Abstract
Objective:
HIV virological failure remains a major threat to programme success in sub-Saharan Africa. While HIV drug resistance (HIVDR) and inadequate adherence are the main drivers of virological failure, the individual, clinical and health system characteristics that lead to poor outcomes are not well understood. The objective of this paper is to identify those characteristics among people failing first-line antiretroviral therapy (ART)
Methods:
We enrolled a cohort of adults in HIV care experiencing virologic failure on first-line antiretroviral therapy (ART) at five sites and used standard statistical methods to characterize them with a focus on three domains: individual/demographic, clinical, and health system, and compared each by country of enrollment.
Results:
Of 840 participants, 51% were women, the median duration on ART was 3.2 years (IQR, 1.1, 6.4) and the median CD4 cell count prior to failure was 281/mm3 (IQR 121, 457). Half of participants (53%, 95% CI, 0.49-0.56) stated they had greater than 90% adherence and 75% (95% CI, 0.72-0.77) take their ART on time all or most of the time. Conversely, the vast majority (90%, 95% CI, 0.86-0.92) with a completed genotypic drug resistance test had any HIVDR. This population had high health system use, reporting a median of 3 (IQR, 2,6) healthcare visits and a median of 1 (IQR, 1,1) hospitalization in the preceding 6 months.
Conclusion:
Patients failing first-line ART in sub-Saharan Africa generally report high rates of adherence to ART, have extremely high rates of HIVDR and utilize significant health-care resources. Health systems interventions to promptly detect and manage treatment failure will be a prerequisite to establishing control of the HIV epidemic.
Keywords: HIV drug resistance, treatment failure, virologic failure, Africa, first-line
Background
Great strides have been made towards achieving global targets for testing, treatment, and viral suppression for people living with HIV (PLWH) (1). The realization of the global “treat all” strategy has provided antiretroviral therapy (ART) to over 25 million of the estimated 38 million PLWH (1). The next stage of the pandemic will require long-term maintenance of virologic suppression particularly in sub-Saharan Africa where the largest proportion of the world’s HIV burden can be found.
With increasing populations of individuals accessing ART and emphasis on virologic monitoring (2), there has been a concomitant increased identification of virologic failure among PLWH. In sub-Saharan Africa, virologic failure has reportedly ranged from 9% to 24% of PLWH on ART, whether recently initiated or after prolonged use (3–8). While the World Health Organization and most endemic countries have drafted guidelines for the management of virologic failure, poor management of virologic failure persists in many contexts (9–11). Moreover, those with persistent failure are known to suffer poor outcomes and higher rates of mortality (12).
To maintain the benefits of global public health investments in HIV treatment and care, a greater understanding of the patient and health-system determinants of virologic failure is needed. Although broadly-speaking, virologic failure is driven by a combination of HIV drug resistance (HIVDR) and sub-optimal adherence, the upstream determinants of these factors are complex and remain poorly understood. Existing characterizations of PLWH at the time of virologic failure are most frequently representative of populations in high-resource settings (13–15). These studies investigate the role of low-level viremia, adherence, CD4 cell count, substance abuse, duration on ART, and HIV visit history, among others. Data from sub-Saharan Africa often focuses on incidence of failure, adherence rates, and viral load (VL) monitoring (3,16,17), with limited characterization of clinical and social characteristics of those failing ART (18). We examined enrollment data from a large, multi-center clinical trial in Uganda and South Africa to explore individual, clinical and health-system characteristics among individuals failing first-line ART. The goal of this analysis was to provide a robust description of each of these three domains for individuals with first-line virologic failure to help inform interventions to improve its prevention and management.
Methods
Study Design
The REVAMP study (Resistance testing versus adherence support for management of patients with virologic failure on first-line antiretroviral therapy in sub-Saharan Africa) was an open, randomized controlled trial designed to evaluate the impact and cost-effectiveness of resistance testing on rates of virologic re-suppression after virologic failure among people in HIV care in sub-Saharan Africa (NCT02787499). Full details of the study design have been described previously (19). The study hypothesized that immediate resistance testing after detection of first-line virologic failure would promote expedited and sustained virologic suppression compared to World Health Organization-based standard of care virologic monitoring guidelines. Results of the primary outcome of the clinical trial are expected in late 2021.
The study enrolled adults 18 years and older on first-line (non-nucleoside reverse transcriptase inhibitor-based) ART for at least five months with evidence of virologic failure, defined as a VL >1,000 copies/mL within the preceding three months. Participants who met a current indication for change to second-line therapy, such as two or more elevated VL results over a six-month period prior to enrollment, were excluded from the study. Consenting participants were randomized into one of two arms: 1) the WHO-based standard of care (SOC) for management of virologic failure arm, or 2) immediate resistance testing (RT) arm. The study was implemented in urban public, government-operated HIV care sites in both Uganda and South Africa. The primary outcome of interest was achievement of viral suppression (<200 copies/milliliter [mL]) 9 months after enrollment.
All data from this analysis were derived from the enrollment visit. Study nurses completed questionnaires to assess demographics, self-reported ART adherence using three scales, and health resource use. They also completed chart reviews to collect laboratory and ART regimen history. In the RT arm, we performed HIV-1 RNA genotypic resistance testing using Sanger sequencing on plasma specimens obtained from the enrollment visit. We interpreted results of genotypic resistance tests using the Stanford algorithm (20). We calculated genotypic susceptibility scores (GSS) for the ART regimen at the time of enrollment for each participant in the RT arm. Scores for each drug in the regimen were assigned with 0, 0.25, 0.5, 0.75, and 1 corresponding to high-level resistance, intermediate resistance, low-level resistance, potential low-level resistance, and susceptible, respectively (21). The sum of the scores for each drug represents the total GSS for the regimen with a possible range of 0 to 3. We defined HIV drug resistance as any Stanford-defined resistance mutation (22).
Data Analysis
We divided descriptive data into three domains: 1) individual patient factors including sex, age, and self-reported adherence patterns (categorical average adherence, categorical frequency of taking ART on time, and likert scale of ability to take pills as directed) (23), 2) clinical factors including most recent CD4 cell count, VL, ART duration, presence and pattern of HIV drug resistance, and opportunistic infection history, and 3) health system factors including health care visitation, providers seen, and measurement of virologic failure over time. For each of these domains we used standard statistical techniques to summarize central tendencies and distributions. We compared characteristics between country by testing the hypothesis that the average characteristic was equal between country, using chi-squared testing for categorical variables, rank sum testing for non-normally distributed continuous variables, and studentized t-tests for normally distributed continuous variables. Finally, we graphically depicted time on ART, genotypic susceptibility scores, and historical number of VLs over and under 1,000 copies/mL in Uganda beginning in 2015 for which historical clinical data were available via electronic medical records (this data was not available for South Africa). Data was analyzed using Stata statistical software version 13 (24).
