Abstract
Background and study aims Surgical gastroenterostomy (SGE) has been the mainstay treatment for gastric outlet obstruction (GOO). The emergence of endoscopic ultrasound-guided gastroenterostomy (EUS-GE) presents a less invasive alternative for palliation of GOO. We conducted a comprehensive review and meta-analysis to compare the effectiveness and safety of EUS-GE compared to SGE.
Methods Multiple electronic databases and conference proceedings up to April 2021 were searched to identify studies that reported on safety and effectiveness of EUS-GE in comparison to SGE. Pooled odds ratios (ORs) of technical success, clinical success, adverse events (AE) and recurrence, and pooled standardized mean difference (SMD) of procedure time and post-procedure length of stay (LOS) were calculated. Study heterogeneity was assessed using I 2 and Cochran Q statistics.
Results Seven studies including 625 patients (372 EUS-GE and 253 SGE) were included. EUS-GE had lower pooled odds of technical success compared with SGE (OR 0.19, 95 % confidence interval [CI] 0.06–0.60, I 2 0 %). Among the technically successful cases, EUS-GE was superior in terms of clinical success (OR 4.73, 95 % CI 1.83–12.25, I 2 18 %), lower overall AE (OR 0.20, 95 % CI 0.10–0.37, I 2 39 %), and shorter procedure time (SMD –2.4, 95 % CI –4.1, –0.75, I 2 95 %) and post-procedure LOS (SMD –0.49, 95 % CI –0.94, –0.03, I 2 78%). Rates of severe AE (0.89, 95 % CI 0.11–7.36, I 2 67 %) and recurrence (OR 0.49, 95 % CI 0.18–1.38, I 2 49 %) were comparable.
Conclusions Our results suggest EUS-GE is a promising alternative to SGE due to its superior clinical success, overall safety, and efficiency. With further evolution EUS-GE could become the intervention of choice in GOO.
Introduction
Gastric outlet obstruction (GOO) refers to restricted emptying of the stomach caused by mechanical obstruction of the distal stomach or proximal duodenum. Malignancy accounts for up to 80 % cases of GOO including cancers of the pancreas, distal stomach, duodenum, ampulla, biliary system, and lymphoma, and metastasis. Benign causes of GOO include peptic strictures, chronic pancreatitis with duodenal stenosis, and post-surgical complications 1 2 . Most patients with malignant GOO have advanced, non-resectable tumors for which symptom palliation and improving quality of life are often the goals of treatment. Surgical gastroenterostomy (SGE) has historically been the standard palliative intervention for GOO 3 4 . While SGE is highly effective in improving tolerance to oral feeds and medications, it is often associated with postoperative complications, delayed gastric emptying, prolonged recovery time, and higher cost 5 6 . Endoscopic self-expandable metal stent placement is an alternative to surgery; however, the clinical course is often complicated by recurrent obstruction caused by stent migration or tumor infiltration, and therefore, not an adequate long-term option 7 8 .
Endoscopic ultrasound (EUS)-guided gastroenterostomy (EUS-GE) is a novel minimally-invasive approach for palliation of GOO 9 10 11 12 . The technique involves insertion of a lumen apposing metal stent across the stomach into the small bowel distal to the obstruction under EUS and fluoroscopic guidance 13 . Because EUS-GE creates a fistulous tract bypassing the obstructed bowel segment, it can be performed regardless of the degree of stenosis or the type of disease (benign or malignant) 14 . Studies have demonstrated the feasibility of this technique with high success rates and few adverse events (AEs), as well as long-term durability and a low rate of reintervention 12 15 16 17 18 . Additional studies have found comparable efficacy and safety to endoscopic stenting but with fewer recurrences requiring reintervention 19 20 .
EUS-GE can be performed either by direct puncture of the desired bowel loop under EUS and fluoroscopic guidance or using assisted techniques such as a balloon passed into the small bowel and filled with fluid to act as a target for penetration 9 21 . The advent of electrocautery-enhanced delivery system (Hot AXIOS; Boston Scientific, Marlborough, Massachusetts, United States) permits stent placement without needle puncture and guidewire placement potentially reducing the risk of missing the target during EUS-GE 9 13 18 . Despite these advancements, EUS-GE remains a technically demanding procedure as access to the small bowel can be difficult and unpredictable. Loss of visualization of the small bowel due to its mobility and subsequent stent mis-deployment into the peritoneum or colon can occur 9 13 .
Individual observational studies have evaluated the performance of EUS-GE against the more established SGE 22 23 24 25 26 27 28 . We conducted a comprehensive review and meta-analysis of these studies to further compare the effectiveness and safety profile of EUS-GE and SGE.
Methods
Search strategy
We conducted a comprehensive search of several databases and conference proceedings, including PubMed, EMBASE, Google Scholar, SCOPUS and Web of Science databases, for publications, e-publications ahead of print, in-process and other non-indexed citations from inception to April 2021. The search was restricted to studies in human subjects published in the English language in peer-reviewed journals. The detailed search methodology is shown in Appendix 1 . Two authors (AK, SC) independently reviewed the title and abstract of studies identified in the primary search and excluded studies that did not address the research question, based on pre-specified exclusion and inclusion criteria. Any discrepancy in article selection was resolved by consensus, in discussion with the senior author (PCB). The bibliographic sections of the selected articles, as well as the systematic and narrative articles were manually searched for additional relevant articles.
All results were exported to Endnote where 162 obvious duplicates were removed leaving 242 citations. We followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines, using a predefined protocol to identify studies reporting outcomes of EUS-GE compared to SGE (Appendix 2) 29 . As the included studies were observational in design, the MOOSE (Meta-analyses Of Observational Studies in Epidemiology) checklist was followed (Appendix 3) 30 . Reference lists of evaluated studies were examined to identify other studies of interest.
Study selection
Only studies that compared EUS-GE and SGE were included (i. e., they needed to have both the EUS-GE and SGE study arms). Studies were included irrespective of the country of origin, whether they were published as full manuscripts or conference abstracts, performed in inpatient or outpatient settings, follow-up duration, or presence of surgically altered anatomy as long as they provided the appropriate data needed for the analysis.
