Abstract
Objectives
Although emerging evidence suggests that hearing loss is an independent risk factor for falls, it is unclear how hearing loss may impact falls risk in adults with vestibular dysfunction and non-vestibular dizziness. The purpose of this study was to characterize the impact of hearing loss on falls in patients with vestibular dysfunction and non-vestibular dizziness relative to a group of patients without dizziness. In addition, this study aimed to evaluate whether there was an interactive effect between hearing loss and vestibular dysfunction or non-vestibular dizziness on the odds of falling.
Design
The authors conducted a retrospective cross-sectional study of 2,750 adult patients with dizziness evaluated at a tertiary care center vestibular clinic between June 1, 2015 and October 7, 2020. Only patients with available self-reported falls status, as extracted from the electronic medical record, were included. Patients were classified into the following diagnostic groups based on rotary chair testing and videonystagmography: Benign Paroxysmal Positional Vertigo (BPPV, n = 255), Unilateral Vestibular Hypofunction (UVH, n = 456), Bilateral Vestibular Hypofunction (BVH, n = 38), Central Dysfunction (n = 208), Multiple Diagnoses (n = 109), and Dizzy, Non-Vestibular (n = 1,389). A control group of patients without dizziness (n = 295) was identified by a random sample of audiology patients. Degree of hearing loss was characterized by the 4-frequency pure-tone average (PTA) (0.5, 1, 2, and 4 kHz) of the better hearing ear. Demographic variables, comorbidities, cognitive impairment status, and falls-associated medications were extracted from the electronic medical record and included as covariates during analysis. Potential associations between PTA and falls status and possible interactions between diagnostic group and PTA were explored using multivariate logistic regression.
Results
The BVH and Central Dysfunction groups had the highest rates of self-reported falls at 26.3% and 26.9% respectively. The control group had the lowest rate of self-reported falls at 6.4%. With the exception of the Multiple Diagnoses group, all diagnostic groups had elevated odds of falling compared to the control group, when adjusting for demographics, comorbidities, cognitive impairment status, and falls-associated medications. There was no significant association between degree of hearing loss and falls status (odds ratio [OR] = 1.02, 95% confidence interval [CI] = 0.93, 1.11, p = .713) when adjusting for diagnostic group and all other covariates. Furthermore, there were no significant interactions between diagnostic group and degree of hearing loss on the odds of falling.
Conclusions
These results indicate that hearing loss was not associated with falls in patients with vestibular dysfunction or non-vestibular dizziness, while adjusting for demographics, comorbidities, and falls-associated medications. There was no significant interactive effect observed between hearing loss and vestibular dysfunction or non-vestibular dizziness on the odds of falling. As previously reported, vestibular dysfunction and non-vestibular dizziness were independently associated with falls relative to a group of patients without dizziness. A population-based study utilizing more robust falls data is needed to explore a potential association between hearing loss and falls in those with vestibular dysfunction.
INTRODUCTION
Among older adults in the United States, falls are the leading cause of fatal and non-fatal injuries (Bergen et al., 2016). Falls also present a significant financial burden—the direct care costs associated with falls totaled an estimated 50 billion dollars in 2015 alone (Florence et al., 2018). Given the significant healthcare and societal burden associated with falls, the identification of risk factors is an issue of great public health concern.
Disorders of balance and vestibular function are among the most prevalent and significant falls risk factors. It is estimated that 35.4% of Americans over the age of 40 suffer some form of balance dysfunction, corresponding with a 12-fold increase in the odds of falling compared to those without balance dysfunction (Agrawal et al., 2009). In addition, those with bilateral vestibular impairment have a 31-fold increase in the odds of falling compared to the overall adult US population (Ward et al., 2013). Disorders of balance and vestibular function become increasingly common with age (Agrawal et al., 2009; Bermúdez Rey et al., 2016; Neuhauser et al., 2005), when falls are also more likely to lead to injury and/or hospitalization (Bergen et al., 2016; O’Loughlin et al., 1993)
There is also emerging evidence to suggest that hearing loss (HL) may be independently associated with falls (Gopinath et al., 2016; Kamil et al., 2016; Lin & Ferrucci, 2012; Viljanen et al., 2009). Though the mechanism of association remains unclear, several theories have been proposed. Individuals with HL may devote additional cognitive resources towards understanding their auditory environment, which may reduce the attention directed towards maintaining balance (Borel & Alescio-Lautier, 2014; Lacour et al., 2008; Pichora-Fuller et al., 2016; Woollacott & Shumway-Cook, 2002). In addition, there is evidence to suggest that spatial information provided by sound cues may contribute to postural control and gait stability (Negahban et al., 2017; Rumalla et al., 2015; Shayman et al., 2017; Vitkovic et al., 2016). Decreases in auditory input could therefore contribute to reduced balance function and increased falls risk. Finally, given that both vestibular and hearing impairment become more prevalent with age, older individuals with HL may suffer concomitant vestibular dysfunction, which may explain the association between HL and falls (Agrawal et al., 2009; Zuniga et al., 2012).
Balance is maintained via the integration of three primary sensory inputs—visual, vestibular, and somatosensory (Horak & Macpherson, 2010). Audition has also been shown to contribute to balance in healthy adults, albeit to a lesser extent than vision, vestibular sensation, and somatosensation (Carpenter & Campos, 2020; Easton et al., 1998; Kanegaonkar et al., 2012; Karim et al., 2018). The relative contributions of the three primary sensory inputs are adaptively weighted as one moves through their environment and in response to disruptions to any of the inputs in a process called “sensory re-weighting” (Assländer & Peterka, 2014; Peterka, 2002). For example, in the setting of vestibular disease or impairment, the vestibular sensory contribution to balance is reduced while other sensory modalities (such as vision and somatosensation) are up-weighted (Maurer et al., 2006; Mergner et al., 2009; Peterka, 2002). Given the evidence that intact sensory cues are up-weighted when one cue is diminished, one might hypothesize that audition may play an increased role in maintaining balance among individuals with vestibular dysfunction. Therefore, among patients with vestibular dysfunction, the presence of HL may confer added risk for falls. Few studies, however, have explored whether hearing loss confers additional falls risk to individuals with known vestibular impairment.
Among studies examining the effects of hearing on balance in patients with vestibular impairment, Berge and colleagues (Berge et al., 2019) found that HL was a significant predictor of postural instability while adjusting for age, sex, and vestibular disease. Vitkovic and colleagues (Vitkovic et al., 2016) found that individuals with unilateral or bilateral vestibular impairment had decreased postural sway in the presence of sound cues, suggesting that auditory contributions to balance are up-weighted in the setting of vestibular impairment to provide a stabilizing influence on balance. They also found that individuals with normal vestibular function did not benefit from sound cues, suggesting that in the setting of normal vestibular function (one of the three primary sensory drivers of balance), auditory cues play a less significant role in maintaining balance. Similarly, Maheu and colleagues (Maheu et al., 2019) found that individuals with combined hearing loss and vestibular impairment had decreased postural sway in the presence of sound cues, an association that was not observed in individuals with normal vestibular function. Both the Vitkovic et al. and Maheu et al. findings would therefore suggest that individuals with vestibular impairment utilize or rely on sound cues to a greater degree than individuals with normal vestibular function. In other words, their results would support the notion that auditory contributions to balance are up-weighted in the setting of vestibular impairment. It would follow that among patients with severe vestibular impairment (e.g., bilateral vestibular hypofunction), the co-occurrence of hearing loss may further impair balance function.
None of these studies, however, evaluated whether HL was associated with falls in patients with vestibular impairment. Furthermore, none have examined the impact of HL in dizzy patients with normal vestibular function (non-vestibular dizziness), which represent a large proportion of patients evaluated in vestibular clinic and a population whose falls risk may be elevated, depending on the specific etiology of their dizziness. Additionally, none of these studies adjusted for medical comorbidities or falls-associated medications and their potential impact on falls. Characterizing the association between HL and falls in patients with vestibular dysfunction or non-vestibular dizziness while adjusting for potential confounders may help identify groups of dizzy patients most at-risk for falls. Additionally, evaluating this association would help determine the clinical utility of HL assessment in the falls-risk assessment of patients with vestibulopathy, especially for high-risk groups, including patients 65 years of age or older or patients with a high falls-associated medication burden.
