Abstract
Objectives:
To provide new information on factors associated with discrepancies between patient-reported and audiometrically-defined hearing loss (HL) in adult-onset cancer survivors after cisplatin-based chemotherapy (CBCT) and to comprehensively investigate risk factors associated with audiometrically-defined HL.
Design:
A total of 1,410 testicular cancer survivors (TCS) ≥6 months post-CBCT underwent comprehensive audiometric assessments (0.25-12 kHz) and completed questionnaires. HL severity was defined using American Speech-Language-Hearing Association (ASHA) criteria. Multivariable multinomial regression identified factors associated with discrepancies between patient-reported and audiometrically-defined HL, and multivariable ordinal regression evaluated factors associated with the latter.
Results:
Overall, 34.8% of TCS self-reported HL. Among TCS without tinnitus, those with audiometrically-defined HL at only extended high frequencies (EHFs) (10-12 kHz) (17.8%) or at both EHFs and standard frequencies (0.25-8 kHz) (23.4%) were significantly more likely to self-report HL than those with no audiometrically-defined HL (8.1%) (OR=2.48; 95%CI, 1.31-4.68 and OR= 3.49; 95%CL,1.89-6.44, respectively). Older age (OR=1.09; 95%CI, 1.07-1.11, P<0.0001), absence of prior noise exposure (OR=1.40; 95%CI, 1.06-1.84, P=0.02), mixed/conductive HL (OR=2.01;95%CI, 1.34-3.02, P=0.0007), no hearing aid use (OR=5.64; 95%CI, 1.84-17.32, P=0.003), and lower education (OR, 2.12; 95%CI, 1.23-3.67, P=0.007 for high school or less education vs. post-graduate education) were associated with greater underestimation of audiometrically-defined HL severity, while tinnitus was associated with greater overestimation (OR, 4.65; 95%CI, 2.64-8.20 for a little tinnitus, OR, 5.87; 95%CI, 2.65-13.04 for quite a bit tinnitus, and OR, 10.57; 95%CI, 4.91-22.79 for very much tinnitus P<0.0001). Older age (OR=1.13; 95%CI, 1.12-1.15, P<0.0001), cumulative cisplatin dose (>300 mg/m2, OR=1.47; 95%CI, 1.21-1.80, P=0.0001), and hypertension (OR=1.80; 95%CI, 1.28-2.52, P=0.0007) were associated with greater ASHA-defined HL severity, whereas post-graduate education (OR=0.58; 95%CI, 0.40-0.85, P=0.005) was associated with less severe HL.
CONCLUSIONS:
Discrepancies between patient-reported and audiometrically-defined HL after CBCT are due to several factors. For survivors who self-report HL, but have normal audiometric findings at standard frequencies, referral to an audiologist for additional testing and inclusion of EHFs in audiometric assessments, should be considered.
Introduction
Testicular cancer (TC) is the most common malignancy among men age 20-39 years (Hayes-Lattin et al. 2009), with 10-year survival rates >95% (Travis et al. 2010), due largely to the introduction of cisplatin-based chemotherapy (CBCT) in the 1970s (C. Fung et al. 2018). Although TC survivors (TCS) can live over 40 years after treatment (Capocaccia et al. 2015), they remain at risk for short-term and long-term CBCT side effects (Travis et al. 2010). Hearing loss (HL), a common cisplatin toxicity, is permanent and irreversible (Frisina et al. 2016; Chunkit Fung et al. 2017; Kerns et al. 2018). No FDA-approved treatments exist to prevent cisplatin-induced HL (Landier 2016) and there are no medical therapies to restore auditory function (Cruickshanks et al. 2015). HL is associated with poorer health-related quality-of-life (HRQOL), depression, cognitive decline, and social isolation (Amieva et al. 2015; Dalton et al. 2003; Li et al. 2014; Stephens et al. 2001). Although the optimal assessment for HL is pure-tone audiometry (Baiduc et al. 2013), evaluation of patient-reported symptoms are increasingly recognized as valid, important (LeBlanc et al. 2017), and time/cost-effective (Oldenburg et al. 2006). However, subjective perceptions of HL differ from audiometrically-defined HL in the general population (Hong et al. 2011; Kamil et al. 2015; Kirk et al. 2012), and in CBCT-treated TCS (Bokemeyer et al. 1998; Frisina et al. 2016). Discrepancies in the former are associated with age (Ikeda et al. 2009; Kamil et al. 2015; Kiely et al. 2012; S. Y. Kim et al. 2017; Ramkissoon et al. 2011), race (Kamil et al. 2015), gender (Kamil et al. 2015; S. Y. Kim et al. 2017), education (Kamil et al. 2015; S. Y. Kim et al. 2017), tinnitus (S. Y. Kim et al. 2017), occupation (S. Y. Kim et al. 2017), middle ear conditions (Louw et al. 2018), anxiety/depression (S. Y. Kim et al. 2017), hearing aid use (S. Y. Kim et al. 2017), smoking (Ramkissoon and Cole 2011), and noise exposure (Ramage-Morin et al. 2019). In contrast, no investigation to date addresses factors associated with discrepancies in patient-reported versus audiometric HL in cancer survivors, in whom adaptation to symptoms over time may lead to underreporting, a phenomenon known as response shift (Ilie et al. 2019).
Audiometrically-defined HL after CBCT in TCS has been associated with age, cumulative cisplatin dose, and hypertension (Frisina et al. 2016). As in de novo HL, additional risk factors may be operational, but have not been examined in adult-onset CBCT-treated cancer survivors. In the general population, these include dyslipidemia (Helzner et al. 2011; J. Shargorodsky et al. 2010), body mass index (BMI) (Curhan et al. 2013), waist circumference (Cruickshanks et al. 2015; Curhan et al. 2013; Engdahl et al. 2015; Linssen et al. 2014), diabetes (Agrawal et al. 2009; Bainbridge et al. 2008; Cruickshanks et al. 2015; Engdahl et al. 2015), alcohol consumption (Engdahl et al. 2015), and physical activity (Curhan et al. 2013; Engdahl et al. 2015). Cardiometabolic factors (e.g., obesity, hypertension, dyslipidemia, diabetes, cerebrovascular disease) are associated with HL due to compromised vascular supply to the stria vascularis, resulting in impaired cochlear function. In contrast, physical activity can improve cochlear vascular endothelial function; thus, protecting against HL (Curhan et al. 2013). Since recommended cisplatin doses to treat metastatic TC are relatively fixed, and since cisplatin can directly increase the risk of ototoxicity through cochlear damage or indirectly through increased risk of cardiometabolic adverse health outcomes (C. Fung et al. 2018), it is important to identify and manage the modifiable cardiometabolic risk factors that may contribute to HL. Additionally, understanding the association between cardiometabolic factors and HL in cisplatin-treated cancer survivors is important to inform cancer care plans.
Our aim was to provide new information on factors associated with discrepancies between patient-reported and audiometrically-defined HL in a large, clinically well-characterized cohort of CBCT-treated TCS and to comprehensively investigate those factors known to be associated with HL in the general population.
Methods
Study Population
Patients were enrolled in the Platinum Study that includes nine sites in the U.S., Canada, and the United Kingdom (see text, Supplemental Digital Content 1, which describes supplementary methods). This study was approved by each institution’s IRB, with patients providing written informed consent. Cohort methods were described previously (Frisina et al. 2016; Chunkit Fung et al. 2017; Kerns et al. 2018). Briefly, eligibility criteria are: histological/serological diagnosis of germ cell tumor (GCT) at age ≤60 years, completion of first-line CBCT for initial GCT or recurrence after active surveillance, and routine follow-up at the enrolling site. This study includes 1,410 TCS ≥6 months postchemotherapy with complete audiometric assessments.
Clinical Characteristics and Patient-reported Outcomes
Standard forms were used by trained personnel to abstract cancer diagnosis and treatment information from medical records. Patient-reported “difficulty hearing over the past four weeks” was assessed with the European Organization for Research and Treatment of Cancer Chemotherapy Induced Peripheral Neuropathy questionnaire (EORTC-CIPN-20) (Postma et al. 2005), and tinnitus was evaluated with the Scale for Chemotherapy-Induced Long-Term Neurotoxicity (SCIN) (Oldenburg et al. 2006). Sociodemographic characteristics, health behaviors, and adverse health outcomes (AHOs) were assessed through validated questionnaires (see text, Supplemental Digital Content 1, which describes supplementary methods) (Ainsworth et al. 2011; Chasan-Taber et al. 1996; Oldenburg et al. 2006; Postma et al. 2005; Taylor et al. 1978; Ventry et al. 1982; Ware et al. 1992).
Audiometric Assessments
Audiometric assessments were designed by a hearing scientist (RDF) and described in detail previously (Frisina et al. 2016). Briefly, bilateral pure-tone air conduction thresholds were conducted at frequencies 0.25-12 kHz. Bone-conduction thresholds (frequencies: 0.25-4 kHz) assessed middle ear dysfunction.
HL classification/severity.
Air-conduction hearing thresholds and HL severity were classified according to American Speech-Language-Hearing Association (ASHA) criteria, with HL defined as thresholds at any frequency that exceeded 20 dB for either ear (Frisina et al. 2016; Le Prell et al. 2011). HL severity criteria were: mild (21-40 dB), moderate (41-55 dB), moderately severe (56-70 dB), severe (71-90 dB), and profound (>91 dB) for ≥ one frequency for either ear (American Speech-Language-Hearing Association (ASHA) ; Frisina et al. 2016). Taking into account middle ear dysfunction, HL was grouped as pure sensorineural HL, pure conductive HL, and mixed HL (Frisina et al. 2016) (see text, Supplemental Digital Content 1, which describes supplementary methods).
Statistical Analysis
Percentages and medians (ranges) were used for discrete and continuous variables, respectively. Proportions of patient-reported difficulty hearing were compared between TCS with no-audiometrically-defined HL and those in each of the following categories by chi-square tests: audiometrically-defined HL at only extended high frequencies (EHFs) (10-12 kHz), at only standard frequencies (0.25-8 kHz), or at both sets of frequencies (see text, Supplemental Digital Content 1, which describes supplementary methods). Odds ratios (OR) and 95%CI were calculated for each comparison (Harrington et al. 2019).
Discrepancies between patient-reported and audiometrically-defined HL using ASHA criteria (0.25-12 kHz) were classified as follows; concordance (matched severity between patient-reported and audiometrically-defined HL), underestimation (less severe patient-reported vs. audiometrically-defined HL), and overestimation (more severe patient-reported vs. audiometrically-defined HL).
The following variables were selected for inclusion in the crude analysis of discrepancies between patient-reported and audiometrically-defined HL since they were associated with differences between subject-reported and audiometrically-defined HL in the general population: age (Ikeda et al. 2009; Kamil et al. 2015; Kiely et al. 2012; S. Y. Kim et al. 2017; Ramkissoon and Cole 2011), race (Kamil et al. 2015), education (Kamil et al. 2015; S. Y. Kim et al. 2017), tinnitus (S. Y. Kim et al. 2017), occupation (S. Y. Kim et al. 2017), middle ear conditions (Louw et al. 2018), anxiety/depression (S. Y. Kim et al. 2017), hearing aid use (S. Y. Kim et al. 2017), smoking (Ramkissoon and Cole 2011), and noise exposure (Ramage-Morin et al. 2019). We additionally tested for the effect of “time since chemotherapy completion” to assess for “response shift” (Ilie et al. 2019), and evaluated the effect of self-reported health on discrepancies between patient-reported and audiometrically-defined hearing loss.