The exclusion criteria included: (1) studies reporting individually on EUS-GE or SGE, or comparing EUS-GE or SGE to only endoscopic stenting, (2) case reports and case series studies with sample size < 10 patients, (3) studies performed in the pediatric population (Age < 18 years), and (4) studies not published in English language. In cases of multiple publications from a single research group reporting on the same or overlapping cohorts, data from the most recent and/or most appropriate comprehensive report were retained.
Data abstraction and quality assessment
Data on study-related outcomes from the individual studies were abstracted independently onto a standardized form by at least two authors (AK, SC). Authors PCB and AJT cross-verified the collected data for possible errors and two authors (PRA, BPM) did the quality scoring independently. The Newcastle-Ottawa scale for cohort studies was used to assess the quality of studies 31 . This quality score consisted of eight questions, the details of which are provided in Supplementary Table 1 .
Outcomes assessed
The primary outcomes were as follows:
Pooled odds ratio (ORs) of technical success (defined as successful creation of a gastroenterostomy)
Pooled OR of clinical success (defined as the ability to tolerate oral intake without vomiting among the technically successful cases)
Pooled OR of all AEs (included infection, bleeding, stent migration, perforation, leak, ileus/gastroparesis etc.)
Pooled OR of recurrence (defined as recurrence of initial symptoms of nausea, vomiting or the need for reintervention due to recurrent GOO).
Secondary outcomes:
Pooled OR of severe AEs (defined by the American Society of Gastrointestinal Endoscopy lexicon for endoscopic AEs and AE subtypes) 32 .
Pooled standardized mean difference (SMD) in procedure time.
Pooled SMD in post-procedure length of stay (LOS)
Statistical analysis
Meta-analysis techniques were used to calculate the pooled estimates in each case following the methods suggested by DerSimonian and Laird using the random-effects model 33 . Summary estimates calculated were either the pooled ORs or the SMD with corresponding 95 % confidence intervals (CIs), as appropriate.
We assessed heterogeneity between study-specific estimates by using Cochran’s Q statistical test for heterogeneity, 95 % prediction interval and the I 2 statistics 34 . In this, values of < 30 %, 30 %–60 %, 61 % to 75 %, and > 75 % were suggestive of low, moderate, substantial, and considerable heterogeneity, respectively. We assessed publication bias, qualitatively, by visual inspection of funnel plot and quantitatively, by the Egger test 35 . Publication bias assessment was deferred if the total number of studies included in the analysis were less than ten. All analyses were performed using Comprehensive Meta-Analysis (CMA) software, version 3 (BioStat, Englewood, New Jersey, United States).
When the original studies only reported median and range or interquartile range, those were converted to mean and standard deviation (SD) for the purpose of meta-analysis of continuous outcome variable 36 37 . If a study did not report the measure of dispersion of a continuous variable, SD was imputed from the sample size and between group SMD and p-value, using the Cochrane RevMan calculator 38 39 .
Results
Search results and population characteristics
A total of seven studies involving 625 patients were included in the final analysis 22 23 24 25 26 27 40 . Single-arm studies on EUS-GE 12 17 18 41 42 43 44 , and/or comparing EUS-GE to only endoscopic stenting 19 45 46 47 were excluded. A schematic diagram demonstrating our study selection is illustrated in Supplementary Fig. 1 . Overall, 372 patients underwent EUS-GE, and 253 patients underwent SGE. There were 289 (55.4 %) males and mean age ranged from 62 to 75 years. Etiology of GOO was malignancy in 87 % (n = 541, most commonly being pancreatic cancer) and benign causes were reported in 13 % (n = 84) of the individuals. Five studies provided data on EUS-GE and SGE techniques. Of those 66 % (n = 131) of EUS-GE were performed by direct puncture technique and the remaining using assisted methods (balloon-assisted or balloon-occlusion). Of the five studies that specified the surgical techniques, 60 % (n = 151) were open and the rest were laparoscopic. Mean follow-up time ranged from 56–234 days for EUS-GE and 166–268 days for SGE. Further details of the study, population characteristics and outcomes are described in Table 1 and Table 2 .
Table 1. Study population characteristics.
