Abstract
Background and study aims In patients with inflammatory bowel disease (IBD), endoscopically visible lesions with distinct borders can be considered for endoscopic resection. The role of endoscopic submucosal dissection (ESD) for these lesions is not well defined because of a paucity of data. We aimed to evaluate the outcomes of colorectal ESD of dysplastic lesions in patients with IBD across centers in the United States.
Patients and methods This was a retrospective analysis of consecutive patients with IBD who were referred for ESD of dysplastic colorectal lesions at nine centers. The primary endpoints were the rates of en bloc resection and complete (R0) resection. The secondary endpoints were the rates of adverse events and lesion recurrence.
Results A total of 45 dysplastic lesions (median size 30mm, interquartile range [IQR] 23 to 42 mm) in 41 patients were included. Submucosal fibrosis was observed in 73 %. En bloc resection was achieved in 43 of 45 lesions (96 %) and R0 resection in 34 of 45 lesions (76 %). Intraprocedural perforation occurred in one patient (2.4 %) and was treated successfully with clip placement. Delayed bleeding occurred in four patients (9.8 %). No severe intraprocedural bleeding or delayed perforation occurred. During a median follow-up of 18 months (IQR 13 to 37 months), local recurrence occurred in one case (2.6 %). Metachronous lesions were identified in 11 patients (31 %).
Conclusions ESD, when performed by experts, is safe and effective for large, dysplastic colorectal lesions in patients with IBD. Despite the high prevalence of submucosal fibrosis, en bloc resection was achieved in nearly all patients with IBD undergoing ESD. Careful endoscopic surveillance is necessary to monitor for local recurrence and metachronous lesions after ESD.
Introduction
Patients with long-standing inflammatory bowel disease (IBD) have an increased risk of developing colorectal dysplasia and cancer 1 2 3 . Historically, patients with IBD and dysplasia have been managed primarily with colectomy. The optimal management of dysplasia in patients with IBD is evolving. According to the 2015 American Society for Gastrointestinal Endoscopy (ASGE) guidelines, endoscopically visible lesions with distinct borders and without features of submucosal invasion should be considered for endoscopic resection. In addition, en bloc resection is preferred because it allows for histologic evaluation of completeness of resection 4 . However, endoscopic resection of these lesions is often not feasible due to impaired submucosal lifting related to significant mucosal and submucosal fibrosis from chronic inflammation.
Endoscopic submucosal dissection (ESD) enables en bloc and potentially curative resection of superficial neoplastic lesions regardless of size, facilitates precise histological evaluation of the resected specimen, and minimizes the risk of local recurrence 5 6 . ESD may overcome limitations of endoscopic mucosal resection (EMR) in achieving en bloc resection of larger size lesions and those with submucosal fibrosis, which is more frequently encountered in patients with IBD.
Although the procedure is technically difficult because of high prevalence of submucosal fibrosis, several recent small studies from Asia and Europe, have indicated that ESD is a safe and effective treatment for dysplastic lesions in the setting of IBD 7 8 9 10 11 12 . ESD is a potential alternative treatment option when EMR is not feasible or does not enable en bloc removal of larger lesions.
In North America, ESD of colorectal neoplasms is gaining traction, but studies specifically addressing the role of ESD for management of dysplastic lesions in IBD are lacking. The aim of this multicenter study was to evaluate the clinical outcomes of ESD in management of dysplastic colorectal lesions in patients with IBD across various centers in the United States.
Patients and methods
Study population
This was a retrospective multicenter cohort study of all consecutive patients with IBD who were referred for ESD of colorectal dysplastic lesions at nine centers across the United States from January 2015 to October 2019. The study was approved by each center’s Institutional Review Board.
The study included patients with dysplastic lesions in a known segment of colitis deemed suitable for endoscopic resection if distinct margins of the lesion were identified without endoscopic features suggestive of submucosal invasion, such as depressions, failure to lift with attempted submucosal injection, or presence of overlying ulceration 4 . We excluded endoscopically invisible dysplastic lesions and those with indistinct borders, as well as lesions occurring within moderate-to-severe active ulcerative colitis.
Relevant clinical data were extracted, including patient demographics, lesion characteristics, prior interventions, procedural details, procedure-related adverse events (AEs) and treatment outcomes, local recurrence, metachronous lesions, and mortality at the last follow-up through October 2020, when available.
