Abstract
A high intrapatient variability (IPV) in tacrolimus exposure is a risk factor for poor long‐term outcomes after kidney transplantation. The main objective of this trial was to investigate whether tacrolimus IPV decreases after switching patients from immediate‐release (IR)‐tacrolimus to either extended‐release (ER)‐tacrolimus or LifeCyclePharma (LCP)‐tacrolimus. In this randomized, prospective, open‐label, cross‐over trial, adult kidney transplant recipients on a stable immunosuppressive regimen, including IR‐tacrolimus, were randomized for conversion to ER‐tacrolimus or LCP‐tacrolimus, and for the order in which IR‐tacrolimus and the once‐daily formulations were taken. Patients were followed 6 months for each formulation, with monthly tacrolimus predose concentration assessments to calculate the IPV. The IPV was defined as the coefficient of variation (%) of dose corrected predose concentrations. Ninety‐two patients were included for analysis of the primary outcome. No significant differences between the IPV of IR‐tacrolimus (16.6%) and the combined once‐daily formulations (18.3%) were observed (% difference +1.7%, 95% confidence interval [CI] −1.1% to ‒4.5%, p = 0.24). The IPV of LCP‐tacrolimus (20.1%) was not significantly different from the IPV of ER‐tacrolimus (16.5%, % difference +3.6%, 95% CI −0.1% to 7.3%, p = 0.06). In conclusion, the IPV did not decrease after switching from IR‐tacrolimus to either ER‐tacrolimus or LCP‐tacrolimus. These results provide no arguments to switch kidney transplant recipients from twice‐daily (IR) tacrolimus formulations to once‐daily (modified‐release) tacrolimus formulations when the aim is to lower the IPV.
Study Highlights.
WHAT IS THE CURRENT KNOWLEDGE ON THE TOPIC?
High intrapatient variability (IPV) in tacrolimus exposure is associated with poor kidney transplant outcomes. Different causes of a high IPV are considered, like drug‐drug interactions, food intake, and adherence and the type of tacrolimus formulation.
WHAT QUESTION DID THIS STUDY ADDRESS?
Does switching from a twice‐daily immediate‐release (IR) formulation to a once‐daily modified‐release tacrolimus formulation with a flatter pharmacokinetic (PK) profile lower the IPV of tacrolimus in stable kidney transplant recipients?
WHAT DOES THIS STUDY ADD TO OUR KNOWLEDGE?
We could not demonstrate a decrease in tacrolimus IPV after switching from IR‐ to modified‐release tacrolimus formulations in stable kidney transplant recipients. Tremor, an important side effect of tacrolimus, seems to occur less frequently with LifeCyclePharma‐tacrolimus compared to IR‐tacrolimus.
HOW MIGHT THIS CHANGE CLINICAL PHARMACOLOGY OR TRANSLATIONAL SCIENCE?
Tacrolimus IPV seems not to be influenced by different PK profiles of the different tacrolimus formulations.
INTRODUCTION
Tacrolimus has a narrow therapeutic index and therapeutic drug monitoring (TDM) is routinely performed to prevent both under‐ and over‐immunosuppression. 1
Besides a high between‐patient variability, tacrolimus concentrations also have a high intrapatient (i.e., within‐patient) variability (IPV). The IPV, often expressed as the coefficient of variation (CV), is defined as the fluctuation of (whole‐) blood tacrolimus concentrations drawn at the same time interval after dose ingestion within an individual over time. A high tacrolimus IPV has been associated with poor clinical outcomes in several studies, including the development of de novo donor‐specific anti‐HLA antibodies, fibrotic damage to the transplanted kidney, and inferior graft survival. 2 , 3 , 4 , 5 , 6 , 7 , 8 In some studies, this association was only present in subgroups of patients with a high immunological risk or a chronic antibody mediated rejection. 9 , 10 Known causes of a high IPV in tacrolimus exposure are medication nonadherence, drug‐drug interactions, concomitant food intake, and gastrointestinal disorders. Other potential determinants of tacrolimus IPV are the pharmaceutical drug formulation and pharmacogenetics (CYP3A5 genotype). 11 , 12 Tacrolimus is metabolized by CYP3A4 and CYP4A5 enzymes. CYP3A5 expressors require higher tacrolimus doses to reach the target level compared with patients who are dependent on CYP3A4 (CYP3A5 nonexpressors). CYP3A4 is more sensitive to induction and inhibition (e.g., by comedication) and it is hypothesized that IPV is higher in CYP4A5 nonexpressors. However, the role of this gene is not fully elucidated and studies on CYP3A5 and IPV show conflicting results. 13 , 14 , 15 , 16
Use of simplified once‐daily, modified‐release tacrolimus formulations may reduce tacrolimus IPV by improving medication adherence. 17 In addition, switching to another tacrolimus formulation may also have a beneficial effect on IPV, by providing a more stable pharmacokinetic (PK) profile due to more stable absorption. Therefore, several investigators performed studies to evaluate the impact on IPV of tacrolimus exposure of switching patients from an immediate‐release (IR) twice‐daily tacrolimus formulation to a once‐daily formulation. In 150 Taiwanese kidney transplant recipients switched from tacrolimus twice‐daily (b.i.d.) to tacrolimus once‐daily (q.d.) the IPV of predose tacrolimus concentrations (C0) decreased significantly from 14.5% to 11.7%. 18 In addition, Stifft et al. converted 40 renal transplant recipients from tacrolimus b.i.d. to tacrolimus q.d. and observed a statistically significant reduction of the IPV of the area under the 24‐h blood concentration‐time curve (AUC0–24) from 14.1% to 10.9%. However, the mean tacrolimus IPV in C0 did not change. 19 Both studies had a before‐and‐after design and lacked a control group that remained on the IR formulation. In both studies the extended‐release (ER) tacrolimus formulation Advagraf was used. Other studies did not show an effect of switching from twice‐daily to once‐daily tacrolimus on IPV. 20 , 21
LifeCyclePharma (LCP)‐tacrolimus (Envarsus) is a more recently registered, ER formulation of tacrolimus designed for once‐daily administration. This formulation is based on the MeltDose drug delivery technology, designed to improve the bioavailability of drugs with a low water solubility. The PK profile of LCP‐tacrolimus is characterized by a higher bioavailability, reduced peak concentrations, and lower peak‐to‐trough fluctuations compared with other tacrolimus formulations, which could have a favorable effect on the IPV. 22 Additionally the different PK characteristics of LCP‐tacrolimus may reduce the incidence and intensity of side effects. Known side effects of tacrolimus are tremor, headache, gastrointestinal complaints, and psychological disturbances.
