Abstract
Salvia miltiorrhiza Burge (Danshen), a member of the Lamiaceae family, has been used in traditional Chinese medicine for many centuries as a valuable medicinal herb with antioxidative, anti-inflammatory, and antifibrotic potential. Several evidence-based reports have suggested that Salvia miltiorrhiza and its components prevent vascular diseases, including myocardial infarction, myocardial ischemia/reperfusion injury, arrhythmia, cardiac hypertrophy, and cardiac fibrosis. Tanshinone IIA (TanIIA), a lipophilic component of Salvia miltiorrhiza, has gained attention because of its possible preventive and curative activity against cardiovascular disorders. TanIIA, which possesses antioxidative, anti-inflammatory, and antifibrotic properties, could be a key component in the therapeutic potential of Salvia miltiorrhiza. Vascular diseases are often initiated by endothelial dysfunction, which is accompanied by vascular inflammation and fibrosis. In this review, we summarize how TanIIA suppresses tissue inflammation and fibrosis through signaling pathways such as PI3K/Akt/mTOR/eNOS, TGF-β1/Smad2/3, NF-κB, JNK/SAPK (stress-activated protein kinase)/MAPK, and ERK/Nrf2 pathways. In brief, this review illustrates the therapeutic value of TanIIA in the alleviation of oxidative stress, inflammation, and fibrosis, which are critical components of cardiovascular disorders.
1. Introduction
Salvia miltiorrhiza Bunge, known as Danshen in Chinese, is a member of the Labiatae family. The dried root of the rhizome of Salvia miltiorrhiza Burge has been widely used in traditional medicine in China and other oriental regions, especially for treating cardiovascular diseases like coronary heart disease, myocardial infarction (MI), angina pectoris, and atherosclerosis [1–3]. The adverse effects of the therapeutic components are often mild [4, 5]. Although dried roots have been used as herbal medicine for more than a thousand years, the study of therapeutic content in the plant did not start until the early 20th century [1].
Endothelial dysfunction describes a series of pathogenics promoting the development of hypertension and atherosclerosis, including oxidative stress, vascular endothelium injury, inflammation, and loss of smooth muscle elasticity [6, 7]. In hyperglycemic or diabetic conditions, mitochondria lose their functions and the electron transport chain is uncoupled, generating reactive oxygen species (ROS) [8]. ROS promote ER stress and mitochondrial alterations, causing apoptosis of endothelial cells. Additionally, ROS-injured endothelial cells generate less nitric oxide (NO) but more endothelin-1 (ET-1), promoting vasoconstriction [9, 10]. Notably, imbalanced proinflammatory mediators also induce leakage of endothelial walls and increase leukocyte adhesion [11].
Tanshinones are a group of nonpolar lipid-soluble components in Salvia miltiorrhiza extract, recognized for their therapeutic activity in the treatment and control of various diseases, including cardiovascular diseases, hepatitis, chronic kidney injury, and even dysmenorrhea [12]. TanIIA is the most well-studied and pharmacologically active compound among lipid-soluble tanshinones [2, 13]. TanIIA has been shown to control the symptoms of cardiovascular disease models such as neointima hyperplasia, atherosclerotic calcification, diet-induced atherosclerosis, and aortic aneurysm [2]. Furthermore, TanIIA controls the development of cardiovascular disorders through multiple mechanisms, including reduction of lipid oxidation and ROS generation, antiapoptosis, anti-inflammation, and antifibrosis. These pathophysiological mechanisms are closely related to endothelial dysfunction. Here, we aimed to summarize the current understanding of the pharmacological activities of TanIIA in cardiovascular diseases with a focus on the antioxidation, anti-inflammatory, and antifibrotic effects of TanIIA.
2. Salvia miltiorrhiza and Its Derivatives
2.1. Compounds Derived from Salvia miltiorrhiza
Although Salvia miltiorrhiza has a long history of application in traditional medicine, the search for possible pharmacological components began in the 1930s [1]. More than 200 compounds have been identified [2]. These derivatives can be mainly classified into two categories: water-soluble phenolic compounds (such as salvianolic acid A, salvianolic acid B, protocatechuic aldehyde, lithospermic acid, danshensu, caffeic acid, and rosmarinic acid) [14–16] and lipid-soluble compounds (such as tanshinone I, tanshinone IIA, tanshinone IIB, cryptotanshinone, and dihydrotanshinone I) (Figure 1) [1, 17, 18]. Water-soluble compounds, such as salvianolates, are widely used in the treatment of coronary heart disease [19, 20]. In contrast, lipid-soluble compounds such as tanshinones are more effective against cardiovascular diseases (CVDs) and cerebrovascular diseases, including atherosclerosis, myocardial infarction, and cardiac hypertrophy [13, 21]. These ingredients from Salvia miltiorrhiza were reported to have benefits in microcirculation and could increase blood flow, dilate coronary arteries, and prevent myocardial ischemia, atherosclerosis, calcification, and aortic aneurysm formation. Owing to their different chemical structures, their pharmacological activities, pharmacokinetics, and clinical applications are different. Among these active ingredients, tanshinones are one of the most well studied compounds [17]. In this review, we specifically focused on the lipid-soluble tanshinone, tanshinone IIA.
Figure 1.
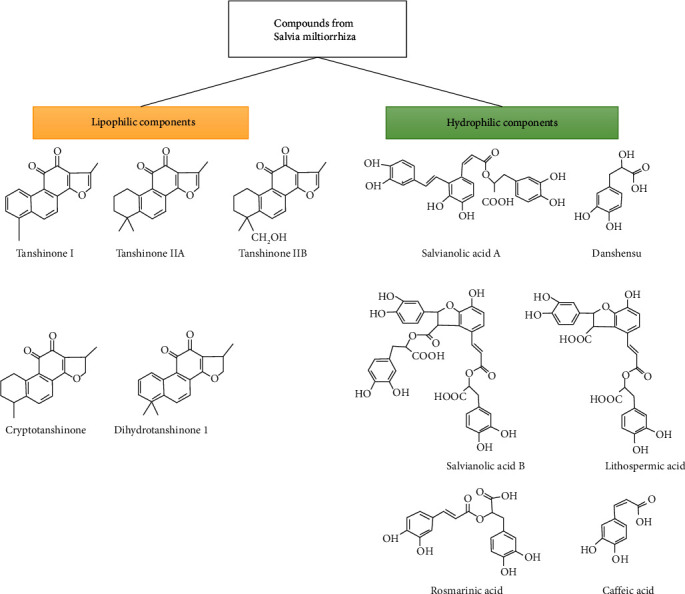
Major lipophilic and hydrophilic components of Salvia miltiorrhiza, obtained after modification from [21].
2.2. The Tanshinones
Tanshinones are quinone diterpenes that were first isolated from Salvia miltiorrhiza roots by Nakao in 1930 [22], and more diterpene compounds have been separated and identified since then. Most of them are diterpene quinone compounds such as tanshinone I, tanshinone IIA, tanshinone IIB, and cryptotanshinone [23]. Tanshinones are synthesized from the five-carbon precursors, isopentenyl pyrophosphate (IPP) and dimethylallyl pyrophosphate (DMAPP), which are produced by the mevalonate (MVA) and the 2-C-methyl-D-erythritol 4-phosphate (MEP) pathways (Figure 2(a)) [24, 25]. TanIIA was discovered considered the main active lipophilic constituent of Salvia miltiorrhiza [14]. The core structure of tanshinones contains four rings, including naphthalene or tetrahydronaphthalene rings A and B, ortho- or para-naphthoquinone or lactone ring C, and a furan or dihydrofuran ring D [14]. TanIIA and cryptotanshinone, which contain an ortho-quinone C-ring, are the most intensively studied compounds. However, their yield from cultured roots is low, and biotechnological approaches are needed to increase their productivity [24, 26]. Another issue with TanIIA is its low water solubility. Sodium TanIIA sulfonate (STS) is a derivative of TanIIA with a sodium sulfonate addition to the dihydrofuran ring at the C-16 position and hence exhibits increased polarity and water solubility (Figure 2(b)) [27–29]. STS often serves as a substitute for TanIIA, and it has been utilized interchangeably in many previous studies [28–30]. Both TanIIA and STS have been widely used in preclinical studies because of their anti-inflammatory, antioxidative, and antifibrotic properties [31]. However, some studies have claimed that the modification of the molecular structure changes the chemical properties and bioactivity [32–35].
Figure 2.
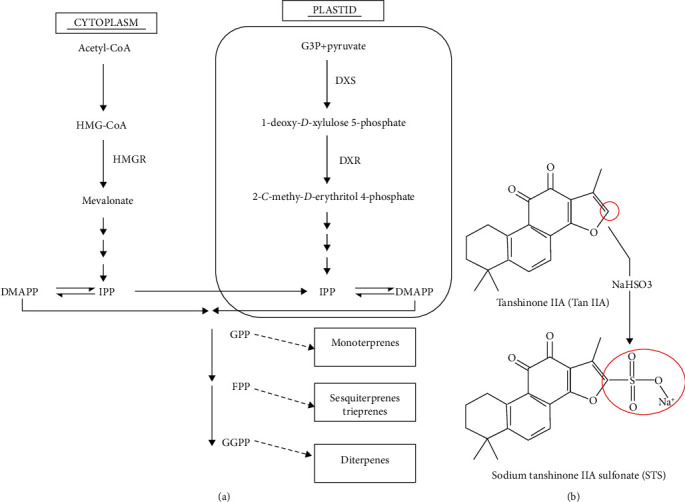
Biogenesis of tanshinones and the synthesis of STS. (a) Biogenesis of tanshinones. Obtained from [24] and modified. (b) Structure of TanIIA and STS. Obtained after modification from [27].
In detail, the metabolic reaction of TanIIA and STS is similar in rat bile except for the oxidation in side chain, which occurred in TanIIA but not STS [35]. STS suppresses atorvastatin-driven cerebral hemorrhage in zebrafish embryos but not TanIIA [32]. Human Purinergic Receptor P2X7 is blocked by STS but not TanIIA [36]. On the other hand, TanIIA inhibits the phosphorylation of Akt when used with anticancer drug epirubicin and increase the apoptosis of breast cancer cell line BT-20. Yet, STS failed to be uptaken by the BT-20 [34]. TanIIA boosts the excretion of anticoagulant warfarin by structural modification. However, STS elevates warfarin concentration by dissociation of albumin-warfarin complex [33].
Notably, TanIIA and STS exhibit different pharmacokinetic patterns. For intravenous injection, TanIIA showed elimination half-life of 1.0 ± 0.7 hour at 20 mg/kg and 1.8 ± 0.6 hour at 60 mg/kg dosage in the serum of male Sprague–Dawley rats [37]. STS showed terminal half-life for about 21.6 ± 2.4 minutes at 50 mg/kg dosage in the serum of Kunming male mice [38]. It seems that STS may exhibit a shorter half-life in animals based on the results above. Yet, the animal models, dosage, and the sensitivity of detection affect the calculated half-life in each model. In one study, STS exhibits a “distribution half-life (t1/2β)” of 0.91 ± 0.21 hour but “elimination half-life” (t1/2γ) for 13.45 hours when 2 mg/kg of STS were injected into male Sprague–Dawley rats [39], while the other study claimed only 26.57 minutes of elimination half-life at the dosage of 6 mg/kg [40]. The extended elimination half-life is partly due to the sensitivity of liquid chromatography-electrospray ionization-tandem mass spectrometry. Above all, a parallel comparison between the pharmacokinetics of TanIIA and STS is required to ascertain the faster turnover of STS.
For the tissue distribution, both TanIIA and STS can both found in organs like the heart, liver, spleen, and lung. For intravenous injection, TanIIA tends to reside in the lung and liver and STS tend to distribute in the kidney and liver [37, 38]. Interestingly, oral gavage of TanIIA resulted in accumulation of the compound in the gastrointestinal tract, reflecting the poor bioavailability of TanIIA [37].
Despite there being differences in pharmacokinetics and some molecular activities, both TanIIA and STS suppress endothelial dysfunction, the focus in this review. In this review, we summarized the studies using STS and TanIIA in vitro and in vivo in order to further explore the functional properties of these compounds (Tables 1 and 2).
Table 1.