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author and will be published on a public data repository after completion and publication of the parent clinical trial.
Ethical Considerations
Study procedures were approved by Mbarara University of Science and Technology Research Ethics Committee, the Ugandan National Council of Science and Technology, the University of KwaZulu-Natal Biomedical Research Ethics committee, and the Partners Human Research Committee. All study participants gave written informed consent.
Results
Individual Factors
Participants were enrolled in both Uganda and South Africa at the time of virologic failure, with 420 participants enrolled in each country. The median age in Uganda was 37 (interquartile range [IQR] 30,45) and 38 (IQR, 31, 44) in South Africa (Table 1). Approximately half of participants were female (57% [238/420] in Uganda, 95% CI, 0.52 – 0.61, and 46% [192/420] in South Africa, 95% CI, 0.41 – 0.51.
Table 1.
Demographic characteristics and self-reported adherence among individuals failing first-line ART in South Africa and Uganda enrolled in the REVAMP clinical trial
| Demographic and Adherence Characteristics | Uganda (n=420) | South Africa (n=420) | P-value |
|---|---|---|---|
| Age | |||
| <30 (%, n) | 20.5% (86) | 19.3% (81) | 0.103 |
| 30-50 (%, n) | 64.5% (271) | 70.2% (295) | |
| >50 (%, n) | 15.0% (63) | 10.5% (44) | |
| Median age (IQR) | 37 (30-45) | 38 (31-44) | 0.695 |
| Sex | |||
| Female (%, n) | 56.7% (238) | 45.7% (192) | 0.001 |
| Self-reported adherence | |||
| Average adherence | |||
| >90% (%, n) | 50.7% (213) | 54.8% (230) | 0.297 |
| 50-90% (%, n) | 45.0% (189) | 42.9% (180) | |
| <50% (%, n) | 4.1% (17) | 2.4% (10) | |
| Decline to answer (%, n) | 0.2% (1) | 0.0% (0) | |
| Frequency of taking ART on time | |||
| All or most of the time (%, n) | 76.0% (319) | 73.3% (308) | 0.007 |
| A good bit or some of the time (%, n) | 22.1% (93) | 20.5% (86) | |
| A little or none of the time (%, n) | 1.9% (8) | 6.2% (26) | |
| Ability to take all tablets as directed | |||
| Excellent or very good (%, n) | 59.8% (251) | 30.2% (127) | <0.001 |
| Good or fair (%, n) | 35.7% (150) | 64.3% (270) | |
| Poor or very poor (%, n) | 4.1% (17) | 5.5% (23) | |
| Decline to answer (%, n) | 0.5% (2) | 0.0% (0) |
More than half of respondents in both Uganda and South Africa reported greater than 90% adherence (52.7% [443/840], 95% CI, 0.49 – 0.56) in the preceding one month. Similarly, close to three out of four respondents in each country reported taking their antiretroviral therapy (ART) on time all or most of the time in the past month (74.6% [627/840], 95% CI, 0.71 – 0.77). However, there were notable differences between sites when asked about ability to take all of the ART tablets as directed, with approximately 60% (251/420) of respondents in Uganda reporting they had excellent or very good adherence (95% CI, 0.54 – 0.64), while only 30% (127/420) of respondents in South Africa stated the same (P-value for difference by country <0.001, 95% CI, 0.26 – 0.435).
Clinical Factors
ART History
The median duration of ART at the time of virologic failure was 5.0 years (IQR, 2.5, 7.8) in Uganda and 1.7 years (IQR, 0.8, 4.1) in South Africa, with 50.2% (211/420) and 20.2% (85/420) of individuals in each country being on ART for five or more years (Table 2, Figure 1). Approximately 76% (639/840) of participants were on tenofovir, emtricitabine/lamivudine, efavirenz at time of virologic failure, although that percentage was higher in South Africa (94% [396/420]) than Uganda (58% [243/420], P-value <0.001). In Uganda an additional 42% of individuals were on zidovudine-based therapy. ART side effects were commonly reported – 15% (64/420) of respondents in Uganda and 12% (42/420) of respondents in South Africa reported at least one.
Table 2.
Clinical and virologic characteristics of individuals failing first-line ART in South Africa and Uganda enrolled in the REVAMP clinical trial
| Clinical and Virologic Characteristics | Uganda (n=420) | South Africa (n=420) | P-value |
|---|---|---|---|
| Duration on ART at baseline | |||
| Median (IQR) | 5.0 (2.5, 7.8) | 1.7 (0.8, 4.1) | <0.001 |
