Abstract
Purpose:
To investigate real-world safety and efficacy of voretigene neparvovec gene therapy administration in pediatric patients with biallelic RPE65 disease-causing variants.
Methods:
A retrospective study of 27 eyes of 14 patients with RPE65-associated Leber congenital amaurosis examined post-operative complications and longitudinal changes in photoreceptor function following treatment with subretinal injection of voretigene neparvovec. Full-field stimulus threshold testing (FST), Goldmann visual fields (GVF), best corrected visual acuity (BCVA), and central subfield thickness (CST) on optical coherence tomography (OCT) scans were collected pre-operatively and up to 12 months post-treatment.
Results:
Baseline through 6-12 month follow-up FST and GVF data were obtained for 13 eyes of 7 patients. FST improved for each eye after treatment with mean improvement of 2.1 log-units (P < .001) and GVF improved for each eye with mean improvement of 221 sum-degrees (P <.001). BCVA improved from logMAR 0.98 at baseline to logMAR 0.83 at last follow-up (P < .001). Across 19 eyes of 10 patients included in CST analysis, there was a small but statistically significant 9 micron decrease in mean CST from baseline to last follow-up (P < .001). The most common postoperative issues included elevation in intraocular pressure (59%), persistent intraocular inflammation (15%) and vitreous opacities (26%) that resolved over a period of months.
Conclusion:
This report provides some of the earliest longitudinal real-world evidence of the pediatric safety and efficacy of voretigene neparvovec using multiple functional and structural measures of the retina. Outcomes demonstrate significant improvements in visual function consistent with clinical trial results.
Keywords: FST, Gene therapy, GVF, retinal degeneration, retinal dystrophy
Introduction
Causative variants for inherited retinal diseases (IRDs) have been identified in more than 270 genes [1], and clinically manifest as vision loss with varied age of onset, rate of disease progression, and severity. The RPE65 gene which encodes the all-trans retinyl ester isomerase, is crucial for normal functioning of the visual phototransduction cascade [2–4]. Biallelic disease-causing variants in RPE65 most frequently cause Leber congenital amaurosis (LCA), whereas other patients may have clinical findings consistent with autosomal recessive retinitis pigmentosa [5–7]. RPE65-associated LCA commonly causes night blindness, reduced light sensitivity, loss of visual acuity, and nystagmus, with progressive retinal dystrophy that renders most affected individuals legally blind by age twenty [8–10].
In 2017, Spark Therapeutics obtained FDA approval for voretigene neparvovec-rzyl (brand name LUXTURNA), the first gene therapy targeted for treating patients with biallelic disease-causing variants in RPE65 [11]. Voretigene neparvovec is an adeno-associated viral (AAV) vector-based gene therapy that preferentially delivers a functional copy of human RPE65 cDNA to retinal pigment epithelium cells [12, 13]. The therapy is administered through subretinal injection, with transduction and transgenic gene expression typically occurring 2 to 4 weeks post-subretinal injection [2, 14]. In a randomized and controlled pivotal trial, treated patients demonstrated marked functional visual improvement 1 year post-treatment as measured by the multi-luminance mobility test (MLMT) and full-field stimulus test (FST), with minimal adverse reactions [15]. These results informed approval of voretigene neparvovec for treatment of patients >1 years of age with confirmed biallelic RPE65 disease-causing variants who are affected with an associated retinal dystrophy and have viable retinal cells as determined by the treating physician.
Following FDA approval, we treated and collected longitudinal data pre- and post-treatment of 27 eyes across 14 patients under the age of 18. Here we report real-world data on clinical outcomes of voretigene neparvovec treatment in the pediatric population.
Materials and Methods
We performed a single-center retrospective chart review of patients under the age of 18 treated with subretinal voretigene neparvovec for confirmed biallelic disease-causing variants in RPE65 at the University of Michigan Kellogg Eye Center from January 2019 and September 2020. A total of 14 patients who met the eligibility criteria and received treatment were examined for outcomes including: perioperative and post-operative complications, longitudinal changes in photoreceptor functional measures including full-field stimulus threshold testing (FST), GVF, best-corrected visual acuity (BCVA), and structural measure of central subfield thickness (CST) from optical coherence tomography (OCT) scans. The chart review was approved by the University of Michigan Institutional Review Board prior to initiation of the study. This study adhered to the tenets of the Declaration of Helsinki.
