Abstract
Objective Primary goal in spheno-orbital meningioma (SOM) surgery still remains complete resection. Nevertheless, given their highly infiltrative nature, a growing body of literature suggests to shift toward function-sparing surgeries. We here present our experience in the management of SOMs through the endoscopic superior eyelid approach (SEA).
Methods Surgical database from our multidisciplinary work group was retrospectively reviewed to identify patients treated for SOMs in the last 10 years by our senior authors, analyzing and correlating clinical, radiological, and outcome variables among the different approaches used.
Results There were 35 patients (mean age of 57.3 ± 12.86 years), with a mean follow-up of 31.5 months (range: 6–84 months). The most common preoperative complaint was proptosis (62.9%) followed by diplopia and visual deficit. Greater and lesser sphenoid wings were the areas mainly involved by the pathology (91.4% and 88.6%, respectively), whereas orbital invasion was evidenced in one-third of cases. Patients were operated on through craniotomic (48.6%), endoscopic superior eyelid (37.1%), and combined cranioendoscopic (14.3%) approaches. Simpson grades 0 to II were accomplished in 46.2% of SEA and 76.5% of craniotomies. All patients with a preoperative visual deficit improved in the postoperative period, independently from the approach used. On patients who underwent endoscopic SEA, there was improved their short-/long-term postoperative Karnofsky Performance Status.
Conclusions Endoscopic SEA is a safe and effective alternative to transcranial approaches in very selected cases of SOMs, where the planned primary objective was to obtain a maximally safe resection, aimed at symptom relief, rather than a gross total resection at any cost.
Keywords: meningiomas, transorbital approach, superior eyelid, spheno-orbital meningioma
Introduction
Spheno-orbital meningiomas (SOMs) are skull base lesions originally described in 1938 by Cushing and Eisenhardt as a variation of en plaque meningiomas. 1 2 3 4 They actually represent 2 to 9% of all intracranial meningiomas and are characteristically hallmarked by pathological hyperostosis of the sphenoid ridge. 3 5 6 7 Bone invasion along with possible inglobation of critical neurovascular structures such as the orbit, optic canal, cranial nerves, and cavernous sinus contribute to the common clinical presentation that includes proptosis, visual-field deficit, and ocular motility defects.
Since the pioneering work of Simpson in 1957, the gold standard for all intracranial meningiomas, including SOMs, is represented by surgery aiming to obtain a Simpson grade 0/1 8 ; patients are usually referred to radiotherapy (RT) or radiosurgery in cases of residual tumor or recurrences. 9 10 Aggressive surgical management aiming for a gross total resection (GTR) was initially proposed for SOMs through the common pterional and fronto-orbito-zigomatic craniotomies and their variations. 2 Nevertheless, given the intrinsic anatomical complexity of such lesions, which are characterized by morbidity and mortality rates up to 6% in many series, 11 complete surgical resection is often not possible. 2 3 4 5 On the other hand, subtotal resection (STR) results in high recurrence and persistence rates.
Starting from this framework, a growing body of literature suggests more conservative treatments for those cases where a radical resection may be obtained only at a high cost in terms of morbidity. In fact, in the last years, many studies have investigated various minimally invasive approaches to manage cranial base lesions, and, actually, resection with functional preservation appears to be the main treatment option. 1 12 13 14
Keeping in mind such considerations, 10 years ago, our group started approaching selected cases of SOMs through the minimally invasive endoscopic superior eyelid approach (SEA), obtaining interesting preliminary results. 13 Given also that for SOM series, long-term results regarding clinical deficits and eventual morbidities are rarely reported, we here present in detail our 10-year clinical experience in treating such tumors, analyzing 35 cases operated on by our senior authors at three referral centers in Italy, through both a craniotomic and an endoscopic transorbital approach. Pre- and postoperative clinical characteristics along with precise location, eventual complications, morbidity outcomes at early and late postoperative follow-up, and recurrence rates were recorded, analyzed, and correlated among the different approaches used, trying to better clarify specific indications and contraindications along with the safety profile of the application of endoscopic SEA for the management of selected SOMs.
Materials and Methods
Data Collection and Patient Selection
An analysis of the institutional “Orbital Pathology” database was performed for this study. From a cohort of 120 patients affected by lesions involving the orbit operated on by our senior authors (D. L. and P. C.) in the past 10 years in three different hospitals in Italy, we selected and included in this analysis adult patients treated for primary or recurrent SOMs.
Clinical Analysis
Patient charts were analyzed to identify demographics, pharmacological anamnesis, and past and current medical/surgical history. Data from pre- and postoperative neurological examinations were obtained for all patients, with particular attention to proptosis assessment and visual and cranial nerve function examinations. Rosenbaum near-vision chart and visual-field test were used to evaluate visual acuity and visual-field deficits, respectively. Postoperative clinical and ophthalmological analyses were performed both in the immediate postoperative period and at follow-up visits, analyzing eventual differences between pre- and postoperative evaluations.
Imaging Review
All patients underwent detailed preoperative imaging studies through high-resolution computed tomography (CT) scan with bone windows and multiplanar reconstructions and magnetic resonance imaging (MRI) with T1, T2, FAT-SAT (fat saturation), and T1 sequences after contrast administration. Such examinations were double-blindly reviewed by two neuroradiologists, determining the exact extension of the pathology according to the involvement of the following structures: intra- and extraconal orbital spaces; frontal, parietal, zygomatic, and maxillary bones; great and lesser sphenoid wings (GSW and LSW, respectively); anterior and middle cranial fossa (ACF and MCF, respectively); ACF and MCF dura; brain parenchyma; pterygopalatine; and infratemporal fossa (cutis or subcutis). CT imaging with bone windows allowed the study of eventual hyperostosis. Proptosis was quantified through the exophthalmos index (EI) and measured on immediate preoperative CT scan, immediate postoperative CT scan, and images obtained during follow-up. Differences in EI between pre- and postoperative periods were evaluated as well ( Fig. 1 ).
Fig. 1.
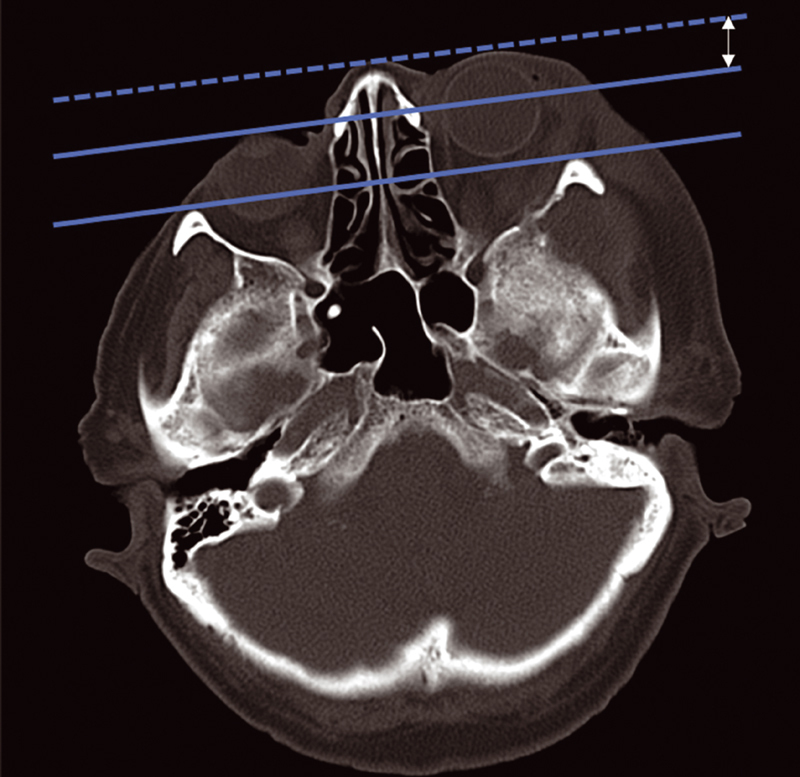
Proptosis assessment through the exophthalmos index (EI). On axial computed tomography (CT) scan with bone window, a line was drawn between the most anterior part of the frontal process of both zygomatic bones. The distance between this line and the most anterior part of both globes was calculated. Such difference was expressed in millimeters and considered as the EI.