| 5+ years (%, n) | 50.2% (211) | 20.2% (85) | <0.001 |
| ART regimen at baseline | |||
| TDF (3TC) EFV/TDF (FTC) EFV (%, n) | 57.9% (243) | 94.3% (396) | <0.001 |
| AZT (3TC) EFV/AZT (3TC) NVP/AZT (FTC) NVP (%, n) | 41.7% (175) | 0.7% (3) | |
| Other (%, n) | 0.5% (2) | 5.0% (21) | |
| Any self-reported side effects to ART | |||
| Yes (%, n) | 15.2% (64) | 12.4% (52) | 0.306 |
| Most recent CD4 count | |||
| Median (IQR) | 344 (163, 526) | 226 (103, 372) | <0.001 |
| <200 (%, n) | 25.7% (108) | 45.5% (191) | <0.001 |
| 200-500 (%, n) | 35.7% (150) | 40.5% (170) | |
| >500 (%, n) | 38.6% (162) | 14.1% (59) | |
| Lowest CD4 count on record | |||
| <200 (%, n) | 42.6% (179) | 61.4% (258) | <0.001 |
| 200-500 (%, n) | 32.9% (138) | 32.4% (136) | |
| >500 (%, n) | 1.7% (7) | 4.8% (20) | |
| Missing/no prior CD4 (%, n) | 22.9% (96) | 1.4% (6) | |
| Any prior opportunistic infection | |||
| Yes (%, n) | 20.2% (85) | 51.2% (215) | <0.001 |
| Types of opportunistic infections | |||
| Tuberculosis (%, n) | 13.1% (55) | 44.3% (186) | <0.001 |
| Cryptococcal meningitis (%, n) | 1.9% (8) | 4.1% (17) | 0.069 |
| Pneumonia (%, n) | 0.5% (2) | 16.4% (69) | <0.001 |
| Esophageal candidiasis (%, n) | 1.0% (4) | 4.8% (20) | 0.001 |
| Kaposi’s sarcoma (%, n) | 1.0% (4) | 0.0% (0) | 0.045 |
| Taking medication for any other issue | |||
| Yes (%, n) | 26.0% (109) | 37.9% (159) | <0.001 |
| HIV-1 RNA viral load prior to enrollment (copies/mL) | |||
| Median (IQR) | 8,817 (2257, 48900) | 13,184 (3297, 68062) | 0.007 |
| 1,000-10,000 (%, n) | 52.4% (220) | 44.8% (188) | 0.087 |
| 10,001-100,000 (%, n) | 31.2% (131) | 36.4% (153) | |
| >100,000 (%, n) | 16.4% (69) | 18.8% (79) | |
| HIV subtype | |||
| A1 (%, n) | 54.8% (80) | Presumed 0% | NA |
| B (%, n) | 11.6% (17) | Presumed 0% | |
| C (%, n) | 7.5% (11) | Presumed 100% | |
| D (%, n) | 24.7% (36) | Presumed 0% | |
| Other/recombinant (%, n) | 1.4% (2) | Presumed 0% | |
| Outcome of resistance testing | N=210 | N=207 | |
| Resistance testing completed (%, n) | 69.5% (146) | 85.0% (176) | 0.001 |
| HIV resistance with completed test | 61.2% (128) | 77.3% (160) | |
| No HIV resistance with completed test | 8.6% (18) | 7.7% (16) | |
| VL quantity not sufficient for sequencing (%, n) | 30.0% (63) | 15.0% (31) | |
| Invalid or lost result (%, n) | 0.5% (1) | 0.0% (0) | |
| Prevalence of resistance, among those with sequencing completed | |||
| NNRTI resistance (%, n) | 87.7% (128) | 90.3% (159) | 0.444 |
| NRTI resistance (%, n) | 79.5% (116) | 75.6% (133) | 0.407 |
| Multi-class drug resistance (n, %) | 78.8% (115) | 75.4% (135) | 0.476 |
| M184V (%, n) | 64.4% (94) | 68.8% (121) | 0.408 |
| K103N (%, n) | 50.0% (73) | 52.8% (93) | 0.612 |
| TAMS (%, n) | 30.1% (44) | 15.3% (27) | 0.001 |
| K65R (%, n) | 26.7% (39) | 32.4% (57) | 0.268 |
| Y181C (%, n) | 19.9% (29) | 17.8% (26) | 0.227 |
| K65R and M184V (%, n) | 17.8% (26) | 29.0% (51) | 0.019 |
Figure 1.
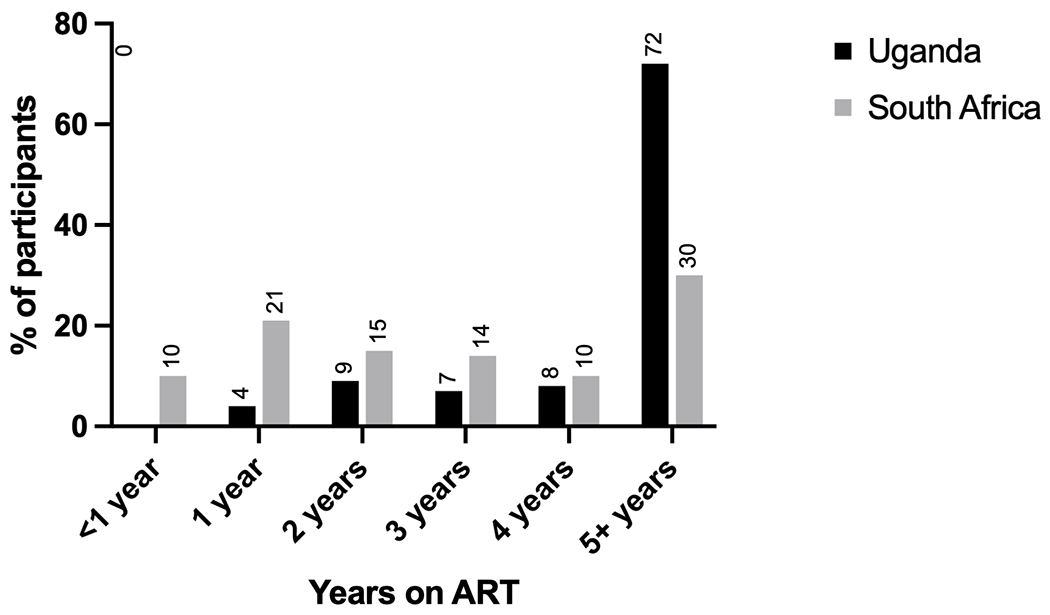
Duration of antiretroviral therapy at time of detection of virologic failure in the REVAMP clinical trial
CD4 and Opportunistic Infection History
The median of the most recent CD4 cell count at time of virologic failure was 344 cells/uL (IQR, 163, 526) for Ugandan participants and 226 cells/uL (IQR, 103, 372) for South African participants (Table 2). Most had achieved some immune reconstitution, with nadir CD4 cell counts less than 200 cells/uL in over 50% (437/640) of all participants. More respondents in South Africa reported a prior opportunistic infection compared to the Ugandan participants (51% [215/420], 95% CI, 0.46 – 0.56, vs 20% [85/420], 95% CI, 0.16 – 0.24, respectively, P-value <0.001). Among those who previously had an opportunistic infection, the most-commonly reported infection in both countries was tuberculosis, reported in 44% (186/420) of respondents in South Africa and 13% (55/420) of respondents in Uganda (P-value <0.001). Apart from HIV treatment, 32% of respondents reported taking at least one other medication for a separate health issue (26% [109/420] in Uganda and 38% [159/420] in South Africa, P-value <0.001).