Summarized in vitro mechanism of action of tanshinone IIA and STS.
| Biological activity | Cell type | Model | Drug used | Drug dose | Timing | Key regulated factors | Physiological effect | Ref. |
|---|---|---|---|---|---|---|---|---|
| Antioxidative | Neonatal cardiac myocytes | H2O2-driven oxidative stress | TanIIA | 0.01-0.1 μM | 24 h prior to oxidative stimuli | Prohibitin (↓) | Attenuates cell death | [146] |
| Adult cardiac myocytes | I/R model Hypoxia |
TanIIA | 0.5-5 μM | 2 h before hypoxia | lncRNA AK003290 (↑) miR-124-5p (↓) |
Decreases apoptosis rate and ROS production through activation of lncRNA AK003290 | [68] | |
| Human umbilical vein endothelial cells (HUVECs) | Acrolein-induced oxidative cell injury | TanIIA | 10-40 μg/mL | 3-12 h | Cystathionine γ-Lyase (CSE) (↑) H2S (↑) cAMP signaling (↑) |
Attenuates oxidative endothelial injury through H2S | [67] | |
|
| ||||||||
| Anti-inflammatory | Rheumatoid arthritis, fibroblast-like synoviocytes (RA-FLS) | TNF-α induction | TanIIA | 2.5-20 μM | 8-48 h | IL-6, IL-8, IL-1β, TNF-α (↓) MMPs (↓) p-p38, p-JNK, p-AKT, p-NF-κB p65 (↓) |
Reduces the viability, migration, and invasion of RA-FLSs | [71] |
| RAW264.7 macrophages | LPS induction | TanIIA | 0.1-10 μM | 30 min prior to LPS treatments | TLR4, COX-2, IL-1β, TNF-α (↓) | Alters microRNA profiles and reduces proinflammatory gene expression | [72] | |
| Bone marrow-derived endothelial progenitor cells from rats | TNF-α induction | TanIIA | 1-20 μM | 18 h prior to TNF-α stimulation | VCAM-1, ICAM-1 (↓) p-NF-κB, p-IκB (↓) |
Inhibits the adhesion of endothelial progenitor cells | [74] | |
| RAW264.7 macrophages | LPS induction | TanIIA | 0.1-10 μM | 24 h | TLR4, HMGB1, iNOS (↓) IL-1β, TNF-α (↓) Arg1, FIZZ1, CEBP (↑) IL-10 (↑) |
Elongates RAW264.7 cells, reduces mitochondrial Ca2+ level, and promotes M2 phenotypes. | [77] | |
|
| ||||||||
| Antifibrotic | Neonatal cardiac fibroblasts | TGF-β1 stimulation | TanIIA | 1-100 μM | 2 h prior to TGFβ1 stimulation | Fibronectin (↓) p-SMAD2/3 (↓) |
Blocks nuclear translocation of pSmad-2/3 | [83] |
| Rat cardiac fibroblasts | X-rays irradiation | STS | 10 μM | 1 h prior to irradiation | GRP78, CHOP (↓) ROS, IGF-1, p-SMAD2/3, Collagen-1 (↓) |
Reduces irradiation-driven ROS generation and ER stress | [147] | |
| Rat atrial fibroblasts | TGF-β1 stimulation | STS | 3-30 μM | 30 min prior to TGF-β1 treatments | p-SMAD2/3, p-ERK1/2 (↓) Collagens, α-SMA (↓) |
Reduces TGF-β1-induced fibrotic markers | [99] | |
| Rat cardiac fibroblasts | Ang II stimulation | STS | 3-30 μM | 30 min prior to Ang II treatments | p47phox (↓) Col1 (↓) MMP-1 (↑) |
Attenuates Ang II-induced collagen expression and ROS generation | [85] | |
| Rat cardiac fibroblasts | TGF-β1 stimulation | STS | 10-100 μM | Pretreat | p-SMAD3 (↓) SMAD7 (↑) CTGF, COLI, α-SMA, vimentin (↓) |
Reduces TGF-β1-driven fibrotic markers | [84] | |
| Human astrocytoma U-87MG cells | None | TanIIA | 1-50 μM | 24-96 h | Notch-1, Casp-3/9 (↑) p-Myc, p-MMP-9, p-Bcl2 (↓) |
Reduces proliferation and migration, but promotes cell death | [107] | |
| HUVEC cells | Cyclic strain | TanIIA | 1-10 μM | Cotreatment | ET-1 (↓) eNOS (↑) ATF3 (↑) |
Attenuates cyclic strain-induced ET-1 expression | [124] | |
| Mouse neural stem cells (C17.2), Rat pheochromocytoma cells (PC12), embryonic Corticalneural stem cells |
None | TanIIA | 0.01-3 μM | 1-7 d | BDNF, NGF, GAP-43 (↑) p-MAPK42/44, p-CREB (↑) CAV-1 (↑) |
Promotes neural differentiation | [134] | |
Table 2.
Summarized in vivo mechanism of action of tanshinone IIA and STS.
| Biological activity | Animal | Disease model | Drug used | Drug dose | Timing | Key regulated factors | Physiological effect | Ref. |
|---|---|---|---|---|---|---|---|---|
| Antioxidative | Male Sprague–Dawley rats | High cholesterol diet-driven atherosclerotic calcification | TanIIA | 35-70 mg/kg, oral | 12 weeks | Cu/Zn SOD (↑) LDL (↓) Superoxide anion (↓) |
Reduces atherosclerotic calcification | [148] |
| Male–Sprague Dawley rats | Two-kidney, two-clip (2K2C) hypertensive rats | TanIIA | 35-70 mg/(kg·d), oral | 6 weeks (starting from 4 weeks after surgery) |
Superoxide (↓) NOX2, NOX4, p47phox (↓) |
Reduces myocardial fibrosis, cardiac hypertrophy and dysfunction | [149] | |
| Male C57BL/6 mice | LPS-induced cardiac fibrosis | TanIIA | 10 mg/(kg·d), i.p. | 2 weeks | gp91phox (↓), p67phox (↓) Collagens (↓), MMP2/9 (↓) TIMP1/2 (↓) |
Suppresses cardiac fibrosis | [150] | |
| Male Sprague–Dawley rats | Isoproterenol-induced myocardial infarction | STS | 4-16 mg/kg, i.v. | 7 d (prior to ISO injection) |
p-ERK1/2 (↓) SOD, GSH, GPx (↑) Nrf2, HO-1 (↑) AMPK/CPT-1 (↓) |
Maintains the levels of circulating lipids and stabilizes cardiac functions | [64] | |
| Male ICR mice | Acute pancreatitis (caerulein, taurocholate injection) | STS | 25 mg/kg, i.p. | 2 h prior to surgery | Nrf2 (↑) | Ameliorates acute pancreatitis | [66] | |
| Male C57BL/6 mice | Lewis lung carcinoma + intermittent hypoxia | STS | 10 mg/(kg·d), i.p. | 5 weeks (starting from 5-7 d After tumor implantation) |
Nrf2 (↑) MDA, SOD (↓) NF-κB (↓) |
Reduces tumor size driven by intermittent hypoxia | [65] | |
|
| ||||||||
| Anti-inflammatory | Male C57BL/6 mice | Adjuvant-induced arthritis (AIA) | TanIIA | 30 mg/(kg·d), intragastric | 2-31 d After arthritis induction |
IL-6, IL-17, TNF-α (↓) | Ameliorates arthritis severity in AIA models | [71] |
| Male ApoE−/− mice | High-fat diet | TanIIA | 10-90 mg/(kg·d), gavage | 13-26 weeks | TLR4, MyD88, NF-κB (↓) MCP-1, TNF-α (↓) |
Reduces atherosclerosis severity, stabilizes plaque, and decreases the blood lipid levels | [73] | |
| Male beagle dogs | Occlusion of left anterior descending | STS | 1.3-5.2 mg/kg, i.v. | 15 min After occlusion |
TXNIP (↓), NLRP3 inflammasome, IL-18 (↓), p-JAK2, p-STAT3 (↓) SOSC3 (↑), p-insulin receptor, p-AkT, p-ERK1/2 (↑), PPARα (↑) | Reduces myocardial infarct size and attenuates inflammatory cells infiltration | [76] | |
|
| ||||||||
| Anti-fibrotic | Spontaneously hypertensive rats | None | TanIIA | 1-10 mg/(kg·week), i.p. | From 21 weeks | cTn-I, ADMA, Col1a1, Col3a1 (↓), NOX4 (↓), eNOS, NO (↑), Cys-C/Wnt signaling (↓) | Reduces systolic blood pressure and cardiac fibrosis in spontaneously hypertensive rats | [120] |
3. Oxidative Stress and Endothelial Dysfunction in Tissue Inflammation and Fibrosis
3.1. Oxidative Stress in Tissue Inflammation and Fibrosis
Reactive oxygen species (ROS) are essential components of metabolism, immune responses, and cell signaling. However, excessive ROS threatens the normal function of cells. For example, oxidative stress damages DNA and alters protein structure into misfolded forms, causing stress to the cells and leading to cell senescence, apoptosis, or even necrosis [41, 42]. In endothelial cells, the nicotinamide adenine dinucleotide phosphate (NADPH) oxidase system (NOX) is the major source of ROS production, determined by NOX4 RNA within the cells [43]. In addition to endothelial cells, other cell types such as fibroblasts and leukocytes also express NOXs [44]. Notably, other enzymes such as cyclooxygenases, cytochrome P450 enzymes, and lipoxygenases, as well as organelles such as the endoplasmic reticulum (ER) and peroxisomes, generate ROS [41, 43].
NOX expression can be triggered by physical stimuli, such as shear stress, growth factors, cytokines, and metabolic factors such as hyperglycemia. Downstream metabolites include (1) ONOO− free radicals from NO oxidation, (2) increased ICAM/VCAM expression, (3) increased intracellular Ca2+ levels activating the MAPK and Akt pathways, and (4) increased collagen disposition [44].
ROS promote vascular fibrosis through several mechanisms. In fibroblasts, NOX4 promotes survival but suppresses cell death in models such as hypoxia [44, 45]. Furthermore, ROS and its generators, such as H2O2 and xanthine oxidase, reduced the RNA level of procollagens but boosted MMP-2, MMP-9, MMP-13, and fibronectin in cardiac fibroblasts [46]. In endothelial cells, NOX2 promotes endothelial-mesenchymal transition in the heart interstitium, worsening cardiac fibrosis if NOX2 transgenic mice were treated with angiotensin II (AngII) [47]. Finally, the ROS-generating enzyme gp91phox boosts ET-1 expression in vascular fibroblasts under AngII stimuli [48], indicating the involvement of oxidative stress in endothelial dysfunction and fibroblast activation. Oxidative stress-mediated endothelial dysfunction has recently been linked to the pathogenesis of COVID-19 [49]. This may explain the cardiovascular complications of COVID-19, considering the role of ROS in immune responses and tissue inflammation [50].
3.2. Endothelial Dysfunction Promotes Chronic Inflammation and Tissue Fibrosis
Endothelial cells are critical regulators of vascular tension. Mechanistically, the endothelium releases vasoconstrictors such as endothelin-1 (ET-1) and vasodilators such as nitric oxide (NO) [7]. Endothelial dysfunction describes a series of imbalanced endothelial regulations concerning redox status, vascular contraction, inflammation, and coagulation, leading to reduced elasticity of blood vessels and enhanced development of vascular plaque [51].
The endothelium of blood vessels plays a critical role in inflammatory responses. For example, NO suppresses ET-1 expression and TNF-driven NF-κB activation in endothelial cells [52]. Endothelial cells regulate inflammation through several mechanisms. First, the transmigration of leukocytes requires cell–cell interactions between leukocytes and endothelial cells. ICAM-1 is induced on the endothelial surface and binds to integrins (such as CD11b, CD18, and LFA-1) on the surface of leukocytes upon challenge with inflammatory stimuli [53]. Furthermore, activated NF-κB in endothelial cells upregulates VCAM-1, which binds to very late antigen-4 (VLA-4, composed of CD49d and CD29) to recruit leukocytes [54]. Mechanical shear stress also synergizes with inflammatory cytokines to boost E-selectin expression, increasing the affinity of neutrophils to cultured human umbilical vein endothelial cells (HUVECs) [55]. Second, endothelial cells express chemokines required for leukocyte recruitment [56]. A classic example is interleukin-8, which is expressed in endothelial cells cultured under plaque-forming physical conditions [57]. Targeting the dysregulation of endothelial inflammation may be a potential treatment strategy for tissue fibrosis [58].
Below, we summarize the mechanism of action of TanIIA in the regulation of endothelial dysfunction and its potential implications for treating tissue inflammation and fibrosis (Figure 3 and Tables 1 and 2).
Figure 3.
