Abstract
An accurate understanding of the changes in height and weight of children with age is critical to the development of models predicting drug concentrations in children (i.e., physiologically‐based pharmacokinetic models). However, curves describing the growth of a typical population of children may not accurately characterize growth of children with various conditions, such as obesity. Therefore, to develop height and weight versus age growth curves for youth who were diagnosed with type 2 diabetes, we extracted data from electronic medical records. Robust nonlinear models were parameterized to the equations describing height and weight versus age as defined by the Centers for Disease Control and Prevention (CDC). CDC z‐scores were calculated using an internal program. The growth curves and z‐scores were compared to CDC norms. Youth with type 2 diabetes were increasingly heavier than CDC norms from early childhood. Except for a period around puberty, youth with type 2 diabetes were, on average, shorter than CDC norms, resulting in shorter average adult height. Deviations in growth were apparent in youth who develop type 2 diabetes; such deviations may be expected for other conditions as well, and disease‐specific growth curves should be considered during development of model‐informed drug development for pediatric conditions.
Study Highlights.
WHAT IS THE CURRENT KNOWLEDGE ON THE TOPIC?
The Centers for Disease Control and other agencies have developed growth curves that represent typical children, but they do not extend beyond the 97th percentile. The growth of many children with type 2 diabetes is therefore not represented by these curves.
WHAT QUESTION DID THIS STUDY ADDRESS?
How does the height and weight of children who are diagnosed with type 2 diabetes change with age relative to a population of typically developing children?
WHAT DOES THIS STUDY ADD TO OUR KNOWLEDGE?
Children who develop type 2 diabetes have growth patterns that deviate from the norm.
HOW MIGHT THIS CHANGE CLINICAL PHARMACOLOGY OR TRANSLATIONAL SCIENCE?
Given that physiologically‐based pharmacokinetic scaling factors, such as liver volume, are based on body surface area, which is, itself, derived from height and weight, disease‐specific growth curves should be considered for modeling and simulation of dosing for pediatric drug development and clinical applications.
INTRODUCTION
Physiologically‐based pharmacokinetic (PBPK) models are systems‐based simulation tools that can help predict pharmacokinetics in a population for which studies have not yet been conducted, or for whom data are largely unavailable. These models can be particularly helpful in children, for whom large scale clinical trials can be challenging to implement. 1 PBPK models integrate physiological and anatomic information for the population of interest with physicochemical information for the drug. Some physiological processes, such as hepatic drug clearance, trend with patient size, and equations have been developed to provide estimates of corresponding anatomic features, such as liver volume, as a function of body size to assist with extrapolation of in vitro enzyme activity to in vivo clearance. 2 , 3 Because children continuously grow and develop during childhood, incorporation of growth curves into these PBPK models is critical for accurately simulating pharmacokinetics across the pediatric age range. Pediatric growth trajectories have been incorporated into PBPK models, but these are largely representative of healthy‐weight children and do not accurately capture the obesity epidemic of the 21st century. 4 , 5
In 2016, over 340 million children worldwide, aged 5 to 18 years, met the World Health Organization (WHO) body mass index (BMI) criteria for overweight/obesity. By 2019, 38 million more children under the age of 5 years were diagnosed with overweight or obesity. 6 These children are at the top of, or exceed, the growth trajectories predicted by the standard pediatric growth curves developed by the Centers for Disease Control and Prevention (CDC) or the WHO. This growing population of children with overweight or obesity is also at highest risk for comorbidities that require management with medications, such as type 2 diabetes mellitus (T2DM). The efficacy of medications used to treat T2DM in children varies substantially 7 and warrants further study. Yet, it is difficult to recruit and retain youth with T2DM in clinical studies, 8 therefore, PBPK can be a useful tool in supplementing or simulating clinical studies for this patient population. We, therefore, developed and evaluated growth curves specific to children who develop T2DM, most of whom have overweight/obesity. These modified growth curves are intended to be used as population‐specific scaling factors to enhance the accuracy of PBPK models that simulate the pharmacokinetics of drugs used to treat children with T2DM (e.g., metformin). This population‐specific modification to pediatric PBPK models is of particular importance given the challenges associated with recruitment of children into T2DM‐drug studies that, in turn, may limit the availability of robust, prospective pharmacokinetic data in this vulnerable and growing pediatric population. 8
METHODS
Dataset
Height, weight, age, and sex for children 2–18 years of age were extracted from an institutional review board‐approved deidentified dataset of visits to providers at Children’s Mercy Kansas City, a Midwest tertiary pediatric health system with hospitals and clinics located in Kansas and Missouri. Data were from visits at Children’s Mercy Clinics recorded between January 1, 2010 and August 24, 2016. Additionally, for some patients with data recorded in 2010 or later, data as early as May 30, 1997 was converted from an older system. Extracted data included visits before and/or after T2DM diagnosis based on International Classification of Disease (ICD)‐9 or ICD‐10 codes (e.g., ICD‐9: 250.00, etc.). Children with a diagnosis of type 1 diabetes were excluded. Electronic health record‐reported height less than 12 or greater than 96 inches, weight = 0 or greater than 600 pounds, and/or BMI less than 7 or greater than 80 kg/m2 were also excluded. Whereas we primarily expect that BMIs greater than 80 kg/m2 are due to measurement or data entry error, we do rarely see patients above these BMI limits. Therefore, we additionally evaluated each of these 13 excluded datapoints in the context of other datapoints for that patient and in all cases found one outlying data point not in line with other points for that patient and clinically implausible, classifying these as data entry errors versus the outlying extremes of real data.