Therefore, the purpose of this study was 1) to determine whether HL is associated with falls in patients with vestibular dysfunction and non-vestibular dizziness relative to a group of patients without dizziness, and 2) to evaluate whether HL confers additional falls risk in patients with specific types of vestibular dysfunction and in patients with non-vestibular dizziness (i.e., to evaluate potential interactions between HL and specific diagnoses on the odds of falling). We hypothesized that HL would be associated with falls overall and that this association would be higher among patients with vestibular dysfunction, given that those with vestibular impairment may have an increased reliance on sound cues to maintain balance.
MATERIALS AND METHODS
This retrospective cross-sectional study was deemed exempt by the Duke University Institutional Review Board (Pro00106930).
Setting and Participants
Patients presenting with a chief complaint of dizziness or vertigo to the Duke Vestibular Clinic were identified via the Duke Vestibular Quality Improvement (QI) database, which contains the laboratory testing results of every patient evaluated at Duke Vestibular Clinic. Patients were included if they had a new patient evaluation (considered the index visit for this study) between June 1, 2015 and October 7, 2020 and they had available falls status. Out of 3,657 eligible patients, 2,976 patients (81.4%) had available falls status (Figure 1). Patients were also excluded if they were under the age of 18 years at the time of their evaluation or if they had unspecified laboratory findings that did not fit criteria for any of the diagnostic categories. Patients with missing audiometric data were excluded from primary regression analyses.
Figure 1:
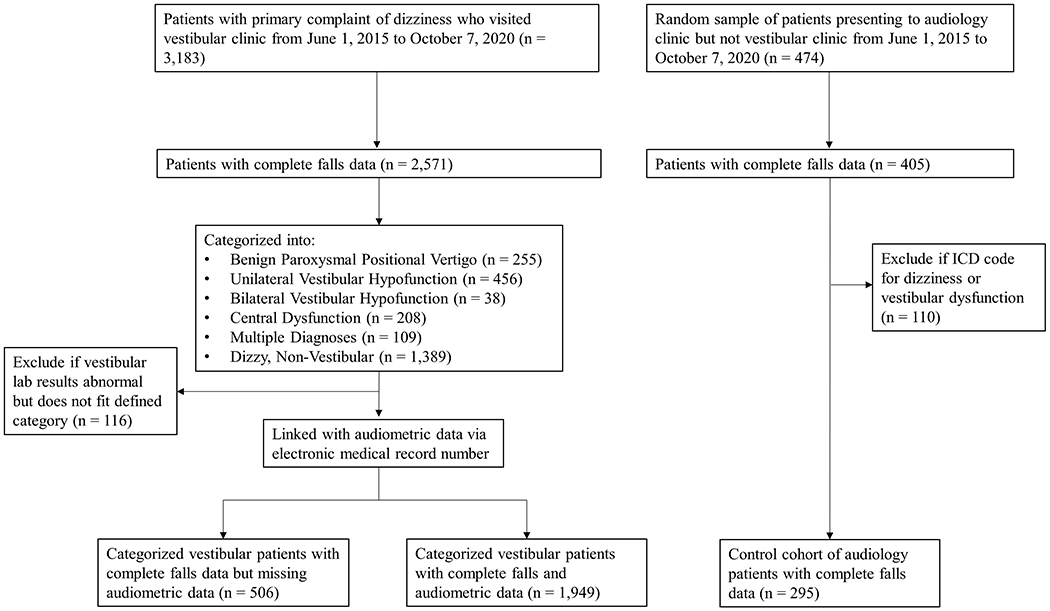
Diagram illustrating cohort development.
Primary Outcome
The primary outcome of this study was a positive answer to the falls screening question of “Have you fallen in the last 90 days?” that is obtained at clinical encounters. The primary outcome was binary (“Yes” or “No”). This self-reported status was abstracted from the electronic medical record using automated extraction. If falls status was unavailable on the date of the index visit, then the closest available falls status within two months prior to the vestibular visit date was used. Only patients with available falls status were included for analysis.
Diagnostic Categorization
Based on rotary chair testing and videonystagmography, (Supplemental Table 1), patients were classified into the following diagnostic groups: Benign Paroxysmal Positional Vertigo (BPPV), Unilateral Vestibular Hypofunction (UVH), Bilateral Vestibular Hypofunction (BVH), and Central Dysfunction. For this study, we categorized patients as having UVH based on the presence of caloric unilateral weakness, which is often considered the “gold standard” for determining presence of unilateral hypofunction (Jacobson et al., 2021) and was recently the standard used in the American Physical Therapy Association definition of unilateral weakness seen in peripheral vestibular hypofunction (Hall et al., 2016). Patients whose laboratory results met the criteria for multiple groups were categorized separately as the Multiple Diagnoses group. Patients with normal vestibular laboratory testing were categorized as the Dizzy, Non-Vestibular group. Patients with unspecified laboratory findings who did not meet criteria for any of the above categories (“Other”, n = 116) were excluded from the present analysis. Etiology of vestibular dysfunction (other than BPPV) was not considered in the categorization of patients.
In addition, a control group of non-dizzy patients presenting with a chief complaint of hearing loss was identified by a random sample of audiology patients and their records were queried using the audiology clinic database, Audbase (Audsoft Inc., Hanover, Maryland). To remove any patients with symptomatic dizziness or vertigo from the control group, any audiology patients who visited Duke Vestibular Clinic were removed from the control group. In addition, any patients with International Classification of Diseases, Ninth and Tenth Revisions (ICD-9 and ICD-10) codes for dizziness or vestibular dysfunction (Supplemental Table 2) occurring prior to their index visit were excluded. The date of audiometric evaluation was considered the index visit date for the control group. Figure 1 illustrates our process for cohort development.
Audiometry
Patients who undergo vestibular evaluation at Duke also routinely receive audiometric evaluation in addition to their vestibular appointment. To extract audiometric data for our cohort, the patient medical record number was used to link vestibular laboratory results with their corresponding audiometric data in Audbase. The closest available audiogram within 6 months of the index visit was used. In addition, audiograms with reliability characterized as “Poor” or “None” by the examining audiologist were excluded (“Methods for Manual Pure-Tone Threshold Audiometry,” 2004). Of 2,244 patients with available audiometric data, 1,656 patients (73.8%) had available audiometric data on the same day as their index visit and 1,905 patients (84.9%) had available audiometric data within two weeks of their index visit. Degree of hearing loss was then characterized by the four frequency pure tone average (PTA) at 0.5, 1, 2, and 4 kHz in the better hearing ear (“WHO | Grades of hearing impairment”).
Demographics, Comorbidities, and Medications
Demographics, comorbidities, and medications for our cohort were extracted using the Duke Enterprise Data Unified Content Explorer (DEDUCE), a web-based clinical research query tool that facilitates automated extraction of EMR data (Horvath et al., 2011). Comorbidities, as characterized by the Functional Comorbidity Index (FCI) (Groll et al., 2005), were extracted based on the occurrence of corresponding ICD-9 and ICD-10 codes in the EMR using strategies described by Sears and Rundell (2020) (Supplemental Table 3). The FCI is commonly used to predict functional outcomes in patients, such as mobility and the ability to complete activities of daily living (Groll et al., 2006; Groll et al., 2005). Higher scores indicate a greater burden of medical comorbidity and are associated with poorer functional outcomes (Groll et al., 2005; Kabboord et al., 2020). Due to the inclusion of PTA in our analysis, HL was omitted from the calculation of the FCI. Additionally, cognitive impairment status (“Yes” or “No”) was extracted based on the occurrence of specific ICD-9 and ICD-10 codes (Supplemental Table 4) at any time point between January 1 2015 and the index visit date (Deardorff et al., 2019; Fujiyoshi et al., 2017; Wilkinson et al., 2018). Patients were considered to have active falls-associated medications if they were prescribed falls-associated medications at the time of their index visit (Supplemental Table 5). Target falls-related medication classes were: Anticholinergics, Sedative/hypnotics, Anti-Hypertensives, Anticonvulsants, Benzodiazepines, Hypoglycemics, Antipsychotics, Antidepressants, Opioids, and Muscle Relaxants. Medication classes were assigned based on American Hospital Formulary Service classifications in DEDUCE (AHFS Pharmacologic-Therapeutic Classification). These medication groupings were then summated into a single measure of medication burden, number of falls-associated medications.
