Abstract
Inflammatory bowel disease (IBD) is a chronic and relapsing disease with multiple underlying influences and notable heterogeneity among its clinical and response‐to‐treatment phenotypes. There is no cure for IBD, and none of the currently available therapies have demonstrated clinical efficacies beyond 40%–60%. Data collected about its omics, pathogenesis, and treatment strategies have grown exponentially with time making IBD a prime candidate for artificial intelligence (AI) mediated discovery support. AI can be leveraged to further understand or identify IBD features to improve clinical outcomes. Various treatment candidates are currently under evaluation in clinical trials, offering further approaches and opportunities for increasing the efficacies of treatments. However, currently, therapeutic plans are largely determined using clinical features due to the lack of specific biomarkers, and it has become necessary to step into precision medicine to predict therapeutic responses to guarantee optimal treatment efficacy. This is accompanied by the application of AI and the development of multiscale hybrid models combining mechanistic approaches and machine learning. These models ultimately lead to the creation of digital twins of given patients delivering on the promise of precision dosing and tailored treatment. Interleukin‐6 (IL‐6) is a prominent cytokine in cell‐to‐cell communication in the inflammatory responses’ regulation. Dysregulated IL‐6‐induced signaling leads to severe immunological or proliferative pathologies, such as IBD and colon cancer. This mini‐review explores multiscale models with the aim of predicting the response to therapy in IBD. Modeling IL‐6 biology and generating digital twins enhance the credibility of their prediction.
INTRODUCTION
Inflammatory bowel diseases (IBDs), comprising Crohn’s disease (CD) and ulcerative colitis (UC), are complex, multifactorial, multifaceted, remitting diseases; makes it challenging to reliably predict the behaviors of key biomarkers and clinical outcomes. 1 Research into IBD has identified various factors and molecular agents involved in its development, but our understanding of its etiology and progression remains incomplete, and its treatment is far from precise. CD is a segmental, transmural disease occurring in any part of the gastrointestinal tract, whereas UC mainly affects the rectal mucosal and submucosal layers, with eventual extension to the colon. Current therapeutic approaches do not optimally integrate IBD heterogeneity and complexity and fail to specifically and durably answer related medical needs.
There has been a recent increase in interest in the application of big data in gastroenterology, including IBD, to better understand disease history, predictors of development or severity, and treatment sensitivities. However, this has presented with several of its own unique challenges, including how to process the data and create reliable classification and prediction algorithms.
Artificial intelligence (AI) refers to the ability of computers to perform tasks requiring human intelligence, such as learning, problem solving, and prediction. AI is a very broad set of technologies with adaptative and anticipatory capacity to deal with a defined problem. It includes machine learning (ML) and subsets as supervised, unsupervised, reinforcement and deep learnings. AI algorithms are developed using input data to train the prediction model, and the more numerous and diverse the data, the more accurate the resulting prediction model will be in real‐world clinical settings. Thus, AI can certainly benefit precision medicine for IBD by improving diagnosis and therapeutic approaches (Figure 1). 2
FIGURE 1.
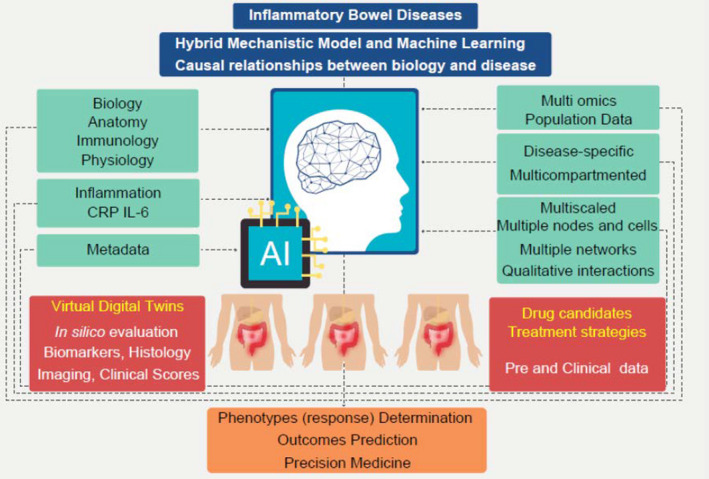
Artificial intelligence in inflammatory bowel disease
Although antibody‐based therapies against tumor necrosis factor (TNF), interleukins (ILs), or integrins have demonstrated efficacy in moderate‐to‐severe IBD, they remain chronic inflammatory conditions with no known cure. The occurrence of nonresponders and long‐term inefficacy rates for the currently available biological treatments may be a consequence of targeting only one signaling pathway. The current lack of biomarkers limits clinicians to treatment selections based only on the current clinical features of any individual patient. 3 Thus, it is necessary to promote the use of precision medicine via the production of improved predictive models that phenotype patients’ response to treatments to guarantee optimal outcomes. 4
This mini‐review provides an overview of the existing multiscale models in IBD and introduces that they optimize treatment strategies by enhancing diseases’ phenotyping and discretizing the IL‐6 pathway. The generation of virtual digital twins is a notable asset into this demonstration of accuracy in prediction and in personalized medicine.