| Study, year | Design, data collection period | Etiology of GOO (M = malignant, B = benign) | Type of malignancy (gastric = 1, duodenal = 2, ampullary = 3, pancreatic = 4, biliary = 5, others = 6) | EUS-GE technique (DP = direct puncture, AT = assisted)/ stent type | SGE technique (O = open, L = laparoscopic) | Prior interventions; Altered anatomy | Total patients | Gender (male) | Age (years) [mean (SD) unless specified] | |||
| EUS-GE | SGE | EUS-GE | SGE | EUS-GE | SGE | |||||||
| Perez-Miranda, 2017 | Retrospective, multicenter, Mar 2014 to Nov 2015 | M = 46/54, B = 8/54 | NR | DP = 6, AT = 19; Axios (hot 12, cold 13) | O = 2, L = 27 | EUS-GE: 18, SGE: 0; EUS-GE: 7, SGE: 0 | 25 | 29 | 11/25 | 22/29 | 63.9 | 75.8 |
| Khashab, 2017 | Retrospective, multicenter, Jan 2013 to Aug 2015 | M = 93, B = 0 | EUS-GE: G 5, A 2, P 17, B 2, O 4; SGE: A 9, D 1, P 53 | DP = 2, AT = 28; Axios (hot 21, cold 7), Niti-S Spaxus 2 | O = 63, L = 0 | NR | 30 | 63 | 17/30 | 32/63 | 70 (13.3) | 68 (9.6) |
| Widmer, 2019 (abs) | Retrospective, single-center, Jan 15 to Nov 18 | M = 24, B = 0 | EUS: P 4, D 1, G 1, O 3; SGE: P 4, D 3, G 1, A 1, B 2, O 2. | DP = 6, AT = 4; S; Axios (hot) 10 | O = 11, L = 3 | NR | 10 | 14 | 5/10 | 5/14 | 63 (range 40–94) | 68 (range 47–86) |
| Marya, 2020 (abs) | Retrospective, multicenter, Jun 2005 to Nov 2019 | EUS-GE: M = 137, B = 34; SGE: M = 24, B = 15 | NR | NR | NR | EUS-GE: 22, SGE: 21; EUS-GE: 8, SGE: 8 | 172 | 39 | 104/172 | 20/39 | 62.4 (11.8) | 63.9 (13.7) |
| Bondi, 2020 (abs) | Retrospective, single-center, 2000 to 2019 | M = 52, B = 0 | EUS-GE: G 2, D 1, P 4, B 5, O 6; SGE: G 8, D 4, P 11, B 1, O 10 | NR; Axios (hot) 18 | NR | NR | 18 | 34 | 8/18 | 16/34 | 64 (11) | 61.3 (14) |
| Kouanda, 2021 | Retrospective, single-center, Jan 2014 to Feb 2020 | M = 50, B = 16 | EUS-GE: P 26, B 3, G 0, D 1, A 1, O 5; SGE: P 3, B 1, G 8, D 1, A 0, O 1 | DP = 40, AT = 0; NR | O = 26, L = 0 | EUS-GE: 9, SGE: 7; NR | 40 | 26 | 23/40 | 15/26 | 70.5 (11.5) | 69.7 (15.4) |
| Bronswijk, 2021 | Retrospective, multi-center, Jan 2015 to May 2020 | EUS-GE: 74; SGE: 41 | EUS-GE: P 37, B 9, G 7, D 11, A 0, O 8 ; SGE: P 14, B 2, G 5, D 10, A 1, O 4 | DP = 77, AT = 0; Axios (hot) 77 | O = 0, L = 48 | NR | 77 | 48 | 41/77 | 29/48 | 65 (12.3) | 66 (11.6) |
EUS-GE, endoscopic ultrasound-guided gastroenterostomy; NR, not reported; SD, standard deviation; SGE, surgical gastroenterostomy.
Table 2. Study results and adverse events.
| Study, year | Technical success | Clinical success | AEs | Severe AE | Recurrence or re-intervention | Procedure Time (min) | LOS (days) | Follow up period (days) | ||||||||
| EUS-GE | SGE | EUS-GE | SGE | EUS-GE | SGE | EUS-GE | SGE | EUS-GE | SGE | EUS-GE | SGE | EUS-GE | SGE | EUS-GE | SGE | |
| Perez-Miranda, 2017 | 22/25 | 29/29 | 21/22 | 28/29 | 3/25 | 12/29 | 1/25 | 2/29 | NR | NR | 77/66 1 | 178/66 1 | 9.4/5.7 1 | 8.9/5.7 1 | Mean 56 | Mean 268.8 |
| Khashab, 2017 | 26/30 | 63/63 | 26/26 | 57/63 | 5/30 | 16/63 | 3/30 | 0/63 | 1/26 | 9/63 | NR | NR | 11.6/6.6 1 | 12/8.2 1 | Mean/SD 115 (63) | Mean/SD 196 (155) |
| Widmer, 2019 (abs) | 10/10 | 14/14 | 10/10 | 8/14 | 0/10 | 4/14 | NR | NR | 1/10 | 0/14 | NR | NR | 4.7/1.9 | 6/3.5 | Mean/range 90 (30–180) | Mean/range 240 (30–1050) |
| Marya, 2020 (abs) | 168/172 | 39/39 | 167/168 | 32/39 | 8/172 | 7/39 | 3/172 | 0/39 | 3/168 | 5/39 | NR | NR | NR | NR | Median 234 | Median 235 |
| Bondi, 2020 (abs) | 17/18 | 34/34 | 17/17 | 33/34 | 4/18 | 16/34 | NR | NR | 6/17 | 12/34 | NR | NR | 10/10 | 13/8 | NR | NR |
| Kouanda, 2021 | 37/40 | 26/26 | 34/37 | 22/26 | 9/40 | 23/26 | NR | NR | 8/37 | 5/26 | 57.0/14.6 | 227.5/55.5 | 4.7/3.8 1 | 13.5/7.8 1 | Median/IQR 98.0 (35.5–288.5) | Median/IQR 166.5 (74–728) |
| Bronswijk, 2021 | 73/77 | 48/48 | 71/73 | 42/48 | 5/77 | 15/48 | 2/77 | 9/48 | 0/73 | 3/48 | 56/33 1 | 96/33 1 | 5.6/6.4 1 | 11.2/11.5 1 | Median/IQR 76 (36–136) | Median/IQR 122 (35–274) |
AE, adverse events; EUS-GE, endoscopic ultrasound-guided gastroenterostomy; LOS, post-procedure length of hospital stay; NR, not reported; SGE, surgical gastroenterostomy.
imputed values using Cochrane RevMan calculator [38,39].
Characteristics and quality of included studies
All the included cohort studies were retrospective in design. Four studies were published as full manuscripts 25 26 27 28 , while three were published as conference abstracts 22 23 24 . Three studies were performed in the United States 22 23 27 , and four were multicenter, multinational studies (Spain, USA, France 26 ; USA, Japan 25 ; USA, Belgium 24 ; Italy, Belgium, Netherland 28 ). Based on the New-Castle Ottawa scoring system, six studies 23 24 25 26 27 28 were considered to be of high quality and one study 22 was of medium quality. There were no low-quality studies (Supplementary Table 1).
Meta-analysis outcomes
The primary outcomes were as follows:
Technical success
The pooled OR of technical success with EUS-GE vs SGE was 0.19 (95 % CI [0.06–0.60]; I 2 0 %; Q = 2.1 [ P = 0.90]); P = 0.005; Fig. 1 . The pooled rate of technical success for EUS-GE was 93.6 % (95 % CI [89.3–96.2]) and 98.5 % (95 % CI [95.9–99.5]) for SGE.