Statistical Analysis
Descriptive statistics were used to summarize patient characteristics (age, sex, race, FCI, number of falls-associated medications, and PTA) as well as falls status by diagnostic group. Characteristics were presented as mean with standard deviation (SD), median with interquartile range (IQR), or frequency with percentage. Additionally, a one-way analysis of variance (ANOVA) or Chi-Square (χ2) test of independence was used to detect diagnostic group differences with respect to patient characteristics.
To address the primary aim of this study, four multivariate logistic regression models were constructed. For all models, the control group (without dizziness) was used as the reference diagnostic group. To examine the unadjusted impact of vestibular dysfunction and non-vestibular dizziness on falls, Model 1 evaluated the unadjusted association between each diagnostic group and falls without consideration of PTA or adjustment for potential confounders. Covariates were then added in clusters in order to understand which factors were driving any observed impact of diagnostic group on falls. In Model 2, we added demographic characteristics (age, race, sex), FCI, cognitive impairment status, and number of falls-associated medications to the unadjusted model (i.e., these covariates were adjusted for in Model 2). In Model 3, we added PTA to characterize the overall impact of HL on falls. In Model 4, we added an interaction term between each diagnostic group and PTA to evaluate whether the impact of HL on falls differed between patients with vestibular dysfunction or non-vestibular dizziness and patients without dizziness. Interaction terms were calculated via multiplication of PTA and diagnostic group. The interaction terms would characterize the impact of HL on falls for each diagnostic group (e.g., whether HL was associated with falls for patients with BVH but not for patients with BPPV). A significant interaction term would suggest that HL has a different impact on patients in a particular diagnostic group compared to those without dizziness (control).
To address the potential for a Type I error arising from the addition of multiple covariates in each successive model, likelihood-ratio tests were used to verify our primary findings. The likelihood-ratio test compares a more complex regression model (i.e., more variables) to a simpler model (i.e., fewer variables). A significant test statistic (p ≤ .05) suggests that the more complex model was a better fit for the data compared to the simpler model (i.e., at least one of the added variables was significantly associated with the dependent variable). An insignificant test statistic (p > .05) suggests that there was no significant difference in goodness-of-fit between the simple model and more complex model. Likelihood-ratio test results that are congruent with our primary findings would suggest that a Type I error is not being committed.
At the conclusion of the primary analyses, we also evaluated whether our primary findings would remain consistent for patient groups at high risk for falls, including patients 65 years of age or older, patients with a high falls-associated medication burden, patients with cognitive impairment, or patients with more severe hearing loss. To accomplish this aim, a series of subgroup analyses were performed. Patients were stratified by age (< or ≥ 65 years of age), medication burden (< or ≥ 2 falls-associated medications), cognitive impairment status (“Yes” or “No”), or PTA (≤ or > 25 dB HL). Similar to the primary analysis, logistic regression was then used to evaluate the association between PTA and falls for each subgroup while adjusting for the same covariates included in Models 2-4. A total of eight models were evaluated, corresponding to the eight subgroups listed above.
To account for potential selection bias arising from the omission of patients without audiometric data, a sensitivity analysis was performed including patients without audiometric data. Instead of PTA, hearing status was characterized into the following three categories: audiometrically-confirmed HL (PTA > 25 dB HL), audiometrically-confirmed normal hearing (PTA ≤ 25 dB HL), or no available audiometric data. Logistic regression was then used to evaluate the potential association between the three categories of hearing status and falls status. Analyses were performed using R 3.5.3 (R Core Team, 2019) and a p-value ≤ 0.05 was used to determine statistical significance.
RESULTS
A total of 2,750 patients with available falls status were included for analysis. Patients were categorized into the following diagnostic groups: Control (n = 295), BPPV (n = 255), UVH (n = 456), BVH (n = 38), Central (n = 208), Multiple Diagnoses (n = 109), and Dizzy, Non-Vestibular (n = 1,389).
Patient Characteristics
Table 1 lists patient characteristics, FCI, number of falls-associated medications, and PTA as a function of diagnostic group. The mean age for all patients was 58.7 (SD = 16.0) years; 63% were female, and 75% were white. Patients had a mean of 2.45 (SD = 2.70) comorbidities on the FCI and were prescribed a mean of 1.44 (SD = 1.69) falls-associated medications at the time of their vestibular clinic visit. The mean PTA for all patients was 22.2 (SD = 16.5) dB HL (Supplemental Table 6), with 18.4% of patients missing audiometric data. Statistical comparisons found significant differences with respect to all characteristics across diagnostic groups with the exception of race (Table 1) and confirmed the importance of including them in our regression models.
Table 1:
Patient Characteristics for each Diagnostic Group
| Control n = 295 |
BPPV n = 255 |
UVH n = 456 |
BVH n = 38 |
Central n = 208 |
Multiple Diagnoses n = 109 |
Dizzy, Non-Vestibular n = 1,389 |
Overall n = 2,750 |
p | |
|---|---|---|---|---|---|---|---|---|---|
| Age, years | <0.001 | ||||||||
| Mean (SD) | 59.1 (17.7) | 65.4 (12.0) | 59.4 (15.8) | 62.3 (17.0) | 60.8 (15.0) | 66.6 (12.7) | 56.1 (16.0) | 58.7 (16.0) | |
| Median [Q1,Q3] | 62.5 [47.0,71.9] | 66.4 [58.4,72.7] | 60.1 [49.4,71.9] | 64.7 [55.8,73.6] | 63.6 [51.5,72.4] | 68.4 [59.2,74.0] | 57.0 [44.5,68.8] | 60.5 [47.8,70.7] | |
| Female, No. (%) | 159 (53.9%) | 178 (69.8%) | 260 (57.0%) | 16 (42.1%) | 117 (56.2%) | 71 (65.1%) | 928 (66.8%) | 1729 (62.9%) | <0.001 |
| Race, No. (%) | 0.392 | ||||||||
| White | 223 (75.6%) | 185 (72.5%) | 358 (78.5%) | 27 (71.1%) | 162 (77.9%) | 87 (79.8%) | 1,027 (73.9%) | 2069 (75.2%) | |
| Afr. Am. | 48 (16.3%) | 45 (17.6%) | 69 (15.1%) | 6 (15.8%) | 31 (14.9%) | 11 (10.1%) | 219 (15.8%) | 429 (15.6%) | |
| Asian | 10 (3.4%) | 7 (2.7%) | 7 (1.5%) | 0 (0.0%) | 4 (1.9%) | 3 (2.8%) | 51 (3.7%) | 82 (3.0%) | |