Standard pre-operative workup included clinical exam, ultra-wide-field fundus imaging and autofluorescence, OCT, and electroretinogram. Visual acuity was recorded by a trained ophthalmic technician, using pinhole visual acuity as a surrogate for refraction. Patients determined to have findings consistent with congenital or early onset inherited retinal disease (e.g. LCA, or severe early childhood onset retinal dystrophy) received genetic counseling and subsequently underwent genetic testing through a Clinical Laboratory Improvement Amendments (CLIA)-certified laboratory. Patients confirmed to have biallelic pathogenic or likely pathogenic variants in RPE65 were scheduled for surgery, following informed consent. Some patients completed additional GVF and FST testing prior to surgery. The III4e isopter of each GVF was quantified using both a sum total degrees method [15] and an area method [16]. To calculate sum total degrees, we measured number of degrees from central fixation to the point of isopter intersection for each meridian and summed over the 24 meridians. To calculate area, we used a previously validated method with a scale factor to convert from degrees to millimeters, and digitally measured the area using Photoshop (Adobe Inc., San Jose, California).
Perioperatively, patients received oral prednisone 1 mg/kg/day starting 3 days prior to surgery on the first eye. The standard surgical approach consisted of 3-port pars plana vitrectomy. After core vitrectomy, dilute preservative-free triamcinolone (Alcon, Geneva, Switzerland) was instilled to assist with induction of posterior vitreous detachment. Then, 1.5×1011 vg voretigene neparvovec in a volume of 0.3 mL was injected subretinally in one to four subretinal blebs. The fovea was detached in seventeen eyes (seven eyes with >1000 µm foveal elevation). Scleral depression was performed to inspect the retinal periphery for retinal breaks. After fluid-fluid exchange, fluid-air exchange was performed to remove excess virus in the vitreous cavity. Intravenous methylprednisolone was given at the end of surgery, and the patient was maintained in supine position for 4 hours post-procedure in the pediatric post-anesthesia care unit. The procedure was repeated for the second eye within 1-3 weeks of surgery on the first eye. Patients received 1 day, 1 week, and 1 month standard post-operative follow-up, with additional visit timing at the discretion of the surgeon and availability of the patient and family. Oral and topical steroid therapy were tapered, and additional treatment of intraocular inflammation or intraocular pressure elevation was provided on an as-needed basis. Post-operative follow-up included clinical exam, fundus imaging and autofluorescence, and OCT. Additionally, most patients had a manifest or cycloplegic refraction performed at follow-up in addition to standard visual acuity testing. For patients who obtained pre-operative FST and GVF testing, additional FST and GVF testing were performed approximately 6-12 months after surgery.
Statistical analyses were performed using SPSS (IBM, Armonk, New York). All statistical tests were two-sided and results were considered significant for P < .05. Analyses using data from both eyes used linear mixed models to account for inter-eye correlation.
Results
A total of 27 eyes of 14 patients age 4 to 17 years (mean 9.1, SD 4.0) were treated at the University of Michigan Kellogg Eye Center during the study period (Table 1). Thirteen patients underwent bilateral treatment, with the second eye treated between 6 and 22 days (mean: 14 days) following treatment of the first eye. One eye of one patient did not receive treatment because the eye had previously undergone subretinal gene therapy for a clinical trial (NCT02781480, OPTI-RPE65 vector; MeiraGTx, London, United Kingdom). The most common genetic variant in the cohort was the c.11+5G>A pathogenic variant, identified as a homozygous or heterozygous variant in eight patients. Seven patients were part of one large consanguineous pedigree. Another two patients were related and shared compound heterozygous disease-causing variants c.11+5G>A and c.962dupA; p.(Asn321Lysfs*15).
Table 1.
