Abstract
The coexistence of cardiovascular disease and erectile dysfunction is widespread, possibly owing to underlying endothelial dysfunction in both diseases. Millions of patients with cardiovascular disease are prescribed phosphodiesterase-5 (PDE5) inhibitors for the management of erectile dysfunction. Although the role of PDE5 inhibitors in erectile dysfunction therapy is well established, their effects on the cardiovascular system are unclear. Preclinical studies investigating the effect of PDE5 inhibitors on ischemia-reperfusion injury, pressure overload-induced hypertrophy, and chemotoxicity suggested a possible clinical role for each of these medications; however, attempts to translate these findings to the bedside have resulted in mixed outcomes. In this review, we explore the biologic preclinical effects of PDE5 inhibitors in mediating cardioprotection. We then examine clinical trials investigating PDE5 inhibition in patients with heart failure, coronary artery disease, and ventricular arrhythmias and discuss why the studies have yet to show positive results and efficacy with PDE5 inhibition despite no safety concerns.
Keywords: Cardiovascular disease, Phosphodiesterase 5 inhibitor, Heart failure, Cardioprotection
Introduction
Cardiovascular disease remains the leading cause of death internationally. The World Health Organization (WHO) estimated the number of cardiovascular deaths in 2016 to be approximately 18 million people, and lifetime risk of cardiovascular disease exceeds 60% [1, 2]. In the USA, about 700,000 people die annually of cardiovascular disease [3, 4]. The estimated economic burden of heart disease in the USA is $219 billion per year [4].
Erectile dysfunction (ED) is a widespread, often underreported medical condition. Surveys in the USA have estimated the national prevalence of ED at 30% of men aged 50–59 years, with rising prevalence associated with increasing age [5, 6]. Generally perceived as a vascular complication due to poor perfusion, ED is commonly found coexisting with other medical comorbidities including cardiovascular disease, diabetes, and obesity [7].
The discovery of oral phosphodiesterase-5 (PDE5) inhibitors that revolutionized management of ED in the late 1990s was an incidental observation during cardiac research [8, 9]. Since PDE5 hydrolyzes cyclic guanosine monophosphate (cGMP) in the cardiopulmonary vasculature, researchers aimed to establish a new anti-anginal agent using PDE5 inhibitors to prolong cGMP activity and promote vasodilation of the coronary arteries. However, with early unconvincing results suggesting PDE5 is minimally present in cardiomyocytes, this pursuit was abandoned [9–12]. During these studies, however, patients with ED reported improved erectile function, leading to extensive research culminating with the United States Food and Drug Administration (FDA) ultimately approving PDE5 inhibitors for ED treatment.
PDE5 inhibitors modulate the cardiovascular system through the interplay of cGMP and nitric oxide (NO), a potent vasodilator facilitating smooth muscle relaxation. NO, produced by the vascular endothelium, upregulates intracellular cGMP, triggering a cyclical pathway propagating further NO production [13]. PDE5 degrades cGMP, reversing the vasodilatory effects described. Therefore, modulation of PDE5 plays a crucial role in circulatory regulation and vascular tone.
Due to the common coexistence of cardiovascular disease and ED, the cardiac impact of PDE5 inhibition has since been revisited, and daily PDE5 inhibitor use as a dual-pronged approach as management of ED and cardiovascular disease has been proposed [14]. This literature review examines the background research, preclinical animal studies, and clinical trials of PDE5 inhibitors in patients with cardiovascular disease.
Methods
This is a narrative review of the literature discussing the evidence behind and potential implications of use of PDE5 inhibitors in cardiovascular disease. Data and manuscripts reported here were identified through the United States National Library of Medicine PubMed/MEDLINE database, with keywords including “phosphodiesterase-5 inhibitors,” “cardiovascular,” “ischemia-reperfusion,” “myocardial infarction,” “volume overload,” “heart failure,” “arrythmia,” and “cardioprotection”. Ongoing clinical trials were identified using the United States Clinical Trials website using the search terms “phosphodiesterase-5 inhibitors” and “cardiac” and restricting results to “recruiting” or “active, not recruiting” status.
PDE5 Expression
The general consensus is that cardiomyocytes likely normally express a minimal, basal level of PDE5 [9–12]. PDE5 upregulation has been reported in diseased cardiac tissue such as in the setting of heart failure [15–18]. However, the degree to which PDE5 is upregulated in cardiovascular disease is unclear and likely varies. The limited effect of PDE5 inhibitors in the cardiovascular system may be explained, at least in part, by the basal level of PDE5 in healthy cardiomyocytes compared to the degree of upregulation of PDE5 expression in patients with cardiovascular disease. To some extent, it could be reasonable to assume that the conflicting data from clinical studies were derived from patients with a varying degree of upregulated PDE5 among those with cardiovascular disease.
Pharmacokinetics of PDE5 Inhibitors
The most common PDE5 inhibitors are sildenafil, vardenafil, and tadalafil, each of which presents differences in pharmacokinetics. Sildenafil is categorized as class 1 by the Biopharmaceutical Classification System, suggesting high solubility and high permeability. Sildenafil is rapidly absorbed and reaches peak plasma concentration within 0.5–2.5 h, and it is primarily metabolized by the cytochrome P-450 isoenzyme CYP3A4, with a half-life of approximately 3–5 h [19]. Vardenafil is considered class 2 by the Biopharmaceutical Classification System, suggesting low solubility and high permeability. Vardenafil is rapidly absorbed achieving peak plasma concentration within 0.25–3 h, and it is primarily metabolized by CYP3A4, with a half-life of approximately 4–5 h [19]. Tadalafil is also a class 2 agent by the Biopharmaceutical Classification System, and it is similarly rapidly absorbed reaching peak plasma concentration at an average of 2 h. Tadalafil is primarily metabolized by CYP3A4 as well, but it has a half-life of approximately 17–20 h [19].
Preclinical Studies of PDE5 Inhibition in Cardiovascular Disease
In preclinical studies, cardioprotective effects of PDE5 inhibitors have been identified following ischemia-reperfusion injury, pressure overload-induced hypertrophy, and chemotoxicity. PDE5 inhibition in ischemia-reperfusion injury has improved cardiac function and decreased cardiomyocyte apoptosis and necrosis [20]. In addition, PDE5 is upregulated in cardiac pressure overload, with PDE5 being directly associated with pro-hypertrophic effects [21]. Via cGMP and protein kinase G (PKG) subtype I-alpha, PDE5 inhibition likely mediates an anti-remodeling response to left ventricular pressure overload [22]. Furthermore, doxorubicin-induced chemotoxicity has been significantly reduced by PDE5 inhibition, likely by reducing cardiomyocyte death via upregulation of NO synthase and activation of PKG [23, 24]. Taken together, these biologic effects have been particularly revealing given the general consensus that cardiomyocytes normally express minimal PDE5 [9–12].
Preclinical Studies in Myocardial Infarction
Ischemia-reperfusion injury occurs due to an interval of ischemia inducing downstream reactive oxygen species overproduction. This reduces NO release, leading to an imbalance causing inflammation and apoptosis despite reperfusion [25]. With lower levels of NO available during ischemia, less cGMP is produced, contributing to negative effects on cardiac function and vascular circulation.
PDE5 inhibitors demonstrated a protective, anti-apoptotic effect in isolated cardiomyocytes exposed to ischemia-reperfusion injury [20, 23]. The cardioprotective effects of PDE5 inhibition were mediated, at least in part, by increased NO production and activation of protein kinase C [26, 27]. It is worth noting that different isoforms of protein kinase C appear to have opposing mechanistic roles in cardiac ischemia-reperfusion injury [28]. The interplay of these mechanisms leads to downstream phosphorylation of additional intermediary factors including extracellular signal-regulated kinases (ERK) and glycogen synthase kinase 3 beta before ultimately opening ATP-sensitive potassium (KATP) channels [20, 29]. The critical step of opening mitochondrial KATP channels limits against ischemia-reperfusion injury through regulation of intracellular calcium and ATP and may represent the final step in the mechanism by which PDE5 inhibitors convey cardioprotection.
Early studies in a rat model showed improved ventricular recovery and decreased myocardial infarction following ischemia-reperfusion injury and PDE5 inhibition [30]. In another study, PDE5 inhibition in rabbits showed significantly reduced ventricular infarct size following ischemia-reperfusion injury [29]. These findings were essentially consistent over two time intervals of analysis whereby treatment was administered either acutely before ischemia or 24 h prior, suggesting that PDE5 inhibition could convey a sustained cardioprotective effect against ischemia [29].