Crude multinomial logistic regression models evaluated the relation between each independent variable of interest and discrepancy between patient-reported and audiometrically-defined HL as the dependent variable (concordance as reference category). Variables with Wald chi-square P≤0.25 were selected for inclusion in the initial set of predictors for the multivariable model (Stoltzfus 2011). The final multivariable model was constructed through backward elimination of variables with Wald chi-square P>0.10. The rationale for a two-stage (crude and adjusted) approach was two-fold. First, both crude results (which answers whether this is an association) and adjusted results (which answers whether there is a unique association controlling for other predictors including potentially confounding covariates) can help interpret findings in the context of prior literature. Second, using crude associations to screen variables for inclusion in the subsequent variable-selection modeling was helpful because too many independent variables in the early steps of the backward-elimination process may lead to unstable results, multicollinearity problems, and reduced generalizability beyond the study sample; (Stoltzfus 2011) further, variables screened at a liberal 0.25 alpha are unlikely to be significant in multivariable models.
To assess associations between independent variables of interest and HL severity (dependent variable), age-adjusted ordinal logistic regression models were used (since age is a known HL risk factor (National Academies of Sciences 2016b)). The following variables were included in the age-adjusted analysis of factors associated with ASHA-defined HL severity since they are associated with HL in the general population: age (Cruickshanks et al. 2015; Hoffman et al. 2017; Linssen et al. 2014), race (Hoffman et al. 2017), education (Cruickshanks et al. 2015; Hoffman et al. 2017), hypertension (Brant et al. 1996; Przewozny et al. 2016), dyslipidemia (Helzner et al. 2011; J. Shargorodsky et al. 2010), body mass index (BMI) (Curhan et al. 2013), waist circumference (Cruickshanks et al. 2015; Curhan et al. 2013; Engdahl et al. 2015; Linssen et al. 2014), diabetes (Agrawal et al. 2009; Bainbridge et al. 2008; Cruickshanks et al. 2015; Engdahl et al. 2015), smoking (Agrawal et al. 2009; Cruickshanks et al. 2015; Engdahl et al. 2015; Helzner et al. 2011), alcohol consumption (Engdahl et al. 2015), noise exposure (Agrawal et al. 2009; Hoffman et al. 2017); and physical activity (Curhan et al. 2013; Engdahl et al. 2015). In addition, age (Brydoy et al. 2009; Frisina et al. 2016; Glendenning et al. 2010), cumulative cisplatin dose (Bokemeyer et al. 1998; Brydoy et al. 2009; Chen et al. 2006; Frisina et al. 2016; Glendenning et al. 2010; Haugnes et al. 2018; Theunissen et al. 2015), hypertension (Frisina et al. 2016), a history of noise exposure (Bokemeyer et al. 1998), educational level (Brydoy et al. 2009), and smoking (Brydoy et al. 2009) have been reported to be associated with cisplatin-induced HL.
ASHA-defined HL severity categories were used based on frequencies previously shown to exhibit significant dose-dependent relations with cumulative cisplatin amount (4-12 kHz) (Frisina et al. 2016). Variables with Wald chi-square P≤0.25 were included in the multivariable model (Stoltzfus 2011). The final multivariable model was constructed through backward elimination of variables with Wald chi-square P>0.10. For all ordinal logistic regression models, the proportional odds assumption was evaluated with the Score chi-square test (Gameroff 2005).
There was no multicollinearity between independent variables in the final models using the variance inflation factor (VIF<5) (Allison 2012; J. H. Kim 2019). All tests were two-sided, and ORs (95%CIs) were reported for all models. A 95% CI that did not overlap 1.00 and 2-tailed P < 0.05 were considered statistically significant. All analyses were performed using SAS version-9.4 (SAS Institute).
Results
Population Characteristics
Median age at audiometry for 1,410 TCS was 37 years (range=18-74 years) and median time since chemotherapy-completion, 3.1 years (range=0.5-31.9 years). Overall median cumulative cisplatin dose was 400 mg/m2 and 92.7% of participants received etoposide and cisplatin with/without bleomycin (BEP/EP). The population was largely white (87.4%), college-educated (67.5%), and never-smokers (61.3%) (Table 1). Overall, 34.8% reported difficulty hearing, while 77.8% had audiometrically-defined HL (Table 2).
Table 1.
Clinical and Sociodemographic Characteristics, Health Behaviors, and Physical Examination Findings for 1,410 Survivors of Cisplatin-Treated Germ-Cell Tumors (GCT) with Comprehensive Audiometric Assessments
| Characteristic | No. (%) |
|---|---|
| Clinical characteristic | |
| Age at GCT diagnosis, years | |
| Median (range) | 31 (10-60) |
| <20 | 95 (6.7) |
| 20-29 | 535 (37.9) |
| 30-39 | 465 (33.0) |
| ≥ 40 | 315 (22.3) |
| Age at audiometry, years | |
| Median (range) | 37 (18-74) |
| <20 | 6 (0.4) |
| 20-29 | 298 (21.1) |
| 30-39 | 501 (35.5) |
| 40-49 | 347 (24.6) |
| 50-59 | 214 (15.2) |
| ≥ 60 | 44 (3.1) |
| Calendar year of GCT diagnosis a | |
| Before 2000 | 141 (10.1) |
| 2000-2009 | 431 (30.8) |
| 2010-2017 | 827 (59.1) |
| Histologic type b | |
| Seminoma | 380 (27.2) |
| Nonseminoma or mixed GCT | 1007 (72.0) |
| GCT, not otherwise specified | 12 (0.9) |
| Site of GCT c | |
| Testis | 1256 (89.8) |
| Extragonadal | 143 (10.2) |
| Cisplatin-based chemotherapy d | |
| BEP e | 792 (56.2) |
| EP f | 514 (36.5) |
| Otder g | 104 (7.4) |
| Cumulative dose of cisplatin, mg/m2 h | |
| Median (range) | 400 (100-1402.8) |
| <300 | 73 (5.2) |
| 300 | 527 (37.4) |
| 301-399 | 58 (4.1) |
| 400 | 685 (48.7) |
| >400 | 65 (4.6) |
| Time since completion of chemotderapy, years i | |
| Median (range) | 3.1 (0.5-31.9) |
| <2 | 537 (38.5) |
| 2-<5 | 350 (25.1) |
| 5-<10 | 250 (17.9) |
| ≥ 10 | 258 (18.5) |
| Sociodemographic characteristic | |
| Race j | |
| White | 1184 (87.4) |
| African American | 12 (0.9) |
| Asian | 63 (4.7) |
| Otder | 96 (7.1) |
| Education k | |
| High school or less | 146 (10.5) |
| After high school but not college graduate | 308 (22.1) |
| College or university graduate | 602 (43.2) |
| Post-graduate | 338 (24.3) |
| Employment status l | |
| Unemployed | 96 (7.0) |
| Employed | 1224 (88.9) |
| Retired | 27 (2.0) |
| On disability leave | 30 (2.2) |
| Healtd behavior | |
| Smoking status m | |
| Never | 854 (61.3) |
| Former | 453 (32.5) |
| Current | 87 (6.2) |
| Average no. alcoholic drinks in past year n | |
| Rarely or never | 266 (19.1) |
| ≤ 4 per week | 634 (45.6) |
| 5 per week to 1 per day | 322 (23.2) |
| ≥2 daily | 169 (12.2) |
| Physical activity (total MET-hours/week) quartiles o | |
| Quartile 1, Median (range) | 4.28 (0-10.23) |
| Quartile 2, Median (range) | 17.5(10.24-26.0) |
| Quartile 3, Median (range) | 34.5 (26.1-48.4) |
| Quartile 4, Median (range) | 72.9 (48.5-300) |
| Engage in vigorous physical activity (≥ 6 METs) p | |
| Yes | 941 (67.5) |
| No | 453 (32.5) |
| Physical examination finding | |
| Body mass index (kg/m2) q | |
| Median (range) | 27.3 (18.0-66.6) |
| < 25 (normal) | 399 (28.5) |
| 25-29 (overweight) | 592 (42.4) |
| ≥ 30 (obese/morbidly obese) | 407 (29.1) |
| Waist circumference, cm r | |
| Median (range) | 94 (62-190) |
| <102 | 980 (71.1) |
| ≥ 102 | 399 (28.9) |
Abbreviations: BEP, bleomycin, etoposide, and cisplatin; BMI, body mass index; CBCT, cisplatin-based chemotherapy; EP, etoposide and cisplatin; GCT, germ cell tumor; MET, metabolic equivalent task; VeIP, vinblastine, ifosfamide, and cisplatin; VIP, etoposide, ifosfamide, and cisplatin.
Calendar year of diagnosis was not available for 11 participants.
Histologic type was not available for 11 participants.
Germ cell tumor site was not available for 11 participants.
Thirty-eight (2.7%), 577 (41%), 758 (53.8%), and 36 (2.6%) participants received ≤ two, three, four, and five or more cycles of CBCT, respectively. Number of cycles was not available for one participant.
Includes 173 (21.8%) participants with modification of dosing and schedules of BEP. The remaining 619 participants (78.2%) received the standard dosing and standard BEP schedule: each chemotherapy cycle consisted of bleomycin, 30,000 IU days 1, 8, and 15; etoposide 100 mg/m2 days 1 through 5; and cisplatin 20 mg/m2 days 1 through 5. In addition, 541 (68.3%) and 223 (28.2%) participants received three and four cycles of BEP, respectively. Median cumulative cisplatin doses for the BEP group were 300 mg/m2 (range, 100 to 800 mg/m2).
Includes 205 (39.9%) participants with modification of dosing and schedules of EP. The remaining 309 participants (60.1%) received the standard dosing and standard EP schedule: each chemotherapy cycle consisted of etoposide 100 mg/m2 days 1 through 5 and cisplatin 20 mg/m2 days 1 through 5. In addition, 456 (88.7%) participants received four cycles of EP. The median cumulative cisplatin doses for the EP group were 400 mg/m2 (range, 200 to 1402.8 mg/m2).
Of 104 participants, 55 received VIP, 32 received cisplatin and ifosfamide; 6 received cisplatin, bleomycin, etoposide, and ifosfamide; one received VeIP. For the remaining 10, other combinations of CBCT were applied.
Cisplatin dose information was not available for 2 participants.
Information on time since completion of chemotherapy was not available for 15 participants.
Race not stated for 55 participants.
Educational status not stated for 16 participants.
Employment status not stated for 33 participants.
Smoking status not stated for 16 participants.
Average no. of alcoholic drinks in past year not stated for 19 participants.