ESD procedure
The ESD procedures were performed under moderate sedation, deep sedation or general anesthesia with endotracheal intubation, at the discretion of the endoscopist and anesthesiologist. Carbon dioxide was routinely used for insufflation in all cases. A transparent distal attachment cap was applied at the tip of the endoscope. ESD was performed as previously described ( Fig. 1 ) 7 8 . In brief, the procedures were performed using either an adult or pediatric colonoscope, or an upper endoscope for lesions within reach. The targeted lesion was carefully examined under high-definition white light imaging, in addition to near-focus mode, electronic chromoendoscopy and/or dye-based chromoendoscopy. Marking with coagulation dots 5 mm outside the lesion boundary was performed with the tip of an ESD knife using a soft coagulation setting. The mucosa was incised along the periphery of the marker dots using the ESD knife following submucosal fluid injection with a solution containing methylene blue or indigo carmine admixed with normal saline or a viscous agent, with or without dilute epinephrine. The submucosal space was expanded further by injection of the solution and the lesion dissected using the ESD knife until en bloc resection was achieved. The type of ESD knife (or knives) selected was at the discretion of the endoscopist.
Fig. 1 .
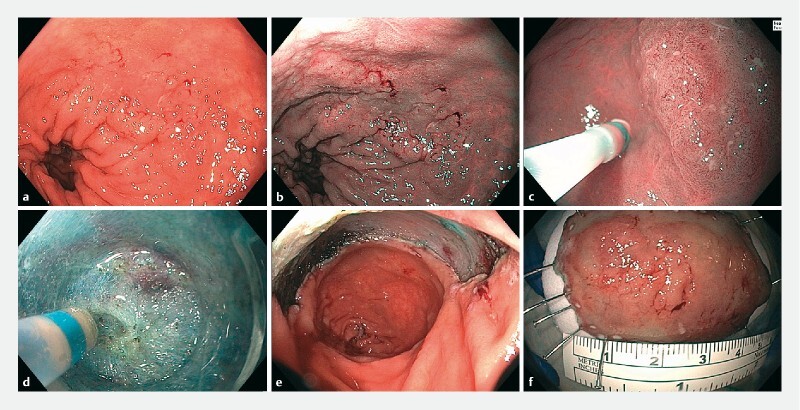
Endoscopic submucosal dissection (ESD) of a dysplastic lesion in a 73-year-old patient with ulcerative colitis. The 45 mm × 30-mm, slightly elevated lesion (Paris 0-IIa) in the rectum is seen with granular background mucosa in a standard white light, on b narrow-band imaging, and c in near-focus mode (C). d An image taken during ESD shows severe fibrosis. e An image of the resected area after ESD was performed. f The resected specimen, with at least 5 mm of normal-appearing mucosa around the suspected neoplastic lesion.
The degree of submucosal fibrosis at the time of ESD was classified as F0 (no fibrosis), F1 (mild fibrosis), or F2 (severe fibrosis), as previously described 13 . When performed, endoscopic closure of the resection bed using clips and/or endoscopic suturing was performed at the discretion of the endoscopist.
Specimens were stretched and pinned on foam and immediately fixed in formalin solution. The resected specimens were sectioned serially at 2-mm intervals and embedded for histological examination. Histopathological examination was performed using hematoxylin and eosin staining. Dysplasia was defined according to Vienna criteria 14 .
Surveillance colonoscopy typically was performed 3 to 6 months after ESD. The interval to subsequent follow-up endoscopies was at the discretion of the endoscopist.
Outcome measures
Primary outcome measures were the rates of en bloc resection (defined as excision of the targeted lesion in a single specimen) and complete (R0) resection (defined as resection with lateral and deep margins free of neoplasia on histopathology). Secondary outcome measures were the rates of AEs, post-ESD surgery, lesion recurrence, and metachronous lesions.
Local recurrence was defined as presence of endoscopic and/or histological evidence of neoplastic tissue at the resection site during follow-up colonoscopy. A metachronous lesion was defined as a new colorectal neoplasm in an area other than the site of the primary lesion diagnosed at least 6 months after the ESD procedure.
Severe intraprocedural bleeding was defined as overt bleeding resulting in a drop in hemoglobin > 2 g/dL and/or need for blood transfusion. Delayed bleeding was defined as clinical evidence of bleeding following ESD completion to 14 days after the procedure with a drop in hemoglobin > 2 g/dL and/or requiring blood transfusion and/or endoscopic treatment or other intervention. Procedure time was defined as the time from incision with the needle-knife until complete removal of the lesion.