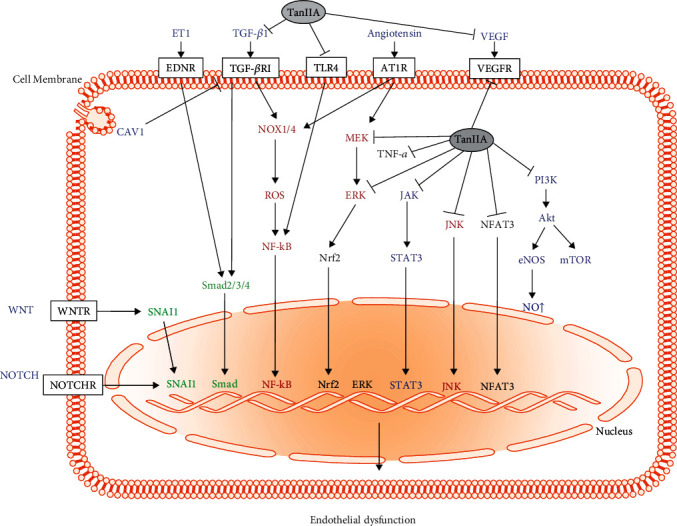
Summarized mechanism of action of tanshinone IIA in the prevention of endothelial dysfunction. Arrows (→) indicate the route of signaling involved in endothelial dysfunction. Factors suppressed by TanIIA are linked with (─┤). Endothelial dysfunction is a combination of oxidative stress, inflammation, and fibrosis of vascular endothelium. Aside from the antioxidative activity, TanIIA inhibits inflammatory signaling like the TLR4-NF-κB axis and the MAPK pathway. TanIIA also blocks profibrotic components like TGF-βR1 and AT1R and the Wnt, Notch, and ET-1 signaling pathways.
4. Mechanism of Action of TanIIA in Tissue Inflammation and Fibrosis
4.1. Antioxidation
The antioxidative activity of Salvia miltiorrhiza is well documented in cardiovascular diseases and anticancer therapy [59, 60]. Previous studies have pointed out that TanIIA quenches ROS through several mechanisms. First, TanIIA suppresses lipid peroxidation and DNA damage in mitochondria in the liver and heart [61, 62]. Notably, TanIIA directly scavenges adriamycin semiquinone free radicals when treated in heart homogenates in vitro [62]. In mitochondria, TanIIA can accept one electron from NADH dehydrogenase in complex I, which can be transferred to oxygen molecules or cytochrome c [63]. TanIIA also triggers redox-sensitive ERK/Nrf2/HO1 and AMPK/ACC (acetyl-coenzyme A carboxylase)/CPT1 (carnitine palmitoyltransferase-1) pathways, governing cell signaling through modulation of redox equilibrium [64].
The antioxidative effect of TanIIA has therapeutic potential in diseases other than CVD. For example, TanIIA reduced oxidative stress in the serum of tumor-bearing mice by combining intermittent hypoxia. TanIIA promotes apoptosis of tumor cells, which may be related to the activation of Nrf2 [65]. Similar protective effects were also observed in mice with experimental pancreatitis [66]. TanIIA shared protective signaling with the ROS scavenger NAC [66]. TanIIA may also contribute to antioxidative activity through other routes. For example, TanIIA induces the cAMP pathway to boost the expression of cystathionine-lyase C (CSE), which synthesizes hydrogen sulfide (H2S) and promotes antioxidative activity in HUVECs [67]. Additionally, TanIIA induced the lncRNA AK003290 in primary myocardial tissue from I/R-injured mice. AK003290 sponges the miRNA miR-124-5p and suppresses proapoptotic proteins such as BAX and ROS in cardiomyocytes. However, how TanIIA boosts lncRNA and how lncRNA regulates ROS levels are still elusive [68]. Our recent study revealed that Salvia miltiorrhiza aqueous extract could alleviate ROS-dependent cell apoptosis in adriamycin-induced cardiomyopathy [69], which further supports the antioxidative activity.
4.2. Anti-Inflammation
Tanshinones possess anti-inflammatory effects and ameliorate many diseases. For example, tanshinone I possesses potency similar to that of celecoxib to suppress IL-1β-driven chondrocyte apoptosis, inflammation, and extracellular matrix degradation in cellular models and protects against bone erosion in an osteoarthritis model [70]. TanIIA also ameliorates the invasiveness of RA fibroblast-like synoviocytes by suppressing the PI3K-Akt, MAPK, and HIF-1α signaling pathways, which are downstream of TNF-α [71].
TanIIA also suppresses inflammatory responses in blood vessels, which are known to exacerbate endothelial dysfunction, atherosclerotic plaque formation, and vascular injury. The NF-κB pathway, a potent proinflammatory circuit, can be inhibited by TanIIA, leading to the reduction of inflammatory mediators such as MCP-1, TGF-β, and TNF-α. The suppression of these cytokines also reduced macrophage infiltration into the infarcted myocardium [72, 73]. NF-κB blockade also prevents the adhesion of TNF-α-driven endothelial progenitor cells (EPCs) by lowering the expression of ICAM-1 and VCAM-1 on the cell surface [74]. In addition, the NLRP3 inflammasome releases mature IL-1β and IL-18 in response to oxidative stress and danger signals upon myocardial infarction [75]. TanIIA was shown to block the NLRP3 inflammasome in a canine myocardial infarction model by restoring JAK-STAT and insulin signaling in the heart [76]. Moreover, a recent study found that TanIIA skews macrophages toward the M2 phenotype in vitro, boosting markers such as Fizz-1, Arginase-1, and CD206. The M2 polarization effect may be exerted by inhibition of the TLR4-HMGB1/CEBP-β pathway and reduction of miR-155 [77]. TanIIA exerts anti-inflammatory activity by blocking NF-κB and NLRP3 inflammasomes but restores other signaling pathways.
4.3. Inhibition of Canonical TGF-β-Mediated Fibrotic Pathway
TGF-β binds to a heterodimeric receptor composed of TGF-βRI and TGF-βRII. Upon TGF-β activation, TGF-βRI is phosphorylated by the kinase activity of TGF-βRII, providing a docking site for Smad2/3. Smad2/3 is phosphorylated by TGF-βRI and then binds to the co-Smad-like Smad4, which forms a complex and then translocates into the nucleus [78]. Profibrotic genes are actively transcribed once the Smad complex interacts with the Smad-binding element [79]. The Smad2/3 complex induces Foxm1, which boosts the expression of Snail [80]. Snail, Twist, and Slug are key transcription factors that suppress endothelial markers but boost mesenchymal markers [81]. For example, Snail suppresses E-cadherin and occludin expression but elevates mesenchymal markers FSP-1 and α-SMA (alpha-smooth muscle actin) [82]. In contrast, there is also a regulatory pathway mediated by Smad7, which blocks signal transduction from TGF-βRI [78].
TanIIA controls fibrosis by interfering with the Smad-dependent TGF-β pathway. In rat cardiac fibroblasts, TanIIA inhibits the phosphorylation of Smad2/3, leading to the reduction of nuclear translocation of Smads and downregulated expression of fibronectin genes [83, 84]. Similar regulatory mechanisms have also been found in STS-treated human atrial fibroblasts. In detail, TanIIA reduced the protein expression level of fibroblastic markers such as α-SMA, collagen type I and III, periostin, and TGF-β but elevated matrix metalloproteinase-1 (MMP-1) in AngII-treated cardiac fibroblasts [85–87], suggesting that TanIIA suppresses cardiac fibrosis [27].
The suppressive effect of TanIIA on Smad phosphorylation may be Nrf2-dependent [88]. TanIIA also demonstrated protective effects in animal models through Smad regulation. For example, TanIIA also protects against cardiac hypertrophy in hypertensive rats by downregulating Smad3 but upregulating Smad7 [89]. TanIIA-containing “Shensong Yangxin Capsule” suppresses Smad3 phosphorylation but boosts Smad7 expression, reduces cardiac fibrosis, and partly rescues cardiac function in the diabetic rodent model [90]. Notably, the antifibrotic effect of TanIIA can be expanded to other models. Recently, TanIIA was found to ameliorate silica-induced pulmonary fibrosis through the removal of triggering of the Nrf2 pathway, dephosphorylation of Smad3, and elevation of Smad7 [91–93].
4.4. Inhibition of Noncanonical TGF-β-Mediated Pathways
In addition to Smad phosphorylation, TGF-β also transmits noncanonical signals through the phosphorylation of MAPK family members, PI3K, Rho A, Rac, c-Abl, and PKC. Downstream genes include Snail1, Snail 2, and Twist1 [79]. For example, TGF-β2 drives EndMT in human cutaneous microvascular endothelial cells via collaborating with the Smad, MEK, PI3K, and p38 MAPK signaling pathways [81]. JAK2 also promotes pulmonary vascular remodeling through Smad3 sensitization in idiopathic pulmonary fibrosis models [94].
STS has been shown to inhibit AngII-induced cardiomyocyte hypertrophy and lower systolic blood pressure in cultured neonatal rat myocardial cells in vitro and in rats in vivo, mainly through the suppression of c-Fos, c-Jun, ERK, and MEK [95–98]. The antifibrotic activity of STS has been demonstrated to inhibit TGF-β1-activated human atrial fibroblast-to-myofibroblast differentiation by suppressing both phosphorylation of Smad3 and ERK1/2 [99], suggesting that the antifibrotic functions of STS are tightly linked to its antioxidant activity. Notably, the antifibrotic effect of TanIIA is not limited to the cardiovascular system. A review article has summarized the antifibrotic effects of TanIIA on organs such as the lungs, kidneys, uterus, peritoneum, and retina [31].
4.5. Notch Pathway
The Notch pathway is essential for the development of vascular smooth muscle [100] and cardiac valves [101] in the embryonic stage. There are five transmembrane ligands (Jagged (Jag) 1 and Jag2, and Dll (delta-like) 1, Dll3, and Dll4) and four Notch receptors (Notch-1, Notch-2, Notch-3, and Notch-4) expressed on the cell surface. Binding of the ligand and Notch receptor triggers cleavage of the extracellular domain by ADAM and release of the Notch intracellular domain (NICD) by gamma-secretase, respectively. When released from the plasma membrane, NICD translocates into the nucleus and binds to the CSL (CBF1/Su(H)/LAG1) complex and MAML (mastermind-like protein), promoting the transcription of downstream genes such as NF-κB, Akt, and p21 [78, 102]. In a fully grown cardiovascular system, Notch may promote EndMT in cardiovascular ECs [103]. For example, Notch-1 activation by Jag-1 induces Ca2+-sensing receptor (CASR) expression in pulmonary arterial smooth muscle cells (PASMCs), sensitizing right ventricular myocardial fibrosis in hypoxia-driven pulmonary hypertension rodent models [104]. By using hESC-derived endothelial cells, high-density culture activates Notch by Dll4 and Jag-1 expressed in other cells, promoting EndMT markers such as SMA, while suppressing CD31 expression [105].
TanIIA is known to suppress tumor progression through activation of the Notch signal pathway [106]. At an in vitro dosage of 1–50 μM, TanIIA limits the growth, migration, and invasion of astrocytoma cells. At the molecular level, TanIIA boosts the expression of Notch-1 and caspase-3/9 but reduces the phosphorylation of c-Myc, MMP-9, and Bcl2 [107]. In the gastric cancer cell line SGC7901, TanIIA limits tumor proliferation and migration through suppression of FOXM1, a transcription factor that governs cell fate and promotes tumor progression and metastasis in multiple cancers [108]. Moreover, TanIIA protects spinal cord endothelium stability in injury models. In detail, STS at 3–10 μM was found to promote the survival of spinal cord endothelial cells (SCMEC) in an oxygen-glucose deprivation model by boosting Notch signaling. In this case, Notch upregulation decreases inflammatory cytokines such as IL-6, TNF, and IL-1β in SCMECs. In the murine spinal cord injury model, STS can (1) boost the expression of Notch, (2) rescue the microvessel, (3) maintain the blood-spinal cord barrier, and (4) protect the structural and functional integrity of the nervous system [109]. However, whether the TanIIA-driven activation of Notch is beneficial or harmful for cardiovascular disorders is still uncertain. Further investigation of the TanIIA-Notch-EndMT crosstalk is required.