Patient Demographics
| Age (yrs) | |
|---|---|
| Mean (SD) | 9.1 (4.0) |
| Range | 4 - 17 |
| Gender, no. (%) | |
| Male | 9 (64%) |
| Female | 5 (36%) |
| Race, no. (%) | |
| White | 11 (79%) |
| Black | 3 (21%) |
| Laterality, no. (%) | |
| Right Eye | 13 (48%) |
| Left Eye | 14 (52%) |
| RPE65 Variants, no. (%) | |
| c.11+5G>A homozygous | 8 (57%) |
| c.11+5G>A and c.962dupA, p.Asn321Lysfs | 2 (14%) |
| c.11+5G>A and c.1249G>C, p.Glu417Gln | 1 (7%) |
| c.74C>T, p.Pro25Leu and c.893delA, p.298Lysfs | 1 (7%) |
| c.118G>A, p.Glu40Lys and c.1102T>C, p.Tyr368His | 1 (7%) |
| c.271C>T, p.Arg91Trp and c.1334A>G, p.Asp445Gly | 1 (7%) |
Patient follow-up varied depending on patient and scheduling availability for specialized testing (GVF, FST). Patients who did not receive baseline GVF or FST testing could not be included in the analysis since there was no baseline with which to compare post-treatment results. Median follow-up time was 531 days, with a range of 167 to 677 days. Baseline and follow-up full-field stimulus threshold testing (FST) were obtained for 13 eyes of 7 patients, and follow-up FST was obtained approximately 6 to 12 months post-treatment based on patient availability (Figure 1A). In some cases, the baseline data may have been the patients’ first time receiving FST and GVF testing. FST improved for each eye after surgery, with mean baseline FST of −2.0 log cd.s/m2 (SD: 0.7, median: −1.8, range: −1.2 to −3.5), and mean follow-up FST of −4.1 log cd.s/m2 (SD: 0.9, median: −3.9, range: −2.9 to −5.8). This improvement after treatment was statistically significant (P < .001).
Fig. 1.

Baseline and post-treatment results for individual patients. (A) White full-field stimulus threshold (FST) test showed improvement in retinal sensitivity in 13 eyes of 7 patients. (B) Goldmann visual field (GVF) testing showed variable increase in total sum degrees on the III4e isopter in 13 eyes of 7 patients.
Baseline and follow-up Goldmann visual fields (GVFs) were obtained for 13 eyes of 7 patients (Figure 1B). GVF improved for each eye after surgery, although the degree of improvement varied among different patients (Table 2). There was an expansion of the III4e isopter, often within the central 20 degrees of visual field, although the direction of expansion appeared to vary. Using a sum-degrees quantification method [15], mean baseline GVF III4e isopter size was 163 sum degrees (SD: 253, median: 45, range: 0 to 767). Mean follow-up isopter size was 384 sum degrees (SD: 328, median: 318, range: 17 to 1047). Using an area quantification method [16], mean baseline area was 791 mm2 (SD: 1529, median: 67, range: 0 to 5280), and mean follow-up area was 2101 mm2 (SD: 2889, median: 811, range: 68 to 8617). These differences were statistically significant by both methods (P < .001 and P = .003, respectively).
Table 2.
GVF IIIe4 by Sum-Degrees and Area Methods
| Patient #, Eye | Baseline (Sum°) | Post-treat (Sum°) | Baseline (mm2) | Post-treat (mm2) |
|---|---|---|---|---|
| #1, OS | 766 | 1047 | 5820 | 8617 |
| #2, OD | 16 | 318 | 18 | 812 |
| #2, OS | 32 | 160 | 86 | 268 |
| #3, OD | 0 | 106 | 0 | 121 |
| #3, OS | 0 | 17 | 0 | 68 |
| #4, OD | 512 | 783 | 1895 | 5263 |
| #4, OS | 487 | 884 | 2088 | 6838 |
| #5, OD | 45 | 431 | 374 | 2098 |
| #5, OS | 84 | 350 | 439 | 1519 |
| #6, OD | 92 | 423 | 67 | 1060 |
| #6, OS | 90 | 255 | 43 | 469 |
| #7, OD | 0 | 111 | 0 | 83 |
| #7, OS | 0 | 107 | 0 | 98 |
All patients had baseline and follow-up visual acuity measured. Two eyes of one patient were excluded from further analysis because visual acuity was non-specifically documented as fix and follow at age 4 pre-operatively. At last follow-up for this patient, visual acuity was 20/125 in both eyes. We therefore included 25 eyes of 13 patients for analysis (Figure 2). Mean visual acuity at baseline was logMAR 0.98 (SD: 0.40, median: 1.10, range: 0.40 to 1.70; Snellen equivalent, 20/191). Mean visual acuity at last follow-up was logMAR 0.83 (SD: 0.35, median: 0.80, range: 0.10 to 1.60; Snellen equivalent, 20/135), corresponding to 7.5 letters improvement on the Early Treatment Diabetic Retinopathy Study eye chart. This improvement was statistically significant (P < .001) (Figure 2). Additionally, manifest or cycloplegic refraction was performed which improved the mean visual acuity to logMAR 0.74 (SD: 0.35, median: 0.60, range: 0.10 to 1.60; Snellen equivalent, 20/110), or an additional 5 letters improvement. Because refraction was not routinely performed prior to surgery, no statistical comparison could be performed between the pre-operative visual acuity and the post-operative, post-refraction visual acuity. Online supplementary material 1 illustrates visual acuity data for individual patients at each follow-up visit.
