ABSTRACT
India approved COVID-19 vaccine called Covaxin, developed by the Indian Council of Medical Research and Bharat Biotech Ltd. The primary objective of the study was to estimate the effectiveness of Covaxin in preventing breakthrough SARS-CoV-2 infection in healthcare workers (HCWs). A test-negative matched case-control study was conducted among HCWs of tertiary care hospital in Eastern India. Any HCW who tested positive for COVID-19 using RT-PCR during April and May 2021 was taken as the case. The HCWs who tested negative for COVID-19 by RT-PCR were considered as controls after matching with the date of testing and profession of the cases. Vaccination data were collected from the institution’s vaccine database and recall. In case of discrepancy, it was confirmed from the CoWIN portal (cowin.gov.in). The sample size was 670 participants (335 pairs). Conditional logistic regression models were used to calculate the adjusted odds ratio for breakthrough SARS-CoV-2 infection. Vaccine effectiveness was calculated using the following formula: VE = (1-aOR) × 100%. Sensitivity analysis was done for effectiveness of Covaxin, excluding Covishield vaccination. The mean age of participants was 29.1 years (SD = 7.1), and the majority were males (55.2%). Among the study participants, 60% were completely vaccinated, 18.51% were partially vaccinated, and 21.49% were unvaccinated. After adjusting for age, gender, type of household and past history of COVID-19 disease in conditional logistic models, the vaccine effectiveness was 22% (aOR 0.78, 95% CI: 0.52–1.17; p = .233). Sensitivity analysis with Covaxin showed an effectiveness of 29% (aOR 0.71, 95% CI: 0.47–1.08; p = .114) for preventing breakthrough SARS-CoV-2 infection.
KEYWORDS: COVID-19, vaccine, effectiveness, covaxin, India
Introduction
The COVID-19 pandemic, which was declared in early 2020, is still going on since its origin.1 Worldwide, millions of people have died due to the pandemic, with hundreds of millions being infected.2,3 The COVID-19 cases in India are not increasing and a third wave is yet to hit.4,5 The emergence of mutant strains of the virus has further complicated the situation.6 As per the World Health Organization (WHO) reports, the strain B.1.617 found in India is now a variant of global concern.7
The primary prevention and control measures against COVID-19 are wearing face masks, hand hygiene, and social distancing. With the reports emerging that the virus is airborne, the impact of these measures in COVID-19 prevention and control is likely to be lower than previously thought.8,9 Similarly, many drugs that were repurposed to be used for COVID-19 have been shown to have a low to almost nil effect.10,11 At this point, a vaccine is the real game-changer. Vaccines were developed and approved with unprecedented speed and deployed with emergency use authorization.12 New vaccine technologies like m-RNA were developed and approved in record time.13 In India, COVID-19 vaccines approved for use were Covaxin and Covishield. Covaxin is an indigenously developed vaccine by the joint venture of Indian Council of Medical Research (ICMR), National Institute of Virology and Bharat Biotech.14 As of December 2, 2021, 83.5% of the adult population of India (117 million doses) are vaccinated with at least a single dose and 46.7% with a double dose of vaccine. Among the vaccine doses administered, 11.1% were Covaxin.15
Covaxin uses time-tested Whole-Virion Inactivated Vero cell-derived platform technology that used Alhydroxiquim-II as adjuvant. On 16th January 2021, it was given emergency use authorization in a clinical trial mode. The interim analysis showed it to have an efficacy of 81%.16 Covaxin-induced antibodies were also found to neutralize the variants B.1.1.7, B.1.1.28, B.1.1.28, B.1.617, B.1.351 & B.1.617.2 of the SARS-CoV-2 virus.16,17 Subsequently, the interim results from Phase 3 trials showed an overall interim clinical efficacy of 81% and 100% efficacy against SARS-CoV-2 infection and severe COVID-19 disease, respectively.16 It is crucial now to understand the effectiveness of these vaccines in the real-world scenario.18,19 The effectiveness of a vaccine may differ from its efficacy based on the disease outcome studied, population administered, and the characteristics of the new strains of the virus. The primary objective of the study was to estimate the effectiveness of Covaxin in preventing breakthrough SARS-CoV-2 infection in healthcare workers (HCWs).