Data were binned in 6‐month intervals and pruned using the modified Thompson‐Tau algorithm (α = 1 × 10−4 for height; α = 1 × 10−5 for weight) to identify and eliminate any outliers (e.g., data‐entry errors). To minimize the removal of real data, data belonging to a patient with greater than three measurements were retained.
To facilitate the development of a smooth curve (i.e., no sudden model “dropoffs”) for the older pediatric age‐range and to evaluate final adult height, data from patients 19–25 years were also included in model development. Datapoints greater than three SDs from the mean height or weight of this older age group were removed.
Model development
The data were randomized (Figure 1). Models were developed on 90% of the data (training set); 10% of the data were reserved for validation after model development. The coefficients (e.g., a, p) of CDC growth functions 5 were fit to the height and weight of children in our dataset. An iteratively reweighted least squares model, which is a preferred model for data with increasing variability (i.e., weight variance increases with age), was fitted using an M‐estimator by the nlrob function in the robustbase package (version 0.93–8). 9 ) in R Studio 10 (1.4.1717 “Juliet Rose,” R version 4.1.1) using the below equations and the coefficients of the 50th percentile CDC growth curves for children older than 2 years 5 as starting parameters. When model convergence could not be reached using these starting parameters, a preliminary model was fitted by the Levenberg‐Marquadt algorithm (nlsLM function in minpack.lm version 1.2‐1 package 11 ); the resulting coefficients were used as starting parameters for the iteratively reweighted least squares model. Height is obtained as a standing measurement (stature).
FIGURE 1.
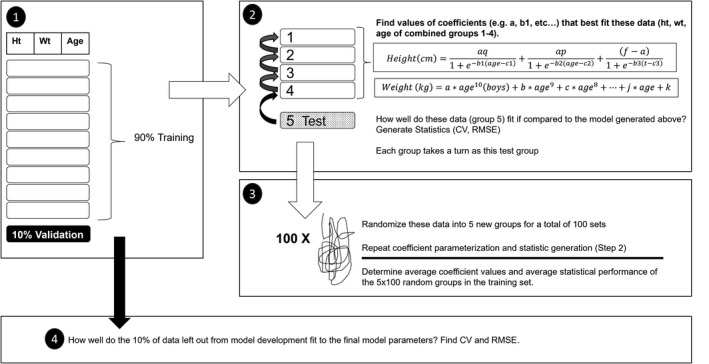
Model development scheme. The dataset was split (step 1) into training (90%) and validation sets (10%). The coefficients for each model were parameterized for the training set using 100 × 5‐fold cross validation (steps 2 and 3), and the performance of the validation set on the final model was determined (step 4). CV, coefficient of variation; RMSE, root mean squared error
To obtain well‐fitted parameters, 100 × 5‐fold cross validation was used. In this process, the training set was randomized into five approximately equally sized groups. Four of the five groups (~80% of the training set) were combined and model coefficients were fit to these data. Then the coefficient of variation (CV) and root mean squared error (RMSE) were calculated for the remaining group’s data (~20% of the training set) fit to the model described by the set of those coefficients. This process of combining four of the five groups to determine the best‐fit model coefficients, and then testing how well the resulting model fits (using CV and RMSE) on the remaining group, was repeated five times, with each group serving once to test. The entire training set was then randomized into five new, comparably sized groups, and the process was repeated. In total, 100 dataset randomizations, each split into five groups, were conducted (100 × 5‐fold cross validation). The means of the CV and RMSE for these 500 groups were calculated (Figure 1).
Because the height model is not additive, heights were predicted for every model at 1‐month intervals and averaged. A final model (as described above) was then fitted through the average predicted heights. The coefficients generated in 100 × 5‐fold cross validation were averaged for the final weight model.
Model performance
The CV and RMSE were calculated for the validation set fit to the final model to establish how well this model might perform on an external dataset. Growth model performance was assessed by comparing the RMSE and CV between the training set and validation set. Similar errors (RMSE and CV) between the training and validation sets on the developed model indicate that the model is not overfit to the data on which it was developed and, therefore, should be applicable to external datasets.
Comparison to CDC growth curves
To compare the fit of actual data of patients with T2DM to the CDC growth curves and the T2DM growth curves developed in this study, the RMSE and CV of the validation set fitted to the 50th percentile CDC equations and were compared to the errors of the validation set for the T2DM‐specific model. Larger values indicate a poorer fit to the model. The final height of the model output was compared with the final height of the CDC 50th percentile height‐growth curves, and the means of final height between 18.5 and 19 years (women) or 18.0 and 18.5 years (men, due to sample size) for the T2DM dataset and the CDC dataset were compared using the Student’s t‐test.
