Abstract
Background
Sub-Saharan Africa (SSA) has the highest age-adjusted burden of hypertension and cardiovascular disease (CVD). SSA also experiences many viral infections due to unique environmental and societal factors. The purpose of this narrative review is to examine evidence around how hypertension, CVD and emerging viral infections interact in SSA.
Methods
In September 2021, we conducted a search in MEDLINE, Embase, and Scopus, limited to English-language studies published since 1990, and found a total of 1169 articles. Forty-seven original studies were included, with 32 on COVID-19 and 15 on other emerging viruses.
Results
Seven articles, including those with the largest sample size and most robust study design, found an association between pre-existing hypertension or CVD and COVID-19 severity or death. Ten smaller studies found no association, and seventeen did not calculate statistics to compare groups. Two studies assessed the impact of COVID-19 on incident CVD, with one finding an increase in stroke admissions. For other emerging viruses, three studies did not find an association between pre-existing hypertension or CVD on West Nile and Lassa fever mortality. Twelve studies examined other emerging viral infections and incident CVD, with four finding no association and eight not calculating statistics.
Conclusions
Growing evidence from COVID-19 suggests viruses, hypertension and CVD interact on multiple levels in SSA, but research gaps remain especially for other emerging viral infections. SSA can and must play a leading role in the study and control of emerging viral infections, with expansion of research and public health infrastructure to address these interactions.
Keywords: virus diseases, hypertension, cardiovascular disease, sub-Saharan Africa, narrative review
INTRODUCTION
Emerging viral infections threaten global health, from the bubonic plague in the 14th century, smallpox in the 16th century, Spanish influenza in the 20th century, to the COVID-19 pandemic.1 Most recent examples of emerging infectious diseases are due to interactions between humans, animals, and their ecosystems, including HIV, SARS, West Nile, Ebola, Zika, and dengue.1–3 It is not surprising, therefore, that many recently emerged viruses originated from Africa, due to unique environmental and social factors such as human-wildlife conflict, poverty, and limited resources for surveillance.2
Simultaneously cardiovascular diseases (CVD) are rapidly rising in sub-Saharan Africa (SSA), resulting in 59 million deaths and 2.8 billion disability adjusted life years in 2019.4 SSA has the highest age-adjusted rates of hypertension, which is the major driver of CVD.4 This epidemiologic transition is linked to rapid urbanization and changing health behaviors toward more sedentary lifestyles.5 HIV infection might also be contributing to this increasing burden of hypertension and CVD in SSA, as we have previously described.6
Research on COVID-19 from high income countries has shown emerging viral infections can have profound bi-directional relationships with hypertension or CVD. Pre-existing hypertension or CVD increases susceptibility for viral infections in one direction, and viral infections can result in long-term consequences for incident hypertension or CVD in the other direction. Pre-existing hypertension, stroke, and coronary artery disease are major predictors of COVID-19 severity and mortality, and COVID-19 can also lead to new CVD events including heart failure, myocardial infarction, and stroke.7 For SSA, though, reviews of interactions between viral infections, hypertension and CVD have focused on HIV.6 The purpose of this narrative review, therefore, is to examine the evidence and knowledge gaps around emerging viral infections and hypertension or CVD in SSA so as to provide a springboard for further research and public health efforts in SSA.
METHODS
Search strategy
A library research specialist constructed a comprehensive search strategy on emerging viral infections and their association with hypertension or CVD in SSA. Using both controlled vocabulary and keywords (Supplemental Materials), the search was conducted in September 2021 across three databases: MEDLINE, Embase, and Scopus. A standard list of emerging viral infections were included in our narrative review with both terms for virus families (e.g., Coronaviridae, Filoviridae) and for virus names (e.g., COVID-19, Ebola).8 HIV was excluded given its interaction with CVD in SSA has been described by other reviews.6 For CVD, terms included variations of hypertension, stroke, heart failure, myocardial infarction, arrhythmias, cardiomyopathies, pericarditis, endocarditis and cardiovascular diseases. The results were limited to English-language studies published from 1990 to present.
Data extraction
After deduplication, a total of 1169 references were reviewed for relevance using titles and abstracts, with 78 selected for full-text review. 31 were excluded because they were reviews (17), case series with <10 participants (5), or did not present data on hypertension, CVD, or an emerging viral infection (9). Articles were assessed for quality using the Joanna Brigg’s Institute critical appraisal checklists for cohort studies, cross-sectional studies, case-control studies, and case series.
Due to the limited number of studies and the heterogeneity of study design, study population, exposure, and outcomes across studies, a narrative review was performed in lieu of a systematic review.
RESULTS
Overall, there were 47 total primary articles included in this review, with 44 original research manuscripts and 3 abstracts. Articles examined the interaction between emerging viral infections and CVD in SSA in two general ways: 1) viral infection as the exposure and CVD as the outcome, or 2) CVD as the exposure and viral infection as the outcome (Table S1). Table S2 summarizes the main finding from each study which provided statistical analysis of the association of emerging viral infections with hypertension and/or CVD. Table S3 summarizes findings from studies which did not provide this statistical analysis. Table S4 details the study characteristics of all included studies.
I. COVID-19
A. Influence of pre-existing hypertension and CVD on COVID-19
There were 32 articles on pre-existing hypertension and/or CVD on COVID-19 outcomes, with 22 examining COVID-19 infection or severity, and 16 examining COVID-19 mortality.
COVID-19 infection and severity
Four large studies found a statistically significant association between pre-existing hypertension, CVD and higher COVID-19 infection rates or severity with median age ranging from 28 to 41 years, and female sex ranging from 30% to 37%.9–12 In a retrospective cohort of 2,617 patients identified through community screening in Ethiopia, 3% had hypertension and/or CVD, and these were associated with a higher risk ratio (RR) for severe COVID-19 (RR 2.53, 95% CI 1.5 – 4.2).9 A cohort study among isolation centers in urban Nigeria found among 2071 patients, 18% had hypertension and 0.7% CVD. Those with hypertension had an adjusted hazard ratio (HR) of 2.41 (95% CI 1.4–4.0) for severe illness, while those with hypertension and additional comorbidities (CVD or diabetes) had an adjusted HR of 3.76 (95% CI 2.1–6.4) for critical illness.10 In a cross-sectional study in treatment centers in Burkina Faso and Guinea (n=1805), 21% had hypertension, with an adjusted odds ratio (OR) of 1.7 (95% CI 1.3–2.2) for clinical worsening.11 Finally, in a cross-sectional study of 279 COVID-19 patients identified through community screening in Ethiopia, 6% had hypertension, which was associated with an adjusted OR 5.26 (95% CI 1.5 – 18.5) of symptomatic COVID-19.