Methodology
This was a hospital-based test-negative case-control study conducted among the HCWs at the All India Institute of Medical Sciences (AIIMS), Bhubaneswar during February 2021 to July 2021. AIIMS Bhubaneswar is a tertiary care hospital from eastern India, which provides both COVID and non-COVID care. The institute started vaccination of HCWs from 16th January 2021. The HCWs of the institute were given Covaxin. However, some HCWs who took vaccine outside the institute received Covishield. The institute had a robust policy in place related to COVID-19 appropriate behavior (CAB) in the workplace (including office orders, sensitization programs, and financial penalties). The primary objective was to estimate the vaccine effectiveness of the COVID-19 vaccine (Covaxin) in protecting against RT-PCR positive SARS-CoV-2 infection. We followed the methods recommended by WHO to evaluate vaccine effectiveness.20 Assuming vaccine effectiveness of 70% and expected COVID-19 vaccine coverage among controls as 75% with a desired precision-width of 20% with a case-control ratio of 1:1, a sample size of 670 participants (335 pairs) was calculated. Assuming the non-response rate as 15%, the final sample size required was 385 pairs (770 participants). In order to estimate the sample size, the WHO sample size calculator for the evaluation of COVID-19 vaccine effectiveness was used.21
Any HCW who tested positive for COVID-19 using RT-PCR during April and May 2021 was taken as the case. HCWs who tested negative for COVID-19 by RT-PCR were considered as controls and selected after matching with date of testing and profession of the cases. When more than one controls were available, the control with the closest age was selected. The severity of COVID-19 disease was classified based on the Govt. of India and AIIMS, New Delhi guidelines.22 Vaccine breakthrough infection for COVID-19 is defined as the detection of SARS-CoV-2 RNA or antigen in a respiratory specimen collected from a person ≥14 days after receipt of all recommended doses of authorized COVID-19 vaccine.23 The vaccination status of the participants was classified as “completely vaccinated” if they have taken two doses of vaccine and completed 14 days before getting tested. A partially vaccinated individual was defined as one who completed 14 days after the first dose of vaccine and does not fulfil the criteria of complete immunization. Those who had not received any vaccine dose or had not completed 14 days after first dose of vaccination were defined as “not immunised.” Vaccination data were collected from the institution’s vaccine database and self-report of the study participants. In case of any discrepancy, it was confirmed from the CoWIN portal (cowin.gov.in) and another verification call to the participants. A telephonic interview was done for each participant. The participants who could not be contacted even after three attempts were labeled as non-responders and excluded from the study. A semi-structured questionnaire prepared in Epicollect5 was used to collect the information on age, gender, designation, family type (alone/nuclear/extended), symptoms of COVID-19, RT-PCR test details, disease status (mild, moderate, and severe) if COVID-19 positive, type of isolation, involvement in patient care, contact history, and past history of COVID-19 disease.
Data was analyzed using STATA 15.0 software after data cleaning in MS Excel. Categorical variables were presented as proportion and continuous variables as mean and standard deviation. The association of breakthrough SARS-CoV-2 infection and vaccination status was calculated using odds ratio. Conditional logistic regression models were used to estimate adjusted odds ratio. In model 1, age, gender, and family type were included. In model 2, the past history of COVID-19 disease was added along with other variables of model 1. In model 3, the vaccination status was included along with variables of model 2. Vaccine effectiveness was calculated using the following formula: VE = (1-aOR) × 100%. The sensitivity analysis was done for vaccine effectiveness of Covaxin, excluding the Covishield vaccination.
Ethical approval was taken from the Institute Ethics Committee of All India Institute of Medical Sciences, Bhubaneswar (Reference no: AIIMS/CMFM/IM-NF/156). Informed consent (Telephonic) was taken from the participants after explaining the details of the study in the language they understood. The ethical committee approved this consent procedure considering the COVID-19 situation.
Results
From April 1, 2021 till May 31, 2021, 1822 HCWs of AIIMS Bhubaneswar were tested for COVID-19 by RT-PCR as per Govt. of India testing guideline.24 Out of 1822, 402 HCWs were positive for COVID-19. After matching the date of diagnosis and profession, 390 matched pairs were prepared from the hospital database. Ten HCWs did not give consent for participation, and there were 45 non-responders. Finally, 335 pairs (670 participants) were included in the analysis (Figure 1).
Figure 1.
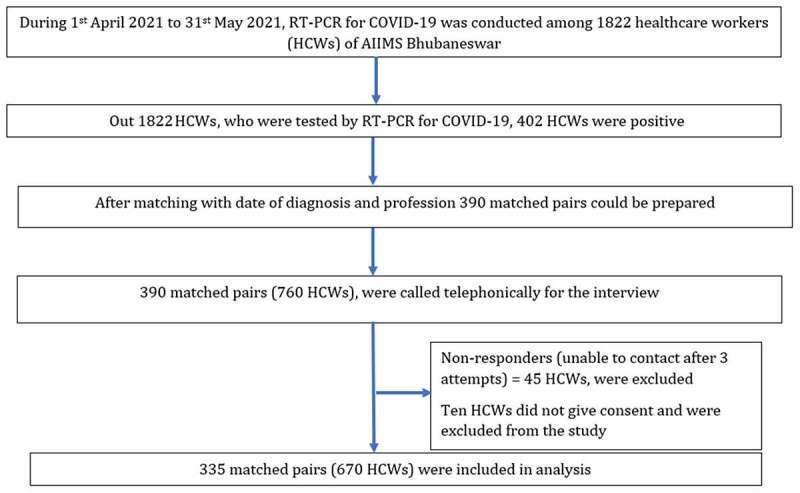
The flow diagram of study participants selection, data collection and analysis.
