ABSTRACT
Autoimmune diseases are caused when immune cells act against self-protein. This biological self–non-self-discrimination phenomenon is controlled by a distinct group of lymphocytes known as regulatory T cells (Tregs), which are key inflammatory response regulators and play a pivotal role in immune tolerance and homeostasis. Treg-mediated robust immunosuppression provides self-tolerance and protection against autoimmune diseases. However, once this system fails to operate or poorly operate, it leads to an extreme situation where immune system reacts against self-antigens and destroys host organs, thus causing autoimmune diseases. Tregs can target both innate and adaptive immunity via modulating multiple immune cells such as neutrophils, monocytes, antigen-presenting cells, B cells, and T cells. This review highlights the Treg-mediated immunosuppression, role of several markers and their interplay during Treg development and differentiation, and advances in therapeutic aspects of Treg cells to reduce severity of autoimmunity-related conditions along with emphasizing limitations and challenges of their usages.
KEYWORDS: Autoimmunity, autoimmune diseases, Foxp3, regulatory T cell (Treg), self versus non-self, Treg therapy
Introduction
Autoimmune diseases are no longer neglected and selected practitioners treat them using immune therapy. Autoimmune disorders, which lead to chronic illnesses, are one of the leading causes of death. The primary cause of autoimmune disorders is unknown; however, a lack of proper immunological response is a general cause of all autoimmune conditions.1 The development of autoimmune diseases is a subject of scientific debate with several tricky questions and incomplete answers to be addressed appropriately. Presently, the numbers of cases of autoimmune diseases have increased. However, while all individuals do not suffer from autoimmune diseases, a considerable population contract some form of this disease. Autoimmune diseases are long-term diseases initiated due to an autoimmune state of affairs that leads to the destruction of organs due to humoral and cell-mediated immune mechanisms. Each autoimmune disorder is unique in nature. In autoimmune disease, the most affected parts involve joints in rheumatoid arthritis (RA), pancreatic cells in type 1 diabetes mellitus (T1D), skin, kidney, serous membranes in systemic lupus erythematosus (SLE), mucosal cells of intestine in inflammatory bowel disease and hepatic cells in autoimmune hepatitis. Autoimmune diseases, such as multiple sclerosis, systemic lupus erythematosus, Guillain–Bare syndrome, and Crohn’s disease, which cause chronic immune response against host cells, are some of the most problematic and challenging disease conditions handled by general practitioners. Though success is uncertain, all efforts are given to cure the disease with targeted treatment.
Human immune system recognizes and reacts against foreign protein, but rarely toward its own (self). The immune system sustains this great responsibility because incidentally originating self-reacting lymphocytes are removed by a process known as programmed cell death, also known as apoptosis, beginning from the embryonic stage. However, we cannot expect all self-reactive lymphocytes to be eliminated as the process to distinguish self from non-self is not 100% full proof. Under physiological selection processes, some self-reacting lymphocytes still evade apoptosis, which may affect in the future. Similarly, it is also difficult to explain why a large number of people never contract autoimmune disease in the presence of autoreactive lymphocytes in circulation, while a few individuals definitely suffer and occasionally respond to clinical treatments.2 Adequate justification has never been delivered to address this complex issue. However, regulatory T cells (Tregs) have been reported to significantly control this auto reactivity and reduce the adverse effects, so that humans do not suffer from autoimmunity-mediated pathological ailments.
Tregs are a specialized T cell subpopulation with specific regulatory mechanisms that inhibit the core components of adaptive and innate immune responses. Human Tregs are characterized with Forkhead box P3 (Foxp3) protein along with low and high expression of CD45RA marker. Functionally and phenotypically three distinct subpopulations of Tregs are (1) CD45RA+FoxP3 low, resting Tregs (2) CD45RA-FoxP3, high activated Tregs, both are suppressive Tregs and the last one (3) is cytokine secreting CD45RA-FoxP3, low nonsuppressive T cells.3
Tregs also play a crucial role in immune homeostasis and self-tolerance.4,5 As Treg cells with Foxp3 and CD4+ maintain immune homeostasis, several studies have reported that Tregs can help to develop efficient and reliable therapeutic regimens to cure autoimmune disorders and several life-threatening allergic reactions.1,6–10 Furthermore, the efficient functioning of Treg requires the expression of not only Foxp3, but also several other Treg signature genes, including CD25 and cytotoxic T lymphocyte antigen 4 (CTLA-4). Moreover, antigen-specific Tregs efficiently control immune response severity for treating autoimmune disorders.
Whether Treg-based cellular therapies may offer a reliable and productive therapeutic regimen for treating autoimmune diseases remains questionable despite the strong evidence supporting this claim. However, many disagreements and major knowledge gaps, particularly related to the mechanism of Treg suppression, necessitate the need of further detailed studies. This review summarizes current knowledge on several Treg-related biological aspects, focusing on their morphogenesis, modes of action, clinical strategies for isolation and expansion, and therapeutic abilities in treating autoimmune diseases. Furthermore, several challenges have been discussed, which need to be addressed in future studies.
Dispute on T suppressor cells versus Treg cells
There are two schools of thoughts; one supports T suppressor cells, while the other advocates Tregs that control autoimmune disorders. However, proponents of T suppressor cells have now realized that these cells are indeed a type of Tregs. Tregs are a T cell subtype that suppresses inflammatory events. Scientific evidence suggests that Treg deficiency contributes to autoimmunity.11,12
Place and time of Treg cell birth
Differentiation of thymus-derived Tregs (tTregs) relies on TCR signaling, particularly the strength and duration of the signal decide the fate of T cells in circulation.13 These self-reacting non-eliminated T cells remain in the circulation for lifetime, but may not aggressively react. Tregs, like other lymphocyte sets, are generated from multipotent hematopoietic stem cells. During embryonic development in the presence of cytokines and under the guidance of several transcription factors, double positive T cell expressing both CD4 and CD8 undergo extensive positive and negative selection to express either CD4 or CD8 markers. However, few cells acquire distinct phenotypic markers and are designated as Tregs. Thymic stromal cells, including cortical medullary thymic epithelial cells and dendritic cells inside thymus, contribute to Treg differentiation and selection. Additionally, both interleukin (IL)-2 and IL-7 are essential for T cell development in the thymic microenvironment.14 The process of Treg development is a complex phenomenon and has been discussed in detail previously.15 A previous report has indicated that a subset of thymus-derived cells expressing CD4 marker along with high expression of IL-2 receptor alpha (IL-2Rα) chain (CD25) protect thymectomized (surgically removal of thymus) mice from autoimmunity.16 These specialized cells quench adaptive immune response and inhibit the proliferation of other cells, thereby suppressing autoreactive immune responses. Tregs leave the thymus to enter the circulation for patrolling and regulate the autoimmune reaction. Further, several inflammatory cytokines, such as IL-7 and IL-15, are also essential for Treg differentiation, and they appear to partly substitute IL-2, leading to reduced Foxp3 Treg formation.17
Apart from thymus-derived Tregs, extrathymic origin of Tregs has been confirmed. Tregs can still be divided into natural/thymic and effector Tregs.18,19 The anti-inflammatory intensity of naïve Tregs, which have just left the thymus, is inadequate to exert its complete potential. Naive Treg cells propagate as well as develop into the hyper repressive and immunologically differentiated effector Tregs (eTregs) following TCR induction within the lymph node. These eTregs then inhibit antigen-specific maturation of APCs, such as dendritic cells (DCs). Effector Tregs, on the contrary, consume IL-2 via an increased sensitivity to IL-2 receptors and secrete several inhibitory cytokines, such as IL-10, transforming growth factor beta (TGF-β), and IL-35, to inhibit immune system function in an antigen-nonspecific way. In the animal model, antigen-specific Tregs outperform antigen nonspecific Tregs in terms of immune suppression, while antigen nonspecific Treg cells also suppress immune response.20 Therefore, while Tregs suppression is partly antigen nonspecific or non-dependent, antigen-dependent Treg activation affects the immune system effectively.21 Furthermore, the control mechanism of this selection has not been fully discovered yet; however, it can be concluded that two population of Treg cells exists, natural Tregs and induced/adaptive Tregs, which can be generated under the influence of specific cytokines, such as TGF-β and IL-10.22
Tissue-resident Tregs and their possible origin
Tregs develop multiple phenotypes and functions depending on their surroundings and microenvironment due to plasticity. Previous studies have revealed that Tregs reside in several tissues/organs, including the visceral adipose tissue (VAT), colon, skeletal muscle, and skin, in addition to being produced in lymphoid tissues and serve several purposes. Tregs residing in adipose tissue are primarily responsible for maintaining the balance between immunity and metabolism, while Tregs residing in colon tissues sustain intestinal homeostasis and protect the body from harmful microbes. Mostly under inflammatory settings, Tregs present in skeletal muscle cells essentially preserve muscle cell homeostasis and stimulate skeletal muscle repair. Tregs in the skin influence hair regrowth. Even though the phenotypes and activities of tissue-specific Tregs differ, they are all linked to autoimmune disorders.7,23,24 Tregs residing in several physiological niches share fundamental phenotypic markers, perform immunosuppression, and may serve as therapeutic targets for treating specific autoimmune diseases in a given circumstance. On the contrary, Tregs adapt to their surroundings and express tissue-specific factors that play different roles in tissue repair.8,23
Intestinal Tregs are mixed origin as indicated by their considerable phenotypic variability, though >70% colonic Tregs do not possess thymic markers like Helios. Compared to spleen and lymph node Tregs, colonic Tregs exhibit reduced Nrp1 (a critical thymic marker) expression. This indicates that the thymus is not necessary for the origin of intestinal Tregs and implies peripherally derived Treg (pTreg) origin.25,26
Skin Tregs are considered to derive from the thymus that proliferates throughout a particular neonatal timeframe (6–13 post-natal days). Blocking T cell migration from the thymus or lymph nodes during this period with FTY720, a sphingosine-1-phosphate receptor antagonist, ten-fold reduces the cutaneous Treg count. It is also linked to an elevation in thymic Tregs, suggesting that skin Tregs are produced from the thymus. However, the CDR3 sequence in skin Tregs, skin Teffs, and their respective analogs in the spleen or lymph nodes have not been studied thoroughly. Therefore, the origin of skin Tregs remains a mystery.27
Identity mark on Treg
The constitutively expressed transcriptional regulator Foxp3 and CD25 (IL-2Rα) and CTLA4 can be considered as distinguishing cell-specific marker for CD4+ Treg cells. Out of these markers, Foxp3 is more specific, while CD25 and CTLA4 are relatively less specific and also expressed on conventional T cells. However, CD25 and CTLA4 are crucial factors in some Treg-mediated suppression mechanisms that maintain immune homeostasis.28 Foxp3 acts as a transcription factor with a 100 amino acid long conserved DNA binding domain that folds like a winged helix structure, termed the forkhead box, and plays an essential role in self-tolerance maintenance and autoimmune disease prevention by regulating the function of CD4+ Treg cells. These molecules have distinct roles in T cell survival and regulate important biological activities. Cell surface markers, such as CD25, are essential for autocrine IL-2 absorption on its own surface. CTLA4 is involved in Treg-mediated suppression by downregulating CD80 and CD86 on APCs, whereas Foxp3 is essential for high CD25 and CTLA4 expression. Despite the improvement in Treg biology, no exact markers have been designated to characterize human Tregs. However, the identification of a conserved non-coding element within the Foxp3 gene locus using gene sequencing technique has been identified to be most appropriate target for “real” human Tregs.29
Tregs are found in the thymus at 13 weeks of gestation in humans and express forkhead/winged helix family transcription factor Foxp3 protein. These cells are functionally suppressive when co-cultured with Teffs.30 Currently, CD4 and CD25 expression and absence of the IL-7R α-chain (CD127) is considered to be an authentic marker for Treg purification.31
Furthermore, while human T lymphocytes temporarily express Foxp3 in response to TCR stimulus, Foxp3+ T cells are functionally and phenotypically heterogeneous in humans. CD127 is expressed at small concentrations by CD25+ CD4+ Tregs, and some Tregs express CD4, CD25, and CD127. However, CD127 is downregulated in naïve T (nT) cells activated by TCR signaling and Foxp3 expression upregulation, implying that the CD4+ CD25+ CD127+ T cell fraction may be contaminated by a few other stimulated non-Treg cells expressing all the markers.31,32 Recent reports also suggest the absence of CD127 on Tregs; however, it is necessary to identify the exact marker proteins on Tregs to develop effective therapeutic strategies. Moreover, Foxp3-expressing Tregs should be distinguished from conventional Foxp3-expressing T cells. The classification, phenotypes, functional characteristics, and balance of Treg cells in the immune response are shown in Figure 1.