Six studies found no association between pre-existing hypertension, CVD and COVID-19 infection or severity but, compared to the studies above, all six were limited by methodologic issues with hypertension/CVD ascertainment, small sample size, and/or recruitment from a single site.13–18 The median age ranged from 18 to 55 years old, and the samples were 32% to 51% female. Out of 4258 COVID-19 patients found through a cross-sectional community survey in Zambia, neither hypertension (found in 3.8% of COVID-19 patients) nor CVD (found in 0.4%) were associated with COVID-19 infections but the diagnoses of hypertension and CVD were apparently determined by patient report which is notoriously unreliable in Africa where many such cases are undiagnosed.13 In a cohort study of 259 COVID-19 patients in a Cameroonian hospital, 19% had hypertension, and 8% had CVD, but neither was associated with viral severity or death.14 In a Cameroonian study with 177 COVID-19 patients, 27% had hypertension, 4.5% heart failure, and 1% prior stroke, but none of these were associated with COVID-19 neurological symptoms.15 In a cross-sectional study in Burkina Faso with 442 patients, 22% had hypertension, 17% CVD, but neither was associated with severe hypoxemia.16 In a cross-sectional study of 274 patients with long-COVID in Nigeria, 16% had hypertension, but this was not statistically significantly associated with persistent COVID-19 symptoms (aOR 1.89, 95% CI 0.3–10.8)17, similar to findings from a case-control study in Sudan with 100 COVID-19 cases with hypertension and/or CVD and 100 controls.18
Two studies were ecological designs that examined country-level indicators with COVID-19 infection rates.19,20 Among 54 countries, the spearman correlation coefficient between country-level CVD death rate and total COVID-19 cases per million was not significant,19 as was a smaller study, including South Africa.20 Authors of both studies noted that the findings were limited by the poor quality of country level indicators for CVD in SSA.
Ten studies did not calculate statistics to compare those with and without pre-existing hypertension or CVD on COVID-19 outcomes.21–30
COVID-19 mortality
Four studies found pre-existing CVD was associated with higher COVID-19 mortality, including two high quality studies using databases from South Africa to pool together large samples.10,11,31,32
In a national hospital registry across South Africa with 219,265 COVID-19 patients, 39% had hypertension and 23% died.32 Both hypertension and CVD were associated with a higher adjusted odds of COVID-19 mortality (hypertension aOR 1.07, 95% CI 1.0–1.1, CVD aOR 2.2, 95% CI 2.1–2.3) after adjusting for age, race, diabetes, chronic cardiac disease, chronic renal disease, malignancy, TB, HIV, admission month, health sector, and province.32
In a prospective cohort of 22,308 COVID-19 patients attending all public sector health facilities in Western Cape Province, South Africa, 23% of those who did not die had hypertension compared to 58% of those who did die.31 After adjusting for age, sex, and location, hypertension had an increased hazard ratio for death (aHR 1.3 95% CI 1.1–1.6).31
In the other two smaller studies, the cohort study in Nigerian COVID-19 isolation centers also found a higher hazard ratio of death among those with hypertension alone (aHR 2.3, 95% CI 1.2–4.6) or hypertension plus additional comorbidities (aHR 6.63, 95% CI 3.4–12.6).10 Lastly, the study pooling data from Burkina Faso and Guinea found hypertension was associated with death from COVID-19 infection an adjusted odds ratio of 2.1 (aOR 2.1 95% CI 1.2–3.4).11
Three studies did not find an association between pre-existing CVD and COVID-19 mortality but all were limited by weaker study designs, smaller sample sizes, and fewer outcomes.9,14,33 In a retrospective cohort study among 2617 COVID-19 patients identified via community screening in Ethiopia, CVD was not associated with death from COVID-19 but only 0.8% of participants died.9 In a cross-sectional study of 259 COVID-19 patients admitted to a Cameroonian hospital, of which 15% had severe COVID-19 and 2% died, neither hypertension nor CVD was associated with severity or death.14 In a case-control study of 49 COVID-19 death cases and 98 COVID-19 survivors in an Ethiopian hospital, hypertension was associated with higher unadjusted odds of COVID-19 death but this became statistically non-significant after adjustment.33
One ecological study across 211 countries found country level rates of ischemic heart disease and ischemic stroke were significantly correlated with COVID-19 deaths (ischemic heart disease r = 0.28 (p = 0.002) and ischemic stroke r = 0.25 (p = 0.01)).34
Nine further studies reported descriptive statistics on COVID-19 mortality and hypertension or CVD, but did not calculate statistics to formally compare groups.15,24,26,29,35–39
B. Influence of COVID-19 infection on CVD
Two small studies examined the impact of the COVID-19 pandemic on incident CVD and CVD mortality.30,40 In an observational comparison of stroke admissions before (n=401) and after (n=431) the COVID-19 pandemic in a hospital in Ghana, the stroke admission rate was +7.5% higher in the COVID-19 era (95% CI 5.1–10.5%), but the stroke mortality rate was similar (aOR 1.2, 95% CI 0.9–1.7).40 A case series from Nigeria with 10 COVID-19 patients found 6 developed new heart failure, including 1 with heart failure with reduced ejection fraction, 1 fulminant myocarditis, and 1 stress cardiopathy.30
II. Emerging viral infections other than COVID-19
There were surprisingly only 15 studies on other emerging viral infections (virus families arenaviridae, bunyaviridae, filoviridae, flaviviridae, togaviridae, poxviridae, paramyxoviridae, orthomyxoviridae, picornaviridae, herpesviridae).41–55
A. Influence of pre-existing hypertension and CVD on emerging viral infection
There were only three studies examining the association between pre-existing hypertension or CVD and other emerging viral infections, with two on West Nile virus and one on Lassa fever.42,47,51
Viral infection or severity
One study found a history of CVD was not associated with West Nile virus infection. In a cross-sectional hospital study in urban Nigeria, having hypertension was not associated with higher odds of having West Nile but, once again, the diagnosis of hypertension appears to have relied on self-reporting.51 In another cross-sectional hospital study in rural Nigeria, 13 out of 15 patients with West Nile Virus had pre-existing hypertension, but no formal statistical comparison with uninfected participants was performed.47
Viral mortality
One abstract used a cross-sectional analysis of a national database in Nigeria to examine 578 cases of Lassa fever, with 31% with hypertension, but again no formal statistical comparison between groups was performed.42
B. Influence of other emerging viral infection on CVD
Twelve studies described the association between an emerging viral infection and incident CVD,41,43,44,46,48–50,52–56 but only four conducted formal statistical analysis to compare those with and without the viral infection.41,46,50,52 No studies counted endocarditis within their definitions of incident CVD.
