Abstract
Randomized controlled trials (RCTs) remain the gold standard to evaluate clinical interventions, producing the highest level of evidence while minimizing potential bias. Inadequate recruitment is a commonly encountered problem that undermines the completion and generalizability of RCTs—and is even more challenging when enrolling amidst a pandemic. Here, we reflect on our experiences with virtual recruitment of non‐hospitalized patients in the United States for ColCorona, an international, multicenter, randomized, placebo‐controlled coronavirus disease 2019 (COVID‐19) drug trial. Recruitment challenges during a pandemic include constraints created by shelter‐in‐place policies and targeting enrollment according to national and local fluctuations in infection rate. Presenting a study to potential participants who are sick with COVID‐19 and may be frightened, overwhelmed, or mistrusting of clinical research remains a challenge. Strategies previously reported to improve recruitment include transparency, patient and site education, financial incentives, and person‐to‐person outreach. Active measures taken during ColCorona to optimize United States recruitment involved rapid expansion of sites, adjustment of recruitment scripts, assessing telephone calls versus text messages for initial contact with participants, institutional review board‐approved financial compensation, creating an infrastructure to systematically identify potentially eligible patients, partnering with testing sites, appealing to both self‐interest and altruism, and large‐scale media efforts with varying degrees of success.
INTRODUCTION
Low enrollment in studies leads to inadequate power, inability to balance unmeasured confounders, and protocol modifications/deviations that may raise cost with nominal scientific benefit. 1 Recruitment is even more challenging in pandemic settings. We reflect on our recruitment experience during ColCorona‐US, the United States arm of ColCorona, a multinational, randomized, placebo‐controlled trial sponsored by Montreal Heart Institute (MHI) testing whether oral colchicine started within 2 days of receiving an outpatient coronavirus disease 2019 (COVID‐19) diagnosis could reduce hospitalization and death. 2 A 30‐day course of colchicine or placebo was delivered to the participant’s home and participants were followed by telephone assessment. ColCorona‐US recruitment began in New York city (NYC) in March 2020, followed quickly by San Francisco. The NYC (New York University [NYU]) site later assumed responsibility for all US trial activities through a single institutional review board (IRB) and US Food and Drug Administration investigational new drug application.
DESIGN OPTIMIZATION OF AN OUTPATIENT COVID‐19 RANDOMIZED CONTROLLED TRIAL
The ColCorona trial was pragmatically designed to be conducted entirely from home. The remote study center had a 24/7 toll‐free hotline for eligibility screening and provision of informed electronic consent. Regional centers delivered a paper consent to patients without internet access (reviewed with the study center via telephone) and delivered the study drug to the participant’s door via no‐contact courier (Supplementary Material S1). Participants could call the hotline for safety concerns at any time.
Patients may decline participation in clinical trials if enrollment will exclude them from taking established treatments. 3 ColCorona permitted use of other therapies (e.g., hydroxychloroquine and monoclonal antibodies), but did not allow enrollment in other outpatient COVID‐19 trials. The number of participants on other outpatient therapies were small (<1%), equal in both arms, and unlikely to affect study outcome. However, such open strategies need to be carefully considered and have the potential to confound the analyses. Because hospital admission met the primary end point, patients who progressed to hospitalization were free to enroll in any inpatient trial.
During a pandemic, mid‐trial study design adaptations to promote recruitment may be needed as knowledge is gained. Access to testing was an early obstacle. ColCorona eligibility criteria were, therefore, adjusted to include both individuals with a positive severe acute respiratory syndrome‐coronavirus 2 (SARS‐CoV‐2) polymerase chain reaction (PCR) test and (outside flu season) those with no PCR but a clinical diagnosis based on symptoms. 4 Although the latter comprised only 7% of the final study cohort, at least some participants may not have had COVID‐19. Ultimately, ColCorona met its predefined level of significance explicitly in the prespecified subgroup with a positive SARS‐CoV‐2 PCR test.