Fig. 1.
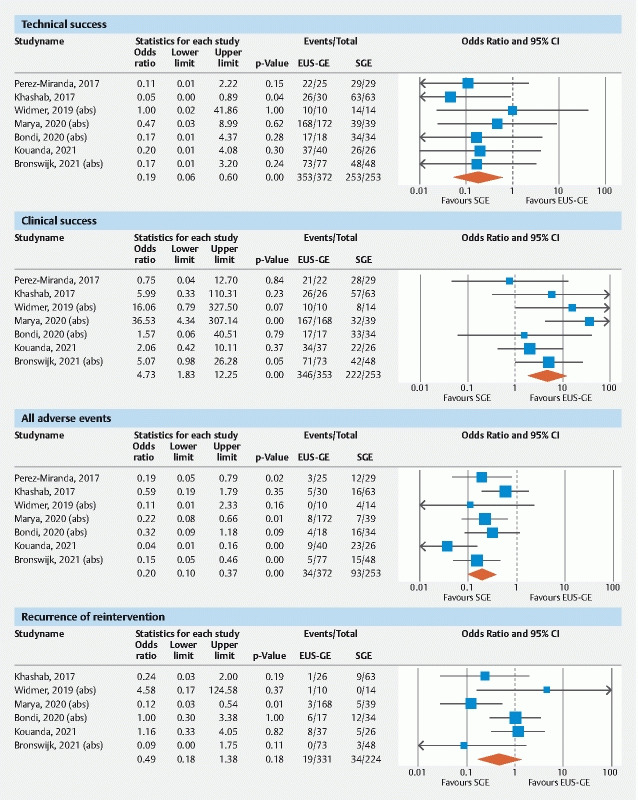
Forest Plots of primary outcomes: technical Success, clinical success, all adverse events, recurrence.
Clinical Success
The pooled OR of clinical success with EUS-GE vs SGE was 4.73 (95 % CI [1.83–12.25]; I 2 18 %; Q = 7.3 [p = 0.29]); P = 0.001, Fig. 1 . The pooled rate of clinical success for EUS-GE was 96.4 % (95 % CI [93.2–98.2]) and for SGE was 86.4 % (95 % CI [77.0–92.4]).
All AEs
The pooled OR of all AEs with EUS-GE vs SGE was 0.20 (95 % CI [0.10–0.37]; I 2 39 %; Q = 9.8 [ P = 0.13]); p < 0.001, Fig. 1 . The pooled rate of all AEs for EUS-GE was 11.5 % (95 % CI [6.4–19.9]) and 38.5 % (95 % CI [24.8–54.3]) for SGE.
Recurrence
Six studies provided data on recurrence/re-intervention. The pooled OR of recurrence with EUS-GE vs SGE was 0.49 (95 % CI [0.18–1.38]; I 2 49 %; Q = 9.8 [p = 0.08]); p = 0.18, Fig. 1 ). The pooled proportion of patients with recurrence after EUS-GE was 10.1 % (95 % CI [2.8–30.2]) and after SGE was 18.2 % (95 % CI [10.4–29.9]).
Secondary outcomes
Severe AEs
Four studies reported proportions of severe AEs separately. The pooled OR of severe AE w i th EUS-GE vs SGE was 0.89 (95 % CI [0.11–7.36]; I 2 67 %; Q = 9.1 [ P = 0.03]); p = 0.99, Fig. 2 . The pooled proportion of patients with a severe AE for EUS-GE was 3.7 % (95 % CI [1.5–8.6]) and 5.4 % (95 % CI [1.3–20.4]) after SGE.
Fig. 2.
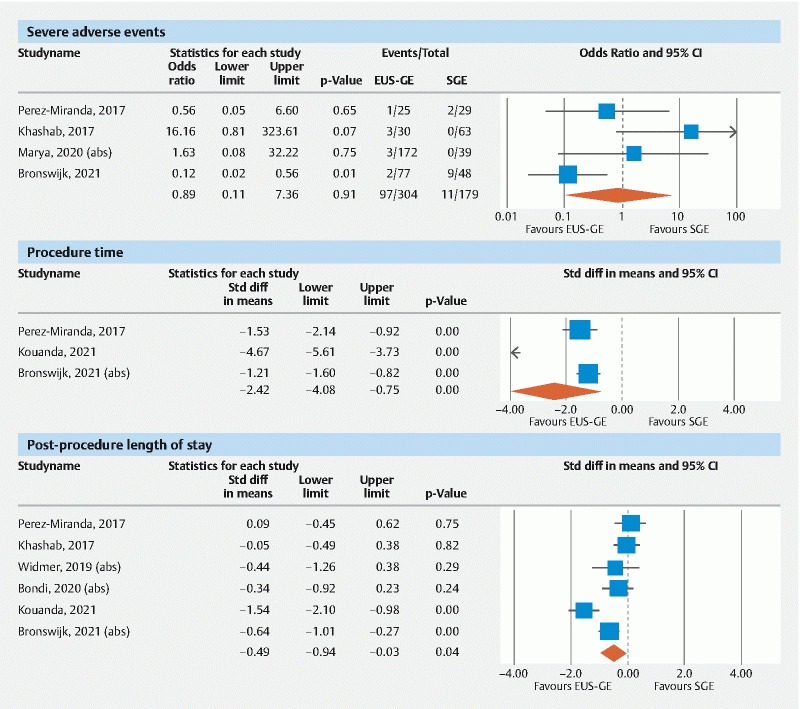
Forest Plots of severe adverse events, procedure time, and post-procedure length of stay.
Procedure time
Based on the three studies that reported on this metric, the pooled SMD in procedure time for EUS-GE vs SGE was –2.4 (95% CI [–4.1, –0.75]; I 2 95 %; Q = 41.8 [ P < 0.01]); P = 0.004, Fig. 2 . The pooled mean procedure time for EUS-GE was 57 mins (95 % CI [53–62]) and for SGE was 167 mins (95 % CI [80–254]).