4.6. Wnt Pathway
There are more than 10 Wnt genes in mammals, which share a conserved feature: palmitoylation on their polypeptide chain [110]. The lipid moiety is critical for the binding of Wnt proteins to frizzled receptors, a group of seven transmembrane proteins that provide a cysteine-rich ligand-binding site [111]. Without the Wnt ligand, the key signaling protein β-catenin is constantly quenched by a destruction complex, comprising proteins such as disheveled and GSK-3β, and then subjected to proteasomal degradation. Upon Wnt signal activation, β-catenin accumulates and translocates into the nucleus [112]. In the nucleus, β-catenin binds to T cell factors (TCFs) to induce the transcription of genes such as Twist [103]. Wnt signaling is crucial in the heart development, such as Wnt-β-catenin signaling is crucial for heart cushion formation through EndMT activity [113]. Nevertheless, Wnt signaling may result in pathogenic fibrosis in the adult heart. Wnt activation favors EndMT in the heart, which may be the cause of cardiac fibrosis. For example, increased Wnt ligands were found in the injured murine heart after acute myocardial infarction. In addition, GSK-3 inhibition and LAD ligation boost the endothelial expression of β-catenin, TCFs, and SMA in vitro and in vivo, respectively [114]. In clinical settings, cardiac samples from patients with idiopathic dilated cardiomyopathy (DCM) exhibit higher levels of Wnt, β-catenin, and snail expressions than those from normal subjects. The results fit the elevation of fibrosis markers such as SMA and FSP-1 in DCM patients [115].
The therapeutic potential of the TanIIA-Wnt axis can be found in skin transplantation and cancer studies. At a dosage of approximately 5 μM, TanIIA ameliorates ischemic skin flap mice by inducing the expression of β-catenin and stem cell markers such as SOX2, Nanog, and OCT4 in epidermal cells [116]. Nevertheless, at a dosage of 20 μM, STS inhibited the expression levels of COX-2, β-catenin, and VEGF, resulting in growth inhibition of HC8693 colon cancer cells [117]. The regulatory role of TanIIA in the Wnt pathway seems contradictory, and the dosage and cell type should be taken into account.
TanIIA exerts protective effects by boosting the Wnt pathway in endothelial cells. For example, STS (~10 μM) rescues the expression of β-catenin and the phosphorylation of GSK-3β in high-glucose-treated HUVECs. In contrast, STS treatment reduced apoptosis and the expression of CXCL1 in HUVEC [118]. Further improvement of HUVEC survival, inflammatory suppression, and production of NO can be found when STS is combined with ghrelin [119]. However, the antifibrotic effect of TanIIA can be demonstrated in spontaneously hypertensive rats (SHRs) by inhibiting the Wnt signaling pathway [120]. Intraperitoneal injection of 1 or 10 mg/kg TanIIA reduced cardiac Wnt2, β-catenin, and WISP-1. In addition, TanIIA readily reduces hypertrophy and fibrosis of the heart, especially the left ventricle. At the molecular level, TanIIA reduced the mRNA levels of Col1a1 and Col3a1. Moreover, TanIIA reduced cardiac injury markers such as troponin, NOX4, and ADMA, but elevated cardioprotective NO and eNOS. Functionally, TanIIA controls systolic blood pressure in SHR animals, regardless of dosage [120]. Although TanIIA is effective in many cardiovascular disorder models, it may not exert a simple molecular action to raise or lower the activity of the Wnt pathway. The therapeutic effect of Wnt regulation may depend on the cell type and disease conditions.
4.7. ET-1 Pathway
Endothelin-1 (ET-1) is encoded by EDN1. Full-length preproendothelin-1 is processed by furin, chymase, and neprilysin cleavage, leaving a 21-amino-acid peptide. ET-1 is the most potent member of the three endothelins found in mammals, largely derived from vascular endothelial cells in all types of blood vessels, although other cells such as macrophages, enteric glial cells, and some neurons express ET-1 as well. In addition to a constant expression, ET-1 in endothelial cells can be further boosted by pathophysiological stimuli. After release from the endothelium, ET-1 largely binds to a GPCR named ETA, but less so to the other GPCRs, ETB [121, 122]. ETA activation results in vasoconstriction of smooth muscle cells, making ET-1 a major culprit in cardiovascular disorders. However, the autocrine action of ET1 binds to ETB on endothelial cells, leading to the induction of the vasodilator NO. This may serve as a feedback mechanism to reduce vasoconstriction [121, 122]. The downstream pathway of ET includes phosphatidylinositol-specific phospholipase C (PI-PLC), digesting PIP2 into IP3 and diacylglycerol (DAG). IP3 induces cytosolic Ca2+ and then cell contraction, and DAG activates protein kinase C (PKC) signaling. In addition, ET activates RTK like Ras, transmitting the signal through RAF/MEK/MAPK [123].
TanIIA suppresses ET-1-driven endothelial dysfunction at both the cellular and organ levels. In endothelial cells, TanIIA inhibits the expression of ET-1 in HUVEC under cyclic strain [124]. In smooth muscle cells, TanIIA reduced smooth muscle proliferation by blocking the ET-1/PDK1/AKT pathway [125]. In addition, TanIIA reduced cardiac fibroblast expansion by inhibiting AngII-driven ERK phosphorylation [126]. In chronic intermittent hypoxia models, TanIIA reduces ET-1 and ETA expressions but elevates ETB expression in the heart and aorta, thereby controlling blood pressure and ameliorating apoptosis and fibrosis of myocardium and blood vessels [127, 128].
4.8. CAV-1 Pathway
Caveolin-1 (CAV-1) is essential for the formation of caveolae and secretion of substances in the apical direction of vesicles. CAV-1 oligomerizes and is coated onto the surface of invaginated membrane structures [129]. In the vascular endothelium, CAV-1 is required for the transportation of LDL from the lumen to the vessel walls, forming atherosclerotic plaques [130, 131]. Genetic ablation of CAV-1 results in several physiological alterations. First, the lack of CAV-1 increased NO release and desensitized AngII, ET-1, and PMA-driven vasoconstriction, providing insight into the role of CAV-1 in maintenance of vascular stress [132]. Moreover, CAV-1 siRNA suppressed lipid transcytosis through the epithelium and diminished VCAM-1 expression and phosphorylation of NF-κB p65 [131]. CAV-1 and TGF-β crosstalk can be found in vascular smooth muscle cells (VSMCs). CAV-1 is phosphorylated by c-Src upon TGF-β stimulation. Y14-phosphorylated CAV-1 activates Rho-GTP/ROCK signaling, which inhibits PTEN and phosphatase PPM1A. As a result, Smad2/3 phosphorylation is maintained and PAI-1 expression is increased in atherosclerotic plaques [133].
TanIIA is known to control the downstream TGF-β pathway. Whether TanIIA directly controls the activity of CAV-1 in endothelial cells or VSMCs remains unknown. A previous study suggested the role of TanIIA in CAV-1-related endocytosis. In neuron progenitor cell lines, CAV-1 is essential for the endocytosis of TanIIA, promoting its biological activity in boosting MAPK42/44 and CREB activities. Furthermore, the entry of TanIIA elevates the expression levels of brain-derived neurotrophic factor (BDNF) and nerve growth factor (NGF) in these cell lines and promotes neuronal differentiation [134].
4.9. Other Mechanisms
As a broad-spectrum therapeutic compound, TanIIA also protects cardiovascular function through other mechanisms. In the hearts of the canine MI model, TanIIA restored PPAR-alpha expression but limited lipid accumulation [76]. TanIIA was found to protect against myocardial injury through boosting the expression of Bim, CHOP, and PDCD4 proteins and maintaining the phosphorylation of Akt, thus preventing apoptosis of cardiac tissues [135, 136]. Moreover, TanIIA-loaded nanoparticle was found to penetrate the blood–brain barrier and demonstrated a preventive effect against cerebral ischemia/reperfusion injury in rat models [137]. TanIIA was also found to reduce the expression and membrane translocation of intracellular chloride channel 1 (CLIC1), which is known responding to cellular oxidative stress and induces the expression of inflammatory cytokines. Furthermore, TanIIA treatment attenuates inflammatory cytokine expression, cellular ROS levels, ICAM-1/VCAM-1 expression, and atherosclerotic plaque formation [138]. TanIIA has also been shown to regulate vascular fibrosis through KLF4, which suppresses vascular remodeling, and consequently attenuates vascular neointimal hyperplasia in left common carotid artery-ligated mice [139]. Finally, a meta-analysis focusing on Salvia miltiorrhiza for the treatment of coronary heart disease was conducted after integrating the protein–protein interaction data. Tanshinones are proposed to regulate blood circulation through guanylate cyclase soluble subunit alpha-1 and guanylate cyclase soluble subunit beta-1 [140], thus providing insights for future studies on the regulatory effects of TanIIA.
5. Our Perspective
Recently, the medicinal value of Tan IIA, especially water-soluble STS, has been intensively explored. Cardiovascular application of TanIIA in combination with classical treatments dominates recent clinical trials. TanIIA has been found to improve the prognosis of coronary artery diseases by reducing injury markers, such as cardiac troponin-I, and the incidence of major adverse cardiovascular events [141–143]. As TanIIA inhibits high-sensitivity C-reactive protein (hs-CRP) and cytokines, such as MCP-1, in the blood, the anti-inflammatory role of TanIIA should be a key factor in ameliorating disease progression [141]. TanIIA also promotes vascular elasticity. Sanghuang–Danshen, a mixture of drugs containing TanIIA, reduced blood pressure and arterial stiffness in healthy smokers [144]. A recent meta-analysis revealed that TanIIA reduces blood pressure in patients with hypertensive nephropathy and improves renal function due to cardiac-renal crosstalk [145]. As detailed in the previous sections, cell and animal studies suggest that the antioxidative, anti-inflammatory, and antifibrotic effects of TanIIA may be the major contributing factors to its protective effect. The better understanding on the mechanism of action of these pathways may lead to identification of novel applications of TanIIA.
6. Conclusion
Taken together, TanIIA, which possesses multifaceted roles involving antioxidation, anti-inflammation, and antifibrosis, could be a key component of Salvia miltiorrhiza with substantial therapeutic potential for cardiovascular disorders (Tables 1 and 2). In this review, we have summarized how TanIIA mediates its therapeutic activities against cardiovascular disorders via the suppression of endothelial dysfunction, tissue inflammation, and fibrosis through multiple signaling pathways. An improved understanding of the action of TanIIA in the amelioration of endothelial cell dysfunction may help to shed light on the molecular mechanisms and clinical implications of TanIIA for treating cardiovascular diseases associated with endothelial dysfunction and fibrosis.
Acknowledgments
This study was funded by the Ministry of Science and Technology (MOST) of Taiwan (108-2320-B-182-022-MY2 and 109-2320-B-182-024-MY2 to Y-C.H.) and the Chang Gung Medical Foundation (CMRPG8K1151 and CMRPG8M0461 to W-Y.C.).