Statistical analysis
Frequencies and percentages were calculated for categorical variables. Means and standard deviations, as well as medians and interquartile ranges (IQRs) were calculated for continuous variables. Comparative analyses using Fisher’s exact test for categorical variables and the t test for continuous variables were performed, with P < 0.05 considered significant. All descriptive analyses were performed with the SPSS software v22 (IBM, SPSS Statistics, Armonk, New York, United States).
Results
Patients, lesions and procedural characteristics
A total of 45 dysplastic lesions in 41 patients were included ( Table 1 ). The mean age was 60.4 years (range, 38 to 81 years) and 61 % of patients were men. Most patients were designated as American Society of Anesthesiologists (ASA) class II (56 %), followed by ASA class III in 27 %. The median duration of colitis was 25 years (range, 1 to 50 years). None of the patients had concomitant stricturing disease or primary sclerosing cholangitis. All procedures were performed by experienced endoscopists who had previous experience with over 80 to 200 ESD procedures.
Table 1. Patient (n = 41) and lesion (n = 45) characteristics.
| Gender (male, n, %) | 25/41 (61 %) |
| Age (years, mean, SD) | 60.4 (± 11) |
| Disease duration (years), median (range) | 25 (1–50) |
| Type of IBD, n (%) | |
|
33 (80 %) |
|
8 (20 %) |
| ASA class, n (%) | |
|
5/41 (12 %) |
|
23/41 (56 %) |
|
11/41 (27 %) |
|
2/41 (5 %) |
| Primary sclerosing cholangitis, n (%) | 0 |
| Stricturing disease, n (%) | 0 |
| Preceding failed endoscopic treatment, n (%) | 9 (20 %) |
| Location of tumor, n (%) | |
|
23 (51.1 %) |
|
10 (22.2 %) |
|
5 (11.1 %) |
|
4 (8.8 %) |
|
3 (6.6 %) |
| Tumor size (mm, median, IQR) | 30 (23–42) |
| Paris classification, n (%) | |
|
2 (4 %) |
|
24 (53 %) |
|
17 (38 %) |
|
1 (2 %) |
|
1 (2 %) |
| Pre-ESD diagnosis based on biopsy, n (%) | |
|
27 (62.7 %) |
|
9 (20.9 %) |
|
5 (11.6 %) |
|
2 (4.7 %) |
|
2 |
| Presence of ulcer at the lesion, n (%) | 0 |
| Degree of submucosal fibrosis, n (%) | |
|
12 (26.7 %) |
|
15 (33.3 %) |
|
18 (40.0 %) |
| Procedure time (min, median, IQR) | 93 (IQR 66–123) |
| Procedure setting, n (%) | |
|
22/45 (49 %) |
|
18/45 (40 %) |
|
5/45 (11 %) |
SD, standard deviation; IBD, inflammatory bowel disease; ASA, American Society of Anesthesiologists; ESD, endoscopic submucosal dissection; IQR, interquartile range.
Background inflammation per Mayo Clinic Endoscopic Subscore at the lesion site was graded as none in 22 (54 %), mild in 15 (37 %), and moderate in four patients (11 %). For patients in whom lesion biopsies (n = 43) were taken before ESD, histopathological diagnoses based on biopsy were low-grade dysplasia (LGD) or adenoma in 27 lesions (62.7 %), high-grade dysplasia (HGD) in nine (20.9 %), sessile serrated polyps/adenomas in five (11 %), and intramucosal cancer in two (4.4 %). Two of the 45 initial lesions did not undergo biopsy prior to ESD. EMR had been attempted previously on nine lesions (20 %).
Lesions were macroscopically non-polypoid (Paris 0-II) in 94%, with a median size of 30 mm (IQR 23 to 42 mm). Twenty-two lesions (48.9 %) were located in the colon and 23 (51.1 %) in the rectum. Submucosal fibrosis was observed in most lesions (n = 33, 73.3 %). Of these 45 lesions, 15 (33.3 %) and 18 (40.0%) were categorized as mild (F1) and severe (F2), respectively.