Figure 1.
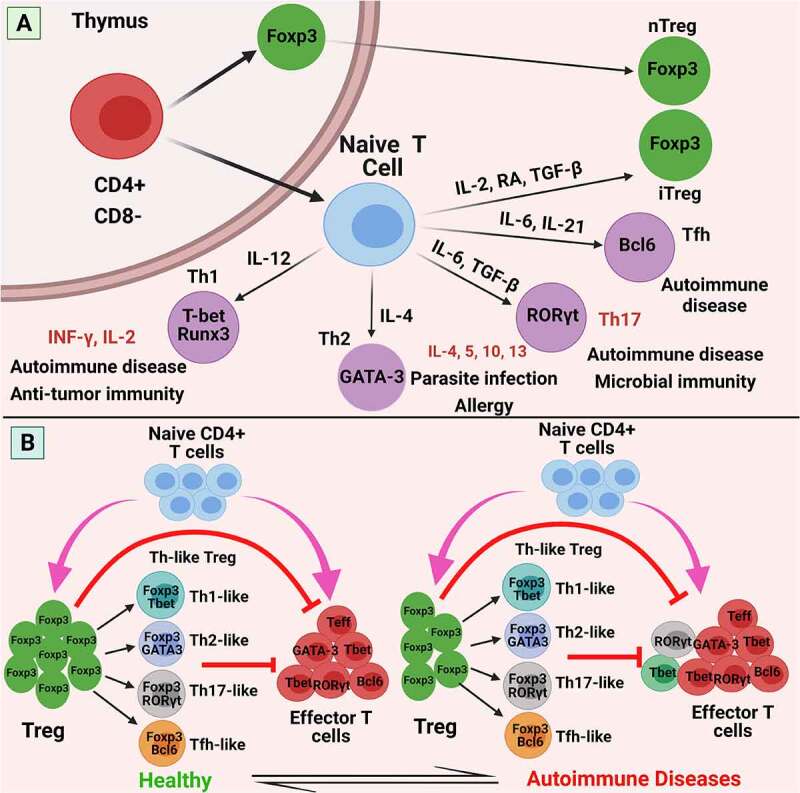
Classification, phenotypes, functional characteristics, and balance of Treg cells in the immune response. (a) Differentiation of naive CD4 + T cells into Tregs or effector T cells. The selection of naïve CD4 + T cells and natural Tregs occurs in the thymus. Naïve CD4 + T cells, subsequently, can differentiate into various different T cell subsets: Th1, Th2, Th17, induced Tregs (iTregs), in the periphery, all heralding distinct immunological functions. These differentiation programs are regulated by different cytokines. The transcription factors T-bet and Runx3, GATA3, or RORγt, are required to differentiate naive T cells into Th1, Th2, or Th17 cells, respectively. For example: T-bet (Th1 cells), GATA3 (Th2 cells), RORγt (Th17 cells), FOXP3 (Tregs). (b) In a healthy individual, the immune system is under regular homeostasis, can suppress autoreactive effector T cells and control a fine balance (left). Aberrant Treg plasticity, quantitative and functional deficiencies of Treg impair immune homeostasis and result in autoimmune diseases (right). Abbreviations: nTreg: natural Treg; iTreg: induced Treg; FOXP3: forkhead Box P3; RA: retinoic acid; CD: cluster of differentiation; IFN: interferon; IL: interleukin; RORγt: retinoid related orphan receptor γ; T-bet: T box transcription factor; TCR: T cell receptor; TGF-β: transforming growth factor-β; Th: T helper cell; GATA3: GATA-binding factor 3; Tfh: follicular helper T cells; Bcl: B-cell lymphoma 2. Figure was designed by Biorender.com program (https://biorender.com/). Accessed on 13 April 2021.
Experimental evidence of Treg-mediated suppression
Treg-mediated immunosuppression involves several immune-suppressive mechanisms, including CTLA4-mediated APC suppression, IL-2 consumption, and immunosuppressive cytokine and metabolite production.28 Furthermore, the Treg-mediated inhibitory pathways impeding various immune cells have been classified as direct pathways, where Tregs induce a direct response on the immune cell, and indirect pathways, under which another cell or molecule is influenced, which in turn inhibit the immune cells. The release of various cytokines, including IL-10, TGF-β, and IL-35, as well as the synthesis of lytic enzymes such as granzyme and perforin, causes target immune cell death, usually by apoptosis. In addition, Tregs directly suppress target cells by expressing CD39/CD73, which in turn reduces extracellular ATP concentration by producing adenosine and AMP.33 Tregs, on the contrary, impact changes in the microenvironment due to their increased CD25 expression. Tregs with increased IL-2 receptor expression will bind to the IL-2, effectively depriving the neighboring tissues.34 While determining the mechanism of Treg-mediated suppression, it is necessary to mention that different interventions are used selectively by the wide range of Treg-related target cells and microenvironments.35 In this context, Treg-mediated suppression mechanisms to maintain homeostasis in the immune response have been discussed in detail.
Several findings indicate that immunosuppression is caused by the direct interaction of Tregs with effector T and B cells or suppressive cytokine secretion. It has been observed that Tregs suppress CD4+ T cell activation and proliferation both contact-dependently and -independently. Co-culturing Tregs (CD4+, CD25+) with APCs and other antigen-specific responder T cells (CD4+ and CD8+) in presence of specific antigen, suppresses responder cell proliferation and reduced IL-2 production. Tregs expressing increased IL-2 receptor on its surface bind to the IL-2 produced from responding T cells, thereby preventing their proliferation.
Tregs may interfere with the antigen-presenting ability of DCs or promote the secretion of suppressive factors, such as indolamine 2,3 dioxygenase (IDO), a potent immunosuppressive enzyme, from DC. IDO induction is dependent upon the high expression of CTLA4, an inhibitory receptor on Treg cell. The mechanism is complex and the conclusive effect of CTLA4 is difficult to prove. Blocking CTLA4 does not affect Tregs either in vitro or in vivo. Tregs isolated from CTLA4-deficient mice have a suppressive effect similar to that of wild mice. On the contrary, in vivo CTLA4 activity blocking in mice can abrogate Treg-mediated suppression of inflammatory bowel disease (IBD), autoimmune gastritis, and transplantation.36 Two Treg subpopulation, naïve (nTreg) and others effective (eTreg), are operational in the body. The nTregs express increased levels of immunosuppressive molecules, particularly IL-10.37 Treg cell depletion acts as an adjuvant effect to enhance DCs and hyperactivity is indicated, speculated for check and balance activity. Treg distribution varies according to the physiological requirements in peripheral location, being abundant in the gut mucosa, where tolerance to residential normal microbiota is essentially required.38,39
Tregs inhibit autoreactive B cell ability to develop detrimental autoantibodies, which also destroys autoreactive B cells. Several markers, such as PD-1 expression on autoreactive B cells and the two PD-1 ligands (PDL-1 and −2) expression on Treg cells, are necessary for autoreactive B cell inhibition.40 Furthermore, Tregs also use granzyme B (granular enzyme) and perforin (cause perforation) to destroy effector B cells, which in turn reduces autoantibody production.41
It is well-established that NK cells, which are innate lymphocytes, destroy tumor cells and remain vigilant throughout human life to reduce tumor burden. Unfortunately, Tregs suppress NK cell activity, resulting in tumor progression. Neutrophil dysfunction results in sustained inflammation and autoimmune disease progression.42 Direct interaction of Tregs on polymorphs limits granulocyte infiltration by suppressing the expression of chemokines that act as chemoattractants. Co-culturing neutrophils with Tregs under culture vessel enhances IL-10 and IL-6 production, which are considered to be immunosuppressive.43 In addition, Tregs promote heme oxygenase-1 and IDO expression, and cytokine signaling three molecule (SOCS3) inhibition.43 These findings confirm that human Tregs are effective in preventing graft-versus-host disease (GVHD) and autoimmune diseases, while delaying graft rejection.35
Tregs work specifically on monocytes, preventing their differentiation, cytokine secretion, and antigen expression. Following co-culture with Tregs, monocytes display classic M2 macrophage characteristics such as increased CD206 (mannose scavenger receptor) and CD163 (hemoglobin scavenger receptor) expression, as well as a reduced capacity to respond to pro-inflammatory stimuli as evidenced by decreased IL-6 and TNF-β production and NF-κB activation.44 Ex vivo extended Tregs are effective at skewing monocytes toward a tolerogenic phenotype. Compared to monocytes co-cultured freshly isolated Tregs, those co-cultured with expanded Tregs have a lower capacity to increase harmful IL-17-emitting T cells.45 The suppression mechanism correlates with the reduced CD86 expression by Treg-conditioned monocytes.
These immunosuppressive mechanisms should be explored in the future to establish the immunosuppressive mechanisms of Tregs under various other autoimmune disorders. The mechanisms underlying Treg-mediated immunosuppression is shown in Figure 2.
Figure 2.
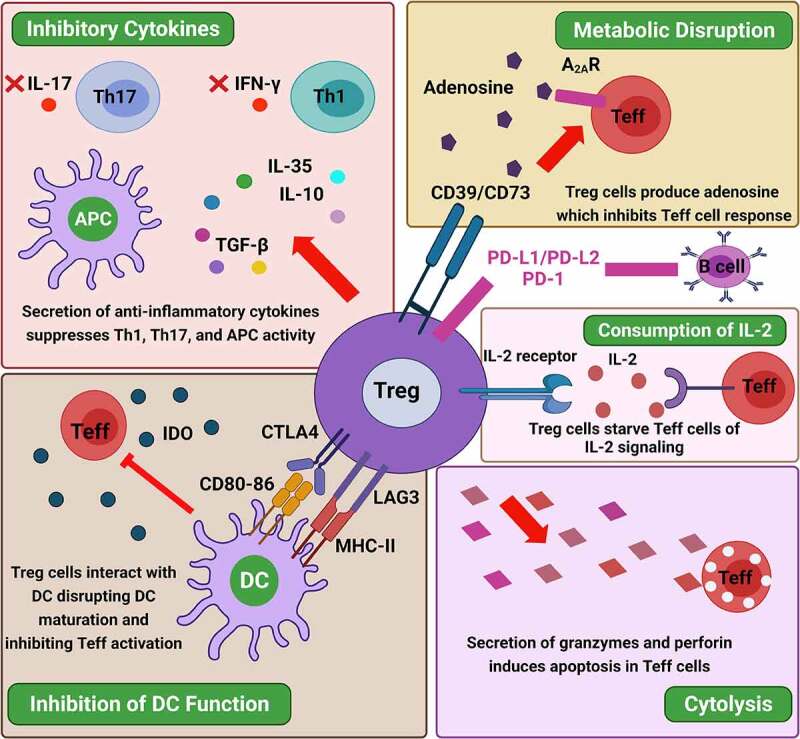
Mechanisms underlying Treg-mediated immunosuppression. Treg cells regulate immune responses by suppressing the functions of effector T cells (Teff) and antigen-presenting cells (APCs) through various mechanisms, including (i) modulation of dendritic cell (DC) function and prevention of DC maturation by the interaction of CTLA4 and LAG3 expressed by Treg cells and the CD80/86 costimulatory molecules and MHC class II expressed by DC, respectively, leading to IDO generation and inhibition of Teff cell activation; (ii) metabolic disruption, Treg cells can disrupt metabolic roles by the expression of the ectoenzymes CD39/73 allowing adenosine generation and binding of adenosine to the adenosine receptor 2A (A2AR) exposed on Teff cells, or by IL-2 deprivation; (iii) generation and secretion of the anti-inflammatory cytokines IL-10, IL-35, and TGF-β that restrain Th1 and Th17 immune responses and the production of IFN-γ and IL-17, respectively; and (iv) direct cytotoxicity, Treg cells can also induce direct killing of effector cells by the release of granzyme A, granzyme B, and perforin, which induce apoptosis in the target cells. Tregs have also been detected to have a direct effect on B-cells via PD-L1/PD-1 interaction. Abbreviations: APC: antigen presenting cell; TGF-β: transforming growth factor-β; A2AR: Adenosine receptor 2A; IL: interleukin; IFN: interferon; Teff: effector T cells; Treg: regulatory T cells; CD: cluster differentiation; IDO: indoleamine 2,3-dioxygenase; DC: dendritic cell; CTLA-4: cytotoxic T lymphocyte antigen-4; TGF-β: transforming growth factor-β; LAG3: lymphocyte-activation gene 3; MHC: major histocompatibility complex; PD-1: programmed death-1; PD-Ls: programmed cell death ligands. Figure was designed by Biorender.com program (https://biorender.com/) Accessed on 13 April 2021.