Mean (SD) age of our study participants was 29.1 (7.1) years. A majority of the participants, i.e., 408 (60.90%), were below 30 years of age. Out of all the participants, 370 (55.22%) were male. Half of the participants 321 (47.91%) lived in the nuclear family, followed by those living alone 217 (32.39%). More than one-third of participants i.e., 244 (36.42%) were nursing staff, followed by 196 (29.25%) supporting staff, 98 (14.63%) doctors and 76 (11.34%) students. Out of the 670, 375 (55.97%) had symptoms suggestive of COVID-19 at the time of testing. Out of 670 participants, 52 (7.76%) had past h/o of COVID-19 disease. Of the 52 participants, who had past h/o of COVID-19 disease, 21 participants had breakthrough SARS-CoV-2 infection. Of the 21 participants, 15 received Covaxin, 13 were completely immunized, and two were partially immunized. Among the 21 participants, two received Covishield. One of them was partially immunized, and the other was completely immunized. Data regarding the severity of past COVID-19 disease was not collected. A large proportion, i.e., 402 (60.00%) of the participants, had completed vaccination, 144 (21.49%) were unvaccinated, and the remaining 124 (18.51%) were partially vaccinated (Table 1). Out of 670 participants, most had received Covaxin, i.e., 554 (82.69%), only 25 (3.73%) participants had received Covishield. Out of 335 cases, 330 (98.5%) had mild COVID-19 disease, 5 (1.5%) had moderate and there were no severe cases. Among the 335 cases, 320 (95.5%) had undergone home isolation, 9 (2.69%) had undergone institutional isolation, while 6 (1.79%) cases got admitted to the hospital. Out of 335 cases, 228 HCWs could not identify the source of infection. Of those who could give contact history, 77 (22.1%) had workplace contact, 22 (6.6%) had social contact, and 11 (3.3%) had household contact (Table 2).
Table 1.
Characteristics of study participants (n = 670)
| Control (n = 335) |
Case (n = 335) |
Total (n = 670) |
P-value | |
|---|---|---|---|---|
| Mean age in years (Mean ± SD) | 29.2 ± 7.2 | 28.9 ± 7.1 | 29.1 ± 7.1 | .647 |
| Age groups (in years) | ||||
| <30 | 205 (61.19) | 203 (60.60) | 408 (60.90) | .797 |
| 30–39 | 98 (29.25) | 106 (31.64) | 204 (30.45) | |
| 40–49 | 22 (6.57) | 17 (5.07) | 39 (5.82) | |
| ≥50 | 10 (2.99) | 9 (2.69) | 19 (2.84) | |
| Gender | ||||
| Male | 184 (54.93) | 186 (55.52) | 370 (55.22) | .877 |
| Female | 151 (45.07) | 149 (44.48) | 300 (44.78) | |
| Type of household | ||||
| Extended family | 8 (2.39) | 15 (4.48) | 23 (3.43) | .273 |
| Nuclear family | 169 (50.45) | 152 (45.37) | 321 (47.91) | |
| With friend or roommate | 56 (16.72) | 53 (15.82) | 109 (16.27) | |
| Living alone | 102 (30.45) | 115 (34.33) | 217 (32.39) | |
| Profession | ||||
| Nursing staff | 122 (36.42) | 122 (36.42) | 244 (36.42) | 1.000 |
| Supporting staff | 98 (29.5) | 98 (29.5) | 196 (29.25) | |
| Doctors | 49 (14.63) | 49 (14.63) | 98 (14.63) | |
| Students | 38 (11.34) | 38 (11.34) | 76 (11.34) | |
| Others* | 24 (7.16) | 24 (7.16) | 48 (7.16) | |
| Administrative staff | 4 (1.19) | 4 (1.19) | 8 (1.19) | |
| Do you have symptoms** suggestive of COVID-19 at the time of diagnosis? | ||||
| Yes | 117 (34.93) | 258 (77.01) | 375 (55.97) | .000 |
| No | 218 (65.07) | 77 (22.99) | 295 (44.03) | |
| Whether you are involved in direct patient care***? | ||||
| Yes | 171 (51.04) | 171 (51.04) | 342 (51.04) | 1.000 |
| No | 164 (48.96) | 164 (48.96) | 328 (48.96) | |
| Do you have ****past h/o of COVID-19 disease? | ||||
| Yes | 31 (9.25) | 21 (6.27) | 52 (7.76) | .149 |
| No | 304 (90.75) | 314 (93.73) | 618 (92.24) | |
| Vaccination status | ||||
| Completely immunized | 208 (62.09) | 194 (57.91) | 402 (60.00) | .468 |
| Partial immunized | 61 (18.21) | 63 (18.81) | 124 (18.51) | |
| Not immunized | 66 (19.70) | 78 (23.28) | 144 (21.49) | |
*Other profession includes Electrical engineer, audiologist, cashier, clerk, cook, CSSD helper, data entry operator, Dietician, fire safety department staff, health educator, lab technician, lift technician, plumber, psychologist, pump operator and swasthya Mitra.
**Symptoms for COVID-19 as per WHO guideline (https://www.who.int/health-topics/coronavirus#tab=tab_3).
***doctors and nurses were involved direct patient care.
****Within last 12 months.
Table 2.
Profile of the COVID-19 cases (n = 335)
| Variables | Number of participants (n = 335) |
% |
|---|---|---|
| COVID-19 disease | ||
| Mild | 330 | 98.5 |
| Moderate | 5 | 1.5 |
| Severe | 0 | 0 |
| Type of isolation opted | ||
| Home Isolation | 320 | 95.5 |
| Institutional Isolation | 9 | 2.7 |
| Admitted to hospital | 6 | 1.8 |
| Testing reason | ||
| Symptoms suggestive of COVID-19 | 257 | 76.7 |
| High risk contact* | 49 | 14.6 |
| Traveling purpose | 21 | 6.3 |
| Others** | 8 | 2.4 |
| Types of contact | ||
| Workplace | 74 | 22.1 |
| Social | 22 | 6.6 |
| Home | 11 | 3.3 |
| Contact h/o cannot be identified | 228 | 68.0 |
*High risk contact (https://aiimsbhubaneswar.nic.in/writereaddata/upload/Documents/COVID.pdf).