Z‐scores for the entire dataset, the number of SDs from the CDC mean of height and weight for sex and age, were calculated using an internal program. 12 Z‐scores were binned in 6‐month intervals and the mean, SD, and percent of patients with z‐scores greater than 1 or less than −1 were calculated.
Height‐growth velocity
The velocity of T2DM height‐growth was calculated as the first‐derivative of the final T2DM‐specific height models—a calculus‐derived equation describing the rate‐of‐change of height‐growth as a function of age (cm/year).
RESULTS
The final dataset used to develop the T2DM growth model included 211 women and 145 men (Table 1). There were 13 datapoints (of 12,602 total BMI datapoints) from 13 patients excluded as a result of BMI greater than 80 kg/m2; all 13 points were inconsistent with other BMIs reported for the patients, clinically implausible points, and were thus excluded as data‐entry errors. Figure 2 shows the measured height or weight versus age and the model overlay. Truncated versions of the final equations are as follows:
TABLE 1.
Description of dataset
| Female | Male | |
|---|---|---|
| Height | ||
| Number of training points | 3973 | 2610 |
| Number of validation points | 458 | 306 |
| Years of data per patient | ||
| Range | 0–15 | 0–15.67 |
| Mean (SD) | 5.41 (4.43) | 5.70 (4.55) |
| Datapoints per patient | ||
| Range | 1–230 | 1–199 |
| Mean (SD) | 21.62 (30.51) | 20.53 (28.07) |
| Weight | ||
| Number of training points | 5189 | 3350 |
| Number of validation points | 576 | 380 |
| Years of data per patient | ||
| Range | 0–15.49 | 0–15.67 |
| Mean (SD) | 6.13 (4.66) | 6.17 (4.65) |
| Datapoints per patient | ||
| Range | 1–293 | 1–143 |
| Mean (SD) | 27.99 (39.97) | 26.08 (34.83) |
FIGURE 2.
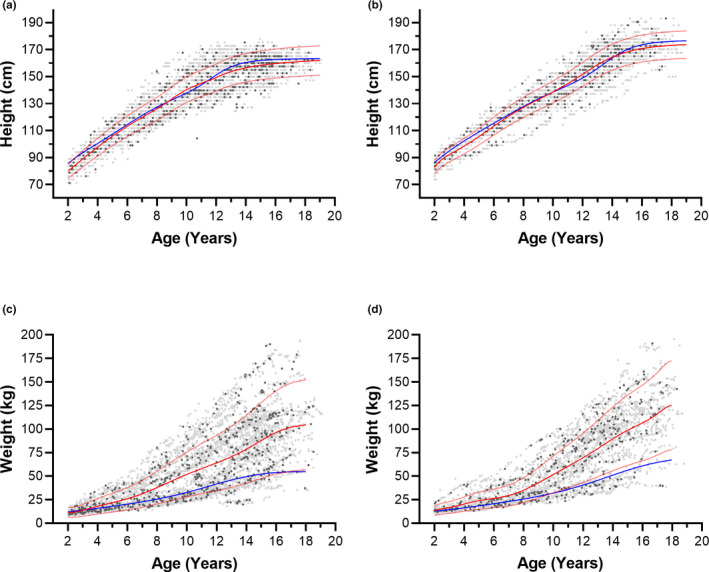
Height or weight of children who develop type 2 diabetes mellitus versus age compared with patient‐specific model of growth (red) or the 50th percentile CDC model (blue). (a) height, females (b) height, males (c) weight, females (d) weight, males. Grey symbols, training set; black symbols, testing set. Faint red lines correspond to the standard deviation, derived from the coefficient of variation (%) of the datapoints from the model. CDC, Centers for Disease Control and Prevention
Male height (cm, age in years)
Female height (cm, age in years)
Male weight (kg, age in years)
Female weight (kg, age in years)
The coefficients for weight reporting six significant figures are in Supplemental Information S1. Due to the high‐degree polynomial, and some very small coefficients, at least six significant figures appear to be needed in the regression coefficients to accurately estimate the weights as a function of age. This is in line with the CDC estimates of weight at each percentile, which are reported to ten significant figures. 5
The training and validation sets had similar CVs and RMSEs, indicating that the growth model was not overfit to the training data. The error rates (CV and RMSE) of the height and weight of children who develop T2DM fitted to the CDC model were substantially larger for weight and marginally larger for height than when they were fitted to T2DM growth models, indicating that CDC growth models are less accurate in describing the growth of children who develop T2DM (Table 2). Absolute residuals of the datapoints from the model, as well as percent error from the model are shown in Supplemental Information [Link], [Link]. Heights and weights of children who develop T2DM, as simulated in Simcyp version 20 13 (n = 1000 simulations) using the reported equations and %CV are shown in Supplemental Information S3, overlayed with the measured heights and weights in the reported dataset.
TABLE 2.