Chemical messengers involved in suppression
Although no consensus has been established to register all the known suppressive agents and their hierarchy of action during immunosuppression, the key molecules governing the suppressive activity of Tregs include IL-10, TGF-β, CTLA4, granzyme, perforin, interferon gamma (IFN-γ), IL-9, cAMP, heme oxygenase-1, CD39, galectins, and IL-35. Treg executes suppressive effect due to IL-10 and TGF-β secretion. However, neutralizing antibodies against these two cytokines may not block Tregs activity and mice lacking these two genes also have similar suppressive activity. No abysmal effect was observed in Treg-deficient mice after the genes for TGFβ were knocked out. In vivo models have suggested the requirement of these two cytokines in Tregs, whereas others have advocated that these cytokines are secreted by other cells as well.46
Furthermore, since Tregs are more vulnerable to oxidative stress than Teffs, NRF2 has been investigated as a primary transcription factor that protects Tregs against oxidative distress. Tregs undergoing apoptosis due to oxidative damage release a considerable amount of ATP. Hence, Tregs containing high levels of CD37 and CD39 represent an important role in catalysis and adenosine generation due to enhanced extracellular ATP or ADP secretion. Adenosine then binds to the A2A receptors of Teffs, leading to immunosuppression.47
Forkhead character of Foxp3 protein
During 1949, a spontaneous scurfy mutation in mice colony was observed at Oak Ridge National Laboratory, Tennessee, USA. Subsequent studies have revealed that the mutation is X-linked, thus affecting only males. Homozygous males carrying the mutant X chromosome exhibit this syndrome, whereas heterozygous female carriers of the mutant X chromosome allele are healthy. Scurfy mice have thick and scaly ears, eyelids, feet, and tail and severe runting.48,49 Internal organs of such mice have characteristic lymphadenopathy, splenomegaly, hepatomegaly, and massive lymphocytic infiltration in the skin and liver. These lesions closely resemble those after graft-versus-host reaction. Initial study uncovered that mutation in scurfy mice affects Foxp3-encoding gene and can be rescued by introducing wild-type Foxp3 allele transgene. Conventional T cells do not express Foxp3 protein under normal physiological conditions, whereas some CD25+/CD4+ population highly expresses Foxp3. Ectopic Foxp3 protein expression in isolated CD4+/CD25+ T cells acquires the suppressor function in non-Tregs. Similarly, foxp3 introduction in naïve T cell confers suppressive function. Adaptive Treg transfer rescues neonatal Foxp3-deficient mice from the disease. Mice experimentally subjected to germ line foxp3 delineation progressively express autoimmune lesions. Even foxp3 knockout from thymic and dendritic cells outlines the repertoire that developing T cells do not affect dysregulation or alteration in T cell differentiation. Similarly, foxp3 knockout macrophage does not affect tumor growth. These data indicate that foxp3 does not affect any other cells except T cells.14
Moreover, hyperinflammation in various organs and a severe autoimmune response have been reported in scurfy mice with a mutant foxp3. The hyperinflammatory response has been linked with the lack of Tregs, which in turn leads to enhanced production of inflammatory cytokines.50 Besides that, in Homo sapiens, mutated foxp3 tends to cause X chromosome-linked immune dysregulation, polyendocrinopathy, and enteropathy (IPEX) syndrome.51 The overexpression of foxp3 in nT cells enhances the immunosuppressive activities of T cells. The nT cells with CD4+ and CD25− characteristics transform into Treg-like cells expressing both CD4 and CD25 markers when transfected with foxp3 gene. These cells mimic Tregs by producing anti-inflammatory cytokines, exhibiting classic Treg‐cell characteristics and their marker proteins, including CTLA‐4 and TNF receptor-related proteins, and showing significant immunosuppressive activities.52 Furthermore, it has been concluded that foxp3 is a lineage-specific marker and a key factor in the development, repair, and autoimmune suppressive roles of Tregs. Hence, foxp3 can be presumed to be a key regulatory gene in developing Tregs.
Strategic expansion of Treg cells and its therapeutic applications
Trials are being conducted since last 10 years to verify the safety and feasibility of Treg therapy. The first clinical trials with transferred Tregs were initiated in 2009.11 Medical practitioners are now interested in the clinical application of Tregs following all safety guidelines to reduce autoimmune disorders as an alternative approach to anti-inflammatory drugs.53,54
Adhering to ethical guidelines, Tregs can be isolated from either peripheral blood or umbilical cord blood, while the thymus is also a rich source of Tregs in experimental animals.55–57 Using standard cell isolation procedure employing gradient centrifugation technique, mononuclear cells comprising lymphocytes and monocytes can be isolated. From the isolated mononuclear cell population, further purification and enrichment process is adapted to sort out desired T cell population. The classical approach involves the depletion of CD8+and/or CD19 + T cells, followed by an enrichment of the CD25 + fraction. This protocol can enrich FOXP3 + expressing T cells to 80% purity due to the presence of cell contaminants. Moreover, flow cytometry-based purification technique is an advanced method of cell isolation with increased purity in the clinics. With this technique, cells are not only selected, but also sorted out for utmost purity (> 99%) and sterility, fulfilling the requirement of in vitro culture. Cells are selected according to CD4, CD25, and CD127 marker expression.58 Treg concentration in cord blood and thymus is high, however it is difficult to isolate. Therefore, post-enrichment Treg expansion in cell culture vessel is essential to achieve optimum concentration for therapeutic applications. Hence, ex vivo polyclonal Treg expansion is now routinely practiced using anti-CD3/CD28-coated beads in the presence of IL-2.59 Protocol refinement using rapamycin and/or IL-2 cytokine to increase Treg’s yield with increased suppressive effect has been followed by several researchers. However, another option is to incorporate TGFβ and all trans retinoic acid (ATR) in culture medium for upregulating chemokine receptor expression on Tregs, which may facilitate Treg homing in the gut.
Recently, adoptive cell therapy using genetically engineered Tregs has been attempted to combat transplant rejection and autoimmune diseases.60 However, the lack of standardized protocols for expanding and transducing Tregs from mice is a significant drawback of this technique. Purifying, extending, and retrovirally transducing mouse Tregs with a vector encoding a chimeric antigen receptor as a model transgene has been described for obtaining considerable improvement in genetically engineered Tregs. It has been discovered that isolating Tregs based on GFP expression yields appropriately pure cells. Although rapamycin expansion inhibits Treg expansion, it does not influence the suppressive activities of Tregs. This approach enables the study of transduced Tregs in animal models, as well as the investigation of Treg therapy based on genetic engineering for multiple inflammatory diseases.61 Moreover, in the future, genetically engineered Tregs can solve the issues associated with Treg expansion and purification along with enhancing the suppressive activities of Tregs.
Therapies targeting Treg-mediated suppressive mechanisms have been introduced in several conditions, including organ transplantation and autoimmune disorders. In addition, Tregs have been introduced to cure other clinical manifestations such as cardiovascular disease, obesity, and systemic inflammatory disorders.62,63 The therapeutic potentials of Tregs to manage a range of autoimmune diseases have been described in following section and findings of clinical trials are summarized in Table 1.
Table 1.
Clinical trials of Tregs against autoimmune diseases
| Disease | Expansion: autologous or polyclonal Treg cells | Description of application and dose | Outcome of clinical trial and final status | References |
|---|---|---|---|---|
| Crohn`s disease | PBMC derived ovalbumin specific Treg cells expanded under in vitro condition designated as ovasve NCT02327221 | Single infusionof cells ranging from 106 to 109in number per patients | Well tolerated yet adverse effect has been observed in 54 patients, all recovered without showing any further adverse effect | 64 |
| Croh`n disease | Polyclonally expanded autologous CD4+ CD25+ CD127lowCD45RA+Tregs treated with rapamycin and retinoic acid receptor agonistNCT03185000 | 0.5–1 x 10 6 cells up to 8–0 × 10 6 cells /kg body weight | Expressed α4β7 and exhibit suppressive function with migratory activities in cell culture system. Final results not posted till Nov. 2021 | 65 |
| Type1 diabetes T1D |
Autologous ex vivo Tregs CD4+ CD25+ CD127, lowISRCTN06128462 | 10 x 106–30 × 06per kg body weight | Absence of serious adverse effect without insulin dependency. Clinical trials completed | 58 |
| T1D | Autologous enriched Tregs derived from PBMC, ex vivo expandedNCT01210664 | 0.5 x 10 8 to 26 × 10 8 Tregs infused as single dose via i/v route per patient | About 25% of peak level of circulatory Tregs persisted for one year after transfer. Transient increase in FOXP3(+)CD4(+)CD25(hi)CD127(lo) phenotype Tregs in recipients was observed. Adverse effect was observed in 3 out of 14 patients, not related to cell infusion. Ddeuterium labeling was absent in non-Treg cells indicating Tregs stability. C-peptide indicator of insulin production persisted up to 2 years of Treg cell transfer. Clinical trials completed | 66 |
| T1D | CD4 + CD25 + CD127 lowTregs derived from autologous PBMCenriched under in-vitro condition, under Phase-I. Clinical trial NCT02772679 |
3 x 10 6–20 × 10 6 cells per kg, s/c route | No result posted as on November 2021 | 35 |
| T1D | Umbilical cord blood derived TregsPhase-I and Phase-II trialNCT02932826 | Tregs with insulin and insulin alone | 40 participants were incorporated in the trial and result not published till Nov 2021 | 35 |
| Active cutaneous lupus | Autologous ex vivo expanded and enriched Tregs, under Phase-I. Clinical trials NCT02428309 | 1 x 10 8 deuterium labeled Tregs cells per patients | Increase population of Tregs and IL-17 production by both CD4 and CD8 cells recorded in skin biopsies. Terminated due to participantrecruitment feasibility | 67 |
| Pemphigus Vulgaris |
Ex vivo expanded autologous CD4+ CD127lo/negCD25polyclonal TregsNCT03239470 | 1.0 x 10 8 and2.5 x 10 8 cells per patients of 18 to 75 years age | Recovery criteria was to detect CD4+, CD25++, CD127 low Treg in patients using cell shorting system. Results submitted during October 2021 but detail is under quality control process for further recruiting, | 68 |
| Auto immune hepatitis | Polyclonally expanded autologous Tregs for phase I/II clinical trials NCT02704338 | 10–20 × 10 6 cells in a single infusion to patients aged between 10–70 years | Initiated at Nanjing Medical University China. Further information not yet published | 35 |
Inflammatory bowel disease /Crohn`s disease