Operative Reports and Surgical Techniques
Operative reports were studied, extrapolating data about surgical approaches, primary surgical aim (i.e., to obtain a maximal safe resection, decompress optic apparatus, debulk the intraorbital portion of the pathology, and so on), extent of resection (EOR) achieved, eventual management of hyperostosis, length of surgeries, and possible intraoperative complications. Intraoperative macroscopic resection, considering also hyperostosis and dura involvement, was classified according to both GTR/STR grade and Simpson grading scale (SGS). 8 In particular, SGS 0 to II was considered as GTR, whereas SGS ≥ III was considered as an STR, as commonly considered in the literature. 1 6
After detailed multidisciplinary discussion, treatment alternatives were given and explained to the patients who were operated through either craniotomic routes, including pterional (PA), frontotemporal (FTA, comprehending all modifications of the specific PA described by Yasargil), and fronto-orbito-temporozygomatic (FOTZA) approaches, or endoscopic SEA. In the latter ones, patients were always operated on by a team of a neurosurgeon and an otorhinolaryngologist, as this is the mainstream of the philosophy of our entire group. 13 14 15 16 Detailed descriptions of PA, FTA, and FOTZA are beyond the scope of this work. Looking at SEA, we here present a brief summary of the procedure, as it is commonly performed by our senior authors. 13 14 The skin incision is made on a lid crease in the superior eyelid ( Fig. 2A ). The orbicularis oculi muscle is then identified and the skin–muscle flap is raised. Stitches and small silastic tubes help in creating an adequately wide access and reducing the risk of iatrogenic damage to the skin ( Fig. 2B ). The orbital rim is reached, and, at this point, the sparing of the upper eyelid retractor system must be a primary objective ( Fig. 2C ). To avoid the protrusion of the orbital fat into the operative field, care must be taken to avoid the opening of the periorbita. If such an event occurs during surgery, orbital fat should be managed with retractors. In the superomedial dissection, the superior oblique muscle tendon must be identified and spared. Once the orbital rim is skeletonized, a careful subperiosteal dissection of the periorbit from the orbital bones is performed until the superior and inferior orbital fissures (SOF and IOF, respectively) are clearly identified. Bridging vessels can be seen during this dissection, and they can be safely sacrificed. In particular, close to SOF, Hyrtl's foramen or meningo-orbital foramen can be found in 50 to 60% of patients. 17 It usually accommodates the recurrent meningeal artery (meningolacrimal branch) that represents a connection between the anterior (orbital) branch of the middle meningeal artery and the lacrimal artery ( Fig. 2D ). If the surgical target resides in the MCF, the GSW should be drilled out as far as the dura mater. During GSW drilling, venous bleeding may be controlled with hemostatic agents or by avoiding irrigation during diamond burr work. At this point, the superomedial boundary of the approach is defined by the SOF, the inferomedial border is defined by the IOF, and the lateral boundary is delineated by the temporalis muscle. Superiorly, the approach can be partially extended to the LSW, toward the anterior clinoid process. If necessary, the frontal bone can be partially resected, and the spheno-orbital sinus can be coagulated. This approach allows adequate exposure of the floor of the MCF as well as direct access to the gasserian ganglion and to the cavernous sinus (lateral wall). When the pathology involves the intracranial compartment, the dura may be safely opened, and both the anterior pole and the medial surface of the temporal lobe can be exposed. Instead, for an ACF approach, the craniotomy is aimed to remove the orbital part of the frontal bone along with partial removal of the LSW; GSW is left untouched. The dura is exposed and opened according to individual requirements to gain access to the frontal lobe ( Fig. 2E ).
Fig. 2.
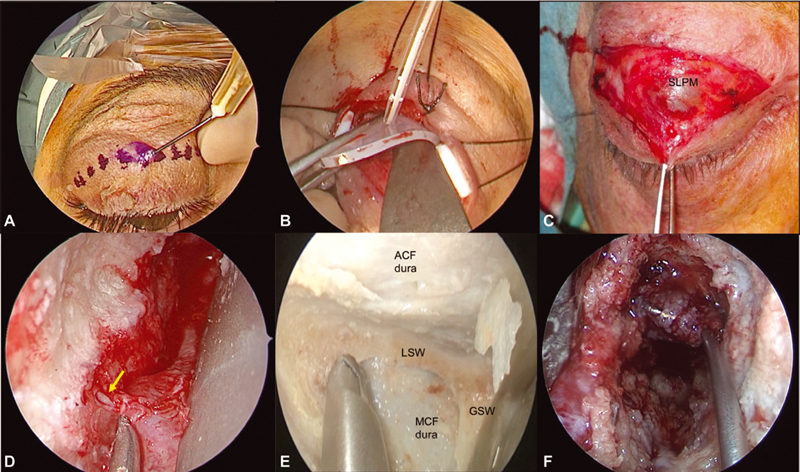
The endoscopic superior eyelid transorbital approach. Skin incision is made on a lid crease superior to the eyelid ( A ), elevating the skin–muscle flap and preserving the upper eyelid retractor system ( B,C ). Stitches and small silastic tubes may help during the first stages of the surgery ( B ). The orbital rim is reached and the subperiosteal dissection begins. Close to superior orbital fissure (SOF), Hyrtl's foramen or meningo-orbital foramen can be found, and the recurrent meningeal artery ( yellow arrow , D ) may be safely coagulated and cut. When reaching SOF and inferior orbital fissure (anatomical dissection image, E ), depending on the surgical target, the drilling of LSW or GSW may open the ACF or MCF (as in F ), respectively. ACF, anterior cranial fossa; GSW, greater sphenoid wing; LSW, lesser sphenoid wing; MCF, middle cranial fossa; SLPM, superior levator palpebrae muscle.
During closure, when the dura mater is partially or completely removed as part of the oncological rationale of the procedure, it is then reconstructed in a standard multilayer fashion with dural substitutes and the help of fibrin glue. Looking to orbital compartment, usually no bony reconstruction is necessary; orbital empty space may be partially filled with fat to support eventual cranial base reconstruction and to reduce the risk of postoperative enophthalmos; small periorbital openings may be safely reapproximated with gentle bipolar coagulation or, in extensive ones, with the help of fibrin glue. Extra care must be placed in preserving the surgical planes to avoid pathological retractions of the palpebrae.
Surgeries were performed in every case with neuronavigation assistance (Stealth S7 and S8, Medtronic, Minneapolis, Minnesota, United States), craniotomic approaches were performed using Pentero microscopes (Zeiss, Oberkochen, Germany), and endoscopic SEA was performed with a rigid endoscope system with 0-, 30- and 45-degree optics (Karl Storz, Tuttlingen, Germany).
Follow-Up and Outcome Measures
Clinical and radiological follow-up consisted of immediate postoperative CT scan (within 48 hours from the surgery) and postoperative MRI at 1 week from the surgery (same sequences as preoperatively), clinical and oculistic assessment at 10 days, and 3-month MRI evaluated at a clinical control (short-term follow-up). Patients were then followed by our dedicated neuro-oncological service with clinical or radiological studies performed according to the specific cases.
Tumor recurrence or progression was defined respectively as radiological return of lesion or progression of tumor remnants. For those cases, time to recurrence/progression was calculated. The eventual administration of an RT regimen was discussed and tailored for the single cases.
Outcome measures were studied comparing pre- and postoperative clinical conditions of patients, including the study of the following: the presence of orbital asymmetry, ptosis or eyelids malpositions, extrinsic ocular movement deficits, diplopia, decline in visual acuity, eventual worsening of the preoperative eye field test, and eventual cerebrospinal fluid (CSF) leaks, among others. Clinicians who were not directly involved in the patients' care (F. R. and T. A.) performed short- and long-term outcome assessments using the modified Rankin Scale (mRS) and Karnofsky Performance Status (KPS).
Statistical Analysis
Calculations and statistical analysis were performed using commercial statistic software (IBM SPSS Statistics, version 26, IBM Corp.). Statistical significance was set at p < 0.05 . As far as continuous variables were concerned, normal value distribution was tested using Kolmogorov–Smirnov and Levene's tests. However, supposedly due to the limited number of cases within our series, value distributions within each of the studied groups proved to be non-normal; thus, nonparametric Mann–Whitney U test was used for data analysis. As for categorical variables, Fischer's exact tests were used and pairwise comparisons with Bonferroni correction were performed in case of polytomous variables. When analyzing functional outcome variables such as KPS and mRS, Friedman's two-way analysis of variance test with pairwise comparisons with Bonferroni correction and Wilcoxon signed-rank tests were used.