Abbreviations
- ACC:
Acetyl-coenzyme A carboxylase
- ADAM:
A disintegrin and metalloproteinase
- ADMA:
Asymmetric dimethylarginine
- Akt:
Protein kinase B, AKT serine/threonine kinase
- AMPK:
AMP-activated protein kinase
- AngII:
Angiotensin II
- ATF3:
Activating transcription factor 3
- Bcl2:
B cell lymphoma 2
- BDNF:
Brain-derived neurotrophic factor
- Bim:
Bcl-2-like protein 11
- cAMP:
Cyclic AMP
- CAV-1:
Caveolin-1
- CEBP-β:
CCAAT/enhancer-binding protein beta
- CHOP:
C/EBP homologous protein
- CLIC1:
Intracellular chloride channel 1
- c-Myc:
Cellular Myc protooncogene
- COVID-19:
Coronavirus disease 2019
- CPT1:
Carnitine palmitoyltransferase-1
- CSE:
Cystathionine γ-lyase
- CSL:
CBF1/Su(H)/LAG1
- cTn:
Cardiac troponin
- DAG:
Diacylglycerol
- DCM:
Dilated cardiomyopathy
- Dll:
Delta-like
- EndMT:
Endothelial-to-mesenchymal transition
- eNOS:
Endothelial nitric oxide synthase
- ER:
Endoplasmic reticulum
- ERK:
Extracellular signal-regulated kinase
- ET-1:
Endothelin-1
- ETA and ETB:
Endothelin receptors, type-A and type-B
- Fizz1:
Resistin-like alpha
- FOXM1:
Forkhead box protein M1
- FSP-1:
Fibroblast-specific protein 1
- GAP43:
Growth-associated protein 43
- GSH:
Glutathione
- GSK:
Glycogen synthase kinase
- GUCY1A1:
Guanylate cyclase soluble subunit alpha-1
- GUCY1B1:
Guanylate cyclase soluble subunit beta-1
- H2S:
Hydrogen sulfide
- hESCs:
Human embryonic stem cells
- HIF-1α:
Hypoxia-inducible factor 1-alpha
- HMGB:
High-mobility group box
- HO-1:
Heme oxygenase-1
- HUVECs:
Human umbilical vein endothelial cell
- ICAM:
Intercellular adhesion molecule
- IGF-1:
Insulin-like growth factor-1
- IL-8:
Interleukin-8
- JAK:
Janus kinases
- JNK:
c-Jun N-terminal kinases
- KLF4:
Kruppel-like factor 4
- LAD:
Left anterior descending coronary artery
- LDL:
Low-density lipoprotein
- LFA-1:
Lymphocyte function-associated antigen 1, integrin, alpha L
- lncRNA:
Long noncoding RNA
- MAML:
Mastermind-like protein
- MAPK:
Mitogen-activated protein kinase
- MCP-1:
Monocyte chemoattractant protein-1, CCL2
- MDA:
3,4-Methylenedioxyamphetamine
- MEK:
MAPK/ERK kinase
- MI:
Myocardial infarction
- MMP:
Matrix metalloproteinase
- mTOR:
Mammalian target of rapamycin
- MyD88:
Myeloid differentiation primary response 88
- NADPH:
Nicotinamide adenine dinucleotide phosphate
- NF-κB:
Nuclear factor kappa B
- NGF:
Nerve growth factor
- NICD:
Notch intracellular domain
- NLRP3:
NOD-, LRR-, and pyrin domain-containing protein 3
- NO:
Nitric oxide
- NOX:
NADPH-oxidase
- Nrf2:
Nuclear factor erythroid 2-related factor 2
- OCT4:
Octamer-binding transcription factor 4
- ONOO−:
Nitrate free radical
- PAI-1:
Plasminogen activator inhibitor 1
- PDCD4:
Programmed cell death 4
- PDK1:
Phosphoinositide-dependent kinase-1
- PI3K:
Phosphoinositide 3-kinase
- PI-PLC:
Phosphatidylinositol-specific phospholipase C
- PKC:
Protein kinase C
- PPAR:
Peroxisome proliferator-activated receptor
- PPM1A:
Protein phosphatase, Mg2+/Mn2+-dependent 1A
- PTEN:
Phosphatase and tensin homolog
- RAF:
Rapidly accelerated fibrosarcoma
- ROCK:
Rho-associated protein kinase
- ROS:
Reactive oxygen species
- SAPK:
Stress-activated protein kinase
- SMA:
Smooth muscle actin
- Smad:
Mothers against decapentaplegic homolog
- SOD:
Superoxide dismutase
- SOSC:
Suppressor of cytokine signaling 1
- SOX2:
SRY- (sex determining region Y-) box 2
- STAT:
Signal transducer and activator of transcription proteins
- STS:
Sodium tanshinone IIA sulfonate
- TanIIA:
Tanshinone IIA
- TCF:
T cell factor
- TGF-β:
Transforming growth factor, beta
- TIMP:
Tissue inhibitor of metalloproteinases
- TLR:
Toll-like receptors
- TNF:
Tumor necrosis factor
- TNXNIP:
Thioredoxin-interacting protein
- VCAM:
Vascular cell adhesion protein
- VLA:
Very late antigen
- VSMC:
Vascular smooth muscle cell
- WISP1:
WNT1-inducible signaling pathway protein 1
- Wnt:
Wingless-related integration site
Contributor Information
Wei-Yu Chen, Email: wychen624@cgmh.org.tw.
Yu-Chiang Hung, Email: hungyuchiang@gmail.com.
Data Availability
No data were used to support this study.
Conflicts of Interest
The authors declare that there is no conflict of interest regarding the publication of this paper.
Authors' Contributions
Tsuo-Cheng Lu and Yi-Hsiu Wu contributed equally to this work and they are co-first authors.
References
- 1.Wang L., Ma R., Liu C., et al. Salvia miltiorrhiza: a potential red light to the development of cardiovascular diseases. Current Pharmaceutical Design . 2017;23(7):1077–1097. doi: 10.2174/1381612822666161010105242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li Z. M., Xu S. W., Liu P. Q. _Salvia miltiorrhiza_ Burge (Danshen): a golden herbal medicine in cardiovascular therapeutics. Acta Pharmacologica Sinica . 2018;39(5):802–824. doi: 10.1038/aps.2017.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shang Q., Xu H., Huang L. Tanshinone IIA: a promising natural cardioprotective agent. Evidence-based Complementary and Alternative Medicine . 2012;2012:7. doi: 10.1155/2012/716459.716459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu H. C., Liu H. H. Adverse reactions of tanshinone II (A) sodium sulfonate injection in treating 18 cases: an analysis of clinical features. Zhongguo Zhong xi yi jie he za zhi Zhongguo Zhongxiyi Jiehe Zazhi= Chinese Journal of Integrated Traditional and Western Medicine . 2013;33(9):1287–1289. [PubMed] [Google Scholar]
- 5.Li D., Wang J., Sun D., et al. Tanshinone IIA sulfonate protects against cigarette smoke-induced COPD and down-regulation of CFTR in mice. Scientific Reports . 2018;8(1):p. 376. doi: 10.1038/s41598-017-18745-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Scioli M. G., Storti G., D'Amico F., et al. Oxidative stress and new pathogenetic mechanisms in endothelial dysfunction: potential diagnostic biomarkers and therapeutic targets. Journal of Clinical Medicine . 2020;9(6):p. 1995. doi: 10.3390/jcm9061995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dagher O., Mury P., Thorin-Trescases N., Noly P. E., Thorin E., Carrier M. Therapeutic potential of quercetin to alleviate endothelial dysfunction in age-related cardiovascular diseases. Frontiers in Cardiovascular Medicine . 2021;8, article 658400 doi: 10.3389/fcvm.2021.658400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boudina S., Sena S., Theobald H., et al. Mitochondrial energetics in the heart in obesity-related diabetes: direct evidence for increased uncoupled respiration and activation of uncoupling proteins. Diabetes . 2007;56(10):2457–2466. doi: 10.2337/db07-0481. [DOI] [PubMed] [Google Scholar]
- 9.Rapoport R. M. Nitric oxide inhibition of endothelin-1 release in the vasculature: in vivo relevance of in vitro findings. Hypertension . 2014;64(5):908–914. doi: 10.1161/HYPERTENSIONAHA.114.03837. [DOI] [PubMed] [Google Scholar]
- 10.Cardillo C., Kilcoyne C. M., Cannon R. O., 3rd, Panza J. A. Interactions between nitric oxide and endothelin in the regulation of vascular tone of human resistance vessels in vivo. Hypertension . 2000;35(6):1237–1241. doi: 10.1161/01.HYP.35.6.1237. [DOI] [PubMed] [Google Scholar]
- 11.Papaconstantinou J. The role of signaling pathways of inflammation and oxidative stress in development of senescence and aging phenotypes in cardiovascular disease. Cells . 2019;8(11) doi: 10.3390/cells8111383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fan G. W., Gao X. M., Wang H., et al. The anti-inflammatory activities of Tanshinone IIA, an active component of TCM, are mediated by estrogen receptor activation and inhibition of iNOS. The Journal of Steroid Biochemistry and Molecular Biology . 2009;113(3-5):275–280. doi: 10.1016/j.jsbmb.2009.01.011. [DOI] [PubMed] [Google Scholar]
- 13.Gao S., Liu Z., Li H., Little P. J., Liu P., Xu S. Cardiovascular actions and therapeutic potential of tanshinone IIA. Atherosclerosis . 2012;220(1):3–10. doi: 10.1016/j.atherosclerosis.2011.06.041. [DOI] [PubMed] [Google Scholar]
- 14.Pang H., Wu L., Tang Y., Zhou G., Qu C., Duan J. A. Chemical analysis of the herbal medicine Salviae miltiorrhizae Radix et Rhizoma (Danshen) Molecules . 2016;21(1):p. 51. doi: 10.3390/molecules21010051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ma P., Liu J., Zhang C., Liang Z. Regulation of water-soluble phenolic acid biosynthesis in Salvia miltiorrhiza Bunge. Applied Biochemistry and Biotechnology . 2013;170(6):1253–1262. doi: 10.1007/s12010-013-0265-4. [DOI] [PubMed] [Google Scholar]
- 16.Shi M., Huang F., Deng C., Wang Y., Kai G. Bioactivities, biosynthesis and biotechnological production of phenolic acids in Salvia miltiorrhiza. Critical Reviews in Food Science and Nutrition . 2019;59(6):953–964. doi: 10.1080/10408398.2018.1474170. [DOI] [PubMed] [Google Scholar]
- 17.Zhou L., Zuo Z., Chow M. S. Danshen: an overview of its chemistry, pharmacology, pharmacokinetics, and clinical use. The Journal of Clinical Pharmacology . 2005;45(12):1345–1359. doi: 10.1177/0091270005282630. [DOI] [PubMed] [Google Scholar]
- 18.Ma P., Liu J., Osbourn A., Dong J., Liang Z. Regulation and metabolic engineering of tanshinone biosynthesis. RSC Advances . 2015;5(23):18137–18144. doi: 10.1039/C4RA13459A. [DOI] [Google Scholar]
- 19.Qi J. Y., Yu J., Huang D. H., et al. Salvianolate reduces murine myocardial ischemia and reperfusion injury via ERK1/2 signaling pathways in vivo. Chinese Journal of Integrative Medicine . 2017;23(1):40–47. doi: 10.1007/s11655-016-2621-z. [DOI] [PubMed] [Google Scholar]
- 20.Qiu H., Liu W., Lan T., et al. Salvianolate reduces atrial fibrillation through suppressing atrial interstitial fibrosis by inhibiting TGF-β1/Smad2/3 and TXNIP/NLRP3 inflammasome signaling pathways in post-MI rats. Phytomedicine . 2018;51:255–265. doi: 10.1016/j.phymed.2018.09.238. [DOI] [PubMed] [Google Scholar]
- 21.Ren J., Fu L., Nile S. H., Zhang J., Kai G. Salvia miltiorrhiza in treating cardiovascular diseases: a review on its pharmacological and clinical applications. Frontiers in Pharmacology . 2019;10:p. 753. doi: 10.3389/fphar.2019.00753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dong Y., Morris-Natschke S. L., Lee K. H. Biosynthesis, total syntheses, and antitumor activity of tanshinones and their analogs as potential therapeutic agents. Natural Product Reports . 2011;28(3):529–542. doi: 10.1039/c0np00035c. [DOI] [PubMed] [Google Scholar]
- 23.Zhong G. X., Li P., Zeng L. J., Guan J., Li D. Q., Li S. P. Chemical characteristics of Salvia miltiorrhiza (Danshen) collected from different locations in China. Journal of Agricultural and Food Chemistry . 2009;57(15):6879–6887. doi: 10.1021/jf901462s. [DOI] [PubMed] [Google Scholar]
- 24.Wang J. W., Wu J. Y. Tanshinone biosynthesis in Salvia miltiorrhiza and production in plant tissue cultures. Applied Microbiology and Biotechnology . 2010;88(2):437–449. doi: 10.1007/s00253-010-2797-7. [DOI] [PubMed] [Google Scholar]
- 25.Kai G., Xu H., Zhou C., et al. Metabolic engineering tanshinone biosynthetic pathway in salvia miltiorrhiza hairy root cultures. Metabolic Engineering . 2011;13(3):319–327. doi: 10.1016/j.ymben.2011.02.003. [DOI] [PubMed] [Google Scholar]
- 26.Xu S., Liu P. Tanshinone II-A: new perspectives for old remedies. Expert Opinion on Therapeutic Patents . 2013;23(2):149–153. doi: 10.1517/13543776.2013.743995. [DOI] [PubMed] [Google Scholar]
- 27.Zhou Z. Y., Zhao W. R., Zhang J., Chen X. L., Tang J. Y. Sodium tanshinone IIA sulfonate: a review of pharmacological activity and pharmacokinetics. Biomedicine & Pharmacotherapy . 2019;118, article 109362 doi: 10.1016/j.biopha.2019.109362. [DOI] [PubMed] [Google Scholar]