RECRUITMENT LANDSCAPE IN THE UNITED STATES
The waxing‐waning nature of the pandemic rapidly altered the recruitment landscape. During the initial surge in the NYC tri‐state area, the number of patients eligible for prescreening at NYU numbered in the thousands monthly. As the region’s infection rate decreased, our initial response was to expand our referral base to include nearby institutions and practices (Figure 1a), while continuing to provide all regulatory and pharmacy services. As local case numbers fell further and the epicenter of the pandemic shifted, we rapidly obtained permission from MHI and our US funder (National Heart, Lung, and Blood Institute [NHLBI]) to expand recruitment to other regions.
FIGURE 1.
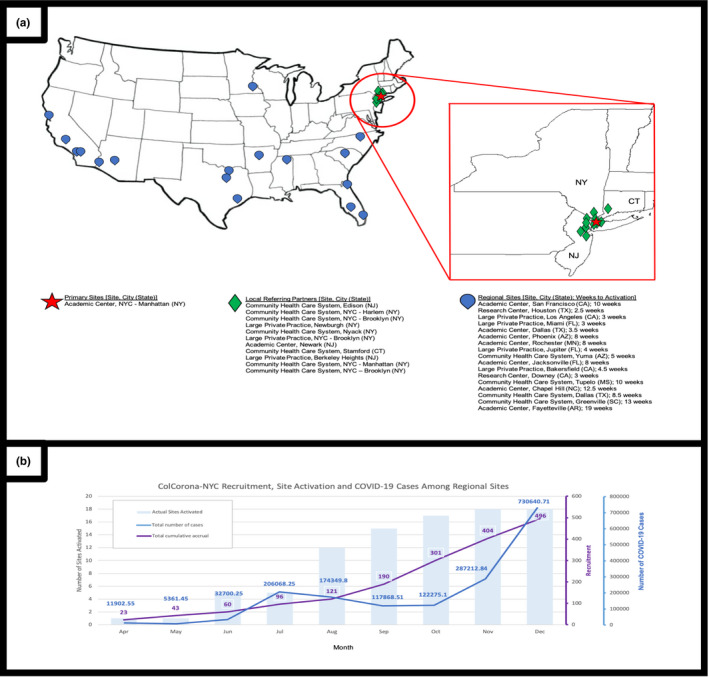
(a) Map and activation of local referring partners and regional recruiting sites in the United States. (b) Activation of regional sites and participant enrollment compared to local infection numbers. The bars represent the number of regional sites with active trial enrollment. The purple line represents the cumulative total of recruited participants in ColCorona‐US, enrolled among all active regional sites at the time. The blue line represents the number of COVID‐19 cases (based on daily rolling averages from the CDC 13 ), among US counties with an active ColCorona‐US recruiting site. CDC, Centers for Disease Control and Prevention; COVID‐19, coronavirus disease 2019; CT, Connecticut; NJ, New Jersey; NY, New York; NYC, New York city
Thereafter, the challenge was identifying rising “hotspots,” which constituted a moving target. Institutions with the highest case numbers were often those whose research infrastructures were less robust and that were unable to rapidly secure the necessary approvals and initiate infrastructure for outpatient recruitment. Although we tried to predict the “next areas” of rising case numbers, institutions we preemptively approached often felt they lacked enough patients, dampening their enthusiasm to participate. By the time the crisis inevitably arrived, they were too clinically overwhelmed to take on the study. Despite attempts to leverage established trial networks, use of a single IRB, and maintenance of administrative and liability burden largely on MHI, this pragmatic trial design with centralized infrastructure and Canadian/United States co‐sponsorship confused some local teams and led to delays in onboarding until after their cases had peaked. We ultimately added 11 local partners and 17 regional US sites with variation in site activation time from 2.5 weeks to 4.5 months (Figure 1).
PARTICIPANT‐LEVEL RECRUITMENT CHALLENGES AND OPTIMIZATION
Financial reimbursement for participation has been shown to increase enrollment. 5 ColCorona’s at‐home trial design minimized participant financial burden. As recruitment numbers declined, ColCorona‐US instituted a modest $50 reimbursement for participation with positive effect. The need for reimbursement in the United States but not in other participating countries raises issues of culture and participation that warrants further study.
