Abstract
Background:
Postural Tachycardia Syndrome (POTS) is characterized by excessive upright tachycardia and disabling pre-syncopal symptoms, which are exacerbated after consuming a high-carbohydrate meal; it is unknown, however, what is the precise underlying mechanism. We seek to investigate the effect of glucose intake on orthostatic hemodynamic changes and gastrointestinal (GI) hormone secretion in POTS.
Methods:
Prospective, case-control study, 12 women with POTS who reported a postprandial worsening of their POTS symptoms and 13 age-matched female controls received 75-gr oral glucose and 20 mg/kg acetaminophen to assess nutrient absorption. Hemodynamic, GI hormone and acetaminophen levels were measured for up to 120-min post-ingestion while supine and standing.
Results:
POTS patients had significant orthostatic tachycardia, 48.7±11.2 vs. 23.3±8.1 bpm, P=0.012 and elevated upright norepinephrine levels, 835.2±368.4 vs. 356.9±156.7 pg/mL, P= 0.004. After oral glucose, upright heart rate significantly increased in POTS, 21.2±11.9 vs. 6.0±19.9 %, P=0.033 with a concomitant decline in upright stroke volume (SV), −10.3±11.90 vs. 3.3±13.7 %, P=0.027; total peripheral resistance, blood pressure and cardiac output remained unaltered. Acetaminophen rate of appearance was similar between groups (P=0.707), indicating comparable nutrient absorption rates. POTS had increased plasma levels of C-peptide (P=0.001), glucose-dependent insulinotropic polypeptide (GIP) (P=0.001), peptide YY (P=0.016) and pancreatic polypeptide (P=0.04) following glucose consumption, but only GIP had a time-dependent association with the worsening upright tachycardia and SV fall.
Conclusions:
the glucose-induced worsening orthostatic tachycardia in POTS was associated with a decline in SV; these changes occurred while GIP, a splanchnic vasodilator, was maximally elevated.
Keywords: Postural Tachycardia Syndrome, POTS, Glucose Dependent Insulinotropic Peptide, GIP, incretin, insulin resistance, tachycardia, meals, autonomics
Graphical Abstract
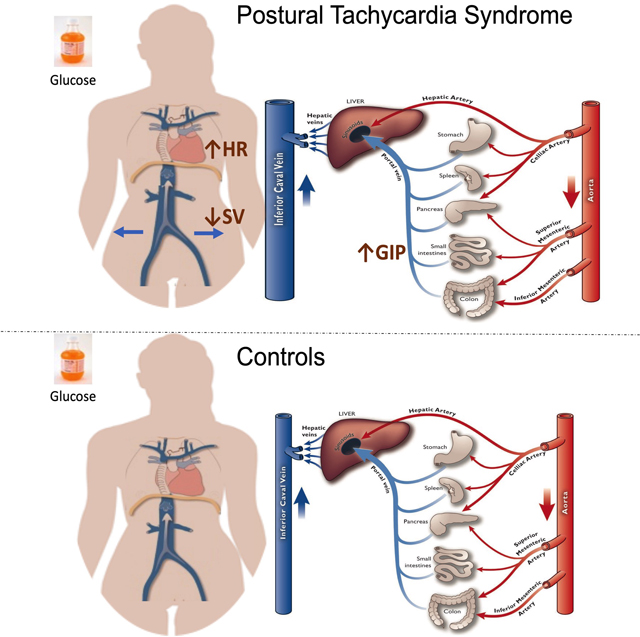
In POTS, oral glucose induced significant GIP secretion which was associated with reduced upright stroke volume and worsening orthostatic tachycardia.
In Postural Tachycardia Syndrome (POTS), 75-gr glucose intake provoked a significant increase in glucose dependent insulinotropic peptide (GIP), a vasodilatory hormone; this change was associated with excessive upright tachycardia and reduced stroke volume, possibly related to decrease venous return (upper panel). Control subjects did not experience these changes (lower panel).
Introduction
Postural Tachycardia Syndrome (POTS) affects ~3 million young women and men in the United States.1 These patients have a poor quality of life because of chronic pre-syncopal symptoms characterized by dizziness, lightheadedness and orthostatic tachycardia that occur while standing.2, 3 POTS primarily affects young women and is characterized by substantial incapacity; a cross-sectional survey found that 25% of these patients filed for disability;4 meals rich in carbohydrates are among the factors that further exacerbate POTS’s symptoms5 and also cause a myriad of gastrointestinal (GI) symptoms.6
As a result of consuming, on average, three meals and three snacks a day, POTS patients spend the majority of their day in an intermittent post-prandial state, experiencing periods of symptoms exacerbation; this has led healthcare providers to prescribe various dietary interventions to alleviate the worsening of POTS symptoms. Among them, eating smalls meals throughout the day and the extremely empirical use of the somatostatin analogue, octreotide®, that suppresses the secretion of all vasodilatory peptides but the risk of inducing malabsorption syndrome.7, 8 These interventions, however, are not supported by any robust scientific evidence. Therefore, understanding the pathophysiology behind the worsening postprandial symptoms in POTS, with the goal of developing targeted therapies, is of the utmost importance.
In POTS patients, one of the main drivers of the orthostatic tachycardia is the excessive sympathetic activation, which mostly occurs in response to blood pooling in the splanchnic veins upon standing.9 The splanchnic circulation is the largest blood volume reservoir of the human body, storing ~25% of the total blood volume and contributing to sudden, and large, fluctuations in the stroke volume. These orthostatic changes in systemic hemodynamics are particularly magnified after meals, due to increased blood volume sequestration triggered by the release of gastrointestinal (GI) peptides with vasodilatory properties. Incretin hormones such as the glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide-2 (GLP-2), which are released after glucose intake, have vasodilatory properties in the splanchnic circulation. Intravenous and subcutaneous administration of GLP-2 in humans decreases the superior mesenteric artery resistance index.10 Similarly, GIP administration increased blood flow to the jejunum using positron emission tomography (PET).11
Accordingly, the overall purpose of this study was to determine the hemodynamic and neurohormonal changes that occur before and after a standard 75-gr oral glucose challenge, while supine and standing at different timepoints and for up to 120-min; we hypothesized that POTS patients would have enhanced GI hormone levels in response to oral glucose compared with age-, body mass index (BMI)-matched controls.