The mechanism by which PDE5 inhibitors exhibit cardioprotection remained unclear, with subsequent experiments focusing on whether preconditioning could be a contributing factor. Several pathways have been proposed to explain this cardioprotective concept, with bradykinin among the important factors [31, 32]. Bradykinin increased NO production resulting in cGMP upregulation and opening of mitochondrial KATP channels in a rabbit model [33]. Further studies identified that PDE5 inhibitors reduced ventricular infarct size in an animal model of ischemia-reperfusion, at least in part, through activation of mitochondrial KATP channels [12]. In addition, selective blockade of mitochondrial KATP channels negated the recovery in infarct size observed with PDE5 inhibition, suggesting that activation of mitochondrial KATP channels is crucial to mediating the cardioprotective effects of PDE5 inhibitors [29]. Importantly, opening of mitochondrial KATP channels not only protects mitochondria from calcium overload induced- and oxidant stress-induced injury, but also triggers redox signals that inhibit glycogen synthase kinase (GSK)-3ß-mediated signaling, which inhibits opening of the mitochondrial permeability transition pore [34–36]. In addition, cardioprotection in the context of improved recovery of ventricular contractile function after ischemia-reperfusion is not necessarily limited to infarct size, as attenuation of myocardial stunning is possibly also involved in the post-ischemic reperfusion process [36]; this latter effect may also be at play in the discussion of the effect of PDE5 inhibitors on heart failure.
Taken together, several studies demonstrated reduced myocardial infarction with PDE5 inhibition when given either prior to occlusion or at reperfusion, and various mechanisms were implicated, including mitochondrial KATP channels, NO, and protein kinase C [12, 27, 29, 37]. A pathway independent of NO/cGMP has also been proposed, with one study reporting reduced myocardial infarct size in eNOS- and iNOS-null animals [37].
While most preclinical studies with PDE5 inhibitors demonstrated a reduction in experimental myocardial infarct size, not all studies were positive. In one study in rabbits, sildenafil did not reduce infarct size but did have a modest effect on improving collateral flow during occlusion and reducing specific vascular resistance and reducing left ventricular end diastolic pressure [38]. In a multicenter, randomized, blinded study, sildenafil reportedly failed to reduce myocardial infarct size in experimental models of infarct size, though final publication of results are still pending [39, 40]. Importantly, the protocol of sildenafil administration employed in this study differed from prior investigations, in that bolus injection was given [40] in place of slow infusion over an hour as was previously reported [12]. This alternative approach to sildenafil administration could significantly alter the impact of PDE5 inhibition in a hemodynamically unstable condition in the setting of myocardial infarction. Taken together, the effect of PDE5 inhibitors on reduction of myocardial infarct size has shown promise but is overall somewhat unclear in experimental animal studies.
Preclinical Studies in Heart Failure
PDE5 is generally believed to be present in minimal amounts or even absent in normal cardiomyocytes; however, PDE5 upregulation has been reported in cardiac tissue in heart failure [15–18]. Dysfunction of the cGMP-PKG axis is one of the primary processes implicated in the progression of heart failure [16, 41]. With upregulation of PDE5 in cardiac hypertrophy, there is increased conversion of cGMP to 5′GMP, and therefore decreased PKG [42]. The downstream effects of these changes are ultimately upregulation of cAMP and increased intracellular calcium [17, 20].
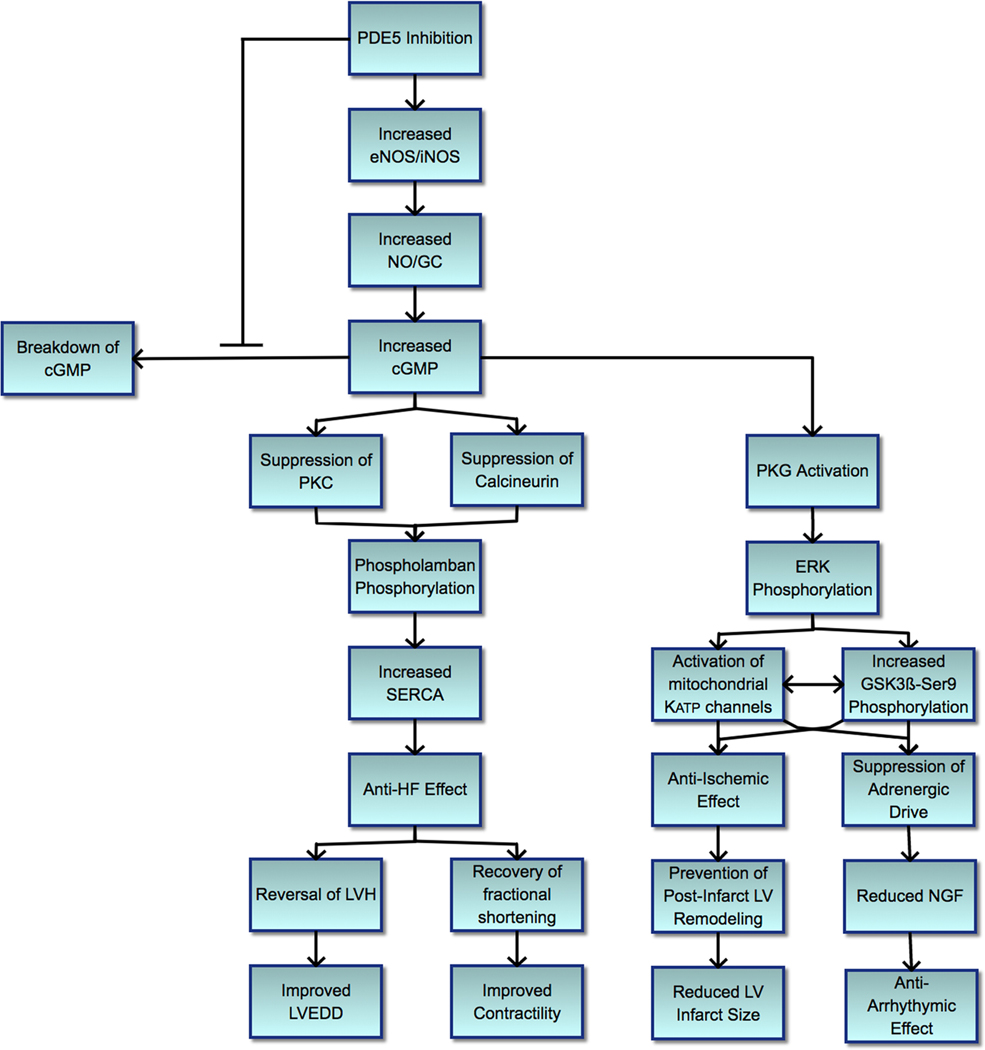
Further research investigating how intracellular calcium imbalance could contribute to heart failure progression suggested these detrimental effects could be a result of increased endoplasmic reticulum stress, and mechanistic studies identified increased sarcoplasmic reticulum calcium ATPase (SERCA) activity to be a mediating factor [43]; and given the direct relationship, phospholamban regulation likely played a role. SERCA improves muscle relaxation by lowering cytosolic calcium while restoring sequestered calcium availability necessary for subsequent muscle contraction [44]. Phospholamban, when dephosphorylated, modulates calcium sequestration by inhibiting SERCA; therefore, phosphorylation of phospholamban leads to increased SERCA activity and improved calcium handling, contributing to improved cardiac contractility [45]. Isolated cardiomyocytes from mice with transverse aortic constriction (TAC)-induced heart failure showed worsening sarcomere shortening and relaxation along with poor intracellular calcium handling, which recovered with PDE5 inhibition [46] (Figure 1).
Figure 1.