- 28.Zhang H., Long M., Wu Z., Han X., Yu Y. Sodium tanshinone IIA silate as an add-on therapy in patients with unstable angina pectoris. Journal of Thoracic Disease . 2014;6(12):1794–1799. doi: 10.3978/j.issn.2072-1439.2014.12.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mao S., Li X., Wang L., Yang P. C., Zhang M. Rationale and design of sodium tanshinone IIA sulfonate in left ventricular remodeling secondary to acute myocardial infarction (STAMP-REMODELING) trial: a randomized controlled study. Cardiovascular Drugs and Therapy . 2015;29(6):535–542. doi: 10.1007/s10557-015-6625-2. [DOI] [PubMed] [Google Scholar]
- 30.Duan H., Ma L., Liu H., et al. Tanshinone IIA attenuates epithelial-mesenchymal transition to inhibit the tracheal narrowing. Journal of Surgical Research . 2016;206(1):252–262. doi: 10.1016/j.jss.2016.04.066. [DOI] [PubMed] [Google Scholar]
- 31.Bi Z., Wang Y., Zhang W. A comprehensive review of tanshinone IIA and its derivatives in fibrosis treatment. Biomedicine & Pharmacotherapy . 2021;137, article 111404 doi: 10.1016/j.biopha.2021.111404. [DOI] [PubMed] [Google Scholar]
- 32.Zhou Z. Y., Huang B., Li S., et al. Sodium tanshinone IIA sulfonate promotes endothelial integrity via regulating VE-cadherin dynamics and Rho A/ROCK-mediated cellular contractility and prevents atorvastatin-induced intracerebral hemorrhage in zebrafish. Toxicology and Applied Pharmacology . 2018;350:32–42. doi: 10.1016/j.taap.2018.04.037. [DOI] [PubMed] [Google Scholar]
- 33.Liu J., Wang X., Cai Z., Lee F. S. Effect of tanshinone IIA on the noncovalent interaction between warfarin and human serum albumin studied by electrospray ionization mass spectrometry. Journal of the American Society for Mass Spectrometry . 2008;19(10):1568–1575. doi: 10.1016/j.jasms.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 34.Chan S. E., Lai H. W., Su C. C., et al. Effect of supplementation of tanshinone IIA and sodium tanshinone IIA sulfonate on the anticancer effect of epirubicin: an in vitro study. Evidence-Based Complementary and Alternative Medicine . 2011;2011:9. doi: 10.1155/2011/841564.841564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sun J., Yang M., Han J., et al. Profiling the metabolic difference of seven tanshinones using high-performance liquid chromatography/multi-stage mass spectrometry with data-dependent acquisition. Rapid Communications in Mass Spectrometry: An International Journal Devoted to the Rapid Dissemination of Up‐to‐the‐Minute Research in Mass Spectrometry . 2007;21(14):2211–2226. doi: 10.1002/rcm.3080. [DOI] [PubMed] [Google Scholar]
- 36.Kaiser M., Sobottka H., Fischer W., Schaefer M., Norenberg W. Tanshinone II A sulfonate, but not tanshinone II A, acts as potent negative allosteric modulator of the human purinergic receptor P2X7. The Journal of Pharmacology and Experimental Therapeutics . 2014;350(3):531–542. doi: 10.1124/jpet.114.214569. [DOI] [PubMed] [Google Scholar]
- 37.Bi H. C., Zuo Z., Chen X., et al. Preclinical factors affecting the pharmacokinetic behaviour of tanshinone IIA, an investigational new drug isolated from Salvia miltiorrhiza for the treatment of ischaemic heart diseases. Xenobiotica . 2008;38(2):185–222. doi: 10.1080/00498250701767675. [DOI] [PubMed] [Google Scholar]
- 38.Mao S., Jin H., Bi Y., Liang Z., Li H., Hou S. Ion-pair reversed-phase HPLC method for determination of sodium tanshinone IIA sulfonate in biological samples and its pharmacokinetics and biodistribution in mice. Chemical and Pharmaceutical Bulletin . 2007;55(5):753–756. doi: 10.1248/cpb.55.753. [DOI] [PubMed] [Google Scholar]
- 39.Hao H., Wang G., Cui N., Li J., Ding Z. Determination of sodium tanshinone IIA sulfonate in plasma by liquid chromatography-electrospray ionisation-tandem mass spectrometry. Biomedical Chromatography . 2007;21(11):1172–1179. doi: 10.1002/bmc.871. [DOI] [PubMed] [Google Scholar]
- 40.Lin G., Jiang J., Yuliang R. Determination of sodium tanshinone II sulfonate in rat plasma by high performance liquid chromatography and its application to pharmacokinetics studies. Pharmaceutica Analytica Acta . 2015;6:p. 383. [Google Scholar]
- 41.Holmstrom K. M., Finkel T. Cellular mechanisms and physiological consequences of redox-dependent signalling. Nature Reviews Molecular Cell Biology . 2014;15(6):411–421. doi: 10.1038/nrm3801. [DOI] [PubMed] [Google Scholar]
- 42.Sack M. N., Fyhrquist F. Y., Saijonmaa O. J., Fuster V., Kovacic J. C. Basic biology of oxidative stress and the cardiovascular system: part 1 of a 3-part series. Journal of the American College of Cardiology . 2017;70(2):196–211. doi: 10.1016/j.jacc.2017.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Senoner T., Dichtl W. Oxidative stress in cardiovascular diseases: still a therapeutic target? Nutrients . 2019;11(9) doi: 10.3390/nu11092090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Touyz R. M., Briones A. M. Reactive oxygen species and vascular biology: implications in human hypertension. Hypertension Research . 2011;34(1):5–14. doi: 10.1038/hr.2010.201. [DOI] [PubMed] [Google Scholar]
- 45.Li S., Tabar S. S., Malec V., et al. NOX4 regulates ROS levels under normoxic and hypoxic conditions, triggers proliferation, and inhibits apoptosis in pulmonary artery adventitial fibroblasts. Antioxidants & Redox Signaling . 2008;10(10):1687–1698. doi: 10.1089/ars.2008.2035. [DOI] [PubMed] [Google Scholar]
- 46.Siwik D. A., Pagano P. J., Colucci W. S. Oxidative stress regulates collagen synthesis and matrix metalloproteinase activity in cardiac fibroblasts. American Journal of Physiology. Cell Physiology . 2001;280(1):C53–C60. doi: 10.1152/ajpcell.2001.280.1.C53. [DOI] [PubMed] [Google Scholar]
- 47.Murdoch C. E., Chaubey S., Zeng L., et al. Endothelial NADPH oxidase-2 promotes interstitial cardiac fibrosis and diastolic dysfunction through proinflammatory effects and endothelial-mesenchymal transition. Journal of the American College of Cardiology . 2014;63(24):2734–2741. doi: 10.1016/j.jacc.2014.02.572. [DOI] [PubMed] [Google Scholar]
- 48.An S. J., Boyd R., Zhu M., Chapman A., Pimentel D. R., Wang H. D. NADPH oxidase mediates angiotensin II-induced endothelin-1 expression in vascular adventitial fibroblasts. Cardiovascular Research . 2007;75(4):702–709. doi: 10.1016/j.cardiores.2007.02.015. [DOI] [PubMed] [Google Scholar]
- 49.Chang R., Mamun A., Dominic A., Le N. T. SARS-CoV-2 mediated endothelial dysfunction: the potential role of chronic oxidative stress. Frontiers in Physiology . 2020;11, article 605908 doi: 10.3389/fphys.2020.605908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pons S., Fodil S., Azoulay E., Zafrani L. The vascular endothelium: the cornerstone of organ dysfunction in severe SARS-CoV-2 infection. Critical Care . 2020;24(1):p. 353. doi: 10.1186/s13054-020-03062-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Daiber A., Steven S., Weber A., et al. Targeting vascular (endothelial) dysfunction. British Journal of Pharmacology . 2017;174(12):1591–1619. doi: 10.1111/bph.13517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ohkita M., Takaoka M., Sugii M., Shiota Y., Nojiri R., Matsumura Y. The role of nuclear factor-kappa B in the regulation of endothelin-1 production by nitric oxide. European Journal of Pharmacology . 2003;472(3):159–164. doi: 10.1016/s0014-2999(03)01903-4. [DOI] [PubMed] [Google Scholar]
- 53.Goncharov N. V., Nadeev A. D., Jenkins R. O., Avdonin P. V. Markers and biomarkers of endothelium: when something is rotten in the state. Oxidative Medicine and Cellular Longevity . 2017;2017:27. doi: 10.1155/2017/9759735.9759735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gimbrone M. A., Jr., Garcia-Cardena G. Endothelial cell dysfunction and the pathobiology of atherosclerosis. Circulation Research . 2016;118(4):620–636. doi: 10.1161/CIRCRESAHA.115.306301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Huang R. B., Eniola-Adefeso O. Shear stress modulation of IL-1beta-induced E-selectin expression in human endothelial cells. PLoS One . 2012;7(2, article e31874) doi: 10.1371/journal.pone.0031874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Williams J. W., Huang L. H., Randolph G. J. Cytokine circuits in cardiovascular disease. Immunity . 2019;50(4):941–954. doi: 10.1016/j.immuni.2019.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dai G., Kaazempur-Mofrad M. R., Natarajan S., et al. Distinct endothelial phenotypes evoked by arterial waveforms derived from atherosclerosis-susceptible and -resistant regions of human vasculature. Proceedings of the National Academy of Sciences . 2004;101(41):14871–14876. doi: 10.1073/pnas.0406073101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hsu T., Nguyen-Tran H. H., Trojanowska M. Active roles of dysfunctional vascular endothelium in fibrosis and cancer. Journal of Biomedical Science . 2019;26(1):p. 86. doi: 10.1186/s12929-019-0580-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chang C. C., Chang Y. C., Hu W. L., Hung Y. C. Oxidative stress and salvia miltiorrhiza in aging-associated cardiovascular diseases. Oxidative Medicine and Cellular Longevity . 2016;2016:11. doi: 10.1155/2016/4797102.4797102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hung Y. C., Pan T. L., Hu W. L. Roles of reactive oxygen species in anticancer therapy with Salvia miltiorrhiza bunge. Oxidative medicine and cellular longevity . 2016;2016:10. doi: 10.1155/2016/5293284.5293284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhou G. Y., Zhao B. L., Hou J. W., Ma G. E., Xin W. J. Protective effects of sodium tanshinone IIA sulphonate against adriamycin-induced lipid peroxidation in mice hearts in vivo and in vitro. Pharmacological Research . 1999;40(6):487–491. doi: 10.1006/phrs.1999.0545. [DOI] [PubMed] [Google Scholar]
- 62.Cao E. H., Liu X. Q., Wang J. J., Xu N. F. Effect of natural antioxidant tanshinone II-A on DNA damage by lipid peroxidation in liver cells. Free Radical Biology & Medicine . 1996;20(6):801–806. doi: 10.1016/0891-5849(95)02211-2. [DOI] [PubMed] [Google Scholar]
- 63.Zhou G., Jiang W., Zhao Y., et al. Sodium tanshinone IIA sulfonate mediates electron transfer reaction in rat heart mitochondria. Biochemical Pharmacology . 2003;65(1):51–57. doi: 10.1016/S0006-2952(02)01447-8. [DOI] [PubMed] [Google Scholar]
- 64.Wei B., You M. G., Ling J. J., et al. Regulation of antioxidant system, lipids and fatty acid β-oxidation contributes to the cardioprotective effect of sodium tanshinone IIA sulphonate in isoproterenol-induced myocardial infarction in rats. Atherosclerosis . 2013;230(1):148–156. doi: 10.1016/j.atherosclerosis.2013.07.005. [DOI] [PubMed] [Google Scholar]