Physical activity was assessed in this study based on a validated questionnaire (Ainsworth et al. 2011; Chasan-Taber et al. 1996; Taylor et al. 1978). Participants were asked about the type of physical activity (walking; jogging (>10 min/mile); running (≤10 min/mile); bicycling; aerobic exercise; lower intensity exercise such as yoga, stretching, or toning; tennis, squash, or racquetball; lap swimming; weight lifting or stretch swimming) and average time per week (during the past year) spent at each of these activities. Participants were also asked about the number of flights of stairs they climb daily. A MET value was assigned to each physical activity based on its energy cost. Walking pace was used to calculate MET-hours/week from walking (Ainsworth et al. 1993). Physical activity (total MET-hours/week) was derived from reported hours per week of each physical activity plus the reported number of climbed flights of stairs per day (Curhan et al. 2013). Physical activity information was not provided by 16 participants.
Vigorous physical activity was defined based on activities with MET value ≥ 6 (Ainsworth et al. 2011). Physical activity information was not provided by 16 participants.
BMI not available for 12 participants. Among 407 participants with BM I≥ 30, 358 and 49 participants had a BMI of 30-39 kg/m2 (obese) and ≥ 40 kg/m2 (morbidly obese), respectively.
Abdominal obesity was defined as waist circumference ≥ 102 for men (D. Kim et al. 2019). Waist circumference not available for 31 participants.
Table 2.
Audiometric Findings and Patient-reported Outcomes for 1,410 Survivors of Cisplatin-Treated Germ-Cell Tumors
| Characteristic | No. (%) |
|---|---|
| Audiometric findings | |
| Audiometrically-defined hearing loss (0.25-12 kHz) a | |
| None, ≤ 20dB | 312 (22.1) |
| Mild, 21-40dB | 342 (24.3) |
| Moderate, 41-55dB | 205 (14.5) |
| Moderately severe, 56-70dB | 284 (20.1) |
| Severe/profound, ≥ 71dB | 267 (18.9) |
| Type of hearing loss | |
| None | 312 (22.1) |
| Pure sensorineural | 810 (57.5) |
| Pure conductive | 11 (0.8) |
| Mixed | 277 (19.7) |
| Patient-reported adverse healtd outcome (AHO) | |
| Difficulty hearing b | |
| Not at all | 904 (65.2) |
| A little | 309 (22.3) |
| Quite a bit | 122 (8.8) |
| Very much | 51 (3.7) |
| Problems hearing speech-in-background-noise c | |
| No | 919 (69.4) |
| Yes | 406 (30.6) |
| Use hearing aid d | |
| No | 1366 (98.3) |
| Yes | 23 (1.7) |
| Tinnitus (ringing in ears) e | |
| Not at all | 802 (57.7) |
| A little | 373 (26.9) |
| Quite a bit | 107 (7.7) |
| Very much | 107 (7.7) |
| Hypertension and on prescription medication f | |
| No | 1222 (88.8) |
| Yes | 154 (11.2) |
| Hypercholesterolemia and on prescription medication g | |
| No | 1240 (88.9) |
| Yes | 155 (11.1) |
| Diabetes and on prescription medication h | |
| No | 1335 (96.7) |
| Yes | 45 (3.3) |
| Cardiovascular disease i | |
| No | 1345 (97.8) |
| Yes | 31 (2.2) |
| Use of medications for anxiety and/or depression j | |
| No | 1287 (91.3) |
| Yes | 123 (8.7) |
| Other | |
| Noise exposure k | |
| No | 802 (57.9) |
| Yes | 582 (42.1) |
| Self-reported healtd l | |
| Excellent | 246 (17.6) |
| Very good | 585 (41.9) |
| Good | 467 (33.5) |
| Fair/poor | 97 (6.9) |
Abbreviations: AHO, adverse health outcome.
Hearing loss was defined using American Speech-Language-Hearing Association criteria (ASHA) classification (American Speech-Language-Hearing Association (ASHA) ; Frisina et al. 2016) for frequencies of 0.25 to 12 kHz with HL defined as thresholds at any frequency that exceeded 20 dB for either ear. Among 267 participants with severe/profound hearing loss, 238 and 29 participants had severe (71-90 dB) and profound (>90 dB) hearing loss, respectively.
Difficulty hearing was assessed with the European Organization for Research and Treatment of Cancer Chemotherapy Induced Peripheral Neuropathy 20-item quality-of-life questionnaire (EORTC-CIPN-20) on the basis of symptoms experienced over the past 4 weeks (Postma et al. 2005). This AHO was not available for 24 participants.
Problems hearing speech-in-background-noise was defined if patients answered “yes” to the following question “Problems hearing words, sounds, or language in crowds?” Data for problems hearing speech-in-background-noise was not available for 28 participants and 57 participants stated to be unsure about it.
Hearing aid use information was not available for 21 participants. Among 23 participants using a hearing aid, 5 and 18 reported using hearing aid for one ear and both ears, respectively.
Tinnitus was assessed with the Scale for Chemotherapy-Induced Long-Term Neurotoxicity (SCIN) questionnaire on the basis of symptoms experienced over the past 4 weeks (Oldenburg et al. 2006). This AHO was not available for 21 participants.
Hypertension and on prescription medication defined as answering “Yes” to (1) have you ever been diagnosed with high blood pressure and “Yes, current” to (2) have you ever taken prescription medications for high blood pressure (including current use) (Chunkit Fung et al. 2017). This AHO was not available for 34 participants.
Hypercholesterolemia and on prescription medication defined as answered “Yes, current” to the following question: have you ever taken prescription medications for high cholesterol (Chunkit Fung et al. 2017). This AHO was not available for 15 participants.
Diabetes and on prescription medication defined as answering “Yes” to either of the following questions: (1) diabetes requiring insulin or (2) diabetes requiring tablets or pills (Chunkit Fung et al. 2017). This AHO was not available for 30 participants.
Includes coronary artery disease, heart failure, or cerebrovascular disease. This AHO was not available for 34 participants.
Based on patient-reported prescription medications taken for at least the past 4 weeks. Medication indication was classified as used for anxiety and/or depression according to both (1) its pharmacological class and (2) if patients indicated its use was for anxiety and/or depression. Participants could report use of more than one medication for anxiety and/or depression. Medications used by 123 participants include alprazolam (n=10), bupropion (n=11), buspirone (n=2), citalopram (n=9), clomipramine (n=1), clonazepam (n=21), desvenlafaxine (n=1), duloxetine (n=8), escitalopram (n=25), fluoxetine (n=8), fluvoxamine (n=1), lorazepam (n=9), mirtazapine (n=2), nortriptyline (n=1), paroxetine (n=12), sertraline (n=17), trazodone (n=5), and venlafaxine (n=12).
Noise exposure information was not available for 26 participants. This include 15 participants who did not reply to questions related to work-related noise exposure and non-work related noise exposure, 1 patient who answered “no” to work-related noise exposure, but did not reply to the question regarding non-work related noise exposure, and 10 patients who answered “no” to non-work related noise exposure, but did not reply to the question regarding work-related noise. Among 582 patients reporting noise exposure, 216, 151, and 215 had work-related only, non-work-related only, and both work-related and non-work-related noise exposure, respectively.
Self-reported health not stated for 15 participants. Among 97 participants with self-reported rated as “fair or poor,” 86 and 11 participants indicated fair and poor heath, respectively.
Audiometrically-defined versus Patient-reported HL
Patients with HL at only EHFs (OR=1.51; 95%CI, 1.02-2.24) or both sets of frequencies (OR=3.68; 95%CI, 2.58-5.25) were significantly more likely to report difficulty hearing vs. TCS without audiometrically-defined HL. But, for patients with HL at only standard frequencies, there was no statistically significant difference between audiometrically-defined HL and self-reported hearing difficulty (OR=1.59; 95%CI, 0.78-3.23; n=48) compared with TCS without audiometrically-defined HL (Table 3).
Table 3.
Patient-Reported Hearing Loss (HL) versus Audiometrically-Defined HL at Extended-High Frequencies (10 and 12 kHz), Standard Audiometric Frequencies (0.25-8 kHz), and Both Sets of Frequencies: All Patients
| Patient-reported Hearing Loss |
No audiome trically- defined HLa |
Audiometrically-defined HL at only EHFs (10 or 12 kHz), but none at standard frequencies (0.25-8 kHz) a |
Audiometrically- defined HL at only standard frequencies (0.25-8 kHz) but none at EHFs (10 and 12 kHz) a |
Audiometrically- defined HL at both EHFs (10 or 12 kHz) and standard frequencies (0.25-8 kHz) a |
|||
|---|---|---|---|---|---|---|---|
| N (%) | N (%) | OR (95%CI) (vs no HL) |
N (%) | OR (95%CI) (vs no HL) |
N (%) | OR (95%CI) (vs no HL) |
|
| Number b | 259 | 368 | 48 | 592 | |||
| Difficulty hearing c | |||||||
| Not at all | 205 (81.0) | 268 (73.8) | 1.51 (1.02-2.24)f | 35 (72.9) | 1.59 (0.78-3.23) | 312 (53.7) | 3.68 (2.58-5.25)f |
| Any degree | 48 (19.0) | 95 (26.2) | 13 (27.1) | 269 (46.3) | |||
| Problems hearing speech-in-background-noise d | |||||||
| No | 201 (83.8) | 266 (78.0) | 1.45 (0.95-2.23) | 37 (78.7) | 1.39 (0.64-3.03) | 330 (59.0) | 3.58 (2.44-5.24)f |
| Yes | 39 (16.3) | 75 (22.0) | 10 (21.3) | 229 (41.0) | |||
| Tinnitus (ringing in ears) e | |||||||
| Not at all | 174 (68.5) | 247 (68.0) | 1.02 (0.72-1.44) | 31 (66.0) | 1.12 (0.58-2.17) | 277 (47.5) | 2.40 (1.76-3.28)f |
| Any degree | 80 (31.5) | 116 (31.9) | 16 (34.0) | 306 (52.5) | |||
Abbreviations: EHF, extended high frequency; HL, hearing loss; N, number.
Audiometrically-defined HL was based on American Speech-Language-Hearing Association (ASHA) criteria that defined HL as hearing thresholds that exceed 20 dB at any frequency for either ear (Frisina et al. 2016; Le Prell et al. 2011).
Among a total of 1,410 patients, 143 had missing audiometry data at frequencies of both 10 kHz and 12 kHz and are excluded from the table.
Difficulty hearing was assessed with the European Organization for Research and Treatment of Cancer Chemotherapy Induced Peripheral Neuropathy 20-item quality-of-life questionnaire (EORTC-CIPN-20) on the basis of symptoms experienced over the past 4 weeks (Postma et al. 2005). Any degree of difficulty hearing includes participant responses of a little, quite a bit, or very much.
Problems hearing speech-in-background-noise was defined if patients answered “yes” to the following question “Problems hearing words, sounds, or language in crowds?”.
Tinnitus was assessed with the Scale for Chemotherapy-Induced Long-Term Neurotoxicity (SCIN) questionnaire on the basis of symptoms experienced over the past 4 weeks (Oldenburg et al. 2006). Any degree of tinnitus includes participant responses of a little, quite a bit, or very much.
Denotes P values of Pearson Chi-square test with significance at P<0.05.