Two studies examined the association between CMV and incident CVD, and both found no association. Using a rural cohort of 2174 adults in Uganda, high CMV IgG titers were not associated with incident hypertension but the analysis was complicated by the ubiquity of CMV infection and missing blood pressure data for half of the cohort.41 The second study was a case-control design in urban Malawi among 139 people with HIV, with 48 cases of ischemic stroke and 91 controls.46 High CMV IgG titers were not associated with ischemic stroke (diagnosed by MRI brain scans, NIHSS, and Oxfordshire Community Stroke Project classification) in adjusted analysis (aOR 0.9, 95% CI 0.3–3.1).46
One study found no association between measles infection and incident ECG abnormalities. In a case control study among children in a hospital in Nigeria with 50 cases and 50 controls, there was no statistically significant differences in ECG abnormalities including T wave inversions, T wave flattening, prolonged PR interval, prolonged QTc, or low RS amplitude.52
For varicella zoster virus, one study comparing 31 cases of stroke to 132 controls in rural Uganda found no statistically significant difference in presence or levels of varicella zoster virus IgG or IgM.50
Three studies described the overlap between Ebola, hypertension and CVD, but did not conduct statistical analysis for group comparison.43,49,56 In a cohort of 334 Ebola survivors with neurologic sequelae in Sierra Leone, 0.6% had new strokes confirmed on CT brain in the outpatient clinic since hospital discharge.43 Stroke was also reported as an important complication of Ebola in a community cohort in urban Liberia56, but the exact number of strokes was not presented. Among 166 Ebola survivors in Sierra Leone, 11% had evidence of incident cardiomyopathy, including valvulopathy, reduced ejection fraction, and myocarditis.49
Among a cohort of 311 febrile patients in a Guinean hospital, 64% of the 22 confirmed cases of Lassa fever had arrhythmias, compared to 9% of the 268 without Lassa fever.44
In a small study of 8 cases of endomyocardial fibrosis, 11 siblings, and 16 matched controls: 1 case had chikungunya antibodies, 5 yellow fever, 6 dengue, and 4 West Nile, while in the matched controls, 3 had chikungunya, 9 yellow fever, 10 dengue, and 9 West Nile.54 No statistics were performed to compare cases versus controls.
DISCUSSION
In this narrative review of interactions between emerging viral infections, hypertension and CVD, we found strong evidence that pre-existing CVD and hypertension increased COVID-19 infection severity and mortality in SSA. Weaker evidence suggests that COVID-19 might also increase CVD incidence in SSA. Although the overall heterogeneity between study findings was high, the heterogeneity between the high-quality studies on COVID-19 with largest sample sizes was relatively low. Based on this evidence and other data from around the world, we envision a bi-directional relationship between emerging viral infections, hypertension and CVD in SSA, noting there may be unique environmental, social, genetic, and health systems factors involved (Figure 1). High quality data describing interactions between hypertension, CVD and non-COVID-19 emerging viral infections were conspicuously lacking although there is some data from the 2013–2016 Ebola outbreak in West Africa indicating that important associations might exist. This lack of data represents a missed opportunity for both improving health outcomes in SSA and increasing preparedness for emerging, re-emerging and deliberately emerging infections worldwide. As previously noted, many emerging viral infections originate from SSA indicating both an urgent need and an obvious opportunity to study interactions with the leading causes of global morbidity and mortality – hypertension and CVD - in this context.
Figure 1:
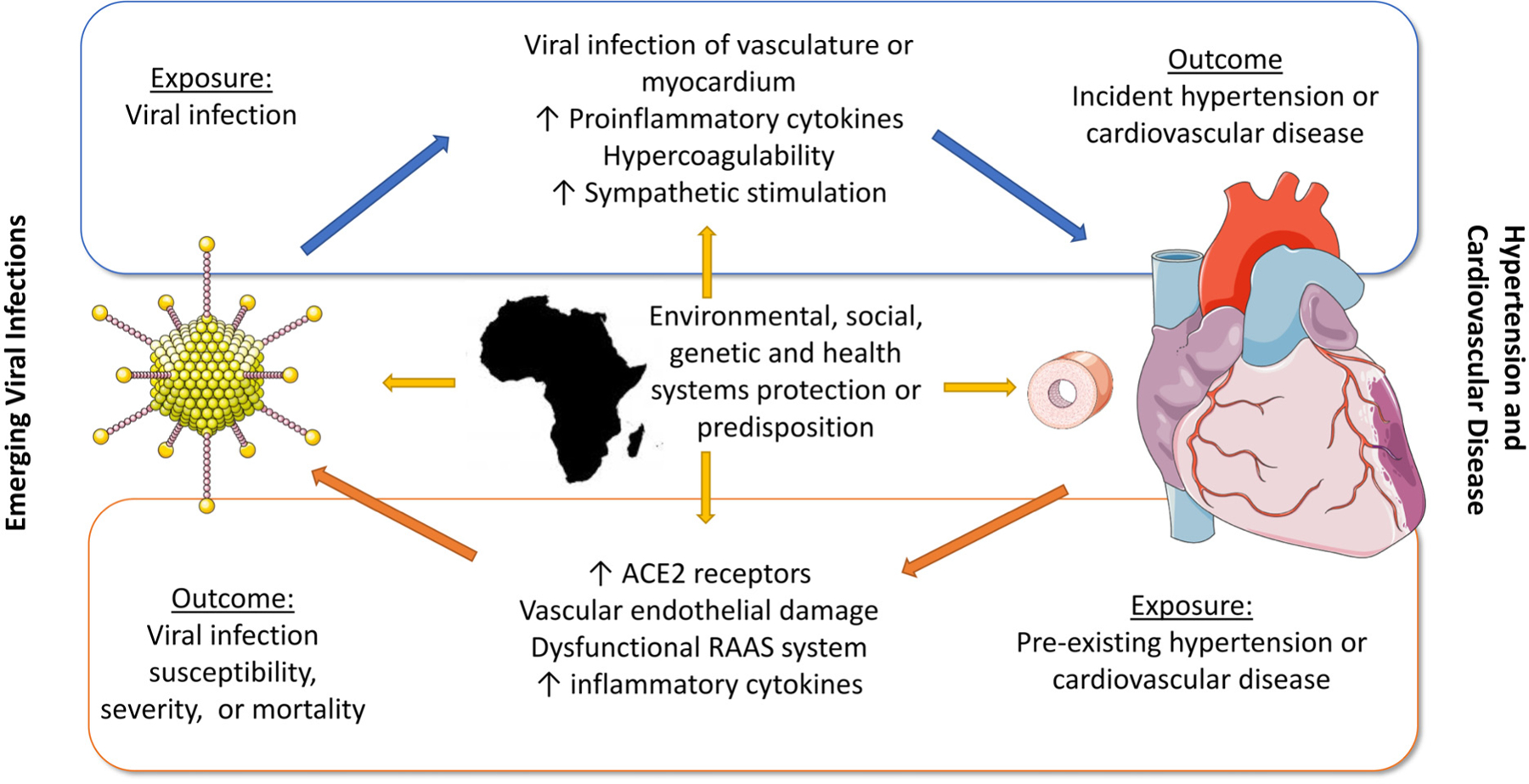
Interactions between emerging viral infections and CVD in sub-Saharan Africa
1). Lessons from existing studies
The largest and highest quality evidence from SSA indicates a strong association between pre-existing hypertension and CVD and COVID-19 severity and death.10,11,31,32 Studies on pre-existing hypertension or CVD on emerging viral infections showed that hypertension and CVD increased the chance of severe COVID-19 (RR 2.5) in studies on the individual level, but no association on the country level in ecological studies, perhaps because average country summary measures are often inaccurate in SSA. The association between hypertension or CVD on COVID-19 mortality was more thoroughly explored, with higher odds and risk ratios for CVD (2.2 to 6.6) than for hypertension (1.1 to 2.3) after multivariable adjustment in South Africa, Nigeria, Burkina Faso, and Guinea.
Unlike with COVID-19, high-quality studies are lacking to assess the association between hypertension and CVD and other emerging viral infections, and only one study had a sample size greater than 1000.41 Notably many studies of emerging viral infections which included indicators for hypertension and/or CVD lacked statistical analyses for these associations. These missing analyses represent missed opportunities, likely driven by a combination of inadequate resources and insufficient awareness of interactions between emerging viral infections with hypertension and CVD.