Methods
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Study Subjects
44 women, in total, were screened for this study. Subjects were excluded if they had a history of type 1 or 2 diabetes mellitus, BMI>30 kg/m2, coronary artery disease, previous bariatric surgery, impaired hepatic or renal function, hematocrit <28%, pregnancy, or chronic use of acetaminophen, 18 subjects did not meet these eligibility criteria, 26 subjects completed the study. The data from one subject was excluded from the analyses because she was using a glucagon-like peptide-1 (GLP-1) agonist for weight control. The data presented consists of 12 POTS patients diagnosed based on current guidelines12 and 13 age/BMI-matched healthy controls. POTS patients were recruited from referrals to the Vanderbilt Autonomic Dysfunction Center. The study was approved by an institutional review board (Vanderbilt Human Research Protection Program) and all participants gave written informed consent. Further, the study was conducted in accordance with institutional guidelines and adhered to the principles of the Declaration of Helsinki and Title 45 of the US Code of Federal Regulations (Part 46, Protection of Human Subjects) and the study was registered in clinicaltrials.gov NCT03263819.
Study Overview
During the screening visit, the participants underwent a medical history review, physical examination, laboratory assessments (cell blood count, comprehensive metabolic panel, lipid profile), supine and standing plasma norepinephrine and autonomic function tests as described in a previous publication.13 All the patients’ medical records were reviewed for additional information about the diagnosis and treatment. Patient were asked to rate their symptoms burden using the Vanderbilt Orthostatic Symptoms Score, developed for symptoms’ evaluation in acute clinical trials.14 Using a scale of 0 to 10 (0 reflects absence of symptoms), the patients were asked to rate nine symptoms (mental clouding, blurred vision, shortness of breath, rapid heartbeat, tremulousness, chest discomfort, headache, lightheadedness, and nausea). POTS patients who reported worsening of these symptoms after meal intake were enrolled in the study.
The participants were asked to maintain their usual diet prior to the study and to arrive to the Vanderbilt Autonomic Dysfunction Center, early in the morning and in fasting conditions. Subsequently, the subjects were asked to ingest 75-gr of glucose dissolved in 300 mL water (TRUTOL®) mixed with acetaminophen (20 mg/kg). The acetaminophen was added to detect differences in glucose absorption between groups which could affect the interpretation of the GI hormone kinetics. Subjects were asked to drink the solution within five minutes. In order to evaluate for the early excursion of gastrointestinal hormones and plasma glucose, we collected blood samples at 5, 10, 15, 30, 60, 90, and 120 minutes post-ingestion. Plasma glucose was assessed at the bedside (YSI Life Sciences, Yellow Springs, OH). Orthostatic vital signs, BP and HR variability, and systemic hemodynamic were obtained in the supine position and after 10 minutes of standing at baseline, 30, 60, 90 and 120 minutes post-glucose ingestion.
Outcomes
Gastrointestinal Hormone Assessment
Plasma utilized for incretin measurements were supplemented with aprotinin (1,000 KIU/ml) and dipeptidyl peptidase-4 inhibitor (20μl/ml plasma; Millipore, St. Charles, MO). Insulin, C-peptide, GIP, glucagon-like peptide-1 (GLP-1), peptide YY (PYY), pancreatic polypeptide (PP), and glucagon were measured with multiplex immunoassays (Luminex xMAP, Millipore). Additional plasma samples were tested for insulin using Radioimmunoassay (Vanderbilt Hormone Core) at time points baseline 0 (baseline), 30, 60, 90, and 120 (Millipore PI-12K). Matsuda index was calculated according to the formula published using insulin and glucose measurements.15
Glucagon-like peptide-2 (GLP-2) measurements: Plasma samples for GLP-2 measurements were extracted in a final concentration of 75% ethanol, GLP-2 was measured using a radio-immuno assay originally described by Hartmann et al.16 The antiserum (code no. 92160) was directed against the N-terminus of GLP-2 and therefore measures only fully processed GLP-2 of intestinal origin. For standards, we used human GLP-2 and the tracer was 125I-labeled rat GLP-2 with an Asp33 -> Tyr33 substitution.
Acetaminophen (paracetamol) was measured at 240 nm using HPLC with a C18 3.9X300 column with guard (Waters uBondapak Milford, MA).15The acetaminophen concentrations obtained at baseline, 30, 60, 90 and 120 minutes were used as a surrogate marker of small intestine nutrient absorption.17, 18 Acetaminophen is not absorbed in the stomach and its absorption depends on the gastric emptying. Given that delayed gastric absorption was reported in POTS patients, we used the acetaminophen test to adjust for this potential confounder.
Hemodynamic Parameters and Autonomic Measurements
Hemodynamic data were recorded using the WINDAQ data acquisition system (DI720, DATAQ, Akron, OH, 14 Bit, 1000Hz), and were processed off-line using a custom written software in PV-Wave language (PV-wave, Visual Numerics Inc., Houston, TX), developed by one of the authors (AD). Detected beat-to-beat values of R-R intervals (RRI) and blood pressure were interpolated and low-pass filtered (cutoff 2 Hz). Data segments of at least 180 seconds were used for spectral analysis. Linear trends were removed, and power spectral density was estimated with the FFT-based Welch algorithm. The total power (TP) and the power in the low (LF: 0.04 to <0.15 Hz), and high (HF: 0.15 to < 0.40 Hz) frequency ranges were calculated. In conjunction, cross spectra, coherence and transfer function analysis were used to capture interrelationships between R-R interval and systolic blood pressure. Along with the baroreflex gain which was determined by calculating the mean magnitude value of the transfer function in the low-frequency band, with negative phase and squared coherence value greater than 0.5. 19
In addition, beat-to-beat stroke volume was estimated by pulse contour analysis of arterial pressure curves (Modelflow algorithm) using a finger photoplethysmographic volume-clamp BP device (Nexfin, BMEYE), as described previously20. An appropriate size cuff was wrapped around the right middle or index finger and a height correction system was used to adjust for hydrostatic height differences between the hand and the heart. Beat-to-beat BP data were calibrated to brachial artery pressure and intermittently checked against, using oscillometric measured brachial blood pressure (Vital-Guard 450C, Ivy Biomedical Systems, Inc., Brandford, CT). Cardiac output (CO) was then calculated by multiplying stroke volume (SV) by the heart rate obtained from oscillometric BP measurements. Systemic total peripheral resistance (TPR) was estimated by dividing oscillometric mean arterial pressure (MAP) by cardiac output.