Viral Loads and HIV Drug Resistance
A VL over 1,000 copies/mL within the preceding three months was an eligibility requirement for inclusion in the study. The median VL was 10,987 copies/mL (IQR, 2,646, 58,386) and approximately 18% (148/420) had a VL over 100,000 copies/mL. HIV resistance testing was attempted for all participants in the RT arm. Sequencing was successful in 77% (322/416) with success rates of 70% [146/210] in Uganda vs 85% [176/207] in South Africa. For those with a successful sequence, 89% (287/322) had at least one Stanford-defined HIV resistance mutation, which was similar between groups (88% [128/146] in Uganda and 91% 160/176] in South Africa). For those with HIVDR, NNRTI resistance was nearly universal (89% [287/322] overall, 88% [128/146] in Uganda vs 91% [159/176] in South Africa, 95% CI, 0.85 – 0.92). NRTI resistance was seen in 80% (116/146) of participants in Uganda and 76% (133/176) in South Africa. The presence of specific mutations such as M184V and K65R was not significantly different between those in Uganda and South Africa (overall prevalence of M184V 67% [215/322], K65R [96/322] 30%). The median GSS score was .5 (IQR, 0.25, 1) with 49% (159/322) having a GSS score of 1 or higher, 23% (74/322) having a GSS score of 2 or higher, and 11% (35/322) with a score of 3 (Figure 2).
Figure 2.
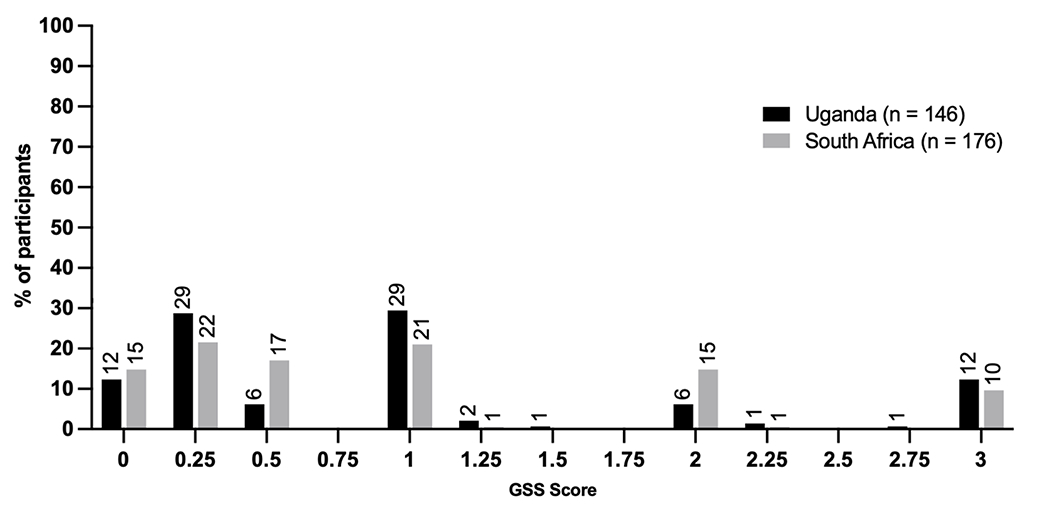
Distribution of genotypic susceptibility scores (GSS), as determined by the Stanford Database, at the time of virologic failure among participants in the REVAMP clinical trial
Health System Factors
Eighty-three percent of participants went to a physician’s office or clinic for any type of healthcare visit in the past six months with a median of 3 healthcare visits (IQR, 2, 6, Table 3). Among those who visited a healthcare facility at least once in the past 6 months, all participants reported routine visits for HIV care, whereas 34% (234/679) reported non-HIV related medical visits and 6% (50/840) reported at least one visit for an opportunistic infection. Seven percent (57/840) of participants were admitted to hospital in the past six months.
Table 3.
Healthcare utilization among individuals failing first-line ART in South Africa and Uganda enrolled in the REVAMP clinical trial
| Healthcare Utilization Characteristics | Uganda (n=420) | South Africa (n=420) | P-value |
|---|---|---|---|
| Median number of healthcare visits in the past 6 months | |||
| Median (IQR) | 3 (2,4) | 4 (0,6) | 0.100 |
| Admitted to the hospital | |||
| Yes (%, n) | 6.9% (29) | 6.7% (28) | 0.600 |
| Median number of hospital admissions among those hospitalized | |||
| Median (IQR) | 1 (1,1) | 1 (1,1) | 0.385 |
| Type of visit in the past 6 months | |||
| Doctor’s room (%, n) | 99.3% (417) | 26.9% (113) | <0.001 |
| Clinic (%, n) | 33.3% (140) | 64.8% (272) | <0.001 |
| Other including community health center and mobile health clinic (%, n) | 8.6% (36) | 5.5% (23) | 0.082 |
| Health care worker seen for HIV/AIDS care in past 9 months | |||
| Nurse (%, n) | 56.6% (237) | 98.8% (415) | <0.001 |
| Counselor (%, n) | 25.2% (106) | 93.6% (393) | <0.001 |
| Doctor (%, n) | 75.7% (318) | 56.2% (236) | <0.001 |
| Physiotherapist, lab technician, radiographer (%, n) | 96.0% (403) | 19.5% (82) | <0.001 |
| Other to include community care giver, medical assistant, clinical officer, specialist physician, pharmacist (%, n) | 52.4% (220) | 20.0% (84) | <0.001 |
| Reasons for visits among those who had a visit in past 6 months | |||
| Routine HIV visit (%, n) | 99.3% (414) | 96.0% (265) | 0.003 |
| Non-HIV related (%, n) | 35.0% (146) | 31.9% (88) | 0.394 |
| HIV-related opportunistic infections (%, n) | 1.9% (8) | 15.2% (42) | <0.001 |
| Median number of VL tests per year | |||
| Median (IQR) | 0.9 (0.5, 1.1) | NA | NA |
| Median result of VL measures over 1,000 copies/mL | |||
| Median (IQR) | 19,517 (3,510, 91,769) | NA | NA |
Viral Load History in Uganda
Since the rollout of routine VL monitoring in Uganda in 2015, participants had a median of 0.9 VL measurements per year (median 0.9, IQR, 0.5, 1.1). Seventy-eight percent (327/420) of participants in Uganda were enrolled after their first VL result over 1000 copies/mL, whereas 16% (67/420) had at least one prior high VL and 6% (26/420) had more than one prior (Figure 3). HIV clinic charts in Uganda also show that close to half had two or more detectable VLs under 1,000 copies/mL in the past.
Figure 3.