Recent evidence suggests that emerging viral infections such as COVID-19 and Ebola might contribute to the rising epidemic of CVD in SSA. One study in Ghana showed a rise in stroke admissions during COVID-19, which is similar to the US where the risk among Black Americans for heart disease deaths (RR 1.19, 95% CI 1.17–1.20) and stroke deaths (RR 1.13, 95% CI 1.10–1.17) increased in the COVID-19 era.57 Data from the recent Ebola epidemic in West Africa indicate that stroke and cardiomyopathy might be important long term consequences of Ebola infection.43,49,56 In robust stroke studies from Uganda and Malawi, though, no association was found for either CMV or VZV and stroke.46,50
2). Comparison to studies from other regions
Data on COVID-19 from high income countries are generally similar to the large, high-quality studies in SSA but suggest that the impact of emerging viral infections on hypertension and CVD may vary by region. A Cochrane review on COVID-19 and cardiovascular effects found a prevalence of hypertension at 36% and ischemic heart disease at 11% in hospitalized patients, both of which were associated with increased risk of death.58 Our studies from South Africa integrating medical record data across large geographic regions demonstrated similar results when similar methodologies were used for data collection.58 For CMV and VZV and stroke in SSA, studies included in this review did not find evidence of an association.46,50 In contrast, a meta-analysis of community-based prospective studies all conducted in high income countries showed exposure to CMV was associated with a RR 1.2 of future CVD, including ischemic heart disease, stroke, and CVD death.59 One potential reason may be that these studies in SSA, although well-designed, were under-powered to detect an association as they had <50 cases of stroke. By comparison, the meta-analysis included multiple studies with sample sizes of hundreds of strokes, and a pooled sample size of thousands of CVD events.59 Other possibilities are that the environmental or genetic factors in SSA are different, modifying the relationship between CMV and VZV and stroke, or that the near ubiquity of these viral infections makes the association more difficult to identify.
3). Clinical implications
African clinicians care for patients with emerging viral infections, hypertension and CVD every day but are rarely prompted to think how these conditions might interact. COVID-19 has demonstrated the impact of epidemic emerging viral infections on the care for hypertension and CVD, with major disruptions observed during the COVID-19 pandemic.60 Shortage of biosafety training, lack of personal protective equipment, inadequate laboratory facilities and health information systems all pose increased risk of exposing healthcare workers in SSA.61 Surveillance and hierarchical reporting systems for emerging viral infectious diseases need to be further strengthened in many parts of Africa, and health systems for management of hypertension and CVD should be given priority.61
4). Limitations of existing studies from SSA and gaps in the literature
The main limitations of existing studies from SSA were the low quality in study design and analysis, and small sample size. All studies were observational and most were cross-sectional or case series, with only a few large, longitudinal studies that were powered to detect the differences in outcomes. This made it impossible to add an objective measure of heterogeneity between studies. We did not explore unpublished sources in the grey literature other than published conference abstracts, thus publication bias may have increased the magnitude of observed associations.
In addition, there was a lack of data on social determinants of health—for example, almost no studies accounted for poverty or education. In the US, the higher rate of COVID-19 infections and mortality among Black Americans is thought to be partially due to lower socioeconomic status, leading to reduced healthcare and insurance access.62 In the US, Black Americans have higher rates of uncontrolled hypertension, and had higher rates of COVID-19 infection (1.5 to 3.5 times higher risk), hospitalization (1.5 to 3 times), and death (3.2 times risk), potentially related to higher rates of comorbidities.63 While socioeconomic status has a different meaning and measurement among Africans compared to Black Americans, there are likely many similarities and lessons that can be learned and applied from each community to the other.
One of the most striking findings of our review was that, of the thousands of recent studies of emerging viral infections in SSA, only a few included standardized measures of blood pressure, let alone CVD. Valid blood pressure measurements, reporting of medications, and description of comorbidities such as diabetes should be included in future studies of emerging viral infections in Africa.