After exclusion of patients with tinnitus (Table 4), the effect size comparing difficulty hearing for patients with HL at only EHFs vs. no audiometrically-defined HL increased (OR=2.48; 95%CI, 1.31-4.68). Only those patients with audiometrically-defined HL at both sets of frequencies reported significantly greater ‘problems hearing speech-in-background-noise’ vs. those without audiometrically-defined HL (OR=2.10; 95%CI, 1.21-3.64).
Table 4.
Patient-Reported Hearing Loss (HL) versus Audiometrically-Defined HL at Extended-High Frequencies (10 and 12 kHz), Standard Audiometric Frequencies (0.25-8 kHz), and Both Sets of Frequencies Among TCS Without Tinnitus
| Patient- reported hearing loss |
No audiometrically- defined HL a |
Audiometrically-defined HL at only EHFs (10 or 12 kHz), but none at standard frequencies (0.25-8 kHz) a |
Audiometrically-defined HL at only standard frequencies(0.25-8 kHz) but noneat EHFs (10 and 12 kHz) a |
Audiometrically-defined HL at both EHFs (10 or 12 kHz)and standard frequencies (0.25-8 kHz)a |
|||
|---|---|---|---|---|---|---|---|
| N (%) | N (%) | OR (95%CI) (vs no HL) |
N (%) | OR (95%CI) (vs no HL) |
N (%) | OR (95%CI) (vs no HL) |
|
| Number b | 179 | 252 | 32 | 286 | |||
| Difficulty hearing c | |||||||
| Not at all | 160 (91.95) | 203 (82.2) | 2.48 (1.31-4.68)f | 27 (84.4) | 2.12 (0.70-6.36) | 213 (76.6) | 3.49 (1.89-6.44)f |
| Any degree | 14 (8.05) | 44 (17.8)e | 5 (15.6) | 65 (23.4) | |||
| Problems hearing speech-in-background-noise d | |||||||
| No | 149 (88.2) | 198 (83.9) | 1.43 (0.80-2.56) | 28 (87.5) | 1.06 (0.34-3.35) | 209 (78.0) | 2.10 (1.21-3.64)f |
| Yes | 20 (11.8) | 38 (16.1) | 4 (12.5) | 59 (22.0) | |||
Abbreviations: EHF, extended high frequency; HL, hearing loss; N, number, TCS, testicular cancer survivors
Audiometrically-defined hearing loss was defined based on American Speech-Language-Hearing Association (ASHA) criteria that defined HL as hearing thresholds that exceeded 20 dB at any frequency for either ear (Frisina et al. 2016; Le Prell et al. 2011).
Among a total of 823 patients without tinnitus, 74 had missing audiometry data at frequencies of both 10 kHz and 12 kHz and are excluded from the table.
Difficulty hearing was assessed with the European Organization for Research and Treatment of Cancer Chemotherapy Induced Peripheral Neuropathy 20-item quality-of-life questionnaire (EORTC-CIPN-20) on the basis of symptoms experienced over the past 4 weeks (Postma et al. 2005). Any degree of difficulty hearing included participant responses of a little, quite a bit, or very much.
Problems hearing speech-in-background-noise was defined if patients answered “yes” to the following question “Problems hearing words, sounds, or language in crowds?”.
Patients with HL at only EHFs who reported any degree of hearing difficulty (N=44) compared to those with no audiometrically HL who reported any degree of hearing difficulty (N=14) had a significantly higher median age at audiometry (37 vs 30 years, P= 0.0002), but there were no significant difference between these two groups in terms of cumulative cisplatin dose as a continuous variable (P=0.68), middle ear deficits (pure conductive/mixed HL vs. no/pure sensorineural HL, P=0.32), noise exposure (P=0.90), and time since chemotherapy (year) as a continuous variable (P=0.47).
Denotes P values of Pearson Chi-square test with significance at P<0.05.
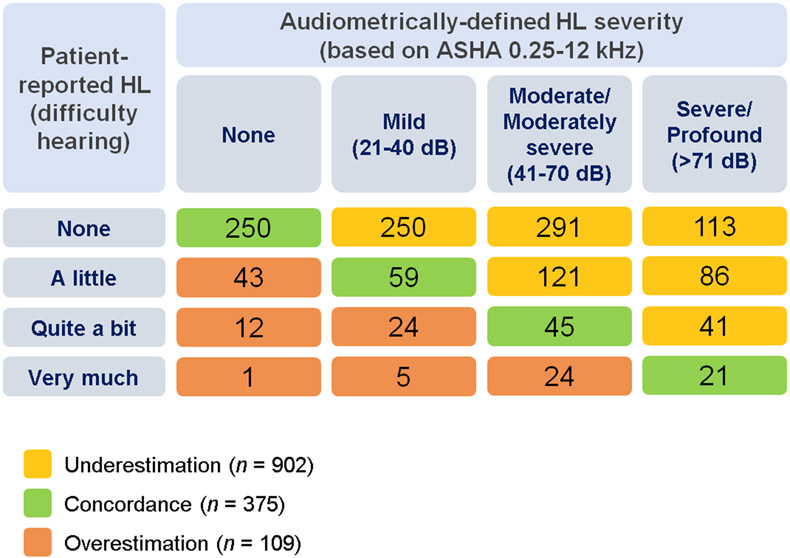
Given the above results and the known importance of EHFs in speech perception (Monson et al. 2014), all frequencies (0.25-12 kHz) were included in analyses of discrepancies between patient-reported and audiometrically-defined HL. Only 375 (27.1%) patients were in the concordance group, whereas 902 (65.1%) underestimated and 109 (7.9%) overestimated HL severity (Figure 1).
Figure 1.
Classification of 1,386 Cisplatin-treated Testicular Cancer Survivors by Patient-reported Hearing Loss (HL) vs. Audiometrically-defined HL: Underestimation, Concordance, and Overestimation. Patient-reported HL (“difficulty hearing”) was assessed with the European Organization for Research and Treatment of Cancer Chemotherapy Induced Peripheral Neuropathy 20-item quality-of-life questionnaire (EORTC-CIPN-20) on the basis of symptoms experienced over the past 4 weeks (Postma et al. 2005). Audiometrically-defined HL was defined following methods in Frisina et al. (Frisina et al. 2016) using ASHA criteria for frequencies of 0.25 to 12 kHz.
Abbreviations: ASHA, American Speech-Language-Hearing Association criteria; HL, hearing loss.
Crude associations of independent variables of interest with discrepancies between patient-reported and audiometrically-defined HL were performed using multinomial logistic regression models (see Table 5). Table 6 shows the final multivariable model. Using the concordant group as reference, the following factors were significantly associated with greater underestimation of audiometrically-defined HL severity: older age (OR=1.09; 95%CI, 1.07-1.11), no prior noise exposure (OR=1.40; 95%CI, 1.06-1.84), mixed/conductive HL (OR=2.01; 95%CI, 1.34-3.02), no hearing aid use (OR=5.64; 95%CI, 1.84-17.32), and lower education vs. post-graduate [including high school or less (OR=2.12; 95%CI, 1.23-3.67) and college or university graduate (OR=1.57; 95%CI, 1.12-2.20)]. Tinnitus was associated with greater overestimation of audiometrically-defined HL severity (OR=4.65, 5.87, and 10.57 for “a little”, “quite a bit”, and “very much”, respectively P each<0.0001). While time since chemotherapy was significantly associated with greater hearing loss underestimation (Table 5), it lost its significance in the multivariable model when adjusting for other variables; thus, it was removed from the final model.
Table 5.
Crude Multinomial Logistic Regression Models of Risk Factors Associated with Discrepancies Between Patient-reported and Audiometrically-defined Hearing Loss (HL) (ASHA Criteria for Frequencies of 0.25 to 12 kHz)
| Variable | Underestimation of Audiometric HL (vs concordance) |
Overestimation of Audiometric HL (vs concordance) |
Omnibus P value |
||
|---|---|---|---|---|---|
| OR (95%CI) | P value | OR (95%CI) | P value | ||
| Age at audiometry | 1.08 (1.07-1.10) | <0.0001 | 1.00 (0.98-1.02) | 0.92 | <0.0001 a |
| Education | 0.15 a | ||||
| Post-graduate | Ref. | Ref. | |||
| College or university graduate | 1.01 (0.74-1.38) | 0.95 | 0.79 (0.46-1.37) | 0.40 | |
| After high school but not college graduate | 0.74 (0.52-1.05) | 0.09 | 0.79 (0.43-1.46) | 0.46 | |
| High school or less | 1.39 (0.86-2.24) | 0.18 | 1.41 (0.65-3.07) | 0.39 | |
| Race | 0.26 | ||||
| White | Ref. | Ref. | |||
| Non-white | 1.03 (0.71-1.50) | 0.88 | 1.58 (0.88-2.85) | 0.13 | |
| Tinnitus b | <0.0001 a | ||||
| Not at all | Ref | Ref. | |||
| A little | 1.11 (0.83-1.47) | 0.49 | 4.44 (2.55-7.76) | <0.0001 | |
| Quite a bit | 0.99 (0.62-1.60) | 0.98 | 5.70 (2.70-12.01) | <0.0001 | |
| Very much | 0.90 (0.55-1.47) | 0.68 | 9.63 (4.84-19.14) | <0.0001 | |
| Noise exposure | 0.007 a | ||||
| Yes | Ref. | Ref. | |||
| No | 1.38 (1.09-1.77) | 0.009 | 0.88 (0.57-1.36) | 0.57 | |
| Use of anti-anxiety and anti-depressant medications c | 0.65 | ||||
| No | Ref. | Ref. | |||
| Yes | 1.19 (0.77-1.86) | 0.43 | 1.34 (0.65-2.78) | 0.43 | |
| Smoking status | 0.03 a | ||||
| Never | Ref. | Ref. | |||
| Former | 1.19 (0.92-1.56) | 0.19 | 1.14 (0.71-1.84) | 0.59 | |
| Current | 0.82 (0.49-1.36) | 0.44 | 2.15 (1.06-4.38) | 0.03 | |
| Employment status | 0.37 | ||||
| Unemployed/Retired/ on disability leave | Ref. | Ref. | |||
| Employed | 1.13 (0.77-1.66) | 0.53 | 0.76 (0.41-1.41) | 0.39 | |
| Use hearing aid | 0.099 a | ||||
| Yes | Ref. | ||||
| No | 1.95 (0.76-4.97) | 0.16 | 0.58 (0.17-1.95) | 0.38 | |
| Self-reported health | 0.19 a | ||||
| Excellent | Ref | Ref. | |||
| Very good | 1.01 (0.72-1.42) | 0.95 | 1.37 (0.69-2.73) | 0.37 | |
| Good | 0.89 (0.63-1.28) | 0.54 | 1.67 (0.84-3.31) | 0.15 | |
| Fair or poor | 1.04 (0.59-1.81) | 0.90 | 2.91 (1.18-7.19) | 0.02 | |
| Time since chemotherapy, years | <0.0001 a | ||||
| < 2 | Ref. | Ref. | |||
| 2-<5 | 0.88 (0.65-1.19) | 0.41 | 1.23 (0.74-2.04) | 0.42 | |
| 5-<10 | 1.77 (1.23-2.55) | 0.002 | 1.51 (0.81-2.79) | 0.19 | |
| ≥ 10 | 1.86 (1.29-2.67) | 0.0008 | 0.74 (0.35-1.57) | 0.43 | |
| Conductive HL (middle ear deficit) | <0.0001 a | ||||
| No HL and/or pure sensorineural HL | Ref. | Ref. | |||
| Mixed and/or pure conductive HL | 2.52 (1.77-3.58) | <0.0001 | 1.63 (0.91-2.94) | 0.10 | |
Abbreviations: ASHA, American Speech-Language-Hearing Association criteria; CI, confidence interval; HL, hearing loss; OR, odds ratio; Ref., reference.