Statistical Methods
Sample size calculation:
This is a pilot study, which limits our sample size to 12 subjects per group. With N=12 per group, the study had 80% power to detect an effect size of 1.2.
Analysis methods:
The primary endpoints were GLP-1, GIP and PYY. Secondary endpoints included hemodynamic orthostatic scores. Values are reported as mean ± standard deviation (SD) unless otherwise stated. In graphics, values are reported as mean ± standard error of the mean (SEM). Standard graphing and screening techniques were used to detect outliers and ensure data accuracy. Summary statistics for both numerical and categorical variables by study groups were provided. Continuous variables are summarized as mean ± standard deviation (SD). We estimated between-group difference in means with standard deviation for the primary endpoints and these differences were tested using Wilcoxon Rank Sum test. The Generalized Least Squares model was used to compare POTS versus controls across various time points for the different GI hormones. P values were reported for group and group x time interaction, and we tested all hypotheses at the level of α=0.05. The open source statistical package R (version 3.5.1, R Development Core Team, 2018) was used for analyses.
Results
Subject’s characteristics
The demographic characteristics of the study subjects are presented in Table 1. Age and BMI were similar in both groups; we found a statistically significant difference in hematocrit levels between POTS and controls (38.3 ± 2.8 vs. 35.9 ± 2.2 %, respectively, P=0.029), which signals lower plasma volume in POTS patients. Of note, in previous studies, 21, 22 hemoconcentration was associated with low plasma volume. Healthy controls scored zero in all nine orthostatic symptoms, whereas POTS patients had the following scores: mental clouding (32±2.74), blurred vision (21±2.02), shortness of breath (11±1.84), rapid heartbeat (19±3.47), tremulousness (29±3.17), chest discomfort (25±3.07), headache (20±2.08), lightheadedness (35±3.03), and nausea (13±1.89).
Table 1.
Subjects Demographic Characteristics
| Parameters | POTS | Controls | P values |
|---|---|---|---|
| N=12 | N=13 | ||
| Age (y) | 36.8 ± 11.7 | 29.9 ± 7.8 | 0.132 |
| Height (cm) | 168.4 ± 5.7 | 168.5 ± 5.3 | 0.817 |
| Weight (kg) | 68.3 ± 14.4 | 63.9 ± 9.6 | 0.750 |
| BMI (kg/m2) | 23.4 ± 4.1 | 22.5 ± 3.2 | 0.401 |
| Fasting Glucose (mg/dL) | 90.2 ± 21.2 | 80.4 ± 12.6 | 0.383 |
| Insulin (mIU/mL) | 8.4 ± 2.8 | 7.5 ± 3.8 | 0.324 |
| Hct/PVC (%) | 38.4 ± 2.8 | 35.9 ± 2.2 | 0.029* |
| Hgb (mg/dL) | 12.9 ± 1.2 | 12.1 ± 0.8 | 0.072 |
| AST (mg/dL) | 22.6 ± 7.6 | 20.2± 5.3 | 0.456 |
| ALT (mg/dL) | 16.1 ± 5.4 | 15.3 ± 9.7 | 0.320 |
| Total Bilirubin (mg/dL) | 0.5 ± 0.2 | 0.7 ± 0.5 | 0.800 |
Values are represented as means ± SD; Postural orthostatic tachycardia syndrome (POTS)
P values obtained using Wilcoxon rank sum test
Medications prescribed for POTS were discontinued 12 hours prior to the study measurements; six patients were on beta blockers, two were alpha-1 adrenergic agonist, midodrine, two were on fludrocortisone, and one on modafinil, droxidopa and amphetamine. The remaining POTS patients followed non-pharmacologic treatment and were not taking any medications.
Baseline Hemodynamic and Neurohormonal Characteristics
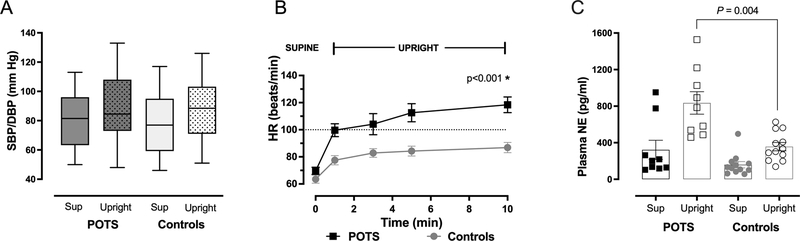
There were no differences in supine and standing systolic (SBP) and diastolic (DBP) blood pressures between groups, figure 1A. Patients with POTS had similar supine NE levels compared with controls (322.2 ± 314.7 vs 159.3 ± 118.2 pg/mL, P=0.171); however, standing NE levels were significantly higher in POTS patients than in controls (835.2 ± 368.4 vs. 356.9 ± 156.7 pg/mL, P= 0.004), which suggests elevated sympathetic activation, figure 1B. Along with this, the standing epinephrine (EPI) levels were also significantly higher in POTS than in controls (49.5 ± 41.6 vs. 13.5 ± 14.9 pg/mL, P= 0.025). POTS patients met the definition of orthostatic tachycardia, they showed a higher standing HR (118.4 ± 13.0 vs. 86.8 ± 13.7 bpm in controls, P=0.002) and higher increase in standing (48.7 ± 11.2 vs. 23.3 ± 8.1 bpm in controls, P=0.012) figure 1C. The results of the standardized autonomic function testing are presented in Table S1, please see http://hyper.ahajournals.org. Cardiovascular autonomic reflexes were normal and similar in both groups. It is noteworthy that the SBP during early phase II of the Valsalva Maneuver significantly decreased in POTS patients compared with controls (−29.3 ± 17.1 vs. −14.0 ± 11.6 mmHg, P=0.03); this response was associated with decreased blood volume.23 Additionally, the spectral analysis of blood pressure and heart rate variability is presented in Table S2; we did not, however, capture differences in sympathetic and parasympathetic activity due the large variability in the measurements.
Figure 1. Hemodynamic and Neurohormonal Changes in POTS vs. Controls During Orthostatic Stress.
Systolic (SBP, top of box) and diastolic (DBP, bottom of box) blood pressure were similar while supine and standing between POTS and controls (A); POTS patients showed a significantly greater increase in HR at 10-min standing compared with controls, P<0.001 (B); supine plasma norepinephrine (NE) was similar between POTS and controls, but significantly elevated while standing is POTS vs. controls, P=0.004 (C). HR- heart rate, SBP- systolic blood pressure, DBP- diastolic blood pressure, NE- norepinephrine. P-values obtained using student t-test.