This prospective, randomized, crossover study investigated whether the IPV in tacrolimus predose concentrations (C0) is reduced by switching stable adult kidney transplant recipients (>1 year post‐transplantation) from maintenance tacrolimus treatment with a twice‐daily IR‐tacrolimus formulation (Prograf; IR‐tacrolimus) to a once‐daily, modified release tacrolimus formulation (either ER‐tacrolimus or LCP‐tacrolimus). Secondary end points include the association between CYP3A5 genotype and IPV and the occurrence of side effects with different formulations.
PATIENTS AND METHODS
Study design
This was an open‐label, randomized, multicenter, crossover trial (the “TacIPV study”; EudraCT 2015‐005559‐29). Patients were included at three sites in The Netherlands (The Erasmus Medical Center in Rotterdam [the Erasmus MC], the Radboud University Medical Center in Nijmegen [Radboudumc], and the Canisius Wilhelmina Hospital in Nijmegen [CWZ]). Stable kidney transplant recipients who were at least 12 months after transplantation and on maintenance treatment with IR‐tacrolimus were eligible for inclusion.
The study design is depicted in Figure 1. At study entry, patients were randomized for (a) the order of treatment:
continuation with IR‐tacrolimus for 6 months followed by a switch to a once‐daily formulation,
immediate switch to a once‐daily tacrolimus formulation followed by a switch back to IR‐tacrolimus after 6 months,
FIGURE 1.
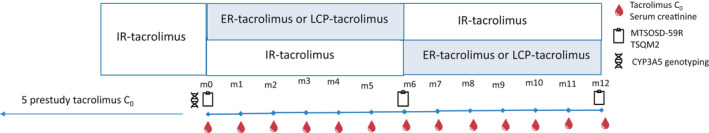
Study design. ER, extended release; IR, immediate release; LCP, LifeCyclePharma
and (b) the once‐daily formulation:
Advagraf (referred to as ER‐tacrolimus)
Envarsus (referred to as LCP‐tacrolimus).
Randomization was done in a 1:1 fashion for both, (a) the order and (b) the formulation with stratification for the study site with the use of sealed opaque envelopes. These envelopes contained randomly generated treatment allocations. The study was approved by the Ethics Committee of the Erasmus MC (METC 2016+180) and by the local institutional review committees of the other two study sites. The trial was conducted in accordance with the Declaration of Helsinki and informed consent was obtained from all participating patients.
Study population
Stable adult kidney transplant recipients were eligible for enrollment if they met the following inclusion criteria: (a) the time after transplantation was at least 12 months; (b) their maintenance regimen included IR‐tacrolimus, and (c) both their immunosuppressive regimen and tacrolimus doses were unchanged for a minimum of 12 weeks prior to enrollment. Patients who had previously been treated with ER‐tacrolimus or LCP‐tacrolimus or those who had previously received a non‐renal organ transplant were not included. Other exclusion criteria included: (a) an acute rejection episode within 6 months prior to enrollment, or an acute rejection episode within the 12 months prior to enrollment that required T lymphocyte‐depleting antibody therapy, (b) proteinuria greater than 2g/24 h, estimated glomerular filtration rate (eGFR; modification of diet in renal disease [(MDRD]) less than 20 ml/min/1.73 m2, (c) deteriorating renal function (defined as an increase of serum creatinine of >20% over the 6 months prior to enrollment), (d) liver cirrhosis or elevated alanine transaminase (ALT), aspartate‐aminotransferase (AST), or bilirubin levels greater than or equal to 2 times the upper value of the reference range, and (e) any psychiatric disorder or any other medical condition that could interfere with the study objectives in the opinion of the investigator.
Baseline characteristics, including available tacrolimus C0 measured prior to inclusion in the study were collected. Tacrolimus C0 measured within the first year after kidney transplantation were not included in the analysis. A blood sample for CYP3A5 genotyping was obtained once during the study period. 23 Individuals with one or more wild‐type alleles (CYP3A5*1) were considered CYP3A5 expressors, whereas individuals homozygous for the CYP3A5*3 allele were considered nonexpressors.
Tacrolimus administration and predose concentrations
Conversion from IR‐tacrolimus to ER‐tacrolimus was performed on a 1:1 mg basis. For conversion from IR‐tacrolimus to LCP‐tacrolimus, the daily dose was reduced by 30% according to the package insert and based on previous studies. 24 , 25 One week after each switch (i.e., at baseline and 6 months after the start of the study), serum creatinine and tacrolimus predose concentrations were measured. The total study duration was 12 months. Patients were followed for 6 months for each tacrolimus formulation, with monthly tacrolimus C0 assessments to calculate the IPV of tacrolimus. Tacrolimus C0 obtained during hospitalization were excluded for analysis.
Blood samples were obtained via venipuncture and tacrolimus concentrations were measured using liquid chromatography‐mass spectrometry (LC‐MS). 1 Dose adjustments were allowed to reach the same target tacrolimus C0 as during the former IR‐tacrolimus treatment. Dose adjustments were calculated based on the linear relationship between tacrolimus dose and concentration, so, generally, by multiplying the current dose by target C0/current C0.
Symptom occurrence, symptom distress, and treatment satisfaction
To assess the quality of life, patients were asked to complete two questionnaires: the Modified Transplant Symptom Occurrence and Symptom Distress Scale (MTSOSD‐59R) and the Treatment Satisfaction Questionnaire for Medication (TSQM version II). Both questionnaires have been validated and are available in Dutch. 26 , 27
The MTSOSDS‐59R includes 59 symptoms associated with side effects of immunosuppressive medication. This questionnaire measures symptom occurrence on a five‐point rating scale from 0 (never) to 4 (always occurring) and symptom distress from 0 (not at all distressing) to 4 (extremely distressing). The TSQM questionnaire includes seven questions to measure patients’ satisfaction regarding their medication.
Patients were asked to fill out the questionnaires at three time points: at study entry, just prior to conversion to the other tacrolimus formulation after 6 months, and at the end of the study.
Study outcomes
Primary outcome
The primary outcome of our study was the change of IPV in dose‐corrected tacrolimus C0 (C0/D) when switching from the twice‐daily tacrolimus formulation to either of the two once‐daily (modified‐release) tacrolimus formulations.
Tacrolimus IPV was quantified by calculating the CV of the C0/D using the following formula: CV% = (SD/mean) × 100%. For dose correction, whole‐blood tacrolimus C0 were divided by the total daily tacrolimus dose (ng/ml per mg). We applied dose correction because dose adjustments were allowed and we aimed to exclude the effects of dose adjustments on IPV as much as possible. Only “true” C0 were included for analysis. “False” C0 were defined as levels that were not obtained from blood samples drawn before the morning dose of tacrolimus, or levels that were more than twice as high as the mean of all the other predose concentrations while the tacrolimus dose was stable.