Effect of phosphodiesterase-5 inhibitors in cardiovascular disease. Schematic summary of the proposed mechanisms by which phosphodiesterase-5 inhibitors exert their cardioprotective effect. The PKG-mediated suppression of calcineurin, leading to suppression of cardiomyocyte hypertrophy, and PKG-mediated phosphorylation of phospholamban at Ser16, leading to restored SERCA activity, are parallel events. Redox signals from mitochondria with activated mitochondrial Katp channels lead to phosphorylation of GSK-3beta-Ser9, which inhibits opening of mitochondrial permeability transition pores, protecting against necrosis. cGMP, cyclic guanosine monophosphate; eNOS, endothelial nitric oxide synthase; ERK, extracellular signal-regulated kinases; GC, guanylate cyclase; GSK3ß-Ser9, glycogen synthase kinase 3 beta serine 9; HF, heart failure; iNOS, inducible nitric oxide synthase; LV, left ventricle; LVEDD, left ventricular end diastolic diameter; LVH, left ventricular hypertrophy; mitochondrial KATP, mitochondrial ATP-sensitive potassium; NGF, nerve growth factor; NO, nitric oxide; PDE5, phosphodiesterase-5; PKG, protein kinase G; SERCA, sarcoplasmic reticulum calcium ATPase
Mechanistic study of how PDE5 inhibition could improve cardiomyocyte calcium cycling showed that TAC-induced heart failure led to SERCA-2A and phospholamban suppression, which was reversed with sildenafil administration, leading to enhanced phospholamban phosphorylation and thereby improved calcium uptake [46]. In addition, chronic high-pressure exposure to cardiomyocytes increased calcineurin, which inhibits protein phosphatase inhibitor-1 activity ultimately leading to decreased phospholamban phosphorylation causing dysregulation of calcium handling [46–48]. A similar mechanism is at play with upregulation of protein kinase C noted in the TAC animal model, leading to phospholamban dephosphorylation (Nagayama). Administration of PDE5 inhibitor showed improved calcium handling in TAC-induced heart failure via suppression of overexpressed calcineurin and protein kinase C [46]. Taken together, PDE5 inhibitors may impart beneficial effects on cardiomyocytes in pressure-overload settings by regulating intracellular calcium cycling, thereby facilitating improved contractility [43, 46].
Persistent pressure and volume overload in the heart inflict maladaptive processes at the molecular, cellular, and functional levels, which progress toward cardiac dysfunction manifesting as congestive heart failure. Hearts of transgenic mice with cardiomyocyte-specific overexpression of PDE5 exhibited more pronounced left ventricular systolic and diastolic dysfunction, increased hypertrophy, and impaired inotropy compared to wild-type mice [49]. PDE5 inhibition showed suppressed chamber and cellular hypertrophy in the pressure-overloaded mouse model of heart failure and reversed pre-established hypertrophy while restoring cardiac function [41]. In addition, early ischemic cardiomyopathy treated with PDE5 inhibitor showed significant recruitment of eNOS/iNOS and recovery of left ventricular end-diastolic diameter and fractional shortening in mice [50].
Preclinical Studies in Ventricular Arrhythmia
PDE5 inhibition has been suggested to reduce the risk of ventricular arrhythmias, and the precise mechanism remains under investigation [51]. Acute suppression of triggered ventricular arrhythmias with PDE5 inhibition was recently demonstrated in vivo, likely mediated by suppression of cellular calcium waves [52].
Increased adrenergic drive has been associated with several cardiac pathologies including the development of ventricular arrhythmias and sudden cardiac death [53, 54]. Effective use of beta blockade has demonstrated reversal of left ventricular dysfunction as well as reduction of ventricular arrhythmias. Therefore, research was undertaken investigating whether PDE5 inhibition could mediate a direct anti-arrhythmic effect through manipulation of beta-adrenergic receptors. While PDE5 inhibition blunted the enhancement in sarcomere shortening caused by isoproterenol in adult cardiomyocytes, such modulation of sarcomere shortening in cardiomyocytes isolated from genetically engineered mice lacking ß3 adrenergic receptors with PDE5 inhibition was prevented. This suggests that suppression of myocardial beta-adrenergic drive may be a plausible pathway by which PDE5 inhibition exerts its anti-arrhythmic effect [51, 55].
Cardiac ischemic injury leads to increased sympathetic nerve regeneration and density mediated by nerve growth factor (NGF) that has been associated with ventricular arrhythmia and sudden cardiac death [56–58]. PDE5 inhibition has been shown to activate KATP channels, which in turn dampens sympathetic drive and inhibits NGF following myocardial infarction [59, 60].
Furthermore, PDE5 inhibitor-induced mitochondrial KATP channel activation suppressed the over-recruited sympathetic innervation and associated arrhythmias [60]. Animals administered PDE5 inhibitor showed a significant decrease in inducible ventricular tachycardia and ventricular fibrillation [60].
The mechanism by which PDE5 inhibition imparts an anti-arrhythmic effect may be via modulation of beta-adrenergic signaling [61, 62], possibly mediated by NGF given the studies described. In addition, PDE5 inhibition has demonstrated protection against ventricular arrhythmias associated with the early stages of cardiac ischemia [63]. There may be an anti-arrhythmic therapeutic range of PDE5 inhibition, since high-dose PDE5 inhibitor administration increased the incidence of ventricular fibrillation [30].
Clinical Studies of PDE5 Inhibition in Cardiovascular Disease
Studies in Myocardial Infarction
The frequency of coexisting CAD and ED has led to extensive study into the safety of PDE5 inhibitor use in these patients. Initial post-marketing reports identified myocardial infarction and sudden death in patients recently started on PDE5 inhibitors, but direct association between the medication and cardiac adverse effects was not possible [64]. However, myocardial infarctions associated with the use of PDE5 inhibitors were rare and may have been related to the increase in oxygen demand that occurs with sexual activity. An early study evaluated the hemodynamic effects of PDE5 inhibitor use in men with stable angina and at least one known severely occluded coronary artery [65]. Investigators assessed the hemodynamic effects of oral sildenafil in 14 men, finding minimal decrease in systemic arterial and pulmonary arterial pressures, no significant effect on pulmonary-capillary wedge pressure, right atrial pressure, heart rate, or cardiac output. Coronary hemodynamics including peak flow velocity and vascular resistance were unchanged. Taken together, no significant adverse cardiovascular effects were reported in this study [65].
In a Swedish study in men with first myocardial infarction, treatment with PDE5 inhibitors was associated with a lower risk of death and cardiovascular events [66]. Limitations of this study included the control group not receiving any treatment for ED, potentially confounding for indication. This led to a recent subsequent study investigating the association between PDE5 inhibition versus prostaglandin E1 (PGE1) in men with stable CAD [67]. Results from this study showed that in men with stable CAD, treatment with PDE5 inhibitor is associated with lower cardiovascular outcomes including death, myocardial infarction, heart failure, and revascularization, compared to treatment with PGE1 [67]. The study was observational and thus, no inferences of causality could be made but the results confirmed the earlier findings.
The effect of PDE5 inhibition on exercise-induced ischemia was studied in symptomatic patients with stable CAD [68]. Several parameters were evaluated including symptom-limited treadmill exercise time, time to first awareness of angina, and time to ischemic threshold during exercise tolerance testing. The exertional metabolic equivalent (MET) goal in this study was 5–10 METs. At peak exercise, PDE5 inhibition did not demonstrate any significant hemodynamic change in blood pressure or heart rate. Similarly, there was no significant change with PDE5 inhibition when assessing treadmill time or time to first awareness of angina. Patients with PDE5 inhibition did exhibit significantly prolonged time to ischemic threshold by approximately 15% [68]. Taken together, findings from this study suggest that PDE5 inhibitor use in patients with stable, symptomatic CAD, does not limit functional capacity at an exertional level of 5–10 METs. However, in another study, the effect of PDE5 inhibition on exercise tolerance times was neutral in patients with stable coronary artery disease [69].
A research team in Denmark retrospectively investigated the risk of cardiovascular disease in patients who had been prescribed PDE5 inhibitors with the end points including acute myocardial infarction and the development of heart failure [70]. In the first 3 years of PDE5 inhibition, in patients who had no prior cardiovascular disease, there was a decreased risk of acute myocardial infarction. In addition, the study reported a trend toward decreased risk of the development of heart failure in the first 3 years of ED therapy. Overall, there was a decrease in the risk of cardiovascular disease in the first 3 years after initiating treatment for ED [70].
Due to the coexistence of cardiovascular disease and ED, the high frequency of PDE5 inhibitor therapy for ED, and the natural progression of CAD, a subset of patients ultimately require evaluation for coronary artery bypass graft (CABG) surgery for CAD management. The safety of PDE5 inhibitors was investigated in a pilot study of patients undergoing CABG surgery, with results suggesting PDE5 inhibitor use prior to CABG surgery is safe [71]. Given its natural biologic effects as described previously, there is evidence to suggest adjunctive use of PDE5 inhibitors in patients with upcoming CABG surgery could be beneficial [72].