For most lesions (n = 28, 62.2 %), ESD was performed under monitored anesthesia care, followed by moderate sedation (n = 10; 22.2 %) and general anesthesia with endotracheal intubation (n = 7; 15.6 %). The DualKnife or DualKnife Jet (Olympus America, Center Valley, Pennsylvania, United States) was the most commonly used electrosurgical knife (n = 37; 82.2 %), followed by the HybridKnife (ERBE USA, Marietta, Georgia, United States) (n = 7; 15.6 %) and FlushKnife (Fujifilm, Stamford, Connecticut, United States) (1; 2.2 %). A combination of ESD knives was used in 15 lesions (33.3 %).
The median procedure time was 93 minutes (IQR 66 to 123 minutes). Almost half of the ESD procedures (n = 22, 49 %) were performed in an outpatient setting, with a median post-procedure hospital stay of 1 day (range 1 to 2 days).
Resection outcomes and adverse events
The en bloc and R0 resection rates were 43 of 45 (95.6 %) and 34 of 45 (75.5 %), respectively ( Table 2 ). En bloc resection was not feasible in two lesions with severe submucosal fibrosis. One of these two lesions had undergone prior EMR before ESD. Both lesions were resected completely in piecemeal fashion. R1 resection (n = 11), excluding two lesions resected in piecemeal fashion, was due to positive lateral resection margins on the resected specimens.
Table 2. Outcomes of colorectal ESD in IBD.
| En bloc resection, n (%) | 43 (95.5 %) |
| R0 resection, n (%) | 34 (75.5 %) |
| Histology, n (%) | |
|
4 (8.9 %) |
|
28 (62.2 %) |
|
9 (20.0 %) |
|
1 (2.2 %) |
|
3 (6.7 %) 1 |
| Adverse events; n (%) | |
|
1 (2.4 %) |
|
0 |
|
0 |
|
4 (9.8 %) |
| Local recurrence on follow-up endoscopy; n (%) | 1/38 (2.6 %) |
ESD, endoscopic submucosal dissection; IBD, inflammatory bowel disease; LGD, low-grade dysplasia; HGD, high-grade dysplasia.
Superficial invasive cancer; all lesions were curatively resected by ESD.
Intraprocedural perforation occurred in one patient (2.4 %), which was treated successfully with clip placement. Delayed bleeding occurred in four patients (9.8 %): two were managed conservatively and two underwent successful endoscopic hemostasis. No severe intraprocedural bleeding or delayed perforation occurred.
The final histopathological diagnoses of the ESD specimens were LGD/adenoma in 28 (62.2 %), HGD in nine (20.0 %), serrated adenomas/polyps in four (8.9 %), intramucosal cancer in one (2.2 %), and superficially invasive cancer in three lesions (6.7 %). Curative resection was achieved in all three cases of superficially invasive adenocarcinoma.
Follow-up and recurrences
Follow-up data were available for 38 resected lesions in 35 patients at a median of 18 months (IQR: 13 to 37 months). During the follow-up period, all patients were alive.
One local recurrent (1/38; 2.6 %) was identified on follow-up colonoscopy 4 months later at the ESD site. This patient had initial R0 resection for HGD. Repeat biopsies at the ESD site on surveillance colonoscopy confirmed HGD, which was completely resected with additional ESD.
Metachronous lesions were identified in 11 of 35 patients (31.4 %) during follow-up endoscopy. Seven metachronous lesions were LGD and four were HGD at other locations in the colon. Eight lesions were treated successfully with endoscopic resection: four lesions were treated with ESD, three with EMR, and one 2-mm polyp with cold biopsy forceps. Two patients with metachronous lesions underwent surgery.
Synchronous neoplasia was found in one patient. This patient was referred for ESD of a serrated sessile lesion with LGD in the sigmoid colon that had undergone prior EMR. At the time of index ESD, a superficial ulceration in the right colon was found (prior biopsies in this area had not shown dysplasia), and this was thought to be an inflammatory or cytomegalovirus-related ulcer. However, at the time of the sigmoid colon ESD, biopsy of the ulcer showed at least HGD, and the patient was referred for surgery. Repeat biopsy of the right colon lesion during a subsequent colonoscopy requested by the surgical team confirmed invasive cancer and no residual dysplasia at the ESD scar. Because the patient was morbidly obese, total proctocolectomy with ileostomy was recommended, but the patient declined the procedure. A total abdominal colectomy with ileorectal anastomosis was performed and pathology showed T3N1 adenocarcinoma.