MODELING IN BIOLOGY
The biological system is a complex ensemble of entities of an individual, which are independent but function as a whole. Models bring confidence in and mastering of a therapeutic ligand and its biological target in the context of a specific disease. A major output of the models are virtual patients and virtual studies that are mimicking actual clinical trials (Figure 2). A virtual digital twin is an in silico individual representation that dynamically reflect her or his biological system status across different treatments and times.
FIGURE 2.
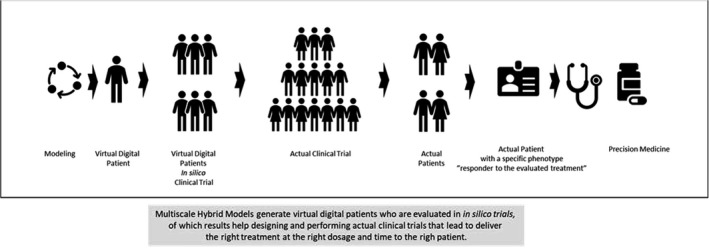
From virtual digital patients to precision medicine
Biological modeling has a long history. 5 Early modeling was using reductional approaches and models were largely based on mathematical equations. Since then, the emergence of more sophisticated computational technologies and computational systems biology approaches have resulted in significant progress in multiscale modeling objectives. Popular modeling techniques in computational immunology include ordinary differential equations (ODEs), stochastic differential equations (SDEs), partial differential equations (PDEs), and agent‐based models (ABMs). ODE is the backbone of most models, but it has some limitations in capturing cell population dynamics assuming deterministic, average behavior by each individual cell, whereas biological systems act stochastically. Given this limitation, ODE models may fail to accurately reflect the complexity of in vivo systems. Therefore, the use or combination of ABMs may be considered to be more appropriate. 6 The ENteric Immunity SImulator (ENISI) models and platforms comprise such techniques, although there are several other tools for this type of application including COmplex PAthway Simulator (COPASI), virtual cell, and the ValueLayer.
ENISI is likely the best and most succinctly described example of a multiscale model structure and its capabilities. It simulates gut mucosal immune responses and models four scales: tissue, cellular, intracellular, and intercellular response. At the tissue scale, the related compartments of the immune system include the lumen, epithelium, lamina propria, lymph nodes, and blood. At the cellular level, this system evaluates the activity and function of the epithelial cells, macrophages, dendritic cells, neutrophils, B cells, T cells, and bacteria and can even model subtypes based on the immune response and microenvironment. The intracellular scale model protein signaling reactions during immune response, and the intercellular scale refers to the cytokines implicated in the engagement of cellular receptors and triggering of signaling in cells.
Only multiscale models are providing the needed level of granularity to represent the individual biological complexity in IBD as well as to foster the required credibility of the model for treatment strategy and decision.
IL‐6 BIOLOGY
As being a master player into the biological complexity observed in IBD, the inhibition of the IL‐6 signaling is a relevant therapeutic target and deserves full attention in modeling IBD. Several treatment candidates with different modes of action and interaction are under evaluation.
