Abstract
Background
Immune checkpoint inhibitors (ICI) are widely used cancer treatments. There are limited data the risk for developing venous thromboembolism (VTE) among patients on an ICI.
Methods
This was a retrospective study of 2854 patients who received ICIs at a single academic center. VTE events, defined as a composite of deep vein thrombosis (DVT) or pulmonary embolism (PE), were identified by individual chart review and were blindly adjudicated using standard imaging criteria. A self-controlled risk-interval design was applied with an “at-risk period” defined as the two-year period after, and the “control period”, defined as the two-year prior to treatment. The hazard ratio (HR) was calculated using a fixed-effect proportional hazards model.
Results
Of the 2854 patients, 1640 (57.5%) were men; mean age was 64±13 years. The risk for VTE was 7.4% at 6 months and 13.8% at 1 year after starting an ICI. The rate of VTE was >4-fold higher after starting an ICI (HR 4.98, 95% CI 3.65 – 8.59, p<0.001). There was a 5.7-fold higher risk for DVT (HR 5.70, 95% CI 3.79 – 8.59, p<0.001) and 4.75-fold higher risk for PE (HR 4.75, 95% CI 3.20 – 7.10, p<0.001). Comparing patients with and without a VTE event, a history of melanoma and older age predicted a lower risk of VTE, while a higher Khorana risk score, history of hypertension, and history of VTE predicted a higher risk.
Conclusions
The rate of VTE among patients on an ICI is high and increases after starting an ICI.
Keywords: Immune Checkpoint Inhibitors, Venous Thromboembolism, Cardio-oncology, Anticoagulation, Cancer, Vascular Medicine
INTRODUCTION
Immune Checkpoint Inhibitors (ICI) are monoclonal antibodies that target specific negative immunological regulators and, as a result, leverage the immune response to treat malignancy1. The use of an ICI was first approved by the Food and Drug Administration for treatment of melanoma in 20112. Since then, the indications for use of ICI have been rapidly increasing and, as of January 2020, ICIs were indicated in treatment of 17 malignancies with over 100 ongoing trials evaluating its efficacy for cancer treatment3, 4. Our knowledge of some of the potential adverse effects of ICI therapy, specifically some of the cardiovascular effects is limited5–10. There are limited data on the risk for venous thromboembolism (VTE) among patients on an ICI. This knowledge gap is important as malignancy and immune activation and inflammation with ICI are both established risk factors for VTE11, 12. In addition, ICIs have been linked to other vascular diseases such as atherosclerosis13. A study of 672 patients treated with ICI observed 47 VTE events with cumulative incidence of 12.9% and another single-center study of 228 patients with melanoma treated with an ICI identified VTE events in 16.2% of study subjects14, 15. However, uncertainties remain as many studies have not reported a control comparison and other studies evaluating the risk of acute vascular events with ICI use have not shown an increase16, 17. Therefore, we aimed to determine whether the risk for VTE after ICI use, whether this risk is increased after starting an ICI, and the risk factors for VTE after ICI use.
METHODS
Study Design, Setting and Population
This was a single center, retrospective cohort study. The database was built upon a protocol approved by institutional review boards at Partners Healthcare (now Mass General Brigham), detailing electronic medical records access in all patients who have received immune checkpoint inhibitors at Massachusetts General Hospital (Boston, MA, USA) until March 2019. Given the limitations in obtaining comparable contemporary controls (patients with similar cancer who were not treated with ICIs), we performed a self-controlled risk-interval design18. The use of an ICI was defined through a pharmacy database. Clinical characteristics and outcome data were extracted from the Research Patient Data Registry (RPDR).
Covariates
Demographics, medical history, medication usage were obtained through RPDR. Anticoagulation history was confirmed through review of electronic medical records. Oncologic data were collected, including date of ICI initiation, the specific type of ICI, cancer diagnosis prior to treatment, prior cardiotoxic cancer treatment, radiation therapy history, corticosteroid use, occurrence of any irAEs. History of cardiovascular events and Khorana score were defined as prior studies13, 19. Specifically, history of cardiovascular events is defined by defined as a composite of myocardial infarction, coronary revascularization, and ischemic stroke. Khorana risk score is a composite score involving five variables. Cancer types associated higher risk of VTE are given two points (stomach, pancreas) or one point (lung, lymphoma, gynecologic, bladder, testicular). Pre-treatment platelet counts ≥ 350 ×109 /L, hemoglobin <10 g/dL, pre-treatment leukocyte count >11 ×109 /L and BMI ≥35 kg/m2 each is assigned one point.