Note: Each row of analysis is derived from a multinomial logistic regression model in which we report the effect for the independent variable of interest and discrepancy between patient-reported and audiometrically-defined HL (concordance, underestimation, and overestimation) as the outcome (dependent) variable.
P values with boldface indicate significance at P<0.05.
Variables with Omnibus (Wald chi-square from type 3 analysis of effects) P≤0.25 are selected to be included in the final multivariable model.
Tinnitus was assessed with the Scale for Chemotherapy-Induced Long-Term Neurotoxicity (SCIN) questionnaire on the basis of symptoms experienced over the past 4 weeks (Oldenburg et al. 2006).
Based on patient-reported prescription medications taken for at least the past 4 weeks. Medication indication was classified as used for anxiety and/or depression according to both (1) its pharmacological class and (2) if patients indicated its use was for anxiety and/or depression.
Table 6.
Final Multivariable Multinomial Logistic Regression Model of Risk Factors Associated with Discrepancies Between Patient-reported and Audiometrically-defined Hearing Loss (HL) (ASHA: Frequencies 0.25-12 kHz)
| Variable | Underestimation of HL (vs concordance) |
Overestimation of HL (vs concordance) |
||
|---|---|---|---|---|
| OR (95%CI) | P value | OR (95%CI) | P value | |
| Age at audiometry | 1.09 (1.07-1.11) | <0.0001 | 0.98 (0.95-1.00) | 0.08 |
| Education | ||||
| Post-graduate | Ref. | Ref. | ||
| College or university graduate | 1.57 (1.12-2.20) | 0.01 | 0.66 (0.36-1.20) | 0.17 |
| After high school but not college graduate | 1.08(0.73-1.61) | 0.68 | 0.64 (0.32-1.26) | 0.19 |
| High school or less | 2.12 (1.23-3.67) | 0.007 | 1.19 (0.50-2.82) | 0.69 |
| Tinnitus a | ||||
| Not at all | Ref. | Ref. | ||
| A little | 1.19 (0.87-1.62) | 0.27 | 4.65 (2.64-8.20) | <0.0001 |
| Quite a bit | 0.74 (0.43-1.27) | 0.28 | 5.87 (2.65-13.04) | <0.0001 |
| Very much | 0.61 (0.35-1.07) | 0.08 | 10.57 (4.91-22.79) | <0.0001 |
| Noise exposure | ||||
| Yes | Ref. | Ref. | ||
| No | 1.40 (1.06-1.84) | 0.02 | 1.13 (0.70-1.83) | 0.61 |
| Use hearing aid | ||||
| Yes | Ref. | Ref. | ||
| No | 5.64 (1.84-17.32) | 0.003 | 0.90 (0.24-3.41) | 0.87 |
| Conductive HL (middle ear deficit) | ||||
| No HL and/or pure sensorineural HL | Ref. | Ref. | ||
| Mixed and/or pure conductive HL | 2.01 (1.34-3.02) | 0.0007 | 1.16 (0.59-2.29) | 0.67 |
Abbreviations: ASHA, American Speech-Language-Hearing Association criteria; CI, confidence interval; HL, hearing loss; OR, odds ratio; Ref., reference.
Note: The ORs and P values are from an adjusted multinomial logistic regression model that includes all variables listed in the table with discrepancy between patient-reported and audiometrically-defined HL (concordance, underestimation, and overestimation) as the outcome (dependent) variable.
Variables with Omnibus (Wald chi-square from type 3 analysis of effects) P>0.1 are removed from the final model in a backward deletion procedure. The Omnibus P value for the variables listed in the table include: age at audiometry (P<0.0001), education (P =0.004), tinnitus (P<0.0001), noise exposure (P=0.053), use hearing aid (P=0.004), conductive hearing loss (middle ear deficit) (P=0.002).
P values with boldface indicate significance of P<0.05.
Tinnitus was assessed with the Scale for Chemotherapy-Induced Long-Term Neurotoxicity (SCIN) questionnaire on the basis of symptoms experienced over the past 4 weeks (Oldenburg et al. 2006).
Factors Associated with ASHA-defined HL Severity
Age-adjusted associations between variables related to HL in the general population as reviewed above (e.g., BMI, waist circumference, alcohol consumption, hypertension, diabetes, hypercholesterolemia, cardiovascular disease (CVD), and noise exposure) and ASHA-defined HL severity were performed using ordinal logistic regression models (see Table 7). In the final multivariable ordinal logistic regression model (Table 8), the following variables showed significant associations with greater ASHA-defined HL severity: older age (OR=1.13; 95%CI, 1.12-1.15), cumulative cisplatin dose (>300 mg/m2) (OR=1.47; 95%CI, 1.21-1.80), and hypertension (OR=1.80; 95%CI,1.28-2.52). Post-graduate education (OR=0.58; 95%CI, 0.40-0.85) was associated with less ASHA-defined HL severity.
Table 7.
Age-Adjusted Logistic Regression Models of Factors Associated with Cisplatin-Associated ASHA-Defined Hearing Loss Severity
| Variable | OR (95% CI) | P value | Omnibus P value |
|---|---|---|---|
| Cumulative dose of cisplatin, mg/m2 | 0.0003 a | ||
| ≤300 | Ref. | ||
| >300 | 1.43 (1.18-1.74) | 0.0003 | |
| Race | 0.28 | ||
| White | Ref. | ||
| African-American | 1.25 (0.44-3.50) | 0.67 | |
| Asian | 1.53 (0.96-2.44) | 0.08 | |
| Other | 1.18 (0.80-1.74) | 0.40 | |
| Education | 0.0032 a | ||
| High school or less | Ref. | ||
| After high school but not college graduate | 0.76 (0.53-1.10) | 0.15 | |
| College or university graduate | 0.83 (0.59-1.15) | 0.26 | |
| Post-graduate | 0.56 (0.39-0.80) | 0.0015 | |
| Smoking status | 0.88 | ||
| Never | Ref. | ||
| Former | 1.04 (0.85-1.29) | 0.70 | |
| Current | 0.95 (0.63-1.42) | 0.79 | |
| Average no. alcoholic drinks in past year | 0.46 | ||
| Rarely or never | Ref. | ||
| ≤ 4 per week | 0.84 (0.65-1.10) | 0.20 | |
| 5 per week to 1 per day | 0.79 (0.59-1.07) | 0.12 | |
| ≥ 2 daily | 0.85 (0.59-1.21) | 0.35 | |
| Body mass index, kg/m2 | 0.11 a | ||
| <25 (normal) | Ref. | ||
| 25-29 (overweight) | 1.25 (0.98-1.58) | 0.07 | |
| ≥ 30 (obese) | 1.29 (0.99-1.67) | 0.06 | |
| Waist circumference, cm | 0.05 a | ||
| <102 | Ref. | ||
| ≥ 102 | 1.25 (1.01-1.56) | 0.05 | |
| Engage in vigorous physical activity (≥ 6 METs) | 0.73 | ||
| No | Ref. | ||
| Yes | 0.96 (0.78-1.19) | 0.73 | |
| Physical activity (total MET-hours/week) quartiles | 0.09 a | ||
| Quartile 1 | Ref. | ||
| Quartile 2 | 0.74 (0.56-0.97) | 0.03 | |
| Quartile 3 | 0.99 (0.76-1.31) | 0.98 | |
| Quartile 4 | 0.84 (0.64-1.11) | 0.23 | |
| Hypertension and on prescription medication (patient-reported) b | 0.0003 a | ||
| No | Ref. | ||
| Yes | 1.86 (1.33-2.59) | 0.0003 | |
| Diabetes and on prescription medication c | 0.76 | ||
| No | Ref. | ||
| Yes | 1.09 (0.62-1.91) | 0.76 | |
| Hypercholesterolemia and on prescription medication d | 0.47 | ||
| No | Ref. | ||
| Yes | 1.13 (0.81-1.58) | 0.47 | |
| Cardiovascular disease e | 0.31 | ||
| No | Ref. | ||
| Yes | 1.44 (0.71-2.90) | 0.31 | |
| Noise exposure | 0.12 a | ||
| No | Ref. | ||
| Yes | 1.17 (0.96-1.42) | 0.12 |
Abbreviations: CI, confidence interval; MET, metabolic equivalent task; OR, odds ratio; Ref., reference.
Note: Each row of analysis is derived from an ordinal regression model in which we report the effect for the primary independent variable of interest and adjust for age at audiometry.
Hearing loss was defined following methods in Frisina et al (Frisina et al. 2016) using American Speech-Language-Hearing Association criteria (ASHA) for frequencies of 4 through 12 kHz for which dose-response relationships with cumulative amount of cisplatin were shown (Frisina et al. 2016).
P values with boldface indicate significance at P<0.05.
Variables with Omnibus (Wald chi-square from type 3 analysis of effects) P≤0.25 were selected for inclusion in the final multivariable model.
Hypertension and on prescription medication was defined as answering “yes” to (1) have you ever been diagnosed with high blood pressure and “yes, current” to (2) have you ever taken prescription medications for high blood pressure (including current use) (Chunkit Fung et al. 2017).
Diabetes and on prescription medication was defined as answering “yes” to either of the following questions: (1) diabetes requiring insulin or (2) diabetes requiring tablets or pills (Chunkit Fung et al. 2017). This adverse health outcome was not available for 30 participants.
Hypercholesterolemia and on prescription medication defined as answered “yes, current” to the following question: have you ever taken prescription medications for high cholesterol (Chunkit Fung et al. 2017).
Includes coronary artery disease, heart failure, or cerebrovascular disease. The age-adjusted association between cardiovascular disease with ASHA-defined hearing loss severity did not meet the proportional odds assumption.
Table 8.
Final Multivariable Ordinal Logistic Regression Model of Factors Associated with Cisplatin-Associated ASHA-Defined Hearing Loss Severity
| Variable | OR (95% CI) | P value |
|---|---|---|
| Age at audiometry | 1.13 (1.12-1.15) | <0.0001 |
| Cumulative dose of cisplatin, mg/m2 | ||
| ≤ 300 | Ref. | |
| > 300 | 1.47 (1.21-1.80) | 0.0001 |
| Education | ||
| High school or less | Ref. | |
| After high school but not college graduate | 0.78 (0.53-1.14) | 0.19 |
| College or university graduate | 0.87 (0.61-1.24) | 0.44 |
| Post-graduate | 0.58 (0.40-0.85) | 0.005 |
| Physical activity (total MET-hours/week) quartiles | ||
| Quartile 1 | Ref. | |
| Quartile 2 | 0.74 (0.56-0.98) | 0.04 |
| Quartile 3 | 1.05 (0.79-1.40) | 0.73 |
| Quartile 4 | 0.88 (0.66-1.17) | 0.36 |
| Hypertension and on prescription medication | ||
| No | Ref. | |
| Yes | 1.80 (1.28-2.52) | 0.0007 |
Abbreviations: ASHA, American Speech-Language-Hearing Association criteria; CI, confidence interval; METs, metabolic equivalent task; OR, odds ratio; Ref., reference.