Comparison of Pre-ESD histologic diagnosis based on biopsy with histologic diagnosis of ESD specimens
The pre-ESD diagnoses and post-ESD histologic diagnoses of the resected specimens are shown in Table 3 . Overall, pre-ESD and post-ESD histologic diagnoses matched in 35 of 43 lesions (81.3 %). Among the 27 lesions with a pre-ESD biopsy diagnosis of LGD/adenoma, the diagnosis was upstaged in two lesions (7.4 %): one lesion was diagnosed as HGD and one was invasive cancer. Of the 11 lesions with HGD or intramucosal cancer on pre-ESD biopsy, two (18.1 %) were diagnosed as having invasive cancer in the ESD specimens.
Table 3. Comparison of pre-ESD diagnoses based on the biopsy and histologic diagnoses of the resected specimens (n = 43).
| Histology from ESD resected specimen | ||||
| LGD/ adenoma | Sessile serrated adenoma/polyp | HGD or intramucosal cancer | Invasive cancer | |
| Pre-ESD diagnoses based on biopsy | ||||
|
25 | 0 | 1 | 1 |
|
1 | 4 | 0 | 0 |
|
1 | 0 | 8 | 2 |
ESD, endoscopic submucosal dissection; LGD, low-grade dysplasia; HGD, high-grade dysplasia.
Discussion
The ASGE practice guidelines recommend en bloc resection of raised, endoscopically visible, dysplastic lesions with distinct borders in regions of chronic active colitis that might be prone to fibrosis by EMR or ESD 4 . However, to date, the outcomes data on colorectal ESD for the management of IBD-associated dysplastic lesions are scarce and limited to studies from Asia and Europe 7 8 9 10 11 12 . In this US multicenter study on clinical outcomes of ESD in IBD, we found that ESD was associated with high en bloc (95.6 %) and R0 resection rates (75.5 %), with a median lesion size of 30 mm. The rate of ESD-related perforation was low (2.4 %) and the AE was managed endoscopically. No emergent surgery was performed for ESD-related AEs. Taking the data together, ESD appears to be a feasible, safe, and effective treatment for IBD-associated dysplastic lesions.
In patients with IBD, long-standing mucosal inflammatory activity can cause significant mucosal and submucosal fibrosis, which may render lesions unresectable using conventional EMR techniques. Furthermore, colorectal EMR has been associated with low en bloc resection rates (27 % to 63 %), and significantly high local recurrence rates (14 % to 50 %) in patients with IBD 15 16 17 . In several cases involving dysplasia with IBD, the margins were partially indistinct, which might have led to positive lateral margins. In many instances, ESD permits dissection of even severe fibrotic tissue using an electrosurgical knife, allowing adequate vertical dissection of the submucosal plane and potentially curative resection of these fibrotic lesions. Submucosal fibrosis (F1 and F2 submucosal fibrosis) was reported in 90 % and 97 % of cases by Iacopini et al 7 and Suzuki et al 11 , respectively. In our study, submucosal fibrosis (F1 and F2) was noted in most lesions (73 %). There were also no significant differences in frequency of submucosal fibrosis between the studies by Iacopini et al 7 and Suzuki et al 11 ( P = 0.79 and 0.49, respectively) and our study.
Smith et al. 18 evaluated the efficacy of a hybrid technique using a combination of circumferential mucosal incision and division of fibrotic tissue, followed by snare resection of colorectal lesions of variable size (8 to 62 mm) in patients with IBD, in whom conventional EMR was unsuccessful due to submucosal fibrosis. The rates of en bloc resection, perforation, and local recurrence were 73 %, 3 %, and 7 %, respectively 18 . Although the hybrid technique appears technically easier and has a safety profile similar to standard ESD, its efficacy, in terms of en bloc resection and local recurrence rates appears, inferior to that of standard ESD. These findings are also line with the data from patients without IBD 19 .
A meta-analysis of 97 studies by Fuccio et al evaluated the efficacy of ESD for treatment of colorectal lesions and demonstrated an en bloc resection rate of 91 %, a R0 resection rate of 82.9 %, a perforation rate of 5.2 %, need for surgery for post-ESD AEs of 1 %, and a local recurrence rate of 2.0 % 19 . A large, multicenter, prospective study from North America reported similar clinical outcomes for colorectal ESD, with an en bloc resection rate of 87 %, a R0 resection rate of 84 %, and a perforation rate of 3.7 % 20 . Our study results compare favorably to the findings from these studies, despite a higher technical difficulty due to the high prevalence of submucosal fibrosis (73 %) and failed prior EMR (20 %) in our patient population.