Results
Epidemiological and Preoperative Clinical Data
Among a total of 120 cases of orbital pathologies, we identified 35 patients operated for SOMs in the past 10 years. Mean age at diagnosis was 57.3 ± 12.86 years (range: 38–80 years). Females represented the 77.1% of patients, whereas males 22.9%, with a female-to-male ratio of 3.4:1. Anamnesis was unremarkable for the great majority of patients, whereas 14% were classified with the American Society of Anesthesiologists (ASA) III or more risk category by the anesthesiologist, mainly due to cardiovascular/metabolic problems. Also, 74.3% presented to our attention for a primary meningioma and 25.7% for an imaging-confirmed recurrence. Right-sided tumors were present in 54.3% of the total ( Table 1 ).
Table 1. Epidemiological and histopathological data.
| Epidemiological data and characteristics of SOMs | n | % |
|---|---|---|
| Age, years | ||
| 30–39 | 2 | 5.7 |
| 40–49 | 10 | 28.5 |
| 50–59 | 8 | 22.9 |
| 60–69 | 7 | 20 |
| 70–79 | 7 | 20 |
| 80–89 | 1 | 2.9 |
| Sex | ||
| Male | 8 | 22.9 |
| Female | 27 | 77.1 |
| Recurrence | ||
| Primary | 26 | 74.3 |
| Recurrence | 9 | 25.7 |
| Histotype | ||
| Meningothelial | 11 | 31.4 |
| Transitional | 5 | 14.3 |
| Fibroblastic | 4 | 11.4 |
| Psammomatous | 3 | 8.6 |
| Atipic | 2 | 5.7 |
| Other | 10 | 28.6 |
| Grading | ||
| WHO I | 31 | 88.6 |
| WHO II | 4 | 11.4 |
| WHO III | 0 | 0 |
| Side | ||
| Right | 19 | 54.3 |
| Left | 16 | 45.7 |
Abbreviations: SOM, spheno-orbital meningioma; WHO, World Health Organization.
The most common presenting complaint was proptosis in 22 (62.9%) patients, with a mean preoperative EI of 1.95 ± 1.31, followed by diplopia (15 patients) and visual deficit (11 patients). Campimetric and ocular extrinsic muscle (OEM) deficits were both present in 17.1% of patients each. Other less common presentations included epileptic events and trigeminal paresthesias or numbness. Table 2 summarizes all preoperative clinical data, subdivided on the basis of the different surgeries performed.
Table 2. Preoperative clinical data.
| Symptoms at diagnosis | Endoscopic SEA (13 patients) | Combined (5 patients) | Craniotomic (17 patients) | |||
|---|---|---|---|---|---|---|
| n | % | n | % | n | % | |
| Proptosis | 13 | 100 | 5 | 100 | 4 | 23.5 |
| Diplopia | 6 | 46.1 | 4 | 80 | 5 | 29.4 |
| Visual deficits | 6 | 46.1 | 1 | 20 | 4 | 23.5 |
| OEM deficits | 4 | 30.8 | 0 | 0 | 3 | 17.6 |
| Visual-field deficits | 3 | 23.1 | 0 | 0 | 3 | 17.6 |
| Pain | 1 | 7.7 | 3 | 60 | 2 | 11.8 |
| Epiphora | 4 | 30.8 | 0 | 0 | 0 | 0 |
| Epileptic seizure | 0 | 0 | 0 | 0 | 2 | 11.8 |
| Other | 2 | 15.4 | 1 | 20 | 5 | 29.4 |
Abbreviations: OEM, ocular extrinsic muscle deficits; SEA, superior eyelid approach.
Imaging Data
The analysis of the exact location of the tumors allowed to classify them according to the invasion of specific anatomical areas. In particular, extraconal space was invaded in almost 43% of cases, whereas intraconal space was invaded in 25.7% of cases. GSW and MCF dura were the areas mainly involved by the pathology (91.4% of cases) followed by LSW (88.6%) dura of ACF (85.7%) and frontal bone (71.4%). Temporal fossa and bone/temporal muscle were invaded in almost 20% of cases, whereas cerebral parenchyma was invaded in 37.15%. In only 8.6%, cutis and/or subcutis was invaded ( Table 3 ).
Table 3. Preoperative radiological data.
| Endoscopic SEA (13 patients) | Combined (5 patients) | Craniotomic (17 patients) | ||||
|---|---|---|---|---|---|---|
| n | % | n | % | n | % | |
| Intraconal | 5 | 38.5 | 2 | 40 | 2 | 11.8 |
| Extraconal | 9 | 69.2 | 3 | 60 | 3 | 17.6 |
| Frontal bone | 9 | 69.2 | 1 | 20 | 15 | 88.2 |
| Zygomatic bone | 5 | 38.5 | 0 | 0 | 1 | 5.9 |
| Maxillary bone | 0 | 0 | 1 | 20 | 1 | 5.9 |
| GSW | 12 | 92.3 | 5 | 100 | 15 | 88.2 |
| LSW | 11 | 84.6 | 5 | 100 | 17 | 100% |
| Parietal bone | 4 | 30.8 | 3 | 60 | 0 | 0 |
| Temporal bone | 2 | 15.4 | 3 | 60 | 2 | 11.8 |
| Pterygoid muscles | 0 | 0 | 3 | 60 | 1 | 5.9 |
| Subcutis/cutis | 2 | 15.4 | 0 | 0 | 1 | 5.9 |
| ACF | 6 | 46.1 | 0 | 0 | 12 | 70.6 |
| MCF | 11 | 84.6 | 4 | 80 | 15 | 88.2 |
| ACF dura | 6 | 46.1 | 0 | 0 | 12 | 70.6 |
| MCF dura | 12 | 92.3 | 4 | 80 | 16 | 94.1 |
| Cerebral parenchyma | 2 | 15.4 | 1 | 20 | 10 | 58.8 |
Abbreviations: ACF, anterior cranial fossa; GSW, greater sphenoid wing; LSW, lesser sphenoid wing; MCF, middle cranial fossa; SEA, superior eyelid approach.
Surgical Strategy, Surgical Results, and Histopathological Analysis
Patients were operated through both transcranial and endoscopic SEA, depending on multiple factors such as patient age, comorbidities, location of the pathology, surgical aim, and patient desire (see the Discussion section). Depending on the different cases, main surgical indications included the obtaining of a GTR to decompress the optic nerve at its entrance in the optic foramen, to laterally decompress optic apparatus, to improve the proptosis, or to improve seizures control. Of the 35 patients, 13 (37.1%) were operated through a purely endoscopic SEA, 17 (48.6%) through a standard craniotomic approach with 11 PA (31.4%), 1 FOTZA (2.9%), and 5 FTA (14.3%), and 5 (14.3%) underwent a combined cranioendoscopic procedure. Independently from the approach, neuronavigation was always used. Looking at transcranial procedures, extradural removal of invaded bone was followed by opening of the dura mater, intradural tumor resection, and then attempt to remove all infiltrated dura. In SEA cases, as also outlined in the previous section, the lateral orbitotomy allowed orbital decompression and management of the intraorbital part of the tumor. In every case when hyperostosis was evidenced, at both preoperative imaging or during the operation, speed drills, bone rongeurs, and Kerrison bone punches were used to manage it, trying to remove all infiltrated bone.
A GTR was obtained in 46.2% of patients operated through an endoscopic SEA against 76.4% of patients operated with a craniotomic approach ( p = 0.005; odds Ratio of STR among SEA: 12.07). At the same time, Simpson grade 0/I was 23.1% in SEA and 64.7% in craniotomic ones, whereas Simpson grades 0 to II were accomplished in 46.2% of SEA patients and 76.5% of craniotomies ( p = 0.026; odds ratio of Simpson > II: 6.45). Simpson grade V was not obtained, whereas Simpson grade IV for SEA was 38.4% against 23.5% for craniotomic approaches ( Table 4 ).
Table 4. Obtained EOR in SOM patients.
| EOR | Endoscopic SEA (13 patients) | Combined (5 patients) | Craniotomic (17 patients) | Endoscopic SEA vs. craniotomic | |||
|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | p -Value | |
| GTR | 6 | 46.2 | 1 | 20 | 13 | 76.4 | 0.005 a |
| STR | 7 | 54 | 4 | 80 | 4 | 23.6 | |
| Simpson grade | |||||||
| 0/I | 3 | 23.1 | 1 | 20 | 11 | 64.7 | 0.026 a |
| II | 3 | 23.1 | 0 | 0 | 2 | 11.8 | |
| III | 2 | 15.4 | 1 | 20 | 0 | 0 | |
| IV | 5 | 38 | 3 | 60 | 4 | 23.5 | |
| V | 0 | 0 | 0 | 0 | 0 | 0 | |
Abbreviations: EOR, extent of resection; GTR, gross total resection; SEA, superior eyelid approach; STR, subtotal Resection.
p < 0.05.