A meta-analysis of randomized, placebo-controlled trials examined whether PDE5 inhibition could indeed impart beneficial cardiac effects [73]. Trials were selected reporting any cardiovascular outcomes, as either primary or secondary endpoints, and independent of the baseline characteristics of the study population. Across 24 trials assessed, nearly 1000 patients were treated with PDE5 inhibitors while approximately 750 were given placebo. Given the criteria for study selection, a significant percentage of these patients had known pulmonary hypertension or congenital heart disease. Several outcomes were evaluated including parameters of cardiac geometry and function as well as overall safety and tolerability of PDE5 inhibitors. The outcomes analyzed included left ventricular mass index, end-diastolic volume index, ventricular transverse diameter, cardiac index, ejection fraction, E/A ratio, and hemodynamics including systemic vascular resistance index. Findings from this meta-analysis suggested that chronic PDE5 inhibitor use imparts a beneficial cardiac inotropic effect together with anti-remodeling properties across different populations [73]. These results favor that PDE5 inhibition could promote positive remodeling and offer potentially promising impact on surrogate endpoints.
Due to the systemic effect of PDE5 inhibition on improving endothelial function, researchers have investigated whether using these medications could improve cardiac risk factors mediated by endothelial dysfunction including diabetes. Since initial proposal of this hypothesis [74], studies with PDE5 inhibitors have led to positive clinical outcomes in patients with cardiac risk factors including diabetes [75]. One trial demonstrated PDE5 inhibition to lower the risk of overall mortality in patients with diabetes and a history of acute myocardial infarction [76]. A non-randomized study reported that PDE5 inhibitors may reduce the occurrence of major adverse cardiac events in patients with coronary artery disease, diabetes, and erectile dysfunction [77]. Taken together, PDE5 inhibition could be cardioprotective by improving outcomes in patients with cardiac risk factors including diabetes, though these studies offered limitations in methodology as well as in assessment of the specifics of PDE5 use in the populations studied.
To the best of our knowledge, PDE5 inhibitors have not been tested in a systematic fashion in clinical trials of acute myocardial infarction. Due to their contraindication in the setting of nitroglycerin use, PDE5 inhibitors are unlikely to ever be tested in humans with acute myocardial infarction.
Studies in Heart Failure
Clinical studies investigating PDE5 inhibition in heart failure have yielded mixed results. Exercise capacity was evaluated in patients with HFrEF using cardiopulmonary exercise testing (CPET), with PDE5 inhibition for 3–6 months showing sustained improvement in exercise ventilation and aerobic efficiency [78]. A trial of patients with HFrEF showed improved functional capacity and left ventricular echocardiographic parameters, including reversal of maladaptive remodeling and left ventricular diastolic function, with PDE5 inhibition [79]. Furthermore, PDE5 inhibition in patients with HFrEF complicated by secondary pulmonary hypertension improved exercise capacity and quality of life, as evidenced by superior peak oxygen uptake (VO2) and 6-minute-walk distance, respectively [80]. However, the utility of PDE5 inhibitors in HFrEF remains unclear due to conflicting reports (Table 1), such as one study reporting no significant functional or quality of life improvement in patients with HFrEF, as measured by 6-minute walk distance and New York Heart Association (NYHA) functional class [81].
Table 1.
Clinical trials investigating the effects of PDE5 inhibitors on cardiac disease
| Author, year [reference no.] | Study design | Number of subjects | Intervention | Targeted disease | Primary endpoint | Overall findings |
|---|---|---|---|---|---|---|
| Guazzi M et al, 2007 [78] | Randomized controlled trial | 46 (23 allocated to sildenafil and 23 to placebo) | Sildenafil 50 mg TID vs placebo | Heart failure with reduced ejection fraction | Assessment of cardiopulmonary exercise testing, echocardiograp hy, and Holter monitoring | Cardiopulmonary exercise testing in patients with HFrEF treated with PDE5 inhibitor for 36 months showed improved exercise ventilation and aerobic efficiency (P<0.01) |
| Guazzi M et al, 2011 [79] | Randomized controlled trial | 45 (23 allocated to sildenafil and 22 to placebo) | Sildenafil 50 mg TID vs placebo | Heart failure with reduced ejection fraction | Assessment of a drug-induced beneficial effect on diastolic function and chamber remodeling | Sildenafil improved echo parameters, exercise capacity, and quality of life in HFrEF patients (P<0.01) |
| Lewis D et al, 2007 [80] | Randomized controlled trial | 34 (17 allocated to sildenafil and 17 to placebo) | Sildenafil 25 mg uptitrated to 75 mg TID vs placebo | Heart failure with reduced ejection fraction complicated by secondary pulmonary hypertension | Assessment of change in peak VO2 from baseline through cardiopulmonary exercise testing | PDE5 inhibition in HFrEF patients complicated by secondary pHTN improved exercise capacity and quality of life (P<0.05) |
| Amin A et al, 2013 [81] | Randomized controlled trial | 106(53 allocated to sildenafil and 53 to placebo) | Sildenafil 25 mg BID to 50 mg TIW vs placebo | Heart failure with reduced ejection fraction | Assessment of change in 6-minute walk distance from baseline | No significant functional or quality of life improvement with PDE5 inhibitor in HFrEF patients (P=0.67) |
| Guazzi M et al, 2011 [82] | Randomized controlled trial | 44 (22 allocated to sildenafil and 22 to placebo) | Sildenafil 50 mg TID vs placebo | Heart failure with preserved ejection fraction | Assessment of pulmonary and left heart hemodynamics | HFpEF treated with PDE5 inhibitor exhibited improved left ventricular structural changes and pulmonary pressures (P<0.01) |
| Redfield MM et al, 2013 [83] | Randomized controlled trial | 216 (113 allocated to sildenafil and 103 to placebo) | Sildenafil 20 mg TID uptitrated to 60 mg TID vs placebo | Heart failure with preserved ejection fraction | Assessment of change in peak oxygen consumption | Chronic PDE5 inhibitor use in HFpEF patients did not improve cardiac functional status (P>0.05) |
| Guay CA et al, 2018 [84] | Meta-analysis | 5448 (over 22 studies) | Pulmonary HTN-directed therapy including PDE5 inhibitor vs placebo | Heart failure with reduced ejection fraction and heart failure with preserved ejection fraction | Assessment of changes in exercise capacity | Pulmonary HTN-directed therapy including PDE5 inhibitor use in HF patients showed improved exercise capacity (P<0.01) |
| Zhuang X et al, 2014 [85] | Meta-analysis | 612 (over 9 studies) | Sildenafil vs placebo | Heart failure with reduced ejection fraction | Assessment of adverse events and peak oxygen consumption (peak VO2) | PDE5 inhibitor use in HFrEF patients improved HF hemodynamic parameters (P<0.01) |
| Thadani U et al, 2002 [68] | Randomized controlled trial | 41 (crossover study) | Vardenafil 10 mg vs placebo | Coronary artery disease | Assessment of effect on total exercise time in patients with exertional angina of moderate severity | PDE5 inhibitor use did not alter functional capacity in stable, symptomatic CAD (P>0.05) |
| Vestergaard N et al, 2017 [70] | Cohort | 71,710 (cohort study) | Erectile dysfunction therapy including PDE5 inhibitor vs general population | Overall risk of cardiovascular disease | Patients were followed until emigration, death, cardiovascular event, or end of follow-up period | Overall risk of cardiovascular disease was decreased in the first 3 years of erectile dysfunction therapy including PDE5 inhibition (P<0.05) |
| Ali A et al, 2013 [71] | Pilot phase II vs retrospective | 57 (10 allocated to vardenafil compared to 47 retrospective ) | Vardenafil 10 mg once prior to CABG vs no vardenafil | Coronary artery disease, pre-surgical candidates | Assessment of drug safety and tolerability (mortality and hypotension) | PDE5 inhibitor use is safe prior to CABG |
| Giannetta E et al, 2014 [73] | Meta-analysis | 1622 (over 24 studies) | Sildenafil, vardenafil, or tadalafil vs placebo | Cardiovascular disease | Studies were selected that reported any cardiovascular outcome as primary or secondary endpoint | Chronic PDE5 inhibitor use improves inotropy and remodeling (P<0.05) |
The effect of PDE5 inhibitors in patients with heart failure with preserved ejection fraction (HFpEF) has been similarly inconclusive. Patients with HFpEF treated with 6 months of PDE5 inhibitor exhibited several beneficial effects including improved left ventricular structural changes and improved pulmonary pressures [82], while another clinical trial also studying patients with HFpEF on 6 months of PDE5 inhibitor did not show significant functional improvement [83]. The disparity in outcomes between these two trials could be at least partly explained by differing therapy regimens.