Fits of reference growth curves versus T2DM‐specific growth curve to data from T2DM children
| Validation set fit to growth model | Model development test set performance (SD) | ||
|---|---|---|---|
| CDC | T2DM | ||
| Coefficients of variation (%) | |||
| Height | |||
| Female | 7.16 | 6.75 | 6.66 ± 0.15 |
| Male | 5.96 | 5.87 | 6.24 ± 0.21 |
| Weight | |||
| Female | 65.1 | 45.9 | 44.6 ± 0.92 |
| Male | 61.4 | 37.8 | 38.6 ± 1.11 |
| Root mean squared error | |||
| Height | |||
| Female | 9.89 | 9.33 | 9.21 ± 0.21 |
| Male | 8.4 | 8.27 | 8.87 ± 0.30 |
| Weight | |||
| Female | 37.4 | 26.3 | 24.8 ± 0.16 |
| Male | 36.6 | 22.6 | 23.3 ± 0.68 |
Abbreviations: CDC, United States Center for Disease Control; T2DM, type 2 diabetes mellitus.
On average, children who develop T2DM were slightly shorter (Figure 3) and substantially heavier than CDC means (Figure 4). Mean height z‐score was less than 0 (shorter than CDC average) in 30 of 34 (female children) and 25 of 34 (male children) age bins. The scores greater than 0 occurred between 8.5 and 11.5 years (female children) and 10.0–14.5 years (male children). A local peak height velocity of T2DM growth curves (Figure 5) occurred at 8.5 years for female children, and 12.5 years for male children. Ultimately, male and female children with T2DM were shorter than CDC averages by 2 and 3 cm, respectively (both p < 0.01). 5 Except for female children between 2 and 4.5 years of age, mean z‐scores were greater than 0 for weight. Weight z‐score increased with age (Spearman R = 0.911 [female children], 0.903 [male children], p < 0.0001). An exploratory analysis suggested that there may be differences in growth depending on self‐reported race or ethnicity (Supplemental Information S4 and S5).
FIGURE 3.
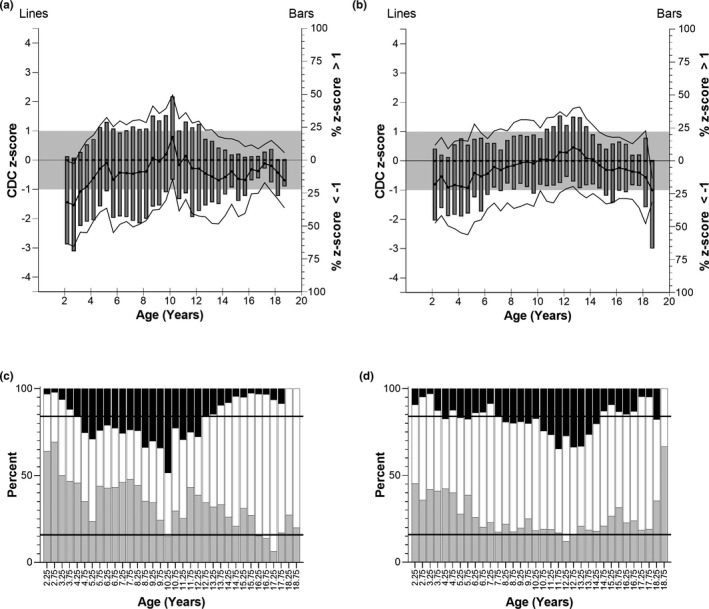
Top panels: CDC z‐scores of height for T2DM children. (a) Female patients, and (b) male patients. Left y‐axis: CDC z‐score of T2DM children (mean (bold lines) ± SD (faint lines)) binned into 6‐month intervals. The grey box indicates one standard deviation above and below the average CDC height (z = 0). Right y‐axis: Bars represent the percent of T2DM children in the age bin with z‐score greater than 1 or less than −1. Bottom panels: Distribution of patient height z‐scores greater than 1 (black bars), between −1 and 1 (white bars), and less than −1 (grey bars). (c) Female patients, (d) male patients. The expected distribution if T2DM patients at each age and sex corresponded to the CDC range would be that 15.9% of patients would fall below −1 (lower horizontal line), 68.2% would fall between the lines, and 15.9% would be greater than 1 (upper horizontal line). CDC, Centers for Disease Control and Prevention; T2DM, type 2 diabetes mellitus
FIGURE 4.