Note: The ORs and P values are from an adjusted ordinal logistic regression model that includes all variables listed in the table with ASHA-defined hearing loss severity based on frequencies of 4 kHz to 12 kHz as the outcome (dependent) variable. Hearing loss was defined following methods in Frisina et al (Frisina et al. 2016) using ASHA for frequencies of 4 to 12 kHz for which dose-response relationships with cumulative amount of cisplatin was shown (Frisina et al. 2016).
Audiometry assessments based on ASHA (4-12 kHz) criteria among a total of 1,410 participants were as follows: 333 (23.6%), 327 (23.2%), 201 (14.3%), 284 (20.1%), 237 (16.8%), and 28 (2.0%) had no hearing loss (HL), mild HL (20-40 dB), moderate HL (41-55 dB), moderately severe HL (56-70 dB), severe HL (71-90 dB), and profound HL (> 90 dB), respectively. The frequency of any degree of audiometric-defined HL in each age category are as follows: 152/304 (50%), 355/501 (70.9%), 317/347 (91.4%), and 253/258 (98.1%) for <30 years, 30-39 years, 40-49 years, and ≥50 years, respectively.
Variables with Omnibus (Wald chi-square from type 3 analysis of effects) P>0.1 have been removed from the final model in a backward deletion procedure. The Omnibus P values for variables listed in the table are: age at audiometry (P<0.0001), cumulative dose of cisplatin (P=0.0001), education (P=0.005), total METS-hours/week (quartiles) (P=0.06), and hypertension and on prescription medication (P=0.0007).
P values with boldface indicate significance of P<0.05.
Discussion
The Platinum Study represents the largest cohort of CBCT-treated patients in which both comprehensive audiometric assessments and patient-reported HL were rigorously evaluated. It thus affords a unique opportunity to provide new information on factors associated with discrepancies between audiometrically-defined and patient-reported HL that could influence the success of post-chemotherapy HL management strategies. To our knowledge, this is the first study to evaluate these factors in adult-onset cancer survivors, with previous investigations conducted only in the general population. An important new finding is that a significantly greater proportion of patients with HL at only EHFs (10-12 kHz) self-reported difficulty hearing vs. patients with no audiometric HL, showing for the first time to our knowledge that deficits restricted to this range are perceptible to about 1 in 4 of all survivors after CBCT. Factors associated with discrepancies between patient-reported and audiometrically-defined HL included age, mixed/conductive HL, absence of prior noise exposure, education, hearing aid use, and tinnitus. Time since chemotherapy completion, was not significantly associated with discrepancies between patient-reported and audiometrically-defined HL in the multivariable model, suggesting that response shift in this relatively young cohort of TCS with median follow-up of 3 years, was not playing a significant role in HL discrepancies. Older age, larger cumulative cisplatin doses, and hypertension were associated with greater ASHA-defined HL severity, while higher education was associated with less severity.
Extended High-Frequency HL
We show that audiometrically-defined HL restricted to EHFs is perceptible to 26.2% of adult-onset cancer survivors after CBCT (Table 3) and 17.8% of those without tinnitus (Table 4), even with normal findings at standard audiometric frequencies. Although the audible range for most individuals extends to 15 kHz (Monson et al. 2014), studies on the perceptual effects of high frequencies are limited. High frequency energy (5.7-22 kHz) is important in the perception of music/speech in terms of sound quality/localization, consonant recognition, and understanding speech in noisy environments (Monson et al. 2014).
Our findings show that for patients with self-reported HL after CBCT but normal audiometric findings (0.25-8 kHz), referral to an audiologist for additional testing and inclusion of EHFs should be encouraged. This is especially important in relatively young TCS, since they are less likely to experience the deficits in EHFs that accompany aging (Skalleberg et al. 2020). To our knowledge, there are no follow-up guidelines for audiometric assessments in CBCT-treated adult-onset cancer survivors. Available guidelines for CBCT-treated childhood cancer survivors recommend testing hearing thresholds at 0.25-8 kHz(Bass et al. 2016) and >8 kHz (Clemens et al. 2019). ASHA guidelines also recommend, but do not require, testing 9-20 kHz after ototoxic insults (e.g., cisplatin) (American Speech-Language-Hearing Association (ASHA) 1994).
Discrepancies Between Patient-reported and Audiometrically-defined HL
Tinnitus.
We show a strong positive association between tinnitus and HL overestimation after CBCT. The effect size of association between even “a little tinnitus” and overestimation of HL in our study was two-fold greater than that in the general population (S. Y. Kim et al. 2017), suggesting that tinnitus might be a strong factor in TCS that could affect the discrepancy between patient-reported and audiometrically defined HL. In a normative Canadian population, patients with tinnitus were less likely to underestimate HL vs. those without tinnitus (Ramage-Morin et al. 2019). Another investigation showed an association of tinnitus with both HL underestimation and overestimation (S. Y. Kim et al. 2017), but no adjustment for confounding factors (e.g., noise exposure) was applied. In contrast, while adjusting for confounders, we found an independent role of tinnitus in HL overestimation. This overestimation may be due to distress associated with tinnitus, or a misunderstanding of the interplay between tinnitus and hearing loss in these patients. For example, tinnitus has been shown to be associated with anxiety (Crocetti et al. 2009; McCormack et al. 2015; Josef Shargorodsky et al. 2010), depression (Crocetti et al. 2009; McCormack et al. 2015), and obsessive thoughts (Pattyn et al. 2016); thus, patients’ concern with regard to related conditions (Bauer 2018), such as HL, may account for the observed overestimation.
Age.
Increasing age was associated with underestimation of HL severity, a finding that mirrors the general population (Kamil et al. 2015; Kiely et al. 2012; S. Y. Kim et al. 2017; Ramkissoon and Cole 2011). Reasons include the progressive nature of HL over time resulting in cumulative small changes that go unnoticed (Gordon-Salant 2005), less demand for communication among older individuals (Gordon-Salant 2005), and HL denial due to its stigma as an age-related condition (Choi et al. 2016). Thus, self-reported HL may not reliably assess age-related HL prevalence (Kiely et al. 2012).
Conductive HL.
We found an association between mixed/conductive HL and greater HL underestimation. Similarly, a Korean study found abnormal tympanic membrane status (a type of conductive HL) associated with less accurate HL reporting compared with audiometric assessments (S. Y. Kim et al. 2017). Conductive HL occurs in the middle or external ear and causes impaired sound transmission to inner ear structures. Contributing factors include infections, tympanic membrane perforation, changes in stiffness of the ossicular chain, and cerumen accumulation (Cunningham et al. 2017; National Research Council 2005), with the latter a common cause (Isaacson et al. 2003). Since some of these factors primarily reduce sound loudness, rather than cause distortion, and are transient (e.g., acute infections), they could result in HL underestimation in some patients.
Noise exposure.
The absence of previous noise exposure was associated with greater HL underestimation, possibly due to patients not expecting HL (Ramage-Morin et al. 2019). Similarly, among a normative Canadian population, individuals who had not worked in noisy environments were more likely to underestimate HL than noise-exposed individuals (Ramage-Morin et al. 2019). On the other hand, noise exposure has been associated with higher levels of patient-reported difficulty hearing, even with normal audiograms (Tremblay et al. 2015).
Education.
Lower education was associated with greater underestimation of HL, consistent with studies in the general population (Kamil et al. 2015; S. Y. Kim et al. 2017). Health literacy may partially account for this observation, since higher education is positively correlated with health literacy (An Issue Brief From the U.S. Department of Health and Human Services). Individuals with lower health literacy are reported to have more difficulty understanding the types of, causes of, and provided services for HL, which adds to the misperception these individuals may have when seeking care for hearing difficulties (National Academies of Sciences 2016a). Conversely, more highly-educated individuals may be more aware of their HL severity, and less likely to underestimate it.
Hearing aid use.
No hearing aid use was associated with more HL underestimation. In the general population, greater levels of handicap caused by HL shorten delays in seeking/adopting hearing aids (Simpson et al. 2019). Reasons for hearing aid non-use in the general population include high cost, difficulties with use/maintenance, and associated stigma (National Academies of Sciences 2016c).
Factors Associated with ASHA-defined HL severity
Few studies have examined HL risk factors in CBCT-treated adult-onset cancer survivors, taking into account a wide range of audiometric frequencies. Limitations of prior investigations included evaluation of only a few frequencies (Brydoy et al. 2009), small sample sizes (<100 survivors) (Bokemeyer et al. 1998; Chen et al. 2006; Haugnes et al. 2018), and confounding by cranial radiotherapy (Chen et al. 2006; Theunissen et al. 2015), vincristine (Bokemeyer et al. 1998; Glendenning et al. 2010), or carboplatin (Chen et al. 2006; Glendenning et al. 2010), Moreover, previous series (Bokemeyer et al. 1998; Brydoy et al. 2009; Chen et al. 2006; Glendenning et al. 2010; Haugnes et al. 2018) did not investigate EHFs (>8 kHz) which are important in speech perception as summarized above. In addition, these investigations did not take into account factors related to HL in the general population, such as BMI, waist circumference, hypercholesterolemia and diabetes (Agrawal et al. 2009; Bainbridge et al. 2008; Cruickshanks et al. 2015; Curhan et al. 2013; Engdahl et al. 2015; Helzner et al. 2011; Linssen et al. 2014; J. Shargorodsky et al. 2010), nor factors that protect against HL, such as physical activity (Curhan et al. 2013; Engdahl et al. 2015). We found no influence of these and other factors in CBCT-treated TCS, possibly because 39% of the patients had less than 2 years of follow-up from completion of chemotherapy which might not provide sufficient time for cisplatin-related cardiometabolic events to be observed. Thus, these factors should be further examined in other cancer survivor studies.
Cumulative cisplatin dose, age, and hypertension were significantly associated with greater HL severity. Our findings support previous studies, in which significant associations were found between either cumulative cisplatin dose (Bokemeyer et al. 1998; Brydoy et al. 2009; Chen et al. 2006; Frisina et al. 2016; Glendenning et al. 2010; Haugnes et al. 2018; Theunissen et al. 2015) or age (Brydoy et al. 2009; Frisina et al. 2016; Glendenning et al. 2010) and greater HL. Significant associations between hypertension and greater HL confirms some previous investigations (Brant et al. 1996; Frisina et al. 2016; Przewozny et al. 2016), but contradicts two studies of TCS (Glendenning et al. 2010; Haugnes et al. 2018), presumably due to differences in cohort characteristics (e.g., treatment) (Glendenning et al. 2010), outcome definition (Glendenning et al. 2010; Haugnes et al. 2018), or small sample size (Haugnes et al. 2018).