Glucose, insulin, C-peptide secretion after 75-gr oral glucose
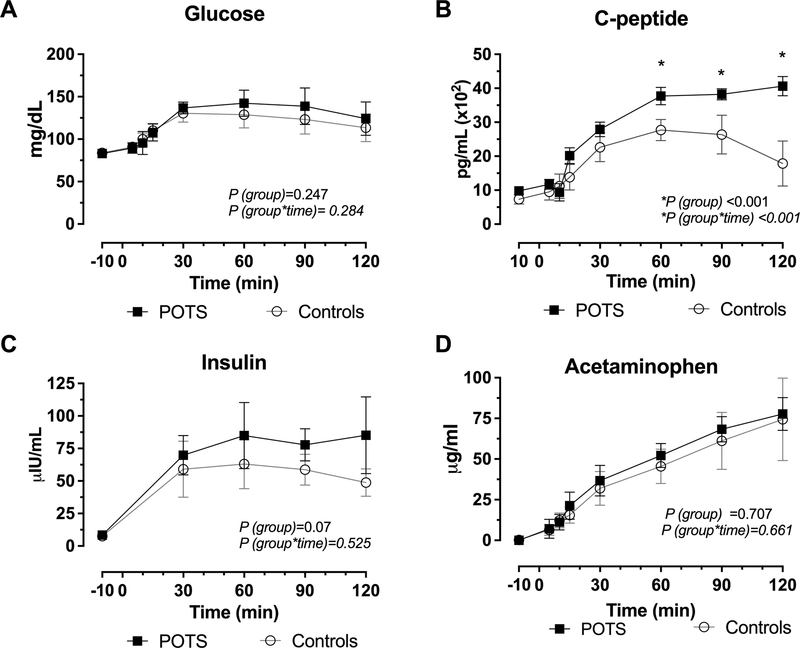
Fasting glucose and insulin levels were normal in POTS and controls. Similarly, after the 75-gr oral glucose intake, the levels increased in a time-dependent manner in both groups, figure 2A. By contrast, there was a significant increase in C-peptide in the POTS cohort (P<0.001 for group) and (P<0.001 for group × time interaction), figure 2B. However, insulin levels were not significantly different between groups (P=0.07 for group), figure 2C, which could be explained by the significant variability in the measurement.
Figure 2. C-peptide Secretion is Increased in POTS following a 75-gr Oral Glucose Consumption.
Blood glucose levels were the same between POTS and controls with similar trends from baseline to 120 minutes (A). POTS patients demonstrated a greater C-peptide secretion compared with controls, P< 0.001 (B). Insulin similarly increased between groups (C). Concentration of acetaminophen in plasma following glucose challenge was the same in POTS and controls (D). P values were calculated using the Generalized Least Squares model, P values were reported for group (POTS versus controls) and group x time (across various time points) for the different GI hormones.
Given that the glucose levels remained similar between POTS and controls despite elevated C-peptide, we postulated that POTS patients were insulin resistant. Using the glucose and insulin levels, we calculated the Matsuda Index (MI) to measure insulin sensitivity. As a result, we could observe that POTS had a greater propensity for insulin resistance than controls (MI, 3.8 ± 1.0 vs. 5.1 ± 1.3, respectively, P=0.009).
Acetaminophen Absorption Test
Fasting acetaminophen levels were undetectable in blood. After the ingestion of 75-gr glucose and 20 mg/kg acetaminophen, acetaminophen levels were detected in plasma in both groups; there was no significant difference between POTS and controls (P=0.707 for group); these results indicated similar nutrient absorption rate, figure 2D.
Effect of Glucose Intake on Systemic Hemodynamics
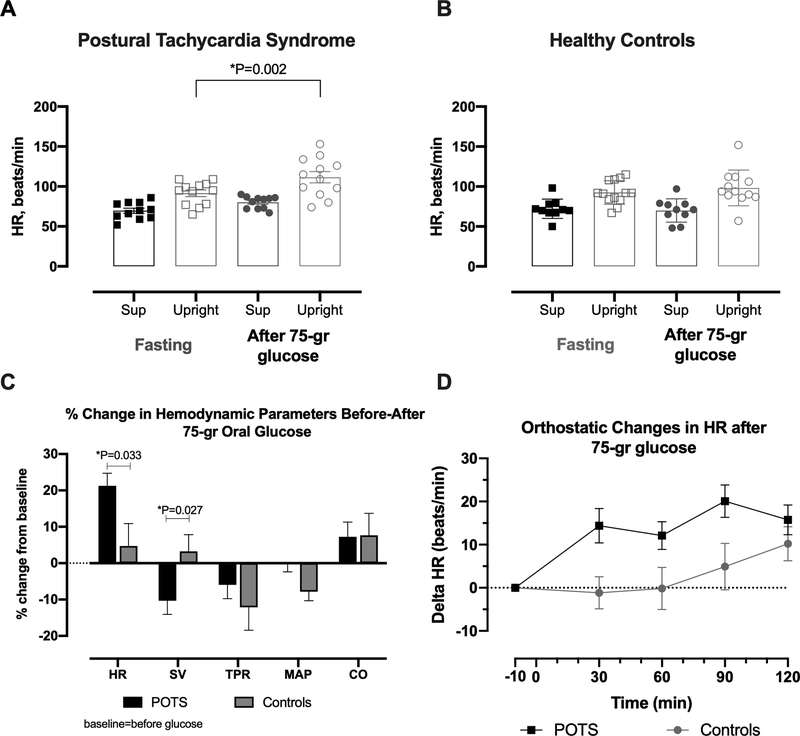
Standing HR significantly increased after the oral glucose ingestion in POTS patients compared to control subjects (figure 3A). The maximal increase from baseline (i.e. fasting) in standing HR in the POTS group was of 19.3±14.2 bpm at 90 minutes post-glucose (P=0.002 for the difference in standing HR between fasting and 90 minutes post-glucose, whereas in the control group, standing HR at the same timepoint was similar to fasting levels (P=0.248, mean difference 6.2±13.0 bpm, figure 3B). Thus, in POTS patients, oral glucose maximally increased standing HR by 21.2±11.9% compared to 6.0±19.9 % in control subjects (P=0.033; figure 3C). This positive chronotropic effect in POTS patients was associated with a significant decrease in standing stroke volume compared with that of healthy controls (−10.3±11.90 vs. 3.3±13.7% in controls, P=0.027; figure 3C), whereas the other hemodynamic measurements (SV, TPR, MAP, CO) did not differ between groups. The absolute changes in HR for POTS and controls are shown in figure 3D. There was a time-dependent increase in HR after glucose ingestion.