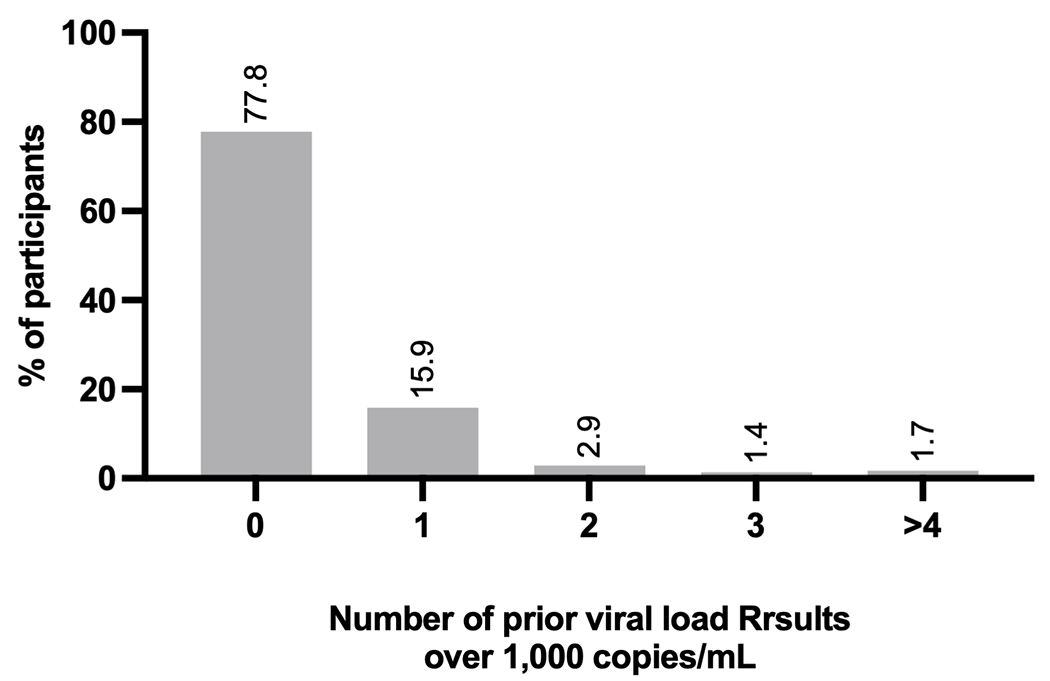
Proportion of participants with prior records of virologic failure in the medical record among participants in Uganda in the REVAMP clinical trial
Discussion
In this analysis of individuals with virologic failure on first-line ART at clinics in Uganda and South Africa, we extremely high rates of HIVDR, that a majority of individuals reported high rates of adherence, and and evidence of significant use of the health system. Overall, these results paint a picture of a patient population that remains highly engaged in care, with virologically-mediated factors for treatment failure. These data, derived from a large cohort of over 800 individuals in ambulatory care in prototypical publicly-supported HIV care centers in sub-Saharan Africa reinforce the critical value of close monitoring for virologic failure and the need to ensure rapid and efficient transitioning to effective drug regimens for this high-risk patient population.
Our data reinforce that HIVDR is extremely common, and seen in a majority of individuals with virologic failure in this population. HIVDR testing was completed for one arm of the study and confirmed a hypothesis of high rates of HIVDR: 90% (95% CI 0.86-0.93) had Stanford-defined HIV drug resistance mutations. These levels of HIVDR are similar to other studies in the region (25,26). Prior studies also found similarly high rates of HIVDR despite optimal adherence (27,28). By contrast, participants reported generally high rates of adherence according to three different scales of self-reported adherence. Nine out of ten respondents said they had “good” to “excellent” ability to take their tablets as directed. Three quarters said they took their ART on time “most of the time” or “all of the time”, and more than half said they took their treatment more than 90% of the time. Poorer self-reported adherence has been shown to be associated with viremia (29,30). While self-reported adherence is not without bias (31), the three measures together suggest generally high rates of perceived treatment adherence in a majority of participants. However, without retrospective and more objective adherence data, we cannot exclude sub-optimal prior adherence that may be under-reported or led to the high rates of HIVDR observed. Nonetheless, the phenomenon of high adherence and HIVDR is noted particularly with NNRTI therapies (32) supporting the need for greater focus on interventions which promptly identify and switch individuals failing NNRTI-based first-line therapy. Importantly, although programmatic switches from NNRTI-based to integrase inhibitor (INSTI)-based first-line ART are underway in much of the region, most of these guidelines prohibit switching to INSTI-based therapy for those with detectable VLs on NNRTI-based therapy (33,34). Consequently, such interventions will be particularly valuable during this transition period.
Our data illuminate region-specific characteristics and challenges of virologic failure. Multiple indicators we reviewed appeared to suggest more advanced disease and greater psychosocial challenges to care in South Africa compared to Uganda. For example, individuals in South Africa had significantly lower CD4 counts, higher rates of opportunistic infections, were failing earlier in the course of treatment, had higher HIV-1 RNA viral loads, were more likely to have HIVDR, and were significantly more likely to report imperfect adherence. These data are consistent with prior work demonstrating lower treatment adherence and higher rates of virologic failure among individuals initiating ART in these two countries (35), and suggest the need for regional approaches to care. Indeed, our data would support a focus on early ART initiation and enhanced support in the period after ART initiation are most crucial in South Africa, whereas persistent, longer-term attention to virologic failure is likely required to detect and support patients failing therapy in Uganda.
These data also highlight the continued presence of advanced HIV disease despite a long duration of ART use for people failing therapy. For example, despite an overall median ART duration of 3.2 years (IQR, 1.1, 6.4), the most recent CD4 count prior to virologic failure in this study was less than 200 cells/uL in approximately one third of participants, and a large proportion had experienced an opportunistic infection at any point (half of participants in South Africa and one-fifth in Uganda). The presence of advanced disease translates into high health system use. The median number of 3 (IQR, 2,6) healthcare visits and a median of 1 (IQR, 1,1) hospitalization in the past 6 months among the 6% with an admission exceeds expected hospitalizations but is in line with 3-6 visits expected due to monthly or bimonthly HIV care visits. We would anticipate people with well-controlled HIV to only have HIV-related visits every six months, although it is clear that some participants seek treatment for other conditions as shown by a third of participants taking medication for a non-HIV issue. These data are in keeping with prior data demonstrating increased healthcare use and expense for PLWH with uncontrolled HIV (36), and further reinforce the benefits of VL monitoring and interventions for this population.