Mean operative time was 285 ± 113 minutes, resulting to be lesser in craniotomic approaches (259 ± 72 minutes) than in endoscopic ones (272 ± 133 minutes), although this difference did not result to be statistically significant ( p = 0.520).
At histological analysis, 31 (88.5%) patients were affected by grade I meningiomas, whereas the rest were grade II tumors. There were no grade III meningiomas. In all cases where bone hyperostosis was present, surgical samples were analyzed, finding tumor cell invasion. Meningothelial variant was the most common histological subtype encountered (31.4%) followed by the transitional (14.3%) and then by other minor subtypes (meningotheliomatous, psammomatous, atipic, etc.) ( Table 1 ).
Postoperative Clinical Outcomes
Detailed postoperative analysis of short- and long-term postoperative results is presented in Tables 5 and 6 . Looking at short-term postoperative deficits, we observed a greater tax of ocular-related deficits such as diplopia, OEM impairment, visual-field deficit, and visual deficit in patients who underwent an endoscopic SEA. In particular, postoperative diplopia was present in 46.1% of patients (vs. 17.6% in craniotomic ones; p = 0 .155 ), OEM deficit in 30.8% (vs. 11.8%; p = 0.225), visual deficit in 46.1% (vs. 17.6%; p = 0.546), and visual-field impairment in 23.1% (vs. 17.6%; p = 0.642). Moreover, postoperative intracranial complications such as hemorrhagic infarction of surgical field or ischemia were found to be higher in craniotomic surgeries (17.6 vs. 7.7% of endoscopic SEA; p = 0.558) as well as systemic complications (three cases in craniotomic patients vs. one case in endoscopic SEA) ( p = 0.528). Proptosis improvement of at least 30% was observed in 17 (77.3%) of the 22 patients; these data remained stable at long-term follow-up. Focusing on long-term postoperative clinical deficits at 1 year, we observed just one case of postoperative stable new-onset visual deficit after a combined procedure, without new deficit regarding OEM and visual field, independently from the procedure performed. We also analyzed how preoperative deficits evolved at long-term follow-up. Of the 13 patients who underwent an endoscopic SEA affected preoperatively by visual (6 patients), OEM (4 patients), and visual-field deficits (3 patients), after surgery we observed a visual improvement in 4 patients ( p = 0 .043 ), an OEM improvement in 3 patients ( p = 0.007) and visual-field improvement in 1 patient (p = 0.001), whereas the remaining 5 patients preoperatively impaired remained stable in the different clinical aspects. No worsening of the preoperative deficits was observed at long-term follow-up. Looking at the 17 craniotomic surgeries, 10 patients harbored a preoperative ocular deficit (4 visual, 3 OEM, and 3 visual-field deficits), of whom, 6 patients improved (3 in visual, p = 0.015; 2 in OEM, p = 0.206; and 1 in visual-field functions, p = 0.001), with the remaining 4 patients clinically stable in their deficit after surgery. Also, in this case, no worsening was observed. Combined procedures were performed in five patients in total, with just one postoperative new-onset visual deficit, which was stable at 1 year.
Table 5. Early postoperative deficits according to the different approaches used.
| Endoscopic SEA (13 patients) | Combined (5 patients) | Craniotomic (17 patients) | Endoscopic SEA vs. craniotomic | ||||
|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | p -Value | |
| Intraoperative complications | 0 | 0 | 0 | 0 | 1 | 5.9 | 0.567 |
| Systemic complications | 1 | 7.7 | 0 | 0 | 4 | 23.5 | 0.528 |
| Surgical scar complications | 1 | 7.7 | 0 | 0 | 0 | 0 | 0.433 |
| Diplopia | 6 | 46.1 | 1 | 20 | 3 | 17.6 | 0.155 |
| OEM deficits | 4 | 30.8 | 1 | 20 | 2 | 11.8 | 0.225 |
| Visual-field deficits | 3 | 23.1 | 0 | 0 | 3 | 17.6 | 0.642 |
| Visual deficits | 6 | 46.1 | 1 | 20 | 3 | 17.6 | 0.546 |
| Other CN deficits | 3 | 23.1 | 2 | 40 | 2 | 11.8 | 0.532 |
| Intracranial complications | 1 | 7.7 | 0 | 0 | 3 | 17.6 | 0.558 |
Abbreviations: CN, cranial nerves; OEM, ocular extrinsic muscles; SEA, superior eyelid approach.
Table 6. Long-term postoperative deficits according to the different approaches performed.
| New-onset postoperative deficits (complications) | |||||||
|---|---|---|---|---|---|---|---|
| n | % | ||||||
| Visual deficits | 1 | 2.8 | |||||
| CSF leak | 1 | 2.8 | |||||
| Hemisyndrome | 1 | 2.8 | |||||
| Mild hypoesthesia in V2 | 2 | 5.7 | |||||
| How preoperative deficits evolved? | |||||||
| Preoperative ( n ) | Surgery | 1-y follow-up ( n , % relative to the same category, preoperatively) | |||||
| Endoscopic SEA | Combined | Craniotomic | Endoscopic SEA | Combined | Craniotomic | ||
| Visual deficits | 6 | 1 | 4 |
4 (66.6) ⇑ (p = 0.043
a
)
2 (33.3) ⇔ |
1 (100) ⇔ |
3 (75) ⇑ (p = 0.015
a
)
1 (25) ⇔ |
|
| OEM deficits | 4 | 0 | 3 |
3 (75) ⇑ (p = 0.007
a
)
1 (25) ⇔ |
0 | 2 (66.6) ⇑ (p = 0.206) 1 (33.3) ⇔ |
|
| Visual-field deficits | 3 | 0 | 3 |
1 (33.3) ⇑
(
p = 0.001
a
)
2 (66.6) ⇔ |
0 |
1 (33.3) ⇑ (p = 0.001
a
)
2 (66.6) ⇔ |
|
| What about new ocular deficits after surgery? | |||||||
| Endoscopic SEA | Combined | Craniotomic | |||||
| Visual deficits | 0 | 1 stable | 1 transitory | ||||
| OEM deficits | 0 | 1 transitory | 0 | ||||
| Visual-field deficits | 0 | 0 | 0 | ||||
Abbreviations: CSF, cerebrospinal fluid; OEM, ocular extrinsic muscles; SEA, superior eyelid approach; ⇑, improved; ⇔, stable.
p < 0.05.
Summing the previous data, new-onset postoperative deficits (complications) were one case of stable visual deficit (see above), one case of CSF leak after a transcranial approach, requiring multiple revision surgeries and actually resolved, one stable hemiparesis due to intraoperative paraclinoid internal carotid artery rupture during a PA, and two cases of stable mild hypoesthesia in the second trigeminal branch omolateral to the endoscopic SEA.
Performance Outcomes
The comparison of preoperative and early/long-term postoperative KPS and mRS between craniotomies and endoscopic SEA is reported in Table 7 . Patients who underwent an endoscopic SEA for SOM removal obtained an improvement from a mean preoperative KPS of 80 ± 10 to 93 ± 5.3 in the immediate postoperative period ( p = 0.008, independently of the EOR) and to 98 ± 4.4 at long-term ( p = 0.00002, independently of the EOR). Craniotomic patients ranged from a preoperative KPS of 79 ± 14.8 to 86 ± 17 and then to 95 ± 12.8 at short- and long-term follow-up, respectively. No statistical differences were found either between preoperative and short-term postoperative or between short- and long-term postoperative performance scores ( p = 1 and p = 0.146, respectively, after adjustment by Bonferroni correction), with a trend of improvement comparing preoperative and long-term postoperative functional state ( p = 0.062). mRS in SEA patients improved from 2.5 ± 0.73 preoperatively to 0.2 ± 0.44 at long-term ( p = 0.00002), whereas in craniotomic ones, it improved from 2.2 ± 1.2 to 0.8 ± 1.1 ( p = 0.012). In combined approaches, functional outcome significantly benefited from surgery, as outlined by mean preoperative and postoperative mRS values of 3 ± 0.707 and 0.2 ± 0.447, respectively ( p = 0.041). As far as KPS is concerned, preoperative, short-term postoperative, and long-term postoperative values were 72 ± 19.235, 86 ± 11.402, and 98 ± 4.472, respectively. A significant improvement was only found when comparing preoperative and long-term postoperative statuses ( p = 0.022).