There was a lack of translational research in SSA to describe the mechanisms linking emerging viral infections to CVD. We know from high income countries that COVID-19 can lead to direct infection of myocardium, increase in proinflammatory cytokines, hypercoagulability leading to plaque rupture, and increased myocardial oxygen demand from sympathetic stimulation.3,58,64 Pre-existing CVD may increase the risk of viral infection through vascular endothelial damage, a dysfunctional renin angiotensin system, and increased cytokines as well.3,58 Genetic variants common in SSA might impact these pathways including sickle cell disease or genetic variants promoting proinflammatory cytokines.65 Epigenetic alterations due to chronic social and environmental stress may also alter immune responses to viral infections,62 whether systemic racism in the United States or a subsistence lifestyle in rural Tanzania. In fact, we are only beginning to understand the genetic diversity of the African genome, and it’s protective or predisposing role in relation to CVD and viral infections.66 In addition, as vividly demonstrated in the COVID-19 pandemic, different viruses may have tropisms for different tissues in the cardiovascular system 730,40,49,53. These tropisms may differ in degree depending on the underlying population genetics66. Research on tropism of emerging viral infections in African populations are lacking.
5). Future directions
Growing evidence from COVID-19 suggests viruses, hypertension and CVD interact on multiple levels in SSA, but research gaps remain for other emerging viral infections. For global preparedness and local public health, SSA can and must play a leading role in the study of interactions between emerging viral infections and CVD.
Our narrative review advances the field in three ways: 1) by describing a conceptual framework to understand such studies (the bidirectional relationship between viruses and hypertension or CVD), 2) summarizing the current state of science on this topic in SSA, and 3) identifying knowledge gaps that future research projects should address. Research concepts, methodologies, and funding mechanisms developed to study the relationship between COVID-19 and CVD should be expanded to other emerging viral infections. The recent push to integrate hypertension and CVD care and research into HIV programs provides a model for public health efforts. Inclusion of these emerging viral infections in ongoing and new studies of CVD in Africa, as well as including standardized measures of CVD in studies of emerging viral infections, are practical next steps to improving both local health in SSA and global preparedness for the next pandemic.
Supplementary Material
Table S1: Published studies on interaction between emerging viral infection and cardiovascular disease in sub-Saharan Africa (1980 – present)
Table S2: Summary of findings organized by sample size for studies with statistical analysis (n=24)
Table S3: Summary of findings organized by sample size for studies without statistical analysis (n=23)
Table S4: Characteristics of published studies on emerging viral infections and cardiovascular disease in Africa organized by type of study and sample size
Sources of Funding
RNP report grants from National Institute of Mental Health R01MH118107, and National Heart, Lung, Blood, and Sleep Research (NHLBI) R01HL160332. SSM reports support from the Fogarty International Center and National Institute of Neurological Disorders and Stroke D43TW009337. MLM reports a grant from NHLBI R01HL143788, NHLBI D43TW011972, and Fogarty International Center R21TW011693. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Disclosures
RNP report grants from the National Heart, Lung, Blood, and Sleep Research (NHLBI) R01HL160332. SSM reports support from the Fogarty International Center and National Institute of Neurological Disorders and Stroke D43TW009337. MLM reports grants from NHLBI R01HL143788, NHLBI D43TW011972, and Fogarty International Center R21TW011693. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Supplemental Material
Search Terms
References
- 1.Bloom DE, Black S, Rappuoli R. Emerging infectious diseases: A proactive approach. Proceedings of the National Academy of Sciences of the United States of America. 2017;114(16):4055–4059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chauhan RP, Dessie ZG, Noreddin A, El Zowalaty ME. Systematic review of important viral diseases in africa in light of the “one health” concept. Pathogens (Basel, Switzerland). 2020;9(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tay MZ, Poh CM, Rénia L, MacAry PA, Ng LFP. The trinity of COVID-19: immunity, inflammation and intervention. Nature Reviews. Immunology 2020;20(6):363–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Global Burden of Disease. GBD Results Tool | GHDx. 2019.
- 5.Keates AK, Mocumbi AO, Ntsekhe M, Sliwa K, Stewart S. Cardiovascular disease in Africa: epidemiological profile and challenges. Nature Reviews. Cardiology 2017;14(5):273–293. [DOI] [PubMed] [Google Scholar]
- 6.Fahme SA, Bloomfield GS, Peck R. Hypertension in HIV-Infected Adults: Novel Pathophysiologic Mechanisms. Hypertension. 2018;72(1):44–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Madjid M, Safavi-Naeini P, Solomon SD, Vardeny O. Potential effects of coronaviruses on the cardiovascular system: A review. JAMA Cardiology. 2020;5(7):831–840. [DOI] [PubMed] [Google Scholar]