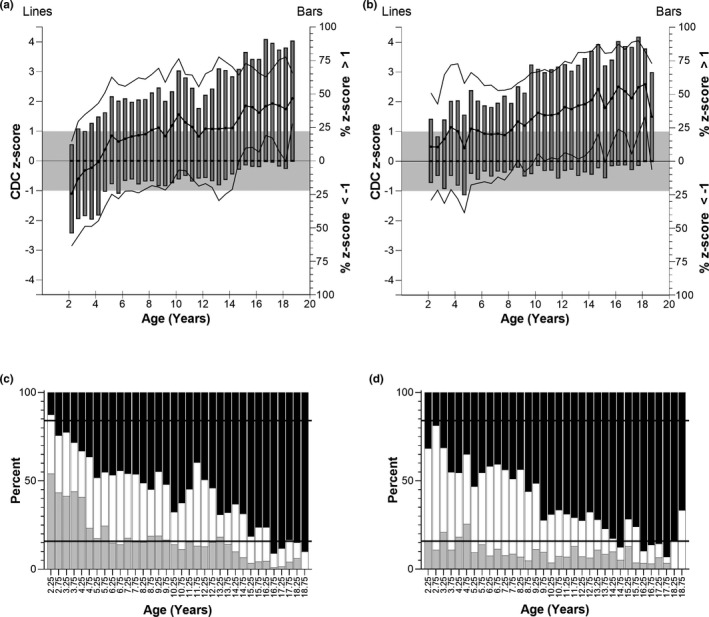
Top panels: CDC z‐scores of weight for T2DM children. (a) Female patients, (b) male patients. Left y‐axis: CDC z‐score of T2DM children (mean (bold lines) ± SD (faint lines)) binned into 6‐month intervals. The grey box indicates one SD above and below the average CDC weight (z = 0). Right y‐axis: Bars represent the percent of T2DM children in the age bin with z‐score greater than 1 or less than −1. Bottom panels: Distribution of patient weight z‐scores greater than 1 (black bars), between −1 and 1 (white bars), and less than −1 (grey bars). (c) Female patients, (d) male patients. The expected distribution if T2DM patients at each age and sex corresponded to the CDC range would be that 15.9% of patients would fall below −1 (lower horizontal line), 68.2% would fall between the lines, and 15.9% would be greater than 1 (upper horizontal line). CDC, Centers for Disease Control and Prevention; T2DM, type 2 diabetes mellitus
FIGURE 5.
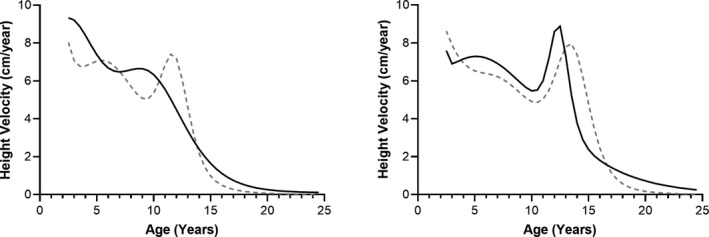
Velocity of height growth per year. Left: female, right: male. The first derivative of the growth models. Grey dashed lines, CDC; black lines, type 2 diabetes. CDC, Centers for Disease Control and Prevention
DISCUSSION
Obesity is a significant risk factor in the development of T2DM, and nearly all children who develop T2DM have overweight or obesity. However, standardized growth patterns in youths with T2DM have not been developed and the dataset presented here suggests that the most recent 2002 CDC‐developed growth curves do not accurately capture the pediatric T2DM growth trajectory. Despite nearly one in five US children having obesity (i.e., BMI >95th percentile for age and sex)—sometimes exceeding BMI of 40 kg/m2 and/or the 99th percentile for age and sex (class III obesity)—the CDC‐developed pediatric growth curves only capture a maximum BMI of 36 kg/m2 and the 97th percentile BMI for age and sex. 5 As a result, there is an inability to specifically describe or capture the growth trajectory of children with severe obesity (about 20% of our dataset, data not shown). Because most children with T2DM have obesity, PBPK models incorporating the CDC‐growth chart data may not accurately simulate pharmacokinetics in children with obesity/T2DM. Given the challenges with recruiting and completing prospective clinical trials in children with T2DM, 8 the use of PBPK simulations could provide a vital tool for evaluating drug disposition in this population. Thus, we developed height and weight versus age growth curves analogous to CDC growth curves, but specific to children who develop T2DM.
Our data indicate there are deviations in the growth patterns between youths ultimately diagnosed with T2DM in childhood and typical healthy‐weight growth trajectories as described by the current CDC standard: the errors (CV and RMSE) of the validation set fitted to the CDC models were larger than when fitted to the T2DM growth model. This observation supports the premise that T2DM‐specific growth curves are required to accurately describe the growth of children who develop youth‐onset T2DM and that these curves have the potential to improve the accuracy of PBPK model predictions for these children during pharmacology trial simulation.