Figure 3. Worsening Orthostatic Tachycardia After 75-gr Oral Glucose.
Supine and upright heart rate (HR) while fasting and after 90 minutes of oral glucose in POTS (A) and healthy controls (B). HR, stroke volume (SV), total peripheral resistance (TPR), mean arterial pressure (MAP) and cardiac output (CO) percent changes calculated from baseline values in POTS patients (black) and controls (gray) (C). Absolute changes in HR from baseline (fasting) at different timepoints up to 120 minutes after glucose consumption (D). P-values were calculated using Mann-Whitney U test.
Gastrointestinal hormones kinetics in response to 75-gr oral glucose
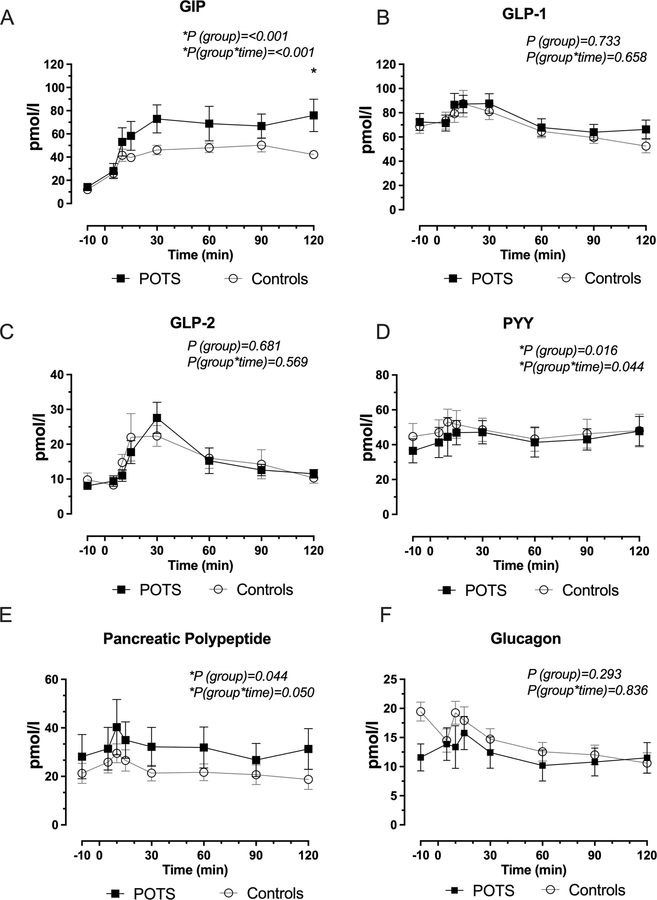
GIP levels were significantly higher in POTS patients after the oral glucose challenge (P=0.001 for group); furthermore, we observed a time-dependent increase in GIP in POTS patients (P=0.001 for groups × time interaction), with a maximum concentration between 90–120 min post-ingestion, figure 4 A. By contrast, GLP-1 and GLP-2 levels were similar between POTS and controls (P=0.733 and P=0.681, respectively), figures 4 B, C.
Figure 4. Secretion of Gastrointestinal Hormones in Response to 75-gr Oral Glucose.
Time-dependent changes in glucose dependent insulinotropic peptide (GIP, A), glucagon-like peptide 1 (GLP-1, panel B); glucagon-like peptide 2 (GLP-2, panel C), peptide YY (PYY, panel D), pancreatic polypeptide (E) and glucagon levels (F). GIP and PYY secretion were significant elevated in POTS but not in controls. P values were calculated using the Generalized Least Squares model, P values were reported for group (POTS versus controls) and group x time (across various time points) for the different GI hormones.
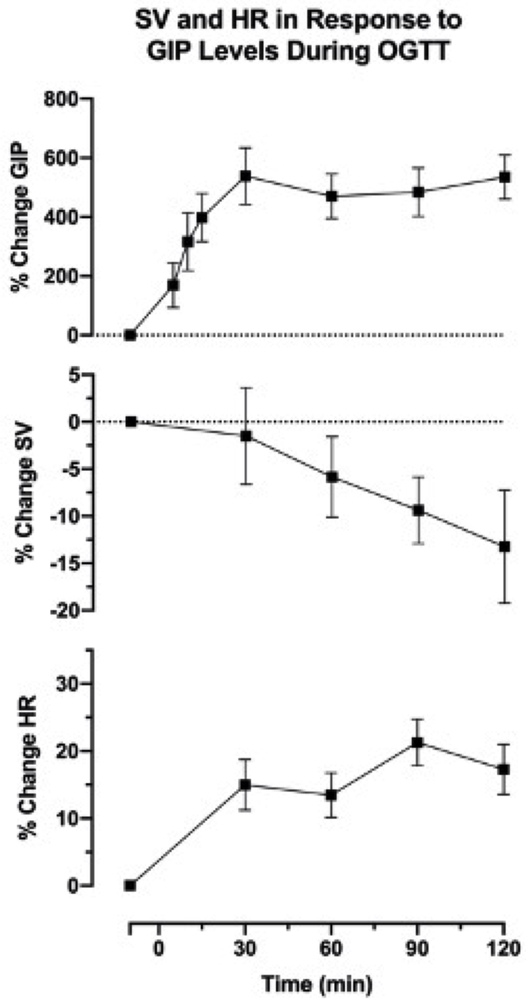
Additionally, POTS patients had a significant increased levels of PYY (P=0.016) and PP (P=0.04) with peak levels of both hormones occurring within the first 30 minutes after glucose ingestion; PPY (P=0.04 for group × time interaction) and PP (P=0.05 for group × time interaction), respectively, figure 4 D and E. In addition, glucagon levels were not different between POTS and controls (P=0.293 for group), figure 4 F. Given that the GIP levels peaked at the same time as the hemodynamic alterations observed in POTS after glucose intake, we plotted the time-dependent, percentage change increase in GIP levels alongside the reduction in standing SV and the increase in standing HR, figure 5. This plot showed that the upright tachycardia in POTS and the decline in SV occurred while GIP was maximally elevated.