| All Other | 14 (4.7%) | 18 (7.1%) | 22 (4.8%) | 5 (13.2%) | 11 (5.2%) | 8 (7.3%) | 92 (6.6%) | 170 (6.0%) | |
| Functional Comorbidity Index | <0.001 | ||||||||
| Mean (SD) | 2.28 (2.34) | 3.23 (2.70) | 1.89 (2.48) | 1.97 (2.97) | 2.92 (3.09) | 3.04 (3.29) | 2.43 (2.67) | 2.45 (2.70) | |
| Median [Q1,Q3] | 2.0 [0.0, 4.0] | 3.0 [1.0,5.0] | 1.0 [0.0,3.0] | 0.0 [0.0,3.0] | 2.0 [0.0, 5.0] | 2.0 [0.0,5.0] | 2.0 [0.0,4.0] | 2.0 [0.0,4.0] | |
| Cog. Imp., No. (%) | 24 (8.1%) | 29 (11.4%) | 28 (6.1%) | 1 (2.6%) | 36 (17.3%) | 12 (11.0%) | 139 (10.0%) | 269 (9.8%) | |
| No. of Falls Associated Medication Classes | <0.001 | ||||||||
| Mean (SD) | 1.41 (1.59) | 1.60 (1.56) | 1.16 (1.52) | 1.32 (1.82) | 1.83 (2.05) | 1.60 (1.76) | 1.45 (1.70) | 1.44 (1.69) | |
| Median [Q1, Q3] | 1.0 [0.0,2.0] | 1.0 [0.0,3.0] | 0.0 [0.0,2.0] | 0.0 [0.0,2.0] | 1.0 [0.0,3.0] | 1.0 [0.0,3.0] | 1.0 [0.0,3.0] | 1.0 [0.0,2.0] | |
| PTA, dB | <0.001 | ||||||||
| Mean (SD) | 24.8 (16.8) | 21.4 (13.6) | 22.8 (17.1) | 31.9 (17.3) | 25.6 (17.3) | 27.4 (17.6) | 20.3 (16.2) | 22.2 (16.5) | |
| Median [Q1,Q3] | 21.3 [11.3,35.6] | 17.5 [11.3,29.7] | 17.5 [11.3,28.8] | 29.4 [21.9,37.8] | 21.3 [13.8,31.9] | 21.3 [14.1,37.2] | 15.0 [10.0,25.0] | 17.5 [11.3,28.8] | |
| Missing | 0 (0.0%) | 61 (23.9%) | 91 (20.0%) | 10 (26.3%) | 53 (25.5%) | 19 (17.4%) | 272 (19.6%) | 506 (18.4%) | |
| Falls, No. (%) | 19 (6.4%) | 38 (14.9%) | 51 (11.2%) | 10 (26.3%) | 56 (26.9%) | 16 (14.7%) | 195 (14.0%) | 385 (14.0%) | <0.001 |
Note: Afr. Am: African-American; BPPV: Benign Paroxysmal Positional Vertigo, BVH: Bilateral Vestibular Hypofunction, Cog. Imp.: Cognitive Impairment, No.: Number, PTA: Pure Tone Average, UVH: Unilateral Vestibular Hypofunction
Q1: 25th percentile, Q3: 75th percentile
Primary Outcome
The primary outcome of falls status with respect to diagnostic group is shown in Table 1. The BVH and Central Dysfunction groups had the highest rates of self-reported falls at 26.3% and 26.9% respectively, while the control group had the lowest rate of self-reported falls at 6.4%. Overall, patients who reported falling had a mean PTA of 23.4 (SD = 15.6) dB HL while patients who did not report falling had a mean PTA of 22.0 (SD = 16.6) dB HL.
In the unadjusted model (Model 1), all diagnostic groups except UVH had increased odds of falling when compared to the control group (Table 2). After adjusting for age, gender, race, cognitive impairment status, FCI, and number of falls-associated medications (Model 2), all diagnostic groups except UVH and Multiple Diagnoses were associated with an increased odds of falling compared to the control group. When PTA was added (Model 3), there was no significant association between degree of HL and falls status (odds ratio [OR] = 1.02, 95% confidence interval [CI] = 0.93, 1.11, p = .713). To compare the impact of hearing loss on falls between those with vestibular dysfunction or non-vestibular dizziness and those without dizziness (control group), an interaction term was added between each diagnostic group and PTA (Model 4). This model revealed no significant interactions between diagnostic group and degree of hearing loss (Table 2). Furthermore, a likelihood-ratio test revealed a significant difference in goodness-of-fit between Models 1 and 2 (p < .001) but not between Models 2 and 3 (p = .714), or Models 3 and 4 (p = .773). Given that our likelihood-ratio test results were congruent with our regression analysis findings, the potential for a type I error is quite low.
Table 2:
Odds Ratios (OR) for Self-Reported Falls, Unadjusted and Adjusted Models
| Diagnostic Group Unadjusted Model 1 |
Diagnostic Group + Covariates Model 2 |
Diagnostic Group + Covariates + HL Model 3 |
Diagnostic Group + Covariates + HL + Interaction Model 4 |
|
|---|---|---|---|---|
|
| ||||
| Estimated OR (95% Confidence Interval) | Estimated OR (95% Confidence Interval) | Estimated OR (95% Confidence Interval) | Estimated OR (95% Confidence Interval) | |
| Diagnostic Group | ||||
| Control | REFERENCE | REFERENCE | REFERENCE | REFERENCE |
| BPPV | 2.32 (1.26, 4.36) ** | 2.06 (1.11, 3.88) * | 2.08 (1.12, 3.93) * | 2.34 (0.75, 7.59) |
| UVH | 1.74 (1.00, 3.13) | 1.71 (0.98, 3.11) | 1.72 (0.98, 3.12) | 1.65 (0.60, 4.77) |
| BVH | 3.63 (1.23, 9.54) * | 3.88 (1.30, 10.37) ** | 3.87 (1.30, 10.34) ** | 1.65 (0.16, 12.86) |
| Central | 6.26 (3.57, 11.39) *** | 5.66 (3.20, 10.35) *** | 5.67 (3.21, 10.37) *** | 6.34 (2.22, 19.12) *** |
| Multiple Diagnoses | 2.21 (1.00, 4.69) * | 1.93 (0.87, 4.14) | 1.93 (0.87, 4.15) | 0.99 (0.20, 4.33) |
| Dizzy, Non-vestibular | 2.34 (1.46, 3.95) *** | 2.30 (1.43, 3.91) ** | 2.31 (1.44, 3.93) *** | 2.01 (0.85, 5.15) |
| Age (10 year increase) | — | 1.10 (1.01, 1.20) * | 1.09 (0.99, 1.20) | 1.09 (0.99, 1.21) |
| Gender (Male) | — | 0.68 (0.51, 0.89) ** | 0.67 (0.51, 0.88) ** | 0.68 (0.51, 0.89) ** |
| Race (non-Black) | — | 1.01 (0.72, 1.44) | 1.00 (0.71, 1.43) | 0.99 (0.71, 1.42) |
| FCI | — | 1.01 (0.94, 1.08) | 1.01 (0.94, 1.07) | 1.01 (0.95, 1.08) |
| Cognitive Impairment | — | 1.43 (0.97, 2.09) | 1.43 (0.97, 2.09) | 1.41 (0.95, 2.06) |
| No. of Falls Associated Medications | — | 1.07 (0.97, 1.18) | 1.07 (0.97, 1.18) | 1.07 (0.97, 1.18) |
| PTA (10 dB increase) | — | — | 1.02 (0.93, 1.11) | 0.97 (0.72, 1.27) |
| Interaction | — | — | — | |
| BPPV * PTA | — | — | — | 0.93 (0.61, 1.43) |
| UVH * PTA | — | — | — | 1.01 (0.72, 1.44) |
| BVH * PTA | — | — | — | 1.30 (0.72, 2.41) |
| Central * PTA | — | — | — | 0.96 (0.67, 1.37) |
| Multiple Diagnoses * PTA | — | — | — | 1.25 (0.81, 1.93) |
| Dizzy, Non-vestibular * PTA | — | — | — | 1.06 (0.80, 1.45) |
BPPV: Benign Paroxysmal Positional Vertigo, BVH: Bilateral Vestibular Hypofunction, FCI: Functional Comorbidity Index, OR: Odds Ratio, PTA: Pure Tone Average, UVH: Unilateral Vestibular Hypofunction
p < .05;
p < .01;
p < .001
Subgroup Analyses
A series of analyses were performed to further explore potential associations between degree of HL and falls in specific subgroups of our cohort, while adjusting for all other covariates. Patients were stratified by age (younger than 65 years of age versus 65 years of age or older), medication burden (<2 falls-associated medications versus ≥ 2 falls-associated medications), cognitive impairment status (“Yes” versus “No”), or PTA (≤25 dB HL versus > 25 dB HL). Separate models were then used to evaluate the association between PTA and falls for each subgroup. Table 3 lists the odds ratios of PTA on falls from eight different models, corresponding to the eight different subgroups. No significant association between degree of hearing loss and falls status was detected in any of our subgroups.