Outcome Measures: Clinical Study
The primary study end-point was the occurrence of VTE event, defined as a composite of deep venous thrombosis or pulmonary embolism using standardized radiographic findings (supplemental document 1). The secondary study end-point was each of the individual components of the primary end-point. These events were searched through RPDR, confirmed through review of electronic medical records and were blindly adjudicated by members of the study team.
Statistical Analysis
Descriptive statistics were used to assess the distribution of variables. Categorical demographic variables were described as counts and percentage. Continuous variables were summarized with mean and standard deviation or median and interquartile ranges. We defined period of ICI exposure (risk period) as two years after initiation of ICI treatment and control period as two years prior to ICI initiation (Supplemental Figure 1). A fixed-effect Cox proportional hazards regression model was used to estimate the hazard ratio (HR) for VTE event during risk period compared to control period20. To address competing risk, cause-specific hazard ratios were used21. Individuals were right-censored if they did not experience a VTE event by the end of follow-up or were lost to follow-up. Univariate and multivariate Cox regression was used to establish association between baseline risk factors and VTE risk. Risk factors that were statistically significant in the univariate model were included in a multivariate analysis. In addition, effect modifier analyses were performed by stratifying the study subjects based on risk factors; interactions between potential effect modifiers were interrogated. Sensitivity analyses were performed by excluding those who have died or lost to follow-up prior to the end of study. Analyses were performed using SAS version 9.4 (SAS Institute, Cary, North Carolina). p-values of < 0.05 were considered statistically significant and all testing was two-sided.
RESULTS
Baseline demographics and cardiovascular risk
Of the 2854 patients, 1640 (57.5%) were men; mean age was 64±13 years old with 2479 (91.7%) patients being Caucasian (Table 1). Many had established cardiovascular risk factors including hypertension (n=1356, 49.2%) and diabetes mellitus (n= 433, 15.7%). A prior VTE was noted in 12.5%; 9.6% of all patients, independent of VTE history, were on anticoagulation at the time of ICI initiation. Most patients had a Khorana score of 0 and 1 (36.5%, 35.5%, respectively).
Table 1.
Baseline Characteristics at the Start of the Immune Checkpoint Inhibitors
| All patients | Patients with VTE events within 2 years from ICI start | Patients with no VTE events within 2 years from ICI start | P value | ||||