The high en bloc resection rate in our study is comparable to previous Asian and European reports of colorectal ESD for dysplastic lesions in IBD, with en bloc resection rates of 80 % to 100 % and R0 resection rates of 69 % to 96 % 7 8 9 10 11 12 . However, the widespread adoption of ESD for management of neoplastic lesions in IBD has partly been limited by the concern for potential serious AEs due to technical difficulty. The rates of perforation (2.2 %) and delayed bleeding (8.8 %) in our study are comparable to those for perforation (0 % to 5.6 %) and bleeding (0 % to 13 %) rates reported in studies from Europe and Japan 7 8 9 10 11 12 . None of our patients required surgery due to ESD-related AEs.
In patients who underwent follow-up at a median time interval of 18 months, the local recurrence rate was low at 2.6 % and metachronous lesions were identified in almost one-third of patients in our cohort. Studies from Japan and Europe have reported local recurrence rates of 0 % to 3 % 7 8 9 10 11 . Metachronous lesions occurred in 0 % to 9 % 7 8 9 11 , except in one Japanese study with follow-up data of 14 years, in which metachronous lesions occurred in five of seven patients after ESD 10 . Thus, careful meticulous endoscopic surveillance in IBD is essential to monitor for local recurrence and metachronous lesions after ESD. Differences in rates of metachronous lesions in this study and previous studies could be due to slight variation in patient selection and the experience of endoscopists in adequately diagnosing dysplasia in patients w2ith IBD during index colonoscopy. Given that endoscopists who performed the ESDs in this study were experienced in lesion identification and lesion resection, we believe that the rate of missed lesions should be low.
ESD can also serve as an important staging method by providing more accurate histopathologic diagnosis than standard biopsies or EMR. Discrepancies in histologic diagnosis between biopsy samples and resected specimens have been reported for colorectal lesions, likely due to the heterogeneity of these lesions 8 . One study showed that biopsy sampling confirmed a final histologic diagnosis of carcinoma with a low sensitivity of 72.2 % and accuracy of only 78 % in IBD 8 . Furthermore, unlike patients without IBD, endoscopic diagnosis of HGD or carcinoma in the colons of patients with IBD using chromoendoscopy and magnifying narrow-band imaging has lower accuracy 8 21 . In our study, 18 % of lesions diagnosed as HGD or intramucosal cancer on pre-ESD biopsy were upstaged to invasive cancer in the resected specimens, which were curatively resected by ESD. Similarly, 7.4 % of lesions initially diagnosed as LGD/adenoma on biopsy were found to have HGD or invasive cancer on the post-ESD specimens. In agreement with the aforementioned studies, our study suggests that histological discrepancies between endoscopic biopsies and ESD specimens are common. Thus, ESD can serve as both a histologic staging procedure and a definitive treatment. In addition, for biopsy-proven lesions with HGD, it is preferable to achieve en bloc resection via ESD rather than piecemeal EMR, given the high probability of covert submucosal invasive cancer.
Our study reports on the first US multicenter experience regarding ESD for the treatment of colorectal neoplastic lesions in patients with IBD with medium-term follow-up. Data are derived from multiple centers with expertise in advanced resection techniques, which increase the generalizability of these results in a referral setting. We suggest that ESD in the setting of IBD should be performed by endoscopists with extensive experience in colorectal ESD, given that the procedure can be technically difficult due to the high prevalence of submucosal fibrosis associated with these lesions. In addition, it is important to emphasize that endoscopists who perform colorectal ESD in patients with IBD be proficient in endoscopic recognition and delineation of dysplastic lesions to ensure complete resection. In patients without IBD, the borders of colorectal polyps are usually obvious and mucosal markings outside the periphery of the lesions are not typically required 22 . In patients with IBD, the lesion margins are sometimes less clear and placement of coagulation dots 5 mm outside the lesion margins is recommended to facilitate lesion identification and complete resection with negative lateral margins 7 . Finally, tattoos should be placed to facilitate localization of the resection sites during colonoscopic surveillance, and in case subsequent surgery is required.