A meta-analysis investigating the role of PDE5 inhibition in patients with heart failure suggested chronic PDE5 inhibition may modestly improve exercise capacity in patients with HFrEF or HFpEF, though significant heterogeneity was noted in the studies analyzed [84, 85]. The marginal benefit is further tempered because increased mortality with PDE5 inhibitor use could not be ruled out [84].
Studies in Ventricular Arrhythmia
Given the association between increased adrenergic drive and ventricular arrhythmia [53], and the link between PDE5 inhibition and suppression of beta-adrenergic drive in vivo [60–63], studies have investigated whether PDE5 inhibition demonstrates similar anti-adrenergic and thereby anti-arrhythmic effects clinically. PDE5 inhibition showed significantly reduced beta-adrenergic response in healthy volunteers, as determined by multiple echocardiographic and contractility indices including suppressed ejection fraction and peak power [61]. These results suggest that PDE5 inhibition could indeed reduce ventricular arrhythmia in the clinical setting by suppressing adrenergic drive. However, in contrast, there have been reports of patients suffering ventricular arrhythmia after initiating PDE5 inhibitor [86, 87]. Subsequent research did not identify any clinically significant difference in QT duration in healthy patients prescribed PDE5 inhibitors, and there have been conflicting reports on the effect of PDE5 inhibitors on cardiac repolarization [88–91]. Taken together, the potential utility of PDE5 inhibitors in an anti-arrhythmic role remains unclear.
Studies of PDE5 Inhibition in LVAD Patients
Given the known effects of PDE5 inhibitors on pulmonary hypertension and the evolution of the left ventricular assist device (LVAD) as an option for end-stage heart failure management, studies have investigated the safety and impact of PDE5 inhibitors pre- and post-LVAD implantation. Although PDE5 inhibitor use in patients with LVADs is thought to be safe and well-tolerated [92], findings from studies evaluating efficacy of PDE5 inhibition pre- and post-LVAD implantation have been inconclusive. A recent report raised concern that pre-LVAD PDE5 inhibition was associated with increased right-sided heart failure in the post-LVAD setting [93]. Another study investigated the effect of PDE5 inhibitors on right ventricular dysfunction in the post-LVAD implantation and found no significant difference in clinical outcomes [94]. In addition, patients with right ventricular dysfunction and pulmonary hypertension requiring LVAD implantation had improved outcomes with perioperative PDE5 inhibition [95]. A systematic review aiming to identify a specific role of PDE5 inhibition in LVAD patients to attenuate right ventricular failure noted mixed results and weak evidence overall [96].
PDE5 Inhibition in Pulmonary Arterial Hypertension
PDE5 inhibitors are one of the major drug categories to treat pulmonary arterial hypertension, a disease process generally characterized by gradual progression of pulmonary vascular resistance ultimately leading to right heart failure. Due to the beneficial effect on smooth muscle in the context of erectile dysfunction, studies have evaluated whether PDE5 inhibitors could have similar improvements in the pulmonary vasculature. In contrast to the previously discussed cardiovascular disease processes, the success of PDE5 inhibition in pulmonary arterial hypertension has been well established, possibly due to a high basal level of PDE5 in healthy pulmonary tissue that is further upregulated in pulmonary hypertension [97, 98]. A full discussion on PDE5 inhibitors on pulmonary hypertension is beyond the scope of this report, but it is important to note that there is strong evidence clearly demonstrating improved functional parameters and quality of life measures with the use of sildenafil or tadalafil in patients with pulmonary arterial hypertension [99–103].
Conclusions
The coexistence of cardiovascular disease and ED is common likely due to the vascular changes contributing to both disease pathologies. The resultant high frequency of patients with cardiovascular disease being prescribed PDE5 inhibitors for ED has led scientists to identify several mechanisms by which these medications may exert cardioprotective effects, and a number of clinical trials have evaluated the role of PDE5 inhibitors in patients with cardiac disease.
Some but not all studies have demonstrated evidence of cardioprotection with PDE5 inhibitors in preclinical models. Chronic administration of PDE5 inhibitors has shown promising results in reducing adverse cardiac outcomes, especially in those with underlying risk factors such as diabetes. However, these findings have not translated consistently as treatment for patients with congestive heart failure, myocardial infarction, or ventricular arrhythmia. Reports convincingly showing that PDE5 inhibitors have potential as cardiovascular therapy is still infrequent. The reasons underlying the lack of translatability of PDE5 inhibitors from bench to bedside remain unclear, though they may be related to in vitro PDE5 inhibitor dosages being used and relevance of animal models, particularly given the known challenges of translating the ischemia-reperfusion animal model. In addition, variable usage of different PDE5 inhibitors and differences in their respective pharmacokinetics could be contributing to conflicting findings. Furthermore, the limited effect of PDE5 inhibitors in the cardiovascular system may be explained, at least in part, that the conflicting data from clinical studies were derived from patients with a varying degree of upregulated PDE5 among those with cardiovascular disease.
Limitations of data interpretation include the observational and retrospective nature of some reports, incomplete information related to medication adherence in some cases, and increased surveillance of blood pressure after initiation of PDE5 inhibitors. Caution is needed in data interpretation because if ED is considered a risk factor for vascular disease, it is difficult to explain an improved outcome with reduction in fatal and non-fatal ischemic events when these patients are treated with PDE5 inhibitors as compared to patients without ED, and there are no randomized trials available to clarify the distinction.
The resultant unclear role of PDE5 inhibition in clinical cardiac pathologies has contributed to the lack of indications for prescribing PDE5 inhibitors in the treatment of cardiovascular disease. Importantly, the safety and tolerability of PDE5 inhibitors in patients with cardiovascular disease have been well established [104], and this review did not identify significant risks to using PDE5 inhibitors as adjunctive therapy in heart failure, coronary disease and myocardial infarction, or ventricular arrhythmia, with the exception of concurrent nitrate use.
Current clinical trials incorporating PDE5 inhibitors are focused on right ventricular dysfunction in patients with LVADs, congenital heart disease, or cystic fibrosis; no studies are investigating the potential utility of PDE5 inhibitors in myocardial infarction, heart failure, or arrhythmia (Table 2). Further trials are warranted to better understand the role of PDE5 inhibitors in patients with cardiovascular disease. Carefully designed dose-dependent and time-course studies to optimize clinical PDE5 inhibition could pave the path toward large-scale, randomized-controlled clinical trials exploring the efficacy of PDE5 inhibitors on cardiac outcomes in coronary artery disease, heart failure, and ventricular arrhythmia Results from such investigations could help reconcile some of the discrepancies in the literature on the role of PDE5 inhibitors in cardiovascular disease.
Table 2.
Current ongoing clinical trials investigating the effects of PDE5 inhibitors on cardiac disease
| Study objective (NCT number) | Study design | Number of subjects | Intervention | Primary outcome(s) |
|---|---|---|---|---|
| Determine whether sildenafil can prevent right heart failure after LVAD placement (NCT03356353) | Open label, single arm | 24 | Sildenafil 40 mg TID | Change in pulmonary vascular resistance (PVR) |
| Determine right ventricular function in LVAD patients before and after discontinuation of phosphodiesterase-5 inhibitor (NCT04117659) | Open label, single arm | 30 | Pre-treatment with PDE5 inhibitor with subsequent discontinuation | Change in right ventricular global longitudinal strain (GLS) |
| Determine whether pre-treatment with sildenafil could significantly impact breath-hold and SCUBA diving-induced pulmonary hypertension in patients with PFO or IPAVA (NCT03945643) | Randomized controlled trial | 80 (40 allocated to sildenafil and 40 to placebo) | Sildenafil 50 mg once, 1 h prior to measurements vs placebo | Change in pulmonary arterial pressure by ultrasound, as well as several cytokine blood tests |
| Determine the safety profile of udenafil in adolescents with single-ventricle congenital heart disease after Fontan palliation (NCT03013751) | Open label, single arm | 300 | Udenafil for 52 weeks | Occurrence of adverse events |
| Determine whether PDE5 inhibition improves right ventricular size and function in adults with congenital subaortic right ventricular positioning (NCT03049540) | Randomized controlled trial | 100 (50 allocated to tadalafil and 50 to placebo) | Tadalafil 20 mg daily for 3 years vs placebo | Change in right ventricle end systolic volume |
| Determine whether PDE5 inhibition improves exercise tolerance in patients with cystic fibrosis (NCT04039087) | Randomized controlled trial | 40 (20 allocated to sildenafil and 20 to placebo) | Sildenafil 40 mg TID vs placebo | Change in 6-minute walk distance |
Acknowledgements
The authors express their gratitude to Jason Miller for his assistance with the figures.