In vitro-generated, IL-10–producing ovalbumin (OVA)-specific Treg cells (Tr1) were reported to prevent colitis induced in SCID mice by CD4+ CD45RBhi T cells. Thus IL-10 secreting CD4+ Treg cells are immunosuppressive and actively downregulate a pathological immune response in vivo.69 Experimental transfer of CD4+CD25+ Treg cells in immunodeficient mice with clinical signs of colitis was able to ameliorate colitis. Recovery was observed after 2 weeks of post-infusion of Treg cells at a dose of 10 6 per mice, as compared to transfer of CD4−CD25− T cells in control group.70 As per published reports, the first clinical trial with single infusion of PBMC-derived freshly isolated Tregs (106–109) to a patient with active Crohn’s disease was safely initiated during 2012 without observation of any severity. Clinical responses were predominantly detected in patients receiving the lowest numbers of Treg cells, suggesting that less suppression may be better.64 Crohn’s disease has been described in details previously.71 It has been observed that the inflamed mucosa of IBD cases are modestly populated with Treg cell as compared to other inflammatory conditions like diverticulitis, therefore the inflammation are poorly controlled due to lack of Treg cell number at the site of inflammation.72 Knock out mice lacking IL-10 gene is considered to be the suitable model for colitis to establish the efficacy of Treg cell producing IL-10 to reverse the pathological condition once infused, supporting the critical role of IL-10.73 Developing humanized mouse by transplanting fetal small bowel followed by developing colitis by Escherichia coli infection has been used as a model for the study of Treg cell function. After transfusing 106 Treg cells along with rIL-10, it has been observed that Treg cells migrate and preferred to home at the site of inflamed lamina propria and suppress the conventional T cells at the site of inflammation.74 Surgically collected specimens from IBD patients have shown defective CD8+ Treg cells in the lamina propria (LP) whereas CD8+ T cells with regulatory activity are present in the LP of healthy individuals. It has been suggested that intestinal epithelial cells play an important role in the generation of such regulatory T cells, because they are able to process and present antigen to T cells. These cells play a central role in maintaining controlled inflammation in the intestinal mucosa.75
Type 1 diabetes (T1D)
T1D is a clear example of autoimmune disease as inflammatory cells infiltrate the pancreatic islets and destroy the insulin-producing cells. Recent report has indicated that children with new-onset T1D have an increased proportion of CD45RA(-)CD25(int)FOXP3(low) Treg cells that are not suppressive and secrete significantly more IL-17 than other FOXP3(+) subsets. Regulatory T cell dysfunctions in T1D have relied on analysis of FOXP3-expressing cells. The number of Tregs reduces during the early phases of the disease.76 Proof-of-concept for the treatment of Type1 diabetes in children by delivering autologous CD3(+)CD4(+)CD25(high)CD127(-) Tregs has been established. Administration of Tregs is safe and tolerable in children with recent-onset of type 1 diabetes.58 A randomized trial with patients with T1D in three groups receiving 10 × 106, 20 × 106, and 30 × 106 ex vivo expanded autologous Tregs/kg body weight have shown no adverse effect under treatment duration. Safety was proved after one year of follow-up, with eight patients showing signs of clinical remission.58 During phase I clinical trials, autologous Tregs derived from patient’s blood were administered to evaluate the clinical efficacy. None of the patients showed any unpleasant events up to 31 months of follow-up program, while only three out of 14 patients developed untoward effect without any opportunistic pathogen infection. Further trial with placebo control has shown positive response of Treg therapy to limit the severity of autoimmune disorders.66
Hemoglobin concentration and insulin requirement was found to be significantly reduced in some T1D patients after receiving Treg cell therapy. Some patients showed complete independence from the exogenous insulin and revealed significant increase in circulating Tregs. Negligible risks of infections or not having any severe adverse effects were reported following Treg therapy.58 Another study has also reported a significant increase in survival of pancreatic β-cells among patients with repeated Treg administration for one year.58 Ongoing clinical trials at phase I/II stage (NCT02932826) with Tregs derived from umbilical cord at high and low dose in a single infusion have not shown any adverse effect and final result are yet to be compiled.77
Pemphigus vulgaris (PV)
PV is a fatal autoimmune disease caused by IgG autoantibodies targeting the desmosomal adhesion protein desmoglein Dsg1 and Dsg3. The disease is characterized by loss of cell adhesion (also known as acantholysis) in skin and mucous membrane. In pemphigoid diseases, the linear deposition of autoantibodies along the dermal-epidermal junction causes sub-epidermal blistering, resulting in blisters.78 Findings of adoptive transfer of splenocytes from Dsg3 -/- mice (mice lacking both the parental gene for Dsg3) immunized with recombinant Dsg3 protein to Rag2-/-Dsg3+/+ mice suggested that adoptively transferred Tregs suppressed the antibody production in a dose-dependent manner and the depletion of endogenous Tregs augmented the antibody production.79 Similarly, using HLA-DRB104:02 transgenic PV mouse model immunized with human Dsg3 established that in vivo, although not statistically significant, Treg cells exert a clear down-regulatory effect on the Dsg3-driven T-cell response and, accordingly, the formation of Dsg3-specific IgG antibodies were detected.80
To evaluate the effects of Tregs on the manifestation of PV, a nonrandomized, open-label, phase 1 ongoing clinical trial (NCT03239470) is operational since 2017. In this trial, 12 pemphigus and or pemphigoid patients received single infusion of autologous expanded Tregs (CD4+ CD127lo/negCD25+) at one of the following doses: either 2.5 × 108 poly Tregs or 10 × 108 poly Tregs. As no adverse effect has been observed, the project is continuing and the final results from this study are awaited.81
Autoimmune hepatitis (AIH)
Tregs have the potential to serve as an effective therapeutic modality in treating autoimmune hepatitis (AIH), a chronic autoimmune inflammatory disease that usually requires life-long immunosuppression for management of this condition. Relapses following therapy discontinuation are common, indicating that current treatments do not regain intrahepatic immune regulation. Immune response homeostasis can be reestablished by increasing the functionally active intrahepatic Tregs. However, steroid therapy preferentially depletes intrahepatic Tregs. In recent clinical trials, Tregs have been used to improve the autoimmune disorders with a low dose of cytokines such as IL-2. Mice with complexed IL-2/anti-IL-2 have been used to improve the Treg selectivity and a significant improvement in intrahepatic and systemic Treg levels along with a decrease in activated, intrahepatic Teffs have been reported. Reduced levels of liver-specific ALT (alanine transaminase) and a molecular pattern close to that observed in healthy people have also been reported. Finally, complexed IL-2/anti-IL-2 improves the equilibrium between Tregs and Teffs within the liver. Treg-specific IL-2 augmentation aids in reestablishing immune tolerance and homeostasis in the immune response.82 It is noteworthy that incorporating several changes in the therapeutic regimens involving Tregs can be an efficient approach to treat autoimmune disorders like AIH.83 In absence of hepatic infection, detection of elevated level of liver enzyme is considered to be presumptive diagnosis of autoimmune hepatitis.84 Divided opinion has been given by researchers to support the role of Tregs in autoimmune hepatitis. A decrease in population of Treg cells in the peripheral blood in few cases of autoimmune hepatitis has been reported,85 on the contrary no such difference in Tregs cells in several other cases has raised controversy in this regard.86
The stability of Treg cells at the site of hepatic inflammation is crucial, however research data suggested that due to low concentration of IL-2 in hepatic cell, human liver tissues may not be the ideal site for Tregs survival and expansion.87 Based on this data, low dose IL-2 was administered in two patients with refractory AIH and an increase in the frequency of Treg cells in the peripheral circulation was observed, which indirectly support the ameliorating role of Tregs for AIH.88
Enrichment of effector T cells (Teffs) within the liver tissues of untreated AIH-1 patients with a constant Treg/Teff ratio but without any change of Tregs in the circulation, is not an uncommon finding. The disproportional decrease of intrahepatic Tregs during therapy might explain high rates of relapse after discontinuation of immunosuppression.89 Under the guidance of National Institute of Health, a phase-1 clinical trials ((NCT02704338) has been registered to evaluate the efficacy of autologous Tregs from peripheral blood of autoimmune hepatitis patients using ex vivo expansion procedure so that each patient should receive 5 × 106 cells /kg body weight. Results of this study have not been posted yet as the project is continuing.90
Systemic lupus erythematosus (SLE)
SLE is an autoimmune, multisystemic disease that may affect virtually any organ due to induction of autoantibodies and the deposition of immune-complexes in tissues that leads to the activation of complement, accumulation of neutrophils and monocytes, and self-reactive lymphocyte.91 In mice model, regulatory T cell numbers have been reported to decrease in lupus-prone mice as the age and the disease progresses.92 Clinical application of Tregs in human patients suffering with SLE have shown variable efficacy and even conflicting reports.93 Such discrepancies in findings may be due to difference in isolation and purification strategies applied for enrichment of Foxp3+ CD25+ Tregs before being infused to the patients.94
Most of the earlier studies on SLE have shown decreased proportion of either CD4 + CD25hi T cells or CD4 + CD25 + FoxP3 + Treg cells once the disease flares.95 Deuterium tracking of infused Treg cells in SLE patient with active skin disease revealed transient presence of cells in peripheral blood, accompanied by increased percentages of highly activated Treg cells in diseased skin. Flow cytometric analysis and whole transcriptome RNA sequencing revealed that Treg cell accumulation in skin was associated with a marked attenuation of the interferon-γ pathway and a reciprocal augmentation of the interleukin-17 (IL-17) pathway. This phenomenon was more pronounced in skin relative to peripheral blood.67 The homeostasis of CD4+ T cell subsets is regulated by IL2, and reduced production of IL-2 by T cells is observed in individuals with SLE. It has been reported that the treatment with low-dose of rIL-2 selectively modulated the abundance of T Regs, follicular helper T (TFH) cells and IL-17-producing helper T (TH17) cells, but not TH1 or TH2 cells.96 The contributing role of Tregs in SLE has been reported from 15 patients in which the proportion of CD45RA−FoxP3hi activated Treg cells was decreased whereas the proportion of CD45RA+FoxP3lo resting Treg cells was increased.97 In a unique case study, single cutaneous lupus adult patient was included in phase I non-randomized clinical trial for evaluating the effects of a single dose of 1 × 108 ex vivo expanded, FACS-sorted, and deuterium-labeled autologous Tregs. The number of circulating labeled cells dropped 4 weeks post-injection, but a marked increase in Tregs and IL-17 production was recorded in skin biopsy.67
Rheumatoid arthritis (RA)