Unfortunately, delays in switching regimens after first-line ART failure in sub-Saharan Africa are well documented (37). Moreover, these delays result in poor outcomes, including reductions in CD4 cell counts, missed clinical visits, incidence of opportunistic infections, lower suppression on second-line regimens, and mortality (37–41). A multi-factorial approach to management of first-line ART failure is needed. The introduction of a regimen containing tenofovir disoproxil fumarate, lamivudine, and dolutegravir (TLD), with a higher barrier to resistance, as a first-line regimen in many countries (42,43) is an important advance. However, PLWH failing NNRTI-based ART are rarely transitioned to TLD due to concerns about the potency of dolutegravir with resistance to the nucleoside reverse transcriptase inhibitor backbone (44) despite growing documented safety of the switch (42). The implications for virologic failure on TLD are not well understood (44), particularly if adherence is not an issue. In fact, recent data suggest that resistance to the NNRTI class of drugs also reduces the efficacy of TLD (45), but data have suggested that dolutegravir with two NRTIs to be an effective second-line treatment (46). The World Health Organization has recently updated treatment guidelines to recommend immediate switching regimens after a single elevated VL measurement (47). Consequently, ongoing vigilance for virologic monitoring for both those on NNRTI and INSTI-based therapy will remain critical, and novel interventions, such as differentiated models of care for high-risk patients (48), point-of-care VL testing (49), and long-acting injectable therapies for those with poor adherence (50) warrant further exploration.
Our study had multiple strengths, including enrollment of a large population of people enrolled in HIV care in prototypical clinics in the public sector in sub-Saharan Africa. Our dataset also includes a rich array of information ranging from healthcare utilization to HIV-1 genotypic resistance testing, enabling us to take a multifactorial approach to characterizing this patient population. Our study should also be interpreted with a number of limitations in its design and generalizability. The study population is one that was already engaged in care and participating in a clinical trial and are assumed to represent the patient population at their respective sites. This population may differ in substantial ways from PLWH who are not as consistently engaged in care. Additionally, questions related to healthcare-seeking behavior and HIV treatment adherence rely on self-report, which have limitations related to social desirability bias and comprehension (51). Finally, medical data in this analysis relied on clinic chart and electronic medical record systems, which are imperfect measures of laboratory and clinical history. This may be particularly true for CD4 count which is no longer checked regularly in these clinics, limiting our ability to assess advanced disease at the time of study participation.
In conclusion, PLWH with first-line virologic failure in sub-Saharan Africa are characterized by generally high rates of self-reported adherence and extremely high prevalence of significant HIVDR, tend to have advanced disease despite years of ART exposure, and consume high levels of health system resources. These data reinforce the public health crisis that HIVDR represents in the sub-Saharan African region, the urgent need for effective monitoring and response systems to HIV virologic failure in the region that go beyond adherence counseling, and the importance of a rapid transition to INSTI-based therapy as first-line therapy. Future research should explore interventions which aid in the prompt detection and support for regimen transition for people with virologic failure in the region and similarly explore the correlates and repercussions of virologic failure among individuals on INSTI-based therapy.
Acknowledgements
This study is funded by the National Institute of Allergy and Infectious Diseases with support from the President’s Emergency Plain for AIDS Relief (NIH R01 AI124718). VCM received support from NIH/NIAID R01AI098558-01A1, R01AI098558-04S1, and the Emory CFAR (P30AI050409). RTG receives grant funding from the Harvard University Center for AIDS Research (NIH P30 AI060354) and the AIDS Clinical Trials Group (NIH/NIAID 2 UMAI069412-09). SMM received support from NIH/NIAID K23 AI143470 and T32 AI007387.
Conflicts of Interest
V.C.M. has received investigator-initiated research grants (to the institution) and consultation fees (both unrelated to the current work) from Eli Lilly, Bayer, Gilead Sciences and ViiV. T.R has received consultation fees (unrelated to the current work) from Abbott. S.M.M is the recipient of a Gilead Sciences Research Scholars Program in HIV award.
References
- 1.UNAIDS data 2020. [Internet]. [cited 2020 Sep 30]. Available from: https://www.unaids.org/en/resources/documents/2020/unaids-data
- 2.WHO | What’s new in treatment monitoring: viral load and CD4 testing [Internet]. WHO. World Health Organization; [cited 2020 Sep 30]. Available from: http://www.who.int/hiv/pub/arv/treatment-monitoring-info-2017/en/ [Google Scholar]
- 3.Kiweewa F, Esber A, Musingye E, Reed D, Crowell TA, Cham F, et al. HIV virologic failure and its predictors among HIV-infected adults on antiretroviral therapy in the African Cohort Study. PLOS ONE. 2019. Feb 5;14(2):e0211344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Iwuji CC, Shahmanesh M, Koole O, Herbst K, Pillay D, Siedner MJ, et al. Clinical outcomes after first-line HIV treatment failure in South Africa: the next cascade of care. HIV Med. 2020;21(7):457–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hermans LE, Carmona S, Nijhuis M, Tempelman HA, Richman DD, Moorhouse M, et al. Virological suppression and clinical management in response to viremia in South African HIV treatment program: A multicenter cohort study. PLOS Med. 2020. Feb 25;17(2):e1003037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Swannet S, Decroo T, de Castro SMTL, Rose C, Giuliani R, Molfino L, et al. Journey towards universal viral load monitoring in Maputo, Mozambique: many gaps, but encouraging signs. Int Health. 2017. 