Figure 5. Time-dependent increase in glucose dependent insulinotropic peptide coincided with a reduction in upright stroke volume and an increase in upright heart rate.
The figure showed the percent (%) change in glucose dependent insulinotropic peptide (GIP), upright stroke volume (SV), and upright heart rate (HR) from baseline.
Discussion
The main findings of our current study are: first, in this subset of POTS patients, we found reduced insulin sensitivity during a standard 75-gr oral glucose tolerance test; second, after glucose ingestion, POTS’s orthostatic tachycardia worsened, which is, in part, due to a reduction in upright stroke volume. Lastly, POTS patients also exhibited higher levels of the GI hormones GIP, PYY and PP compared with controls; however, only GIP, is a splanchnic vasodilator. Altogether, these findings reveal significant postprandial metabolic and cardiovascular alterations in POTS. Given that GIP has vasodilatory properties, we postulate that this peptide may contribute to the pathophysiology of the post-prandial worsening of POTS’s symptoms, which needs to be tested in future studies.
The subset of POTS patients enrolled in this study had evidence of elevated sympathetic activity as shown by a significant increase in plasma NE. Even though POTS patients were not obese, the absence of hypoglycemia in response to an enhanced C-peptide and insulin secretion suggests reduced insulin sensitivity, which was later confirmed by a significantly lower Matsuda index in POTS patients. It is noteworthy that fasting glucose and insulin were normal in POTS and that insulin resistance was only noticeable after the oral glucose tolerance test; this test is not routinely performed in subjects without traditional risk factors such as obesity or family history of type 2 diabetes mellitus; therefore, it could be possible that insulin resistance is an underdiagnosed condition in POTS. In addition, increased sympathetic activity, which was present in our POTS patients as shown by the elevated upright NE, has been shown to contribute to insulin resistance. For example, removal of excessive sympathetic activation in obese subjects with the ganglionic blocker, trimethaphan resulted in an improvement in insulin sensitivity during hyperinsulinemic-euglycemic clamps.24 Alternatively, endothelial dysfunction25 and sub-clinical inflammation26, conditions associated with dysmetabolic states such as insulin resistance and type 2 diabetes mellitus, have been described in POTS and could contribute to its pathogenesis.
One aspect in the clinical management of POTS patients that merits discussion is the worsening pre-syncopal symptoms and tachycardia, particularly after high-carbohydrate meals. There is consensus that the orthostatic tachycardia, innate to POTS, is triggered by an exaggerated sympathetic activation in response to splanchnic blood pooling that occurs upon standing. In these circumstances, meals are known to significantly increase splanchnic arterial blood flow in healthy subjects, a phenomenon that is largely amplified upon standing.9, 27 It is noteworthy, however, that a previous study, 5 and our current data, figure 3A found that glucose ingestion further potentiates the orthostatic tachycardia in POTS patients, but not in controls, figure 3B and this was associated with a significant reduction in upright SV. Thus, we speculate that the worsening upright tachycardia in POTS patients after glucose ingestion may be due to excessive splanchnic venous capacitance, a major regulator of SV.
Since all these orthostatic hemodynamic changes in POTS patients occurred after the oral glucose ingestion; we measured the kinetics of multiple glucose-induced GI peptide hormones with potential cardiovascular effects. Interestingly, among the three most important incretin hormones, GIP but not GLP-1 or GLP-2 was found to be increased in POTS; additionally, members of the neuropeptide Y family of biologically active peptides such as PYY and PP were also significantly elevated. It should be noted that the major source of PYY is the endocrine L cells which occur abundantly in the lower gastrointestinal tract; the same cells are responsible for the production of GLP-1; however, it is unknown why only PYY secretion is elevated. Nevertheless, it is unlikely that this hormone contributes to the POTS’ worsening post-prandial symptoms and upright tachycardia because this peptide is known to be a powerful vasoconstrictor.28
Unexpectedly, we also found an enhanced in PP secretion in POTS patients within the first 30 minutes after glucose ingestion; this peptide is released in a biphasic manner, mostly after high-protein meals; the initial rise in PP levels is regulated by vagal cholinergic stimulation, 29 and, therefore, the enhanced release in POTS could signal an increased vagal stimulation of GI hormonal secretion; PP and PYY play important roles in the brain-gut signaling because they influence food intake, gastric emptying and energy balance and therefore may contribute to GI symptoms and weight management in POTS. 29
The cause of the elevated GIP levels in POTS patients is unknown; it could be possible that alterations in dietary habits contribute to the observed differences in GIP levels. GIP is synthesized in enteroendocrine K cells in the proximal gut, and its secretion can be altered by several nutritional factors, including meal size, diet composition or glycemic index. Specifically, chronic exposure to high fat diet promotes proliferation of K cells, thus resulting in increased GIP synthesis.30
Finally, we postulate that GIP may have a prominent role in the pathophysiology of post-prandial POTS’s symptoms and upright tachycardia; GIP has vasoactive properties, and its receptor (GIPR) is present in the endothelial cells and smooth muscle cells of the arterioles and 31 veins.32Importantly, a previous study found that GIP infusion increased the superior mesenteric artery flow by two-fold, which occurred in a dose-dependent manner.33 Likewise, in humans, GIP at physiological levels, achieved after the consumption of a mixed meal or after an intravenous infusion, significantly increased intestinal blood flow.11 Given that the GIP peak incidentally coincided with the decrease in upright SV; we hypothesize that this peptide, by increasing the splanchnic blood pooling, could contribute to the SV reduction and consequently to the worsening upright tachycardia. Indeed, a previous study34 reported a significant increase in heart rate during infusion of the GIPR agonist GIP(1–42) and this effect was inhibited by the GIPR antagonist (GIP(3–30)N2).
In conclusion, our study showed that patients with POTS have significant cardiovascular and metabolic alterations after a glucose challenge, with reduced insulin sensitivity and worsening orthostatic tachycardia in response to a fall in upright SV. Furthermore, the evaluation of the glucose-induced GI hormone secretion favors an enhanced release of GIP, and the neuropeptide Y family in POTS. However, only GIP has vasodilatory properties and the glucose-induced hemodynamic alterations observed in POTS occurred while this hormone was maximally secreted.