Footnotes
Data Availability
N/A [review article].
Code Availability
N/A.
Declarations
Ethics approval and consent to participate
N/A.
Consent for Publication
N/A.
Competing Interests
RAK is a paid consultant for Sanofi. Dr. Fadi Salloum is funded by the National Institutes of Health (R35 HL155651).
Publisher's Disclaimer: This AM is a PDF file of the manuscript accepted for publication after peer review, when applicable, but does not reflect post-acceptance improvements, or any corrections. Use of this AM is subject to the publisher’s embargo period and AM terms of use. Under no circumstances may this AM be shared or distributed under a Creative Commons or other form of open access license, nor may it be reformatted or enhanced, whether by the Author or third parties. See here for Springer Nature’s terms of use for AM versions of subscription articles: https://www.springernature.com/gp/open-research/policies/accepted-manuscript-terms
References
- 1.Garcia R. and Burkle J, New and future parenteral therapies for the management of lipid disorders. Arch Med Res, 2018. 49(8): p. 538–547. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization. Cardiovascular diseases. 2021. May 17, 2017 [accessed 2021 May 31, 2021]; Available from: https://www.who.int/en/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds).
- 3.Writing Group M, et al. , Heart disease and stroke statistics-2016 update: a report from the American Heart Association. Circulation, 2016. 133(4): p. e38–360. [DOI] [PubMed] [Google Scholar]
- 4.Fryar CD, Chen TC, and Li X, Prevalence of uncontrolled risk factors for cardiovascular disease: United States, 1999–2010. NCHS Data Brief, 2012(103): p. 1–8. [PubMed] [Google Scholar]
- 5.Foster SA, et al. , Erectile dysfunction with or without coexisting benign prostatic hyperplasia in the general US population: analysis of US National Health and Wellness Survey. Curr Med Res Opin, 2013. 29(12): p. 1709–17. [DOI] [PubMed] [Google Scholar]
- 6.Shaeer O. and Shaeer K, The Global Online Sexuality Survey (GOSS): the United States of America in 2011. Chapter I: erectile dysfunction among English-speakers. J Sex Med, 2012. 9(12): p. 3018–27. [DOI] [PubMed] [Google Scholar]
- 7.Goldstein I, et al. , The serendipitous story of sildenafil: an unexpected oral therapy for erectile dysfunction. Sex Med Rev, 2019. 7(1): p. 115–128. [DOI] [PubMed] [Google Scholar]
- 8.Tzoumas N, et al. , Established and emerging therapeutic uses of PDE type 5 inhibitors in cardiovascular disease. Br J Pharmacol, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wallis RM, et al. , Tissue distribution of phosphodiesterase families and the effects of sildenafil on tissue cyclic nucleotides, platelet function, and the contractile responses of trabeculae carneae and aortic rings in vitro. Am J Cardiol, 1999. 83(5A): p. 3C–12C. [DOI] [PubMed] [Google Scholar]
- 10.Corbin J, et al. , Sildenafil citrate does not affect cardiac contractility in human or dog heart. Curr Med Res Opin, 2003. 19(8): p. 747–52. [DOI] [PubMed] [Google Scholar]
- 11.Reffelmann T. and Kloner RA, Therapeutic potential of phosphodiesterase 5 inhibition for cardiovascular disease. Circulation, 2003. 108(2): p. 239–44. [DOI] [PubMed] [Google Scholar]
- 12.Salloum FN, et al. , Sildenafil and vardenafil but not nitroglycerin limit myocardial infarction through opening of mitochondrial K(ATP) channels when administered at reperfusion following ischemia in rabbits. J Mol Cell Cardiol, 2007. 42(2): p. 453–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Inoue T, et al. , cGMP upregulates nitric oxide synthase expression in vascular smooth muscle cells. Hypertension, 1995. 25(4 Pt 2): p. 711–4. [DOI] [PubMed] [Google Scholar]
- 14.Cai Z, Zhang J, and Li H, Two birds with one stone: regular use of PDE5 inhibitors for treating male patients with erectile dysfunction and cardiovascular diseases. Cardiovasc Drugs Ther, 2019. 33(1): p. 119–128. [DOI] [PubMed] [Google Scholar]
- 15.Loughney K, et al. , Isolation and characterization of cDNAs encoding PDE5A, a human cGMP-binding, cGMP-specific 3’,5’-cyclic nucleotide phosphodiesterase. Gene, 1998. 216(1): p. 139–47. [DOI] [PubMed] [Google Scholar]
- 16.Lu Z, et al. , Oxidative stress regulates left ventricular PDE5 expression in the failing heart. Circulation, 2010. 121(13): p. 1474–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nagendran J, et al. , Phosphodiesterase type 5 is highly expressed in the hypertrophied human right ventricle, and acute inhibition of phosphodiesterase type 5 improves contractility. Circulation, 2007. 116(3): p. 238–48. [DOI] [PubMed] [Google Scholar]
- 18.Zhang M. and Kass DA, Phosphodiesterases and cardiac cGMP: evolving roles and controversies. Trends Pharmacol Sci, 2011. 32(6): p. 360–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gupta M, Kovar A, and Meibohm B, The clinical pharmacokinetics of phosphodiesterase-5 inhibitors for erectile dysfunction. J Clin Pharmacol, 2005. 45(9): p. 987–1003. [DOI] [PubMed] [Google Scholar]
- 20.Kukreja RC, Salloum FN, and Das A, Cyclic guanosine monophosphate signaling and phosphodiesterase-5 inhibitors in cardioprotection. J Am Coll Cardiol, 2012. 59(22): p. 1921–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nagayama T, et al. , Pressure-overload magnitude-dependence of the anti-hypertrophic efficacy of PDE5A inhibition. J Mol Cell Cardiol, 2009. 46(4): p. 560–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Blanton RM, et al. , Protein kinase g ialpha inhibits pressure overload-induced cardiac remodeling and is required for the cardioprotective effect of sildenafil in vivo. J Am Heart Assoc, 2012. 1(5): p. e003731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Das A, Xi L, and Kukreja RC, Phosphodiesterase-5 inhibitor sildenafil preconditions adult cardiac myocytes against necrosis and apoptosis. Essential role of nitric oxide signaling. J Biol Chem, 2005. 280(13): p. 12944–55. [DOI] [PubMed] [Google Scholar]
- 24.Fisher PW, et al. , Phosphodiesterase-5 inhibition with sildenafil attenuates cardiomyocyte apoptosis and left ventricular dysfunction in a chronic model of doxorubicin cardiotoxicity. Circulation, 2005. 111(13): p. 1601–10. [DOI] [PubMed] [Google Scholar]
- 25.Carden DL and Granger DN, Pathophysiology of ischaemia-reperfusion injury. J Pathol, 2000. 190(3): p. 255–66. [DOI] [PubMed] [Google Scholar]
- 26.Das A, et al. , Protein kinase C plays an essential role in sildenafil-induced cardioprotection in rabbits. Am J Physiol Heart Circ Physiol, 2004. 286(4): p. H1455–60. [DOI] [PubMed] [Google Scholar]
- 27.Salloum F, et al. , Sildenafil induces delayed preconditioning through inducible nitric oxide synthase-dependent pathway in mouse heart. Circ Res, 2003. 92(6): p. 595–7. [DOI] [PubMed] [Google Scholar]
- 28.Chen L, Shi D, and Guo M, The roles of PKC-delta and PKC-epsilon in myocardial ischemia/reperfusion injury. Pharmacol Res, 2021. 170: p. 105716. [DOI] [PubMed] [Google Scholar]
- 29.Ockaili R, et al. , Sildenafil (Viagra) induces powerful cardioprotective effect via opening of mitochondrial K(ATP) channels in rabbits. Am J Physiol Heart Circ Physiol, 2002. 283(3): p. H1263–9. [DOI] [PubMed] [Google Scholar]
- 30.Das S, et al. , Cardioprotection with sildenafil, a selective inhibitor of cyclic 3’,5’monophosphate-specific phosphodiesterase 5. Drugs Exp Clin Res, 2002. 28(6): p. 213–9. [PubMed] [Google Scholar]
- 31.Yellon DM and Downey JM, Preconditioning the myocardium: from cellular physiology to clinical cardiology. Physiol Rev, 2003. 83(4): p. 1113–51. [DOI] [PubMed] [Google Scholar]
- 32.Kukreja RC, et al. , Pharmacological preconditioning with sildenafil: basic mechanisms and clinical implications. Vascul Pharmacol, 2005. 42(5–6): p. 219–32. [DOI] [PubMed] [Google Scholar]