- 8.Graham BS, Sullivan NJ. Emerging viral diseases from a vaccinology perspective: preparing for the next pandemic. Nature Immunology. 2018;19(1):20–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Abraha HE, Gessesse Z, Gebrecherkos T, Kebede Y, Weldegiargis AW, Tequare MH, Welderufael AL, Zenebe D, Gebremariam AG, Dawit TC, Gebremedhin DW, de Wit TR, Wolday D. Clinical features and risk factors associated with morbidity and mortality among patients with COVID-19 in northern Ethiopia. International Journal of Infectious Diseases. 2021;105:776–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abayomi A, Osibogun A, Kanma-Okafor O, Idris J, Bowale A, Wright O, Adebayo B, Balogun M, Ogboye S, Adeseun R, Abdus-Salam I, Mutiu B, Saka B, Lajide D, Yenyi S, et al. Morbidity and mortality outcomes of COVID-19 patients with and without hypertension in Lagos, Nigeria: a retrospective cohort study. Global Health Research and Policy. 2021;6(1):26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jaspard M, Sow MS, Juchet S, Dienderé E, Serra B, Kojan R, Sivahera B, Martin C, Kinda M, Lang H-J, Bangaly Sako F, Amara Traoré F, Koumbem E, Tinto H, Sanou A, et al. Clinical presentation, outcomes and factors associated with mortality: A prospective study from three COVID-19 referral care centres in West Africa. International Journal of Infectious Diseases. 2021;108:45–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abdela SG, Abegaz SH, Demsiss W, Tamirat KS, van Henten S, van Griensven J. Clinical Profile and Treatment of COVID-19 Patients: Experiences from an Ethiopian Treatment Center. The American Journal of Tropical Medicine and Hygiene. 2020. 10.4269/ajtmh.20-1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mulenga LB, Hines JZ, Fwoloshi S, Chirwa L, Siwingwa M, Yingst S, Wolkon A, Barradas DT, Favaloro J, Zulu JE, Banda D, Nikoi KI, Kampamba D, Banda N, Chilopa B, et al. Prevalence of SARS-CoV-2 in six districts in Zambia in July, 2020: a cross-sectional cluster sample survey. The Lancet. Global health 2021;9(6):e773–e781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fouda Mbarga N, Epee E, Mbarga M, Ouamba P, Nanda H, Nkengni A, Guekeme J, Eyong J, Tossoukpe S, Noumedem Sosso S, Ngono Ngono E, Ntsama LM, Bonyomo L, Tchatchoua P, Vogue N, et al. Clinical profile and factors associated with COVID-19 in Yaounde, Cameroon: A prospective cohort study. Plos One. 2021;16(5):e0251504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fogang YF, Noubom M, Bassong P-Y, Mbonda PC, Mfopou IN, Gams DM, Kuate CT, Kamtchum-Tatuene J. Neurological manifestations in patients with symptomatic COVID-19 admitted to the Bafoussam Regional Hospital, Cameroon. The Pan African medical journal. 2021;38:326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Diendéré EA, Sondo KA, Ouédraogo AR, Dahourou DL, Cissé K, Sawadogo A, Maiga S, Kuiré M, Zida S, Kaboré PR, Minoungou CJW, Habou U, Badalo H, Zoungrana N, Ouédraogo AG, et al. Predictors of severe hypoxemia among COVID-19 patients in Burkina Faso (West Africa): Findings from hospital based cross-sectional study. International Journal of Infectious Diseases. 2021;108:289–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Osikomaiya B, Erinoso O, Wright KO, Odusola AO, Thomas B, Adeyemi O, Bowale A, Adejumo O, Falana A, Abdus-Salam I, Ogboye O, Osibogun A, Abayomi A. “Long COVID”: persistent COVID-19 symptoms in survivors managed in Lagos State, Nigeria. BMC Infectious Diseases. 2021;21(1):304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Faroug Mohamed M, Ahmad A, Mohammed Elmahi O, Babker AM, Ali Waggiallah H. Susceptibility of Blood Groups Infection with COVID-19 Disease Among Sudanese Patients Suffering from Different Chronic Diseases. Pakistan journal of biological sciences: PJBS. 2021;24(7):815–820. [DOI] [PubMed] [Google Scholar]
- 19.Amadu I, Ahinkorah BO, Afitiri A-R, Seidu A-A, Ameyaw EK, Hagan JE, Duku E, Aram SA. Assessing sub-regional-specific strengths of healthcare systems associated with COVID-19 prevalence, deaths and recoveries in Africa. Plos One. 2021;16(3):e0247274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhu J, Yan W, Zhu L, Liu J. COVID-19 pandemic in BRICS countries and its association with socio-economic and demographic characteristics, health vulnerability, resources, and policy response. Infectious diseases of poverty. 2021;10(1):97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chaka B, Mekonnen A, Demilew T, Abdo Y, Bisrat F, Haji AA, Deyessa N. Community-Based Assessment of People with Chronic Diseases and Conditions Worsening the Severity of COVID-19 in Addis Ababa City Administration. Ethiopian Journal of Health Development. 2021;35(2):1–8. [Google Scholar]
- 22.Ahmed MLCB, Zehaf S, El Alem MM, Elbara A, Ely Mahmoud MM, Mohamed Abdellahi MV, Heukelbach J. COVID-19 outbreak in Mauritania: epidemiology and health system response. Journal of infection in developing countries. 2021;15(8):1048–1053. [DOI] [PubMed] [Google Scholar]
- 23.Taha SAH, Osman MEM, Abdoelkarim EAA, Holie MAI, Elbasheir MM, Abuzeid NMK, Al-Thobaiti SA, Fadul SB, Konozy EHE. Individuals with a Rh-positive but not Rh-negative blood group are more vulnerable to SARS-CoV-2 infection: demographics and trend study on COVID-19 cases in Sudan. New microbes and new infections. 2020;38:100763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Geteneh A, Alemnew B, Tadesse S, Girma A. Clinical characteristics of patients infected with SARS-CoV-2 in North Wollo Zone, North-East Ethiopia. The Pan African medical journal. 2021;38:217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ashinyo ME, Duti V, Dubik SD, Amegah KE, Kutsoati S, Oduro-Mensah E, Puplampu P, Gyansa-Lutterodt M, Darko DM, Buabeng KO, Ashinyo A, Ofosu AA, Baddoo NA, Akoriyea SK, Ofei F, et al. Clinical characteristics, treatment regimen and duration of hospitalization among COVID-19 patients in Ghana: a retrospective cohort study. The Pan African medical journal. 2020;37(Suppl 1):9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kaswa R, Yogeswaran P, Cawe B. Clinical outcomes of hospitalised COVID-19 patients at Mthatha Regional Hospital, Eastern Cape, South Africa: A retrospective study. South African family practice : official journal of the South African Academy of Family Practice/Primary Care. 2021;63(1):e1–e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Basu JK, Chauke L, Cert Maternal and Fetal Medicine, Magoro T. Clinical Features and Outcomes of COVID-19 Infection among Pregnant Women in South Africa. International journal of MCH and AIDS. 2021;10(2):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jibrin YB, Okwong OK, Maigari IM, Dunga JA, Ballah AM, Umar MS, Mohammed A, Hassan ZI. Clinical and laboratory characteristics of COVID-19 among adult patients admitted to the isolation centre at Abubakar Tafawa Balewa Teaching Hospital Bauchi, Northeast Nigeria. The Pan African medical journal. 2020;37(Suppl 1):27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Abdalrahman Adamali Ayyad F, Alam Eldeen S, Abdallah T, Noureldaim MK, Ahmed Y, Khalid W, Khalid N, Tagelseed A, Elsheikh E, Hamad S, Alkhider L, Ali O, Badi S, Elamien S, Merghani H, et al. Neurological manifestations and outcomes in Sudanese patients diagnosed with COVID-19 in Khartoum state, Sudan. European Journal of Neurology. 2021;28(SUPPL 1):587–588.33058438 [Google Scholar]