|---|---|---|---|---|---|---|---|
| Demographics | |||||||
| Number of patients | 2854 | 244 | 2610 | ||||
| Male | 1640 (57.5%) | 130 (53.3%) | 1510(57.9%) | 0.18 | |||
| Age (years) | 64±13 | 63±12 | 65±13 | 0.047 | |||
| Data available (n) | Number (%) | Data available (n) | Number (%) | Data available (n) | Number (%) | ||
| Race | |||||||
| White | 2704 | 2479 (91.7%) | 241 | 216 (89.6%) | 2463 | 2263 (91.9%) | 0.12 |
| Asian | 96 (3.6%) | 11 (4.6%) | 85 (3.5%) | ||||
| Black or African American | 57 (2.1%) | 10 (4.2%) | 47 (1.9%) | ||||
| Hispanic | 29 (0.9%) | 1 (0.4%) | 24 (1.0%) | ||||
| Other | 43 (1.6%) | 3 (1.2%) | 44 (1.6%) | ||||
| Baseline Risk Factors | |||||||
| Hypertension | 2756 | 1356 (49.2%) | 244 | 118 (48.4%) | 2512 | 1238 (48.9%) | 0.78 |
| Diabetes mellitus | 2756 | 433 (15.7%) | 244 | 39 (16.0%) | 2512 | 394 (15.7%) | 0.90 |
| Smoking current or prior | 2756 | 429 (15.6%) | 244 | 27 (15.2%) | 2512 | 392 (15.6%) | 0.92 |
| Chronic kidney disease | 2756 | 327 (11.9%) | 244 | 23 (9.4%) | 2512 | 304 (12.1%) | 0.21 |
| History of myocardial infarction | 2854 | 136 (4.5%) | 244 | 11 (4.5%) | 2598 | 125 (4.8%) | 1.00 |
| History of any coronary revascularization | 2854 | 195 (6.9%) | 244 | 7 (2.9%) | 2598 | 188 (7.2%) | 0.008 |
| History of ischemic stroke | 2854 | 82 (2.9%) | 244 | 6 (2.5%) | 2598 | 76 (2.9%) | 0.84 |
| History of any cardiovascular event | 2854 | 322 (11.3%) | 244 | 20 (8.2%) | 2598 | 302 (11.6%) | 0.11 |
| History of venous thromboembolism | 2854 | 358 (12.5%) | 244 | 45 (18.4%) | 2610 | 313 (12.0%) | 0.004 |
| Khorana Risk score | |||||||
| 0 | 2136 | 779 (36.5%) | 195 | 51 (26.2%) | 1941 | 728 (37.5%) | 0.0014 |
| 1 | 759 (35.5%) | 78(40.0%) | 681 (35.1%) | ||||
| 2 | 428 (20.0%) | 41(21.0%) | 387 (19.9%) | ||||
| 3 | 135 (6.3%) | 19(9.7%) | 116(6.0%) | ||||
| 4 | 33 (1.5%) | 5(2.6%) | 28 (1.4%) | ||||
| 5 | 2 (0.09%) | 1(0.5%) | 1 (0.05%) | ||||
| Baseline Cardiovascular Medications | |||||||
| Angiotensin Converting Enzyme Inhibitor or Angiotensin II Receptor Blocker | 2704 | 612 (22.6%) | 239 | 52 (21.8%) | 2465 | 560 (22.7%) | 0.73 |
| Statins | 2704 | 705 (26.1%) | 243 | 89 (36.6%) | 2515 | 772 (30.1%) | 0.057 |
| Aspirin | 2704 | 578 (21.4%) | 239 | 61 (25.5%) | 2465 | 517 (20.1%) | 0.10 |
| Other anti-platelet | 2704 | 66 (2.4%) | 239 | 3 (1.3%) | 2486 | 63 (2.6%) | 0.21 |
| Any anticoagulation | 2730 | 262 (9.6%) | 244 | 27 (11.1%) | 2486 | 235 (9.5%) | 0.42 |
| Low Molecular Weight Heparin (treatment dosing) | 2730 | 139 (5.1%) | 244 | 21 (8.6%) | 2486 | 116 (4.8%) | 0.014 |
| Direct Oral Anticoagulant | 2730 | 60 (2.2%) | 244 | 4 (1.6%) | 2486 | 56 (2.3%) | 0.82 |
| Warfarin | 2730 | 96 (3.5%) | 244 | 6 (2.5%) | 2486 | 90 (3.6%) | 0.46 |