- 65.Zhang X. B., Chen X. Y., Sun P., et al. Sodium tanshinone IIA sulfonate attenuates tumor oxidative stress and promotes apoptosis in an intermittent hypoxia mouse model. Technology in Cancer Research & Treatment . 2020;19 doi: 10.1177/1533033820928073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chen W., Yuan C., Lu Y., et al. Tanshinone IIA protects against acute pancreatitis in mice by inhibiting oxidative stress via the Nrf 2/ROS pathway. Oxidative Medicine and Cellular Longevity . 2020;2020:12. doi: 10.1155/2020/5390482.5390482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yan Q., Mao Z., Hong J., et al. Tanshinone IIA stimulates cystathionine gamma-lyase expression and protects endothelial cells from oxidative injury. Antioxidants . 2021;10(7) doi: 10.3390/antiox10071007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chen L., Wei L., Yu Q., Shi H., Liu G. Tanshinone IIA alleviates hypoxia/reoxygenation induced cardiomyocyte injury via lnc RNA AK003290/mi R-124-5p signaling. BMC Molecular and Cell Biology . 2020;21(1):p. 20. doi: 10.1186/s12860-020-00264-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hung Y. C., Wang P. W., Lin T. Y., Yang P. M., You J. S., Pan T. L. Functional redox proteomics reveal that Salvia miltiorrhiza aqueous extract alleviates adriamycin-induced cardiomyopathy via inhibiting ROS-dependent apoptosis. Oxidative Medicine and Cellular Longevity . 2020;2020:15. doi: 10.1155/2020/5136934.5136934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wang X., Fan J., Ding X., Sun Y., Cui Z., Liu W. Tanshinone I inhibits IL-1β-induced apoptosis, inflammation and extracellular matrix degradation in chondrocytes CHON-001 cells and attenuates murine osteoarthritis. Drug Design, Development and Therapy . 2019;13:3559–3568. doi: 10.2147/DDDT.S216596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Du H., Wang Y., Zeng Y., et al. Tanshinone IIA suppresses proliferation and inflammatory cytokine production of synovial fibroblasts from rheumatoid arthritis patients induced by TNF-alpha and attenuates the inflammatory response in AIA mice. Frontiers in Pharmacology . 2020;11:p. 568. doi: 10.3389/fphar.2020.00568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fan G., Jiang X., Wu X., et al. Anti-inflammatory activity of tanshinone IIA in LPS-stimulated RAW264.7 macrophages via mi RNAs and TLR4-NF-kappa B pathway. Inflammation . 2016;39(1):375–384. doi: 10.1007/s10753-015-0259-1. [DOI] [PubMed] [Google Scholar]
- 73.Chen Z., Gao X., Jiao Y., et al. Tanshinone IIA exerts anti-inflammatory and immune-regulating effects on vulnerable atherosclerotic plaque partially via the TLR4/MyD88/NF-kappa B signal pathway. Frontiers in Pharmacology . 2019;10:p. 850. doi: 10.3389/fphar.2019.00850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yang J. X., Pan Y. Y., Ge J. H., et al. Tanshinone II A attenuates TNF-alpha-induced expression of VCAM-1 and ICAM-1 in endothelial progenitor cells by blocking activation of NF-kappa B. Cellular Physiology and Biochemistry . 2016;40(1-2):195–206. doi: 10.1159/000452537. [DOI] [PubMed] [Google Scholar]
- 75.Takahashi M. NLRP3 inflammasome as a novel player in myocardial infarction. International Heart Journal . 2014;55(2):101–105. doi: 10.1536/ihj.13-388. [DOI] [PubMed] [Google Scholar]
- 76.Hu Q., Wei B., Wei L., et al. Sodium tanshinone IIA sulfonate ameliorates ischemia-induced myocardial inflammation and lipid accumulation in Beagle dogs through NLRP3 inflammasome. International Journal of Cardiology . 2015;196:183–192. doi: 10.1016/j.ijcard.2015.05.152. [DOI] [PubMed] [Google Scholar]
- 77.Gao S., Wang Y., Li D., et al. TanshinoneIIA alleviates inflammatory response and directs macrophage polarization in lipopolysaccharide-stimulated RAW264.7 cells. Inflammation . 2019;42(1):264–275. doi: 10.1007/s10753-018-0891-7. [DOI] [PubMed] [Google Scholar]
- 78.Gonzalez D. M., Medici D. Signaling mechanisms of the epithelial-mesenchymal transition. Science Signaling . 2014;7(344) doi: 10.1126/scisignal.2005189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Piera-Velazquez S., Jimenez S. A. Endothelial to mesenchymal transition: role in physiology and in the pathogenesis of human diseases. Physiological Reviews . 2019;99(2):1281–1324. doi: 10.1152/physrev.00021.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Song S., Zhang R., Cao W., et al. Foxm1 is a critical driver of TGF‐β‐induced EndMT in endothelial cells through Smad2/3 and binds to the Snail promoter. Journal of Cellular Physiology . 2019;234(6):9052–9064. doi: 10.1002/jcp.27583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Medici D., Potenta S., Kalluri R. Transforming growth factor-β2 promotes Snail-mediated endothelial-mesenchymal transition through convergence of Smad-dependent and Smad-independent signalling. Biochemical Journal . 2011;437(3):515–520. doi: 10.1042/BJ20101500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Peinado H., Quintanilla M., Cano A. Transforming growth factor beta-1 induces snail transcription factor in epithelial cell lines: mechanisms for epithelial mesenchymal transitions. The Journal of Biological Chemistry . 2003;278(23):21113–21123. doi: 10.1074/jbc.M211304200. [DOI] [PubMed] [Google Scholar]
- 83.Zhan C. Y., Tang J. H., Zhou D. X., Li Z. H. Effects of tanshinone IIA on the transforming growth factor β1/Smad signaling pathway in rat cardiac fibroblasts. Indian Journal of Pharmacology . 2014;46(6):633–638. doi: 10.4103/0253-7613.144933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zhou D., Li Z., Zhang L., Zhan C. Inhibitory effect of tanshinone II a on TGF II-β1-induced cardiac fibrosis. Journal of Huazhong University of Science and Technology. Medical Sciences . 2012;32(6):829–833. doi: 10.1007/s11596-012-1042-2. [DOI] [PubMed] [Google Scholar]
- 85.Yang L., Zou X. J., Gao X., et al. Sodium tanshinone IIA sulfonate attenuates angiotensin II-induced collagen type I expression in cardiac fibroblasts in vitro. Experimental & Molecular Medicine . 2009;41(7):508–516. doi: 10.3858/emm.2009.41.7.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Yang L., Zou X. J., Yin Z., Hao H. Z. Effect of sodium tanshinone II (A) sulfonate on Ang II -induced atrial fibroblast collagen synthesis and TGF-beta 1 activation. Zhongguo Zhong yao za zhi= Zhongguo Zhongyao Zazhi= China Journal of Chinese Materia Medica . 2014;39(6):1093–1096. [PubMed] [Google Scholar]
- 87.Chen T., Li M., Fan X., Cheng J., Wang L. Sodium tanshinone IIA sulfonate prevents angiotensin II-induced differentiation of human atrial fibroblasts into myofibroblasts. Oxidative Medicine and Cellular Longevity . 2018;2018:10. doi: 10.1155/2018/6712585.6712585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Tsai Y. T., Loh S. H., Lee C. Y., et al. Tanshinone IIA inhibits high glucose-induced collagen synthesis via nuclear factor erythroid 2-related factor 2 in cardiac fibroblasts. Cellular Physiology and Biochemistry . 2018;51(5):2250–2261. doi: 10.1159/000495870. [DOI] [PubMed] [Google Scholar]
- 89.Li Y., Yang Y., Yu D., Liang Q. The effect of tanshinone IIA upon the TGF-beta1/Smads signaling pathway in hypertrophic myocardium of hypertensive rats. Journal of Huazhong University of Science and Technology. Medical Sciences . 2009;29(4):476–480. doi: 10.1007/s11596-009-0417-5. [DOI] [PubMed] [Google Scholar]
- 90.Shen N., Li X., Zhou T., et al. Shensong Yangxin Capsule prevents diabetic myocardial fibrosis by inhibiting TGF-beta 1/Smad signaling. Journal of Ethnopharmacology . 2014;157:161–170. doi: 10.1016/j.jep.2014.09.035. [DOI] [PubMed] [Google Scholar]
- 91.Feng F., Cheng P., Zhang H., et al. The protective role of tanshinone IIA in silicosis rat model via TGF-beta 1/Smad signaling suppression, NOX4 inhibition and Nrf 2/ARE signaling activation. Drug Design, Development and Therapy . 2019;13:4275–4290. doi: 10.2147/DDDT.S230572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Feng F., Li N., Cheng P., et al. Tanshinone IIA attenuates silica-induced pulmonary fibrosis via inhibition of TGF-β1-Smad signaling pathway. Biomedicine & Pharmacotherapy . 2020;121, article 109586 doi: 10.1016/j.biopha.2019.109586. [DOI] [PubMed] [Google Scholar]
- 93.Feng F., Cheng P., Xu S., et al. Tanshinone IIA attenuates silica-induced pulmonary fibrosis via Nrf 2-mediated inhibition of EMT and TGF-beta 1/Smad signaling. Chemico-Biological Interactions . 2020;319, article 109024 doi: 10.1016/j.cbi.2020.109024. [DOI] [PubMed] [Google Scholar]
- 94.Milara J., Ballester B., Morell A., et al. JAK2 mediates lung fibrosis, pulmonary vascular remodelling and hypertension in idiopathic pulmonary fibrosis: an experimental study. Thorax . 2018;73(6):519–529. doi: 10.1136/thoraxjnl-2017-210728. [DOI] [PubMed] [Google Scholar]
- 95.Li S. S., Feng J., Zheng Z., Liang Q. S. Effect of sodium tanshinone II A sulfonate on phosphorylation of extracellular signal-regulated kinase 1/2 in angiotensin II-induced hypertrophy of myocardial cells. Chinese Journal of Integrative Medicine . 2008;14(2):123–127. doi: 10.1007/s11655-008-0123-3. [DOI] [PubMed] [Google Scholar]
- 96.Zhou D., Liang Q., He X., Zhan C. Changes of c-fos and c-jun mRNA expression in angiotensin II-induced cardiomyocyte hypertrophy and effects of sodium tanshinone IIA sulfonate. Journal of Huazhong University of Science and Technology [Medical Sciences] . 2008;28(5):531–534. doi: 10.1007/s11596-008-0509-7. [DOI] [PubMed] [Google Scholar]
- 97.Feng J., Zheng Z. Effect of sodium tanshinone II A sulfonate on cardiac myocyte hypertrophy and its underlying mechanism. Chinese Journal of Integrative Medicine . 2008;14(3):197–201. doi: 10.1007/s11655-008-0197-y. [DOI] [PubMed] [Google Scholar]
- 98.Yang L., Zou X., Liang Q., et al. Sodium tanshinone IIA sulfonate depresses angiotensin II-induced cardiomyocyte hypertrophy through MEK/ERK pathway. Experimental & Molecular Medicine . 2007;39(1):65–73. doi: 10.1038/emm.2007.8. [DOI] [PubMed] [Google Scholar]
- 99.Yang L., Hu J., Hao H. Z., Yin Z., Liu G., Zou X. J. Sodium tanshinone IIA sulfonate attenuates the transforming growth factor-beta 1-induced differentiation of atrial fibroblasts into myofibroblasts in vitro. International Journal of Molecular Medicine . 2015;35(4):1026–1032. doi: 10.3892/ijmm.2015.2087. [DOI] [PubMed] [Google Scholar]
- 100.High F. A., Lu M. M., Pear W. S., Loomes K. M., Kaestner K. H., Epstein J. A. Endothelial expression of the Notch ligand Jagged 1 is required for vascular smooth muscle development. Proceedings of the National Academy of Sciences of the United States of America . 2008;105(6):1955–1959. doi: 10.1073/pnas.0709663105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Chang A. C., Fu Y., Garside V. C., et al. Notch initiates the endothelial-to-mesenchymal transition in the atrioventricular canal through autocrine activation of soluble guanylyl cyclase. Developmental Cell . 2011;21(2):288–300. doi: 10.1016/j.devcel.2011.06.022. [DOI] [PubMed] [Google Scholar]
- 102.Tian D. Y., Jin X. R., Zeng X., Wang Y. Notch signaling in endothelial cells: is it the therapeutic target for vascular neointimal hyperplasia? International journal of molecular sciences . 2017;18(8):p. 1615. doi: 10.3390/ijms18081615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Jackson A. O., Zhang J., Jiang Z., Yin K. Endothelial-to-mesenchymal transition: a novel therapeutic target for cardiovascular diseases. Trends in Cardiovascular Medicine . 2017;27(6):383–393. doi: 10.1016/j.tcm.2017.03.003. [DOI] [PubMed] [Google Scholar]
- 104.Guo Q., Xu H., Yang X., et al. Notch activation of Ca2+-sensing receptor mediates hypoxia-induced pulmonary hypertension. Hypertension Research . 2017;40(2):117–129. doi: 10.1038/hr.2016.118. [DOI] [PubMed] [Google Scholar]
- 105.Reichman D., Man L., Park L., et al. Notch hyper-activation drives trans-differentiation of hESC-derived endothelium. Stem cell research . 2016;17(2):391–400. doi: 10.1016/j.scr.2016.09.005. [DOI] [PubMed] [Google Scholar]
- 106.Fu L., Han B., Zhou Y., et al. The anticancer properties of tanshinones and the pharmacological effects of their active ingredients. Frontiers in Pharmacology . 2020;11:p. 193. doi: 10.3389/fphar.2020.00193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Dong W., Zhang Y., Chen X., Jia Y. High-dose tanshinone IIA suppresses migration and proliferation while promoting apoptosis of astrocytoma cells via Notch-1 pathway. Neurochemical Research . 2018;43(9):1855–1861. doi: 10.1007/s11064-018-2601-0. [DOI] [PubMed] [Google Scholar]