- 30.Lasisi GT, Duro-Emanuel AO, Akintomide TE, Ologunja JO, Amah OE. Cardiac manifestation of corona virus disease 2019: a preliminary report. Cardiovascular Journal of Africa. 2021;32:1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Boulle A, Davies M-A, Hussey H, Ismail M, Morden E, Vundle Z, Zweigenthal V, Mahomed H, Paleker M, Pienaar D, Tembo Y, Lawrence C, Isaacs W, Mathema H, Allen D, et al. Risk factors for COVID-19 death in a population cohort study from the Western Cape Province, South Africa. Clinical Infectious Diseases. 2020. DOI: 10.1093/cid/ciaa1198 [DOI] [Google Scholar]
- 32.Jassat W, Cohen C, Tempia S, Masha M, Goldstein S, Kufa T, Murangandi P, Savulescu D, Walaza S, Bam J-L, Davies M-A, Prozesky HW, Naude J, Mnguni AT, Lawrence CA, et al. Risk factors for COVID-19-related in-hospital mortality in a high HIV and tuberculosis prevalence setting in South Africa: a cohort study. The lancet. HIV 2021;8(9):e554–e567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Leulseged TW, Maru EH, Hassen IS, Zewde WC, Chamiso NW, Abebe DS, Jagema TB, Banegyisa AB, Gezahegn MA, Tefera OS, Shiferaw WG, Admasu TT. Predictors of death in severe COVID-19 patients at millennium COVID-19 care center in Ethiopia: a case-control study. The Pan African medical journal. 2021;38:351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Azarpazhooh MR, Morovatdar N, Avan A, Phan TG, Divani AA, Yassi N, Stranges S, Silver B, Biller J, Tokazebani Belasi M, Kazemi Neya S, Khorram B, Frydman A, Nilanont Y, Onorati E, et al. COVID-19 Pandemic and Burden of Non-Communicable Diseases: An Ecological Study on Data of 185 Countries. Journal of Stroke and Cerebrovascular Diseases. 2020;29(9):105089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mekolo D, Bokalli FA, Chi FM, Fonkou SB, Takere MM, Ekukole CM, Balomoth JMB, Nsagha DS, Essomba NE, Njock LR, Ngowe MN. Clinical and epidemiological characteristics and outcomes of patients hospitalized for COVID-19 in Douala, Cameroon. The Pan African medical journal. 2021;38:246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mucheleng’anga LA, Telendiy V, Hamukale A, Shibemba AL, Zumla A, Himwaze CM. COVID-19 and Sudden Unexpected Community Deaths in Lusaka, Zambia, Africa - A Medico-Legal Whole-Body Autopsy Case Series. International Journal of Infectious Diseases. 2021;109:160–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bezuidenhout MC, Wiese OJ, Moodley D, Maasdorp E, Davids MR, Koegelenberg CF, Lalla U, Khine-Wamono AA, Zemlin AE, Allwood BW. Correlating arterial blood gas, acid-base and blood pressure abnormalities with outcomes in COVID-19 intensive care patients. Annals of Clinical Biochemistry. 2021;58(2):95–101. [DOI] [PubMed] [Google Scholar]
- 38.Sultan M, Kene D, Waganew W, Worku A, Azazh A, Girma B, Seman Y, Tessema N, Yifru S, Teklu S, Argaw R, Tefera M, Walelegn M, Redae B. Clinical Characteristics of COVID-19 Related Deaths in Ethiopia. Ethiopian journal of health sciences. 2021;31(2):223–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Himwaze CM, Telendiy V, Maate F, Mupeta S, Chitalu C, Chanda D, Julius P, Mumba C, Marimo C, Hamukale A, Mulenga L, Shibemba AL, Zumla A, Mucheleng’anga LA. Post-mortem examination of Hospital Inpatient COVID-19 Deaths in Lusaka, Zambia - A Descriptive Whole-body Autopsy Series. International Journal of Infectious Diseases. 2021;108:363–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sarfo FS, Mensah NO, Opoku FA, Adusei-Mensah N, Ampofo M, Ovbiagele B. COVID-19 and stroke: Experience in a Ghanaian healthcare system. Journal of the Neurological Sciences. 2020;416:117044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stockdale L, Nash S, Nalwoga A, Painter H, Asiki G, Fletcher H, Newton R. Human cytomegalovirus epidemiology and relationship to tuberculosis and cardiovascular disease risk factors in a rural Ugandan cohort. Plos One. 2018;13(2):e0192086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mba S, Ukponu W, Adekanye U, Saleh M, Agogo E, Dan-Nwafor C, Amao L, Oparah O, Olajide L, Oyegoke A, Mba N, Ilori E, Ihekweazu C. A description of Lassa Fever mortality during the 2019 outbreak in Nigeria. International Journal of Infectious Diseases. 2020;101:409–410.33075527 [Google Scholar]
- 43.Howlett PJ, Walder AR, Lisk DR, Fitzgerald F, Sevalie S, Lado M, N’jai A, Brown CS, Sahr F, Sesay F, Read JM, Steptoe PJ, Beare NAV, Dwivedi R, Solbrig M, et al. Case Series of Severe Neurologic Sequelae of Ebola Virus Disease during Epidemic, Sierra Leone. Emerging Infectious Diseases. 2018;24(8):1412–1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bausch DG, Demby AH, Coulibaly M, Kanu J, Goba A, Bah A, Condé N, Wurtzel HL, Cavallaro KF, Lloyd E, Baldet FB, Cissé SD, Fofona D, Savané IK, Tolno RT, et al. Lassa fever in Guinea: I. Epidemiology of human disease and clinical observations. Vector Borne and Zoonotic Diseases. 2001;1(4):269–281. [DOI] [PubMed] [Google Scholar]
- 45.Billioux J, Ohayon J, Reich DS, Nath A, Cone K, Eckes R, Diaz G, Hensley L, Higgs E, Lane HC, Sneller M, Tarfeh-Burnette H, Lysander J, Tegli J, Dorbor J, et al. Case series of ebola survivors from liberia with neurological sequelae undergoing in-depth neurological evaluation at the national institutes of health bridgette. Annals of Neurology. 2018;84(Supplement 22):S177–S178. [Google Scholar]
- 46.Kamtchum-Tatuene J, Al-Bayati Z, Mwandumba HC, Solomon T, Christmas SE, Benjamin LA. Serum concentration of anti-Cytomegalovirus IgG and ischaemic stroke in patients with advanced HIV infection in Malawi. Plos One. 2018;13(11):e0208040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kolawole OM, Adelaiye G, Ogah JI. Emergence and associated risk factors of vector borne west nile virus infection in ilorin, nigeria. Journal of arthropod-borne diseases. 2018;12(4):341–350. [PMC free article] [PubMed] [Google Scholar]