Post-procedure length of stay
Six studies provided data on the post-procedure LOS. The pooled SMD of LOS for EUS-GE vs SGE was –0.49 (95 % CI [– 0.94, –0.03]; I 2 78 %; Q = 21.2 [ P < 0.01]); p = 0.037, Fig. 2 . The pooled mean LOS for EUS-GE was 7.3 days (95 % CI [5.2–9.4]) and 10.6 days (95 % CI [8.1–13.2]) for SGE.
A summary of pooled outcomes from the meta-analysis is provided in Supplementary Table 2.
Validation of meta-analysis results
Sensitivity analysis
To assess whether any one study had a dominant effect on the meta-analysis, we excluded one study at a time and analyzed its effect on the main summary estimate. In this analysis, no single study significantly affected the outcome or its heterogeneity. Widmer et al reported unusually low clinical success rate for SGE (57 %) compared with EUS-GE (100 %); however, excluding this study did not affect the statistical significance. Removing the outlier studies by Kouanda et al and Khashab et al for the meta-analysis of all AEs and severe AEs, respectively, did not affect their statistical significance.
In a subgroup analysis of studies with full-text manuscripts (excluding three studies available in only the abstract form), the statistical significance remained unchanged for all meta-analysis outcomes except for post-procedure LOS (which was numerically lower but no longer statistically significant due to reduced sample size)
Heterogeneity
We assessed dispersion of the calculated rates using the I 2 percentage and Cochran’s Q statistics and the values are reported with the pooled outcomes in Supplemental Table 1 . Low to moderate heterogeneity was noted for the analysis of technical success, clinical success, all AEs and recurrence. Substantial to considerable heterogeneity was seen for the outcomes of severe AEs, procedure times and LOS.
Publication bias
Publication bias was not estimated as the number of studies included in the analysis was less than 10.
Discussion
Our meta-analysis demonstrated high pooled technical success rates for both EUS-GE [93.6 % (95 % CI 89.3–96.2)] and SGE [98.5 % (95 % CI 95.9–99.5)]. Although the pooled odds of technical success of EUS-GE was statistically inferior to SGE [OR 0.19 (95 % CI 0.06–0.60)], the overlapping pooled proportions and a wide CI in the OR indicate that the difference may not be clinically relevant. More importantly, among the technically successful cases, EUS-GE was superior to SGE in terms of higher clinical success [pooled OR 4.73 (95 % CI 1.83–12.25)], fewer overall AEs [pooled OR 0.20 (95 % CI 0.10–0.37)] as well as shorter procedure time (pooled SMD –2.4) and post-procedure LOS (pooled SMD –0.49). Rates of recurrence and severe AEs were comparable between the two gastroenterostomy techniques. To the best of our knowledge, this is the first and most comprehensive analysis comparing outcomes of EUS-GE and SGE within a large cohort of patients with GOO.
All attempted SGE cases were technically successful, whereas 19 of 372 (5 %) cases of EUS-GE were technically unsuccessful. Reported causes of technical failure included stent dislodgment during deployment needing retrieval and closure of the access site (n = 3) 26 , stent deployment into the peritoneum (n = 1), inability to distend a limb of jejunum (n = 1), and failure to identify an adequate target loop (n = 1) 27 . It is possible that patients who underwent EUS-GE were sicker and therefore deemed to be poor surgical candidates compared to their SGE counterparts. Similarly, prior surgically altered anatomy could make EUS-GE more challenging. For example, in the study by Perez-Miranda et al, 88 % of patients in the EUS-GJ group achieved technical success, however 28 % of these patients had altered anatomy (prior Whipple, Roux-en-Y or partial gastrectomy) versus zero SGE patients 26 . EUS-GE is still evolving and remains a technically demanding procedure. Further advancements in tools and technology such as use of forward-viewing echoendoscopes and change in view from curved to linear may increase technical success 48 . Despite these limitations, the technical success rate of > 90 % demonstrated in this meta-analysis is still considered excellent in most clinical settings.
Among the cases that were technically successful, a significantly higher odds of clinical success (OR 4.73, 95 % CI 1.83–12.25, P = 0.001) was seen with EUS-GE compared to SGE. Reported causes of clinical failure included inability to tolerate orally despite a patent GJ (n = 2) 26 27 and post-procedure stent migration (n = 2) 27 . For SGE the causes of clinical failure included postoperative death before initiating feeds (n = 2) 26 27 , persistent inability to tolerate any diet (n = 3) 27 , and delayed gastric emptying (n = 6) 23 . Several potential factors could be responsible for the superior clinical success seen with EUS-GE versus SGE. SGE involves percutaneous incision and prolonged anesthesia that may result in postoperative pain, nausea, gastroparesis and ileus. Two of the studies included in this meta-analysis indeed reported a shorter time to resumption of oral feeds in patients with EUS-GE compared with SGE 27 28 . Additionally, access to the posterior wall of the stomach may be challenging by the laparoscopic approach, and as bowel loops are mobilized for creating a surgical anastomosis there is a potential for tension at the anastomosis or creation of anti-peristaltic anastomosis. EUS-GE is for the most part is devoid of these procedural concerns.
EUS-GE was associated with five-fold decrease in the odds of all AEs compared with SGE (OR: 0.20, 95 % CI 0.10–0.37, P < 0.001) – unsurprising given the minimally-invasive and less morbid nature of the endoscopic intervention over a surgical or laparoscopic GE. The relative safety of EUS-GE over SGE is critical in patients with malignant GOO who are already plagued by limited life expectancy and reduced quality of life. As EUS-GE is often offered preferentially to complex patients with a higher baseline surgical risk, the adjusted safety difference may be even larger. Rates of a severe AE were relatively low among both EUS-GE and SGE (≤ 5.4 %) without a significant difference between the two, suggesting both interventions are acceptable in the appropriate clinical setting.
