Abstract
Twenty-three aminooxy compounds have been examined for their ability to inhibit the growth of the malaria parasite Plasmodium falciparum in vitro. Eight of these compounds were found to have 50% inhibitory concentrations less than 10 μM, with the best drugs being canaline (the aminooxy analogue of ornithine) and CGP51905A at 297 ± 23.6 nM and 242 ± 18.8 nM, respectively. Canaline was also assayed in combination with the ornithine decarboxylase inhibitor difluoromethylornithine, and the two drugs were found to be synergistic in antimalarial activity.
Malaria remains one of the world's most frequent causes of mortality and morbidity, with between 200 and 300 million cases and over two million deaths per year (18). With the recent spread of resistance to commonly used antimalarials, such as chloroquine and pyrimethamine, there is an urgent need for new therapeutic compounds. Aminooxy compounds (or O-hydroxylamines) have been known for some time as potent inhibitors of pyridoxal-5-phosphate-dependent enzymes, such as aminotransferases, serine hydroxymethyltransferase, tyrosine decarboxylase, cystathionase, and ornithine decarboxylase, whereby the aminooxy moiety forms a stable oxime with the aldehyde group present on the cofactor (1, 2, 10, 11, 19). One compound in particular, canaline (the aminooxy analogue of ornithine), has been well studied as an antimetabolite generated from canavanine (the guanidinooxy analogue of arginine) present in legumes (13). Despite the demonstrated potential of canaline for inhibiting ornithine aminotransferase and ornithine decarboxylase (4, 10, 12, 17), fairly large doses of the compound are required in order to see substantial mammalian toxicity (4). In this context, canaline has been examined as an anticancer agent based on disrupting polyamine synthesis and other biochemical processes in tumor cells (10). As most parasites and bacteria are rapidly growing organisms with a concomitant requirement for polyamine biosynthesis (8), these organisms are susceptible to compounds which interfere with the relevant pathways. In the present study, a series of aminooxy compounds, including canaline, have been tested as antimalarial agents in vitro and several have been discovered to have potent activity.
Plasmodium falciparum clone 3D7 (chloroquine and pyrimethamine sensitive) was cultured in A+ human erythrocytes at 5% hematocrit by the method of Trager and Jensen (16) with a medium consisting of RPMI 1640 (pH 7.4) plus 2 g of glucose per liter, 1 g of sodium bicarbonate per liter, 5 g of Albumax II (Gibco, Paisley, United Kingdom) per liter, and 26 mg of hypoxanthine per liter. To examine the antimalarial properties of test compounds, stock solutions of a given drug were serially diluted 10-fold from 10 mM down to 10 nM, and 10 μl of each dilution was added to the appropriate wells of a 96-well microtiter plate. Infected blood was diluted to a hematocrit of 1.5% and a parasitemia of 3 to 5%, and then 90 μl was added to the microtiter wells. The plate was then incubated at 37°C in a candle jar for 48 h. After incubation, each well was resuspended, a thin smear was made and stained with Diff-Quik (Dade Behring, Milton Keynes, United Kingdom), and then at least 1,000 erythrocytes were counted per slide. The resulting parasitemia for each drug dilution was used in the Scientist computer program (MicroMath, Salt Lake City, Utah) and subjected to nonlinear fitting to a four-parameter equation to determine the 50% inhibitory concentration (IC50). For synergism studies, two compounds were mixed at fixed ratios of their individual IC50s for P. falciparum 3D7 and then serially diluted and used as described above. The partial IC50s calculated from the mixtures were then used in the construction of an isobologram.
Canaline was obtained from Sigma (Poole, United Kingdom); carboxymethoxylamine, allyl-O-hydroxylamine, nitrobenzyl-O-hydroxylamine, t-butyl-O-hydroxylamine, ethoxyamine, tetrahydropyranyl-O-hydroxylamine, aminooxyethoxyethoxyethyl-O-hydroxylamine, trityl-O-hydroxylamine, pentafluorobenzyl-O-hydroxylamine, trimethylsilyl-O-hydroxylamine, and methoxyamine were obtained from Aldrich (Poole, United Kingdom); phenyl-O-hydroxylamine was obtained from Fluka (Poole, United Kingdom); aminooxyphenylpropionate was obtained from Toronto Research Chemicals (North York, Ontario, Canada); benzyl-O-hydroxylamine was obtained from Acros (Loughborough, United Kingdom); and the CGP compounds were obtained from Novartis Pharma SA (Basel, Switzerland). All compounds were resuspended in either 100 mM HEPES (pH 7.4) or dimethyl sulfoxide.