Fig. 2.

(A) There was a statistically significant improvement in mean visual acuity from baseline through last post-treatment follow-up (P < .001). (B) Mean visual acuity measured at baseline and followed up to 12 months after treatment. (C) Change in visual acuity from baseline pre-treatment to last follow-up for individual patients.
Figure 3 shows change in central subfield thickness (CST) on OCT. A total of 8 eyes of 4 patients were excluded due to poor scan quality at the baseline visit, which precluded further analysis. Therefore, 19 eyes of 10 patients were included. Mean CST at baseline was 215 microns (SD: 15, median: 212, range: 192 to 247). Mean CST at last follow-up was 206 microns (SD: 11, median: 208, range: 185 to 230). This decrease in mean CST was statistically significant (P < .001) (Figure 3). Two eyes of one individual had a notable decrease in CST, from 219 microns at baseline to 193 microns at last follow-up in the right eye, and from 212 microns at baseline to 175 microns at last follow-up in the left eye. This individual had an otherwise uncomplicated operative and post-operative course, and visual acuity remained stable in the right eye at 20/60 and improved slightly in the left eye from 20/1000 to 20/500 through 12 months of follow-up. Additionally, for all eyes we measured the sub-foveal outer nuclear layer thickness which remained stable over time. Online supplementary material 2 illustrates central subfield thickness data for individual patients at each follow-up visit.
Fig. 3.
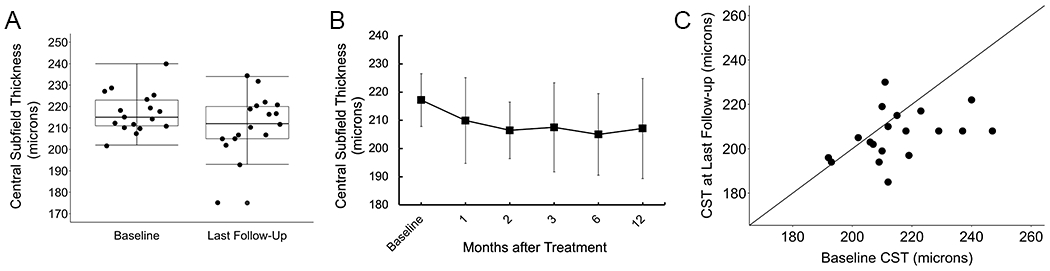
(A) A statistically significant decrease in mean central subfield thickness (CST) from baseline through last post-treatment follow-up was observed (P < .001). (B) Mean CST measured at baseline and followed up to 12 months post-treatment. (C) Change in CST on optical coherence tomography from baseline pre-treatment to last follow-up for individual patients.
No intraoperative complications occurred. The most frequent post-operative issue requiring change in management was elevation of intraocular pressure (IOP) (Table 4). The intraocular pressure elevation was most frequently observed around 1 week after surgery, and 2 out of 27 (7%) eyes treated with pressure-lowering therapy required more than 6 months of IOP-lowering therapy. 4 out of 27 (15%) eyes had IOP elevation requiring oral acetazolamide. Other post-operative issues included persistent or recurrent intraocular inflammation requiring adjustments to the steroid tapering regimen (n = 4, 15%), or vitreous opacities (n = 7, 26%). We did not observe any relationship between the order of surgery (first versus second eye) and any post-operative issues. No cases of endophthalmitis were observed. The patient whose fellow eye had been treated with subretinal gene therapy (OPTI-RPE65, NCT02781480) as part of a previous clinical trial experienced intraocular pressure elevation in the eye treated with voretigene neparvovec, but otherwise had an uncomplicated post-operative course.