01;9(4):206–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Glass TR, Motaboli L, Nsakala B, Lerotholi M, Vanobberghen F, Amstutz A, et al. The viral load monitoring cascade in a resource-limited setting: A prospective multicentre cohort study after introduction of routine viral load monitoring in rural Lesotho. PLOS ONE. 2019. Aug 28;14(8):e0220337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Labhardt ND, Ringera I, Lejone TI, Cheleboi M, Wagner S, Muhairwe J, et al. When patients fail UNAIDS’ last 90-the “failure cascade” beyond 90-90-90 in rural Lesotho, Southern Africa: a prospective cohort study. J Int AIDS Soc [Internet]. 2017. Jul 19 [cited 2020 Sep 30];20(1). Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5577637/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Singini I, Campbell TB, Smeaton LM, Kumarasamy N, La Rosa A, Taejareonkul S, et al. Predictors of late virologic failure after initial successful suppression of HIV replication on efavirenz-based antiretroviral therapy. HIV Clin Trials. 2016. Sep 2;17(5):173–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barth RE, Aitken SC, Tempelman H, Geelen SP, van Bussel EM, Hoepelman AIM, et al. Accumulation of drug resistance and loss of therapeutic options precede commonly used criteria for treatment failure in HIV-1 subtype-C-infected patients. Antivir Ther. 2012;17(2):377–86. [DOI] [PubMed] [Google Scholar]
- 11.Cozzi-Lepri A, Paredes null, Phillips AN, Clotet B,Kjaer J,Von Wyl V, et al. The rate of accumulation of nonnucleoside reverse transcriptase inhibitor (NNRTI) resistance in patients kept on a virologically failing regimen containing an NNRTI*. HIV Med. 2012. Jan;13(1):62–72. [DOI] [PubMed] [Google Scholar]
- 12.Piketty C, Weiss L, Thomas F, Mohamed AS, Belec L, Kazatchkine MD. Long-Term Clinical Outcome of Human Immunodeficiency Virus–Infected Patients with Discordant Immunologic and Virologic Responses to a Protease Inhibitor–Containing Regimen. J Infect Dis. 2001. May 1;183(9):1328–35. [DOI] [PubMed] [Google Scholar]
- 13.Robbins GK, Johnson KL, Chang Y, Jackson KE, Sax PE, Meigs JB, et al. Predicting Virologic Failure in an HIV Clinic. Clin Infect Dis. 2010. Mar 1;50(5):779–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rosenblum M, Deeks SG, Laan M van der, Bangsberg DR. The Risk of Virologic Failure Decreases with Duration of HIV Suppression, at Greater than 50% Adherence to Antiretroviral Therapy. PLOS ONE. 2009. Sep 29;4(9):e7196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Laprise C, de Pokomandy A, Baril J-G, Dufresne S, Trottier H. Virologic Failure Following Persistent Low-level Viremia in a Cohort of HIV-Positive Patients: Results From 12 Years of Observation. Clin Infect Dis. 2013. Nov 15;57(10):1489–96. [DOI] [PubMed] [Google Scholar]
- 16.Hassan AS, Nabwera HM, Mwaringa SM, Obonyo CA, Sanders EJ, Rinke de Wit TF, et al. HIV-1 virologic failure and acquired drug resistance among first-line antiretroviral experienced adults at a rural HIV clinic in coastal Kenya: a cross-sectional study. AIDS Res Ther. 2014. Jan 23;11(1):9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.El-Khatib Z, Katzenstein D, Marrone G, Laher F, Mohapi L, Petzold M, et al. Adherence to Drug-Refill Is a Useful Early Warning Indicator of Virologic and Immunologic Failure among HIV Patients on First-Line ART in South Africa. PLOS ONE. 2011. Mar 9;6(3):e17518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marconi VC, Wu B, Hampton J, Ordóñez CE, Johnson BA, Singh D, et al. Early Warning Indicators for First-Line Virologic Failure Independent of Adherence Measures in a South African Urban Clinic. AIDS Patient Care STDs. 2013. Dec 1;27(12):657–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Siedner MJ, Bwana MB, Moosa M-YS, Paul M, Pillay S, McCluskey S, et al. The REVAMP trial to evaluate HIV resistance testing in sub-Saharan Africa: a case study in clinical trial design in resource limited settings to optimize effectiveness and cost effectiveness estimates. HIV Clin Trials. 2017;18(4):149–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu TF, Shafer RW. Web Resources for HIV Type 1 Genotypic-Resistance Test Interpretation. Clin Infect Dis. 2006. Jun 1;42(11):1608–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rhee S-Y. Human immunodeficiency virus reverse transcriptase and protease sequence database. Nucleic Acids Res. 2003. Jan 1;31(1):298–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wensing AM, Calvez V, Ceccherini-Silberstein F, Charpentier C, Günthard HF, Paredes R, et al. 2019 update of the drug resistance mutations in HIV-1. Top Antivir Med. 2019. Sep;27(3):111–21. [PMC free article] [PubMed] [Google Scholar]
- 23.Musinguzi N, Muganzi CD, Boum Y, Ronald A, Marzinke MA, Hendrix CW, et al. Comparison of subjective and objective adherence measures for preexposure prophylaxis against HIV infection among serodiscordant couples in East Africa. AIDS. 2016. Apr 24;30(7):1121–9. [DOI] [PubMed] [Google Scholar]
- 24.StataCorp. Stata Statistical Software: Release 13. College Station, TX: StataCorp LP; [Google Scholar]
- 25.Inzaule SC, Bertagnolio S, Kityo CM, Siwale M, Akanmu S, Wellington M, et al. The relative contributions of HIV drug resistance, nonadherence and low-level viremia to viremic episodes on antiretroviral therapy in sub-Saharan Africa. AIDS. 2020. Aug 1;34(10):1559–66. [DOI] [PubMed] [Google Scholar]
- 26.Boender TS, Kityo CM, Boerma RS, Hamers RL, Ondoa P, Wellington M, et al. Accumulation of HIV-1 drug resistance after continued virological failure on first-line ART in adults and children in sub-Saharan Africa. J Antimicrob Chemother. 2016. Oct 1;71(10):2918–27. [DOI] [PubMed] [Google Scholar]
- 27.Pascoe SJ, Fox MP, Huber AN, Murphy J, Phokojoe M, Gorgens M, et al. Differentiated HIV care in South Africa: the effect of fast-track treatment initiation counselling on ART initiation and viral suppression as partial results of an impact evaluation on the impact of a package of services to improve HIV treatment adherence. J Int AIDS Soc. 2019;22(11):e25409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Meresse M, March L, Kouanfack C, Bonono R-C, Boyer S, Laborde-Balen G, et al. Patterns of adherence to antiretroviral therapy and HIV drug resistance over time in the Stratall ANRS 12110/ESTHER trial in Cameroon. HIV Med. 2014. Sep;15(8):478–87. [DOI] [PubMed] [Google Scholar]