Although the study protocol specified that IPV could be calculated in individual patients if at least five or more C0 were available, this number was reduced to the availability of at least four C0, as several patients (n = 12) had unexpected missing or “false” levels. In 11 cases, levels were drawn after ingestion of the morning dose of tacrolimus despite clear instructions to the patients. In one patient, a single level was defined “false” based on the fact that it was more than twice as high as the mean value of the other predose levels, indicating that it was not a predose concentration.
Sensitivity analysis
Sensitivity analyses included (a) the change in IPV of the C0 (without dose normalization) and (b) the change in IPV when including only the latest three C0/D of the various study periods (introducing a washout period of >1 month).
Secondary outcomes
Secondary outcome measures were (a) difference between “pre‐study IPV” and IPV during the study (b) the association between tacrolimus IPV and CYP3A5 genotype, (c) the association between tacrolimus IPV and other patient characteristics (age, sex, body mass index [BMI], and ethnicity), (d) changes in self‐reported side‐effects measured by the MTSOSD‐59R questionnaire after switching from tacrolimus formulation, (e) difference in patient satisfaction measured by TSQM questionnaire after switching from tacrolimus formulation, and (f) patients’ preference to continue taking one of the tacrolimus formulations after study closure.
Safety outcomes
Safety outcomes included the incidence of serious adverse events (SAEs), especially the incidence of acute rejection, graft failure, and death. The eGFR values were calculated by using the MDRD‐4 equation. 28
Sample size determination
The mean tacrolimus IPV during IR‐tacrolimus treatment at baseline was expected to be 18%, based on a recent analysis of a large cohort of kidney transplant recipients at the Erasmus MC. 6 A meaningful reduction of the IPV was assumed to be at least 4% based on previous studies. In their survival model for graft failure, Shuker et al. presented a hazard ratio of 1.014 for every percent of increase of IPV. A reduction of IPV of 4% would mean a 5.6% reduction in graft failure. 6 In order to detect a difference of 4% with a power of 80% and an alpha of 0.05 (2‐sided), at least 42 patients were required per group (IR‐tacrolimus vs. either ER‐tacrolimus or LCP‐tacrolimus).
Statistical analysis
Baseline characteristics are presented as means and SDs, medians and interquartile ranges (IQRs), or frequencies and proportions, as appropriate. For continuous variables, an independent t‐test or Mann‐Whitney U test was applied, and for categorical data a chi‐square test was performed to compare baseline characteristics between study groups.
The primary outcome, tacrolimus IPV, was compared between IR‐tacrolimus and the once‐daily formulations (ER‐tacrolimus and LCP‐tacrolimus groups combined) using a paired samples t‐test. An unpaired t‐test was used to compare the IPV of ER‐tacrolimus with the IPV of LCP‐tacrolimus. To compare the IPV of either formulation, ER‐tacrolimus or LCP‐tacrolimus, separately with the IPV of IR‐tacrolimus, again a paired samples t‐test was used. Additionally, the “pre‐study IPV” was compared with the IPV during the study in patients of whom at least four pre‐study tacrolimus C0 were available (measured >12 months post‐transplantation).
To find determinants for a higher IPV, a linear mixed model was used that included the following factors and covariates: age, sex, ethnicity, CYP3A5 (expressor vs. nonexpressor), BMI, eGFR at baseline, and formulation of once‐daily tacrolimus (ER‐tacrolimus vs. LCP‐tacrolimus).
The scores of the MTSOSD‐59R and TSQM were analyzed using a related samples Wilcoxon signed rank test.
Analyses were performed using Statistical Package for the Social Science software (SPSS, version 25).
RESULTS
In total, 102 patients were randomized between October 2016 and March 2019 Erasmus MC: 58 patients, Radboud UMC: 38 patients, CWZ: 6 patients). Ten patients discontinued the study prematurely, and 92 patients completed the trial and were included for analysis of the primary end point (Figure 2). Baseline characteristics are presented in Table 1. The median interval from kidney transplantation to study enrollment was 46.1 months (IQR: 19.5–94.9). CYP3A5 genotyping was performed in 80 of 92 patients and the CYP3A5*3 allele frequency was consistent with the Hardy‐Weinberg equilibrium (22/160) resulting in 21 patients (20.6%) expressing the CYP3A5 enzyme.
FIGURE 2.
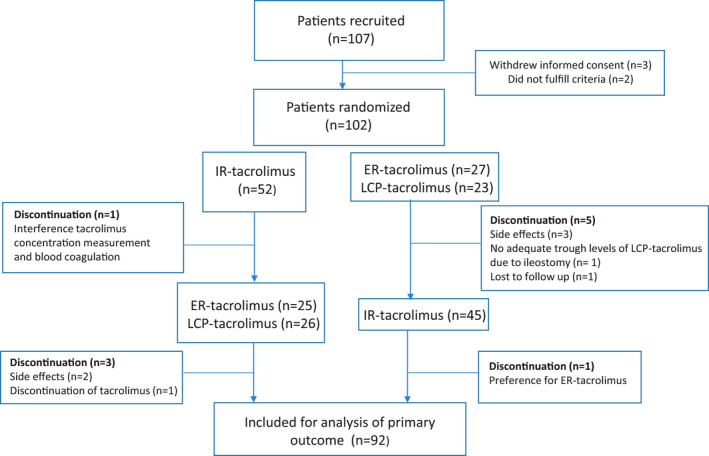
Study population. Flowchart depicting the in‐ and exclusion of patients. ER, extended release; IR, immediate release; LCP, LifeCyclePharma
TABLE 1.
Baseline characteristics (n = 92)
| ER‐tacrolimus (n = 47) | LCP‐tacrolimus (n = 45) | |
|---|---|---|
| Male sex (%) | 33 (70.2) | 27 (60.0) |
| Age in years, mean (SD) | 56.8 (13.5) | 50.6 (14.7) |
| BMI, mean (SD) | 27.0 (4.7) | 27.5 (4.1) |
| Ethnicity (%) | ||
| White | 41 (87.2) | 41 (91.1) |
| Asian | 0 | 1 (2.2) |
| Black | 4 (8.5) | 2 (4.4) |
| Other | 2 (4.3) | 1 (2.2) |
| Transplantation (%) | ||
| First | 34 (72.3) | 37 (82.2) |
| Second | 11 (23.4) | 6 (13.3) |
| ≥3 | 2 (4.3) | 2 (4.4) |
| Donor category (%) | ||
| Living | 33 (70.2) | 38 (84.4) |
| Deceased | 14 (29.8) | 7 (15.6) |
| Time from transplantation to enrollment, months, median (IQR) | 42.8 (20.5–78.3) | 50.0 (17.7–99.8) |
| eGFR (MDRD) in ml/min/1.73 m2 at baseline, mean (SD) | 49.9 (17.0) | 48.3 (14.6) |
| Concomitant immunosuppressive therapy (%) | ||
| Azathioprine | 1 (2.1) | 0 |
| Mycophenolate mofetil | 22 (46.8) | 16 (35.6) |
| Prednisolone | 10 (21.3) | 17 (37.8) |
| Azathioprine + prednisolone | 2 (4.3) | 3 (6.7) |
| Mycophenolate mofetil + prednisolone | 12 (25.5) | 9 (20.0) |
| CYP3A5 (%) | ||
| Expressor | 11 (23.4) | 10 (22.2) |
| *1/*1 | 0 | 1 |
| *1/*3 | 10 | 9 |
| *1/*6 | 1 | 0 |
| Nonexpressor (*3/*3) | 30 (63.8) | 29 (64.4) |
| Unknown | 6 (12.8) | 6 (13.3) |
Abbreviations: BMI, body mass index; eGFR, estimated glomerular filtration rate; ER, extended release; IQR, interquartile range; LCP, LifeCyclePharma; MDRD, modification of diet in renal disease.