**Other for a reason for testing COVID-19 RT-PCR includes-as a requirement for undergoing a surgical procedure and joining the institute.
After adjusting for age, gender, type of household and past h/o COVID-19 disease in conditional logistic models, the vaccine effectiveness was found to be 22% (p = .233) (Table 3). In sensitivity analysis, the vaccine effectiveness for Covaxin was found to be 29% (p = .114) (Table 4). The participants with age group 40–49 years [aOR 0.44, 95% CI: 0.13–1.45] and ≥50 years [aOR 0.58, 95% CI: 0.11–3.13] had a lower risk of SARS-CoV-2 infection compared to less than 30 years participants; however, it was not statistically significant. Female had similar risk [aOR 0.94, 95% CI: 0.61–1.43] of SARS-CoV-2 infection compared to male. Living in nuclear family [aOR 0.43, 95% CI: 0.17–1.08], living with friends [aOR 0.47, 95% CI: 0.17–1.27] and living alone (in hostels or outside) [aOR 0.69, 95% CI: 0.27–1.75] had lower risk of SARS-CoV-2 infection compared to participants living in extended family; however, none of the association was statistically significant. Similarly, the past history of COVID-19 disease [aOR 0.64, 95% CI: 0.35–1.15] and completely immunized status [aOR 0.78, 95% CI: 0.52–1.17] had less risk of SARS-CoV-2 infection without statistical significance (Table 3).
Table 3.
Conditional logistic regression models for associated factors of SARS-CoV-2 infection (n = 670)
| Variable | Unadjusted odds ratio (95% CI) |
p-value | Model-1 |
Model-2 |
Model-3 |
|||
|---|---|---|---|---|---|---|---|---|
| Adjusted odds ratio (95% CI) |
p-value | Adjusted odds ratio (95% CI) |
p-value | Adjusted odds ratio (95% CI) |
p-value | |||
| Age groups (in years) | ||||||||
| <30 | Reference | Reference | Reference | Reference | ||||
| 30–39 | 1.10 (0.65–1.88) | .719 | 1.07 (0.61–1.86) | .809 | 1.06 (0.61–1.85) | .836 | 1.08 (0.61–1.89) | .782 |
| 40–49 | 0.53 (0.17–1.69) | .285 | 0.46 (0.14–1.52) | .204 | 0.44 (0.13–1.44) | .173 | 0.44 (0.13–1.45) | .176 |
| ≥50 | 0.59 (0.11–3.08) | .534 | 0.49 (0.09–2.64) | .407 | 0.51 (0.09–2.72) | .428 | 0.58 (0.11–3.13) | .522 |
| Gender | ||||||||
| Male | Reference | Reference | Reference | Reference | ||||
| Female | 0.95 (0.64–1.44) | .837 | 0.96 (0.63–1.45) | .833 | 0.94 (0.62–1.43) | .776 | 0.94 (0.61–1.43) | .779 |
| Type of household | ||||||||
| Extended family | Reference | Reference | Reference | Reference | ||||
| Nuclear family | 0.45 (0.18–1.09) | .079 | 0.42 (0.17–1.03) | .058 | 0.41 (0.17–1.01) | .052 | 0.43 (0.17–1.08) | .072 |
| With friend or roommate | 0.49 (0.19–1.26) | .137 | 0.45 (0.17–1.17) | .101 | 0.44 (0.16–1.15) | .094 | 0.47 (0.18–1.27) | .136 |
| Living alone (either hostels or outside) | 0.69 (0.28–1.72) | .429 | 0.65 (0.26–1.63) | .358 | 0.62 (0.25–1.58) | .322 | 0.69 (0.27–1.75) | .431 |
| Do you have past h/o of COVID-19 disease*? | ||||||||
| No | Reference | Reference | Reference | |||||
| Yes | 0.66 (0.37–1.17) | .152 | 0.63 (0.35–1.15) | .133 | 0.64 (0.35–1.15) | .135 | ||
| Vaccination status | ||||||||
| Not immunized | Reference | Reference | ||||||
| Partial immunized | 0.87 (0.53–1.42) | .577 | 0.88 (0.53–1.46) | .631 | ||||
| Completely immunized | 0.79 (0.53–1.15) | .220 | 0.78 (0.52–1.17) | .233 | ||||
*The h/o of COVID-19 disease within last 12 months was considered.
Table 4.