RA is a systemic and heterogeneous autoimmune disease characterized with clinical manifestation of symmetrical polyarthritis. It is a chronic inflammatory joint disease, which can cause cartilage and bone damage as well as disability condition in patients.98 The pathogenesis of RA is complex, and the dysfunction of Tregs is one of the proposed mechanisms underlying the breakdown of self-tolerance leading to the progression of rheumatoid arthritis. For patients with a poor response to traditional disease-modifying anti-rheumatic drugs, the clinicians adapted Treg therapy with variable success.99 Clinical findings indicated an increase in number of Treg cells in the peripheral blood of several individuals suffering with RA.100 On the contrary, either an unchanged Treg population101 and even low count of Tregs in several other patients manifesting frank clinical cases has been reported by different research groups, which is a subject of clinical concern.102 Variability in the interpretation of data of the above discussed cases mostly depends upon the identification of Tregs based upon the expression of FoxP3 and CD25 marker, which are likely to be mediated by inflammation and T cell activation. Invariably, Tregs of RA patients don’t secrete copious amount of IL-2 and IFNγ in their peripheral circulation as compared to healthy individuals, suggestive of FoxP3 expression to be a hallmark of Tregs.103 Recovery in RA patients due to anti-TNF antibody therapy was attributed to the generation of novel Treg subsets expressing FoxP3 marker but not CD-62 ligand.104 Whether TNF-α plays a direct role to modulate Treg function is still under debate.105 This indicates that the use of a more stringent method to define Treg cells will reveal decreased number of Treg cells in peripheral blood and increased number in synovial fluid, but whether Treg cells in the synovial fluid function normally is yet to be confirmed.106 Transfer of Treg cells can slow down the disease process, confirming that these cells have the potential to treat autoimmune diseases.107 Adaptive transfer of Tregs in mice model using collagen-induced arthritis (CIA) showed inhibitory effects on arthritis, which significantly prevents the development of CIA.108 Importantly, when arthritis is inhibited in these models, not only T and B cells are inhibited by Treg cells but osteoclast-mediated bone destruction is also directly diminished, preventing joint injury.109 Adaptive Treg cell therapy supplements the functional activity of existing residential Tregs impaired by the highly inflamed synovial milieu.110
Systemic sclerosis (SSc)
SSc is an autoimmune inflammatory condition that results in potentially widespread fibrosis and vascular abnormalities, which can affect the skin, lungs, gastrointestinal tract, heart, and kidneys. The skin becomes thickened and hard (sclerotic). It is characterized by clinical features that resemble GVH disease. The classification of systemic sclerosis is based on skin involvement. Limited cutaneous systemic sclerosis (LcSSc), formerly known as the CREST syndrome, is associated with skin thickening distal to the elbows, distal to the knees, and/or face without trunk involvement.111
The majority of the studies reported decreased frequencies and/or impaired function of circulating Tregs in SSc patients compared to controls.112 Reduced frequency of Tregs, together with that of total TGFβ1 and IL-10, may be responsible for the loss of tolerance observed in SSc.113 Contrary to this, other studies have reported decreased frequency of circulating Tregs in peripheral blood but not reaching statistical significance.114,115 Success of adaptive therapy of Tregs in SSc is not so promising, however uncontrolled studies in SSc have demonstrated that hematopoietic stem cell therapy (HSCT) can halt disease progression and reverse skin fibrosis, and might even reduce mortality.116 In a clinical trial, 26 SSc patients were selected for CD34 + autologous hematopoietic stem cell transplantation to compare its effectiveness and safety to intravenous cyclophosphamide, the only treatment option for progressive SSc. Sustained improvement of skin thickening and stabilization of organ function for up to 7 years after transplantation was recorded in this clinical trial.117
Primary Sjogren’s syndrome (pSS)
pSS is a systemic autoimmune disease characterized by lymphocytic infiltration of the salivary and lacrimal glands. In addition to the exocrine glands, many other organs can be affected by the disease as well. Hyperactivity of B cells is thought to play a central role in the pathogenesis of pSS. Available evidence strongly indicates that this B cell hyperactivity is mediated by T cells. Loss of self-tolerance and secretion of many pro-inflammatory cytokines including IFN-γ, IL-17 and IL-21 associated with local inflammation in pSS, are mediated by T-cell activity.118 However, circulating Treg cells in pSS is controversial since either diminished, normal or increased proportions are reported by different workers.105 Report indicating localization of Treg cells in target organ, i.e. salivary gland is also conflicting because of either the absence of Treg cells119 or presence of infiltrating FoxP3+CD4+T cells positively correlated to the biopsy scores in lymphocytic sialadenitis but without any change in circulating Tregs in periphery.120
Psoriasis
Tregs have also been employed as crucial pla yers in psoriasis, which is a systemic inflammatory disorder with significant involvement of genetic factors induced by environmental influence. Disease pathogenicity is mainly driven by type 1 and Th17 cytokine-producing cells, which are tightly controlled by Tregs in normal people. Tregs play critical roles in maintaining immune homeostasis via blocking inflammatory cells, which in turn prevent autoimmune disorders like psoriasis and other skin-related autoimmune diseases. It has been postulated that Treg-mediated suppression is disrupted in psoriasis, changing the Th17/Treg ratio. While Treg dysfunction is linked to disease worsening in patients with psoriasis, it is unclear how they have been biologically controlled.121 Recent research suggests that Tregs are essential in psoriasis treatment. While the significance of Treg concentration is unknown, attenuated Treg activity and a disrupted Th17/Treg equilibrium have been linked to psoriasis pathogenesis and exacerbation.121–125
Other auto immune diseases
Recently, Huang et al. have reported the critical roles of Tregs in the initiation, treatment, and recovery of uveitis, which is a recurrent autoimmune disease characterized by relapsing and remitting ocular attacks that necessitates corticosteroids and systemic immunosuppression to avoid serious vision loss. Both Th17 (pro-inflammatory cells) and Tregs mediate uveitis, and it has been suggested that modulating Tregs could be an effective potential therapeutic strategy against uveitis.126
Further, the protective role of Tregs has been described in Myasthenia gravis (MG), which is a T cell-dependent, B-lymphocyte-mediated autoimmune disease caused by the production of antibodies against the nicotinic acetylcholine receptors and other post-synaptic components at the neuromuscular junction. Adoptive transfer of Tregs has been found to suppress the autoimmune response in the rodent model and the protective role of these cells has been suggested in MG. However, any disturbances in the ratio of T-helper (Th) cells and Tregs might be linked with the poor prognosis of the autoimmune diseases, such as MG.127
Herrnstadt et al. postulated the potential roles of Tregs in providing protection against any renal tissue injury, which has been linked with pathogenic driving force of Th cells such as Th1 and Th17, also known as T effector cells, in glomerulonephritis (GN).128 Tregs overcome the pathogenic behavior of vital innate immunity components in inflamed kidney cells or tissues. Hence, these findings can be utilized to further elucidate the immunosuppressive function of Treg cell population in renal injuries and kidney inflammation.129
Several clinical trials for addressing autoimmune disorders have used polyclonal Tregs and antigen-specific Tregs designed with TCR or chimeric antigen receptor (CAR) constructs that could dramatically boost their performance in the host. Furthermore, it has been proposed that antigen-specific Tregs may be injected into patients with autoimmune skin diseases to restore Treg content and provide resistance against the exacerbated immune response.12
Briefly, the present therapies adapted by the clinicians to control the autoimmune disease in population are primarily aimed to restore immune tolerance in the patients. The drugs used to tame down autoimmune diseases are targeted to encourage in vivo Treg induction and expansion. Drugs or biologicals such as mTOR inhibitor rapamycin and cytokines IL-10, tiny dose of IL-2, TNF receptor 2 agonist, or tyrosine kinase 3 ligand are used universally to manage the autoimmune disease under control. Besides these, further explorative researches into Treg persistence and the optimal requirements for Treg survival in peripheral blood are expected in the coming years to properly promote the clinical use of Tregs in immunotherapeutics.
Challenges in clinical applications
Application of Tregs in clinics must follow several stringent guidelines imposed by Food and Drug Administration (FDA). It is mandatory that the cell therapy products should pass the sterility, identity, purity, and potency standards before administration. Demonstration of purity and identity is most grim part of the test system. Foxp3 marker expression is a universally accepted marker for Treg identity. However, ex vivo Treg expansion may not yield 100% Foxp3+ cells, but it should adhere the acceptable limit of contaminating cells, which must satisfy the qualitative as well as quantitative bench marks. Contaminating cells cause allogenic reaction in the recipient; hence, it should be at extremely low concentration. Currently, investigators are looking forward to develop disease-specific models to establish the potency of expanded enriched Treg products, as one product is not suitable for all diseases.130 However, some other limitations associated with the use of Treg cells in clinical conditions include the stability of the Treg population in peripheral blood, and, therefore, also limit their effects. Furthermore, Treg cryopreservation is required for its robust use among the patients in clinical settings, which may damage Tregs by reducing its durability, triggering unusual cytokine secretion, and interfering with the expressions of several surface proteins necessary for Treg functioning and processing. Hence, optimum Treg culture techniques, cryopreservation extension, and storage for medicinal use must also be explored and established.131
Conclusion and future perspectives
Recent advancements in technology have enabled scientists to identify and classify Tregs and their immunosuppressive mechanisms, allowing a better comprehension of the relationship among Treg plasticity, which is needed for optimal Treg immune suppression and Treg destabilization or vulnerability from various factors that leads to autoimmune diseases. The plasticity and heterogeneity in the mice- and human-derived Treg population have given new information in Treg biology. Due to the conflicting findings in different studies, the contribution of Tregs to different autoimmune disease models is yet unclear. However, it has been confirmed that Tregs express specific transcription factors, such as Foxp3 protein, which is considered to be a master regulator. Treg isolation is not easy due to the lack of a specific marker for experimental purposes. Human Tregs expressing a low, intermediate, or high CD25 molecule label l are difficult to isolate as these boundaries are thin. Using human Tregs as therapeutic agents is a new area of research in clinical application. Clinical findings have provided some hope to use Tregs as an adjunct with approved drugs to support the treatment regimen for reducing the reoccurrence of autoimmune diseases and can add few years of quality life in an individual.