Study Limitations:
The study has some limitations; because of ethical concerns, it was not feasible to discontinue all the medications for five half-lives due to the risk of exacerbating POTS symptoms. Thus, we withheld the medications for only 12 hours prior to the study and, therefore, it could be possible that medications with longer half-lives had a residual effect on the measurements obtained. We only included POTS patients with post-prandial symptoms and, therefore, our findings may not apply to everyone with the POTS diagnosis. Also, local changes in splanchnic blood pooling after oral glucose and while upright were not obtained given the difficulty of assessing the splanchnic venous capacitance. Finally, we did not measure body composition and physical fitness, which could potentially contribute to the differences in insulin resistance between POTS and normal controls.
Perspective
POTS is a chronic, disabling condition that mostly affects young women; however, there are no approved drugs for the treatment of POTS in the US. Our findings provide insights into the pathophysiology of the worsening of POTS’s symptoms and tachycardia after glucose intake suggesting a potential link between post-prandial orthostatic changes and the GI hormone GIP, a splanchnic vasodilator. Currently, GIPR antagonists are under development, thus opening up a wide range of therapeutic possibilities.
Supplementary Material
Novelty and Relevance.
What is New?
POTS exacerbation after oral glucose is associated with worsening orthostatic tachycardia and a decrease in stroke volume.
Glucose-induced gastrointestinal hormone secretion favors an enhance release of GIP, which has vasodilatory properties in the splanchnic circulation
What is Relevant?
Our findings provide insights into the pathophysiology of the worsening post-prandial POTS symptoms suggesting a potential link between the worsening tachycardia and GIP, a splanchnic vasodilator.
Clinical/Pathophysiological Implicaitons
The pathophysiological phenomen demonstrated in this study provides potential mechanism by which POTS symptoms are exacerbated following meal consumption. Thus, GIPR antagonists in development have potential utility in these patients.
Acknowledgements
David Wright, Copy Editor; Bianca M Plunkett
Sources of Funding
The study was supported by gift funds from Dysautonomia International (East Moriches, NY 11940), NIH grants DK059637 (MMPC), DK020593 (DRTC), T32GM007569 Clinical Pharmacology Training Program. A.D. and L.E.O are supported by the National Heart, Lung, and Blood Institute of the National Health, Award Number NIH 1R01 HL142583 and NIH R01 HL144568, respectively.
Abbreviations
- BMI
Body Mass Index
- BP
Blood Pressure
- BPM
Beats per Minute
- CO
Cardiac Output
- DBP
Diastolic Blood Pressure
- EPI
Epinephrine
- GI
Gastrointestinal
- GIP
Glucose-dependent Inulinotropic Polypeptide
- GLP
Glucagon Like Peptide
- HR
Heart Rate
- MAP
Mean Arterial Pressure
- MI
Matsuda Index
- NE
Norepinephrine
- PET
Postive Emission Tomography
- POTS
Postural Tachycardia Syndrome
- PP
Pancreatice Polypeptide
- PYY
Peptide YY
- SBP
Systolic Blood Pressure
- SD
Standard Deviation
- SEM
Standard Error of the Mean
- SV
Stroke Volume
- TP
Total Power
- TPR
Total Peripheral Resistance
Footnotes
Disclosures
LSG is a co-founder of Antag Therapeutics.
References
- 1.Collins FS. Postural Orthostatic Tachycardia Syndrome (POTS): State of the Science, Clinical Care, and Research. Report prepared for the National Institute of Health, National Heart, Lung, and Blood Institute & National Institute of Neurological Disorders and Stroke. 2020. [Google Scholar]
- 2.Anderson JW, Lambert EA, Sari CI, Dawood T, Esler MD, Vaddadi G and Lambert GW. Cognitive function, health-related quality of life, and symptoms of depression and anxiety sensitivity are impaired in patients with the postural orthostatic tachycardia syndrome (POTS). Front Physiol. 2014;5:230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moon J, Kim DY, Byun JI, Sunwoo JS, Lim JA, Kim TJ, Shin JW, Lee WJ, Lee HS, Jun JS, Park KI, Jung KH, Lee ST, Jung KY, Chu K and Lee SK. Orthostatic intolerance symptoms are associated with depression and diminished quality of life in patients with postural tachycardia syndrome. Health Qual Life Outcomes. 2016;14:144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shaw BH, Stiles LE, Bourne K, Green EA, Shibao CA, Okamoto LE, Garland EM, Gamboa A, Diedrich A, Raj V, Sheldon RS, Biaggioni I, Robertson D and Raj SR. The face of postural tachycardia syndrome - insights from a large cross-sectional online community-based survey. J Intern Med. 2019;286:438–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Habek M, Ruska B, Crnosija L, Adamec I, Junakovic A and Krbot Skoric M. Effect of Food Intake on Hemodynamic Parameters during the Tilt-Table Test in Patients with Postural Orthostatic Tachycardia Syndrome. J Clin Neurol. 2019;15:205–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mehr SE, Barbul A and Shibao CA. Gastrointestinal symptoms in postural tachycardia syndrome: a systematic review. Clin Auton Res. 2018;28:411–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hoeldtke RD, Bryner KD, Hoeldtke ME and Hobbs G. Treatment of autonomic neuropathy, postural tachycardia and orthostatic syncope with octreotide LAR. Clin Auton Res. 2007;17:334–340. [DOI] [PubMed] [Google Scholar]
- 8.Khan M, Ouyang J, Perkins K, Somauroo J and Joseph F. Treatment of Refractory Postural Tachycardia Syndrome with Subcutaneous Octreotide Delivered Using an Insulin Pump. Case Rep Med. 2015;2015:545029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Takala J Determinants of splanchnic blood flow. British Journal of Anaesthesia. 1996;77:50–58. [DOI] [PubMed] [Google Scholar]
- 10.Hansen LB. GLP-2 and mesenteric blood flow. Dan Med J. 2013;60:B4634. [PubMed] [Google Scholar]