Table 7. Report of perioperative KPS and mRS scales.
| Endoscopic SEA | Combined | Craniotomic | |||||||||||||
| Preoperative KPS | Early postoperative KPS | 1-y postoperative KPS | Preoperative mRS | 1-y postoperative mRS | Preop KPS | Early postoperative KPS | 1-y postoperative KPS | Preoperative mRS | 1-y postoperative mRS | Preop KPS | Early postoperative KPS | 1-y postoperative KPS | Preoperative mRS | 1-y postoperative mRS | |
| 80 | 100 | 100 | 2 | 0 | 70 | 60 | 70 | 3 | 2 | 70 | 100 | 100 | 3 | 0 | |
| 80 | 90 | 100 | 2 | 0 | 60 | 100 | 100 | 3 | 0 | 100 | 100 | 100 | 1 | 0 | |
| 70 | 90 | 90 | 3 | 1 | 70 | 100 | 100 | 3 | 0 | 80 | 80 | 100 | 3 | 0 | |
| 90 | 100 | 100 | 2 | 0 | 80 | 90 | 100 | 3 | 0 | 100 | 40 | 50 | 1 | 4 | |
| 80 | 90 | 100 | 3 | 0 | 80 | 90 | 90 | 3 | 2 | 80 | 100 | 100 | 3 | 0 | |
| 90 | 90 | 90 | 2 | 1 | 70 | 70 | 90 | 3 | 1 | ||||||
| 80 | 100 | 100 | 2 | 0 | 80 | 80 | 100 | 3 | 0 | ||||||
| 90 | 100 | 100 | 2 | 0 | 60 | 80 | 100 | 3 | 0 | ||||||
| 60 | 90 | 100 | 4 | 0 | 100 | 100 | 100 | 0 | 0 | ||||||
| 80 | 100 | 100 | 2 | 0 | 40 | 90 | 100 | 4 | 0 | ||||||
| 80 | 90 | 100 | 3 | 0 | 20 | 80 | 100 | 5 | 0 | ||||||
| 80 | 90 | 100 | 3 | 0 | 70 | 60 | 80 | 3 | 2 | ||||||
| 80 | 80 | 90 | 2 | 1 | 100 | 100 | 100 | 0 | 0 | ||||||
| 90 | 90 | 90 | 1 | 1 | |||||||||||
| 100 | 100 | 100 | 0 | 0 | |||||||||||
| 100 | 100 | 100 | 0 | 0 | |||||||||||
| 80 | 90 | 100 | 2 | 2 | |||||||||||
| Mean | 80 ± 10 | 93 ± 5.3 | 98 ± 4.4 | 2.5 ± 0.73 | 0.2 ± 0.44 | 72 ± 8.4 | 88 ± 16.4 | 92 ± 13 | 3 ± 0 | 0.8 ± 1.1 | 79 ± 14.8 | 86 ± 17 | 95 ± 12.8 | 2.2 ± 1.2 | 0.8 ± 1.1 |
| Median | 80 | 90 | 100 | 2 | 0 | 70 | 90 | 100 | 3 | 0 | 80 | 90 | 100 | 3 | 0 |
| p-Value | 0.008 a | 0.00002 a | 0.00002 a | 0.140 | 0.022 a | 0.041 a | 1 | 0.062 | 0.012 a | ||||||
Abbreviations: KPS, Karnofsky Performance Status; mRS, modified Ranking Scale; SEA, superior eyelid approach.
p < 0.05.
Follow-up, Additional Treatments, and Recurrence/Progression Rate
Seven (20%) patients were lost during follow-up. The mean follow-up was 31.5 months (range: 6–84 months).
Looking at recurrences in transcranial approaches, among 17 GTRs, we observed three (17%) cases of recurrence after a mean of 42.6 ± 14.2 months. Of these patients, two were operated a second time, whereas one is under strict imaging and clinical control, appearing to be stable at the last follow-up. Among STR cases, two cases of tumor progression were observed after a mean of 27.5 ± 24.7 months: one patient underwent an RT treatment and was then operated a second time, and the other patient is under follow-up with a progressively growing tumor remnant. Looking at purely endoscopic SEA, among GTR patients, just one case of tumor recurrence after 30 months was observed, which is still not considered worthy of surgery, whereas among STR patients, three tumor progressions were observed (22.66 ± 15.3 months): one patient had a remnant in infratemporal fossa, not causing symptoms and intentionally left in place during the first surgery, one patient was reoperated and she is now under follow-up, and the third patient was originally operated through an endoscopic SEA to control proptotic symptoms (already performed three transcranial surgeries in other centers), and his tumor remnant is progressively growing. Out of such patients, just one more was addressed to RT in the postoperative period (combined surgery with programmed proton therapy in the postoperative period).
Discussion
SOMs are a group of heterogeneous meningiomas that arise from the spheno-orbital region and may have characteristics of hyperostosis with histologically proven bone invasion. Such tumors, with their common extension to the ACF, MCF, SOF, IOF, cavernous sinus, frontal and temporal convexities, and temporal and infratemporal fossa, still represent a challenge for neurosurgeons. Although the primary goal remains the complete removal of the lesion along with its dural and osseous parts, it is nowadays accepted to aim for maximally safe STR, trying to preserve patient quality of life. 1 6 11 18 19
Typically, these tumors are addressed through classical frontotemporal craniotomies, such as the pterional and orbitozygomatic variants. 2 Along with the recent evolution of minimally invasive techniques to manage skull base lesions, in the last years the endoscopic superior eyelid transorbital corridor was proposed to manage orbital lesions laterally placed to the optic nerve. 20 Moe et al in 2010 and then Ramakrishna et al described the SEA as a part of “TONES” (transorbital neuroendoscopic surgeries) procedures to the anterior skull base, outlining such corridor for laterally and paramedian placed lesions in the anterior skull base. 20 21 Later on, in 2014, Lew et al proposed an exclusive transorbital endoscopic approach for the treatment of extradural cranial base lesions, 22 whereas other authors proposed in the same years lateral orbitotomies to manage lateral orbital wall pathologies. 23 24 Almeida et al reported on two cases of SOMs treated through both a transorbital and endonasal corridor in 2018. 25 Such clinical work was also supported by various cadaver dissection studies, confirming the possibility to consider the orbit both as a “surgical corridor” and as a “surgical target.” 12 26 27 28
With this framework in mind, and considering the paucity of data available on SOMs management through transorbital corridors, 25 29 our group in 2018 presented the preliminary results of the application of a purely transorbital approach to the management of SOMs, suggesting the possible role for this approach in the treatment of very selected lesions, especially when proptotic symptoms are the main complaint. 13 We thus tried to better clarify surgical indications and applications of such surgical route for SOMs, presenting our results in a cohort of 35 patients operated on in the last 10 years. To the best of our knowledge, this study comprehends the largest available cohort of SOMs treated through an endoscopic SEA.
Looking at the clinical outcomes analyzed, in the immediate postoperative period, we observed in the craniotomic group a higher occurrence of intracranial and systemic complications, but statistical analysis did not show the data as statistical significant. Moreover, the SEA group demonstrated a higher rate of immediate postoperative ocular complications, such as visual-field, OEM, and visual acuity deficits, if compared with craniotomic surgeries; however, no statistical difference was found. Nevertheless, focusing on long-term postoperative clinical deficits, these data were not confirmed.
To note, we included in the analysis the eventual presence at preoperative time of neurovisual deficits, examining separately patients harboring a new-onset postintervention ophthalmological deficit, considering it a complication of the procedure, from patients with already present ocular deficits at the time of presentation (the great majority of patients). At 1-year follow-up, we observed just one case of postoperative stable new-onset visual deficit after a combined procedure, without new deficit regarding OEM and visual field, independently from the procedure performed. Moreover, when looking at how preoperative deficits evolved at long-term follow-up, we did not observe any worsening of the preoperative ocular deficits in both SEA and craniotomic patients; on the contrary, with both approaches, we observed statistical improvement of preoperative visual and visual-field deficits, with OEM function improvement only in SEA group ( Table 6 ). Considering new-onset non-ophthalmological postoperative deficits, we obtained a complication rate consistent with the current literature. 1 6 30 Thus, we can conclude that SEA is an approach at least as safe as craniotomic ones.