The IL‐6 is a pleiotropic cytokine known to play a central role in both inflammation and immune regulation, with data linking its expression to pivotal roles in both innate and adaptive immune responses. IL‐6 production and signaling are increased in IBD‐inflamed mucosa, and IL‐6 is the main inducer of C‐reactive protein (CRP). Serum levels of IL‐6 and its complex with its soluble receptor (sIL‐6R) are elevated in both UC and CD and are well‐correlated with CRP levels. 7
Cytokines exert a wide range of biological effects, and their dysregulation may result in a variety of autoimmune and inflammatory disorders, including IBD. Cytokines are grouped into families based on their structure, specificity, and composition of their receptor complexes. The IL‐6 family currently includes eight cytokines using a common signaling receptor subunit, glycoprotein 130 (gp130) kDA. 8 The cytokines in the IL‐6 family signal via two mechanisms: classic signaling and trans‐signaling. In classic signaling, IL‐6 binds to a specific cell membrane receptor, whereas during trans‐signaling, IL‐6 binds to its soluble receptor. This trans‐signaling drives the IL‐6 pro‐inflammatory activities, whereas classic signaling (membrane bound IL‐6) drives their anti‐inflammatory activities. Different therapeutic strategies have been evaluated which either neutralize the classic pathway using monoclonal anti‐IL‐6 receptor antibodies, such as tocilizumab, or target IL‐6 trans‐signaling using compounds like olamkicept. 9 , 10 Molecules, such as anti‐TNF, anti‐other ILs, anti‐integrins, and JAK‐inhibitors also impact the IL‐6‐CRP pattern through different, often indirect, mechanisms.
Such different binding patterns lead to different transcriptional signatures, IL‐6 inhibition phenotypes, and biological effects. Tocilizumab and olamkicept have different blood transcriptome signatures. Sarilumab and tocilizumab bind with different affinities and express different kinetics and levels of CRP reduction. Olamkicept, vedolizumab, and infliximab have different mucosal transcriptome signatures. 10 , 11 , 12
The prototypical and multifunctional aspects of the IL‐6 require a multiscale modeling approach to better phenotype the patients for better treatment strategies and clinical benefits.
MODELS REVIEW
This paper included an exhaustive literature review using Medical Subject Heading (MeSH) terms across three databases: PubMed, EMBase, and the Cochrane Library from inception with the search date terminated on October 7, 2021. MeSH terms focused on a combination of keyword and free words, including IBD, UC, CD, IL‐6, model, platform, AI, computer‐aided, neural network, ML, deep learning, hybrid computational, and multiscale. Studies that used AI for IBD diagnosis or prediction, IBD severity assessment, IBD treatment response, and clinical outcome prediction were all selected for final inclusion and reviews. Animal models or AI applications and models with no objective measures of efficacy were excluded. This search yielded nine multiscale models using AI in IBD which met the inclusion and exclusion criteria (Table 1). 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 Eight of these (89%) were published within the past 10 years (2011 or later) and five of them (55%) within the past 5 years (2016 or later). Four were not included here as they were focused on a single scale, were developed for a non‐IBD pathology, were designed to explore the genetic architecture of these conditions rather than their cytokine biology, or were not published in extenso. 13 , 14 , 15 , 16
TABLE 1.
Brief summary of the current models in use
| Lead author | Year | Multiscale | Disease | IL‐6 biology included | Digital twins | Treatment evaluated |
|---|---|---|---|---|---|---|
| Moya C. 13 | 2011 | No | NAFLD | Yes | No | None |
| Peter L. 14 | 2017 | Yes | IBD | Yes | No | None |
| Park A. 15 | 2020 | No | CD | Yes | No | Anti IL12, anti TNFα |
| Iwakiri R. 16 | 2021 | Yes | CD | Yes | Yes | Vedolizumab |
| Rogers K. 17 | 2021 | Yes | IBD | Yes | Yes | Brazikumab, infliximab, PF‐04236921, ustekinumab |
| Wendelsdorf K. 18 | 2010 | Yes | IBD | Yes | No | None |
| Dwivedi G. 19 | 2014 | Yes | CD | Yes | No | Tocilizumab |
| Mei Y. 20 | 2015 | Yes | IBD | Yes | No | None |
| Balbas‐Martinez V. 21 | 2018 | Yes | IBD | Yes | No | Ustekinumab |
Abbreviations: CD, Crohn’s disease; IBD, inflammatory bowel disease; NAFLD, non‐alcoholic fatty liver disease; PF‐04236921, anti‐IL‐6 antibody.