Because nearly all recruitment for ColCorona‐US was conducted remotely, effective telephone/email outreach scripts were essential for successful enrollment. Our scripts underwent multiple iterations, including review by a communications specialist (Supplementary Material [Link], [Link], [Link], [Link]). Site team members were trained in conversational delivery, and we periodically inserted a recruitment specialist acting as a patient into the call list to provide performance feedback. Appealing to a person’s altruistic tendencies by emphasizing the importance of trial participation as a service to the community may also enhance enrollment efforts. 6 , 7 Over time, ColCorona messaging evolved from one of potential personal benefit to one that reflected trial participation to “help defeat COVID‐19.”
Potential participants from vulnerable communities may distrust research institutions given historical violations of ethical standards. 8 With a goal for diversity, equity, and inclusion in ColCorona‐US, we carefully considered feedback from one of our sites with a largely Hispanic patient population. Patients at that site implicitly trusted their own physicians but were hesitant to provide consent to remote study staff. That site was given an exemption to assess eligibility and obtain informed consent on site prior to remote follow‐up, which greatly increased their enrollment numbers. About 40% of US participants in ColCorona were of Hispanic ethnicity, many from that site. Although we hoped for similar outcomes in Black and Indigenous populations, the timeframe of the study did not allow us to develop and cultivate relationships with sites with established connections to these communities. There remains a need to financially and logistically support the building of a network of medical institutions and practices that serve the underserved and minority populations so that there may be greater access and representation of diverse populations in research.
A practical obstacle to recruitment lay in potential participants declining an unrecognized telephone number. We worked with our technology team to ensure all study team telephone calls were identified on the recipient’s end as NYU. We also created recruitment material that could be delivered via text messaging (Supplementary Material S4). We conducted a substudy during enrollment, in which potential participants were randomized to be initially contacted via telephone call versus text message with a follow‐up call. Although recruitment outcomes did not differ between the two groups (46% and 45% of patients in the text and call cohorts declined enrollment, respectively), the study team’s effort spent on an individual’s recruitment decreased with the incorporation of text messaging.
Retention is as important as recruitment. We found that retention of eligible participants from the time of initial contact to enrollment via MHI’s hotline was most successful when patients gave permission for MHI to contact them directly, or allowed regional sites to perform a “warm” hand‐off to the hotline using a three‐way call application. Simply giving the hotline number to interested patients and asking them to call the study center was least successful.
Only about 10% of patients contacted enrolled in ColCorona‐US (Figure 2). Many expressed reluctance to participate in a study while they were asymptomatic or had minimal symptoms, not acknowledging that progression to a symptomatic stage might render them ineligible for outpatient therapy. A smaller cohort declined enrollment for the opposite reason: they felt too ill to take the time to listen to a study team member explain the trial. Others felt overwhelmed from managing multiple medical comorbidities, processing either their own diagnoses or recent loss of a loved one to COVID‐19, or being approached for multiple COVID‐19 trials, to contemplate the risks/benefits of enrolling in ColCorona‐US.
FIGURE 2.
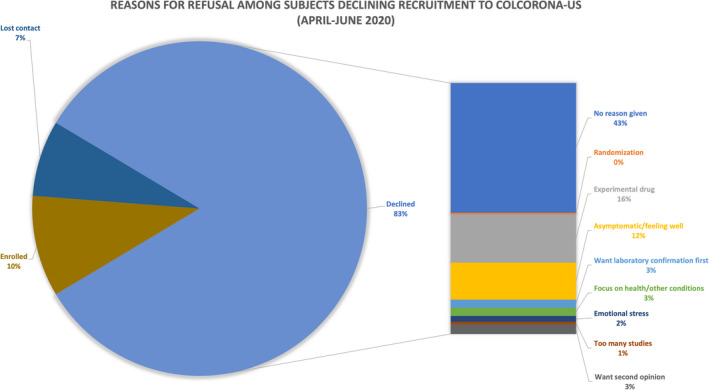
Reasons eligible participants approached from the New York regional site gave in declining enrollment in ColCorona‐US. Contact was attempted of 1710 eligible participants from the New York regional site during the time period of April through June. Initial contact was unable to be made with 687 patients, whereas 451 were determined to be not eligible based on further review of eligibility criteria during the screening process. There was a total of 572 eligible patients for which initial contact was successfully made (although 42 of those patients were lost to follow‐up before they could be enrolled)
SITE‐LEVEL RECRUITMENT CHALLENGES AND OPTIMIZATION
Physicians on a patient’s care team may play an important role in recruitment. However, with overwhelming clinical demands during surges in infection rates, many of our potential sites cited lack of physician time to support site activation and rapidly introduce outpatients to clinical trial options. Some systems also lacked infrastructure to systematically identify and contact eligible patients. The Canadian arm of ColCorona, which enrolled more than half of the patients in the global trial, utilized a provincial single‐electronic medical record (EMR) system that allowed patients to be identified quickly in the disease process. Unfortunately, such strategies are not feasible in the United States.