Table 3:
Odds Ratios of PTA on Self-Reported Falls, Results of Eight Subgroup Analyses
| Estimated OR (95% Confidence Interval), PTA (10 dB increase) | |
|---|---|
| Subgroup† | |
| Age < 65 years | 1.02 (0.89, 1.15) |
| Age ≥ 65 years | 1.01 (0.89, 1.15) |
| Cognitive Impairment | 1.13 (0.90, 1.42) |
| No Cognitive Impairment | 0.99 (0.90, 1.09) |
| < 2 falls-associated medications | 0.98 (0.87, 1.09) |
| ≥ 2 falls-associated medications | 1.07 (0.94, 1.22) |
| PTA ≤ 25 dB HL | 1.07 (0.79, 1.45) |
| PTA > 25 dB HL | 0.96 (0.82, 1.10) |
PTA: Pure Tone Average
A total of eight logistic regression analyses were performed to evaluate the potential associations between degree of HL and falls in each subgroup of our cohort. Analyses were adjusted for vestibular group, age, race, sex, Functional Comorbidity Index, cognitive impairment status, and number of falls associated medications.
Sensitivity Analysis
To account for potential selection bias arising from the omission of patients without audiometric data, an additional sensitivity analysis was performed including patients without audiometric data. Rather than PTA, hearing status was characterized into the following categories: audiometrically-confirmed HL (PTA > 25 dB HL), audiometrically-confirmed normal hearing (PTA ≤ 25 dB HL), or no available audiometric data. No significant association was detected between any hearing group and falls status after adjusting for covariates (Table 4).
Table 4:
Odds Ratios for Self-Reported Falls, Sensitivity Analysis
| Estimated OR (95% Confidence Interval) | |
|---|---|
| Diagnostic Group | |
| Control | REFERENCE |
| BPPV | 2.22 (1.24, 4.07) ** |
| UVH | 1.87 (1.09, 3.32) * |
| BVH | 5.43 (2.22, 12.78) *** |
| Central | 4.84 (2.79, 8.72) *** |
| Multiple Diagnoses | 2.15 (1.04, 4.39) * |
| Dizzy, Non-vestibular | 2.31 (1.44, 3.92) *** |
| Age (10 year increase) | 1.08 (1.00, 1.18) |
| Gender (Male) | 0.73 (0.58, 0.93) * |
| Race (non-Black) | 0.97 (0.72, 1.32) |
| FCI | 1.03 (0.98, 1.09) |
| Cognitive Impairment | 1.54 (1.09, 2.14) * |
| No. of Falls Associated Medications | 1.08 (0.99, 1.17) |
| Hearing Status† | |
| Audiometrically-Confirmed Normal Hearing | REFERENCE |
| Audiometrically-Confirmed Hearing Loss | 1.08 (0.81, 1.45) |
| No Audiometric Data Available | 1.10 (0.82, 1.46) |
BPPV: Benign Paroxysmal Positional Vertigo
BVH: Bilateral Vestibular Hypofunction
FCI: Functional Comorbidity Index
UVH: Unilateral Vestibular Hypofunction
Hearing status was characterized into the following categories: audiometrically-confirmed HL (PTA > 25 dB HL), audiometrically-confirmed normal hearing (PTA ≤ 25 dB HL), or no available audiometric data.
p < .05;
p < .01;
p < .001
DISCUSSION
Our study characterized the association between HL and falls in patients with vestibular dysfunction or non-vestibular dizziness relative to patients without dizziness. In addition, our study evaluated whether HL confers additional falls risk in patients with specific types of vestibular dysfunction and in patients with non-vestibular dizziness. We had hypothesized that HL would be associated with falls overall and that this association would be higher among dizzy patients with vestibular dysfunction, given that patients with vestibular impairment may have an increased reliance on sound cues to maintain balance. Our results do not support this hypothesis. In our sample, HL was not significantly associated with falls overall when adjusting for diagnostic group, demographics, comorbidities, and falls-associated medications among our cohort of patients. Furthermore, there was no significant interaction between any of our diagnostic groups and PTA, signifying that HL was not associated with falls in patients with dizziness due to vestibular dysfunction or non-vestibular dizziness. This lack of a significant interaction was even observed in the BVH group, a group which previous studies suggest may have had a greater reliance on sound cues (Maheu et al., 2019; Vitkovic et al., 2016). Similarly, there was no significant interaction between HL and the other diagnostic groups (UVH, BPPV, Central, Multiple Diagnoses, Non-Vestibular Dizziness), indicating that HL was not associated with falls within any of the other diagnostic groups. However, as expected, our analysis confirms prior findings showing that vestibular function is a significant predictor of falls (Agrawal et al., 2009; Gazzola et al., 2006; Ward et al., 2013). In addition, those presenting with non-vestibular dizziness also had an elevated odds of falling compared to our control group.
Whereas previous literature demonstrated an association between hearing and laboratory measures of static balance in patients with vestibular impairment (Berge et al., 2019; Maheu et al., 2019; Vitkovic et al., 2016), our study did not reveal a significant association when examining real world falls. Our investigation differed from previous studies in several ways. First, previous studies adjusted for vestibular function and patient demographics; however, they did not account for the potential confounding effect of medical comorbidities and falls-associated medications. These variables are critically important to control for when examining falls and our analysis has adjusted for these important covariates. Second, the differences in findings illustrate a fundamental gap between laboratory assessments of falls risk and actual falls events. Although measures of postural sway may help quantify falls risk, they are performed in controlled settings that do not necessarily reflect the more dynamic contexts in which most falls occur. Dynamic elements may include changing the speed and direction of gait, responding to environmental hazards (such as a loose rug or cord on the floor), or completing other tasks while walking (e.g., carrying groceries, talking with a friend). Furthermore, laboratory measures alone may not capture patient efforts to mitigate falls risk with walking aids or reductions in activity perceived to put one at risk of falling. These real world considerations may reduce the impact of factors found to be significant in the laboratory setting. Finally, while previous studies may have demonstrated statistically significant associations between HL and laboratory measures of falls risk in patients with vestibular dysfunction, these associations may not necessarily indicate clinical significance. For example, none of the aforementioned studies (Berge et al., 2019; Maheu et al., 2019; Vitkovic et al., 2016) discussed whether the observed stabilizing nature of audition (and destabilizing contribution of HL) on balance (measured via postural sway) would cause postural sway to exceed a functional threshold that would have clinical significance. Moreover, some associations were only detected in challenging conditions (i.e., on a foam surface and/or with eyes closed). Therefore, while HL may contribute to postural sway in the laboratory, HL may be less important when considering more significant drivers of falls in a real world context. For example, nearly all of our groups with vestibular impairment had elevated odds of falling compared to our control group after adjustment for demographics, comorbidities, and medications. Our study thus reiterates the clinical value of vestibular evaluation in characterizing falls risk and highlights the important role of vestibular rehabilitation in addressing vestibular impairment to reduce falls risk (Herdman et al., 2007; McDonnell & Hillier, 2015; Scherer et al., 2008) in conjunction with measures to improve mobility (Steadman et al., 2003), optimize functional and cognitive health (Mirelman et al., 2012; Resnick & Boltz, 2019), and reduce high-risk medication use (Campbell et al., 1999).