Multiscale quantitative models are required to properly explore the dynamics of the immune system. Such models should integrate the most current knowledge about each immune system for the specific disease to be used for mechanistic assessment, and there should preferably be some comparison between several potential and/or already available therapeutic strategies in terms of target selection, candidate optimization, and dosage(s) selection. However, working models should balance the details required for the model with the quality and quantity of available data to accurately incorporate such details. That is why these models can only be as complex as the available reliable data allows.
Modeling IBD as a network and equation simplifies complex multi‐signal pathways and helps identify treatment responders and nonresponders as no two patients behave identically.
Rogers et al. recently reported the successful development of an IBD ODE‐based mathematical model and its application to CD with a focus on cytokines and biomarkers. 17 , 22 The observed and model‐predicted longitudinal changes in CRP and IL‐6 under different treatment conditions illustrate the need, relevance, and limitations of such simplification. 17 However, because the simplification from Rogers et al. more accurately considers the ability of the model to simulate both UC and CD using the same underlying mechanisms but different initial conditions, including the absence of intestinal epithelial barrier, the absence of spatial consideration at the tissue level, and a reduced number of compartments (colon and blood), some limitations in prediction may occur. 23
The suite of causal biological network models assembled by Ruiz Castro et al. offers a comprehensive visualization of the underlying IBD processes. It includes four independent models: intestinal permeability, barrier defense, inflammation, and wound healing. Their network highly objectifies existing divergences in the underlying molecular mechanisms of UC and CD. Most of these divergences appear in the barrier defense and wounding healing models, which is consistent with clinical data that suggest that patients with UC are likely to experience increased barrier and inflammation recovery dysfunction than patients with CD. It is also worth noting that this model identified PPARG, IFN, and IL‐6 associated patterns as particularly important to the regulation of both diseases. 24
A compromised gut epithelial barrier is also a widely recognized predictor of disease relapse and remission, and one recent AI‐assisted approach has helped to identify potential barrier‐protective agents. 25 Similarly, as different molecules inhibit the different IL‐6 signaling pathways in different ways, evaluating these differences among existing therapies, such as tocilizumab, sarilumab, olamkicept, or tofacitinib, with PF‐04236921 may have helped to demonstrate the full relevance of the simplification. It would have also facilitated improved adjustments of these algorithms as needed. Beyond efficacy, differences in the mechanisms of action are important for safety, especially when gastrointestinal perforation occurs. 9
The model of Rogers et al. also facilitates in silico evaluation of multiple treatment strategies, including combination therapies, which is a notable step forward in terms of benefit to patients for these kinds of applications. This provides a glimpse into the expected heterogeneity of different therapeutic classes and may be useful in identifying potential therapeutic synergies. 17
The generation of digital twins, a realistic virtual population, brings better confidence to the predictions, clinical trial simulations, and analyses. 26 One example of this is the use of one set of digital twins to help produce a series of novel therapies for CD. 16 , 22 Here, a digital twin was calibrated to match an individual (real) patient, and virtual populations were then produced to correspond to specific variations within the mechanistic model while maintaining the majority of the model as a duplicate. This facilitated much more nuanced evaluations of specific pathological processes. The parameters that are likely to differ among patients were determined using a sensitivity analysis, which allowed the researchers to identify the most influential parts of each of the selected dynamics (biomarkers, mucosal healing histology or imaging, and clinical scores) and their impact on treatment response for inclusion in the model. Thus, these models usually produce novel core virtual population libraries for each disease using literature‐based estimates for parameter ranges, clinical case reports, and experience from IBD practitioners to develop more comprehensive clinical evaluation models.