Five studies described the AEs. Perez-Miranda et al found EUS-GE to be associated with postprocedural bleeding managed with transfusion (n = 2) and peritonitis (n = 1), and SGE to be associated with ileus or gastroparesis (n = 3), anastomotic edema (n = 1), bacteremia (n = 1), pneumonia (n = 1), urinary tract infection (n = 2), and anastomotic leaks requiring surgical revision (n = 2) 26 . In the study by Khashab et al, the AEs with EUS-GE were mis-deployment of the stent flange in the peritoneum (n = 3), abdominal pain requiring hospitalization (n = 2), and with SGE were infection (n = 8), anastomotic leak (n = 4), persistent ileus (n = 1), agitation/delirium (n = 2), and pulmonary embolism (n = 1) 25 . Widmer et al reported no AE in the EUS-GE group, and 4 AEs in the SGE group including non-ST elevation myocardial infarction (n = 1), pneumonia (n = 1), gastro-cutaneous fistula (n = 1), wound hematoma (n = 1) 23 . Kouanda et al reported the following AEs in their study: EUS-GE- Infection (n = 2), venous thromboembolism (n = 1), perforation (n = 1), bleeding (n = 1), stent migration (n = 4); SGE- infection (n = 9), ileus (n = 7), VTE (n = 1), bleeding (n = 2), AKI (n = 3), delirium (n = 1) 27 . In the recently published study by Bornswijk et al, the AEs with EUS-GE included fever (n = 2), sepsis (n = 1), intraperitoneal stent deployment (n = 2) and those who underwent SGE: anastomotic leak (n = 3), anastomotic bleeding (n = 2), need for endoscopy (n = 4), and surgical reintervention (n = 3) 28 .
Post-procedure mortality was reported variably in four studies in our meta-analysis. Perez-Miranda et al reported deaths within the 1–11 days following the intervention (1 in EUS-GE and 2 in SGE) 26 . Kounada et al reported death within 30 days (12.5 % vs 3.8 %, P = 0.84) 27 . Two other studies reported post-procedure death rates without specifying the follow-up period 22 25 . Patients with malignant GOO have a high mortality rate regardless of gastroenterostomy.
The mean procedure time and post-procedure LOS were both significantly shorter with EUS-GE versus SGE, albeit with considerable heterogeneity in the analysis of these outcomes. Two studies provided a cost analysis and found significantly lower cost of EUS-GE vs SGE. First study reported procedural cost based on physician fee and facility fee (EUS-GE vs SGE: $4515 vs $14,778.80; P < 0.001) 26 and the second study reported based on the facility fee ($19,785 vs $42,716, P < 0.001) 27 . Time-to-oral intake was reported in two studies and both individually found it to be significantly shorter with EUS-GE than SGE (mean 1.3 vs 4.7 days, P < 0.001) 27 and (median 1 day vs 3 days; P < 0.001) 28 , respectively.
The strengths of our review include systematic literature search with rigorous process accounting for study quality, limitations and heterogeneity, well-defined inclusion and exclusion criteria, high-quality studies with detailed extraction of data. A large cohort of GOO patients were included in the analysis. All included studies in our analysis reported head-to-head comparison of both interventions i. e. EUS-GE and SGE, which allowed us to perform a comparative meta-analysis between the two techniques. This is the most up-to-date systematic review and meta-analysis of available studies on this topic.
There are several limitations to this study, most of which are inherent to meta-analysis. Three of the included studies in our analysis were published only as abstracts. Although the primary outcomes remained statistically unchanged in the subgroup analysis of full-text studies. Second, the included studies were mostly performed in tertiary-care referral centers by expert endoscopists and therefore may not be entirely representative of the general population and community practice. All the studies included in our analysis were retrospective in nature and are subject to inherent bias. There are limitations due to significant heterogeneity observed in the analysis of severe AE, procedure duration and post-procedure LOS. The observed heterogeneity could be related to combining outcomes of different EUS-GE techniques (direct puncture and balloon-assisted; electrocautery-enhanced and non-enhanced) and SGE techniques (open and laparoscopic), and malignant and benign etiologies of GOO (while majority of GOO in our analysis had a malignant etiology and only 15 % had a benign etiology). Furthermore, only three studies provided data on prior interventions and altered anatomy, therefore we were unable to assess if this had any influence on the technical and clinical success of either technique. Finally, combining multicenter data has limitations due to differences in practices across centers/countries and inter-operator variability.
Conclusions
In conclusion, our meta-analysis demonstrates that in expert hands EUS-GE is safe and highly effective. It provides superior clinical effectiveness and safety profile with a shorter procedure time and post-procedure LOS compared to SGE. Further advancements in the techniques of EUS-GE are needed to achieve technical success rates at par with SGE. These characteristics make EUS-GE an attractive minimally-invasive option for palliation of GOO, particularly where peri-operative risk with SGE is considerable. Prospective randomized controlled trials are needed to validate our findings.
Footnotes
Competing interests Dr. Trindade is a consultant to Pentax Medical and Olympus America and receives research support from NinePoint Medical. Dr. Benias is a consultant for Olympus America, Apollo Endosurgery, Boston Scientific, and FujiFilm.