To support our primary findings, we also conducted a series of supplemental analyses to assess whether our findings would remain consistent for different patient populations, especially patient groups at elevated risk of falling. Many previous studies exploring HL and falls risk have done so only in individuals over the age of 65 (Jiam et al., 2016) or only in individuals with moderate-to-severe HL (Negahban et al., 2017; Rumalla et al., 2015; Shayman et al., 2017). We therefore assessed whether HL was associated with falls within specific subgroups of our cohort. After stratifying patients by age, medication burden, cognitive impairment status, and PTA, we found that HL was not associated with falls within any subgroup when adjusting for vestibular function, demographics, comorbidities, and medications. The consistency of our subgroup analyses further bolsters our primary finding that HL was not associated with falls in patients with vestibular impairment and/or non-vestibular dizziness.
Since patients without audiometric data were omitted from analyses utilizing PTA, our study also faced the potential for selection bias. In other words, patients without audiometric data may have demonstrated systematic differences in falls compared to patients with available audiometric data, thereby limiting the generalizability of our findings. To address the potential for selection bias, we performed a sensitivity analysis that included patients without audiometric data. We found no differences in the odds of falling among those with audiometrically-confirmed normal hearing, those with audiometrically-confirmed hearing loss, and those without audiometric data. This analysis would therefore suggest overall generalizability of our findings to a wider clinical population of patients seeking vestibular evaluation.
As a whole, our results suggest that HL does not confer additional falls risk to individuals with co-existing vestibular impairment or non-vestibular dizziness. We accounted for vestibular semicircular canal function based on the results of rotary chair testing and videonystagmography and found that HL had essentially no effect on the odds of falling in our cohort of dizzy patients (OR = 1.02, 95% CI = 0.93, 1.11, p = .713). While our study was restricted to a clinical population of vestibular and audiology patients, our findings were strengthened by a large sample size, adjustment for potential confounders, and subgroup and sensitivity analyses that reaffirm the results of our primary analysis. Our findings would therefore suggest that assessments of hearing loss would be less clinically helpful in determining the falls risk of vestibular patients and decision-making regarding falls prevention. Nevertheless, audiometric evaluation remains essential in the diagnosis and management of patients with suspected vestibular pathology.
Although, this study was not designed to address the association between HL and falls directly, our results may provide some indirect insights into this association. In a systematic literature review of the association between HL and falls, Jiam and colleagues (Jiam et al., 2016) found that none of the twelve reviewed studies accounted for vestibular function. Unmeasured concomitant vestibular loss, they noted, could potentially explain some of the association between HL and falls. As such, our data may provide some indirect evidence that vestibular dysfunction may be a possible explanatory mechanism of the association between HL and falls. Future investigations designed to address the association between HL and falls in the hearing-impaired population should continue to utilize direct measures of vestibular function to characterize the independent effects of auditory mediated factors and vestibular function on falls.
This study has limitations. First, our study utilized self-reported falls as its primary outcome, which may be subject to recall bias as well as misclassification bias. In particular, patients may tend to under-report falls (Hoffman et al., 2018), which could potentially bias our results towards null findings. Despite the limitations of self-reported falls, however, it has been used widely in the literature (Agrawal et al., 2009; Bergen et al., 2016; Gopinath et al., 2016; Kamil et al., 2016; Lin & Ferrucci, 2012; Ward et al., 2013). Second, our study also did not identify whether patients with more severe hearing loss were using hearing interventions such as hearing aids or whether certain patients were using mobility aids. Although neither hearing aids (Gopinath et al., 2016; Kamil et al., 2016; Riska et al., 2021; Riska et al., In Press) nor mobility aids (Gell et al., 2015; O’Hare et al., 2013) have been shown to reduce falls, we acknowledge that both factors could potentially impact the findings of our study and should be addressed in future work. Hearing aid and mobility aid usage are often not well-charted in the electronic medical record and are thus difficult to adjust for in a retrospective study. The best way to determine whether these factors modulate the impact of HL on falls would be via a prospective study design that carefully quantifies hearing aid and mobility aid use for each patient. Third, our study cohort represented a clinical population of vestibular and audiology patients, which may over-sample patients with more severe vestibular impairment and may not be representative of the overall population. A population-based study could more accurately characterize potential associations between HL and falls in those with vestibular dysfunction or non-vestibular dizziness. Fourth, this study characterized hearing loss via four-frequency PTA. This definition has been widely used in the literature, especially in the context of HL and falls (Berge et al., 2019; Gopinath et al., 2016; Kamil et al., 2016; Lin & Ferrucci, 2012). Within the vestibular clinic, many etiologies present with certain patterns of hearing loss. For example, older adults and patients with acoustic neuroma may present with predominantly high-frequency hearing loss whereas patients with Meniere’s may present with low-to-mid frequency hearing loss. As such, 4-frequency PTA may not fully characterize hearing loss for these patients and it would be worthwhile to test this association between high- and low-frequency HL and falls in a future study. Moreover, how we define hearing in research is part of a broader question that has recently been playing out in our scientific literature ((Gatlin & Dhar, 2021; Humes & Weinstein, 2021; Lin & Reed, 2021). How we define HL is particularly salient in the context of important public health domains and one that remains undecided. Finally, this study did not take into account the chronicity of vestibular dysfunction, otolithic function, or unmeasured potential confounders (such as vision impairment not captured by the FCI) and their potential impact on falls.
CONCLUSIONS
Our results indicate that HL was not associated with falls in patients with vestibular dysfunction or non-vestibular dizziness, while adjusting for demographics, comorbidities, and falls-associated medications. Furthermore, HL did not show any interactive effect as it was not associated with falls among patients with any specific type of vestibular dysfunction or patients with non-vestibular dizziness. A population-based study utilizing more robust falls data is needed to explore a potential association between HL and falls in those with vestibular dysfunction. Furthermore, future investigations of the association between HL and falls in the hearing-impaired population should consider utilizing direct measures of vestibular function to characterize the independent effects of hearing loss and vestibular function on falls.
Supplementary Material
Supplemental Table 1: Diagnostic Group Categorization Criteria
Supplemental Table 2: ICD Codes to Identify Dizziness and Vestibular Dysfunction
Supplemental Table 3: ICD Codes to Identify Comorbidities in the Functional Comorbidity Index (FCI)
Supplemental Table 4: ICD Codes to Identify Cognitive Impairment
Supplemental Table 5: Categorization of DEDUCE Pharmaceutical Classes into Falls-Associated Medication Classes
Supplemental Table 6: World Health Organization Grades of Hearing Impairment for each Diagnostic Group
ACKNOWLEDGMENTS
The authors would like to acknowledge Sherri Smith, AuD, PhD for her thoughtful feedback on the drafting and revising of this manuscript.
R.J.H. was supported by a Pfizer Foundation grant and the Duke Clinical Translational Science Institute (CTSI). The content of this publication is solely the responsibility of the authors and does not necessarily represent the official views of the Pfizer Foundation or the Duke CTSI. This publication reflects work conducted in fulfillment of a Master’s thesis by R.J.H. at the Duke University School of Medicine Clinical Research Training Program (CRTP) and has not been published previously. K.M.R. receives funding from the NIH (Riska, PI, NIH Grant Number: 1R21DC018616). The contents of this research are solely the responsibility of the authors and do not necessarily represent the official views of NCATS or NIH.
Footnotes
Portions of this article were presented at the American Balance Society 13th Annual Meeting, Virtual, March 3, 2021.
Conflicts of Interest and Source of Funding: none declared.