| Cancer Types | |||||||
| Non-small cell lung | 2754 | 781 (28.4%) | 244 | 82 (33.6%) | 2511 | 699 (27.8%) | 0.056 |
| Melanoma | 778 (28.2%) | 49 (20.1%) | 729 (29.0%) | 0.003 | |||
| Head and neck | 334 (12.1%) | 27 (11.1%) | 307 (12.2%) | 0.59 | |||
| Renal and genitourinary | 174 (6.3%) | 23 (9.4%) | 151 (6.0%) | 0.036 | |||
| Breast | 119 (4.3%) | 11 (4.5%) | 108 (4.3%) | 0.88 | |||
| Gastrointestinal | 109 (3.9%) | 12 (4.9%) | 97 (3.9%) | 0.42 | |||
| Gynecologic | 107 (3.9%) | 11 (4.5%) | 96 (3.8%) | 0.60 | |||
| Non-Hodgkin Lymphoma | 76 (2.7%) | 6 (2.5%) | 70 (2.8%) | 0.76 | |||
| Hepatocellular | 58 (2.1%) | 4 (1.6%) | 54 (2.2%) | 0.60 | |||
| Cholangiocarcinoma | 39 (1.4%) | 2 (0.8%) | 37 (1.5%) | 0.41 | |||
| Pancreatic | 37 (1.3%) | 5 (2.1%) | 32 (1.3%) | 0.32 | |||
| Other | 142 (5.2%) | 12 (4.9%) | 130 (5.2%) | 0.86 | |||
| Prior Cancer Therapies | |||||||
| Radiation therapy | 2756 | 572 (20.8%) | 244 | 51 (20.9%) | 2512 | 521 (20.7%) | 0.93 |
| Anthracyclines | 2723 | 151 (5.5%) | 242 | 15 (6.2%) | 2481 | 136 (5.5%) | 0.64 |
| Tyrosine kinase inhibitors | 2723 | 61 (2.2%) | 242 | 7 (2.9%) | 2481 | 54 (2.2%) | 0.47 |
| 5- Fluorouracil | 2723 | 284 (10.4%) | 242 | 22 (9.1%) | 2481 | 262 (9.6%) | 0.58 |
| Platinum-based chemotherapy | 2723 | 1022 (37.5%) | 242 | 94 (38.8%) | 2481 | 928 (37.4%) | 0.68 |
| Janus kinase Inhibitors | 2723 | 1 (0.04%) | 242 | 0 | 2481 | 1 (0.04%) | 1.00 |
| Tumor necrosis factor inhibitors | 2723 | 11 (0.4%) | 242 | 0 | 2481 | 11 (0.4%) | 0.62 |
| Mitogen-activated protein kinase kinase inhibitor | 2723 | 44 (1.6%) | 242 | 2 (0.84%) | 2481 | 42 (1.7%) | 0.43 |
| Anaplastic lymphoma kinase inhibitor | 2723 | 11 (0.4%) | 242 | 1 (0.41%) | 2481 | 10 (0.40%) | 1.00 |
| Immune Checkpoint Inhibitor Type | |||||||
| Single Therapy | |||||||
| Programmed death-ligand-1 | 2854 | 283 (9.9%) | 244 | 30 (12.3%) | 2610 | 253 (9.7%) | 0.21 |
| Cytotoxic-T-Lymphocyte associated protein 4 | 2854 | 225 (7.9%) | 244 | 14 (5.7%) | 2610 | 211 (8.1%) | 0.22 |
| Programmed death-protein 1 | 2854 | 2147 (75.2%) | 244 | 180 (73.8%) | 2610 | 1968 (75.4%) | 0.58 |
| Cytotoxic-T-Lymphocyte associated protein 4 or programmed death protein 1 | 2854 | 2(0.1%) | 244 | 0 (0%) | 2610 | 2 (0.08%) | 1.00 |
| Combination Therapy | |||||||
| Cytotoxic-T-Lymphocyte associated protein 4/Programmed death protein 1 | 2854 | 197 (6.9%) | 244 | 20 (8.2%) | 2610 | 176 (6.7%) | 0.36 |
| Corticosteroid treatment at start of ICI | 2342 | 417 (17.8%) | 208 | 41 (19.7%) | 2134 | 376 (17.6%) | 0.45 |
| Time from cancer diagnosis to ICI initiation – days (IQR) | 2745 | 652 (195–1972) | 243 | 550 (170–1870) | 2502 | 665 (199–1975) | 0.15 |
| Immune Related Adverse Events (irAE) with ICI Use | |||||||