- 11.Honka H, Koffert J, Kauhanen S, Teuho J, Hurme S, Mari A, Lindqvist A, Wierup N, Groop L and Nuutila P. Bariatric Surgery Enhances Splanchnic Vascular Responses in Patients With Type 2 Diabetes. Diabetes. 2017;66:880–885. [DOI] [PubMed] [Google Scholar]
- 12.Raj SR. The Postural Tachycardia Syndrome (POTS): pathophysiology, diagnosis & management. Indian Pacing Electrophysiol J. 2006;6:84–99. [PMC free article] [PubMed] [Google Scholar]
- 13.Cheshire WP Jr. and Goldstein DS. Autonomic uprising: the tilt table test in autonomic medicine. Clin Auton Res. 2019;29:215–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Raj SR, Black BK, Biaggioni I, Paranjape SY, Ramirez M, Dupont WD and Robertson D. Propranolol decreases tachycardia and improves symptoms in the postural tachycardia syndrome: less is more. Circulation. 2009;120:725–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Matsuda M and DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care. 1999;22:1462–1470. [DOI] [PubMed] [Google Scholar]
- 16.Hartmann B, Johnsen AH, Orskov C, Adelhorst K, Thim L and Holst JJ. Structure, measurement, and secretion of human glucagon-like peptide-2. Peptides. 2000;21:73–80. [DOI] [PubMed] [Google Scholar]
- 17.Holt S, Heading RC, Clements JA, Tothill P and Prescott LF. Acetaminophen absorption and metabolism in celiac disease and Crohn’s disease. Clin Pharmacol Ther. 1981;30:232–238. [DOI] [PubMed] [Google Scholar]
- 18.Willems M, Quartero AO and Numans ME. How useful is paracetamol absorption as a marker of gastric emptying? A systematic literature study. Digest Dis Sci. 2001;46:2256–2262. [DOI] [PubMed] [Google Scholar]
- 19.Kim JA, Park Y-G, Cho K-H, Hong M-H, Han H-C, Choi Y-S and Yoon DJTJotABoFP. Heart rate variability and obesity indices: emphasis on the response to noise and standing. 2005;18:97–103. [DOI] [PubMed] [Google Scholar]
- 20.Diedrich A, Jordan J, Tank J, Shannon JR, Robertson R, Luft FC, Robertson D and Biaggioni I. The sympathetic nervous system in hypertension: assessment by blood pressure variability and ganglionic blockade. J Hypertens. 2003;21:1677–1686. [DOI] [PubMed] [Google Scholar]
- 21.Jacob G, Robertson D, Mosqueda-Garcia R, Ertl A, Robertson RM and Biaggioni I. Hypovolemia in syncope and orthostatic intolerance. Role of the renin-angiotensin system. AmJMed. 1997;103:128–133. [DOI] [PubMed] [Google Scholar]
- 22.Jacob G, Ertl AC, Shannon JR, Furlan R, Robertson RM and Robertson D. Effect of standing on neurohumoral responses and plasma volume in healthy subjects. Journal of Applied Physiology. 1998;84:914–921. [DOI] [PubMed] [Google Scholar]
- 23.Stewart JM, Medow MA, Bassett B and Montgomery LD. Effects of thoracic blood volume on Valsalva maneuver. Am J Physiol Heart Circ Physiol. 2004;287:H798–804. [DOI] [PubMed] [Google Scholar]
- 24.Gamboa A, Okamoto LE, Arnold AC, Figueroa RA, Diedrich A, Raj SR, Paranjape SY, Farley G, Abumrad N and Biaggioni I. Autonomic blockade improves insulin sensitivity in obese subjects. Hypertension. 2014;64:867–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chopoorian AH, Wahba A, Celedonio J, Nwazue V, Smith EC, Garland EM, Paranjape S, Okamoto LE, Black BK, Biaggioni I, Raj SR and Gamboa A. Impaired Endothelial Function in Patients With Postural Tachycardia Syndrome. Hypertension. 2021;77:1001–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Okamoto LE, Raj SR, Gamboa A, Shibao CA, Arnold AC, Garland EM, Black BK, Farley G, Diedrich A and Biaggioni I. Sympathetic activation is associated with increased IL-6, but not CRP in the absence of obesity: lessons from postural tachycardia syndrome and obesity. Am J Physiol Heart Circ Physiol. 2015;309:H2098–2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Matheson PJ, Wilson MA and Garrison RN. Regulation of intestinal blood flow. J Surg Res. 2000;93:182–196. [DOI] [PubMed] [Google Scholar]
- 28.Lundberg JM, Tatemoto K, Terenius L, Hellstrom PM, Mutt V, Hokfelt T and Hamberger B. Localization of peptide YY (PYY) in gastrointestinal endocrine cells and effects on intestinal blood flow and motility. Proc Natl Acad Sci U S A. 1982;79:4471–4475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Asakawa A, Inui A, Yuzuriha H, Ueno N, Katsuura G, Fujimiya M, Fujino MA, Niijima A, Meguid MM and Kasuga M. Characterization of the effects of pancreatic polypeptide in the regulation of energy balance. Gastroenterology. 2003;124:1325–1336. [DOI] [PubMed] [Google Scholar]
- 30.Gniuli D, Calcagno A, Dalla Libera L, Calvani R, Leccesi L, Caristo ME, Vettor R, Castagneto M, Ghirlanda G and Mingrone G. High-fat feeding stimulates endocrine, glucose-dependent insulinotropic polypeptide (GIP)-expressing cell hyperplasia in the duodenum of Wistar rats. Diabetologia. 2010;53:2233–2240. [DOI] [PubMed] [Google Scholar]
- 31.Zhong Q, Bollag RJ, Dransfield DT, Gasalla-Herraiz J, Ding KH, Min L and Isales CM. Glucose-dependent insulinotropic peptide signaling pathways in endothelial cells. Peptides. 2000;21:1427–1432. [DOI] [PubMed] [Google Scholar]
- 32.Ding KH, Zhong Q, Xu J and Isales CM. Glucose-dependent insulinotropic peptide: differential effects on hepatic artery vs. portal vein endothelial cells. Am J Physiol Endocrinol Metab. 2004;286:E773–779. [DOI] [PubMed] [Google Scholar]
- 33.Kogire M, Inoue K, Sumi S, Doi R, Takaori K, Yun M, Fujii N, Yajima H and Tobe T. Effects of synthetic human gastric inhibitory polypeptide on splanchnic circulation in dogs. Gastroenterology. 1988;95:1636–1640. [DOI] [PubMed] [Google Scholar]
- 34.Gasbjerg LS, Bari EJ, Stensen S, Hoe B, Lanng AR, Mathiesen DS, Christensen M, Hartmann B, Holst JJ, Rosenkilde MM and Knop FK. The dose-dependent efficacy of the glucose-dependent insulinotropic polypeptide receptor antagonist, GIP(3–30)NH2, on GIP actions in humans. Diabetes Obes Metab. 2020. doi: 10.1111/dom.14186 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.