The study limitations include the retrospective design and lack of long-term follow-up data. Furthermore, data from this study were derived from tertiary centers with experience in ESD and the results may not be applicable to the community setting. Despite the involvement of multiple centers across the United States, the cohort of patients was relatively small. These limitations notwithstanding, our findings add to the growing body of literature showing the efficacy and safety of ESD for IBD-associated dysplastic lesions and its potential as an alternative to colectomy in select cases.
Conclusions
In conclusion, this study represents the first US multicenter report on colorectal ESD for neoplastic lesions in the setting of IBD. For endoscopically visible lesions, ESD appears to be safe and effective for en bloc removal of these lesions, regardless of size and the presence of submucosal fibrosis. Prospective studies that define the long-term outcomes and that compare ESD to EMR for resection of IBD-associated dysplastic lesions are awaited.
Footnotes
Competing interests Dr. Kumta is a consultant for Apollo Endosurgery, Boston Scientific, and Olympus. Dr. Yang is a consultant for Boston Scientific, Lumendi and Steris. Dr. Draganov is a consultant for Boston Scientific, Lumendi, Cook, Olympus and Microtech. Dr. Ngamruengphong is a consultant for Boston Scientific. Dr. Aihara is a consultant for Olympus America, Boston Scientific, Fujifilm Medical Systems, Auris Health, Lumendi, Medtronic, ConMed, and 3 D Matrix. Dr. Nishimura is a consultant for Boston Scientific, Lumendi, and Olympus America. Dr. Marion is on the Advisory Board for Janssen. Dr. Song is a consultant for Olympus America and US Endoscopy. Dr. Friedland is a consultant for Capsovision Inc., Dr. Charabaty is on the advisory board of and/or a consultant for and/or receives educational grants from Abbvie, Janssen, Pfizer, and Takeda.
References
- 1.Jess T, Simonsen J, Jorgensen K T.Decreasing risk of colorectal cancer in patients with inflammatory bowel disease over 30 years Gastroenterol 2012143375–381 e371.quiz e313–374 [DOI] [PubMed] [Google Scholar]
- 2.Rutter M D, Saunders B P, Wilkinson K H et al. Thirty-year analysis of a colonoscopic surveillance program for neoplasia in ulcerative colitis. Gastroenterol. 2006;130:1030–1038. doi: 10.1053/j.gastro.2005.12.035. [DOI] [PubMed] [Google Scholar]
- 3.Laine L, Kaltenbach T, Barkun A et al. SCENIC international consensus statement on surveillance and management of dysplasia in inflammatory bowel disease. Gastroenterol. 2015;148:639–651 e628. doi: 10.1053/j.gastro.2015.01.031. [DOI] [PubMed] [Google Scholar]
- 4.American Society for Gastrointestinal Endoscopy Standards of Practice C . Shergill A K, Lightdale J R et al. The role of endoscopy in inflammatory bowel disease. Gastrointest Endosc. 2015;81:1101–1121 e1101–1113. doi: 10.1016/j.gie.2014.10.030. [DOI] [PubMed] [Google Scholar]
- 5.Draganov P V, Wang A Y, Othman M O et al. AGA Institute Clinical Practice Update: Endoscopic Submucosal Dissection in the United States. Clin Gastroenterol Hepatol. 2019;17:16–25 e11. doi: 10.1016/j.cgh.2018.07.041. [DOI] [PubMed] [Google Scholar]
- 6.Pimentel-Nunes P, Dinis-Ribeiro M, Ponchon T et al. Endoscopic submucosal dissection: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy. 2015;47:829–854. doi: 10.1055/s-0034-1392882. [DOI] [PubMed] [Google Scholar]
- 7.Iacopini F, Saito Y, Yamada M et al. Curative endoscopic submucosal dissection of large nonpolypoid superficial neoplasms in ulcerative colitis (with videos) Gastrointest Endosc. 2015;82:734–738. doi: 10.1016/j.gie.2015.02.052. [DOI] [PubMed] [Google Scholar]