- 29.Phillips TK, Wilson IB, Brittain K, Zerbe A, Mellins CA, Remien RH, et al. Decreases in Self-Reported ART Adherence Predict HIV Viremia Among Pregnant and Postpartum South African Women. J Acquir Immune Defic Syndr 1999. 2019. 01;80(3):247–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bijker R, Jiamsakul A, Kityo C, Kiertiburanakul S, Siwale M, Phanuphak P, et al. Adherence to antiretroviral therapy for HIV in sub-Saharan Africa and Asia: a comparative analysis of two regional cohorts. J Int AIDS Soc. 2017;20(1):21218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alcaide ML, Ramlagan S, Rodriguez VJ, Cook R, Peltzer K, Weiss SM, et al. Self-Report and Dry Blood Spot Measurement of Antiretroviral Medications as Markers of Adherence in Pregnant Women in Rural South Africa. AIDS Behav. 2017. Jul 1;21(7):2135–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.von Wyl V, Klimkait T, Yerly S, Nicca D, Furrer H, Cavassini M, et al. Adherence as a predictor of the development of class-specific resistance mutations: the Swiss HIV Cohort Study. PloS One. 2013;8(10):e77691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Uganda Ministry of Health. Consolidated Guidelines for Prevention and Treatment of HIV in Uganda [Internet]. Kampala, Uganda: Ministry of Health; 2016. Dec [cited 2021 Jan 8]. Available from: file:///C:/Users/zr049/Downloads/Consolidated%20Guidelines%20for%20Prevention%20and%20Treatment%20of%20HIV%20in%20Uganda%202016.pdf [Google Scholar]
- 34.Republic of South Africa Health Department. 2019 ART Clinical Guidelines for the Management of HIV in Adults, Pregnancy, Adolescents, Children, Infants and Neonates [Internet]. Pretoria, South Africa; 2019. Oct [cited 2021 Jan 8]. Available from: https://www.knowledgehub.org.za/system/files/elibdownloads/2020-05/2019%20ART%20Guideline%2028042020%20pdf.pdf [Google Scholar]
- 35.Haberer JE, Bwana BM, Orrell C, Asiimwe S, Amanyire G, Musinguzi N, et al. ART adherence and viral suppression are high among most non-pregnant individuals with early-stage, asymptomatic HIV infection: an observational study from Uganda and South Africa. J Int AIDS Soc. 2019/02/13 ed. 2019. Feb;22(2):e25232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gonah L, Moodley I, Hlongwana K. Effects of HIV and non-communicable disease comorbidity on healthcare costs and health experiences in people living with HIV in Zimbabwe. South Afr J HIV Med. 2020;21(1):1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Petersen ML, Tran L, Geng EH, Reynolds SJ, Kambugu A, Wood R, et al. Delayed switch of antiretroviral therapy after virologic failure associated with elevated mortality among HIV-infected adults in Africa. AIDS Lond Engl. 2014. Sep 10;28(14):2097–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ssempijja V, Nakigozi G, Chang L, Gray R, Wawer M, Ndyanabo A, et al. Rates of switching to second-line antiretroviral therapy and impact of delayed switching on immunologic, virologic, and mortality outcomes among HIV-infected adults with virologic failure in Rakai, Uganda. BMC Infect Dis. 2017. Dec;17(1):582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Haas AD, Keiser O, Balestre E, Brown S, Bissagnene E, Chimbetete C, et al. Monitoring and switching of first-line antiretroviral therapy in adult treatment cohorts in sub-Saharan Africa: collaborative analysis. Lancet HIV. 2015. Jul;2(7):e271–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Levison JH, Orrell C, Losina E, Lu Z, Freedberg KA, Wood R. Early outcomes and the virological effect of delayed treatment switching to second-line therapy in an antiretroviral roll-out programme in South Africa. Antivir Ther. 2011;16(6):853–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bell-Gorrod H, Fox MP, Boulle A, Prozesky H, Wood R, Tanser F, et al. The Impact of Delayed Switch to Second-Line Antiretroviral Therapy on Mortality, Depending on Definition of Failure Time and CD4 Count at Failure. Am J Epidemiol. 2020. Aug 1;189(8):811–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vitoria M, Hill A, Ford N, Doherty M, Clayden P, Venter F, et al. The transition to dolutegravir and other new antiretrovirals in low-income and middle-income countries: what are the issues? AIDS. 2018. Jul;32(12):1551–61. [DOI] [PubMed] [Google Scholar]
- 43.World Health Organization. Update of Recommendations on First- and Second-Line Antiretroviral Regimens [Internet]. Geneva, Switzerland; 2019. Jul [cited 2020 Dec 18]. (HIV Treatment). Available from: https://apps.who.int/iris/bitstream/handle/10665/325892/WHO-CDS-HIV-19.15-eng.pdf?ua=1 [Google Scholar]
- 44.Inzaule SC, Hamers RL, Doherty M, Shafer RW, Bertagnolio S, Rinke de Wit TF. Curbing the rise of HIV drug resistance in low-income and middle-income countries: the role of dolutegravir-containing regimens. Lancet Infect Dis. 2019. Jul;19(7):e246–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Siedner MJ, Moorhouse MA, Simmons B, de Oliveira T, Lessells R, Giandhari J, et al. Reduced efficacy of HIV-1 integrase inhibitors in patients with drug resistance mutations in reverse transcriptase. Nat Commun. 2020. Dec 1;11(1):5922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Paton Nicholas. Dolutegravir with recycled tenofovir and lamivudine performs well second-line: primary results from the NADIA trial. In Virtual; 2021. Available from: https://i-base.info/htb/40165 [Google Scholar]
- 47.World Health Organization. Updated Recommendations on HIV Prevention, Infant Diagnosis, Antiretroviral Initiation and Monitoring [Internet]. Geneva, Switzerland: World Health Organization; 2021. Mar. Available from: ISBN 978-92-4-002224-9 [PubMed] [Google Scholar]
- 48.Grimsrud A, Bygrave H, Doherty M, Ehrenkranz P, Ellman T, Ferris R, et al. Reimagining HIV service delivery: the role of differentiated care from prevention to suppression. J Int AIDS Soc. 2016. Jan;19(1):21484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Drain PK, Dorward J, Violette LR, Quame-Amaglo J, Thomas KK, Samsunder N, et al. Point-of-care HIV viral load testing combined with task shifting to improve treatment outcomes (STREAM): findings from an open-label, non-inferiority, randomised controlled trial. Lancet HIV. 2020. Apr;7(4):e229–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.D’Amico R, Margolis DA. Long-acting injectable therapy: an emerging paradigm for the treatment of HIV infection. Curr Opin HIV AIDS. 2020. Jan;15(1):13–8. [DOI] [PubMed] [Google Scholar]
- 51.Mooney AC, Campbell CK, Ratlhagana M-J, Grignon JS, Mazibuko S, Agnew E, et al. Beyond Social Desirability Bias: Investigating Inconsistencies in Self-Reported HIV Testing and Treatment Behaviors Among HIV-Positive Adults in North West Province, South Africa. AIDS Behav. 2018. Jul;22(7):2368–79. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author and will be published on a public data repository after completion and publication of the parent clinical trial.