We then analyzed the obtained EOR. For craniotomic surgeries, we obtained a GTR of 76.4%/SGS 0-II grade of 76.5%, which is in line with the current literature. 1 4 Looking at SEA, a statistically significant lower EOR rate was observed (46.2% of GTR and 46.2% of SGS grades 0–II; p = 0.005 and p = 0.026, respectively). These data are not surprising at all and may be explained by the fact that endoscopic SEA was mainly performed in patients where the primary surgical goal was not a GTR but symptoms improvement, trying to avoid significant morbidities. In fact, five out of the seven STR patients (53.8% of SEA approaches) represented already preoperatively planned STR patients. Focusing on this aspect and following the main current thinking, we are strongly convinced that given the highly infiltrative nature of this kind of meningioma, trying to remove entirely tumor tissue (especially at dural margins) may not be the best option for all patients. Thus, our actual philosophy is to relieve patient symptoms and removing the tumor as safely as possible rather than obtaining GTR in all patients, regardless of the possible (and likely) postoperative morbidities. For such reasons, surgical strategy should be precisely tailored for each patient.
Looking at our results, we then tried to sum up the preoperative factors that we consider of leading importance in choosing the correct surgical approach and surgical aim for each patient.
Starting from a purely anatomical point of view , it is clear that transcranial and transorbital approaches have different benefits and limitations. While PA and common craniotomic approaches are usually adequate to obtain a good exposure of the lateral wall and roof of the orbit, to resect diffuse intradural tumors, and to reach cavernous sinus portions especially when the FOTZA variant is performed, endoscopic SEA may not be the best option to manage extensive intracranial pathologies with cavernous sinus or sellar region invasion. Instead, it may be an optimal option if a patient's major complaint is proptosis, and pathology has extensively spread to the lateral orbital compartment. In our experience, endoscopic SEA allows to early assess periorbital invasion and to remove tumor-infiltrated, hyperostotic bone from the sphenoid wing with decompression of the SOF. 13 14 25 We can also suggest to use endoscopic SEA to manage intradural portions of meningiomas (frontal or temporal) within more or less 1 cm from the dural opening. We think that going further than this may be dangerous due to the imprecise control of complications that a long and narrow corridor like this may offer. Nevertheless, endoscopic SEA is being studied in anatomical and clinical works to approach cavernous sinus, Meckel's cave, and MCF, with early but promising results. 31 32 33 Thus, eventual extensive intradural pathology represents another factor to keep in mind when choosing the surgical strategy. Fig. 3 reports the anatomical criteria that we usually adopt when choosing among transorbital and transcranial routes to further highlight the purely surgical viewpoint. Still looking to the pathology itself, SEA may be a great option in all those patients already operated multiple times through transcranial corridors or who underwent RT/radiosurgery regimens. Both patients with intraorbital recurrence complaining of ophthalmological symptoms and patients affected by intracranial recurrence (i.e., toward the temporal pole) causing intracranial symptoms (i.e., seizures or brain edema) may be candidates to endoscopic SEA, avoiding to retrace previous corridors hallmarked by extensive surgical scars. Finally, patients with very extensive pathologies with locations in the ACF, MCF, LSW, MSW, and beyond, or patients with extensive hyperostosis with the so-called “carpet-like” tumors, may beneficiate from a symptom relief aimed surgery through endoscopic SEA. Many epidemiological data also need to be taken into account when choosing the most suitable approach for each patient: those who are in advanced age (>75 years), with many comorbidities that may raise ASA score, without clinical signs of intracranial pathology, or with a not-evolving intracranial lesion may be more appropriately treated through an endoscopic SEA, without the need to submit those patients to extensive and long surgeries, raising the risk of systemic problems (resulted to be higher in our craniotomy group but not statistically different from SEA patients). And, last but not least, are patient preferences . In our experience, it is not so uncommon that patients in advanced age, with comorbidities, but still in relative good health, complaining only mild proptosis and ocular muscle deficits, if offered, decide to undergo a less invasive surgery, with a lesser risk of major postoperative morbidities. The conjunct analysis of the previous factors allowed to offer and recommend tailored surgeries to different patients with different needs and health status. As an example, Fig. 4 reports a case of SOMs treated through an endoscopic SEA. Of course, anatomical location of the pathology represents the real “conditio sine qua non” to propose endoscopic SEA, but it is always considered in conjunction with the analysis of the other factors mentioned previously. To note, we consider endoscopic SEA as a valid way to accomplish such symptom-centered strategies, never “scotomizing” that craniotomic approaches may actually be similarly adequate in obtaining such objective. We are convinced that the real point, and the new perspective, should be to focus on the identification of surgical aim, in conjunction with clear identification of surgical strategy for the specific patient, rather than merely on choosing the most suitable and rational surgical approach. This is the reason why we are actually giving space in selected cases to strategies aimed at symptom relief. Thus, in such cases, where an STR is the aim, we usually consider performance indexes, such as KPS and mRS, as the most important outcome score to be considered in the postoperative period rather than a mere analysis of Simpson grade. The observation of the greater improvement in KPS and mRS after SEA surgeries corroborated such thinking: while the analysis of the long-term mRS showed a statistical improvement in both approaches ( p = 0.00002 vs. p = 0.012 for SEA and craniotomics, respectively), when analyzing KPS, a statistically significant improvement between preoperative and both short- and long-term follow-up was seen in patients operated through endoscopic SEA ( p = 0.008 and 0.00002, respectively, both independently of the EOR obtained); in craniotomic patients, a trend of improvement was found only at the long term, without reaching statistical significance ( p = 0.062). Thus, in Fig. 5 , flow-chart proposal for the management of SOMs is presented.
Fig. 3.
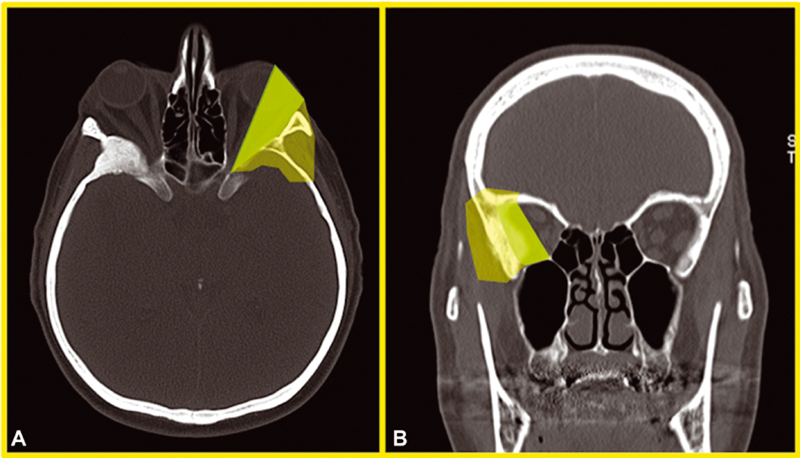
Anatomical criteria for endoscopic superior eyelid approach (SEA) choice. ( A,B ) Axial and coronal bone window computed tomography (CT) scans of an spheno-orbital meningioma (SOM) patient demonstrating a green area (heavily shaded in black-and-white version) and a more transparent periphery area in the left orbital region. The darker area comprehends the intraorbital area, lateral to the optic nerve, that may be easily accessed through an endoscopic SEA for the removal of intra- and extraconal pathologies or for the control of the intraorbital growing of a symptomatic SOM. When a patient complains of ocular-related symptoms that are reasonably linked to the osseous or parenchymatous growing of an SOM in such area, it eventuality represents a strong indication for an endoscopic SEA from a purely anatomical point of view. The more peripheral area, comprehending anterior and middle cranial base, lateral orbital wall with temporalis muscle, up to the most anterior part of temporal lobe, may be also accessed from a transorbital route, although for such cases, the decision to perform an endoscopic SEA should be even more carefully counterbalanced in light of the other factors discussed in the text (age, eventual comorbidities, desired extent of resection, etc.) that may favor a craniotomic approach with a consequent higher chance of obtaining a gross total resection.
Fig. 4.