- 108.Yu J., Wang X., Li Y., Tang B. Tanshinone IIA suppresses gastric cancer cell proliferation and migration by downregulation of FOXM1. Oncology Reports . 2017;37(3):1394–1400. doi: 10.3892/or.2017.5408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Li X., Luo D., Hou Y., et al. Sodium tanshinone IIA silate exerts microcirculation protective effects against spinal cord injury in vitro and in vivo. Oxidative medicine and cellular longevity . 2020;2020:16. doi: 10.1155/2020/3949575.3949575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Miller J. R. The Wnts. Genome biology . 2002;3(1):p. REVIEWS3001. doi: 10.1186/gb-2001-3-1-reviews3001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Nusse R. Wnt signaling in disease and in development. Cell Research . 2005;15(1):28–32. doi: 10.1038/sj.cr.7290260. [DOI] [PubMed] [Google Scholar]
- 112.Clevers H., Nusse R. Wnt/β-Catenin Signaling and Disease. Cell . 2012;149(6):1192–1205. doi: 10.1016/j.cell.2012.05.012. [DOI] [PubMed] [Google Scholar]
- 113.Liebner S., Cattelino A., Gallini R., et al. Beta-catenin is required for endothelial-mesenchymal transformation during heart cushion development in the mouse. The Journal of cell biology . 2004;166(3):359–367. doi: 10.1083/jcb.200403050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Aisagbonhi O., Rai M., Ryzhov S., Atria N., Feoktistov I., Hatzopoulos A. K. Experimental myocardial infarction triggers canonical Wnt signaling and endothelial-to-mesenchymal transition. Disease models & mechanisms . 2011;4(4):469–483. doi: 10.1242/dmm.006510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Xie Y., Liao J., Yu Y., et al. Endothelial‑to‑mesenchymal transition in human idiopathic dilated cardiomyopathy. Molecular Medicine Reports . 2018;17(1):961–969. doi: 10.3892/mmr.2017.8013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Xu Z., Wu L., Sun Y., et al. Tanshinone IIA pretreatment protects free flaps against hypoxic injury by upregulating stem cell-related biomarkers in epithelial skin cells. BMC Complementary and Alternative Medicine . 2014;14(1):p. 331. doi: 10.1186/1472-6882-14-331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ma S., Lei Y., Zhang L., Wang J. Research on the inhibiting effect of tanshinone IIA on colon cancer cell growth via COX-2-Wnt/beta-catenin signaling pathway. Journal of the Balkan Union of Oncology (JBUON) . 2018;23(5):1337–1342. [PubMed] [Google Scholar]
- 118.Li F. Q., Zeng D. K., Jia C. L., et al. The effects of sodium tanshinone IIa sulfonate pretreatment on high glucose-induced expression of fractalkine and apoptosis in human umbilical vein endothelial cells. International Journal of Clinical and Experimental Medicine . 2015;8(4):5279–5286. [PMC free article] [PubMed] [Google Scholar]
- 119.Zhu Q., Zeng D., Li F. Ghrelin combined with sodium tanshinone IIA sulfonate pretreatment reduces apoptosis and fractalkine expression induced by high-dose glucose in human umbilical vein endothelial cells. Minerva Endocrinologica . 2020;45(1):36–42. doi: 10.23736/S0391-1977.19.02964-X. [DOI] [PubMed] [Google Scholar]
- 120.Feng J., Chen H. W., Pi L. J., Wang J., Zhan D. Q. Protective effect of tanshinone IIA against cardiac hypertrophy in spontaneously hypertensive rats through inhibiting the Cys-C/Wnt signaling pathway. Oncotarget . 2017;8(6):10161–10170. doi: 10.18632/oncotarget.14328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Davenport A. P., Hyndman K. A., Dhaun N., et al. Endothelin. Pharmacological Reviews . 2016;68(2):357–418. doi: 10.1124/pr.115.011833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Jankowich M., Choudhary G. Endothelin-1 levels and cardiovascular events. Trends in Cardiovascular Medicine . 2020;30(1):1–8. doi: 10.1016/j.tcm.2019.01.007. [DOI] [PubMed] [Google Scholar]
- 123.Khimji A. K., Rockey D. C. Endothelin and hepatic wound healing. Pharmacological Research . 2011;63(6):512–518. doi: 10.1016/j.phrs.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Hong H. J., Hsu F. L., Tsai S. C., et al. Tanshinone IIA attenuates cyclic strain-induced endothelin-1 expression in human umbilical vein endothelial cells. Clinical and Experimental Pharmacology & Physiology . 2012;39(1):63–68. doi: 10.1111/j.1440-1681.2011.05637.x. [DOI] [PubMed] [Google Scholar]
- 125.Yu Z. L., Wang J. N., Wu X. H., et al. Tanshinone IIA prevents rat basilar artery smooth muscle cells proliferation by inactivation of PDK1 during the development of hypertension. Journal of cardiovascular pharmacology and therapeutics . 2015;20(6):563–571. doi: 10.1177/1074248415574743. [DOI] [PubMed] [Google Scholar]
- 126.Chan P., Liu J. C., Lin L. J., et al. Tanshinone IIA inhibits angiotensin II-induced cell proliferation in rat cardiac fibroblasts. The American Journal of Chinese Medicine . 2011;39(2):381–394. doi: 10.1142/S0192415X11008890. [DOI] [PubMed] [Google Scholar]
- 127.Chen L., Guo Q. H., Chang Y., Zhao Y. S., Li A. Y., Ji E. S. Tanshinone IIA ameliorated endothelial dysfunction in rats with chronic intermittent hypoxia. Cardiovascular Pathology . 2017;31:47–53. doi: 10.1016/j.carpath.2017.06.008. [DOI] [PubMed] [Google Scholar]
- 128.Wang N., Chang Y., Chen L., et al. Tanshinone IIA protects against chronic intermittent hypoxia-induced myocardial injury via activating the endothelin 1 pathway. Biomedicine & Pharmacotherapy . 2017;95:1013–1020. doi: 10.1016/j.biopha.2017.08.036. [DOI] [PubMed] [Google Scholar]
- 129.Liu P., Rudick M., Anderson R. G. Multiple functions of caveolin-1. The Journal of Biological Chemistry . 2002;277(44):41295–41298. doi: 10.1074/jbc.R200020200. [DOI] [PubMed] [Google Scholar]
- 130.Fernández-Hernando C., Yu J., Suárez Y., et al. Genetic evidence supporting a critical role of endothelial caveolin-1 during the progression of atherosclerosis. Cell Metabolism . 2009;10(1):48–54. doi: 10.1016/j.cmet.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Pavlides S., Gutierrez-Pajares J. L., Iturrieta J., Lisanti M. P., Frank P. G. Endothelial caveolin-1 plays a major role in the development of atherosclerosis. Cell and Tissue Research . 2014;356(1):147–157. doi: 10.1007/s00441-013-1767-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Drab M., Verkade P., Elger M., et al. Loss of caveolae, vascular dysfunction, and pulmonary defects in caveolin-1 gene-disrupted mice. Science . 2001;293(5539):2449–2452. doi: 10.1126/science.1062688. [DOI] [PubMed] [Google Scholar]
- 133.Samarakoon R., Chitnis S. S., Higgins S. P., Higgins C. E., Krepinsky J. C., Higgins P. J. Redox-induced Src kinase and caveolin-1 signaling in TGF-β1-Initiated SMAD2/3 activation and PAI-1 expression. PLoS One . 2011;6(7, article e22896) doi: 10.1371/journal.pone.0022896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Zhao Y., Xu P., Hu S., et al. Tanshinone II A, a multiple target neuroprotectant, promotes caveolae-dependent neuronal differentiation. European Journal of Pharmacology . 2015;765:437–446. doi: 10.1016/j.ejphar.2015.09.006. [DOI] [PubMed] [Google Scholar]
- 135.Fang Y., Duan C., Chen S., et al. Tanshinone IIA inhibits myocardial infarct via decreasing of the mitochondrial apoptotic signaling pathway in myocardiocytes. International Journal of Molecular Medicine . 2021;48(2) doi: 10.3892/ijmm.2021.4991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Deng H., Yu B., Li Y. Tanshinone IIA alleviates acute ethanol-induced myocardial apoptosis mainly through inhibiting the expression of PDCD4 and activating the PI3K/Akt pathway. Phytotherapy Research . 2021;35(8):4309–4323. doi: 10.1002/ptr.7102. [DOI] [PubMed] [Google Scholar]
- 137.Wang L., Xu L., Du J., et al. Nose-to-brain delivery of borneol modified tanshinone IIA nanoparticles in prevention of cerebral ischemia/reperfusion injury. Drug Delivery . 2021;28(1):1363–1375. doi: 10.1080/10717544.2021.1943058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Zhu J., Xu Y., Ren G., et al. Tanshinone IIA sodium sulfonate regulates antioxidant system, inflammation, and endothelial dysfunction in atherosclerosis by downregulation of CLIC1. European Journal of Pharmacology . 2017;815:427–436. doi: 10.1016/j.ejphar.2017.09.047. [DOI] [PubMed] [Google Scholar]
- 139.Lou G., Hu W., Wu Z., et al. Tanshinone II A attenuates vascular remodeling through klf4 mediated smooth muscle cell phenotypic switching. Scientific reports . 2020;10(1, article 13858) doi: 10.1038/s41598-020-70887-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Wu D., Huo M., Chen X., Zhang Y., Qiao Y. Mechanism of tanshinones and phenolic acids from Danshen in the treatment of coronary heart disease based on co-expression network. BMC complementary medicine and therapies . 2020;20(1):p. 28. doi: 10.1186/s12906-019-2712-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Li S., Jiao Y., Wang H., et al. Sodium tanshinone IIA sulfate adjunct therapy reduces high-sensitivity C-reactive protein level in coronary artery disease patients: a randomized controlled trial. Scientific reports . 2017;7(1, article 17451) doi: 10.1038/s41598-017-16980-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Mao S., Wang L., Zhao X., et al. Efficacy of sodium tanshinone IIA sulfonate in patients with non-ST elevation acute coronary syndrome undergoing percutaneous coronary intervention: results from a multicentre, controlled, randomized trial. Cardiovascular Drugs and Therapy . 2021;35(2):321–329. doi: 10.1007/s10557-020-07077-8. [DOI] [PubMed] [Google Scholar]
- 143.Mao S., Taylor S., Chen Q., Zhang M., Hinek A. Sodium tanshinone IIA sulfonate prevents the adverse left ventricular remodelling: focus on polymorphonuclear neutrophil-derived granule components. Journal of Cellular and Molecular Medicine . 2019;23(7):4592–4600. doi: 10.1111/jcmm.14306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Lim Y., Song T. J., Hwang W., et al. Synergistic effects of Sanghuang–Danshen bioactives on arterial stiffness in a randomized clinical trial of healthy Smokers: an integrative approach to in silico network analysis. Nutrients . 2019;11(1):p. 108. doi: 10.3390/nu11010108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Xu J., Zhang C., Shi X., et al. Efficacy and safety of sodium tanshinone IIA sulfonate injection on hypertensive nephropathy: a systematic review and meta-analysis. Frontiers in Pharmacology . 2019;10:p. 1542. doi: 10.3389/fphar.2019.01542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Ping Y., Yu-hua J. I. A., Jie L. I., Feng-hua Z., Li-jun L. I. Anti-oxidation of tanshinone IIA and prohibitin on cardiomyocytes. Chinese Herbal Medicines (CHM) . 2022;2(3):204–210. [Google Scholar]
- 147.Gu J., Li H. L., Wu H. Y., et al. Sodium tanshinone IIA sulfonate attenuates radiation-induced fibrosis damage in cardiac fibroblasts. Journal of Asian Natural Products Research . 2014;16(9):941–952. doi: 10.1080/10286020.2014.935769. [DOI] [PubMed] [Google Scholar]
- 148.Tang F., Wu X., Wang T., et al. Tanshinone II A attenuates atherosclerotic calcification in rat model by inhibition of oxidative stress. Vascular Pharmacology . 2007;46(6):427–438. doi: 10.1016/j.vph.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 149.Wang P., Wu X., Bao Y., et al. Tanshinone IIA prevents cardiac remodeling through attenuating NAD (P) H oxidase-derived reactive oxygen species production in hypertensive rats. Pharmazie . 2011;66(7):517–524. [PubMed] [Google Scholar]
- 150.Huang L., Zhu J., Zheng M., Zou R., Zhou Y., Zhu M. Tanshinone IIA protects against subclinical lipopolysaccharide induced cardiac fibrosis in mice through inhibition of NADPH oxidase. International Immunopharmacology . 2018;60:59–63. doi: 10.1016/j.intimp.2018.04.036. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data were used to support this study.