Conditional logistic regression models for associated factors of SARS-CoV-2 infection for COVAXIN vaccination (n = 624)
| Variable | Unadjusted odds ratio (95% CI) |
p-value | Model-1 |
Model-2 |
Model-3 |
|||
|---|---|---|---|---|---|---|---|---|
| Adjusted odds ratio (95% CI) |
p-value | Adjusted odds ratio (95% CI) |
p-value | Adjusted odds ratio (95% CI) |
p-value | |||
| Age groups (in years) | ||||||||
| <30 | Reference | Reference | Reference | Reference | ||||
| 30–39 | 1.22 (0.70–2.13) | .492 | 1.19 (0.67–2.13) | .553 | 1.17 (0.65–2.10) | .596 | 1.22 (0.68–2.20) | .508 |
| 40–49 | 0.58 (0.17–1.93) | .372 | 0.48 (0.14–1.67) | .247 | 0.45 (0.12–1.56) | .206 | 0.44 (0.12–1.57) | .208 |
| ≥50 | 0.40 (0.06–2.60) | .335 | 0.29 (0.04–1.97) | .207 | 0.31 (0.05–2.05) | .222 | 0.35 (0.05–2.35) | .280 |
| Gender | ||||||||
| Male | Reference | Reference | Reference | Reference | ||||
| Female | 0.98 (0.64–1.49) | .914 | 0.99 (0.64–1.55) | .982 | 0.98 (0.63–1.52) | .936 | 0.98 (0.62–1.53) | .917 |
| Type of household | ||||||||
| Extended family | Reference | Reference | Reference | Reference | ||||
| Nuclear family | 0.34 (0.13–0.89) | .028 | 0.30 (0.11–0.80) | .017 | 0.29 (0.11–0.80) | .016 | 0.31 (0.11–0.85) | .023 |
| With friend or roommate | 0.35 (0.12–1.01) | .051 | 0.31 (0.11–0.91) | .034 | 0.31 (0.10–0.91) | .033 | 0.33 (0.11–1.00) | .050 |
| Living alone (either hostels or outside) | 0.54 (0.20–1.48) | .231 | 0.49 (0.18–1.36) | .170 | 0.48 (0.17–1.34) | .160 | 0.53 (0.19–1.50) | .231 |
| Do you have past h/o of COVID-19 disease*? | ||||||||
| No | Reference | Reference | Reference | |||||
| Yes | 0.61 (0.33–1.11) | .105 | 0.60 (.32–1.12) | .108 | 0.60 (0.33–1.12) | .112 | ||
| Vaccination status | ||||||||
| Not Immunized | Reference | Reference | ||||||
| Partial immunized | 0.82 (0.50–1.37) | .450 | 0.82 (0.49–1.39) | .470 | ||||
| Completely immunized | 0.72 (0.48–1.08) | .116 | 0.71 (0.47–1.08) | .114 | ||||
*The h/o of COVID-19 disease within last 12 months was considered.
Discussion
The primary purposes of any vaccine are disease prevention and reduction in disease severity.25–28 The present study found low effectiveness of COVID-19 vaccine (Covaxin) in prevention of breakthrough SARS-CoV-2 infection. However, our study had not studied the effectiveness of vaccination for preventing severe COVID-19 disease due to absence of severe COVID-19 cases among the study participants. Bharat Biotech announced the interim results from the phase three trials of Covaxin which claimed as the first indigenous COVID-19 vaccine developed by the Indian Council of Medical Research (ICMR) and the Bharat Biotech, having an efficacy of 81% for prevention of SARS-CoV-2 infection. However, this was reported in March 2021, before the peak of second wave of COVID-19 in India during which the study was conducted.(16) In addition, the COVID mutant strains from India have been announced as ‘Variants of Concern’ (VoC) by the World Health Organization, meaning that these mutant strains are global public health risks.29–31 These mutant strains might not be susceptible to the currently available vaccines, although few studies have claimed immunogenicity against the mutant strains too.32–34 Covaxin-induced antibodies were found to neutralize against beta and delta variants in-vitro, and its effect on real world vaccine effectiveness is unknown.17,35 This may be a possible reason for the low effectiveness of COVID-19 vaccine (Covaxin) in preventing breakthrough SARS-CoV-2 infection in the vaccinated health care workers as found in our study. Our finding is also consistent with literature from India and other parts of the world that the development of breakthrough infections after vaccination is a major concern.36–39
Our findings suggest that even natural infections may not protect against SARS-CoV-2 infection for a year in view of the emergence of mutant strains of SARS-CoV-2. The SARS-CoV-2 infection is associated with a variable immune response in the infected population. The maximum number of SARS-CoV-2 infected persons (87.9%) had positive IgG antibodies after 2 months of infection; however, it declined to 50% after 4 months which explained short-lived protective immunity and the possibility of reinfection.40 In contrast to our findings, a study from Italy suggests that reinfections are rare events and patients who have recovered from COVID-19 have a lower risk of reinfection for a year.41
Vaccine can reduce COVID-19 related hospitalization, severe disease and death.42 In a recent multicentric study by ICMR, the vaccine effectiveness of Covaxin for preventing severe COVID-19 disease was 69% (95% CI: 54%–79%).43 The number of severe COVID-19 cases in our study was nil, and only five had moderate COVID-19 disease. Majority of our participants were from younger age-groups and had received the vaccination which may explain the findings. Notably, the majority of the participants were nursing staff and support staff, and most were symptomatic at the time of diagnosis with no previous history of COVID-19. The previous studies have also found that healthcare workers, medical support staff and nursing professionals are at the highest risk of getting infected with COVID-19. Other studies conducted on the factors associated with COVID-19 vaccines effectiveness have reported variable findings. A quantitative synthesis of COVID-19 vaccine efficacy trials found that the type of vaccine, age and sex of the participants, and the rate of COVID-19 transmission in the population were not associated with vaccine effectiveness against SARS-CoV-2 infection.44 On the other hand, there is a rising concern due to vaccine hesitancy among the public; however, it is lower in the lower-middle-income countries.45,46
Our study’s strengths are adequate sample size, verification of vaccination status from multiple sources (institute vaccine database, CoWIN and recall) and RT-PCR confirmed all the COVID-19 cases. Our study had several limitations. Vaccine effectiveness for prevention of severe COVID-19 disease could not be studied due to the absence of severe COVID-19 disease in the study sample. We did not do genomic sequencing to study the strain of SARS-COV-2 virus. Anti-SARS-COV-2 antibodies titers were not measured and its association with vaccine effectiveness could not be studied. Since the study design is observational, there can be unknown risk factors that cannot be factored in and controlled in analysis. A further sizable multicentric study is required to understand the vaccine effectiveness of COVID-19 vaccine, overcoming these limitations.