Supplementary material :
References
- 1.Chowdhury A, Dhali G K, Banerjee P K. Etiology of gastric outlet obstruction. Am J Gastroenterol. 1996;91:1679. [PubMed] [Google Scholar]
- 2.Johnson C D. Gastric outlet obstruction malignant until proved otherwise. Am J Gastroenterol. 1995;90:1740. [PubMed] [Google Scholar]
- 3.Takeno A, Takiguchi S, Fujita J et al. Clinical outcome and indications for palliative gastrojejunostomy in unresectable advanced gastric cancer: multi-institutional retrospective analysis. Ann Surg Oncol. 2013;20:3527–3533. doi: 10.1245/s10434-013-3033-3. [DOI] [PubMed] [Google Scholar]
- 4.Bahra M, Jacob D. Surgical palliation of advanced pancreatic cancer. Recent Results Cancer Res. 2008;177:111–120. doi: 10.1007/978-3-540-71279-4_13. [DOI] [PubMed] [Google Scholar]
- 5.Khashab M, Alawad A S, Shin E J et al. Enteral stenting versus gastrojejunostomy for palliation of malignant gastric outlet obstruction. Surg Endosc. 2013;27:2068–2075. doi: 10.1007/s00464-012-2712-7. [DOI] [PubMed] [Google Scholar]
- 6.Jeurnink S M, Steyerberg E W, van Hooft J E et al. Surgical gastrojejunostomy or endoscopic stent placement for the palliation of malignant gastric outlet obstruction (SUSTENT study): a multicenter randomized trial. Gastrointest Endosc. 2010;71:490–499. doi: 10.1016/j.gie.2009.09.042. [DOI] [PubMed] [Google Scholar]
- 7.Storm A C, Ryou M. Advances in the endoscopic management of gastric outflow disorders. Curr Opin Gastroenterol. 2017;33:455–460. doi: 10.1097/MOG.0000000000000403. [DOI] [PubMed] [Google Scholar]
- 8.No J H, Kim S W, Lim C H et al. Long-term outcome of palliative therapy for gastric outlet obstruction caused by unresectable gastric cancer in patients with good performance status: endoscopic stenting versus surgery. Gastrointest Endosc. 2013;78:55–62. doi: 10.1016/j.gie.2013.01.041. [DOI] [PubMed] [Google Scholar]
- 9.Itoi T, Baron T H, Khashab M A et al. Technical review of endoscopic ultrasonography-guided gastroenterostomy in 2017. Dig Endosc. 2017;29:495–502. doi: 10.1111/den.12794. [DOI] [PubMed] [Google Scholar]
- 10.Khashab M A, Baron T H, Binmoeller K F et al. EUS-guided gastroenterostomy: a new promising technique in evolution. Gastrointest Endosc. 2015;81:1234–1236. doi: 10.1016/j.gie.2014.12.053. [DOI] [PubMed] [Google Scholar]
- 11.Binmoeller K F, Shah J N. Endoscopic ultrasound-guided gastroenterostomy using novel tools designed for transluminal therapy: a porcine study. Endoscopy. 2012;44:499–503. doi: 10.1055/s-0032-1309382. [DOI] [PubMed] [Google Scholar]
- 12.Tyberg A, Perez-Miranda M, Sanchez-Ocana R et al. Endoscopic ultrasound-guided gastrojejunostomy with a lumen-apposing metal stent: a multicenter, international experience. Endosc Int Open. 2016;4:E276–E281. doi: 10.1055/s-0042-101789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Irani S, Baron T H, Itoi T et al. Endoscopic gastroenterostomy: techniques and review. Curr Opin Gastroenterol. 2017;33:320–329. doi: 10.1097/MOG.0000000000000389. [DOI] [PubMed] [Google Scholar]
- 14.Carbajo A Y, Kahaleh M, Tyberg A. Clinical review of EUS-guided gastroenterostomy (EUS-GE) J Clin Gastroenterol. 2020;54:1–7. doi: 10.1097/MCG.0000000000001262. [DOI] [PubMed] [Google Scholar]
- 15.Kerdsirichairat T, Irani S, Yang J et al. Durability and long-term outcomes of direct EUS-guided gastroenterostomy using lumen-apposing metal stents for gastric outlet obstruction. Endosc Int Open. 2019;7:E144–E50. doi: 10.1055/a-0799-9939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Iqbal U, Khara H S, Hu Y et al. EUS-guided gastroenterostomy for the management of gastric outlet obstruction: A systematic review and meta-analysis. Endosc Ultrasound. 2020;9:16–23. doi: 10.4103/eus.eus_70_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Itoi T, Ishii K, Ikeuchi N et al. Prospective evaluation of endoscopic ultrasonography-guided double-balloon-occluded gastrojejunostomy bypass (EPASS) for malignant gastric outlet obstruction. Gut. 2016;65:193–195. doi: 10.1136/gutjnl-2015-310348. [DOI] [PubMed] [Google Scholar]
- 18.Khashab M A, Kumbhari V, Grimm I S et al. EUS-guided gastroenterostomy: the first U.S. clinical experience (with video) Gastrointest Endosc. 2015;82:932–938. doi: 10.1016/j.gie.2015.06.017. [DOI] [PubMed] [Google Scholar]
- 19.Chen Y I, Itoi T, Baron T H et al. EUS-guided gastroenterostomy is comparable to enteral stenting with fewer re-interventions in malignant gastric outlet obstruction. Surg Endosc. 2017;31:2946–2952. doi: 10.1007/s00464-016-5311-1. [DOI] [PubMed] [Google Scholar]
- 20.Chandan S, Khan S R, Mohan B P et al. EUS-guided gastroenterostomy versus enteral stenting for gastric outlet obstruction: Systematic review and meta-analysis. Endosc Int Open. 2021;9:E496–E504. doi: 10.1055/a-1341-0788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Itoi T, Itokawa F, Uraoka T et al. Novel EUS-guided gastrojejunostomy technique using a new double-balloon enteric tube and lumen-apposing metal stent (with videos) Gastrointest Endosc. 2013;78:934–939. doi: 10.1016/j.gie.2013.09.025. [DOI] [PubMed] [Google Scholar]
- 22.Bondi G, Bazarbashi A N, Abbas A M et al. Su1269 Endoscopic gastroenterostomy versus surgical gastrojejunostomy for the treatment of gastric outlet obstruction in patients with peritoneal carcinomatosis: a retrospective comparative study. Gastrointest Endosc. 2020;91:AB303. [Google Scholar]
- 23.Widmer J L, Winner M, Allendorf J et al. Su1154 Single center comparative study of endoscopic gastrojejunostomy versus surgical gastrojejunostomy for malignant gastric outlet obstruction. Gastrointest Endosc. 2019;89:AB291. [Google Scholar]
