstruation in COVID-19 women of child-bearing age. Reprod Biomed Online. 2021;42(1):260–67. Epub 2020 Sep 29. doi: 10.1016/j.rbmo.2020.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data supporting this study can be obtained upon request from corresponding author.