Our data indicate that female children who developed T2DM were below average weight during early childhood, yet rapidly and consistently increased in weight z‐score beyond 4.5 years (Figure 3). Conversely, the male children who developed T2DM were increasingly heavy relative to CDC standards at all ages. Although we were unable to evaluate birthweight, the observed patterns suggest that some of these young female children with below average weights may have been born with low birth weight and/or small for gestational age (SGA), and could represent a subset of the 15% of SGA children who do not catch up by 2 years of age. 14 Both low and high birthweight are associated with the development of T2DM in adults, and multiple studies have shown this association is stronger in female compared to male children, 15 , 16 supporting our conclusions. The mechanism behind these sex‐based differences is undetermined, although postulated to be due to in utero sex hormones, particularly testosterone. 16
Prior studies have shown that adults who develop T2DM tend to experience rapid weight gain between 2 and 12 years of age. 17 , 18 Our data indicate that this weight gain may actually be prolonged until 16–17 years of age among those with youth‐onset T2DM. Because of the strong association between overweight/obesity and T2DM, it is plausible that this prolonged period of rapid weight gain is representative of a more severe obesity phenotype, contributing to earlier onset of T2DM in pediatrics. The treatment of T2DM in our cohort may have also influenced growth trajectories, especially if patients were treated with insulin. Although insulin therapy is effective at improving glycemic control in diabetes, it is also associated with weight gain. 19 Information regarding patient‐specific treatments was beyond the scope of this dataset, yet it is conceivable that insulin initiated for T2DM treatment may have impacted trends in weight gain among the study population. Conversely, treatment with metformin is not anticipated to contribute to rapid weight gain, and if anything may have curtailed the rapid weight gain captured in our model, albeit with inconsistent success. 20
The modest deviations observed in height were not anticipated. It was expected that the mean height z‐scores would approximate zero, similar to CDC growth curves. Instead, both male and female children who developed T2DM were—on average—shorter than expected as represented by CDC curves (z‐score <0) during the majority of their early childhood and later adolescence. Based on our incorporation of continued T2DM growth trajectories for young adulthood (i.e., 19–25 years), these children ultimately achieved a shorter final adult height (3 cm shorter in female patients, 2 cm shorter in male patients) than expected from averages reported by the CDC. Robust analysis of height‐specific data among T2DM populations are lacking, although some studies suggest adults with glucose intolerance and T2DM are modestly, but significantly shorter than control subjects. 21 , 22 One limitation of our deidentified dataset is the possibility that concomitant syndromic conditions associated with T2DM and short stature (e.g., Turner syndrome and Prader Willi Syndrome) were inadvertently included in this analysis, potentially skewing the final adult height results. Variation in the racial/ethnic makeup of our population compared to those used for CDC growth curves may have also contributed to discrepancies in height. Indeed, an exploratory analysis revealed significant differences in the heights and weights, as well as preliminary fitted curves for children in our dataset based on self‐identified race (Supplemental Information S4). However, evidence suggests that children have similar growth potential, with differences attributed to primarily environmental factors versus genetic. 5 Finally, differences in pubertal timing may have impacted epiphyseal fusion, particularly in female patients, and thus impacted obtainment of the expected adult height, as discussed below.
One exception to the overall tendency toward shorter stature for children who develop T2DM compared to CDC‐standards was from ages 8.5–11.5 years for girls and 10.0–14.5 years for boys. During this time, thought to coincide with puberty onset, T2DM‐specific growth curves demonstrated taller stature than expected based on CDC curves. We hypothesize this is related to slightly earlier timing of the pubertal growth spurt. Indeed, a local peak height‐growth velocity occurred at 8.5 years for girls who develop T2DM, and 12.5 years for boys who develop T2DM, which is earlier than the accepted standard ages of pubertal growth spurt 11–11.5 23 , 24 , 25 , 26 for healthy girls and 13–14 for boys. 23 , 24 , 25 , 26 Obesity 27 and hyperinsulinemic insulin resistance 28 may shift puberty earlier, which could potentially result in the early growth spurt observed here. In female patients, but not male patients, earlier growth spurts and puberty 28 , 29 , 30 are associated with decreased adult height.
Although this study was not designed to assess the pubertal timeline, our data and others suggest the potential for earlier puberty in children with T2DM. Puberty is accompanied by behavioral changes that may affect drug compliance and lifestyle (e.g., diet, exercise, and illicit drug use), and is associated with increased insulin resistance. Furthermore, puberty has the potential to affect pharmacokinetics 31 by altering factors such as plasma protein concentrations, fat distribution, and high concentrations of sex steroids, and therefore may affect the systemic exposure and distribution, and thus effect of antidiabetic drugs and other therapeutics. An earlier progression to puberty is therefore not trivial and additional therapeutic and clinical monitoring may be advisable based on our data.
The trends characterized in this study are important for the development of PBPK models that accurately capture growth trajectories across the pediatric age range. Nevertheless, our study has several limitations. First, our findings are representative of a single regional pediatric hospital, and variability in these trends will need to be studied in a larger dataset, including other hospitals with different patient demographics, local resources, regional cultures, etc. Second, metformin—the first‐line pharmaceutical treatment for children with T2DM—may ameliorate height deficits and delay puberty in children with early puberty. 28 , 29 We acknowledge that the introduction of this necessary treatment has the potential to confound a complete understanding of the effects of T2DM on pubertal timing. Additionally, while we suspect that our growth models support a hypothesis of premature puberty, this could not be confirmed by Tanner staging, given the nature of our dataset. The initiation of T2DM treatments, (e.g., metformin and insulin) in this sample population could confound a complete understanding of the effects of T2DM on growth. Last, in the United States, after the age of 2 years, weight is generally evaluated as a function of stature (standing height), as opposed to age. However, pediatric PBPK models often report weight as a function of age and thus our models were built to best represent the input required for these models.