It is also worth noting that when we move beyond single‐scale models, there are very few alternatives to the Rogers mechanistic and multiscale models at the cellular level, which explore IL‐6 biology in IBD. 18 , 19 , 20 , 21 Wendelsdorf et al. developed a colonic inflammation model describing the interaction, traffic, and differentiation of immune, dendritic, and epithelial cells in response to cytokines and bacteria in the lumen, lamina propria, and lymph nodes. The proposed in silico model of IBD considered various cytokines, such as IL‐6, IL‐10, IL‐12, and TNF‐α, and was designed to better understand gut homeostasis in healthy subjects. Focus was given to identifying the critical motifs of mucosal immunity, but none of them was specifically related to UC or CD or IL‐6 biology. No treatment candidates were tested, although the model was later used to elaborate on an ABM counterpart, the ENISI. 18
Interestingly, the dimensionless mathematical model proposed by Park et al. was derived from Wendelsdorf et al., simplified by Lo et al., and is substituted with an additional compartment for drug intervention. The IL‐6 pathway is integrated within the mathematical model to evaluate the dynamics of the immune system of patients with CD treated with biologics. However, only anti‐TNFα and anti‐IL‐12 agents, which are known to inhibit the pro‐inflammatory cytokines (Infγ, TNFα, IL‐2, IL‐6, IL‐12, and IL‐21), have been evaluated using this model. No formal conclusion has been drawn on the efficacy of these drugs, but the model can identify the key beneficial compounds in a therapeutic assay based on the individual features of the patient’s immune system, most notably the ratio of pro to anti‐inflammatory cytokines. 15
Dwivedi et al. constructed an ODE‐based multiscale model of IL‐6 signaling in CD, comprising three structural modules. The first module describes the IL‐6 mediated cellular signal transduction and is embedded into the second module, which is made up of the target organs—the gastrointestinal tract and liver. These two organs are connected by a third compartment, which facilitates the exchange of various serum soluble molecules. The third module is the general monoclonal antibody PK model. Data from a previous study evaluating tocilizumab in CD were used to validate this model. 7 As developed, the model allowed Dwivedi et al. to evaluate four different IL‐6 signaling therapeutic strategies, including IL‐6, IL‐6Rα, IL‐6/sIL‐6Rα, and sgp130Fc as antagonists of IL‐6 trans‐signaling. The results demonstrated that IL‐6 inhibition and the dose‐response characteristics of each strategy were clearly distinct. One notable limitation of this model is that it underestimates the CRP response to the evaluated therapies because of the limited data available to train and validate the model and the limited consideration of the cell and cytokine networks involved within CD physiopathology.
The model developed by Dwivedi et al. is circumscribed to the IL‐6 mediated immune pathway, whereas inflammation is led by a network of cytokines, not a sole contributor. More recent models include and simulate additional pathways. 20 , 21 The complexity of the IL‐6 network was further explored by fusing experimental data and dynamic computational modeling. 27 Results highlighted the dependencies among the IL‐6, the Janus Kinase (JAK), and the transcription factor signal transducer and activator of transcription (STAT) signaling, reinforcing the necessity of individualized and computer‐aided approaches in treatment strategies.
Mei et al. reported the development of an ENISI‐based multiscale model of gut inflammation but did not explicitly consider UC or CD. The proposed platform is derived from the CD4+ T cell differential model and implements six cytokines as inputs (IL‐6, IL‐12, and TGF‐β) and outputs (IL‐10, IL‐17, and INFγ). Although this model has been set up to run in silico simulations, IBD clinical situations and treatment candidate evaluations have not been reported. 20
Balbas‐Martinez et al. proposed a systems pharmacology (logic) model that considers 43 nodes and 298 qualitative interactions within the lymph nodes, the blood, and the lymph circulatory system irrigating the intestinal cells, and the gut lumen. This network integrates multiple ILs, including IL‐6, and several specific cells, such as fibroblasts, macrophages, and naïve CD4+ T cells, making it more inclusive than many others. This model has also been validated using the clinical trial results for infliximab, ustekinumab, fontolizumab, secukinumab, basiliximab, and daclizumab in IBD. The outcomes of these challenges were similar to those of the clinical studies’ outputs, but not as detailed. 21
Full details of the computational platform for predicting the time course of mucosal healing in CD developed by Iwakiri et al. are not yet available, but they present some similarities with Rogers et al., in that they both integrate a response classifier with digital twin simulations. 16 , 17 Whereas trained using vedolizumab, a gut selective agent, the model also considers CRP as an essential biomarker and factor, confirming the central role of IL‐6 biology in such predictive models.