| Gastrointestinal | 2748 | 500 (18.2%) | 243 | 36 (14.8%) | 2505 | 464 (18.5%) | 0.15 |
| Skin | 2748 | 429 (15.6%) | 243 | 33 (13.6%) | 2505 | 396 (15.8%) | 0.36 |
| Pulmonary | 2748 | 189 (6.9%) | 243 | 26 (10.7%) | 2505 | 163 (6.5%) | 0.014 |
| Hepatic | 2748 | 179 (6.5%) | 243 | 14 (5.8%) | 2505 | 165 (6.6%) | 0.62 |
| Endocrine | 2748 | 175 (6.4%) | 243 | 21 (8.6%) | 2505 | 154 (6.2%) | 0.13 |
| Renal | 2748 | 120 (4.4%) | 243 | 8 (3.3%) | 2505 | 112 (4.5%) | 0.39 |
| Neuromuscular | 2815 | 98 (3.8%) | 241 | 8 (3.3%) | 2574 | 90 (3.5%) | 0.88 |
| Pancreas | 2748 | 61 (2.2%) | 243 | 2 (0.8%) | 2505 | 59 (2.4%) | 0.12 |
| Any of the above adverse events | 2748 | 1179 (42.9%) | 243 | 103 (42.4%) | 2505 | 1076 (43.0%) | 0.86 |
| Corticosteroids use for irAE | 2748 | 734 (26.7%) | 243 | 69 (28.4%) | 2505 | 665 (26.6%) | 0.53 |
| Baseline Clinical Parameters and Lab Values | |||||||
| Data available (n) | Mean±SD | Data available (n) | Mean±SD | Data available (n) | Mean±SD | ||
| Body mass index - (kg/m2) | 2271 | 27.0±6.4 | 206 | 27.0±5.6 | 2065 | 27.0±6.5 | 0.81 |
| Systolic blood pressure (mmHg) | 2297 | 127.6±18.6 | 207 | 126.6±17.0 | 2090 | 127.7±18.7 | 0.40 |
| Hemoglobin (g/dL) | 2599 | 11.9±2.0 | 231 | 11.7± 1.9 | 2368 | 11.9±2.0 | 0.11 |
| Platelets (thousands/uL) | 2598 | 253.8± 113.0 | 230 | 284.1±130.7 | 2368 | 251.0±110.8 | <0.001 |
| High density lipoprotein (mg/dL) | 810 | 50.0±20.0 | 74 | 51.6±20.6 | 736 | 49.8±20.0 | 0.44 |
| Low density lipoprotein (mg/dL) | 785 | 100.0±36.9 | 73 | 107.5±38.4 | 712 | 99.2±36.6 | 0.067 |
| Glomerular filtration rate (mL/min/1.73m2) | 1035 | 68.2±23.9 | 88 | 67.6±23.2 | 947 | 68.3±24.0 | 0.81 |
| Data available (n) | Median (IQR) | Data available (n) | Median (IQR) | Data available (n) | Median (IQR) | ||
| White blood count (thousand/uL) | 2701 | 7.1 (5.5–9.3) | 239 | 7.6(5.7–10.2) | 2462 | 7.1(5.5–9.2) | 0.0081 |
Oncologic History
The most common cancer types were non-small cell lung cancer (n=781, 28.4%) and melanoma (n=778, 28.2%, Table 1). About one fifth of the patients had received radiation therapy (n=572, 20.8%) and 10.4% of the patients had received fluorouracil (n=284). Overall, ICIs were started a median of 652 days (IQR 195–1972) after cancer diagnosis. Checkpoint inhibitor-based immunotherapies that target the programmed death protein 1 (PD1) pathway were the most commonly prescribed (n=2147, 75.2%, Table 1). Corticosteroid therapy was prescribed in 417 (17.8%) patients within 30 days prior to ICI initiation. Across the entire cohort, 1179 (42.9%) patients had irAEs; within these 1179 patients, 734 (62.3%) received corticosteroid therapy for treatment of these immune mediated adverse effects.