Table 2 shows mean daily dose, mean C0, and number of available C0 and dose adjustments for each formulation.
TABLE 2.
Tacrolimus pharmacokinetics: daily dose, C0, and dose adjustments
| Baseline |
IR‐tacrolimus n = 92 |
ER‐tacrolimus n = 47 |
LCP‐tacrolimus n = 45 |
|
|---|---|---|---|---|
| Tacrolimus dose, mg/day | 5.2 (3.7) | 5.0 (3.9) | 5.6 (4.1) | 3.3 (2.6) |
| Tacrolimus C0, ng/ml | 6.0 (1.5) | 6.0 (1.7) | 5.4 (1.5) | 5.7 (1.6) |
| Tacrolimus C0/dose, ng/ml/mg | 1.6 (1.0) | 1.7 (1.1) | 1.4 (0.9) | 2.4 (1.4) |
| Number of tacrolimus C0 levels | ||||
| 4 | 7 (7.6%) | 2 (4.3%) | 4 (8.9%) | |
| 5 | 28 (30.4%) | 9 (19.1%) | 10 (22.2%) | |
| 6 | 46 (50%) | 26 (55.3%) | 24 (53.3%) | |
| ≥7 | 11 (12%) | 10 (21.3%) | 7 (15.6%) | |
| No. of dose adjustments | ||||
| 0 | 62 (67.4%) | 22 (46.8%) | 27 (60%) | |
| 1 | 22 (23.9%) | 19 (40.4%) | 9 (20%) | |
| 2 | 6 (6.5%) | 5 (5.4%) | 5 (11.1%) | |
| ≥3 | 2 (2.2%) | 1 (2.1%) | 4 (8.9%) | |
| First tacrolimus C0 | ||||
| In target range (%) | 84 (91.3) | 38 (80.9) | 36 (80) | |
| Below target range (%) | 1 (1.1) | 9 (19.1) | 3 (6.7) | |
| Above target range (%) | 7 (7.6) | 0 | 6 (13.3) |
Data is presented as mean ± SD.
Data represent tacrolimus dose and tacrolimus trough level at the end of the 6‐month treatment period.
Abbreviations: ER, extended release; IR, immediate release; LCP, LifeCyclePharma.
Figure 3a shows that there was no significant difference between the IPV of patients using IR‐tacrolimus (16.6%) and the IPV in patients using one of the once‐daily formulations (18.3%; difference +1.7%, 95%confidence interval [CI] −1.1% to 4.5%, p = 0.24). The IPV of LCP‐tacrolimus (20.1%) was higher compared to that of ER‐tacrolimus (16.5%), but this difference was not statistically significantly different (difference +3.6%, 95% CI −0.1% to 7.3%, p = 0.06; Figure 3b). In patients randomized to ER‐tacrolimus (n = 47), the IPV was not different between IR‐tacrolimus (17.6%) and ER‐tacrolimus (16.4%), as depicted in Figure 3c. In patients randomized to LCP‐tacrolimus (n = 45), the IPV was lower for IR‐tacrolimus (15.5%) compared to LCP‐tacrolimus (20.1%; difference +4.6%, 95% CI: 2.1% to 8.1%, p = 0.01; Figure 3c).
FIGURE 3.
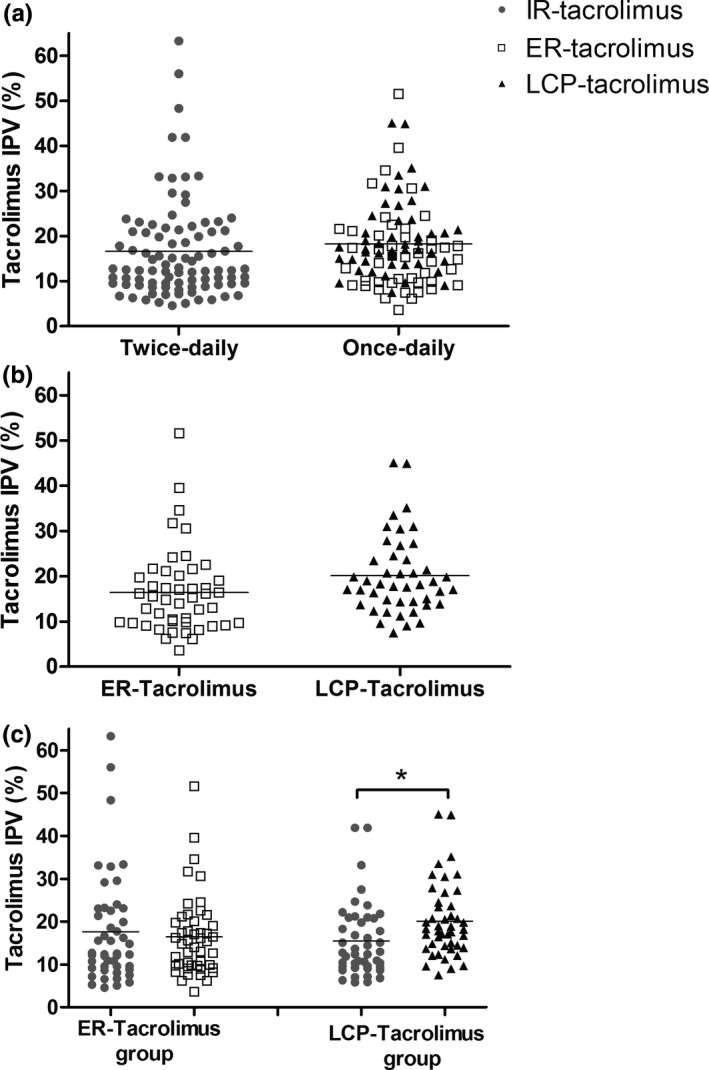
Tacrolimus intra‐patient variability. Panel (a) Tacrolimus IPV in percentage of once daily versus twice daily formulations. Panel (b) Tacrolimus IPV in percentage of ER‐tacrolimus versus LCP‐tacrolimus. Panel (c) Tacrolimus IPV in percentage of different tacrolimus formulations divided in subgroups according to the once‐daily formulation. ER, extended release; IR, immediate release; IPV, intrapatient variability; LCP, LifeCyclePharma
The IPV of C0 (without dose‐correction) did not differ between IR‐tacrolimus (19.1%) and the once‐daily formulations (20.8%) (change +1.7%, 95% CI: −1.6% to +5.0%, p = 0.31). IPV of C0 in the ER‐tacrolimus group was 19.4% and 18.6%, respectively, for IR‐ and ER‐tacrolimus (change −0.8%, 95% CI: −2.1% to +3.3%, p = 0.68). IPV of C0 in the LCP‐tacrolimus group was 18.7% and 23.1%, respectively, for IR‐ and LCP‐tacrolimus (change +4.8%, 95% CI: −0.8% to +9.5%). None of these differences was statistically significant. Analysis of only the last three C0/D of each formulation, and thus creating a “washout period” of at least 1 month, showed a slightly lower IPV for all formulations. The primary outcome, the change in IPV when switching to a once‐daily formulation, was similar to our analysis of all available C0/D.