CONCLUSIONS
AI, and, more specifically, multiscale hybrid computational and ML models or platforms, may foster improved drug development and bring increased accuracy to the design of clinical studies, which may facilitate the best possible understanding of the clinical benefits of these technologies. This improved accuracy may help health authorities to further consider in silico clinical trials during drug registration processes, especially for precision medicine. This is a necessary step for overcoming the treatment plateau observed with specific biological therapies.
Standards for mechanistic model accuracy and credibility evaluation should be established to value in silico methods in drug development and evaluation and to aid precision medicine to deliver the optimal clinical benefit to patients.
The models proposed by Rogers et al. and Iwakiri et al. are the latest generation of hybrid platforms combining mathematics and ML. They include libraries consisting of thousands of virtual patients simulated over a period of multiple years, providing the highest accuracy for predicting the efficacy of various therapeutic strategies. Capturing the causal relationships between biology and disease, hybrid multiscale computational and ML models focus on data interpretation and simultaneously provide a natural way of incorporating continuous time variables, which are critical for generating a time‐resolved picture of how patients respond to different treatment scenarios, including combination treatments. Anchoring ML in physiology also improves prediction interpretability and overall efficacy.
Other types of predictive computational tools are also available. However, most of them do not explore how patients develop the specific outcomes they predict nor do they allow for what‐if treatment strategies and scenario simulations.
This review suggests that multiscale multicomponent mechanistic models combined with ML and digital twin’s generation provide the best approach to gain the needed understanding of biology and various omics leading to different treatment outcomes and to predict the response in patients with IBD to treatments.
CONFLICT OF INTEREST
P.P. is an employee of Ferring Pharmaceuticals and owns stocks in Takeda Pharmaceutical Company Limited.
ACKNOWLEDGEMENT
The author would like to thank Wiley Editing Services for editorial support, which was funded by Ferring Pharmaceuticals.
Pinton P. Computational models in inflammatory bowel disease. Clin Transl Sci. 2022;15:824‐830. doi: 10.1111/cts.13228
Funding information
No funding was received for this work
REFERENCES
- 1. Abraham C, Cho J. Inflammatory Bowel disease. N Engl J Med. 2021;361(21):2066‐2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gubatan J, Levitte S, Patel A, Balabanis T, Wei MT, Sinha SR. Artificial intelligence applications in inflammatory bowel disease: emerging technologies and future directions. World J Gastroenterol. 2020;17:1920‐1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Fischer S, Neurath MF. Precision medicine in inflammatory Bowel diseases. Clin Pharmacol Ther. 2017;102:623‐632. [DOI] [PubMed] [Google Scholar]
- 4. Fiocchi C, Dragoni G, Iliopoulos D, et al. Results of the seventh scientific workshop of ECCO: Precision Medicine in IBD – What, why and how. J Crohns Colitis. 2021;15(9):1410‐1430. [DOI] [PubMed] [Google Scholar]
- 5. DeLisi C. Mathematical modeling in immunology. Annu Rev Biophys Bioeng. 1983;12:117‐138. [DOI] [PubMed] [Google Scholar]
- 6. Bauer A, Beauchemin CPA. Agent‐based modeling in host‐pathogen systems: the successes and challenges. Inf Sci. 2009;179:1379‐1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ito H, Takazoe M, Fukuda Y, et al. A pilot randomized trial of a human anti–interleukin‐6 receptor monoclonal antibody in active Crohn’s disease. Gastroenterology. 2004;126:989‐996. [DOI] [PubMed] [Google Scholar]