- 33.Oldenburg O, et al. , Bradykinin induces mitochondrial ROS generation via NO, cGMP, PKG, and mitoKATP channel opening and leads to cardioprotection. Am J Physiol Heart Circ Physiol, 2004. 286(1): p. H468–76. [DOI] [PubMed] [Google Scholar]
- 34.Jankowski M, Broderick TL, and Gutkowska J, The role of oxytocin in cardiovascular protection. Front Psychol, 2020. 11: p. 2139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Paggio A, et al. , Identification of an ATP-sensitive potassium channel in mitochondria. Nature, 2019. 572(7771): p. 609–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lukowski R, et al. , cGMP and mitochondrial K(+) channels-compartmentalized but closely connected in cardioprotection. Br J Pharmacol, 2021. [DOI] [PubMed] [Google Scholar]
- 37.Elrod JW, Greer JJ, and Lefer DJ, Sildenafil-mediated acute cardioprotection is independent of the NO/cGMP pathway. Am J Physiol Heart Circ Physiol, 2007. 292(1): p. H342–7. [DOI] [PubMed] [Google Scholar]
- 38.Reffelmann T. and Kloner RA, Effects of sildenafil on myocardial infarct size, microvascular function, and acute ischemic left ventricular dilation. Cardiovasc Res, 2003. 59(2): p. 441–9. [DOI] [PubMed] [Google Scholar]
- 39.Jones SP, et al. , The NHLBI-sponsored Consortium for preclinicAl assESsment of cARdioprotective therapies (CAESAR): a new paradigm for rigorous, accurate, and reproducible evaluation of putative infarct-sparing interventions in mice, rabbits, and pigs. Circ Res, 2015. 116(4): p. 572–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kukreja RC, et al. , Administration of sildenafil at reperfusion fails to reduce infarct size: results from the CAESAR cardioprotection consortium (LB650). The FASEB Journal, 2014. 28: p. LB650. [Google Scholar]
- 41.Takimoto E, et al. , Chronic inhibition of cyclic GMP phosphodiesterase 5A prevents and reverses cardiac hypertrophy. Nat Med, 2005. 11(2): p. 214–22. [DOI] [PubMed] [Google Scholar]
- 42.Salloum FN, et al. , Phosphodiesterase-5 inhibitor, tadalafil, protects against myocardial ischemia/reperfusion through protein-kinase g-dependent generation of hydrogen sulfide. Circulation, 2009. 120(11 Suppl): p. S31–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gong W, et al. , Chronic inhibition of cGMP-specific phosphodiesterase 5 suppresses endoplasmic reticulum stress in heart failure. Br J Pharmacol, 2013. 170(7): p. 1396–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lipskaia L, et al. , Sarcoplasmic reticulum Ca(2+) ATPase as a therapeutic target for heart failure. Expert Opin Biol Ther, 2010. 10(1): p. 29–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Frank K. and Kranias EG, Phospholamban and cardiac contractility. Ann Med, 2000. 32(8): p. 572–8. [DOI] [PubMed] [Google Scholar]
- 46.Nagayama T, et al. , Sildenafil stops progressive chamber, cellular, and molecular remodeling and improves calcium handling and function in hearts with pre-existing advanced hypertrophy caused by pressure overload. J Am Coll Cardiol, 2009. 53(2): p. 207–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.El-Armouche A, et al. , Role of calcineurin and protein phosphatase-2A in the regulation of phosphatase inhibitor-1 in cardiac myocytes. Biochem Biophys Res Commun, 2006. 346(3): p. 700–6. [DOI] [PubMed] [Google Scholar]
- 48.MacDonnell SM, et al. , Calcineurin inhibition normalizes beta-adrenergic responsiveness in the spontaneously hypertensive rat. Am J Physiol Heart Circ Physiol, 2007. 293(5): p. H3122–9. [DOI] [PubMed] [Google Scholar]
- 49.Pokreisz P, et al. , Ventricular phosphodiesterase-5 expression is increased in patients with advanced heart failure and contributes to adverse ventricular remodeling after myocardial infarction in mice. Circulation, 2009. 119(3): p. 408–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Salloum FN, et al. , Sildenafil (Viagra) attenuates ischemic cardiomyopathy and improves left ventricular function in mice. Am J Physiol Heart Circ Physiol, 2008. 294(3): p. H1398–406. [DOI] [PubMed] [Google Scholar]
- 51.Hutchings DC, et al. , Phosphodiesterase-5 inhibitors and the heart: compound cardioprotection? Heart, 2018. 104(15): p. 1244–1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hutchings DC, et al. , PDE5 inhibition suppresses ventricular arrhythmias by reducing SR Ca(2+) content. Circ Res, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cao JM, et al. , Relationship between regional cardiac hyperinnervation and ventricular arrhythmia. Circulation, 2000. 101(16): p. 1960–9. [DOI] [PubMed] [Google Scholar]
- 54.Lymperopoulos A, Rengo G, and Koch WJ, Adrenergic nervous system in heart failure: pathophysiology and therapy. Circ Res, 2013. 113(6): p. 739–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lee DI, et al. , PDE5A suppression of acute beta-adrenergic activation requires modulation of myocyte beta-3 signaling coupled to PKG-mediated troponin I phosphorylation. Basic Res Cardiol, 2010. 105(3): p. 337–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cao JM, et al. , Nerve sprouting and sudden cardiac death. Circ Res, 2000. 86(7): p. 816–21. [DOI] [PubMed] [Google Scholar]
- 57.Kreusser MM, et al. , Differential expression of cardiac neurotrophic factors and sympathetic nerve ending abnormalities within the failing heart. J Mol Cell Cardiol, 2008. 44(2): p. 380–7. [DOI] [PubMed] [Google Scholar]
- 58.Shelton DL and Reichardt LF, Expression of the beta-nerve growth factor gene correlates with the density of sympathetic innervation in effector organs. Proc Natl Acad Sci U S A, 1984. 81(24): p. 7951–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kang CS, et al. , Effect of ATP-sensitive potassium channel agonists on sympathetic hyperinnervation in postinfarcted rat hearts. Am J Physiol Heart Circ Physiol, 2009. 296(6): p. H1949–59. [DOI] [PubMed] [Google Scholar]
- 60.Lee TM, et al. , Effect of sildenafil on ventricular arrhythmias in post-infarcted rat hearts. Eur J Pharmacol, 2012. 690(1–3): p. 124–32. [DOI] [PubMed] [Google Scholar]
- 61.Borlaug BA, et al. , Sildenafil inhibits beta-adrenergic-stimulated cardiac contractility in humans. Circulation, 2005. 112(17): p. 2642–9. [DOI] [PubMed] [Google Scholar]
- 62.Senzaki H, et al. , Cardiac phosphodiesterase 5 (cGMP-specific) modulates betaadrenergic signaling in vivo and is down-regulated in heart failure. FASEB J, 2001. 15(10): p. 1718–26. [DOI] [PubMed] [Google Scholar]
- 63.Nagy O, et al. , Sildenafil (Viagra) reduces arrhythmia severity during ischaemia 24 h after oral administration in dogs. Br J Pharmacol, 2004. 141(4): p. 549–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Feenstra J, et al. , Acute myocardial infarction associated with sildenafil. Lancet, 1998. 352(9132): p. 957–8. [DOI] [PubMed] [Google Scholar]
- 65.Herrmann HC, et al. , Hemodynamic effects of sildenafil in men with severe coronary artery disease. N Engl J Med, 2000. 342(22): p. 1622–6. [DOI] [PubMed] [Google Scholar]
- 66.Andersson DP, et al. , Association between treatment for erectile dysfunction and death or cardiovascular outcomes after myocardial infarction. Heart, 2017. 103(16): p. 1264–1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Andersson DP, et al. , Association of phosphodiesterase-5 inhibitors versus alprostadil with survival in men with coronary artery disease. J Am Coll Cardiol, 2021. 77(12): p. 1535–1550. [DOI] [PubMed] [Google Scholar]
- 68.Thadani U, et al. , The effect of vardenafil, a potent and highly selective phosphodiesterase-5 inhibitor for the treatment of erectile dysfunction, on the cardiovascular response to exercise in patients with coronary artery disease. J Am Coll Cardiol, 2002. 40(11): p. 2006–12. [DOI] [PubMed] [Google Scholar]