With an “opt‐in” approach, patients must actively indicate in advance their willingness to be contacted about ongoing trials. ColCorona‐US sites with “opt‐in” approaches struggled to find acceptable ways to contact potential participants who had not previously opted in. Our NYU site has an established “opt‐out” policy, wherein patients permit outreach to hear about studies unless they have indicated otherwise, allowing us to contact all potentially eligible outpatients based on the EMR, probably resulting in higher recruitment rates. 9 , 10 Another high‐enrolling ColCorona‐US site developed an efficient model to streamline the recruitment process 11 ; all patients who were COVID‐19‐positive were reviewed by a multidisciplinary panel twice daily, which allocated which clinical trial(s) the patients would be approached for, based on eligibility criteria. These approaches were particularly valuable in ColCorona, where recruiting patients early in an acute outpatient disease was mandatory.
Attempts to partner with external diagnostic testing sites proved bureaucratically challenging, and a costly partnership with one of the largest private national diagnostic centers in the United States yielded fewer than 2% of our US enrollees. Other outreach strategies included low‐cost websites, webinars, and geofencing via social media. ColCorona maintained a central website updated periodically to include logos of newly activated sites and multilingual participant testimonials to better connect with potential participants. Webinars on COVID‐19 providing the scientific data that supported equipoise of outpatient treatment with colchicine were conducted for local medical personnel/press upon activation of a new site in their region. Investigators also promoted the study via posting of IRB‐approved material on personal social media platforms. Larger‐scale paid advertisements were attempted and were both expensive and low‐yield. More effective was snowball sampling, in which active study participants recruit other participants via word‐of‐mouth among personal acquaintances 12 ; in the NYC tri‐state area, 6% of those enrolled were family members of another participant.
Public‐private partnerships, when properly aligned, may add flexibility and effectively support trials with unique needs; in our case, the Gates Foundation supported our outreach and publicity opportunities and forwarded our study results to the World Health Organization for increased dissemination. Partnership with local and national governments may also help reach a wider recruitment sample and promote trial enrollment. In Canada, ColCorona’s toll‐free hotline number was broadcast as a news ticker under the daily COVID‐19 government news conference, identifying ColCorona as a trusted partnership. This component was lacking in the United States despite NHLBI funding and investigator efforts to reach out to local government officials.
CONCLUSIONS
In ColCorona‐US, we ultimately reached our target enrollment contribution to the global trial but were unable to achieve diversity in inclusion of Black and Native populations largely due to our lack of existing ties to an established trusted community network. We found that successful outpatient randomized controlled trial recruitment in the COVID‐19 pandemic setting required the ability to (1) rapidly bring on a large number of collaborating sites; (2) contact a large number of potentially eligible patients; and (3) establish trust between the research enterprise and the potential participants. Local collaborators needed to leverage institutional and local community infrastructure to connect patients to the study center and provide options on methods to obtain informed consent. Finally, in a geographically and temporally fluctuating pandemic, it is of paramount importance to be flexible in trial design and quickly adapt to changing conditions. Future studies should address best practices for rapidly effective participant outreach in stressful situations, and best practices for rapid establishment of multisite research groups. National and governmental institutions should consider the establishment of flexible, latent trial networks that can be called rapidly into action in future pandemics.