To evaluate the effect of study participation on IPV, we calculated a “pre‐study” (baseline) tacrolimus IPV in the 61 patients with at least four available tacrolimus C0 before enrollment. This “pre‐study” IPV was 19.7% and not significantly different from the IPV of IR‐tacrolimus during the study (17.5%) for this subgroup (difference −2.2%, 95% CI: −5.8% to 1.3%).
As expected, CYP3A5 expressors needed a higher dose of tacrolimus to reach target levels (Table 3). IPV was similar between CYP3A5 expressors and nonexpressors for all three tacrolimus formulations (Table 3). The linear mixed model detected a small positive association between BMI (in kg/m2) and IPV (estimate +0.37, 95% CI: 0.04 to 0.7). A higher BMI was significantly associated with a higher IPV with an increase of 0.34% for every kg/m2. Other factors and covariates in our model were not associated with IPV.
TABLE 3.
CYP3A5, tacrolimus dose and IPV
| CYP3A5 nonexpressor, n = 59 | CYP3A5 expressor, n = 21 | |||||
|---|---|---|---|---|---|---|
| IR‐tacrolimus | ER‐tacrolimus | LCP‐tacrolimus | IR‐tacrolimus | ER‐tacrolimus | LCP‐tacrolimus | |
| Tacrolimus daily dose, mg | 3.7 (1.8) | 4.0 (2.1) | 2.6 (1.4) | 9.3 (5.7) | 9.4 (6.0) | 5.9 (4.6) |
| Tacrolimus IPV, % | 16.6 (10.3) | 15.6 (9.5) | 18.1 (7.1) | 14.7 (7.8) | 16.0 (9.0) | 19.4 (6.4) |
Results are expressed as mean (SD).
Abbreviations: ER, extended release; IR, immediate release; IPV, intrapatient variability; LCP, LifeCyclePharma.
The MTSOSD‐59R and TSQM questionnaires for the assessment of self‐reported side effects and patient satisfaction regarding drug use, respectively, were completed at the three time points (baseline, after 6 months, and at the end of the study) by 60 patients; 31 in the ER‐tacrolimus group and 29 in the LCP‐tacrolimus group. Overall, MTSOSD‐59 scores did not differ between formulations, but we observed differences at item levels. Figure 4 shows occurrence scores for the most prevalent side effects. Tremor (baseline prevalence: 67%) and itching (baseline prevalence: 50%) occurred less often when using LCP‐tacrolimus compared to IR‐tacrolimus. Mean occurrence scores (scale from 0 to 4) for, respectively, LCP‐tacrolimus and IR‐tacrolimus were 0.97 versus 1.48 for tremor and 0.59 versus 0.93 for itching. Occurrence scores for diarrhea (baseline prevalence: 48%) were lower when using LCP‐tacrolimus (mean occurrence score 0.41) compared to baseline (mean occurrence score 0.76). However, joint pain (baseline prevalence: 53%) occurred more often when using LCP‐tacrolimus with a mean occurrence score of 1.00 compared to 0.60 for IR‐tacrolimus (Figure 4). Patients’ satisfaction measured by the TSQM questionnaire did not differ between the formulations. The majority of patients continued with a once‐daily formulation at the end of the study; in the ER‐tacrolimus study group, 29 of 47 patients (62%) continued with ER‐tacrolimus, and in the LCP‐tacrolimus study group, 34 of 45 patients (76%) continued with LCP‐tacrolimus.
FIGURE 4.
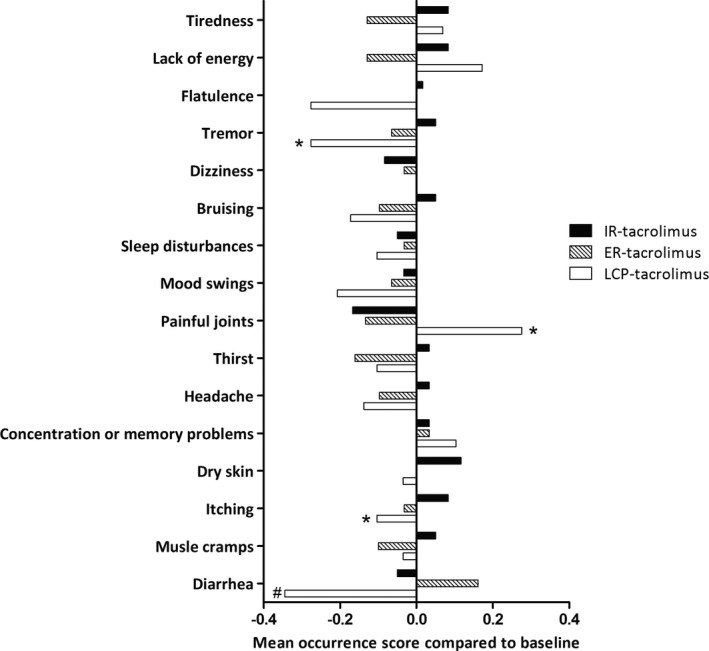
Occurrence of self‐reported side‐effects. The X‐axis presents the change in occurrence score compared to baseline. On the Y‐axis the most prevalent side‐effects are presented in order of their prevalence with the most prevalent side‐effect at the top. *Statistical difference between IR‐tacrolimus and LCP‐tacrolimus (Wilcoxon signed rank test without correction for multiple tests). #Statistical difference between baseline and LCP‐tacrolimus (Wilcoxon signed rank test without correction for multiple tests). ER, extended release; IR, immediate release; LCP, LifeCyclePharma
The eGFR at study entry was 49.1 (SD = 15.7) ml/min/1.73 m2, and remained stable during the 6‐month treatment period (IR‐tacrolimus 48.4 [SD = 14.7] ml/min/1.73 m2; ER‐tacrolimus 50.0 [SD = 17.0] ml/min/1.73 m2; LCP‐tacrolimus 46.7 [SD = 13.7] ml/min/1.73 m2). The incidence of SAEs was similar for both treatment groups. No acute rejections, graft losses, or deaths occurred. During follow‐up, 15 SAEs were reported in 12 patients (23%) in the ER‐tacrolimus group and 20 SAEs were reported in 12 patients (24%) in the LCP‐tacrolimus group. The most frequently reported SAEs were urinary tract infections (n = 5), respiratory tract infections (n = 5), and gastrointestinal infections (n = 4). Two patients had a recurrence of their primary kidney disease (focal segmental glomerulosclerosis and monoclonal gammopathy of renal significance). Adverse events leading to discontinuation of the study included depressive feelings with joint pains (n = 1) and diarrhea (n = 2) in the ER‐tacrolimus group, and depressive feelings (n = 1) and hair loss with tinnitus (n = 1) in the LCP‐tacrolimus group.