- 8. Rose‐John S. Interleukin‐6 family cytokines. Cold Spring Harb Perspect Biol. 2018;10(2):a028415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Danese S, Vermeire S, Hellstren P, et al. Randomised trial and open‐label extension study of an anti‐interleukine‐6 antibody in Crohn’s disease (ANDANTE I and II). Gut. 2019;68:40‐48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Schreiber S, Aden K, Bernardes JP, et al. Therapeutic interleukin‐6 trans‐signaling inhibition by olamkicept (sgp130Fc) in patients with active inflammatory Bowel disease. Gastroenterology. 2021;160:2354‐2366. [DOI] [PubMed] [Google Scholar]
- 11. Mihara M, Kasutani K, Okazaki M, et al. Tocilizumab inhibits signal transduction mediated by both mIL‐6R and SIL‐6R, but not by the receptors of other members of IL‐6 cytokine family. Int Immunopharmacol. 2005;5:1731‐1740. [DOI] [PubMed] [Google Scholar]
- 12. Nishimoto N. Interleukin‐6 as a therapeutic target in candidate inflammatory diseases. Clin Pharmacol Ther. 2010;87(4):483‐487. [DOI] [PubMed] [Google Scholar]
- 13. Moya C, Huang Z, Cheng P, Jayaraman A, Hahn J. Investigation of IL‐6 and IL‐10 signaling via mathematical modelling. IET Syst Biol. 2011;1:15‐26. [DOI] [PubMed] [Google Scholar]
- 14. Peters LA, Perrigoue J, Mortha A, et al. A functional genomics predictive network model identifies regulators of inflammatory bowel disease. Nat Genet. 2017;49(10):1437‐1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Park A, Kim S, Jung IH, Byun JH. An immune therapy model for effective treatment on inflammatory bowel disease. PLoS One. 2020;15(9):e0238918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Iwakiri R, Udagawa E, Kobayashi T, et al. Evaluation of a computational platform for predicting temporal progression of mucosal damage and healing in patients with Crohn’s Disease. United Eur Gastroenterol J. 2021;9(S8):482, P0388. [Google Scholar]
- 17. Rogers KV, Martin SW, Bhattacharya I, Singh RSP, Nayak S. A dynamic quantitative Systems pharmacology model of inflammatory bowel disease: part 1 – Model Framework. Clin Transl Sci. 2021;14:239‐248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wendelsdorf K, Bassaganya‐Riera J, Hontecillas R, Eubank S. Model of colonic inflammation: Immune modulatory mechanisms in inflammatory bowel disease. J Theor Biol. 2010;264(4):1225‐1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Dwivedi G, Fitz L, Hegen M, et al. A multiscale model of interleukin‐6 mediated immune regulation in Crohn’s Disease and its application in drug and development. CPT Pharmacometrics Syst Pharmacol. 2014;3:e89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mei Y, Abedi V, Carbo A, et al. Multiscale modeling of mucosal immune responses. BMC Bioinformatics. 2015;16(Suppl 12):S2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Balbas‐Martinez V, Ruiz‐Cerdá L, Irurzun‐Arana I, et al. A systems pharmacology model for inflammatory bowel disease. PLoS One. 2018;13(3):e0192949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rogers KV, Martin SW, Bhattacharya I, Singh RSP, Nayak S. A dynamic quantitative Systems pharmacology model of inflammatory bowel disease: part 2 – application to current therapies in Crohn’s disease. Clin Transl Sci. 2021;14:249‐259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Pinton P. Predictive approaches in Inflammatory Bowel Disease [published online ahead of print October 30, 2021]. Clin Transl Sci. 10.1111/cts.13179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ruiz Castro PA, Yepiskoposyan H, Gubian S, et al. Systems biology approach highlights mechanistic differences between Crohn’s disease and ulcerative colitis. Sci Rep. 2021;11:11519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sahoo D, Swanson L, Sayed I, et al. Artificial Intelligence guided discovery of a barrier protective therapy in inflammatory Bowel disease. Nat Commun. 2021;12(1):4246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Allen RJ, Rieger TR, Musante CJ. Efficient generation and selection of virtual populations in quantitative systems pharmacology models. CPT Pharmacometrics Syst Pharmacol. 2016;5:140‐146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Reeh H, Rudolph N, Billing U, et al. Response to IL‐6 trans and IL‐6 classic signaling is determined by the ratio of the IL‐6 receptor α to gp130 expression: fusing experimental insights and dynamic modeling. Cell Commun Signal. 2019;17:46. [DOI] [PMC free article] [PubMed] [Google Scholar]