Further, the results of studies on the patients with inflammation and autoimmune diseases are still conflicting and misleading due to a lack of reliable Treg-specific markers, which in turn play a pivotal role in providing immunosuppressive functionality to the Tregs. It is the ultimate need of the time to elucidate the specific markers on the Tregs for designing efficient therapeutic strategies. A vivid elucidation of the Treg-mediated immune suppression and the identification of changes in the immune response of patients with functional Treg deficiency is necessary for efficient therapeutic regimes to control autoimmune diseases. Furthermore, in-depth and specific studies are required to establish the roles of Tregs under specific inflammatory responses, which will pave a path toward a personalized therapeutic strategy to control specific clinical manifestations. Various advanced techniques, such as single-cell RNA sequencing, can discern Treg subsets and provide knowledge on TCR repertoires. Specific Tregs against specific antigens, as well as their heterogeneity in stable and diseased states, will open up several possibilities, including the identification of TCR-transduced cells for medicinal purposes. In addition, using genetically engineered Tregs and chimeric antigen receptor-Tregs is promising in pre-clinical autoimmune disorder models, and may have medical applications, particularly when extracellular receptors are important. Finally, it can be well stated that identifying and deciphering immunosuppressive pathways and their interlinked markers will lead to the development of efficient and reliable therapeutic regimes to treat autoimmune diseases.
Acknowledgments
All the authors acknowledge and thank their respective Institutes and Universities.
Funding Statement
The author(s) reported there is no funding associated with the work featured in this article.
Author contributions
Conceptualization, T.K.G., M.S., M.D., and K.D.; investigation and resources, T.K.G., A.A.R., M.S., M.D., S.M., T.B.E., and K.D.; writing—original draft preparation, T.K.G., M.S., M.D., and K.D; writing—review, updation and editing, T.K.G., M.D., T.B.E., A.A.R., A.A.M., Z.A.I., S.A., and K.D.; visualization and supervision, T.K.G. and K.D.; project administration, T.K.G. and K.D.; funding acquisition, A.A.R. All authors have read and agreed to the published version of the manuscript.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- 1.Arellano B, Graber DJ, Sentman CL.. Regulatory T cell-based therapies for autoimmunity. Discov Med. 2016;22:73–16. [PMC free article] [PubMed] [Google Scholar]
- 2.Matzinger P. The danger model: a renewed sense of self. Science (80-). 2002;296:301–05. doi: 10.1126/science.1071059. [DOI] [PubMed] [Google Scholar]
- 3.Miyara M, Yoshioka Y, Kitoh A, Shima T, Wing K, Niwa A, Parizot C, Taflin C, Heike T, Valeyre D, et al. Functional delineation and differentiation dynamics of human CD4+ T cells expressing the FoxP3 transcription factor. Immunity. 2009;30:899–911. doi: 10.1016/j.immuni.2009.03.019. [DOI] [PubMed] [Google Scholar]
- 4.Gliwiński M, Iwaszkiewicz-Grześ D, Trzonkowski P. Cell-based therapies with T regulatory cells. BioDrugs. 2017;31:335–47. doi: 10.1007/s40259-017-0228-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mikami N, Kawakami R, Sakaguchi S. New Treg cell-based therapies of autoimmune diseases: towards antigen-specific immune suppression. Curr Opin Immunol. 2020;67:36–41. doi: 10.1016/j.coi.2020.07.004. [DOI] [PubMed] [Google Scholar]
- 6.Palomares O, Yaman G, Azkur AK, Akkoc T, Akdis M, Akdis CA. Role of Treg in immune regulation of allergic diseases. Eur J Immunol. 2010;40:1232–40. doi: 10.1002/eji.200940045. [DOI] [PubMed] [Google Scholar]
- 7.Wang S, Zou X, Zhang Y, Wang X, Yang W, Li Y. The generation and regulation of tissue-resident Tregs and their role in autoimmune diseases. J Immunol Res. 2020;2020:1–13. doi: 10.1155/2020/8815280. [DOI] [Google Scholar]
- 8.Rocamora-Reverte L, Melzer FL, Würzner R, and Weinberger B. The complex role of regulatory T cells in immunity and aging. Front Immunol 11 . 2021;616949. doi: 10.3389/fimmu.2020.616949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sampani E, Stangou M, Daikidou DV, Nikolaidou V, Asouchidou D, Dimitriadis C, Lioulios G, Xochelli A, Fylaktou A, Papagianni A. Influence of end stage renal disease on CD28 expression and T-cell immunity. Nephrology. 2021;26:185–96. doi: 10.1111/nep.13784. [DOI] [PubMed] [Google Scholar]
- 10.Sampani E, Daikidou DV, Lioulios G, Xochelli A, Mitsoglou Z, Nikolaidou V, Dimitriadis C, Fylaktou A, Papagianni A, Stangou M. CD28null and regulatory T cells are substantially disrupted in patients with end-stage renal disease due to diabetes mellitus. Int J Mol Sci. 2021;22:1–13. doi: 10.3390/ijms22062975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Trzonkowski P, Bieniaszewska M, Juścińska J, Dobyszuk A, Krzystyniak A, Marek N, Myśliwska J, Hellmann A. First-in-man clinical results of the treatment of patients with graft versus host disease with human ex vivo expanded CD4+CD25+CD127- T regulatory cells. Clin Immunol. 2009;133:22–26. doi: 10.1016/j.clim.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 12.Mukhatayev Z, Ostapchuk YO, Fang D, and Le Poole IC. Engineered antigen-specific regulatory T cells for autoimmune skin conditions. Autoimmun Rev 20 3 . 2021;102761. doi: 10.1016/j.autrev.2021.102761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Singer A, Adoro S, Park JH. Lineage fate and intense debate: myths, models and mechanisms of CD4- versus CD8-lineage choice. Nat Rev Immunol. 2008;8:788–801. doi: 10.1038/nri2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Josefowicz SZ, Lu LF, Rudensky AY. Regulatory T cells: mechanisms of differentiation and function. Annu Rev Immunol. 2012;30:531–64. doi: 10.1146/annurev.immunol.25.022106.141623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nazzal D, Gradolatto A, Truffault F, Bismuth J, and Berrih-Aknin S. Human thymus medullary epithelial cells promote regulatory T-cell generation by stimulating interleukin-2 production via ICOS ligand. Cell Death Dis 5 9 . 2014;e1420. doi: 10.1038/cddis.2014.377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sakaguchi S, Sakaguchi N, Asano M, Itoh M, and Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol. 1995;155 3 :1151–64. Accessed date 1 August 1995. http://www.ncbi.nlm.nih.gov/pubmed/7636184 [PubMed] [Google Scholar]
- 17.Burchill MA, Yang J, Vogtenhuber C, Blazar BR, Farrar MA. IL-2 receptor β-Dependent STAT5 activation is required for the development of Foxp3 + regulatory T cells. J Immunol. 2007;178:280–90. doi: 10.4049/jimmunol.178.1.280. [DOI] [PubMed] [Google Scholar]
- 18.Shevach EM, Thornton AM. tTregs, pTregs, and iTregs: similarities and differences. Immunol Rev. 2014;259:88–102. doi: 10.1111/imr.12160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Adeegbe DO, Nishikawa H. Natural and induced T regulatory cells in cancer. Front Immunol. 2013;4:4. doi: 10.3389/fimmu.2013.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tang Q, Adams JY, Tooley AJ, Bi M, Fife BT, Serra P, Santamaria P, Locksley RM, Krummel MF, Bluestone JA. Visualizing regulatory T cell control of autoimmune responses in nonobese diabetic mice. Nat Immunol. 2006;7:83–92. doi: 10.1038/ni1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Allos H, Al Dulaijan BS, Choi J, Azzi J. Regulatory T cells for more targeted immunosuppressive therapies. Clin Lab Med. 2019;39:1–13. doi: 10.1016/j.cll.2018.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bluestone JA, Abbas AK. Natural versus adaptive regulatory T cells. Nat Rev Immunol. 2003;3:253–57. doi: 10.1038/nri1032. [DOI] [PubMed] [Google Scholar]
- 23.Lui PPW, Cho I, Ali N. Tissue regulatory T cells. Immunology. 2020;161:4–17. doi: 10.1111/imm.13208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Panduro M, Benoist C, Mathis D. Tissue Tregs. Annu Rev Immunol. 2016;34(1):609–33. doi: 10.1146/annurev-immunol-032712-095948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weiss JM, Bilate AM, Gobert M, Ding Y, de Lafaille MAC, Parkhurst CN, Xiong H, Dolpady J, Frey AB, Ruocco MG, et al. Neuropilin 1 is expressed on thymus-derived natural regulatory T cells, but not mucosagenerated induced Foxp3+ T reg cells. J Exp Med. 2012;209:1723–42. doi: 10.1084/jem.20120914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Atarashi K, Tanoue T, Shima T, Imaoka A, Kuwahara T, Momose Y, Cheng G, Yamasaki S, Saito T, Ohba Y, et al. Induction of colonic regulatory T cells by indigenous Clostridium species. Science (80-). 2011;331:337–41. doi: 10.1126/science.1198469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Scharschmidt TC, Vasquez KS, Truong HA, Gearty SV, Pauli ML, Nosbaum A, Gratz IK, Otto M, Moon JJ, Liese J, et al. A wave of regulatory T cells into neonatal skin mediates tolerance to commensal microbes. Immunity. 2015;43:1011–21. doi: 10.1016/j.immuni.2015.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ohue Y, Nishikawa H. Regulatory T (Treg) cells in cancer: can Treg cells be a new therapeutic target? Cancer Sci. 2019;110:2080–89. doi: 10.1111/cas.14069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Toker A, Engelbert D, Garg G, Polansky JK, Floess S, Miyao T, Baron U, Düber S, Geffers R, Giehr P, et al. Active demethylation of the Foxp3 locus leads to the generation of stable regulatory T cells within the thymus. J Immunol. 2013;190:3180–88. doi: 10.4049/jimmunol.1203473. [DOI] [PubMed] [Google Scholar]
- 30.Darrasse J, Mardon G, Salomon BL, Catala MKD, Klatzmann D. Ontogeny of CD4+CD25+ regulatory/suppressor T cells in human fetuses. Blood. 2005;105:4715–21. doi: 10.1182/blood-2004-10-4051. [DOI] [PubMed] [Google Scholar]
- 31.Liu W, Putnam AL, Xu-yu Z, Szot GL, Lee MR, Zhu S, Gottlieb PA, Kapranov P, Gingeras TR, Barbara BF, et al. CD127 expression inversely correlates with FoxP3 and suppressive function of human CD4+ T reg cells. J Exp Med. 2006;203:1701–11. doi: 10.1084/jem.20060772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Onizuka S, Tawara I, Shimizu J, Sakaguchi S, Fujita T, Nakayama E. Tumor rejection by in vivo administration of anti-CD25 (interleukin-2 receptor α) monoclonal antibody. Cancer Res. 1999;59:3128–33. [PubMed] [Google Scholar]
- 33.Vignali DAA, Collison LW, Workman CJ. How regulatory T cells work. Nat Rev Immunol. 2008;8:523–32. doi: 10.1038/nri2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sojka DK, Huang YH, Fowell DJ. Mechanisms of regulatory T-cell suppression - A diverse arsenal for a moving target. Immunology. 2008;124:13–22. doi: 10.1111/j.1365-2567.2008.02813.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Romano M, Fanelli G, Albany CJ, Giganti G, Lombardi G. Past, present, and future of regulatory T cell therapy in transplantation and autoimmunity. Front Immunol. 2019;10:10. doi: 10.3389/fimmu.2019.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wing K, Sakaguchi S. Regulatory T cells exert checks and balances on self tolerance and autoimmunity. Nat Immunol. 2010;11:7–13. doi: 10.1038/ni.1818. [DOI] [PubMed] [Google Scholar]
- 37.Feuerer M, Hill JA, Kretschmer K, Von Boehmer H, Mathis D, Benoist C. Genomic definition of multiple ex vivo regulatory T cell subphenotypes. Proc Natl Acad Sci U S A. 2010;107:5919–24. doi: 10.1073/pnas.1002006107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Omenetti S, Pizarro TT, Bancu I, Lauzurica-Valdemoros R, Borràs FE. The Treg/Th17 axis: a dynamic balance regulated by the gut microbiome. Front Immunol. 2015;6:6. doi: 10.3389/fimmu.2015.00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu Y, Tran DQ, Lindsey JW, Rhoads JM. The association of gut microbiota and treg dysfunction in autoimmune diseases. Adv Exp Med Biol. 2021;1278:191–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gotot J, Gottschalk C, Leopold S, Knolle PA, Yagita H, Kurts C, Ludwig-Portugall I. Regulatory T cells use programmed death 1 ligands to directly suppress autoreactive B cells in vivo. Proc Natl Acad Sci U S A. 2012;109:10468–73. doi: 10.1073/pnas.1201131109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Iikuni N, Lourenço EV, Hahn BH, La Cava A. Cutting edge: regulatory T cells directly suppress B cells in systemic lupus erythematosus. J Immunol. 2009;183:1518–22. doi: 10.4049/jimmunol.0901163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Richards H, Williams A, Jones E, Hindley J, Godkin A, Simon AK, Gallimore A. Novel role of regulatory T cells in limiting early neutrophil responses in skin. Immunology. 2010;131:583–92. doi: 10.1111/j.1365-2567.2010.03333.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lewkowicz N, Klink M, Mycko MP, Lewkowicz P. Neutrophil - CD4+CD25+ T regulatory cell interactions: a possible new mechanism of infectious tolerance. Immunobiology. 2013;218:455–64. doi: 10.1016/j.imbio.2012.05.029. [DOI] [PubMed] [Google Scholar]