Table 4.
Frequency of Post-operative Events and Management
| Intraocular pressure elevation | |
|---|---|
| IOP ever ≥ 30 mmHg after surgery | 16/27 (59%) |
| Tmax (median, range) | 32, 12 - 44 |
| # days to Tmax after surgery (median, range) | 12, 1 - 58 |
| IOP treatment ≥ 6 months | 2/27 (7%) |
| # of IOP-lowering drops | 2.4 |
| Required PO acetazolamide | 4/27 (15%) |
| Other post-operative events | |
| Persistent or recurrent inflammation with tapering of steroid drops | 4/27 (15%) |
| Vitreous opacities | 7/27 (26%) |
Discussion
Our real-world experience with voretigene neparvovec in pediatric patients demonstrates significant post-treatment improvements in FST at 6-12 months follow-up. There were also notable improvements of GVF across all eyes. Visual acuity improved and a mild decrease in CST was observed. We did not find any associations between treatment outcomes and the RPE65 genotype or patient age. Overall, the real-world outcomes of voretigene neparvovec administration presented in this study demonstrates a strong safety profile consistent with the randomized controlled clinical trial findings.
Across 13 eyes of 7 patients, we observed a statistically significant post-treatment improvement in FST by 2.1 log-units, similar to an average of 2.3 log-unit improvement observed by Maguire and Russell et al in their phase 1 follow-on and phase 3 randomized, controlled clinical trials at one-year follow-up [15, 17]. Patients in the control group of the phase 3 clinical trial exhibited no significant change in FST over 1 year. These changes reflect increased rod photoreceptor function. Although multi-luminance mobility testing (MLMT) was specifically designed and utilized as the primary efficacy endpoint measure in the phase 3 trial [15, 18], MLMT is not a feasible test in real-world follow-up and FST is a reliable surrogate measure for MLMT performance. Our results support the conventional use of FST as a surrogate marker for MLMT performance and indicator of overall treatment efficacy.
We also observed significant improvements in GVFs with a mean (SD) change of +221 (121) sum total degrees measured in 13 eyes of 7 patients that mirrors the +267 (276) gain among phase 3 treated subjects at 1 year follow-up [17]. In comparison, there was a mean −77 (259) sum total degrees loss among control subjects in the randomized controlled trial [15, 17, 19]. Similarly, in a natural history study of 70 individuals between 1 and 43 years of age (mean 15 years) with biallelic RPE65 mutation-associated inherited retinal dystrophies, III4e GVF decreased with age, with a 1-year increase in cohort age resulting in a loss of approximately −25 sum total degrees in each eye [20].
While BCVA is not a primary measurement of efficacy given that the therapy targets rod photoreceptors, we observed stable BCVA in the majority of eyes, with mild improvement in mean acuity. In the natural history of RPE65-mediated retinal dystrophies, visual acuity is typically impaired but stable during the first decade of life and gradually deteriorates starting around the age of 15 [20]. OCT revealed a mild but significant decrease in CST post-treatment, usually occurring within the first 1-2 months after treatment. The thickness of the foveal outer nuclear layer remained stable through our study follow-up. Prior studies have reported a correlation between retinal thickness and visual function in retinitis pigmentosa, as well as evidence that CST is a measure of both current central field function and future macular dysfunction [21]. In patients with Leber congenital amaurosis, the specific cause of the decrease in CST is not known and there is limited data given the rarity of the condition. However, the decrease in CST does not appear to be clinically significant since visual acuity is not negatively impacted by treatment. As part of the natural course of LCA, retinal thickness does decline with age due to retinal degeneration and loss of cells. However, we cannot exclude the intervention as a cause of decreased CST. Long-term follow-up of CST is required to determine whether CST continues to decrease over time.