In summary, we have shown that the development of T2DM during childhood or adolescence is associated with growth patterns that deviate from those characterized by CDC growth curves. As expected, children with T2DM exhibited greater‐than‐average weights and prolonged duration of abnormal weight gain. Unexpectedly, children who develop T2DM appear to attain a slightly shorter stature than their peers by adulthood, but may have an early growth spurt, potentially due to earlier onset of puberty. Further longitudinal research on larger populations will be necessary to confirm these observations and to determine whether the observed differences in growth trajectories are similar across pediatric centers. PBPK simulations of drug concentrations in children rely on scaling factors, such as liver volume, which can be estimated for children with normal weight, as well as obesity, as a function of body surface area, 2 , 3 which can be calculated with height and weight. Therefore, reliable estimates of height and weight may be critical to accurately estimating drug concentrations in the pediatric population of interest, 32 and the development of growth curves accurately reflecting the pediatric patient population of interest is an essential preliminary step in pediatric PBPK model development when growth trajectories for the population differ substantially from typical healthy peers (e.g., children with obesity and children who develop T2DM). Differences observed between the T2DM and CDC growth curves suggest that disease conditions in children may deviate from the “usual” patterns of growth, with potential implications for modeling and simulation in those diseases (e.g., cerebral palsy and cystic fibrosis). The use of disease‐specific population growth curves should be considered for pediatric drug development and model‐informed precision dosing.
CONFLICT OF INTEREST
All authors declared no competing interests for this work.
AUTHOR CONTRIBUTIONS
C.M.H., K.H., V.S., and J.S.L. wrote the manuscript. C.M.H., C.B., B.S., Y.Y., and J.S.L. designed the research. C.M.H. performed the research. C.M.H., K.H., and J.S.L. analyzed the results. C.B. and J.S.L. contributed the z‐score calculation tool for this study.
Supporting information
Supplementary Material S1
Supplementary Material S2a
Supplementary Material S2b
Supplementary Material S3
Supplementary Material S4
Supplementary Material S5
ACKNOWLEDGEMENTS
The authors would like to thank Dr. Susan Abdel‐Rahman, Dr. Mark Hoffman, Mr. Warren Teachout, and Dr. Vincent Staggs for fruitful discussions regarding the development of growth curves, use of electronic health records, and statistical methods.
Hosey CM, Halpin K, Shakhnovich V, et al. Pediatric growth patterns in youth‐onset type 2 diabetes mellitus: Implications for physiologically‐based pharmacokinetic models. Clin Transl Sci. 2022;15:912‐922. doi: 10.1111/cts.13207
Funding information
Salary support for C.M.H. was provided by institutional pediatric clinical pharmacology training funds, Children’s Mercy Kansas City. This research was partially supported by grant 1 P50HD090258 (J.S.L.) and by a Children’s Mercy Hospital Fellow Research Grant (C.M.H.).
REFERENCES
- 1. Shakhnovich V, Hornik CP, Kearns GL, Weigel J, Abdel‐Rahman SM. How to conduct clinical trials in children: a tutorial. Clin Transl Sci. 2019;12(3):218‐230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hosey‐Cojocari C, Chan SS, Friesen CS, et al. Are body surface area based estimates of liver volume applicable to children with overweight or obesity? An in vivo validation study. Clin Transl Sci. 2021;14:2008‐2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Johnson TN, Tucker GT, Tanner MS, Rostami‐Hodjegan A. Changes in liver volume from birth to adulthood: a meta‐analysis. Liver Transpl. 2005;11(12):1481‐1493. [DOI] [PubMed] [Google Scholar]
- 4. Wright C, Booth I, Buckler J, et al. Growth reference charts for use in the United Kingdom. Arch Dis Child. 2002;86:11‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kuczmarski RJ, Ogden CL, Guo SS, et al. 2000 CDC growth charts for the United States: methods and development. Vital Health Stat 11. 2002;246:1‐190. [PubMed] [Google Scholar]
- 6. Obesity and Overweight World Health Organization 2020. Available from: https://www.who.int/news‐room/fact‐sheets/detail/obesity‐and‐overweight.
- 7. Today Study Group . Effects of metformin, metformin plus rosiglitazone, and metformin plus lifestyle on insulin sensitivity and beta‐cell function in TODAY. Diabetes Care. 2013;36(6):1749‐1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Nadeau KJ, Anderson BJ, Berg EG, et al. Youth‐onset type 2 diabetes consensus report: current status, challenges, and priorities. Diabetes Care. 2016;39(9):1635‐1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Maechler M, Rousseeuw P, Croux C, et al. Basic Robust Statistics. 0.93‐8 ed2021. https://cran.irsn.fr/web/packages/robustbase/robustbase.pdf.
- 10. RStudio Team . RStudio: Integrated Development for R. RStudio P; 2021. http://www.rstudio.com/. [Google Scholar]
- 11. Elzhov TV, Mullen KM, Spiess A‐N, Bolker B. R Interface to the Levenberg‐Marquardt Nonlinear Least‐Squares Algorithm Found in MINPACK, Plus Support for Bounds. 1.2‐1 ed2016. https://cran.r‐project.org/web/packages/minpack.lm/minpack.lm.pdf.
- 12. Bi C, Leeder JS, editors. Large‐Scale Computation of Pediatric Growth Percentiles with Fuzzy Logic Justification of Parameter Selection. Proceedings of The IEEE Computational Intelligence Symposium on Bioinformatics & Computational Biology (CIBCB); 2012. IEEE Press; 2012:43‐46. [Google Scholar]
- 13. Certara UK Limited . Simcyp. 20 ed2020.