- 44.Tiemessen MM, Jagger AL, Evans HG, Van Herwijnen MJC, John S, Taams LS. CD4+CD25+Foxp3+ regulatory T cells induce alternative activation of human monocytes/macrophages. Proc Natl Acad Sci U S A. 2007;104:19446–51. doi: 10.1073/pnas.0706832104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Romano M, Fanelli G, Tan N, Nova-Lamperti E, McGregor R, Lechler RI, Lombardi G, Scottà C. Expanded regulatory T cells induce alternatively activated monocytes with a reduced capacity to expand T helper-17 cells. Front Immunol. 2018;9. doi: 10.3389/fimmu.2018.01625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kullberg MC, Hay V, Cheever AW, Mamura M, Sher A, Letterio JJ, Shevach EM, Piccirillo CA. TGF-β1 production by CD4+CD25+ regulatory T cells is not essential for suppression of intestinal inflammation. Eur J Immunol. 2005;35:2886–95. doi: 10.1002/eji.200526106. [DOI] [PubMed] [Google Scholar]
- 47.Maj T, Wang W, Crespo J, Zhang H, Wang W, Wei S, Zhao L, Vatan L, Shao I, Szeliga W, et al. Oxidative stress controls regulatory T cell apoptosis and suppressor activity and PD-L1-blockade resistance in tumor. Nat Immunol. 2017;18:1332–41. doi: 10.1038/ni.3868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tang Q, Bluestone JA. The Foxp3+ regulatory T cell: a jack of all trades, master of regulation. Nat Immunol. 2008;9:239–44. doi: 10.1038/ni1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ziegler SF. FOXP3: of mice and men. Annu Rev Immunol. 2006;24:209–26. doi: 10.1146/annurev.immunol.24.021605.090547. [DOI] [PubMed] [Google Scholar]
- 50.Brunkow ME, Jeffery EW, Hjerrild KA, Paeper B, Clark LB, Yasayko SA, Wilkinson JE, Galas D, Ziegler SF, Ramsdell F. Disruption of a new forkhead/winged-helix protein, scurfin, results in the fatal lymphoproliferative disorder of the scurfy mouse. Nat Genet. 2001;27:68–73. doi: 10.1038/83784. [DOI] [PubMed] [Google Scholar]
- 51.Wildin RS, Ramsdell F, Peake J, Faravelli F, Casanova J-L-R, Buist N, Levy-Lahad E, Mazzella M, Goulet O, Perroni L, et al. The immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) is caused by mutations of FOXP3. Nat Genet. 2001;27:20–21. doi: 10.1038/83713. [DOI] [PubMed] [Google Scholar]
- 52.Shimizu J, Yamazaki S, Takahashi T, and Ishida YSS. Stimulation of CD25+CD4+ regulatory T cells through GITR breaks immunological self-tolerance. Nat Immunol. 2002;3 2 135–142. doi: 10.1038/ni759 [DOI] [PubMed] [Google Scholar]
- 53.Longhi MS, Ma Y, Mieli-Vergani G, Vergani D. Regulatory T cells in autoimmune hepatitis. J Hepatol. 2012;57:932–33. doi: 10.1016/j.jhep.2012.05.022. [DOI] [PubMed] [Google Scholar]
- 54.Aghabi YO, Yasin A, Kennedy JI, Davies SP, Butler AE, and Stamataki Z. Targeting enclysis in liver autoimmunity, transplantation, viral infection and cancer. Front Immunol. 2021;12 662134. doi: 10.3389/fimmu.2021.662134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Brunstein CG, Miller JS, Cao Q, McKenna DH, Hippen KL, Curtsinger J, DeFor T, Levine BL, June CH, Rubinstein P, et al. Infusion of ex vivo expanded T regulatory cells in adults transplanted with umbilical cord blood: safety profile and detection kinetics. Blood. 2011;117:1061–70. doi: 10.1182/blood-2010-07-293795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dijke IE, Hoeppli RE, Ellis T, Pearcey J, Huang Q, McMurchy AN, Boer K, Peeters AMA, Aubert G, Larsen I, et al. Discarded human thymus is a novel source of stable and long-lived therapeutic regulatory T cells. Am J Transplant. 2016;16:58–71. doi: 10.1111/ajt.13456. [DOI] [PubMed] [Google Scholar]
- 57.Safinia N, Vaikunthanathan T, Fraser H, Thirkell S, Lowe K, Blackmore L, Whitehouse G, Martinez-Llordella M, Jassem W, Sanchez-Fueyo A, et al. Successful expansion of functional and stable regulatory T cells for immunotherapy in liver transplantation. Oncotarget. 2016;7:7563–77. doi: 10.18632/oncotarget.6927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Marek-Trzonkowska N, Myśliwiec M, Dobyszuk A, Grabowska M, Derkowska I, Juścińska J, Owczuk R, Szadkowska A, Witkowski P, Młynarski W, et al. Therapy of type 1 diabetes with CD4+CD25highCD127-regulatory T cells prolongs survival of pancreatic islets - Results of one year follow-up. Clin Immunol. 2014;153:23–30. doi: 10.1016/j.clim.2014.03.016. [DOI] [PubMed] [Google Scholar]
- 59.Fraser H, Safinia N, Grageda N, Thirkell S, Lowe K, Fry LJ, Scottá C, Hope A, Fisher C, Hilton R, et al. A rapamycin-based GMP-compatible process for the isolation and expansion of regulatory T cells for clinical trials. Mol Ther - Methods Clin Dev. 2018;8:198–209. doi: 10.1016/j.omtm.2018.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Janssens I, and Cools N. Regulating the regulators: is introduction of an antigen-specific approach in regulatory T cells the next step to treat autoimmunity? Cell Immunol. 2020;358 104236. doi: 10.1016/j.cellimm.2020.104236 [DOI] [PubMed] [Google Scholar]
- 61.Wu D, Wong MQ, Vent-Schmidt J, Boardman DA, Steiner TS, and Levings MK. A method for expansion and retroviral transduction of mouse regulatory T cells. J Immunol Methods. 2021;488 112931. doi: 10.1016/j.jim.2020.112931 [DOI] [PubMed] [Google Scholar]
- 62.Roth-Walter F, Adcock IM, Benito-Villalvilla C, Bianchini R, Bjermer L, Boyman O, Caramori G, Cari L, Fan Chung K, Diamant Z, et al. Immune modulation via T regulatory cell enhancement: disease-modifying therapies for autoimmunity and their potential for chronic allergic and inflammatory diseases—An EAACI position paper of the Task Force on Immunopharmacology (TIPCO). Allergy Eur J Allergy Clin Immunol. 2021;76:90–113. doi: 10.1111/all.14478. [DOI] [PubMed] [Google Scholar]
- 63.Eggenhuizen PJ, Ng BH, Ooi JD. Treg enhancing therapies to treat autoimmune diseases. Int J Mol Sci. 2020;21:1–18. doi: 10.3390/ijms21197015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Desreumaux P, Foussat A, Allez M, Beaugerie L, Hébuterne X, Bouhnik Y, Nachury M, Brun V, Bastian H, Belmonte N, et al. Safety and efficacy of antigen-specific regulatory T-cell therapy for patients with refractory Crohn’s disease. Gastroenterology. 2012:143. doi: 10.1186/1471-230X-12-143 [DOI] [PubMed] [Google Scholar]
- 65.Goldberg R, Scotta C, Cooper D, Nissim-Eliraz E, Nir E, Tasker S, Irving PM, Sanderson J, Lavender P, Ibrahim F, et al. Correction of defective T-Regulatory cells from patients with crohn’s disease by ex vivo ligation of retinoic acid receptor-α. Gastroenterology. 2019;156:1775–87. doi: 10.1053/j.gastro.2019.01.025. [DOI] [PubMed] [Google Scholar]
- 66.Bluestone JA, Buckner JH, Fitch M, Gitelman SE, Gupta S, Hellerstein MK, Herold KC, Lares A, Lee MR, Li K. Type 1 diabetes immunotherapy using polyclonal Tregs. Sci Transl Med. 2015. Nov 25;7:315ra189. doi: 10.1126/scitranslmed.aad4134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dall’Era M, Pauli ML, Remedios K, Taravati K, Sandova PM, Putnam AL, Lares A, Haemel A, Tang Q, Hellerstein M, et al. Adoptive Treg cell therapy in a patient with systemic lupus erythematosus. Arthritis Rheumatol. 2019;71:431–40. doi: 10.1002/art.40737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Terry LV, Oo YH. The next frontier of regulatory T cells: promising immunotherapy for autoimmune diseases and organ transplantations. Front Immunol. 2020;11:11. doi: 10.3389/fimmu.2020.00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Groux H, O’Garra A, Bigler M, Rouleau M, Antonenko S, De Vries JE, Roncarolo MG. A CD4+ T-cell subset inhibits antigen-specific T-cell responses and prevents colitis. Nature. 1997;389:737–42. doi: 10.1038/39614. [DOI] [PubMed] [Google Scholar]
- 70.Mottet C, Uhlig HH, Powrie F. Cutting edge: cure of colitis by CD4 + CD25 + Regulatory T Cells. J Immunol. 2003;170:3939–43. doi: 10.4049/jimmunol.170.8.3939. [DOI] [PubMed] [Google Scholar]
- 71.Goswami TK, Joardar SN, Ram GC, Banerjee R, Singh DK. Association of mycobacterium paratuberculosis in Crohn’s disease and Johne’s disease: a possible zoonotic threat. Curr Sci. 2000;79:1076–81. [Google Scholar]
- 72.Maul J, Loddenkemper C, Mundt P, Berg E, Giese T, Stallmach A, Zeitz M, Duchmann R. Peripheral and intestinal regulatory CD4+CD25high T cells in inflammatory bowel disease. Gastroenterology. 2005;128:1868–78. doi: 10.1053/j.gastro.2005.03.043. [DOI] [PubMed] [Google Scholar]
- 73.Foussat A, Cottrez F, Brun V, Fournier N, Breittmayer J-P, Groux H. A comparative study between T regulatory Type 1 and CD4 + CD25 + T cells in the control of inflammation. J Immunol. 2003;171:5018–26. doi: 10.4049/jimmunol.171.10.5018. [DOI] [PubMed] [Google Scholar]
- 74.Canavan JB, Scottà C, Vossenkämper A, Goldberg R, Elder MJ, Shoval I, Marks E, Stolarczyk E, Lo JW, Powell N, et al. Developing in vitro expanded CD45RA+ regulatory T cells as an adoptive cell therapy for Crohn’s disease. Gut. 2016;65:584–94. doi: 10.1136/gutjnl-2014-306919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Brimnes J, Allez M, Dotan I, Shao L, Nakazawa A, Mayer L. Defects in CD8 + regulatory T cells in the lamina propria of patients with inflammatory bowel disease. J Immunol. 2005;174:5814–22. doi: 10.4049/jimmunol.174.9.5814. [DOI] [PubMed] [Google Scholar]
- 76.Marwaha AK, Crome SQ, Panagiotopoulos C, Berg KB, Qin H, Ouyang Q, Xu L, Priatel JJ, Levings MK, Tan R. Cutting edge: increased IL-17–secreting T cells in children with new-onset type 1 diabetes. J Immunol. 2010;185:3814–18. doi: 10.4049/jimmunol.1001860. [DOI] [PubMed] [Google Scholar]
- 77.Baeten P, Van Zeebroeck L, Kleinewietfeld M, Hellings N, Broux B. Improving the efficacy of regulatory T cell therapy. Clin Rev Allergy Immunol. 2021. doi: 10.1007/s12016-021-08866-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sugiyama H, Matsue H, Nagasaka A, Nakamura Y, Tsukamoto K, Shibagaki N, Kawamura T, Kitamura R, Ando N, Shimada S. CD4+CD25high regulatory T cells are markedly decreased in blood of patients with pemphigus vulgaris. Dermatology. 2007;214:210–20. doi: 10.1159/000099585. [DOI] [PubMed] [Google Scholar]
- 79.Yokoyama T, Matsuda S, Takae Y, Wada N, Nishikawa T, Amagai M, Koyasu S. Antigen-independent development of Foxp31 regulatory T cells suppressing autoantibody production in experimental pemphigus vulgaris. Int Immunol. 2011;23:365–73. doi: 10.1093/intimm/dxr020. [DOI] [PubMed] [Google Scholar]
- 80.Schmidt T, Willenborg S, Hünig T, Deeg CA, Sonderstrup G, Hertl M, Eming R. Induction of T regulatory cells by the superagonistic anti-CD28 antibody D665 leads to decreased pathogenic IgG autoantibodies against desmoglein 3 in a HLA-transgenic mouse model of pemphigus vulgaris. Exp Dermatol. 2016;25:293–98. doi: 10.1111/exd.12919. [DOI] [PubMed] [Google Scholar]
- 81.Izumi K, Bieber K, Ludwig RJ. Current clinical trials in pemphigus and pemphigoid. Front Immunol. 2019;10:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Buitrago-Molina LE, Pietrek J, Noyan F, Schlue J, Manns MP, Wedemeyer H, Hardtke-Wolenski M, and Jaeckel E. Treg-specific IL-2 therapy can reestablish intrahepatic immune regulation in autoimmune hepatitis. J Autoimmun. 2021;117 102591. doi: 10.1016/j.jaut.2020.102591 [DOI] [PubMed] [Google Scholar]
- 83.Longhi MS, Mieli-Vergani G, and Vergani D. Regulatory T cells in autoimmune hepatitis: un updated overview. J Autoimmun. 2021;119 102619. doi: 10.1016/j.jaut.2021.102619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sebode M, Hartl J, Vergani D, Lohse AW. Autoimmune hepatitis: from current knowledge and clinical practice to future research agenda. Liver Int. 2018;38:15–22. doi: 10.1111/liv.13458. [DOI] [PubMed] [Google Scholar]
- 85.Longhi MS, Ma Y, Bogdanos DP, Cheeseman P, Mieli-Vergani G, Vergani D. Impairment of CD4+CD25+ regulatory T-cells in autoimmune liver disease. J Hepatol. 2004;41:31–37. doi: 10.1016/j.jhep.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 86.Peiseler M, Sebode M, Franke B, Wortmann F, Schwinge D, Quaas A, Baron U, Olek S, Wiegard C, Lohse AW, et al. FOXP3+ regulatory T cells in autoimmune hepatitis are fully functional and not reduced in frequency. J Hepatol. 2012;57:125–32. doi: 10.1016/j.jhep.2012.02.029. [DOI] [PubMed] [Google Scholar]