REFERENCES
- Agrawal Y, Carey JP, Della Santina CC, Schubert MC, & Minor LB (2009). Disorders of balance and vestibular function in US adults: data from the National Health and Nutrition Examination Survey, 2001-2004. Arch Intern Med, 169(10), 938–944. 10.1001/archinternmed.2009.66 [DOI] [PubMed] [Google Scholar]
- AHFS Pharmacologic-Therapeutic Classification. Retrieved May 25 from https://www.ahfsdruginformation.com/ahfs-pharmacologic-therapeutic-classification/
- Assländer L, & Peterka RJ (2014). Sensory reweighting dynamics in human postural control. Journal of neurophysiology, 111(9), 1852–1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berge JE, Nordahl SHG, Aarstad HJ, & Goplen FK (2019). Hearing as an independent predictor of postural balance in 1075 patients evaluated for dizziness. Otolaryngology–Head and Neck Surgery, 161(3), 478–484. [DOI] [PubMed] [Google Scholar]
- Bergen G, Stevens MR, & Burns ER (2016). Falls and fall injuries among adults aged≥ 65 years—United States, 2014. Morbidity and Mortality Weekly Report, 65(37), 993–998. [DOI] [PubMed] [Google Scholar]
- Bermúdez Rey MC, Clark TK, Wang W, Leeder T, Bian Y, & Merfeld DM (2016). Vestibular Perceptual Thresholds Increase above the Age of 40. Front Neurol, 7, 162. 10.3389/fneur.2016.00162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borel L, & Alescio-Lautier B (2014). Posture and cognition in the elderly: interaction and contribution to the rehabilitation strategies. Neurophysiol Clin, 44(1), 95–107. 10.1016/j.neucli.2013.10.129 [DOI] [PubMed] [Google Scholar]
- Campbell AJ, Robertson MC, Gardner MM, Norton RN, & Buchner DM (1999). Psychotropic Medication Withdrawal and a Home-Based Exercise Program to Prevent Falls: A Randomized, Controlled Trial. Journal of the American Geriatrics Society, 47(7), 850–853. 10.1111/j.1532-5415.1999.tb03843.x [DOI] [PubMed] [Google Scholar]
- Carpenter MG, & Campos JL (2020). The effects of hearing loss on balance: A critical review. Ear and Hearing, 41, 107S–119S. [DOI] [PubMed] [Google Scholar]
- Deardorff WJ, Liu PL, Sloane R, Van Houtven C, Pieper CF, Hastings SN, Cohen HJ, & Whitson HE (2019). Association of sensory and cognitive impairment with healthcare utilization and cost in older adults. Journal of the American Geriatrics Society, 67(8), 1617–1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Easton R, Greene AJ, DiZio P, & Lackner JR (1998). Auditory cues for orientation and postural control in sighted and congenitally blind people. Experimental brain research, 118(4), 541–550. [DOI] [PubMed] [Google Scholar]
- Florence CS, Bergen G, Atherly A, Burns E, Stevens J, & Drake C (2018). Medical Costs of Fatal and Nonfatal Falls in Older Adults. Journal of the American Geriatrics Society, 66(4), 693–698. 10.1111/jgs.15304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiyoshi A, Jacobs DR Jr, Alonso A, Luchsinger JA, Rapp SR, & Duprez DA (2017). Validity of death certificate and hospital discharge ICD codes for dementia diagnosis: the Multi Ethnic Study of Atherosclerosis. Alzheimer disease and associated disorders, 31(2), 168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazzola JM, Ganança FF, Aratani MC, Perracini MR, & Ganança MM (2006). Circumstances and consequences of falls in elderly people with vestibular disorder. Braz J Otorhinolaryngol, 72(3), 388–392. 10.1016/s1808-8694(15)30974-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gell NM, Wallace RB, LaCroix AZ, Mroz TM, & Patel KV (2015). Mobility Device Use in Older Adults and Incidence of Falls and Worry About Falling: Findings from the 2011-2012 National Health and Aging Trends Study. Journal of the American Geriatrics Society, 63(5), 853–859. 10.1111/jgs.13393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gopinath B, McMahon CM, Burlutsky G, & Mitchell P (2016). Hearing and vision impairment and the 5-year incidence of falls in older adults. Age and Ageing, 45(3), 409–414. 10.1093/ageing/afw022 [DOI] [PubMed] [Google Scholar]
- Groll DL, Heyland DK, Caeser M, & Wright JG (2006). Assessment of Long-Term Physical Function in Acute Respiratory Distress Syndrome (ARDS) Patients: Comparison of the Charlson Comorbidity Index and the Functional Comorbidity Index. American Journal of Physical Medicine & Rehabilitation, 85(7), 574–581. 10.1097/01.phm.0000223220.91914.61 [DOI] [PubMed] [Google Scholar]
- Groll DL, To T, Bombardier C, & Wright JG (2005). The development of a comorbidity index with physical function as the outcome. Journal of clinical epidemiology, 58(6), 595–602. [DOI] [PubMed] [Google Scholar]
- Hall CD, Herdman SJ, Whitney SL, Cass SP, Clendaniel RA, Fife TD, Furman JM, Getchius TS, Goebel JA, Shepard NT, & Woodhouse SN (2016). Vestibular Rehabilitation for Peripheral Vestibular Hypofunction: An Evidence-Based Clinical Practice Guideline: FROM THE AMERICAN PHYSICAL THERAPY ASSOCIATION NEUROLOGY SECTION. J Neurol Phys Ther, 40(2), 124–155. 10.1097/npt.0000000000000120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herdman SJ, Hall CD, Schubert MC, Das VE, & Tusa RJ (2007). Recovery of dynamic visual acuity in bilateral vestibular hypofunction. Archives of Otolaryngology–Head & Neck Surgery, 133(4), 383–389. [DOI] [PubMed] [Google Scholar]
- Hoffman GJ, Ha J, Alexander NB, Langa KM, Tinetti M, & Min LC (2018). Underreporting of fall injuries of older adults: implications for wellness visit fall risk screening. Journal of the American Geriatrics Society, 66(6), 1195–1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horak FB, & Macpherson JM (2010). Postural orientation and equilibrium. Comprehensive Physiology, 255–292. [Google Scholar]
- Horvath MM, Winfield S, Evans S, Slopek S, Shang H, & Ferranti J (2011). The DEDUCE Guided Query tool: providing simplified access to clinical data for research and quality improvement. Journal of biomedical informatics, 44(2), 266–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson GP, Shepard NT, Barin K, Burkard RF, Janky K, & McCaslin DL (2021). Balance Function Assessment and Management (3rd ed.). Plural Publishing. [Google Scholar]
- Jiam NT-L, Li C, & Agrawal Y (2016). Hearing loss and falls: A systematic review and meta-analysis. The Laryngoscope, 126(11), 2587–2596. 10.1002/lary.25927 [DOI] [PubMed] [Google Scholar]
- Kabboord AD, Godfrey D, Gordon AL, Gladman JR, Van Eijk M, van Balen R, & Achterberg WP (2020). The modified functional comorbidity index performed better than the Charlson index and original functional comorbidity index in predicting functional outcome in geriatric rehabilitation: a prospective observational study. BMC geriatrics, 20, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamil RJ, Betz J, Powers BB, Pratt S, Kritchevsky S, Ayonayon HN, Harris TB, Helzner E, Deal JA, Martin K, Peterson M, Satterfield S, Simonsick EM, & Lin FR (2016). Association of Hearing Impairment With Incident Frailty and Falls in Older Adults. Journal of Aging and Health, 28(4), 644–660. 10.1177/0898264315608730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanegaonkar R, Amin K, & Clarke M (2012). The contribution of hearing to normal balance. Journal of Laryngology and otology, 126(10), 984. [DOI] [PubMed] [Google Scholar]
- Karim AM, Rumalla K, King LA, & Hullar TE (2018). The effect of spatial auditory landmarks on ambulation. Gait & posture, 60, 171–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacour M, Bernard-Demanze L, & Dumitrescu M (2008). Posture control, aging, and attention resources: models and posture-analysis methods. Neurophysiol Clin, 38(6), 411–421. 10.1016/j.neucli.2008.09.005 [DOI] [PubMed] [Google Scholar]