One of the key messages conveyed by our study is that vaccination did not prevent breakthrough SARS-CoV-2 infection, i.e., infection after 14 days of vaccination. Various recent national and international guidelines have mentioned the exemption of travelers who are vaccinated against COVID-19 from home quarantine following travel, besides other exemptions.47–49 Such policies might further increase the transmission of SARS-CoV-2 infection, especially the variant strains. However, they might significantly affect the impact of the pandemic by reducing hospitalization, and deaths.
Funding Statement
The author(s) reported there is no funding associated with the work featured in this article.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- 1.Timeline of WHO’s response to COVID-19 [Internet]. [accessed Aug 5]. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/interactive-timeline.
- 2.WHO Coronavirus Disease (COVID-19) Dashboard | WHO Coronavirus Disease (COVID-19) Dashboard [Internet]. [accessed 2021 Sep 20]. https://covid19.who.int/
- 3.COVID-19 Map - Johns Hopkins Coronavirus Resource Center [Internet]. [accessed 2021 Aug 3]. https://coronavirus.jhu.edu/map.html.
- 4.MoHFW | home [Internet]. Ministry of Health & Family Welfare Government of India; 2021. [accessed 2021 Dec 2]. https://www.mohfw.gov.in/. [Google Scholar]
- 5.Mondal S, Ghosh S.. Mapping First to Second wave transition of covid19 Indian data via Sigmoid function and prediction of Third wave. medRxiv [Internet]. 2021. Jan 1. http://medrxiv.org/content/early/2021/07/14/2021.07.11.21260325.abstract.
- 6.Mahase E. Covid-19: what new variants are emerging and how are they being investigated? BMJ [Internet]. 2021. Jan;372:n158: https://www.bmj.com/content/bmj/372/bmj.n158.full.pdf. [DOI] [PubMed] [Google Scholar]
- 7.WHO Says Indian COVID Strain ‘a Variant of Concern’ [Internet]. 2021. [accessed 2021 Aug 2]: https://www.webmd.com/lung/news/20210510/who-says-indian-covid-strain-a-variant-of-concern
- 8.Noorimotlagh Z, Jaafarzadeh N, Martínez SS, Mirzaee SA. A systematic review of possible airborne transmission of the COVID-19 virus (SARS-CoV-2) in the indoor air environment. Environ Res [Internet]. 2021. Feb;193:110612. doi: 10.1016/j.envres.2020.110612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Greenhalgh T, Jimenez JL, Prather KA, Tufekci Z, Fisman D, Schooley R. Ten scientific reasons in support of airborne transmission of SARS-CoV-2. Lancet. 2021. May;397(10285):1603–7. http://www.thelancet.com/article/S0140673621008692/fulltext. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Siemieniuk RAC, Bartoszko JJ, Ge L, Zeraatkar D, Izcovich A, Kum E, Pardo-Hernandez H, Qasim A, Martinez JP, Rochwerg B, et al. Drug treatments for covid-19: living systematic review and network meta-Analysis. BMJ. 2020. Jul;370:1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.WHO Solidarity Trial Consortium . Repurposed antiviral drugs for Covid-19 - Interim WHO solidarity trial results. N Engl J Med. 2021. Feb;384(6):497–511. doi: 10.1056/NEJMoa2023184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kashte S, Gulbake A, El-Amin SF III, Gupta A. COVID-19 vaccines: rapid development, implications, challenges and future prospects. Hum Cell. 2021. May;34(3):711. doi: 10.1007/s13577-021-00512-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim YC, Dema B, Reyes-Sandoval A. COVID-19 vaccines: breaking record times to first-in-human trials. Npj Vaccines. 2020. Apr;5(1):1–3. doi: 10.1038/s41541-020-0188-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.COVAXIN - India’s First Indigenous Covid-19 Vaccine | bharat Biotech [Internet]. 2021. [accessed 2021 Aug 1]. https://www.bharatbiotech.com/covaxin.html.
- 15.COWIN. Largest Vaccination Drive . [Internet]. Ministry of Health & Family Welfare Government of India. [accessed Dec 2]. https://www.cowin.gov.in/home.