- 24.Marya N, Jaruvongvanich V, Abu Dayyeh BK et al. Su1268 A multicenter international study comparing clinical outcomes of eus-guided gastrojejunostomy, surgical gastrojejunostomy, and enteral stenting for patients with gastric outlet obstruction. Gastrointest Endosc. 2020;91:AB302–AB303. [Google Scholar]
- 25.Khashab M A, Bukhari M, Baron T H et al. International multicenter comparative trial of endoscopic ultrasonography-guided gastroenterostomy versus surgical gastrojejunostomy for the treatment of malignant gastric outlet obstruction. Endosc Int Open. 2017;5:E275–E81. doi: 10.1055/s-0043-101695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Perez-Miranda M, Tyberg A, Poletto D et al. EUS-guided gastrojejunostomy versus laparoscopic gastrojejunostomy: an international collaborative study. J Clin Gastroenterol. 2017;51:896–899. doi: 10.1097/MCG.0000000000000887. [DOI] [PubMed] [Google Scholar]
- 27.Kouanda A, Binmoeller K, Hamerski C et al. Endoscopic ultrasound-guided gastroenterostomy versus open surgical gastrojejunostomy: clinical outcomes and cost effectiveness analysis. Surg Endosc. 2021;35:7058–7067. doi: 10.1007/s00464-020-08221-z. [DOI] [PubMed] [Google Scholar]
- 28.Bronswijk M, Vanella G, van Malenstein H et al. Laparoscopic versus EUS-guided gastroenterostomy for gastric outlet obstruction: an international multicenter propensity score-matched comparison (with video) Gastrointest Endosc. 2021;94:526–53600. doi: 10.1016/j.gie.2021.04.006. [DOI] [PubMed] [Google Scholar]
- 29.Moher D, Liberati A, Tetzlaff J et al. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stroup D F, Berlin J A, Morton S C et al. for the Meta-analysis Of Observational Studies in Epidemiology (MOOSE) Group. Meta-analysis of Observational Studies in Epidemiology. A Proposal for Reporting. JAMA. 2000;283:2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 31.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25:603–605. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- 32.Cotton P B, Eisen G M, Aabakken L et al. A lexicon for endoscopic adverse events: report of an ASGE workshop. Gastrointest Endosc. 2010;71:446–454. doi: 10.1016/j.gie.2009.10.027. [DOI] [PubMed] [Google Scholar]
- 33.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 34.Higgins J P, Thompson S G, Spiegelhalter D J. A re-evaluation of random-effects meta-analysis. J R Stat Soc Ser A Stat Soc. 2009;172:137–159. doi: 10.1111/j.1467-985X.2008.00552.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Higgins J P, Thompson S G, Deeks J J et al. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wan X, Wang W, Liu J et al. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. 2014;14:135. doi: 10.1186/1471-2288-14-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Higgins J P, White I R, Wood A M. Imputation methods for missing outcome data in meta-analysis of clinical trials. Clin Trials. 2008;5:225–239. doi: 10.1177/1740774508091600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Furukawa T A, Barbui C, Cipriani A et al. Imputing missing standard deviations in meta-analyses can provide accurate results. J Clin Epidemiol. 2006;59:7–10. doi: 10.1016/j.jclinepi.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 39.Higgins J PT, Thomas J, Chandler J . Chichester (UK): John Wiley & Sons; 2019. Cochrane Handbook for Systematic Reviews of Interventions. 2 ed. [Google Scholar]
- 40.Bronswijk M, Vanella G, Van Malenstein H et al. VDM. Laparoscopic or EUS-guided gastroenterostomy for gastric outlet obstruction: A propensity score matched analysis. United Europ Gastroenterol J. 2020;8:804–805. [Google Scholar]
- 41.Xu G, Shen Y, Lv Y et al. Safety and efficacy of endoscopic ultrasound-guided gastroenterostomy using double balloon occlusion methods: a clinical retrospective study in 36 patients with malignant gastric outlet obstruction. Endosc Int Open. 2020;8:E1690–E1697. doi: 10.1055/a-1221-9656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kastelijn J B, Moons L MG, Garcia-Alonso F J et al. Patency of endoscopic ultrasound-guided gastroenterostomy in the treatment of malignant gastric outlet obstruction. Endosc Int Open. 2020;8:E1194–E201. doi: 10.1055/a-1214-5659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Amateau S K, Lim C H, McDonald N M et al. EUS-guided endoscopic gastrointestinal anastomosis with lumen-apposing metal stent: feasibility, safety, and efficacy. Obes Surg. 2018;28:1445–1451. doi: 10.1007/s11695-018-3171-6. [DOI] [PubMed] [Google Scholar]
- 44.Kerdsirichairat T, Yang J, Gutierrez O IB et al. Long-term outcomes of endoscopic ultrasound-guided gastroenterostomy using lumen-apposing metal stents for gastric outlet obstruction: A 4-year cohort. Gastrointest Endosc. 2018;87:AB320–AB1. [Google Scholar]
- 45.Ge P S, Young J Y, Dong W et al. EUS-guided gastroenterostomy versus enteral stent placement for palliation of malignant gastric outlet obstruction. Surg Endosc. 2019;33:3404–3411. doi: 10.1007/s00464-018-06636-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Iqbal U, Berger A, Confer B et al. Endoscopic ultrasound-guided gastroenterostomy vs. enteral stenting for treatment of gastric outlet obstruction: a retrospective review: 918. Am J Gastroenterol. 2019;114:S536–S537. [Google Scholar]
- 47.Vazquez-Sequeiros E, Sanchez-Aldehuelo R, de Santiago E R et al. Su1286 Endoscopic ultrasound-guided gastrojejunostomy is superior to duodenal self expandable metal stent for pallitaive treatment of malignant gastric outlet obstructtion: a comparative case control study. Gastrointest Endosc. 2020;91:AB312–AB313. [Google Scholar]
- 48.Larghi A, Ibrahim M, Fuccio L et al. Forward-viewing echoendoscope versus standard echoendoscope for endoscopic ultrasound-guided tissue acquisition of solid lesions: a randomized, multicenter study. Endoscopy. 2019;515:444–451. doi: 10.1055/a-0790-8342. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