The commercially available aminooxy compounds were tested over a final concentration range of 1 nM to 1 mM to determine their ability to inhibit the growth of P. falciparum in vitro (Table 1). Of the 15 compounds examined, six were found to have IC50s lower than 10 μM: allyl-O-hydroxylamine, phenyl-O-hydroxylamine, ethoxyamine, canaline, carboxymethoxylamine, and trimethylsilyl-O-hydroxylamine. Only canaline had a submicromolar IC50 (297 ± 23.6 nM). A series of eight proprietary aminooxy compounds were also made available by Novartis Pharma (Table 2). Of these, only CGP54382A and CGP51905A had IC50s lower than 10 μM. However, CGP51905A, with an IC50 of 242.1 ± 18.8 nM, was found to be as potent as canaline.
TABLE 1.
Antimalarial activity of aminooxy compoundsa
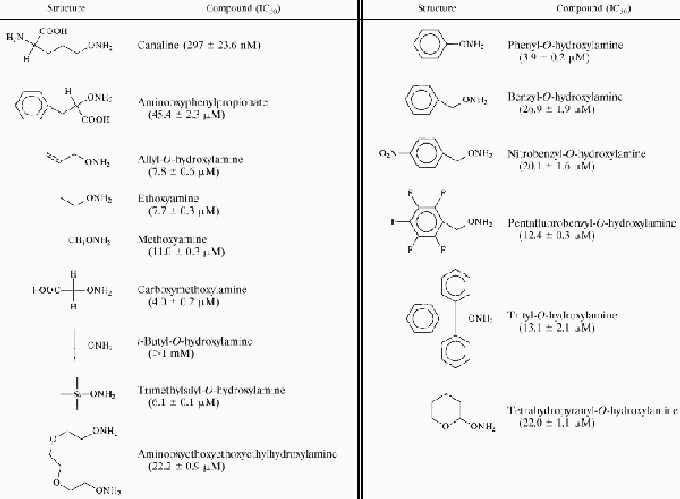 |
Inhibitors were screened in vitro against P. falciparum 3D7 over a final concentration range of 1 nM to 1 mM, as described in the text.
TABLE 2.
Antimalarial activity of novel aminooxy compoundsa
| Structure | Compound (IC50) |
|---|---|
 |
CGP48393A (34.6 ± 1.7 μM) |
| CGP50206A (40.8 ± 2.0 μM) | |
| CGP52430A (19.6 ± 0.2 μM) | |
| CGP51905A (242 ± 18.8 nM) | |
| CGP54381A (26.3 ± 2.6 μM) | |
| CGP54382A (7.0 ± 1.8 μM) | |
| CGP55573A (>1 mM) |
Inhibitors were tested in vitro against P. falciparum 3D7 over a final concentration range of 1 nM to 1 mM, as described in the text.
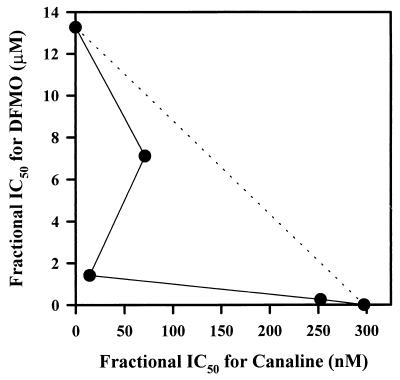
Canaline is a known inhibitor of mammalian ornithine aminotransferase (4); we have found that it also inhibits malarial ornithine aminotransferase (unpublished data) and parasite aspartate aminotransferases involved in methionine regeneration from methylthioadenosine (3, 7; unpublished data). As interference with this methionine recycling pathway is known to be lethal for malaria organisms (9, 15) and ornithine aminotransferase plays a key role in regulating ornithine levels (5, 14), canaline was screened in combination with the ornithine decarboxylase inhibitor difluoromethylornithine (DFMO) in order to assess the possibility of therapeutic synergism. As seen in Fig. 1, the isobologram for canaline plus ornithine is clearly synergistic.