- 16.Press Information Bureau . Phase 3 Clinical Trial of COVAXIN, developed by ICMR & Bharat Biotech, shows 81% efficacy [Internet]. Press Information Bureau. 2021. [accessed 2022 May 1]. https://www.bharatbiotech.com/images/press/covaxin-phase3-clinical-trials-interim-results.pdf.
- 17.Sapkal GN, Yadav PD, Ella R, Deshpande GR, Sahay RR, Gupta N, Vadrevu K M, Abraham P, Panda S, Bhargava B. Inactivated COVID-19 vaccine BBV152/COVAXIN effectively neutralizes recently emerged B.1.1.7 variant of SARS-CoV-2. J Travel Med. 2021. Jun;28(4):1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Olliaro P, Torreele E, Vaillant M. COVID-19 vaccine efficacy and effectiveness-the elephant (not) in the room. Lancet Microbe. 2021;2(7):e279–e280. doi: 10.1016/S2666-5247(21)00069-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hodgson SH, Mansatta K, Mallett G, Harris V, Emary KRW. Pollard A j. What defines an efficacious COVID-19 vaccine? A review of the challenges assessing the clinical efficacy of vaccines against SARS-CoV-2. Lancet Infect Dis. 2021. Feb;21(2):e26–35. doi: 10.1016/S1473-3099(20)30773-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.World Health Organization . Evaluation of COVID-19 vaccine effectiveness. Geneva: World Health Organization . [Internet]. Vol. 70. 2021. [accessed 2021 May 15]. https://www.who.int/publications/i/item/WHO-2019-nCoV-vaccine_effectiveness-measurement-2021.1. [Google Scholar]
- 21.Sample size calculator for evaluation of COVID-19 vaccine effectiveness (Excel) [Internet]. [accessed 2021 Aug 5]: https://www.who.int/publications/i/item/WHO-2019-nCoV-vaccine_effectiveness-measurement_tool-2021.1.
- 22.Clinical Guidance for Management of Adult COVID-19 Patients | AIIMS Covid Information Portal [Internet]. 2021. [accessed 2021 Aug 5]. https://covid.aiims.edu/clinical-guidance-for-management-of-adult-covid-19-patients/.
- 23.Centre for Disease Control . COVID-19 Vaccine Breakthrough Infections Reported to CDC. Morb Mortal Wkly Rep. 2021;70(21):792–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Indian Council of Medical Research . Strategy for COVID-19 testing in India [Internet]. Vol. Version 5, National Task Force on COVID-19. 2020. https://www.mohfw.gov.in/pdf/AdvisoryonstrategyforCOVID19TestinginIndia.pdf. [Google Scholar]
- 25.Vaccines and immunization [Internet]. [accessed 2021 Aug 5]. https://www.who.int/health-topics/vaccines-and-immunization#tab=tab_1.
- 26.Basics of Vaccines | CDC [Internet] . [accessed 2021 Aug 5]. https://www.cdc.gov/vaccines/vpd/vpd-vac-basics.html.
- 27.Coronavirus disease (COVID-19): vaccines [Internet]. [accessed 2021 Aug 5]. https://www.who.int/news-room/q-a-detail/coronavirus-disease-(covid-19)-vaccines
- 28.Bhattacharya A, Ranjan P, Ghosh T, Agarwal H, Seth S, Maher GT, Upadhyay AD, Kumar A, Baitha U, Gupta G, et al. Evaluation of the dose-effect association between the number of doses and duration since the last dose of COVID-19 vaccine, and its efficacy in preventing the disease and reducing disease severity: a single centre, cross-sectional analytical study from In. Diabetes Metab Syndr. 2021;15(5):102238. doi: 10.1016/j.dsx.2021.102238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.WHO | SARS-CoV-2 Variants [Internet]. WHO. World Health Organization ; 2021. [accessed 2021 Aug 5]. http://www.who.int/csr/don/31-december-2020-sars-cov2-variants/en/.
- 30.WHO classifies triple-mutant Covid variant from India as global health risk [Internet]. [accessed 2021 Aug 6]. https://www.cnbc.com/2021/05/10/who-classifies-triple-mutant-covid-variant-from-india-as-global-health-risk-.html.