Prior reports in TCS (Brydoy et al. 2009) and the general population (Cruickshanks et al. 2015; Hoffman et al. 2017) have also shown associations between higher education and decreased HL severity. As noted by Hoffman et al.,(Hoffman et al. 2017) lower educational level poses a HL risk factor, and could be related to influences associated with lower socioeconomic status, including greater noise exposure (Hoffman et al. 2017).
Strengths and Limitations
Strengths of our study include the large sample size, comprehensive audiometric testing, use of validated instruments (Postma et al. 2005) to capture HL severity, and analysis of clinical and sociodemographic factors. Patients received homogenous CBCT, with >90% given BEP/EP regimens at uniform doses. Well-defined approaches were used to select independent variables and build multivariable models (Stoltzfus 2011).
Limitations in our analysis of factors associated with HL severity include sparse numbers of TCS with diabetes, CVD, use of hearing aids, hypertension, and hypercholesterolemia (<12%), limiting our ability to analyze these HL risk factors. Any cross-sectional design, such as ours, precludes causal inferences of associations between risk factors and HL.
In conclusion, after CBCT, 1 in 4 of all patients with audiometrically-defined HL restricted to EHFs report difficulty hearing, as do 1 in 5 of those without tinnitus. Thus, for patients with self-reported HL but normal audiometric findings at standard frequencies (0.25-8 kHz), referral to an audiologist for additional testing and examination of EHFs should be strongly considered. Given that patient-reported HL is associated with poorer health-related quality-of-life, depression, cognitive decline, and social isolation (Amieva et al. 2015; Dalton et al. 2003; Li et al. 2014; Stephens et al. 2001), additional studies within our cohort are being undertaken to further investigate the impact of HL on physical, social and emotional functioning. In the only 2 small audiometric studies of cisplatin-related HL progression to date (restricted to children), HL worsened in 33% and 29% of 21 and 36 patients, respectively, after a median follow-up of 3.4 and 7 years, respectively (Al-Khatib et al. 2010; Bertolini et al. 2004). Thus, longitudinal studies are planned in our cohort to investigate the extent to which HL after CBCT might accelerate age-related HL.
Our findings indicate that age, education, mixed/conductive HL, absence of prior noise exposure, hearing aid use, and tinnitus can result in discrepancies between patient-reported and audiometrically-defined HL. Understanding these factors will enable healthcare providers to better interpret patient-reported HL and its use as a surrogate for audiometrically-defined HL.
Given the significant association we found between hypertension and HL severity, routine follow-up should include hypertension control. In the interim, given the relationships reported in the general population between HL and cardiometabolic diseases (e.g., diabetes (Agrawal et al. 2009; Bainbridge et al. 2008; Cruickshanks et al. 2015; Engdahl et al. 2015) dyslipidemia (Helzner et al. 2011; J. Shargorodsky et al. 2010)), BMI (Curhan et al. 2013) and tobacco use (Agrawal et al. 2009; Cruickshanks et al. 2015; Engdahl et al. 2015; Helzner et al. 2011), TCS should be encouraged to adopt behaviors consistent with a healthy lifestyle, including smoking cessation, weight control and physical activity. Patients should avoid ototoxic drugs that may exacerbate hearing deficits (Centers for Disease Control and Prevention 2020) and be advised to wear hearing protection in noisy environments (The National Institute for Occupational Safety and Health (NIOSH)).
Supplementary Material
Supplemental Digital Content 1. Text that describes supplementary methods. docx
Acknowledgements
This work was supported by the National Cancer Institute (1R01 CA157823 to LBT). S.A., S.D.F., R. H., C.F., M.E.D., R.D.F., and L.B.T. conceptualized the study. S.A. did the formal analysis, validated the data, and wrote the original draft of the paper. S.A., P.O.M., Y.S., R.D.F., and L.B.T. contributed to study design and methodology. L.B.T. supervised the study, provided funding and resources, and is the principal investigator of the study. R.J.H., C.F., D.R.F, R.H., D.V., N.E.M., C.K., L.E., collected data at the respective enrolling sites. All authors reviewed, critically revised, and approved the final article.
Conflicts of Interest and Source of Funding
This work was supported by the National Cancer Institute (1R01 CA157823 to LBT).
Robert Huddart reports non-financial support from Janssen, grants and personal fees from MSD, personal fees from Bristol Myers Squibb, grants from CRUK, other fees from Nektar, grants, personal fees and non-financial support from Roche, personal fees from Astellas. All remaining authors have declared no conflict of interest.
References
- Agrawal Y, Platz EA, Niparko JK (2009). Risk factors for hearing loss in US adults: data from the National Health and Nutrition Examination Survey, 1999 to 2002. Otol Neurotol, 30, 139–145. [DOI] [PubMed] [Google Scholar]
- Ainsworth BE, Haskell WL, Herrmann SD, et al. (2011). 2011 Compendium of Physical Activities: a second update of codes and MET values. Med Sci Sports Exerc, 43, 1575–1581. [DOI] [PubMed] [Google Scholar]
- Al-Khatib T, Cohen N, Carret AS, et al. (2010). Cisplatinum ototoxicity in children, long-term follow up. Int J Pediatr Otorhinolaryngol, 74, 913–919. [DOI] [PubMed] [Google Scholar]
- Allison P (2012). Logistic Regression Using SAS: Theory and Application. (2nd ed.). Cary, NC: SAS Institute Inc. [Google Scholar]
- American Speech-Language-Hearing Association (ASHA). Degree of Hearing Loss. Retrieved September 28, 2019, 2019. from https://www.asha.org/public/hearing/Degree-of-Hearing-Loss/.
- American Speech-Language-Hearing Association (ASHA). (1994). Audiologic Management of Individuals Receiving Cochleotoxic Drug Therapy [Guidelines]. Retrieved January 15, 2020, 2020 from https://www.asha.org/policy/gl1994-00003.htm.
- Amieva H, Ouvrard C, Giulioli C, et al. (2015). Self-Reported Hearing Loss, Hearing Aids, and Cognitive Decline in Elderly Adults: A 25-Year Study. J Am Geriatr Soc, 63, 2099–2104. [DOI] [PubMed] [Google Scholar]
- An Issue Brief From the U.S. Department of Health and Human Services. America's Health Literacy: Why We Need Accessible Health Information. Retrieved November 15, 2019, 2019. from http://www.aaaceus.com/courses/nl0610/article2.html.
- Baiduc RR, Poling GL, Hong O, et al. (2013). Clinical measures of auditory function: the cochlea and beyond. Dis Mon, 59, 147–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bainbridge KE, Hoffman HJ, Cowie CC (2008). Diabetes and hearing impairment in the United States: audiometric evidence from the National Health and Nutrition Examination Survey, 1999 to 2004. Ann Intern Med, 149, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bass JK, Knight KR, Yock TI, et al. (2016). Evaluation and Management of Hearing Loss in Survivors of Childhood and Adolescent Cancers: A Report From the Children's Oncology Group. Pediatric blood & cancer, 63, 1152–1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer CA (2018). Tinnitus. N Engl J Med, 378, 1224–1231. [DOI] [PubMed] [Google Scholar]
- Bertolini P, Lassalle M, Mercier G, et al. (2004). Platinum compound-related ototoxicity in children: long-term follow-up reveals continuous worsening of hearing loss. J Pediatr Hematol Oncol, 26, 649–655. [DOI] [PubMed] [Google Scholar]
- Bokemeyer C, Berger CC, Hartmann JT, et al. (1998). Analysis of risk factors for cisplatin-induced ototoxicity in patients with testicular cancer. Br J Cancer, 77, 1355–1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brant LJ, Gordon-Salant S, Pearson JD, et al. (1996). Risk factors related to age-associated hearing loss in the speech frequencies. J Am Acad Audiol, 7, 152–160. [PubMed] [Google Scholar]
- Brydoy M, Oldenburg J, Klepp O, et al. (2009). Observational study of prevalence of long-term Raynaud-like phenomena and neurological side effects in testicular cancer survivors. J Natl Cancer Inst, 101, 1682–1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capocaccia R, Gatta G, Dal Maso L (2015). Life expectancy of colon, breast, and testicular cancer patients: an analysis of US-SEER population-based data. Ann Oncol, 26, 1263–1268. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. (2020). Preventing Hearing Loss Caused by Chemical (Ototoxicity) and Noise Exposure. Retrieved May/3/2020, from https://www.cdc.gov/niosh/docs/2018-124/pdfs/2018-124.pdf. [Google Scholar]
- Chasan-Taber S, Rimm EB, Stampfer MJ, et al. (1996). Reproducibility and validity of a self-administered physical activity questionnaire for male health professionals. Epidemiology, 7, 81–86. [DOI] [PubMed] [Google Scholar]
- Chen WC, Jackson A, Budnick AS, et al. (2006). Sensorineural hearing loss in combined modality treatment of nasopharyngeal carcinoma. Cancer, 106, 820–829. [DOI] [PubMed] [Google Scholar]
- Choi JS, Betz J, Deal J, et al. (2016). A Comparison of Self-Report and Audiometric Measures of Hearing and Their Associations With Functional Outcomes in Older Adults. Journal of aging and health, 28, 890–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemens E, van den Heuvel-Eibrink MM, Mulder RL, et al. (2019). Recommendations for ototoxicity surveillance for childhood, adolescent, and young adult cancer survivors: a report from the International Late Effects of Childhood Cancer Guideline Harmonization Group in collaboration with the PanCare Consortium. The Lancet Oncology, 20, e29–e41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crocetti A, Forti S, Ambrosetti U, et al. (2009). Questionnaires to evaluate anxiety and depressive levels in tinnitus patients. Otolaryngol Head Neck Surg, 140, 403–405. [DOI] [PubMed] [Google Scholar]
- Cruickshanks KJ, Nondahl DM, Dalton DS, et al. (2015). Smoking, central adiposity, and poor glycemic control increase risk of hearing impairment. J Am Geriatr Soc, 63, 918–924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham LL, Tucci DL (2017). Hearing Loss in Adults. N Engl J Med, 377, 2465–2473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curhan SG, Eavey R, Wang M, et al. (2013). Body mass index, waist circumference, physical activity, and risk of hearing loss in women. Am J Med, 126, 1142.e1141–1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalton DS, Cruickshanks KJ, Klein BE, et al. (2003). The impact of hearing loss on quality of life in older adults. Gerontologist, 43, 661–668. [DOI] [PubMed] [Google Scholar]
- Engdahl B, Aarhus L, Lie A, et al. (2015). Cardiovascular risk factors and hearing loss: The HUNT study. Int J Audiol, 54, 958–966. [DOI] [PubMed] [Google Scholar]