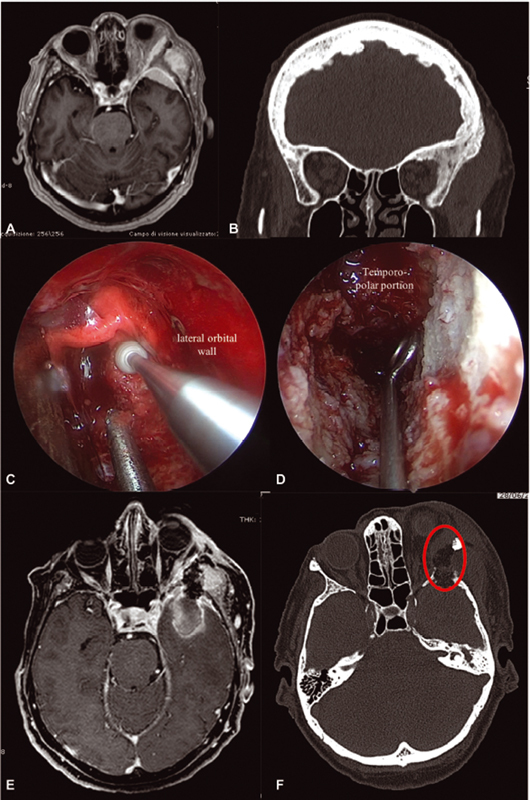
Exemplificative case of a left spheno-orbital meningioma (SOM) treated through an endoscopic superior eyelid approach (SEA). This 75 year–old-woman presented complaining of recent visual acuity reduction, left sixth cranial nerve deficit, diplopia, proptosis, and temporal region tumefaction. Magnetic resonance imaging (MRI) and computed tomography (CT) disclosed a left SOM characterized by extensive pterional, lateral orbital, and bifrontal bone invasion, with meningiomatosis spread to the middle cranial fossa (MCF), temporal and infratemporal fossa, and lateral orbital compartment ( A,B ). Anamnesis revealed triple cardiac stenting, obstructive chronic bronchitis, renal insufficiency, and severe smoking history. When proposed, the patient accepted to undergo an endoscopic SEA aimed at subtotal resection, controlling her visual symptoms. Surgery was then performed, removing the intraorbital part of the lesion along with the lateral orbital wall ( red circle in F ); a small opening in the temporopolar dura permitted to partially resect the meningioma in MCF ( C,D ). Postoperative imaging demonstrated an acceptable lateral orbital decompression, with subtotal resection of the temporopolar portion of the meningioma. The portion of the lesion in the temporal/infratemporal fossa was left untouched ( E,F ). The patient went home on fifth postoperative day, without any new neurological deficit. Progressive improvement of both cranial nerve deficit and visual acuity at 6 months was noticed. Her Karnofsky Performance Status improved from a preoperative value of 80 to 90 at 1 year, mainly due to improvement in visual acuity.
Fig. 5.
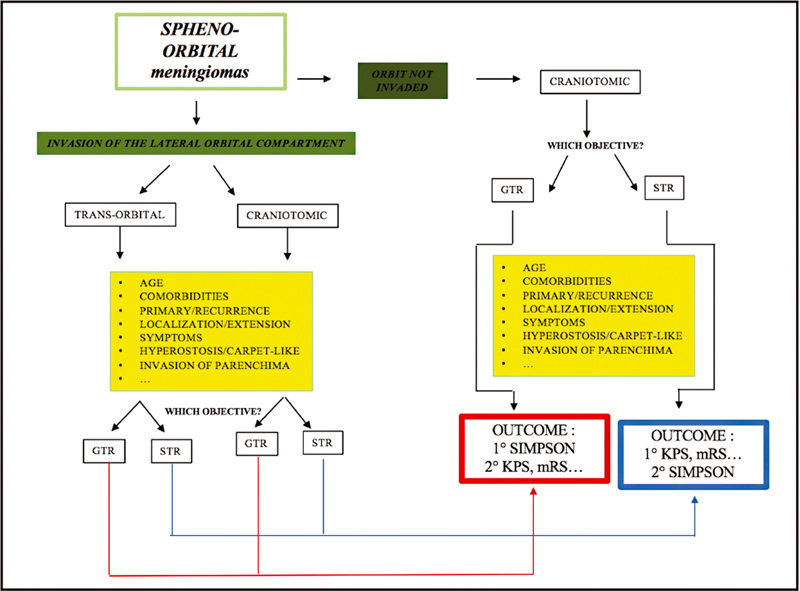
Proposal for a new treatment allocation algorithm for the management of spheno-orbital meningiomas (SOMs). Invasion of lateral orbital compartment is the “conditio sine qua non” for the possible proposal of an endoscopic superior eyelid approach in the management of an SOM. When both craniotomic and transorbital corridors may be chosen from an anatomical point of view, a series of factors ( yellow boxes ) may help in determining surgical aim and most appropriate surgical corridor. When a planned subtotal resection is the objective, the analysis of performance indexes such as KPS and mRS should be the outcome that deserves to be primarily considered; in cases where a GTR is considered safely achievable, Simpson grade 0/I should be the objective. GTR, gross total resection; KPS, Karnofsky Performance Status; mRS, modified Rankin Scale; STR, subtotal resection.
To note, in our department, it is not uncommon to manage ACF and MCF lesions through combined cranioendoscopic approaches, given the wide angles of view that such a collaboration may offer. 16 34 In this report, we described five cases of SOMs that were considered to be suitable for such a management, but they were not included in the statistical analysis due to the paucity of their number and because we are convinced that they deserve to be discussed in a different optic in further papers, analyzing the role of such combined approaches in the management of ACF and MCF lesions in general.
Looking at the limitations, such a study has all the strong limits that are usually encountered when performing retrospective studies. Moreover, the patient sample is small, and thus statistical power is low.
In conclusion, nothing further from intentions is to propose a symptom-relief surgery for all SOMs, elevating it as a gold standard. Obtaining a grade 0/I SGS still remains the primary objective in meningioma surgery. Nevertheless, we are convinced that in very selected cases, endoscopic SEA may have a complementary role to classical craniotomic approaches, that is, helping in the management of complex patients complaining primarily of ocular and proptotic symptoms and operated multiple times or with important comorbidities, and yielding a maximally safe STR rather than obtaining a GTR at any cost.
Conclusions
SOMs are a specific type of cranial base meningiomas characterized by a strong dura mater and bony infiltrative nature. Nowadays, the treatment paradigm is shifting from the objective of obtaining GTR at any cost to a primarily symptom-centered surgery. Among various minimally invasive approaches to the cranial base, endoscopic SEA has been proposed for the management of selected SOMs. With this work, we presented our 10-year experience analyzing pre- and postoperative clinical, radiological, anatomical, and surgical characteristics of patients affected by SOMs managed through both transcranial and endoscopic SEA corridors. Our results suggest that such a minimally invasive approach may be a safe and effective alternative to transcranial approaches in very selected cases where the primary objective is to obtain as safe and extensive resections as possible aimed at symptom relief.
Conflict of Interest None declared.
These authors contributed equally to this work.