CONFLICT OF INTEREST
The content of this paper is solely the responsibility of the authors and does not necessarily reflect the views of the National Heart, Lung, and Blood Institute, the National Institutes of Health, or the United States Department of Health and Human Services. For the purposes of full disclosure, B.S. reports grants from NIH NHLBI and the VA Office of Research and Development, serves on the advisory of Philips Volcano, and serves as a consultant for Terumo Medical. M.H.P. reports grants from Hikma Pharmaceuticals and Horizon Therapeutics, and from the NIH NCATS, and personal fees from Horizon Therapeutics and Fortress Biotech. J.‐C.T. reports grants from Government of Quebec, the Montreal Heart Institute Foundation, the Bill & Melinda Gates Foundation, Amarin, Esperion, Ionis, Servier, and RegenXBio, along with grants and personal fees from AstraZeneca, Sanofi, and Servier, and grants, personal fees, and minor equity interests from Dalcor. In addition, J.‐C.T.’s institution has submitted a pending patent for a method of treating a coronavirus infection using colchicine, and a pending patent on early administration of low‐dose colchicine after myocardial infarction. J.‐C.T. has waived his rights in all patents related to colchicine and does not stand to benefit financially if colchicine becomes used as a treatment for COVID‐19. All other authors declared no competing interests for this work.
Supporting information
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
ACKNOWLEDGEMENTS
The authors would like to acknowledge Steven A. Gogel, Associate Director of Marketing in the Office of Communications & Marketing at NYU Langone Health, for consultation about potential strategies to advertise the trial, as well as the US site investigators: NYU Grossman School of Medicine: Binita Shah, MD; Michael H. Pillinger, MD; Melanie Huber, Maria Daly with referrals from NYU Langone Health (Brooklyn and Long Island), NYC Health and Hospitals, Hackensack Meridian Health (Martha Radford, MD), Rutgers Newark (Diana Finkel, DO); Newburgh Horizon/Mid‐Hudson Medical (Sashi Makam, MD), Summit Medical Group (Lauren Kennish, MD), Valley Health (Evan Leibowitz, MD), Montefiore Nyack (Anthony Matejicka, MD), Stamford Health (Joseph Costanzo), Maimonides Hospital (Felix Yang, MD), Woodhull Hospital (Konstantin Brodetskiy, MD), Harlem Hospital (Farbod Raiszadeh, MD), Yoav Borsuk, MD Practice; South Florida Research Organization: Giralt Yanez, MD; Alexander Martinez; Mayo Clinic Rochester: Avni Joshi, MD; Jasmine Sexton; Mayo Clinic Phoenix: Matthew Rank, MD; Vy Nguyen; Mayo Clinic Jacksonville: Leigh Speicher, MD; Federico Rey L. Simon; University of North Carolina at Chapel Hill: Saira Z. Sheikh, MD; Beth Jonas, MD; Julie Walker; Westside Medical Associates of Los Angeles: Norman E. Lepor, MD; Edith Flores; Spring Clinical Research: Thu A Hoang, MD; Aijaz Hussain, MD; Merzieh Aijaz‐Bajwa; University of Texas Southwestern Medical Center: Jessica Meisner, MD; Nancy Rollins, MD; Stacey Hail, MD; Erika Molina; Yuma Regional Medical Center: Oday Al‐Rabadi, MD; Yesenia Zambrano, Sarah Medina‐Rodriguez, Rwanda Reyes; North Mississippi Health Services: Charles M. King, MD; Lara Blake, Beth Mathews; Rancho Research Institute: Sylvia Shaw, MD; Sheetal Desai, Nicole Bayus, Marielena Meza; Centric Health Resources Inc: William Baker, MD; Ritika Sharma; University of California San Francisco: Priscilla Hsue, MD; Kevin L Moore, Jr; Rebecca Park; Baylor Scott & White Research Institute: Harold Szerlip, MD; Tanqunisha Coleman; Miami Center for Advanced Cardiology: Ralph Nader, MD; Adam Nader, MD; Prisma Health Upstate: Amanda Schnee, MD; Prerana Roth, MD; Ann “Harvey” Shrum; University of Arkansas: Seth Berney, MD; Danielle Evans.