- 69.Patterson D, et al. , The effect of tadalafil on the time to exercise-induced myocardial ischaemia in subjects with coronary artery disease. Br J Clin Pharmacol, 2005. 60(5): p. 459–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Vestergaard N, et al. , Relationship between treatment of erectile dysfunction and future risk of cardiovascular disease: a nationwide cohort study. Eur J Prev Cardiol, 2017. 24(14): p. 1498–1505. [DOI] [PubMed] [Google Scholar]
- 71.Ali A, et al. , The safety of preoperative vardenafil in patients undergoing coronary artery bypass graft surgery. J Cardiovasc Pharmacol, 2013. 62(1): p. 106–9. [DOI] [PubMed] [Google Scholar]
- 72.Fung E, et al. , The potential use of type-5 phosphodiesterase inhibitors in coronary artery bypass graft surgery. Chest, 2005. 128(4): p. 3065–73. [DOI] [PubMed] [Google Scholar]
- 73.Giannetta E, et al. , Is chronic inhibition of phosphodiesterase type 5 cardioprotective and safe? A meta-analysis of randomized controlled trials. BMC Med, 2014. 12: p. 185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rosano GM, et al. , Chronic treatment with tadalafil improves endothelial function in men with increased cardiovascular risk. Eur Urol, 2005. 47(2): p. 214–20; discussion 220–2. [DOI] [PubMed] [Google Scholar]
- 75.Hackett G, et al. , Statin, testosterone and phosphodiesterase 5-inhibitor treatments and age related mortality in diabetes. World J Diabetes, 2017. 8(3): p. 104–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Anderson SG, et al. , Phosphodiesterase type-5 inhibitor use in type 2 diabetes is associated with a reduction in all-cause mortality. Heart, 2016. 102(21): p. 1750–1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gazzaruso C, et al. , Erectile dysfunction as a predictor of cardiovascular events and death in diabetic patients with angiographically proven asymptomatic coronary artery disease: a potential protective role for statins and 5-phosphodiesterase inhibitors. J Am Coll Cardiol, 2008. 51(21): p. 2040–4. [DOI] [PubMed] [Google Scholar]
- 78.Guazzi M, et al. , Long-term use of sildenafil in the therapeutic management of heart failure. J Am Coll Cardiol, 2007. 50(22): p. 2136–44. [DOI] [PubMed] [Google Scholar]
- 79.Guazzi M, et al. , PDE5 inhibition with sildenafil improves left ventricular diastolic function, cardiac geometry, and clinical status in patients with stable systolic heart failure: results of a 1-year, prospective, randomized, placebo-controlled study. Circ Heart Fail, 2011. 4(1): p. 8–17. [DOI] [PubMed] [Google Scholar]
- 80.Lewis GD, et al. , Sildenafil improves exercise capacity and quality of life in patients with systolic heart failure and secondary pulmonary hypertension. Circulation, 2007. 116(14): p. 1555–62. [DOI] [PubMed] [Google Scholar]
- 81.Amin A, et al. , Is chronic sildenafil therapy safe and clinically beneficial in patients with systolic heart failure? Congest Heart Fail, 2013. 19(2): p. 99–103. [DOI] [PubMed] [Google Scholar]
- 82.Guazzi M, et al. , Pulmonary hypertension in heart failure with preserved ejection fraction: a target of phosphodiesterase-5 inhibition in a 1-year study. Circulation, 2011. 124(2): p. 164–74. [DOI] [PubMed] [Google Scholar]
- 83.Redfield MM, et al. , Effect of phosphodiesterase-5 inhibition on exercise capacity and clinical status in heart failure with preserved ejection fraction: a randomized clinical trial. JAMA, 2013. 309(12): p. 1268–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Guay CA, et al. , Pulmonary hypertension-targeted therapies in heart failure: a systematic review and meta-analysis. PLoS One, 2018. 13(10): p. e0204610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zhuang XD, et al. , PDE5 inhibitor sildenafil in the treatment of heart failure: a meta-analysis of randomized controlled trials. Int J Cardiol, 2014. 172(3): p. 581–7. [DOI] [PubMed] [Google Scholar]
- 86.Shah PK, Sildenafil in the treatment of erectile dysfunction. N Engl J Med, 1998. 339(10): p. 699; author reply 701–2. [DOI] [PubMed] [Google Scholar]
- 87.Rasmussen JG, Toft E, and Frobert O, Ventricular tachycardia after administration of sildenafil citrate: a case report. J Med Case Rep, 2007. 1: p. 65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Alpaslan M, et al. , Sildenafil citrate does not affect QT intervals and QT dispersion: an important observation for drug safety. Ann Noninvasive Electrocardiol, 2003. 8(1): p. 14–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Beasley CM Jr., et al. , The combined use of ibutilide as an active control with intensive electrocardiographic sampling and signal averaging as a sensitive method to assess the effects of tadalafil on the human QT interval. J Am Coll Cardiol, 2005. 46(4): p. 678–87. [DOI] [PubMed] [Google Scholar]
- 90.Morganroth J, et al. , Evaluation of vardenafil and sildenafil on cardiac repolarization. Am J Cardiol, 2004. 93(11): p. 1378–83, A6. [DOI] [PubMed] [Google Scholar]
- 91.Varma A, Shah KB, and Hess ML, Phosphodiesterase inhibitors, congestive heart failure, and sudden death: time for re-evaluation. Congest Heart Fail, 2012. 18(4): p. 229–33. [DOI] [PubMed] [Google Scholar]
- 92.Ravichandran AK, et al. , Sildenafil in left ventricular assist device is safe and well-tolerated. ASAIO J, 2018. 64(2): p. 280–281. [DOI] [PubMed] [Google Scholar]
- 93.Gulati G, et al. , Preimplant phosphodiesterase-5 inhibitor use is asssociated with higher rates of severe early right heart failure after left ventricular assist device implantation. Circ Heart Fail, 2019. 12(6): p. e005537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Roberts KL, et al. , Evaluation of clinical outcomes with phosphodiesterase-5 inhibitor therapy for right ventricular dysfunction after left ventricular assist device implantation. ASAIO J, 2019. 65(3): p. 264–269. [DOI] [PubMed] [Google Scholar]
- 95.Hamdan R, et al. , Prevention of right heart failure after left ventricular assist device implantation by phosphodiesterase 5 inhibitor. Artif Organs, 2014. 38(11): p. 963–7. [DOI] [PubMed] [Google Scholar]
- 96.Baker WL, Radojevic J, and Gluck JA, Systematic review of phosphodiesterase-5 inhibitor use in right ventricular failure following left ventricular assist device implantation. Artif Organs, 2016. 40(2): p. 123–8. [DOI] [PubMed] [Google Scholar]
- 97.Corbin JD, et al. , High lung PDE5: a strong basis for treating pulmonary hypertension with PDE5 inhibitors. Biochem Biophys Res Commun, 2005. 334(3): p. 930–8. [DOI] [PubMed] [Google Scholar]
- 98.Montani D, et al. , Phosphodiesterase type 5 inhibitors in pulmonary arterial hypertension. Adv Ther, 2009. 26(9): p. 813–25. [DOI] [PubMed] [Google Scholar]
- 99.Rubin LJ, et al. , Long-term treatment with sildenafil citrate in pulmonary arterial hypertension: the SUPER-2 study. Chest, 2011. 140(5): p. 1274–1283. [DOI] [PubMed] [Google Scholar]
- 100.Pepke-Zaba J, et al. , Sildenafil improves health-related quality of life in patients with pulmonary arterial hypertension. Chest, 2008. 133(1): p. 183–9. [DOI] [PubMed] [Google Scholar]
- 101.Oudiz RJ, et al. , Tadalafil for the treatment of pulmonary arterial hypertension: a double-blind 52-week uncontrolled extension study. J Am Coll Cardiol, 2012. 60(8): p. 768–74. [DOI] [PubMed] [Google Scholar]
- 102.Pepke-Zaba J, et al. , Tadalafil therapy and health-related quality of life in pulmonary arterial hypertension. Curr Med Res Opin, 2009. 25(10): p. 2479–85. [DOI] [PubMed] [Google Scholar]
- 103.Galie N, et al. , Tadalafil therapy for pulmonary arterial hypertension. Circulation, 2009. 119(22): p. 2894–903. [DOI] [PubMed] [Google Scholar]
- 104.Kloner RA, et al. , Cardiovascular safety of phosphodiesterase type 5 inhibitors after nearly 2 decades on the market. Sex Med Rev, 2018. 6(4): p. 583–594. [DOI] [PubMed] [Google Scholar]