- 48.Sadoh WE, Ikhurionan P, Imarengiaye C. Pre-anesthetic echocardiographic findings in children undergoing non-cardiac surgery at the University of Benin Teaching Hospital, Nigeria. Cardiovascular Journal of Africa. 2016;27(5):276–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tiffany A, Vetter P, Mattia J, Dayer J-A, Bartsch M, Kasztura M, Sterk E, Tijerino AM, Kaiser L, Ciglenecki I. Ebola virus disease complications as experienced by survivors in sierra leone. Clinical Infectious Diseases. 2016;62(11):1360–1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Asiki G, Stockdale L, Kasamba I, Vudriko T, Tumwekwase G, Johnston T, Kaleebu P, Kamali A, Seeley J, Newton R. Pilot study of antibodies against varicella zoster virus and human immunodeficiency virus in relation to the risk of developing stroke, nested within a rural cohort in Uganda. Tropical Medicine & International Health. 2015;20(10):1306–1310. [DOI] [PubMed] [Google Scholar]
- 51.Ma’aji JA, Olonitola OS, Ella EE. Seroprevalence of West Nile virus (WNV) infection among febrile patients attending selected hospitals in Kaduna state, Nigeria. Scientific African. 2020;10:e00588. [Google Scholar]
- 52.Olowu AO, Taiwo O. Electrocardiographic changes after recovery from measles. Tropical Doctor. 1990;20(3):123–126. [DOI] [PubMed] [Google Scholar]
- 53.Okonko IO, Adebiyi AA, Ogah OS, Adu FD. Enteroviruses as a possible cause of hypertension, dilated cardiomyopathy (DCM) and hypertensive heart failure (HHF) in South western Nigeria. African health sciences. 2013;13(4):1098–1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ijaola O, Falase AO. Distribution of antibodies against Coxsackie B viruses, arboviruses and Toxoplasma gondii among patients with endomyocardial fibrosis (EMF) compared with normal subjects from EMF endemic and non-endemic zones of Nigeria. African journal of medicine and medical sciences. 1990;19(2):93–103. [PubMed] [Google Scholar]
- 55.Aderele WI, Johnson WB, Osinusi K, Gbadero D, Fagbami AH, Babarinde ZO, Okubanjo OA. Respiratory syncytial virus--associated lower respiratory diseases in hospitalised pre-school children in Ibadan. African journal of medicine and medical sciences. 1995;24(1):47–53. [PubMed] [Google Scholar]
- 56.Billioux BJ, Mith B, Bowen L, Schindler M, Azodi S, Ohayon J, Tarfeh-Burnette H, Dorbor J, Reilly C, Sneller M, Fallah M, Nath A. Longitudinal cohort study of neurological sequelae in ebola virus disease survivors in liberia. Journal of the Neurological Sciences. 2017;381:91–92. [Google Scholar]
- 57.Wadhera RK, Figueroa JF, Rodriguez F, Liu M, Tian W, Kazi DS, Song Y, Yeh RW, Joynt Maddox KE. Racial and Ethnic Disparities in Heart and Cerebrovascular Disease Deaths During the COVID-19 Pandemic in the United States. Circulation. 2021;143(24):2346–2354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pellicori P, Doolub G, Wong CM, Lee KS, Mangion K, Ahmad M, Berry C, Squire I, Lambiase PD, Lyon A, McConnachie A, Taylor RS, Cleland JG. COVID-19 and its cardiovascular effects: a systematic review of prevalence studies. Cochrane Database of Systematic Reviews. 2021;3:CD013879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang H, Peng G, Bai J, He B, Huang K, Hu X, Liu D. Cytomegalovirus Infection and Relative Risk of Cardiovascular Disease (Ischemic Heart Disease, Stroke, and Cardiovascular Death): A Meta-Analysis of Prospective Studies Up to 2016. Journal of the American Heart Association. 2017;6(7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schwartz JI, Muddu M, Kimera I, Mbuliro M, Ssennyonjo R, Ssinabulya I, Semitala FC. Impact of a COVID-19 National Lockdown on Integrated Care for Hypertension and HIV. Global heart. 2021;16(1):9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mangu CD, Manyama CK, Msila H, Sudi L, Chaula G, Ntinginya NE, Sabi I, Maboko L. Emerging viral infectious disease threat: Why Tanzania is not in a safe zone. Tanzania journal of health research. 2016;18(3). [Google Scholar]
- 62.Saini G, Swahn MH, Aneja R. Disentangling the coronavirus disease 2019 health disparities in african americans: biological, environmental, and social factors. Open forum infectious diseases. 2021;8(3):ofab064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mackey K, Ayers CK, Kondo KK, Saha S, Advani SM, Young S, Spencer H, Rusek M, Anderson J, Veazie S, Smith M, Kansagara D. Racial and Ethnic Disparities in COVID-19-Related Infections, Hospitalizations, and Deaths : A Systematic Review. Annals of Internal Medicine. 2021;174(3):362–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chakafana G, Mutithu D, Hoevelmann J, Ntusi N, Sliwa K. Interplay of COVID-19 and cardiovascular diseases in Africa: an observational snapshot. Clinical Research in Cardiology. 2020;109(12):1460–1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nédélec Y, Sanz J, Baharian G, Szpiech ZA, Pacis A, Dumaine A, Grenier J-C, Freiman A, Sams AJ, Hebert S, Pagé Sabourin A, Luca F, Blekhman R, Hernandez RD, Pique-Regi R, et al. Genetic ancestry and natural selection drive population differences in immune responses to pathogens. Cell. 2016;167(3):657–669.e21. [DOI] [PubMed] [Google Scholar]
- 66.Choudhury A, Aron S, Botigué LR, Sengupta D, Botha G, Bensellak T, Wells G, Kumuthini J, Shriner D, Fakim YJ, Ghoorah AW, Dareng E, Odia T, Falola O, Adebiyi E, et al. High-depth African genomes inform human migration and health. Nature. 2020;586(7831):741–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1: Published studies on interaction between emerging viral infection and cardiovascular disease in sub-Saharan Africa (1980 – present)
Table S2: Summary of findings organized by sample size for studies with statistical analysis (n=24)
Table S3: Summary of findings organized by sample size for studies without statistical analysis (n=23)
Table S4: Characteristics of published studies on emerging viral infections and cardiovascular disease in Africa organized by type of study and sample size