- 31.Adam D. What scientists know about new, fast-spreading coronavirus variants. Nature [Internet]. 2021. May. http://www.nature.com/articles/d41586-021-01390-4. [DOI] [PubMed]
- 32.Garcia-Beltran WF, Lam EC, Astudillo MG, Yang D, Miller TE, Feldman J, Hauser BM, Caradonna TM, Clayton KL, Nitido AD, et al. COVID-19-neutralizing antibodies predict disease severity and survival. Cell. 2021. Jan;184(2):476–488.e11. doi: 10.1016/j.cell.2020.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Madhi SA, Baillie V, Cutland CL, Voysey M, Koen AL, Fairlie L, Padayachee SD, Dheda K, Barnabas SL, Bhorat QE, et al. Efficacy of the ChAdOx1 nCoV-19 Covid-19 Vaccine against the B.1.351 Variant. N Engl J Med. 2021. Mar;384(20):1885–98. doi: 10.1056/NEJMoa2102214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Callaway E, Mallapaty S. Novavax offers first evidence that COVID vaccines protect people against variants. Nature. 2021. Jan;590(7844):17. doi: 10.1038/d41586-021-00268-9. [DOI] [PubMed] [Google Scholar]
- 35.Yadav PD, Sapkal GN, Ella R, Sahay RR, Nyayanit DA, Patil DY, Deshpande G, Shete AM, Gupta N, Mohan VK, et al. Neutralization of Beta and Delta variant with sera of COVID-19 recovered cases and vaccinees of inactivated COVID-19 vaccine BBV152/Covaxin. J Travel Med. 2021;28(7):104. doi: 10.1093/jtm/taab104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tyagi K, Ghosh A, Nair D, Dutta K, Bhandari PS, Ansari IA, Misra A. Breakthrough COVID19 infections after vaccinations in healthcare and other workers in a chronic care medical facility in New Delhi, India. Diabetes Metab Syndr Clin Res Rev. 2021;15(3):1007–08. doi: 10.1016/j.dsx.2021.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hacisuleyman E, Hale C, Saito Y, Blachere NE, Bergh M, Conlon EG, Schaefer-Babajew DJ, DaSilva J, Muecksch F, Gaebler C. Vaccine breakthrough infections with SARS-CoV-2 variants. N Engl J Med. 2021;384(23):2212–18. doi: 10.1056/NEJMoa2105000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Farinholt T, Doddapaneni H, Qin X, Menon V, Meng Q, Metcalf G, Chao H, Gingras M-C, Avadhanula V, Farinholt P, et al. Transmission event of SARS-CoV-2 delta variant reveals multiple vaccine breakthrough infections. BMC Med. 2021;19(1):255. doi: 10.1186/s12916-021-02103-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dash GC, Subhadra S, Turuk J, Parai D, Rath S, Sabat J, Rout UK, Kanungo S, Choudhary HR, Nanda RR, et al . Breakthrough SARS-CoV-2 infections among BBV-152 (COVAXIN®) and AZD1222 (COVISHIELDTM) recipients: report from the eastern state of India. J Med Virol. 2022;94:1201–1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mishra M, Chaudhry R, Rana F, Nag DS, and Rai S. Serosurveillance of health care workers in a COVID hospital: immune response, and its longevity. Cureus. 2021;13(3):e14020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vitale J, Mumoli N, Clerici P, De Paschale M, Evangelista I, Cei M, Mazzone A. Assessment of SARS-CoV-2 reinfection 1 year after primary infection in a population in Lombardy, Italy. JAMA Intern Med. 2021; May 28;181(10):1407. doi: 10.1001/jamainternmed.2021.2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Haas EJ, Angulo FJ, McLaughlin JM, Anis E, Singer SR, Khan F, Brooks N, Smaja M, Mircus G, Pan K. Impact and effectiveness of mRNA BNT162b2 vaccine against SARS-CoV-2 infections and COVID-19 cases, hospitalisations, and deaths following a nationwide vaccination campaign in Israel: an observational study using national surveillance data. Lancet. 2021. May 15;397(10287):1819–29. doi: 10.1016/S0140-6736(21)00947-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bhatnagar T, Chaudhuri S, Ponnaiah M, Yadav PD, Sabarinathan R, Sahay RR, Ahmed F, Aswathy S, Bhardwaj P, Bilimale A, et al. Effectiveness of BBV152/Covaxin and AZD1222/Covishield Vaccines Against Severe COVID-19 and B.1.617.2/Delta Variant in India, 2021: a multi-centric hospital-based case-control study. 2021. doi: 10.2139/ssrn.3955739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Calzetta L, Ritondo BL, Coppola A, Matera MG, Di Daniele N, Rogliani P. Factors Influencing the Efficacy of COVID-19 Vaccines: a Quantitative Synthesis of Phase III Trials. Vaccines. 2021;9(4):341. Internet pmc/articles/PMC8065664/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Machingaidze S, Wiysonge CS. Understanding COVID-19 vaccine hesitancy. Nat Med [Internet]. 2021; July 16:1–2. https://www.nature.com/articles/s41591-021-01459-7. [DOI] [PubMed] [Google Scholar]
- 46.Solís Arce JS, Warren SS, Meriggi NF, Scacco A, McMurry N, Voors M. COVID-19 vaccine acceptance and hesitancy in low- and middle-income countries. Nat Med [Internet]. 2021;1–10. https://www.nature.com/articles/s41591-021-01454-y. [DOI] [PMC free article] [PubMed]
- 47.Switzerland plans to lift quarantine for vaccinated arrivals & those who have recovered from COVID-19 - SchengenVisaInfo.com [Internet]. [accessed 2021 Aug 6]. https://www.schengenvisainfo.com/news/switzerland-plans-to-lift-quarantine-for-vaccinated-arrivals-those-who-have-recovered-from-covid-19/.
- 48.Fully vaccinated Germans to be exempt from quarantine - minister | reuters [Internet]. [accessed 2021 Aug 6]. www.reuters.com/world/europe/fully-vaccinated-germans-be-exempt-quarantine-minister-2021-05-03/
- 49.Interim Public Health Recommendations for Fully Vaccinated People | CDC [Internet]. [accessed 2021 Aug 6]. www.cdc.gov/coronavirus/2019-ncov/vaccines/fully-vaccinated-guidance.html.