DISCUSSION
In this randomized, crossover trial the IPV of tacrolimus (measured by %CV of the C0/D) did not change when patients were switched from the twice‐daily immediate‐release tacrolimus formulation (IR‐tacrolimus) to a once‐daily extended‐release formulation (either ER‐tacrolimus or LCP‐tacrolimus). There was no significant difference in IPV between ER‐tacrolimus or LCP‐tacrolimus. The IPV in the 45 patients randomized to LCP‐tacrolimus as once‐daily formulation was lower during use of IR‐tacrolimus as compared to during use of LCP‐tacrolimus. To the best of our knowledge, this is the first randomized, prospective intervention study comparing the IPV of tacrolimus of all formulations that are currently available: IR‐tacrolimus, ER‐tacrolimus, and LCP‐tacrolimus.
In the study by Stifft et al., a significant decrease of 3.2% in the IPV of AUC0–24 was observed when patients were converted from IR‐tacrolimus to ER‐tacrolimus, whereas the IPV for tacrolimus C0 did not change significantly (−1.6%). 19 Wu et al. observed a 2.8% decrease in IPV of C0 after conversion from IR‐tacrolimus to ER‐tacrolimus, which is less than our predefined meaningful decrease of 4%. 18 Yet, in agreement with our results, other studies found no significant change in IPV in tacrolimus C0 when switching from twice‐daily to once‐daily formulations. 20 , 21 Shuker et al. studied the IPV of C0 after switching from IR‐tacrolimus to ER‐tacrolimus in a nonrandomized trial. No difference in IPV was reported (mean 17.3% vs. 16.4%). 20 Van Hooff et al. performed a PK study comparing IR‐ and ER‐tacrolimus and, although IPV was not one of the end points, the authors reported a similar IPV of the AUC0–24 for both formulations (17.2 vs. 17.1%). 21 Like most previous studies, we used C0 instead of AUC to approach tacrolimus exposure. Clearly, this is more feasible in daily practice and there is a good correlation between the AUC and C0 of tacrolimus for both tacrolimus once‐daily and twice‐daily formulations. 19 , 21 Additionally, all studies showing an association between a high IPV in tacrolimus exposure and poor clinical outcomes included C0 and not AUC for calculation of IPV.
The improvement of IPV after conversion to an ER formulation in the studies from Stifft et al. and Wu et al. might have resulted from increased adherence rather than from different PK characteristics of the ER formulations. Kuypers et al. showed improved adherence with once‐daily tacrolimus compared to twice‐daily tacrolimus. 17 If a once‐daily formulation reduces IPV by improving adherence, the impact of conversion is expected to be highest in nonadherent patients. In the current study, baseline IPV was 19.7%, which is in line with the IPV found in other studies that included stable patients more than 1 year after transplantation. We did detect a small, but nonsignificant change of −2.2% in IPV when patients entered the study, possibly reflecting a consciously or unconsciously induced increase in adherence as a result of study participation. In a kidney transplant population that was 99.9% adherent, Leino et al. found a median IPV of C0 of 16.8%, which is only slightly lower compared to the IPV in our study, suggesting we studied an adherent population. 29 We included two essentially different once‐daily formulations to study the effect of formulations with a flatter PK profile on the IPV. In this context, LCP‐tacrolimus was not studied before. We included stable patients who were at least 1‐year post‐transplantation to eliminate factors with a known influence on tacrolimus PKs like changes in medication and complications like infections. Contrary to our hypothesis, a potential benefit of a stable PK profile of ER formulations on the IPV could not be demonstrated.
As expected, CYP3A5 expressors needed higher tacrolimus doses to reach adequate target C0, but their IPV was not different from CYP3A5 nonexpressors. In our study, the CYP3A5 genotype did not influence the change in IPV. Although the number of patients expressing CYP3A5 was relatively small (n = 21), this reflected the proportion of CYP3A5 expressors in the White population. Others, including Wu et al., did find an influence of genotype, with the most pronounced drop in IPV after switching from IR‐tacrolimus to ER‐tacrolimus in CYP3A5 expressers (CYP3A5*1/*1 and CYP3A5*1/*3). 18 Their study was performed in Taiwan, where almost half of the population is CYP3A5 expresser, whereas in our largely White population this is not more than 15%–20%. 30 Stifft et al. also observed a higher reduction in IPV after conversion from IR‐tacrolimus to ER‐tacrolimus in CYP3A5 expressors than in nonexpressors, although the difference was not statistically significant (−5.4% vs. −2.4%, respectively).
Switching from IR tacrolimus to a once‐daily formulation did not reduce the IPV in C0/D, but there was a reduction of certain self‐reported side‐effects. Switching to LCP‐tacrolimus reduced the occurrence of tremor, a highly prevalent side effect reported by 70% of patients. Tremor is assumed to be more related to peak concentrations, and independent of IPV, and our results are in line with those of the STRATO study. 31 However, occurrence of joint pain increased significantly, which was an unexpected finding and was not seen with LCP‐tacrolimus in earlier studies with larger cohorts. 24 , 32 , 33 Our results concerning side effects should be interpreted with caution because the study was not powered to detect differences in self‐reported side effects, no correction for multiple testing was applied, and the open‐label design may have created information bias. Nevertheless, we think that conversion to LCP‐tacrolimus should be considered in patients with tremors.