- Lin FR, & Ferrucci L (2012). Hearing loss and falls among older adults in the United States. Arch Intern Med, 172(4), 369–371. 10.1001/archinternmed.2011.728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maheu M, Behtani L, Nooristani M, Houde MS, Delcenserie A, Leroux T, & Champoux F (2019). Vestibular Function Modulates the Benefit of Hearing Aids in People With Hearing Loss During Static Postural Control. Ear Hear, 40(6), 1418–1424. 10.1097/aud.0000000000000720 [DOI] [PubMed] [Google Scholar]
- Maurer C, Mergner T, & Peterka R (2006). Multisensory control of human upright stance. Experimental brain research, 171(2), 231. [DOI] [PubMed] [Google Scholar]
- McDonnell MN, & Hillier SL (2015). Vestibular rehabilitation for unilateral peripheral vestibular dysfunction. Cochrane Database of Systematic Reviews(1). 10.1002/14651858.CD005397.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mergner T, Schweigart G, Fennell L, & Maurer C (2009). Posture control in vestibular-loss patients. Annals of the New York Academy of Sciences, 1164(1), 206–215. [DOI] [PubMed] [Google Scholar]
- Methods for Manual Pure-Tone Threshold Audiometry. (2004). In American National Standard. American National Standards Institue; S23.1. [Google Scholar]
- Mirelman A, Herman T, Brozgol M, Dorfman M, Sprecher E, Schweiger A, Giladi N, & Hausdorff JM (2012). Executive Function and Falls in Older Adults: New Findings from a Five-Year Prospective Study Link Fall Risk to Cognition. PLOS ONE, 7(6), e40297. 10.1371/journal.pone.0040297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negahban H, Bavarsad Cheshmeh Ali M, & Nassadj G (2017). Effect of hearing aids on static balance function in elderly with hearing loss. Gait Posture, 58, 126–129. 10.1016/j.gaitpost.2017.07.112 [DOI] [PubMed] [Google Scholar]
- Neuhauser HK, von Brevern M, Radtke A, Lezius F, Feldmann M, Ziese T, & Lempert T (2005). Epidemiology of vestibular vertigo. Neurology, 65(6), 898. 10.1212/01.wnl.0000175987.59991.3d [DOI] [PubMed] [Google Scholar]
- O’Loughlin JL, Robitaille Y, Boivin JF, & Suissa S (1993). Incidence of and risk factors for falls and injurious falls among the community-dwelling elderly. Am J Epidemiol, 137(3), 342–354. 10.1093/oxfordjournals.aje.a116681 [DOI] [PubMed] [Google Scholar]
- O’Hare MP, Pryde SJ, & Gracey JH (2013). A systematic review of the evidence for the provision of walking frames for older people. Physical Therapy Reviews, 18(1), 11–23. 10.1179/1743288X12Y.0000000036 [DOI] [Google Scholar]
- Peterka RJ (2002). Sensorimotor integration in human postural control. Journal of neurophysiology, 88(3), 1097–1118. [DOI] [PubMed] [Google Scholar]
- Pichora-Fuller MK, Kramer SE, Eckert MA, Edwards B, Hornsby BW, Humes LE, Lemke U, Lunner T, Matthen M, Mackersie CL, Naylor G, Phillips NA, Richter M, Rudner M, Sommers MS, Tremblay KL, & Wingfield A (2016). Hearing Impairment and Cognitive Energy: The Framework for Understanding Effortful Listening (FUEL). Ear Hear, 37 Suppl 1, 5s–27s. 10.1097/aud.0000000000000312 [DOI] [PubMed] [Google Scholar]
- Resnick B, & Boltz M (2019). Optimizing Function and Physical Activity in Hospitalized Older Adults to Prevent Functional Decline and Falls. Clin Geriatr Med, 35(2), 237–251. 10.1016/j.cger.2019.01.003 [DOI] [PubMed] [Google Scholar]
- Riska KM, Peskoe SB, Gordee A, Kuchibhatla M, & Smith SL (2021). Preliminary Evidence on the Impact of Hearing Aid Use on Falls Risk in Individuals With Self-Reported Hearing Loss. Am J Audiol, 30(2), 376–384. 10.1044/2021_aja-20-00179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riska KM, Peskoe SB, Kuchibhatla M, Gordee A, Pavon J, Kim SE, West JS, & Smith SL (In Press). Impact of hearing aid use on falls and falls-related injury: Results from the Health and Retirement Study. Ear and Hearing. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rumalla K, Karim AM, & Hullar TE (2015). The effect of hearing aids on postural stability. Laryngoscope, 125(3), 720–723. 10.1002/lary.24974 [DOI] [PubMed] [Google Scholar]
- Scherer M, Migliaccio AA, & Schubert MC (2008). Effect of vestibular rehabilitation on passive dynamic visual acuity. Journal of Vestibular Research, 18(2, 3), 147–157. [PMC free article] [PubMed] [Google Scholar]
- Sears JM, & Rundell SD (2020). Development and Testing of Compatible Diagnosis Code Lists for the Functional Comorbidity Index: International Classification of Diseases, Ninth Revision, Clinical Modification and International Classification of Diseases, 10th Revision, Clinical Modification. Medical Care, 58(12), 1044–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shayman CS, Earhart GM, & Hullar TE (2017). Improvements in Gait With Hearing Aids and Cochlear Implants. Otol Neurotol, 38(4), 484–486. 10.1097/mao.0000000000001360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steadman J, Donaldson N, & Kalra L (2003). A Randomized Controlled Trial of an Enhanced Balance Training Program to Improve Mobility and Reduce Falls in Elderly Patients. Journal of the American Geriatrics Society, 51(6), 847–852. 10.1046/j.1365-2389.2003.51268.x [DOI] [PubMed] [Google Scholar]
- Viljanen A, Kaprio J, Pyykkö I, Sorri M, Pajala S, Kauppinen M, Koskenvuo M, & Rantanen T (2009). Hearing as a predictor of falls and postural balance in older female twins. J Gerontol A Biol Sci Med Sci, 64(2), 312–317. 10.1093/gerona/gln015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitkovic J, Le C, Lee SL, & Clark RA (2016). The Contribution of Hearing and Hearing Loss to Balance Control. Audiology and Neurotology, 21(4), 195–202. 10.1159/000445100 [DOI] [PubMed] [Google Scholar]
- Ward BK, Agrawal Y, Hoffman HJ, Carey JP, & Della Santina CC (2013). Prevalence and Impact of Bilateral Vestibular Hypofunction: Results From the 2008 US National Health Interview Survey. JAMA Otolaryngology–Head & Neck Surgery, 139(8), 803–810. 10.1001/jamaoto.2013.3913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO | Grades of hearing impairment. WHO. http://www.who.int/deafness/hearing_impairment_grades/en/ [Google Scholar]
- https://www.who.int/pbd/deafness/hearing_impairment_grades/en/
- Wilkinson T, Ly A, Schnier C, Rannikmäe K, Bush K, Brayne C, Quinn TJ, Sudlow CL, Group UBNO, & UK DP (2018). Identifying dementia cases with routinely collected health data: a systematic review. Alzheimer’s & Dementia, 14(8), 1038–1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woollacott M, & Shumway-Cook A (2002). Attention and the control of posture and gait: a review of an emerging area of research. Gait Posture, 16(1), 1–14. 10.1016/s0966-6362(01)00156-4 [DOI] [PubMed] [Google Scholar]
- Zuniga MG, Dinkes RE, Davalos-Bichara M, Carey JP, Schubert MC, King WM, Walston J, & Agrawal Y (2012). Association between hearing loss and saccular dysfunction in older individuals. Otology & neurotology: official publication of the American Otological Society, American Neurotology Society [and] European Academy of Otology and Neurotology, 33(9), 1586. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table 1: Diagnostic Group Categorization Criteria
Supplemental Table 2: ICD Codes to Identify Dizziness and Vestibular Dysfunction
Supplemental Table 3: ICD Codes to Identify Comorbidities in the Functional Comorbidity Index (FCI)
Supplemental Table 4: ICD Codes to Identify Cognitive Impairment
Supplemental Table 5: Categorization of DEDUCE Pharmaceutical Classes into Falls-Associated Medication Classes
Supplemental Table 6: World Health Organization Grades of Hearing Impairment for each Diagnostic Group