Post-operative adverse events were mild, mostly resolving within weeks to months. The most common adverse events included elevated intraocular pressure and transient inflammation, consistent with clinical trial findings. In this study, post-treatment elevated intraocular pressure occurred in 16/27 (59%) of eyes or 11/14 (79%) of patient cases, higher than the 18-20% of cases previously reported.[15, 17] None of the patients in our study experienced some of the previously reported adverse events including retinal tear, macular hole, endophthalmitis, or cataract. It is interesting to note that vitreous opacities were observed post-operatively in 7 out of 27 (26%) eyes and typically persisted over months, gradually resolving over time. The etiology of these vitreous opacities is not known, but they do not appear to be detrimental to outcomes.
One patient who was previously treated with another subretinal gene therapy vector (OPTI-RPE65, MeiraGTx, NCT02781480) in the right eye received voretigene neparvovec subretinal injection in the left eye two years after the first procedure. The patient experienced early elevation of intraocular pressure that resolved within three months. The OPTI-RPE65 vector is a recombinant adeno-associated virus (AAV) serotype 2/5 in which an AAV2 genome is packaged into an AAV5 capsid, whereas the voretigene neparvovec vector is an AAV serotype 2. It is reassuring that the patient did not develop a deleterious immune response when treated with voretigene neparvovec two years after initial treatment in the fellow eye with OPTI-RPE65.
This study has limitations. It is a single-center, single-surgeon retrospective review with a limited number of patients given the rarity of this inherited retinal dystrophy. Due to FDA approval of voretigene neparvovec, there is no placebo group, so follow-up data can only be compared to baseline pre-treatment data. Furthermore, many patients treated in this study were genetically related to one another, which limits the generalizability of results. We minimized bias by including consecutive patients that met FDA-approved eligibility criteria to receive treatment and analyzed data from both eyes, using linear mixed models to account for inter-eye correlation. For some patients the baseline FST and GVF values may have been a patient’s first experience undergoing these specialized tests. It is important to note that pediatric visual fields expand with increasing age until approximately age 12. The OPTIC study showed improved reliability with increasing age in healthy children without eye disease [22]. For children with inherited retinal dystrophies, visual fields also appear to expand until age 12 despite disease progression [23]. We therefore caution against interpreting the GVF results as a positive treatment effect, since improvement could also be a result of older age or a learning effect with repeated GVF testing.
In conclusion, our results represent some of the earliest longitudinal real-world outcomes of voretigene neparvovec treatment in pediatric patients. Post-operative adverse events, FST, GVF, and BCVA findings reported in this study are generally consistent with clinical trial data, demonstrating safe and effective treatment of patients with biallelic RPE65 disease-causing variants beyond 6 to 12 months follow-up. Additionally, we report previously undescribed post-operative events and a significant trend in central subfield thickness OCT measurements within a pediatric study population. Finally, we also demonstrate the use of FST as a practical and effective surrogate for MLMT performance in monitoring real-world treatment efficacy.
Supplementary Material
Table 3.
Effect of Treatment on Central Subfield Thickness (CST)
| CST (microns) |
|||
|---|---|---|---|
| Mean | SD | P value | |
| Baseline | |||
| OD | 215.9 | 15.9 | |
| OS | 214.9 | 14.3 | |
| Post-treatment | 0.04a | ||
| OD | 208.0 | 12.3 | |
| OS | 204.8 | 10.3 | |
P value calculated using a linear mixed model accounting for inter-eye correlation.
Key Messages.
Voretigene neparvovec-rzyl, approved by the US Food and Drug Administration in 2017 as the first-ever in vivo durable targeted gene therapy for RPE65-associated Leber congenital amaurosis, has limited post-market outcomes data given the rarity of the condition.
Real-world outcomes data of RPE65-associated Leber congenital amaurosis in pediatric patients treated with subretinal voretigene neparvovec show significant improvement in full-field stimulus threshold testing, kinetic visual field, and visual acuity.
Full-field stimulus threshold testing serves as a practical and effective surrogate for multi-luminance mobility testing in monitoring real-world treatment efficacy.