An important strength of this study is the prospective, randomized, crossover study design. Patients were not only randomized for the order in which once‐daily and twice‐daily dosing were compared, but also for either of the two once‐daily formulations. Furthermore, the study was powered to detect a minimum reduction of IPV of 4%, as this change was considered to be necessary to ultimately improve clinical outcomes. Patients were included in a stable situation, at least 1‐year post‐transplant, thus excluding factors associated with high IPV in the first postoperative months.
A limitation of the study was its non‐blinded design. All patients had been treated with the IR formulation for several years, and despite the information they had received during the informed consent procedure, some felt uncomfortable with switching to an alternative formulation. This may have resulted in a few premature discontinuations. Furthermore, in line with the population distribution of the Netherlands, most patients were White. We included only a limited number of patients with the CYP3A5*1 allele. Therefore, our results may not be representative for other populations, especially if CYP3A5 genotype affects the change in IPV following conversion (as suggested by others). 18
In conclusion, IPV did not decrease after switching from IR‐tacrolimus to ER‐tacrolimus or LCP‐tacrolimus. The results of this study provide no arguments to switch kidney transplant recipients from a twice‐daily (IR) tacrolimus formulation to a once‐daily (modified‐release) tacrolimus formulation when the single aim is to lower the IPV.
CONFLICT OF INTEREST
This study was supported by Chiesi Pharmaceuticals B.V. The Netherlands, as local representative of the marketing authorization holder Chiesi Farmaceutici S.p.A. of LCPT (Envarsus). The underlying study represents an investigator‐initiated trial. The study was designed by the authors alone without any external input regarding design, analysis, or approval for the manuscript either by Chiesi Pharmaceuticals B.V. The Netherlands or by any other unmentioned party. The authors themselves did not receive any financial support with regard to this study. D.A.H. has received lecture fees and consulting fees from Astellas Pharma, Chiesi Pharma, Medincell, Novartis Pharma, and Vifor Pharma. He has received grant support from Astellas Pharma, Bristol‐Myers Squibb, and Chiesi Pharma (paid to his institution). D.A.H. does not have employment or stock ownership at any of these companies, and neither does he have patents or patent applications. L.B.H. has received lecture fees and consulting fees from Astellas, Chiesi, and Novartis. He has received grant support from Chiesi and Sandoz (paid to his institution). L.B.H. does not have employment or stock ownership at any of these companies, and neither does he have patents or patent applications. T.vG. has received lecture fees and study grants from Chiesi and Astellas, in addition to consulting fees from Roche Diagnostics, Thermo Fisher, Vitaeris, CSL Behring, Astellas, and Aurinia Pharma. In all cases, money has been transferred to hospital accounts, and none has been paid to his personal bank accounts. T.vG. does not have employment or stock ownership at any of these companies, and neither does he have patents or patent applications. All other authors declared no competing interests for this work.
AUTHOR CONTRIBUTIONS
K.L.W.B., L.A.H., T.vG., L.B.H., M.C.B., D.A.H., M.tD., and R.vS. wrote the manuscript. T.vG., D.A.H., M.C.B., and L.B.H. designed the research. K.L.W.B., G.N., and L.A.H. performed the research. K.L.W.B. analyzed the data.
Bunthof KLW, Al‐Hassany L, Nakshbandi G, et al. A randomized crossover study comparing different tacrolimus formulations to reduce intrapatient variability in tacrolimus exposure in kidney transplant recipients. Clin Transl Sci. 2022;15:930‐941. doi: 10.1111/cts.13206
Funding information
This study was supported by Chiesi Pharmaceuticals B.V. The Netherlands, as local representative of the marketing authorization holder Chiesi Farmaceutici S.p.A. of LCPT (Envarsus). The underlying study represents an investigator‐initiated trial. The study was designed by the authors alone without any external input regarding design, analysis, or approval for the manuscript either by Chiesi Pharmaceuticals B.V. The Netherlands or by any other unmentioned party. The authors themselves did not receive any financial support with regard to this study
Kim L. W. Bunthof and Linda Al‐Hassany contributed equally to this work.
[Correction added on 22 December, 2021, after first online publication: Author contribution statement has been included in this version].
REFERENCES
- 1. Brunet M, van Gelder T, Asberg A, et al. Therapeutic drug monitoring of tacrolimus‐personalized therapy: second consensus report. Ther Drug Monit. 2019;41(3):261‐307. [DOI] [PubMed] [Google Scholar]
- 2. Shuker N, van Gelder T, Hesselink DA. Intra‐patient variability in tacrolimus exposure: causes, consequences for clinical management. Transplant Rev (Orlando). 2015;29(2):78‐84. [DOI] [PubMed] [Google Scholar]
- 3. Borra LC, Roodnat JI, Kal JA, Mathot RA, Weimar W, van Gelder T. High within‐patient variability in the clearance of tacrolimus is a risk factor for poor long‐term outcome after kidney transplantation. Nephrol Dial Transplant. 2010;25(8):2757‐2763. [DOI] [PubMed] [Google Scholar]
- 4. Sapir‐Pichhadze R, Wang Y, Famure O, Li Y, Kim SJ. Time‐dependent variability in tacrolimus trough blood levels is a risk factor for late kidney transplant failure. Kidney Int. 2014;85(6):1404‐1411. [DOI] [PubMed] [Google Scholar]
- 5. Rodrigo E, Segundo DS, Fernández‐Fresnedo G, et al. Within‐patient variability in tacrolimus blood levels predicts kidney graft loss and donor‐specific antibody development. Transplantation. 2016;100(11):2479‐2485. [DOI] [PubMed] [Google Scholar]
- 6. Shuker N, Shuker L, van Rosmalen J, et al. A high intrapatient variability in tacrolimus exposure is associated with poor long‐term outcome of kidney transplantation. Transpl Int. 2016;29(11):1158‐1167. [DOI] [PubMed] [Google Scholar]
- 7. van Gelder T. Within‐patient variability in immunosuppressive drug exposure as a predictor for poor outcome after transplantation. Kidney Int. 2014;85(6):1267‐1268. [DOI] [PubMed] [Google Scholar]
- 8. Vanhove T, Vermeulen T, Annaert P, Lerut E, Kuypers DRJ. High intrapatient variability of tacrolimus concentrations predicts accelerated progression of chronic histologic lesions in renal recipients. Am J Transplant. 2016;16(10):2954‐2963. [DOI] [PubMed] [Google Scholar]