- 8.Kinoshita S, Uraoka T, Nishizawa T et al. The role of colorectal endoscopic submucosal dissection in patients with ulcerative colitis. Gastrointest Endosc. 2018;87:1079–1084. doi: 10.1016/j.gie.2017.10.035. [DOI] [PubMed] [Google Scholar]
- 9.Manta R, Zullo A, Telesca A et al. Endoscopic submucosal dissection for visible dysplasia treatment in ulceratiVe colitis patients: cases series and systematic review of literature. J Crohn Coliti. 2021;15:165–168. doi: 10.1093/ecco-jcc/jjaa158. [DOI] [PubMed] [Google Scholar]
- 10.Matsumoto K, Oka S, Tanaka S et al. Long-term outcomes after endoscopic submucosal dissection for ulcerative colitis-associated dysplasia. Digestion. 2021;201:205–215. doi: 10.1159/000503341. [DOI] [PubMed] [Google Scholar]
- 11.Suzuki N, Toyonaga T, East J E. Endoscopic submucosal dissection of colitis-related dysplasia. Endoscopy. 2017;49:1237–1242. doi: 10.1055/s-0043-114410. [DOI] [PubMed] [Google Scholar]
- 12.Yang D H, Kim J, Song E M et al. Outcomes of ulcerative colitis-associated dysplasia patients referred for potential endoscopic submucosal dissection. J Gastroenterol Hepatol. 2019;34:1581–1589. doi: 10.1111/jgh.14623. [DOI] [PubMed] [Google Scholar]
- 13.Matsumoto A, Tanaka S, Oba S et al. Outcome of endoscopic submucosal dissection for colorectal tumors accompanied by fibrosis. Scand J Gastroenterol. 2010;45:1329–1337. doi: 10.3109/00365521.2010.495416. [DOI] [PubMed] [Google Scholar]
- 14.Schlemper R J, Kato Y, Stolte M. Diagnostic criteria for gastrointestinal carcinomas in Japan and Western countries: proposal for a new classification system of gastrointestinal epithelial neoplasia. J Gastroenterol Hepatol. 2000;15:G49–G57. doi: 10.1046/j.1440-1746.2000.02266.x. [DOI] [PubMed] [Google Scholar]
- 15.Alkandari A, Thayalasekaran S, Bhandari M et al. Endoscopic resections in inflammatory bowel disease: a multicentre european outcomes study. J Crohns Colitis. 2019;13:1394–1400. doi: 10.1093/ecco-jcc/jjz075. [DOI] [PubMed] [Google Scholar]
- 16.Hurlstone D P, Sanders D S, Atkinson R et al. Endoscopic mucosal resection for flat neoplasia in chronic ulcerative colitis: can we change the endoscopic management paradigm? Gut. 2007;56:838–846. doi: 10.1136/gut.2006.106294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yadav S, Loftus E V, Jr., Harmsen W S et al. Outcome of endoscopic resection of colonic polyps larger than 10 mm in patients with inflammatory bowel disease. Endosc Int Open. 2019;7:E994–E1001. doi: 10.1055/a-0953-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Smith L A, Baraza W, Tiffin N et al. Endoscopic resection of adenoma-like mass in chronic ulcerative colitis using a combined endoscopic mucosal resection and cap assisted submucosal dissection technique. Inflamm Bowel Dis. 2008;14:1380–1386. doi: 10.1002/ibd.20497. [DOI] [PubMed] [Google Scholar]
- 19.Fuccio L, Hassan C, Ponchon T et al. Clinical outcomes after endoscopic submucosal dissection for colorectal neoplasia: a systematic review and meta-analysis. Gastrointest Endosc. 2017;86:74–86 e17. doi: 10.1016/j.gie.2017.02.024. [DOI] [PubMed] [Google Scholar]
- 20.Draganov P V, Aihara H, Karasik M S et al. Endoscopic submucosal dissection in North America: a large prospective multicenter study. Gastroenterol. 2021;160:2317–2327 e2312. doi: 10.1053/j.gastro.2021.02.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Matsuda T, Fujii T, Saito Y et al. Efficacy of the invasive/non-invasive pattern by magnifying chromoendoscopy to estimate the depth of invasion of early colorectal neoplasms. Am J Gastroenterol. 2008;103:2700–2706. doi: 10.1111/j.1572-0241.2008.02190.x. [DOI] [PubMed] [Google Scholar]
- 22.Bhatt A, Abe S, Kumaravel A et al. Indications and techniques for endoscopic submucosal dissection. Am J Gastroenterol. 2015;110:784–791. doi: 10.1038/ajg.2014.425. [DOI] [PubMed] [Google Scholar]