FIG. 1.
Isobologram for canaline and DFMO. The two compounds were mixed at a 1:1, 1:5, or 5:1 ratio of their individual IC50s, serially diluted, and then added to in vitro cultures of P. falciparum as outlined in the text. The fractional IC50s were then determined and plotted. The dotted line represents theoretical additivity, with curves below this line being indicative of synergism and curves above being indicative of antagonism.
It is possible to draw some broad structure-activity conclusions from the compounds used in the study. Introduction of an extra methyl group to phenyl-O-hydroxylamine (resulting in benzyl-O-hydroxylamine) led to a 10-fold loss of activity. However, addition of a nitro group to the ring of benzyl-O-hydroxylamine, the substitution of the ring hydrogens for fluorine, or the addition of two extra phenyl groups to the methyl linker had only a minor effect on antimalarial activity, with activity increasing twofold or less. Replacement of the central carbon in t-butyl-O-hydroxylamine with a silicon atom led to a 200-fold increase in activity. Lengthening the side chain of CGP51905A by one carbon led to an 80-fold loss of antimalarial activity, while the stereoisomers CGP54381A and CGP54382A had a fourfold difference in activity. While both canaline and aminooxyphenylpropionate are aminooxy analogues of physiological amino acids (ornithine and phenylalanine, respectively), there was a 150-fold difference in antimalarial activity. This wide variance may be due to a number of different factors. First, aminooxyphenylpropionate contains a substitution in the α position, whereas canaline contains a substitution in the γ position, which may make a large difference in the interaction of the inhibitor with the pyridoxal-5-phosphate of the target enzyme(s). Second, there may be a large difference in the ability of malaria organisms to transport the two compounds. However, the uptake of phenylalanine and ornithine in Plasmodium has not been well characterized. The effect of aminooxy substitution on the rate of uptake of a given amino acid is also completely unknown.
As canaline has been shown to inhibit a number of diverse pyridoxal-5-phosphate-dependent enzymes and can also form stable oximes with α-keto acids important as biochemical intermediates (such as α-ketoglutarate) (6, 10, 13), it is not yet possible to define a single target as the mechanism of antimalarial activity. Nevertheless, the fact that canaline and DFMO are synergistic suggests that the inhibition of polyamine synthesis may play an important role in the action of the aminooxy compound. Since the IC50 of canaline for human MIAPaCa-2 pancreatic carcinoma cells is 600 μM and intraperitoneal injections of 500 mg of canaline/kg of body weight had no obvious toxic effects on rats (aside from sedation) (4, 10), malaria parasites appear to be approximately 2,000 times more susceptible to canaline. In vivo studies, with and without DFMO, are the next logical step in further investigating this class of compound.
Acknowledgments
I thank Louise Berger for technical assistance in the maintenance of the malaria cultures and Alan H. Fairlamb for helpful discussions and the donation of DFMO.
This work was supported by the Wellcome Trust.