- 9. Kim EJ, Kim SJ, Huh KH, et al. Clinical significance of tacrolimus intra‐patient variability on kidney transplant outcomes according to pre‐transplant immunological risk. Sci Rep. 2021;11(1):12114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sablik KA, Clahsen‐van Groningen MC, Hesselink DA, van Gelder T, Betjes MGH. Tacrolimus intra‐patient variability is not associated with chronic active antibody mediated rejection. PLoS One. 2018;13(5):e0196552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kuypers DRJ. Intrapatient variability of tacrolimus exposure in solid organ transplantation: a novel marker for clinical outcome. Clin Pharmacol Ther. 2020;107(2):347‐358. [DOI] [PubMed] [Google Scholar]
- 12. Neuberger JM, Bechstein WO, Kuypers DR, et al. Practical recommendations for long‐term management of modifiable risks in kidney and liver transplant recipients: a guidance report and clinical checklist by the Consensus on Managing Modifiable Risk in Transplantation (COMMIT) Group. Transplantation. 2017;101(4S):S1‐S56. [DOI] [PubMed] [Google Scholar]
- 13. Cheung CY, Chan KM, Wong YT, Chak WL, Bekers O, van Hooff JP. Impact of CYP3A5 genetic polymorphism on intrapatient variability of tacrolimus exposure in Chinese kidney transplant recipients. Transpl Proc. 2019;51(6):1754‐1757. [DOI] [PubMed] [Google Scholar]
- 14. Pashaee N, Bouamar R, Hesselink DA, et al. CYP3A5 genotype is not related to the intrapatient variability of tacrolimus clearance. Ther Drug Monit. 2011;33(3):369‐371. [DOI] [PubMed] [Google Scholar]
- 15. Spierings N, Holt DW, MacPhee IA. CYP3A5 genotype had no impact on intrapatient variability of tacrolimus clearance in renal transplant recipients. Ther Drug Monit. 2013;35(3):328‐331. [DOI] [PubMed] [Google Scholar]
- 16. Seibert SR, Schladt DP, Wu B, et al. Tacrolimus trough and dose intra‐patient variability and CYP3A5 genotype: effects on acute rejection and graft failure in European American and African American kidney transplant recipients. Clin Transplant. 2018;32(12):e13424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kuypers DR, Peeters PC, Sennesael JJ, et al. Improved adherence to tacrolimus once‐daily formulation in renal recipients: a randomized controlled trial using electronic monitoring. Transplantation. 2013;95(2):333‐340. [DOI] [PubMed] [Google Scholar]
- 18. Wu MJ, Chang CH, Cheng CY, et al. Reduced variability of tacrolimus trough level in once‐daily tacrolimus‐based Taiwanese kidney transplant recipients with high‐expressive genotype of cytochrome P450 3A5. Transplant Proc. 2014;46(2):403‐405. [DOI] [PubMed] [Google Scholar]
- 19. Stifft F, Stolk LM, Undre N, van Hooff JP, Christiaans MH. Lower variability in 24‐hour exposure during once‐daily compared to twice‐daily tacrolimus formulation in kidney transplantation. Transplantation. 2014;97(7):775‐780. [DOI] [PubMed] [Google Scholar]
- 20. Shuker N, Cadogan M, van Gelder T, et al. Conversion from twice‐daily to once‐daily tacrolimus does not reduce intrapatient variability in tacrolimus exposure. Ther Drug Monit. 2015;37(2):262‐269. [DOI] [PubMed] [Google Scholar]
- 21. van Hooff J, Van der Walt I, Kallmeyer J, et al. Pharmacokinetics in stable kidney transplant recipients after conversion from twice‐daily to once‐daily tacrolimus formulations. Ther Drug Monit. 2012;34(1):46‐52. [DOI] [PubMed] [Google Scholar]
- 22. Tremblay S, Nigro V, Weinberg J, Woodle ES, Alloway RR. A steady‐state head‐to‐head pharmacokinetic comparison of all FK‐506 (Tacrolimus) Formulations (ASTCOFF): an open‐label, prospective, randomized, two‐arm, three‐period crossover study. Am J Transplant. 2017;17(2):432‐442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Francke MI, Andrews LM, Le HL, et al. Avoiding tacrolimus under‐ and overexposure with a dosing algorithm for renal transplant recipients: a single arm prospective intervention trial. Clin Pharmacol Ther. 2021;110(1):169‐178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gaber AO, Alloway RR, Bodziak K, Kaplan B, Bunnapradist S. Conversion from twice‐daily tacrolimus capsules to once‐daily extended‐release tacrolimus (LCPT): a phase 2 trial of stable renal transplant recipients. Transplantation. 2013;96(2):191‐197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kamar N, Cassuto E, Piotti G, et al. Pharmacokinetics of prolonged‐release once‐daily formulations of tacrolimus in de novo kidney transplant recipients: a randomized, parallel‐group, open‐label, multicenter study. Adv Ther. 2019;36(2):462‐477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Dobbels F, Moons P, Abraham I, Larsen CP, Dupont L, De Geest S. Measuring symptom experience of side‐effects of immunosuppressive drugs: the modified transplant symptom occurrence and distress scale. Transpl Int. 2008;21(8):764‐773. [DOI] [PubMed] [Google Scholar]
- 27. Atkinson MJ, Sinha A, Hass SL, et al. Validation of a general measure of treatment satisfaction, the Treatment Satisfaction Questionnaire for Medication (TSQM), using a national panel study of chronic disease. Health Qual Life Outcomes. 2004;2:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Levey AS, Coresh J, Greene T, et al. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med. 2006;145(4):247‐254. [DOI] [PubMed] [Google Scholar]
- 29. Leino AD, King EC, Jiang W, et al. Assessment of tacrolimus intrapatient variability in stable adherent transplant recipients: Establishing baseline values. Am J Transplant. 2019;19(5):1410‐1420. [DOI] [PubMed] [Google Scholar]
- 30. Tang JT, Andrews LM, van Gelder T, et al. Pharmacogenetic aspects of the use of tacrolimus in renal transplantation: recent developments and ethnic considerations. Expert Opin Drug Metab Toxicol. 2016;12(5):555‐565. [DOI] [PubMed] [Google Scholar]
- 31. Langone A, Steinberg SM, Gedaly R, et al. Switching STudy of Kidney TRansplant PAtients with Tremor to LCP‐TacrO (STRATO): an open‐label, multicenter, prospective phase 3b study. Clin Transplant. 2015;29(9):796‐805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bunnapradist S, Ciechanowski K, West‐Thielke P, et al. Conversion from twice‐daily tacrolimus to once‐daily extended release tacrolimus (LCPT): the phase III randomized MELT trial. Am J Transplant. 2013;13(3):760‐769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Budde K, Bunnapradist S, Grinyo JM, et al. Novel once‐daily extended‐release tacrolimus (LCPT) versus twice‐daily tacrolimus in de novo kidney transplants: one‐year results of Phase III, double‐blind, randomized trial. Am J Transplant. 2014;14(12):2796‐2806. [DOI] [PubMed] [Google Scholar]