Funding:
Partial financial support was received from the Foundation Fighting Blindness Clinical/Research Fellowship Award (CD-CL-0619-0758-UMICH) to Dr. Peter Y. Zhao and Heed Ophthalmic Foundation Award to Dr. Peter Y. Zhao. Dr. Cagri Besirli is supported by an RPB Unrestricted Grant from Research to Prevent Blindness.
Conflicts of interest:
Dr. Cagri G. Besirli has received clinical trial support from Spark Therapeutics.
Footnotes
Ethics approval and consent to participate: This retrospective study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the University of Michigan Institutional Review Board (Date 11/24/2020/No. HUM00104061). All potentially identifying details were omitted and data anonymized in the materials for submission.
Consent for publication: Not applicable
Availability of data and material:
The lead author affirms that this manuscript is an honest, accurate, and transparent account of the study being reported and that no important aspects of the study have been omitted. All data and materials comply with field standards.
References
- 1.Daiger SP, Rossiter BJF, Greenberg J, Christoffels A, Hide W (2021) RetNet: Summaries of genes and loci causing retinal diseases. https://sph.uth.edu/RetNet/. Accessed 2 September 2021
- 2.Gao J, Hussain RM, Weng CY (2020) Voretigene Neparvovec in Retinal Diseases: A Review of the Current Clinical Evidence. Clin Ophthalmol 14:3855–3869. 10.2147/OPTH.S231804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cai X, Conley SM, Naash MI (2009) RPE65: role in the visual cycle, human retinal disease, and gene therapy. Ophthalmic Genet 30(2):57–62. 10.1080/13816810802626399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miraldi Utz V, Coussa RG, Antaki F, Traboulsi EI (2018) Gene therapy for RPE65-related retinal disease. Ophthalmic Genet 39(6):671–677. 10.1080/13816810.2018.1533027 [DOI] [PubMed] [Google Scholar]
- 5.den Hollander AI, Roepman R, Koenekoop RK, Cremers FP (2008) Leber congenital amaurosis: genes, proteins and disease mechanisms. Prog Retin Eye Res 27(4):391–419. 10.1016/j.preteyeres.2008.05.003 [DOI] [PubMed] [Google Scholar]
- 6.Ong T, Pennesi ME, Birch DG, Lam BL, Tsang SH (2019) Adeno-Associated Viral Gene Therapy for Inherited Retinal Disease. Pharm Res 36(2):34. 10.1007/s11095-018-2564-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berger W, Kloeckener-Gruissem B, Neidhardt J (2010) The molecular basis of human retinal and vitreoretinal diseases. Prog Retin Eye Res 29(5):335–75. 10.1016/j.preteyeres.2010.03.004 [DOI] [PubMed] [Google Scholar]
- 8.Kumaran N, Moore AT, Weleber RG, Michaelides M (2017) Leber congenital amaurosis/early-onset severe retinal dystrophy: clinical features, molecular genetics and therapeutic interventions [published correction appears in Br J Ophthalmol. 2019;103(6):862]. Br J Ophthalmol 101(9):1147–1154. 10.1136/bjophthalmol-2016-309975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chao DL, Burr A, Pennesi M (2019) RPE65-Related Leber Congenital Amaurosis / Early-Onset Severe Retinal Dystrophy. In: Adam MP, Ardinger HH, Pagon RA, et al. editors GeneReviews®. University of Washington, Seattle, WA. https://www-ncbi-nlm-nih-gov.proxy.lib.umich.edu/books/NBK549574/. Accessed 3 September 2021 [PubMed] [Google Scholar]
- 10.Tsang SH, Sharma T (2018) Leber congenital amaurosis. Adv Exp Med Biol 1085:131–7. 10.1007/978-3-319-95046-4_26 [DOI] [PubMed] [Google Scholar]
- 11.US Food and Drug Administration (2017) FDA approves novel gene therapy to treat patients with a rare form of inherited vision loss. https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm589467.htm. Accessed 3 September 2021
- 12.Spark Therapeutics (2017) LUXTURNA (voretigene neparvovec-rzyl) [package insert]. U.S. Food and Drug Administration website. https://www.fda.gov/media/109906/download. Accessed 3 September 2021 [Google Scholar]