REFERENCES
- 1.Baskaran N, Prakash V, Savithri H S, Radhakrishnan A N, Appaji Rao N. Mode of interaction of aminooxy compounds with sheep liver serine hydroxymethyltransferase. Biochemistry. 1989;28:9613–9617. doi: 10.1021/bi00451a010. [DOI] [PubMed] [Google Scholar]
- 2.Beeler T, Churchich J E. Reactivity of the phosphopyridoxal groups of cystathionase. J Biol Chem. 1976;251:5267–5271. [PubMed] [Google Scholar]
- 3.Berger B J, Dai W-W, Wilson J. Methionine formation from α-ketomethiobutyrate in the trypanosomatid Crithidia fasciculata. FEMS Microbiol Lett. 1998;165:305–312. doi: 10.1111/j.1574-6968.1998.tb13162.x. [DOI] [PubMed] [Google Scholar]
- 4.Bolkenius F N, Knodgen B, Seiler N. dl-Canaline and 5-fluoromethylornithine. Comparison of two inactivators of ornithine aminotransferase. Biochem J. 1990;268:409–414. doi: 10.1042/bj2680409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cataldi A A, Algranati I D. Polyamines and regulation of ornithine biosynthesis in Escherichia coli. J Bacteriol. 1989;171:1998–2002. doi: 10.1128/jb.171.4.1998-2002.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cooper A J. Oxime formation between alpha-keto acids and l-canaline. Arch Biochem Biophys. 1984;233:603–610. doi: 10.1016/0003-9861(84)90485-5. [DOI] [PubMed] [Google Scholar]
- 7.Heilbronn J, Wilson J, Berger B J. Tyrosine aminotransferase catalyzes the final step of methionine recycling in Klebsiella pneumoniae. J Bacteriol. 1999;181:1739–1747. doi: 10.1128/jb.181.6.1739-1747.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marston L J, Pegg A E. Polyamines as targets for therapeutic intervention. Annu Rev Pharmacol. 1995;35:55–91. doi: 10.1146/annurev.pa.35.040195.000415. [DOI] [PubMed] [Google Scholar]
- 9.Riscoe M K, Ferro A J, Fitchen J H. Analogs of 5-methylthioribose, a novel class of antiprotozoal agents. Antimicrob Agents Chemother. 1988;32:1904–1906. doi: 10.1128/aac.32.12.1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rosenthal G A. l-Canaline: a potent antimetabolite and anti-cancer agent from leguminous plants. Life Sci. 1997;60:1635–1641. doi: 10.1016/s0024-3205(96)00595-4. [DOI] [PubMed] [Google Scholar]
- 11.Rosenthal G A. A mechanism of l-canaline toxicity. Eur J Biochem. 1981;114:301–304. doi: 10.1111/j.1432-1033.1981.tb05149.x. [DOI] [PubMed] [Google Scholar]
- 12.Rosenthal G A, Dahlman D L. Interaction of l-canaline with ornithine aminotransferase of the tobacco hornworm, Manduca sexta (Sphingidae) J Biol Chem. 1990;265:868–873. [PubMed] [Google Scholar]
- 13.Rosenthal G A, Hughes C G, Janzen D H. l-Canavanine, a dietary nitrogen source for the seed predator Caryedes brasiliensis (Bruchidae) Science. 1982;217:353–355. doi: 10.1126/science.217.4557.353. [DOI] [PubMed] [Google Scholar]
- 14.Storici P, Capitani G, Muller R, Schirmer T, Jansonius J N. Crystal structure of human ornithine aminotransferase complexed with the highly specific and potent inhibitor 5-fluoromethylornithine. J Mol Biol. 1999;285:297–309. doi: 10.1006/jmbi.1998.2289. [DOI] [PubMed] [Google Scholar]
- 15.Sufrin J R, Meshnick S R, Spiess A J, Garafalo-Hannon J, Pan X-Q, Bacchi C J. Methionine recycling pathways and antimalarial drug design. Antimicrob Agents Chemother. 1995;39:2511–2515. doi: 10.1128/aac.39.11.2511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Trager W, Jensen J B. Human malaria parasites in continuous culture. Science. 1976;193:673–675. doi: 10.1126/science.781840. [DOI] [PubMed] [Google Scholar]
- 17.Van Nieuwenhove S, Schechter P J, Declerq J, Bone G, Burke J, Sjoerdsma A. Treatment of gambiense sleeping sickness in the Sudan with oral DFMO (dl-alpha-difluoromethylornithine), an inhibitor of ornithine decarboxylase; first field trial. Trans R Soc Trop Med Hyg. 1985;79:692–698. doi: 10.1016/0035-9203(85)90195-6. [DOI] [PubMed] [Google Scholar]
- 18.World Health Organization. Tropical disease research: progress 1997–1998. Geneva, Switzerland: World Health Organization; 1999. [Google Scholar]
- 19.Worthen D R, Ratliff D K, Rosenthal G A, Trifonov L, Crooks P A. Structure-activity studies of l-canaline-mediated inhibition of porcine alanine aminotransferase. Chem Res Toxicol. 1996;9:1293–1297. doi: 10.1021/tx9600199. [DOI] [PubMed] [Google Scholar]