- 14. Hokken‐Koelega AC, De Ridder MA, Lemmen RJ, Den Hartog H, De Muinck Keizer‐Schrama SM, Drop SL. Children born small for gestational age: do they catch up? Pediatr Res. 1995;38(2):267‐271. [DOI] [PubMed] [Google Scholar]
- 15. Knop MR, Geng TT, Gorny AW, et al. Birth weight and risk of type 2 diabetes mellitus, cardiovascular disease, and hypertension in adults: a meta‐analysis of 7 646 267 participants from 135 studies. J Am Heart Assoc. 2018;7(23):e008870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zimmermann E, Gamborg M, Sørensen TIA, Baker JL. Sex differences in the association between birth weight and adult type 2 diabetes. Diabetes. 2015;64(12):4220‐4225. [DOI] [PubMed] [Google Scholar]
- 17. Eriksson JG, Osmond C, Kajantie E, Forsén TJ, Barker DJP. Patterns of growth among children who later develop type 2 diabetes or its risk factors. Diabetologia. 2006;49(12):2853‐2858. [DOI] [PubMed] [Google Scholar]
- 18. Bhargava SK, Sachdev HS, Fall CH, et al. Relation of serial changes in childhood body‐mass index to impaired glucose tolerance in young adulthood. N Engl J Med. 2004;350(9):865‐875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jansen HJ, Vervoort GM, de Haan AF, Netten PM, de Grauw WJ, Tack CJ. Diabetes‐related distress, insulin dose, and age contribute to insulin‐associated weight gain in patients with type 2 diabetes: results of a prospective study. Diabetes Care. 2014;37(10):2710‐2717. [DOI] [PubMed] [Google Scholar]
- 20. Friesen CS, Hosey‐Cojocari C, Chan SS, et al. Efficacy of weight reduction on pediatric nonalcoholic fatty liver disease: opportunities to improve treatment outcomes through pharmacotherapy. Front Endocrinol (Lausanne). 2021;12:663351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Brown DC, Byrne CD, Clark PM, et al. Height and glucose tolerance in adult subjects. Diabetologia. 1991;34(7):531‐533. [DOI] [PubMed] [Google Scholar]
- 22. Vangipurapu J, Stancakova A, Jauhiainen R, Kuusisto J, Laakso M. Short adult stature predicts impaired beta‐cell function, insulin resistance, glycemia, and type 2 diabetes in Finnish Men. J Clin Endocrinol Metab. 2017;102(2):443‐450. [DOI] [PubMed] [Google Scholar]
- 23. Kelly A, Winer KK, Kalkwarf H, et al. Age‐based reference ranges for annual height velocity in US children. J Clin Endocrinol Metab. 2014;99(6):2104‐2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Baumgartner RN, Roche AF, Himes JH. Incremental growth tables: supplementary to previously published charts. Am J Clin Nutr. 1986;43(5):711‐722. [DOI] [PubMed] [Google Scholar]
- 25. Roche AF, Himes JH. Incremental growth charts. Am J Clin Nutr. 1980;33(9):2041‐2052. [DOI] [PubMed] [Google Scholar]
- 26. Liu YX, Wikland KA, Karlberg J. New reference for the age at childhood onset of growth and secular trend in the timing of puberty in Swedish. Acta Paediatr. 2000;89(6):637‐643. [DOI] [PubMed] [Google Scholar]
- 27. Ahmed ML, Ong KK, Dunger DB. Childhood obesity and the timing of puberty. Trends Endocrinol Metab. 2009;20(5):237‐242. [DOI] [PubMed] [Google Scholar]
- 28. Ibáñez L, Lopez‐Bermejo A, Diaz M, Marcos MV, de Zegher F. Early metformin therapy to delay menarche and augment height in girls with precocious pubarche. Fertil Steril. 2011;95(2):727‐730. [DOI] [PubMed] [Google Scholar]
- 29. Ibanez L, Valls C, Ong K, Dunger DB, de Zegher F. Metformin therapy during puberty delays menarche, prolongs pubertal growth, and augments adult height: a randomized study in low‐birth‐weight girls with early‐normal onset of puberty. J Clin Endocrinol Metab. 2006;91(6):2068‐2073. [DOI] [PubMed] [Google Scholar]
- 30. Yousefi M, Karmaus W, Zhang H, et al. Relationships between age of puberty onset and height at age 18 years in girls and boys. World J Pediatr. 2013;9(3):230‐238. [DOI] [PubMed] [Google Scholar]
- 31. Carr RR, Ensom MH. Drug disposition and therapy in adolescence: the effects of puberty. J Pediatr Pharmacol Ther. 2003;8(2):86‐96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hosey‐Cojocari CHK, Yan Y, Leeder J. Towards an individualized dosing strategy to reduce pharmacokinetic (PK) variability and improve response to metformin in children with insulin resistance or type 2 diabetes. Clin Pharmacol Ther. 2020;107(Suppl 1):S110. Abstract PIII‐7. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material S1
Supplementary Material S2a
Supplementary Material S2b
Supplementary Material S3
Supplementary Material S4
Supplementary Material S5