- Frisina RD, Wheeler HE, Fossa SD, et al. (2016). Comprehensive Audiometric Analysis of Hearing Impairment and Tinnitus After Cisplatin-Based Chemotherapy in Survivors of Adult-Onset Cancer. J Clin Oncol, 34, 2712–2720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fung C, Dinh P Jr., Ardeshir-Rouhani-Fard S, et al. (2018). Toxicities Associated with Cisplatin-Based Chemotherapy and Radiotherapy in Long-Term Testicular Cancer Survivors. Adv Urol, 2018, 8671832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fung C, Sesso HD, Williams AM, et al. (2017). Multi-Institutional Assessment of Adverse Health Outcomes Among North American Testicular Cancer Survivors After Modern Cisplatin-Based Chemotherapy. Journal of Clinical Oncology, 35, 1211–1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gameroff M (2005). Using the Proportional Odds Model for Health-Related Outcomes: Why, When, and How with Various SAS Procedures. In The 30th annual SAS Users Group conference (pp. 1–8). Philadelphia, PA: SAS Institute. [Google Scholar]
- Glendenning JL, Barbachano Y, Norman AR, et al. (2010). Long-term neurologic and peripheral vascular toxicity after chemotherapy treatment of testicular cancer. Cancer, 116, 2322–2331. [DOI] [PubMed] [Google Scholar]
- Gordon-Salant S (2005). Hearing loss and aging: new research findings and clinical implications. J Rehabil Res Dev, 42, 9–24. [DOI] [PubMed] [Google Scholar]
- Harrington D, D'Agostino RB Sr., Gatsonis C, et al. (2019). New Guidelines for Statistical Reporting in the Journal. N Engl J Med, 381, 285–286. [DOI] [PubMed] [Google Scholar]
- Haugnes HS, Stenklev NC, Brydoy M, et al. (2018). Hearing loss before and after cisplatin-based chemotherapy in testicular cancer survivors: a longitudinal study. Acta Oncol, 57, 1075–1083. [DOI] [PubMed] [Google Scholar]
- Hayes-Lattin B, Nichols CR (2009). Testicular cancer: a prototypic tumor of young adults. Seminars in oncology, 36, 432–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helzner EP, Patel AS, Pratt S, et al. (2011). Hearing sensitivity in older adults: associations with cardiovascular risk factors in the health, aging and body composition study. J Am Geriatr Soc, 59, 972–979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman HJ, Dobie RA, Losonczy KG, et al. (2017). Declining Prevalence of Hearing Loss in US Adults Aged 20 to 69 Years. JAMA Otolaryngol Head Neck Surg, 143, 274–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong O, Ronis DL, Antonakos CL (2011). Validity of self-rated hearing compared with audiometric measurement among construction workers. Nurs Res, 60, 326–332. [DOI] [PubMed] [Google Scholar]
- Ikeda N, Murray CJ, Salomon JA (2009). Tracking population health based on self-reported impairments: Trends in the prevalence of hearing loss in US adults, 1976-2006. Am J Epidemiol, 170, 80–87. [DOI] [PubMed] [Google Scholar]
- Ilie G, Bradfield J, Moodie L, et al. (2019). The Role of Response-Shift in Studies Assessing Quality of Life Outcomes Among Cancer Patients: A Systematic Review. Front Oncol, 9, 783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaacson JE, Vora NM (2003). Differential diagnosis and treatment of hearing loss. Am Fam Physician, 68, 1125–1132. [PubMed] [Google Scholar]
- Kamil RJ, Genther DJ, Lin FR (2015). Factors associated with the accuracy of subjective assessments of hearing impairment. Ear Hear, 36, 164–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerns SL, Fung C, Monahan PO, et al. (2018). Cumulative Burden of Morbidity Among Testicular Cancer Survivors After Standard Cisplatin-Based Chemotherapy: A Multi-Institutional Study. J Clin Oncol, 36, 1505–1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiely KM, Gopinath B, Mitchell P, et al. (2012). Evaluating a dichotomized measure of self-reported hearing loss against gold standard audiometry: prevalence estimates and age bias in a pooled national data set. J Aging Health, 24, 439–458. [DOI] [PubMed] [Google Scholar]
- Kim JH (2019). Multicollinearity and misleading statistical results. Korean J Anesthesiol, 72, 558–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SY, Kim HJ, Kim MS, et al. (2017). Discrepancy between self-assessed hearing status and measured audiometric evaluation. PLoS One, 12, e0182718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirk KM, McGuire A, Nasveld PE, et al. (2012). Comparison of self-reported and audiometrically-measured hearing loss in the Australian Defence Force. Int J Audiol, 51, 294–298. [DOI] [PubMed] [Google Scholar]
- Landier W (2016). Ototoxicity and cancer therapy. Cancer, 122, 1647–1658. [DOI] [PubMed] [Google Scholar]
- Le Prell CG, Hensley BN, Campbell KCM, et al. (2011). Evidence of hearing loss in a 'normally-hearing' college-student population. International journal of audiology, 50, S21–S31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeBlanc TW, Abernethy AP (2017). Patient-reported outcomes in cancer care - hearing the patient voice at greater volume. Nat Rev Clin Oncol, 14, 763–772. [DOI] [PubMed] [Google Scholar]
- Li CM, Zhang X, Hoffman HJ, et al. (2014). Hearing impairment associated with depression in US adults, National Health and Nutrition Examination Survey 2005-2010. JAMA Otolaryngol Head Neck Surg, 140, 293–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linssen AM, van Boxtel MP, Joore MA, et al. (2014). Predictors of hearing acuity: cross-sectional and longitudinal analysis. J Gerontol A Biol Sci Med Sci, 69, 759–765. [DOI] [PubMed] [Google Scholar]
- Louw C, Swanepoel DW, Eikelboom RH (2018). Self-Reported Hearing Loss and Pure Tone Audiometry for Screening in Primary Health Care Clinics. Journal of primary care & community health, 9, 2150132718803156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormack A, Edmondson-Jones M, Fortnum H, et al. (2015). Investigating the association between tinnitus severity and symptoms of depression and anxiety, while controlling for neuroticism, in a large middle-aged UK population. Int J Audiol, 54, 599–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monson BB, Hunter EJ, Lotto AJ, et al. (2014). The perceptual significance of high-frequency energy in the human voice. Front Psychol, 5, 587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Research National Council. (2005). Basics of Sound, the Ear, and Hearing. In Dobie R, Van Hemel S (Eds.), Hearing Loss: Determining Eligibility for Social Security Benefits (pp. 42–68). Washington, DC: The National Academies Press. [PubMed] [Google Scholar]
- National Academies of Sciences, Engineering, and Medicine. (2016a). Hearing Health Care Services: Improving Access and Quality. In Blazer D, Domnitz S, Liverman C (Eds.), Hearing health care for adults: Priorities for improving access and affordability (pp. 75–148). Washington, DC: The National Academies Press. [PubMed] [Google Scholar]
- National Academies of Sciences, Engineering, and Medicine. (2016b). Hearing Loss: Extent, Impact, and Research Needs. In Blazer D, Domnitz S, Liverman C (Eds.), Hearing health care for adults: Priorities for improving access and affordability (pp. 2–74). Washington, DC: The National Academies Press. [PubMed] [Google Scholar]
- National Academies of Sciences, Engineering, and Medicine. (2016c). Hearing Technologies: Expanding Options. In Blazer D, Domnitz S, Liverman C (Eds.), Hearing Health Care for Adults: Priorities for Improving Access and Affordability (pp. 149–203). Washington, DC: The National Academies Press. [PubMed] [Google Scholar]
- Oldenburg J, Fossa SD, Dahl AA (2006). Scale for chemotherapy-induced long-term neurotoxicity (SCIN): psychometrics, validation, and findings in a large sample of testicular cancer survivors. Qual Life Res, 15, 791–800. [DOI] [PubMed] [Google Scholar]
- Pattyn T, Van Den Eede F, Vanneste S, et al. (2016). Tinnitus and anxiety disorders: A review. Hearing Research, 333, 255–265. [DOI] [PubMed] [Google Scholar]
- Postma TJ, Aaronson NK, Heimans JJ, et al. (2005). The development of an EORTC quality of life questionnaire to assess chemotherapy-induced peripheral neuropathy: the QLQ-CIPN20. Eur J Cancer, 41, 1135–1139. [DOI] [PubMed] [Google Scholar]
- Przewozny T, Gojska-Grymajlo A, Kwarciany M, et al. (2016). Hypertension is associated with dysfunction of both peripheral and central auditory system. J Hypertens, 34, 736–744. [DOI] [PubMed] [Google Scholar]
- Ramage-Morin PL, Banks R, Pineault D, et al. (2019). Unperceived hearing loss among Canadians aged 40 to 79. Health Rep, 30, 11–20. [DOI] [PubMed] [Google Scholar]
- Ramkissoon I, Cole M (2011). Self-reported hearing difficulty versus audiometric screening in younger and older smokers and nonsmokers. Journal of clinical medicine research, 3, 183–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shargorodsky J, Curhan GC, Farwell WR (2010). Prevalence and Characteristics of Tinnitus among US Adults. The American Journal of Medicine, 123, 711–718. [DOI] [PubMed] [Google Scholar]
- Shargorodsky J, Curhan SG, Eavey R, et al. (2010). A prospective study of cardiovascular risk factors and incident hearing loss in men. Laryngoscope, 120, 1887–1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson AN, Matthews LJ, Cassarly C, et al. (2019). Time From Hearing Aid Candidacy to Hearing Aid Adoption: A Longitudinal Cohort Study. Ear Hear, 40, 468–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skalleberg J, Smastuen MC, Oldenburg J, et al. (2020). The Relationship Between Cisplatin-related and Age-related Hearing Loss During an Extended Follow-up. Laryngoscope. [DOI] [PubMed] [Google Scholar]
- Stephens D, Gianopoulos I, Kerr P (2001). Determination and classification of the problems experienced by hearing-impaired elderly people. Audiology, 40, 294–300. [PubMed] [Google Scholar]
- Stoltzfus JC (2011). Logistic regression: a brief primer. Acad Emerg Med, 18, 1099–1104. [DOI] [PubMed] [Google Scholar]
- Taylor HL, Jacobs DR Jr., Schucker B, et al. (1978). A questionnaire for the assessment of leisure time physical activities. J Chronic Dis, 31, 741–755. [DOI] [PubMed] [Google Scholar]
- The National Institute for Occupational Safety and Health (NIOSH). Noise and hearing loss prevention. Retrieved May/3/2020, 2020. from www.cdc.gov/niosh/topics/noise/toolbox.html.
- Theunissen EA, Zuur CL, Jozwiak K, et al. (2015). Prediction of Hearing Loss Due to Cisplatin Chemoradiotherapy. JAMA Otolaryngol Head Neck Surg, 141, 810–815. [DOI] [PubMed] [Google Scholar]
- Travis LB, Beard C, Allan JM, et al. (2010). Testicular cancer survivorship: research strategies and recommendations. J Natl Cancer Inst, 102, 1114–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay KL, Pinto A, Fischer ME, et al. (2015). Self-Reported Hearing Difficulties Among Adults With Normal Audiograms: The Beaver Dam Offspring Study. Ear Hear, 36, e290–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ventry IM, Weinstein BE (1982). The hearing handicap inventory for the elderly: a new tool. Ear Hear, 3, 128–134. [DOI] [PubMed] [Google Scholar]
- Ware JE Jr., Sherbourne CD (1992). The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care, 30, 473–483. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Digital Content 1. Text that describes supplementary methods. docx