Hu K, Tardif J‐C, Huber M, et al. Chasing the storm: Recruiting non‐hospitalized patients for a multi‐site randomized controlled trial in the United States during the COVID‐19 pandemic. Clin Transl Sci. 2022;15:831‐837. doi: 10.1111/cts.13211
Michael H. Pillinger and Binita Shah are co‐senior authors.
Funding information
This research was supported in part by the National Heart, Lung, and Blood Institute of the National Institutes of Health (3R01HL146206‐02S1); the NYU CTSA grant from the National Center for Advancing Translational Sciences, National Institutes of Health (UL1 TR001445‐06A1); NYU School of Medicine; the Rudin Family Foundation, Inc; the philanthropist Sophie Desmarais; the Bill & Melinda Gates Foundation; the Government of Quebec; and the Montreal Heart Institute Foundation.
REFERENCES
- 1. Kitterman DR, Cheng SK, Dilts DM, Orwoll ES. The prevalence and economic impact of low‐enrolling clinical studies at an academic medical center. Acad Med. 2011;86:1360‐1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Tardif J‐C, Bouabdallaoui N, L'Allier PL, et al. Colchicine for community‐treated patients with COVID‐19 (COLCORONA): a phase 3, randomised, double‐blinded, adaptive, placebo‐controlled, multicentre trial. Lancet Respir Med. 2021;9(8):924‐932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lara PN Jr, Higdon R, Lim N, et al. Prospective evaluation of cancer clinical trial accrual patterns: identifying potential barriers to enrollment. J Clin Oncol. 2001;19:1728‐1733. [DOI] [PubMed] [Google Scholar]
- 4. Axell‐House DB, Lavingia R, Rafferty M, Clark E, Amirian ES, Chiao EY. The estimation of diagnostic accuracy of tests for COVID‐19: a scoping review. J Infect. 2020;81:681‐697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Treweek S, Pitkethly M, Cook J, et al. Strategies to improve recruitment to randomised trials. Cochrane Database Syst Rev. 2018;2:MR000013‐MR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Truong TH, Weeks JC, Cook EF, Joffe S. Altruism among participants in cancer clinical trials. Clin Trials. 2011;8:616‐623. [DOI] [PubMed] [Google Scholar]
- 7. Jenkins V, Fallowfield L. Reasons for accepting or declining to participate in randomized clinical trials for cancer therapy. Br J Cancer. 2000;82:1783‐1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Unger JM, Cook E, Tai E, Bleyer A. The role of clinical trial participation in cancer research: barriers, evidence, and strategies. Am Soc Clin Oncol Educ Book. 2016;35:185‐198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ahmad MU, Hanna A, Mohamed AZ, et al. A systematic review of opt‐out versus opt‐in consent on deceased organ donation and transplantation (2006–2016). World J Surg. 2019;43:3161‐3171. [DOI] [PubMed] [Google Scholar]
- 10. Junghans C, Feder G, Hemingway H, Timmis A, Jones M. Recruiting patients to medical research: double blind randomised trial of "opt‐in" versus "opt‐out" strategies. BMJ. 2005;331:940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. O’Horo J, Cerhan J, Cahn E, Bauer P, Temesgen Z. Outcomes of COVID‐19 with the Mayo Clinic model of care and research [published online ahead of print December 22, 2020]. Mayo Clin Proc. 2021;96(3):601‐618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Roehr S, Wittmann F, Jung F, et al. Strategies to recruit refugees for intervention studies: lessons learned from the "Sanadak" Trial. Psychother Psychosom Med Psychol. 2019;69:484‐489. [DOI] [PubMed] [Google Scholar]
- 13. COVID Data Tracker . COVID‐19 Integrated County View. CDC: Centers for Disease Control and Prevention. https://covid.cdc.gov/covid‐data‐tracker/#county‐view. Accessed May 4, 2021.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material