References
- 1.Terrier L M, Bernard F, Fournier H D. Spheno-orbital meningiomas surgery: multicenter management study for complex extensive tumors. World Neurosurg. 2018;112:e145–e156. doi: 10.1016/j.wneu.2017.12.182. [DOI] [PubMed] [Google Scholar]
- 2.Ringel F, Cedzich C, Schramm J.Microsurgical technique and results of a series of 63 spheno-orbital meningiomas Neurosurgery 200760(04, Suppl 2): discussion 221–222214–221. [DOI] [PubMed] [Google Scholar]
- 3.Shrivastava R K, Sen C, Costantino P D, Della Rocca R. Sphenoorbital meningiomas: surgical limitations and lessons learned in their long-term management. J Neurosurg. 2005;103(03):491–497. doi: 10.3171/jns.2005.103.3.0491. [DOI] [PubMed] [Google Scholar]
- 4.Bikmaz K, Mrak R, Al-Mefty O. Management of bone-invasive, hyperostotic sphenoid wing meningiomas. J Neurosurg. 2007;107(05):905–912. doi: 10.3171/JNS-07/11/0905. [DOI] [PubMed] [Google Scholar]
- 5.Pompili A, Derome P J, Visot A, Guiot G. Hyperostosing meningiomas of the sphenoid ridge--clinical features, surgical therapy, and long-term observations: review of 49 cases. Surg Neurol. 1982;17(06):411–416. doi: 10.1016/s0090-3019(82)80006-2. [DOI] [PubMed] [Google Scholar]
- 6.Freeman J L, Davern M S, Oushy S. Spheno-orbital meningiomas: a 16-year surgical experience. World Neurosurg. 2017;99:369–380. doi: 10.1016/j.wneu.2016.12.063. [DOI] [PubMed] [Google Scholar]
- 7.Boari N, Gagliardi F, Spina A, Bailo M, Franzin A, Mortini P. Management of spheno-orbital en plaque meningiomas: clinical outcome in a consecutive series of 40 patients. Br J Neurosurg. 2013;27(01):84–90. doi: 10.3109/02688697.2012.709557. [DOI] [PubMed] [Google Scholar]
- 8.Simpson D. The recurrence of intracranial meningiomas after surgical treatment. J Neurol Neurosurg Psychiatry. 1957;20(01):22–39. doi: 10.1136/jnnp.20.1.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bagshaw H P, Burt L M, Jensen R L. Adjuvant radiotherapy for atypical meningiomas. J Neurosurg. 2017;126(06):1822–1828. doi: 10.3171/2016.5.JNS152809. [DOI] [PubMed] [Google Scholar]
- 10.Rockhill J, Mrugala M, Chamberlain M C. Intracranial meningiomas: an overview of diagnosis and treatment. Neurosurg Focus. 2007;23(04):E1. doi: 10.3171/FOC-07/10/E1. [DOI] [PubMed] [Google Scholar]
- 11.Saeed P, van Furth W R, Tanck M. Natural history of spheno-orbital meningiomas. Acta Neurochir (Wien) 2011;153(02):395–402. doi: 10.1007/s00701-010-0878-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Di Somma A, Andaluz N, Cavallo L M. Endoscopic transorbital superior eyelid approach: anatomical study from a neurosurgical perspective. J Neurosurg. 2018;129(05):1203–1216. doi: 10.3171/2017.4.JNS162749. [DOI] [PubMed] [Google Scholar]
- 13.Dallan I, Sellari-Franceschini S, Turri-Zanoni M. Endoscopic transorbital superior eyelid approach for the management of selected spheno-orbital meningiomas: Preliminary experience. Oper Neurosurg (Hagerstown) 2018;14(03):243–251. doi: 10.1093/ons/opx100. [DOI] [PubMed] [Google Scholar]
- 14.Locatelli D, Pozzi F, Turri-Zanoni M. Transorbital endoscopic approaches to the skull base: current concepts and future perspectives. J Neurosurg Sci. 2016;60(04):514–525. [PubMed] [Google Scholar]
- 15.Castelnuovo P, Turri-Zanoni M, Battaglia P, Locatelli D, Dallan I. Endoscopic endonasal management of orbital pathologies. Neurosurg Clin N Am. 2015;26(03):463–472. doi: 10.1016/j.nec.2015.03.001. [DOI] [PubMed] [Google Scholar]
- 16.Dallan I, Castelnuovo P, Locatelli D. Multiportal combined transorbital transnasal endoscopic approach for the management of selected skull base lesions: preliminary experience. World Neurosurg. 2015;84(01):97–107. doi: 10.1016/j.wneu.2015.02.034. [DOI] [PubMed] [Google Scholar]
- 17.Erdogmus S, Govsa F. Importance of the anatomic features of the lacrimal artery for orbital approaches. J Craniofac Surg. 2005;16(06):957–964. doi: 10.1097/01.scs.0000179741.68294.1c. [DOI] [PubMed] [Google Scholar]
- 18.Peron S, Cividini A, Santi L, Galante N, Castelnuovo P, Locatelli D. Spheno-orbital meningiomas: when the endoscopic approach is better. Acta Neurochir Suppl. 2017;124:123–128. doi: 10.1007/978-3-319-39546-3_19. [DOI] [PubMed] [Google Scholar]
- 19.Mirone G, Chibbaro S, Schiabello L, Tola S, George B.En plaque sphenoid wing meningiomas: recurrence factors and surgical strategy in a series of 71 patients Neurosurgery 200965(6, Suppl): discussion 108–109100–108. [DOI] [PubMed] [Google Scholar]
- 20.Moe K S, Bergeron C M, Ellenbogen R G.Transorbital neuroendoscopic surgery Neurosurgery 201067(3, Suppl Operative):ons16–ons28. [DOI] [PubMed] [Google Scholar]
- 21.Ramakrishna R, Kim L J, Bly R A, Moe K, Ferreira M., Jr Transorbital neuroendoscopic surgery for the treatment of skull base lesions. J Clin Neurosci. 2016;24:99–104. doi: 10.1016/j.jocn.2015.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lew H, Rootman D B, Nassiri N, Goh A, Goldberg R A. Transorbital approach without craniotomy to orbital tumors with extradural intracranial extension. Orbit. 2014;33(05):343–351. doi: 10.3109/01676830.2014.904374. [DOI] [PubMed] [Google Scholar]
- 23.Mariniello G, Maiuri F, de Divitiis E.Lateral orbitotomy for removal of sphenoid wing meningiomas invading the orbit Neurosurgery 201066(6, Suppl Operative): discussion 292287–292. [DOI] [PubMed] [Google Scholar]
- 24.Amirjamshidi A, Abbasioun K, Amiri R S, Ardalan A, Hashemi S MR. Lateral orbitotomy approach for removing hyperostosing en plaque sphenoid wing meningiomas. Description of surgical strategy and analysis of findings in a series of 88 patients with long-term follow up. Surg Neurol Int. 2015;6(06):79. doi: 10.4103/2152-7806.157074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Almeida J P, Omay S B, Shetty S R. Transorbital endoscopic eyelid approach for resection of sphenoorbital meningiomas with predominant hyperostosis: report of 2 cases. J Neurosurg. 2018;128(06):1885–1895. doi: 10.3171/2017.3.JNS163110. [DOI] [PubMed] [Google Scholar]
- 26.Chen H I, Bohman L E, Emery L. Lateral transorbital endoscopic access to the hippocampus, amygdala, and entorhinal cortex: initial clinical experience. ORL J Otorhinolaryngol Relat Spec. 2015;77(06):321–332. doi: 10.1159/000438762. [DOI] [PubMed] [Google Scholar]
- 27.Mishra A. Transorbital approach to infratemporal fossa: novel technique. J Laryngol Otol. 2011;125(06):638–642. doi: 10.1017/S002221511100003X. [DOI] [PubMed] [Google Scholar]
- 28.Di Somma A, Andaluz N, Gogela S L. Surgical freedom evaluation during optic nerve decompression: laboratory investigation. World Neurosurg. 2017;101:227–235. doi: 10.1016/j.wneu.2017.01.117. [DOI] [PubMed] [Google Scholar]
- 29.Lubbe D, Mustak H, Taylor A, Fagan J. Minimally invasive endo-orbital approach to sphenoid wing meningiomas improves visual outcomes - our experience with the first seven cases. Clin Otolaryngol. 2017;42(04):876–880. doi: 10.1111/coa.12722. [DOI] [PubMed] [Google Scholar]
- 30.Bikmaz K, Mrak R, Al-Mefty O. Management of bone-invasive, hyperostotic sphenoid wing meningiomas. J Neurosurg. 2007;107(05):905–912. doi: 10.3171/JNS-07/11/0905. [DOI] [PubMed] [Google Scholar]
- 31.Dallan I, Di Somma A, Prats-Galino A. Endoscopic transorbital route to the cavernous sinus through the meningo-orbital band: a descriptive anatomical study. J Neurosurg. 2017;127(03):622–629. doi: 10.3171/2016.8.JNS16465. [DOI] [PubMed] [Google Scholar]
- 32.Jeon C, Hong C K, Woo K I.Endoscopic transorbital surgery for Meckel's cave and middle cranial fossa tumors: surgical technique and early results J Neurosurg 2018(e-pub ahead of print) 10.3171/2018.6.JNS181099 [DOI] [PubMed] [Google Scholar]
- 33.Priddy B H, Nunes C F, Beer-Furlan A, Carrau R, Dallan I, Prevedello D MS. A side door to Meckel's cave: anatomic feasibility study for the lateral transorbital approach. Oper Neurosurg (Hagerstown) 2017;13(05):614–621. doi: 10.1093/ons/opx042. [DOI] [PubMed] [Google Scholar]
- 34.Restelli F, Tabano A, Pozzi F, Castelnuovo P, Locatelli D. Combined multiportal endoscopic endonasal and transcranial approach for recurrent tuberculum sellae meningioma: operative video. World Neurosurg. 2019;127(127):221. doi: 10.1016/j.wneu.2019.04.027. [DOI] [PubMed] [Google Scholar]