- 13.Patel U, Boucher M, de Léséleuc L, Visintini S (2016) Voretigene neparvovec: an emerging gene therapy for the treatment of inherited blindness. In: CADTH issues in emerging health technologies, Canadian Agency for Drugs and Technologies in Health, Ottawa, Ontario, 169. [PubMed] [Google Scholar]
- 14.Auricchio A, Kobinger G, Anand V, Hildinger M, O’Connor E, Maguire AM, Wilson JM, Bennett J (2001) Exchange of surface proteins impacts on viral vector cellular specificity and transduction characteristics: the retina as a model. Hum Mol Genet 10(26):3075–81. 10.1093/hmg/10.26.3075 [DOI] [PubMed] [Google Scholar]
- 15.Russell S, Bennett J, Wellman JA, et al. (2017) Efficacy and safety of voretigene neparvovec (AAV2-hRPE65v2) in patients with RPE65-mediated inherited retinal dystrophy: a randomized, controlled, open-label, phase 3 trial. Lancet 390(10097):849–860. 10.1016/s0140-6736(17)31868-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zahid S, Peeler C, Khan N, Davis J, Mahmood M, Heckenlively JR, Jayasundera T (2014) Digital quantification of Goldmann visual fields (GVFs) as a means for genotype-phenotype comparisons and detection of progression in retinal degenerations. Adv Exp Med Biol 801:131–7. 10.1007/978-1-4614-3209-8_17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maguire AM, Russell S, Wellman JA, et al. (2019) Efficacy, Safety, and Durability of Voretigene Neparvovec-rzyl in RPE65 Mutation-Associated Inherited Retinal Dystrophy: Results of Phase 1 and 3 Trials. Ophthalmology 126(9):1273–1285. 10.1016/j.ophtha.2019.06.017 [DOI] [PubMed] [Google Scholar]
- 18.Chung DC, McCague S, Yu ZF, Thill S, DiStefano-Pappas J, Bennett J, Cross D, Marshall K, Wellman J, High KA (2018) Novel mobility test to assess functional vision in patients with inherited retinal dystrophies. Clin Exp Ophthalmol 46(3):247–259. 10.1111/ceo.13022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bennett J, Wellman J, Marshall KA, et al. (2016) Safety and durability of effect of contralateral-eye administration of AAV2 gene therapy in patients with childhood-onset blindness caused by RPE65 mutations: a follow-on phase 1 trial. Lancet 388(10045):661–72. 10.1016/s0140-6736(16)30371-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chung DC, Bertelsen M, Lorenz B, Pennesi ME, Leroy BP, Hamel CP, Pierce E, Sallum J, Larsen M, Stieger K, Preising M, Weleber R, Yang P, Place E, Liu E, Schaefer G, DiStefano-Pappas J, Elci OU, McCague S, Wellman JA, High KA, Reape KZ (2018) The Natural History of Inherited Retinal Dystrophy Due to Biallelic Mutations in the RPE65 Gene. Am J Ophthalmol. 199:58–70. doi: 10.1016/j.ajo.2018.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fujiwara K, Ikeda Y, Murakami Y, Tachibana T, Funatsu J, Koyanagi Y, Nakatake S, Yoshida N, Nakao S, Hisatomi T, Yoshida S, Yoshitomi T, Ishibashi T, Sonoda KH (2018) Assessment of Central Visual Function in Patients with Retinitis Pigmentosa. Sci Rep 8(1):8070. 10.1038/s41598-018-26231-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Patel DE, Cumberland PM, Walters BC, Russell-Eggitt I, Rahi JS, for the OPTIC Study Group (2015). Study of Optimal Perimetric Testing in Children (OPTIC): Feasibility, Reliability, and Repeatability of Perimetry in Children. PLoS One 10(6): e0130895. 10.1371/journal.pone.0130895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dedania VS, Liu JY, Schlegel D, Andrews CA, Branham K, Khan NW, Musch DC, Heckenlively JR, Jayasundera KT (2017) Reliability of kinetic visual field testing in children with mutation-proven retinal dystrophies: Implications for therapeutic clinical trials. Ophthalmic Genet 22–28. doi: 10.1080/13816810.2017.1329447. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The lead author affirms that this manuscript is an honest, accurate, and transparent account of the study being reported and that no important aspects of the study have been omitted. All data and materials comply with field standards.