- 87.Jeffery HC, Jeffery LE, Lutz P, Corrigan M, Webb GJ, Hirschfield GM, Adams DH, Oo YH. Low-dose interleukin-2 promotes STAT-5 phosphorylation, Treg survival and CTLA-4-dependent function in autoimmune liver diseases. Clin Exp Immunol. 2017;188:394–411. doi: 10.1111/cei.12940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lim TY, Martinez-Llordella M, Kodela E, Gray E, Heneghan MA, Sanchez-Fueyo A. Low-dose interleukin-2 for refractory autoimmune hepatitis. Hepatology. 2018;68:1649–52. doi: 10.1002/hep.30059. [DOI] [PubMed] [Google Scholar]
- 89.Taubert R, Hardtke-Wolenski M, Noyan F, Wilms A, Baumann AK, Schlue J, Olek S, Falk CS, Manns MP, Jaeckel E. Intrahepatic regulatory T cells in autoimmune hepatitis are associated with treatment response and depleted with current therapies. J Hepatol. 2014;61:1106–14. doi: 10.1016/j.jhep.2014.05.034. [DOI] [PubMed] [Google Scholar]
- 90.Esensten JH, Muller YD, Bluestone JA, Tang Q. Regulatory T-cell therapy for autoimmune and autoinflammatory diseases: the next frontier. J Allergy Clin Immunol. 2018;142:1710–18. doi: 10.1016/j.jaci.2018.10.015. [DOI] [PubMed] [Google Scholar]
- 91.Tsokos GC, Lo MS, Reis PC, Sullivan KE. New insights into the immunopathogenesis of systemic lupus erythematosus. Nat Rev Rheumatol. 2016;12:716–30. doi: 10.1038/nrrheum.2016.186. [DOI] [PubMed] [Google Scholar]
- 92.Humrich JY, Morbach H, Undeutsch R, Enghard P, Rosenberger S, Weigert O, Kloke L, Heimann J, Gaber T, Brandenburg S, et al. Homeostatic imbalance of regulatory and effector T cells due to IL-2 deprivation amplifies murine lupus. Proc Natl Acad Sci U S A. 2010;107:204–09. doi: 10.1073/pnas.0903158107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ohl K, Tenbrock K. Regulatory T cells in systemic lupus erythematosus. Eur J Immunol. 2015;45(2):344–55. doi: 10.1002/eji.201344280. [DOI] [PubMed] [Google Scholar]
- 94.Schmidt A, Rieger CC, Venigalla RK, Éliás S, Max R, Lorenz HM, Gröne HJ, Krammer PH, Kuhn A. Analysis of FOXP3+ regulatory T cell subpopulations in peripheral blood and tissue of patients with systemic lupus erythematosus. Immunol Res. 2017;65:551–63. doi: 10.1007/s12026-017-8904-4. [DOI] [PubMed] [Google Scholar]
- 95.Lee HY, Hong YK, Yun HJ, Kim YM, Kim JR, Yoo WH. Altered frequency and migration capacity of CD4+ CD25+ regulatory T cells in systemic lupus erythematosus. Rheumatology. 2008;47:789–94. doi: 10.1093/rheumatology/ken108. [DOI] [PubMed] [Google Scholar]
- 96.He J, Zhang X, Wei Y, Sun X, Chen Y, Deng J, Jin Y, Gan Y, Hu X, Jia R, et al. Low-dose interleukin-2 treatment selectively modulates CD4+ T cell subsets in patients with systemic lupus erythematosus. Nat Med. 2016;22:991–93. doi: 10.1038/nm.4148. [DOI] [PubMed] [Google Scholar]
- 97.Mizui M, Tsokos GC. Targeting regulatory T cells to treat patients with systemic lupus erythematosus. Front Immunol. 2018;9:786. doi: 10.3389/fimmu.2018.00786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Smolen JS, Aletaha D, McInnes IB. Rheumatoid arthritis. Lancet. 2016;388(10055):2023–38. doi: 10.1016/S0140-6736(16)30173-8. [DOI] [PubMed] [Google Scholar]
- 99.Cao D, van Vollenhoven R, Klareskog L, Trollmo C, Malmström V. CD25brightCD4+ regulatory T cells are enriched in inflamed joints of patients with chronic rheumatic disease. Arthritis Res Ther. 2004;6:6. doi: 10.1186/ar1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Han GM, O’Neil-Andersen NJ, Zurier RB, Lawrence DA. CD4+CD25high T cell numbers are enriched in the peripheral blood of patients with rheumatoid arthritis. Cell Immunol. 2008;253(1–2):92–101. doi: 10.1016/j.cellimm.2008.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Liu MF, Wang CR, Fung LL, Lin LH, Tsai CN. The presence of cytokine-suppressive CD4+CD25+ T cells in the peripheral blood and synovial fluid of patients with rheumatoid arthritis. Scand J Immunol. 2005;62:312–17. doi: 10.1111/j.1365-3083.2005.01656.x. [DOI] [PubMed] [Google Scholar]
- 102.Lawson CA, Brown AK, Bejarano V, Douglas SH, Burgoyne CH, Greenstein AS, Boylston AW, Emery P, Ponchel F, Isaacs JD. Early rheumatoid arthritis is associated with a deficit in the CD4+CD25high regulatory T cell population in peripheral blood. Rheumatology. 2006;45:1210–17. doi: 10.1093/rheumatology/kel089. [DOI] [PubMed] [Google Scholar]
- 103.Flores-Borja F, Jury EC, Mauri C, Ehrenstein MR. Defects in CTLA-4 are associated with abnormal regulatory T cell function in rheumatoid arthritis. Proc Natl Acad Sci U S A. 2008;105:19396–401. doi: 10.1073/pnas.0806855105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Nadkarni S, Mauri C, Ehrenstein MR. Anti-TNF-α therapy induces a distinct regulatory T cell population in patients with rheumatoid arthritis via TGF-β. J Exp Med. 2007;204:33–39. doi: 10.1084/jem.20061531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Miyara M, Gorochov G, Ehrenstein M, Musset L, Sakaguchi S, Amoura Z. Human FoxP3 + regulatory T cells in systemic autoimmune diseases. Autoimmun Rev. 2011;10:744–55. doi: 10.1016/j.autrev.2011.05.004. [DOI] [PubMed] [Google Scholar]
- 106.Morita T, Shima Y, Wing JB, Sakaguchi S, Ogata A, and Kumanogoh A. The proportion of regulatory T cells in patients with rheumatoid arthritis: a meta-Analysis. PLoS One. 2016;11 9 e0162306 doi: 10.1371/journal.pone.0162306. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kelchtermans H, De Klerck B, Mitera T, Van Balen M, Bullens D, Billiau A, Leclercq G, Matthys P. Defective CD4+CD25+ regulatory T cell functioning in collagen-induced arthritis: an important factor in pathogenesis, counter-regulated by endogenous IFN-gamma. Arthritis Res Ther. 2005;7:402–15. doi: 10.1186/ar1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Morgan ME, Flierman R, Van Duivenvoorde LM, Witteveen HJ, Van Ewijk W, Van Laar JM, De Vries RRP, Toes REM. Effective treatment of collagen-induced arthritis by adoptive transfer of CD25+ regulatory T cells. Arthritis Rheum. 2005;52:2212–21. doi: 10.1002/art.21195. [DOI] [PubMed] [Google Scholar]
- 109.Zaiss MM, Axmann R, Zwerina J, Polzer K, Gückel E, Skapenko A, Schulze-Koops H, Horwood N, Cope A, Schett G. Treg cells suppress osteoclast formation: a new link between the immune system and bone. Arthritis Rheum. 2007;56:4104–12. doi: 10.1002/art.23138. [DOI] [PubMed] [Google Scholar]
- 110.van Amelsfort JM, van Roon JA, Noordegraaf M, Jacobs KM, Bijlsma JW, Lafeber FP, Taams LS. Proinflammatory mediator-induced reversal of CD4+,CD25+ regulatory T cell-mediated suppression in rheumatoid arthritis. Arthritis Rheum. 2007;56:732–42. doi: 10.1002/art.22414. [DOI] [PubMed] [Google Scholar]
- 111.LeRoy EC, Medsger TJ. Criteria for the classification of early systemic sclerosis. J Rheumatol. 2001;28:1573–76. [PubMed] [Google Scholar]
- 112.Frantz C, Auffray C, Avouac J, Allanore Y. Regulatory T cells in systemic sclerosis. Front Immunol. 2018;9. doi: 10.3389/fimmu.2018.02356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Antiga E, Quaglino P, Bellandi S, Volpi W, Del Bianco E, Comessatti A, Osella-Abate S, De Simone C, Marzano A, Bernengo MG, et al. Regulatory T cells in the skin lesions and blood of patients with systemic sclerosis and morphoea. Br J Dermatol. 2010;162:1056–63. doi: 10.1111/j.1365-2133.2010.09633.x. [DOI] [PubMed] [Google Scholar]
- 114.Kataoka H, Yasuda S, Fukaya S, Oku K, Horita T, Atsumi T, Koike T. Decreased expression of Runx1 and lowered proportion of Foxp3+ CD25+ CD4+ regulatory T cells in systemic sclerosis. Mod Rheumatol. 2015;25:90–95. doi: 10.3109/14397595.2014.899736. [DOI] [PubMed] [Google Scholar]
- 115.Klein S, Kretz CC, Ruland V, Stumpf C, Haust M, Hartschuh W, Hartmann M, Enk A, Suri-Payer E, Oberle N, et al. Reduction of regulatory T cells in skin lesions but not in peripheral blood of patients with systemic scleroderma. Ann Rheum Dis. 2011;70:1475–81. doi: 10.1136/ard.2009.116525. [DOI] [PubMed] [Google Scholar]
- 116.Tsukamoto H, Nagafuji K, Horiuchi T, Mitoma H, Niiro H, Arinobu Y, Inoue Y, To K, Miyamoto T, Iwasaki H, et al. Analysis of immune reconstitution after autologous CD34 + stem/progenitor cell transplantation for systemic sclerosis: predominant reconstitution of Th1 CD4 + t cells. Rheumatology. 2011;50:944–52. doi: 10.1093/rheumatology/keq414. [DOI] [PubMed] [Google Scholar]
- 117.Vonk MC, Marjanovic Z, Van Den Hoogen FHJ, Zohar S, Schattenberg AVMB, Fibbe WE, Larghero J, Gluckman E, Van Laar JM, Farge D, et al. Long-term follow-up results after autologous haematopoietic stem cell transplantation for severe systemic sclerosis. Ann Rheum Dis. 2008;67:98–104. doi: 10.1136/ard.2007.071464. [DOI] [PubMed] [Google Scholar]
- 118.Verstappen GM, Kroese FGM, Bootsma H. T cells in primary Sjögren’s syndrome: targets for early intervention. Rheumatol (United Kingdom). 2021;60:3088–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Liu MF, Lin LH, Weng CT, Weng M. Decreased CD4+CD25+bright T cells in peripheral blood of patients with primary Sjogren’s syndrome. Lupus. 2008;17:34–39. doi: 10.1177/0961203307085248. [DOI] [PubMed] [Google Scholar]
- 120.Christodoulou MI, Kapsogeorgou EK, Moutsopoulos NM, Moutsopoulos HM. Foxp3+ T-regulatory cells in Sjögren’s syndrome: correlation with the grade of the autoimmune lesion and certain adverse prognostic factors. Am J Pathol. 2008;173:1389–96. doi: 10.2353/ajpath.2008.080246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Nussbaum L, Chen YL, Ogg GS. Role of regulatory T cells in psoriasis pathogenesis and treatment. Br J Dermatol. 2021;184:14–24. doi: 10.1111/bjd.19380. [DOI] [PubMed] [Google Scholar]
- 122.Dong F, Li XH, Zhang KM, Yin GH. Functional characterization of CD4+ CD25+ regulatory T cells differentiated in vitro from bone marrow-derived hematopoietic cells of psoriatic patients. J Clin Dermatology. 2008;37:207–09. [DOI] [PubMed] [Google Scholar]
- 123.Zhang L, Yang XQ, Cheng J, Hui RS, Gao TW. Increased Th17 cells are accompanied by FoxP3+ Treg cell accumulation and correlated with psoriasis disease severity. Clin Immunol. 2010;135:108–17. doi: 10.1016/j.clim.2009.11.008. [DOI] [PubMed] [Google Scholar]
- 124.Sugiyama H, Gyulai R, Toichi E, Garaczi E, Shimada S, Stevens SR, McCormick TS, Cooper KD. Dysfunctional blood and target tissue CD4 + CD25 high regulatory T cells in psoriasis: mechanism underlying unrestrained pathogenic effector T cell proliferation. J Immunol. 2005;174:164–73. doi: 10.4049/jimmunol.174.1.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Yun WJ, Lee DW, Chang SE, Yoon GS, Huh JR, Won CH, Lee MW, Kim SE, Kim BJ, Moon KC, et al. Role of CD4+CD25high+FOXP3+ regulatory T cells in psoriasis. Ann Dermatol. 2010;22:397–403. doi: 10.5021/ad.2010.22.4.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Huang Z, Li W, Su W. Tregs in autoimmune uveitis. Adv Exp Med Biol. 2021;1278:205–27. doi: 10.1007/978-981-15-6407-9_11. [DOI] [PubMed] [Google Scholar]
- 127.Wu Y, Luo J, Garden OA, Christensen CB, Lildal T, Pedersen M, Magnusson M, Borghammer P, Ovesen T. Immunoregulatory cells in myasthenia gravis. Front Neurol. 2020;11:11. doi: 10.3389/fneur.2020.00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Herrnstadt GR, Steinmetz OM. The role of Treg subtypes in glomerulonephritis. Cell Tissue Res. 2020;385(2):293–304. doi: 10.1007/s00441-020-03359-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Neumann K, Tiegs G. Immune regulation in renal inflammation. Cell Tissue Res. 2021;385(2):305–22. doi: 10.1007/s00441-020-03351-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Riley JL, June CH, Blazar BR. Human T regulatory cells as therapeutic agents: take a billion or so of these and call me in the morning. Immunity. 2010;30:656–65. doi: 10.1016/j.immuni.2009.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Golab K, Leveson-Gower D, Wang XJ, Grzanka J, Marek-Trzonkowska N, Krzystyniak A, Millis JM, Trzonkowski P, Witkowski P. Challenges in cryopreservation of regulatory T cells (Tregs) for clinical therapeutic applications. Int Immunopharmacol. 2013;16:371–75. doi: 10.1016/j.intimp.2013.02.001. [DOI] [PubMed] [Google Scholar]