Risk of venous thromboembolism after initiation of immune checkpoint inhibitors
The median length of observation for patients post-ICI initiation was 194 days (IQR 65412). The adjusted incidence rate for VTE was higher after starting an ICI. There were 259 events (4.85 per 100 person-years) during the control interval and 244 events (11.75 per 100 person-years) during the at-risk interval. There was a higher incidence rate for both PE and DVT (Table 2). A graph depicting the rate of VTE over time is shown in Figure 1. We observed that rate of VTE minimally increased with cancer progression prior to ICI initiation and was the highest immediately after ICI initiation. From there, it subsequently declined but the risk of VTE never returned to the baseline risk prior to ICI (Figure 1). The absolute risk for VTE was 7.4% at 6 months after starting and ICI and 13.8% at 1 year after an ICI. Analysis with fixed-effect proportional hazards model demonstrated an over 4-fold increase in risk of VTE post ICI compared to pre-ICI (Hazard Ratio [HR] 4.98, 95% confidence interval [CI] 3.65 – 6.79, p<0.001, Table 3). To additionally control for competing risk, we tested the rates of VTE after exclusion of subjects who died within 6 months, 1 year and 2 years of starting ICI treatment in separate analyses and similarly observed an increase in VTE rates after ICI (Table 3, Supplemental Table 1, 2 and 3). The trends of VTE risks relative to ICI initiation remained largely unchanged as well (Figure 2 and 3). Out of 2854 patients, 892 patients died before the study period ended or a VTE event could have occurred. Among the remaining 1962 patients, 1208 were lost to follow-up (defined by last medication change, last new diagnosis code or last appointment) before the 2-year study period ended and 510 patients were followed up to the end of the study and did not experience either death or a VTE event. As the median length of observation in the post-ICI period was significantly shorter than 730 days, we also performed sensitivity analysis by excluding patients who were dead and lost to follow up by the end of 2-year period and found similar results (Table 3, Figure 4). Given that the control period included in the study was 2 years and some patients could have been cancer free in that period of time, we examined incidence rates of VTE events at different intervals pre- and post-ICI (Supplemental Table 7). The incidence rate of DVT, PE, and VTE were 2.26, 2.88, and 4.67 events per 100 person years at 3 months prior to ICI initiation. In comparison, at 3 months post ICI initiation, the rates of DVT, PE, VTE were 12.41, 9.45 and 19.24 per 100 person years, respectively. At 2 years post ICI initiation, the rates of DVT, PE, VTE were 7.67, 7.08 and 12.48 events per 100 person years, suggesting that increase in VTE post ICI is unlikely to be due to patients being cancer free during control period.
Table 2:
Incidence rate ratios for VTE events within two years pre-and post-immune checkpoint inhibitors (n=2854)
| Pre-Immune Checkpoint Inhibitor | Post-Immune Checkpoint Inhibitor | |||
|---|---|---|---|---|
| Number of events | Incidence rate (per 100 person-years) | Number of events | Incidence rate (per 100 person-years) | |
| Deep Vein Thrombosis | 126 | 2.30 | 155 | 7.37 |
| Pulmonary Embolism | 162 | 2.95 | 140 | 6.61 |
| Venous Thromboembolism | 259 | 4.85 | 244 | 11.75 |
Figure 1:
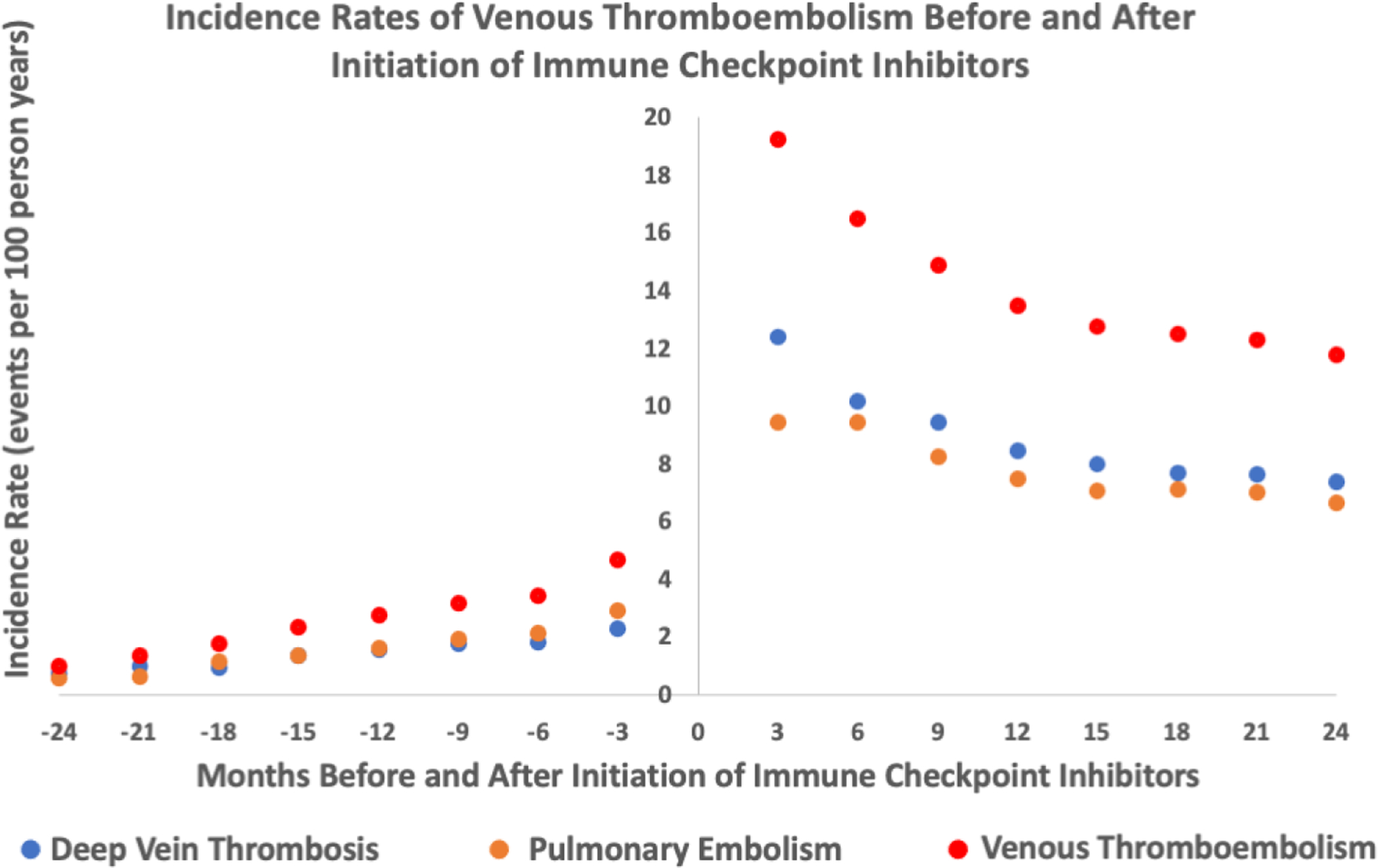
Incidence rates of venous thromboembolism events (deep vein thrombosis, pulmonary embolism, deep vein thrombosis/pulmonary embolism) before and after initiation of immune checkpoint inhibitors (entire cohort n=2854)
Table 3:
Fixed-effect cause-specific proportional hazards regression analysis for VTE events within two years pre-and post-immune checkpoint inhibitors
| Entire Cohort (n=2854) | Patients who are alive at 6 months’ post immune checkpoint inhibitors (n=2287) | Patients who are alive at 1 year post immune checkpoint inhibitors (n=2077) | Patients who are alive and have not been lost to follow up at 2 years post immune checkpoint inhibitors (n=754) | |||||
|---|---|---|---|---|---|---|---|---|
| Hazard ratio (95% confidence Interval) | P value | Hazard ratio (95% confidence Interval) | P value | Hazard ratio (95% confidence Interval) | P value | Hazard ratio (95% confidence Interval) | P value | |
| Deep Vein Thrombosis | 5.70 (3.79, 8.59) | <0.001 | 4.58 (2.99, 7.00) | <0.001 | 4.04 (2.61, 6.26) | <0.001 | 5.73 (3.13, 9.92) | <0.001 |
| Pulmonary Embolism | 4.75 (3.20, 7.10) | <0.001 | 3.76 (2.50, 5.66) | <0.001 | 3.18 (2.08, 4.86) | <0.001 | 4.47 (2.64, 7.56) | <0.001 |
| Venous Thromboembolism | 4.98 (3.65, 6.79) | <0.001 | 3.98 (2.89, 5.48) | <0.001 | 3.49 (2.51, 4.86) | <0.001 | 4.89 (3.26, 7.35) | <0.001 |
Figure 2:
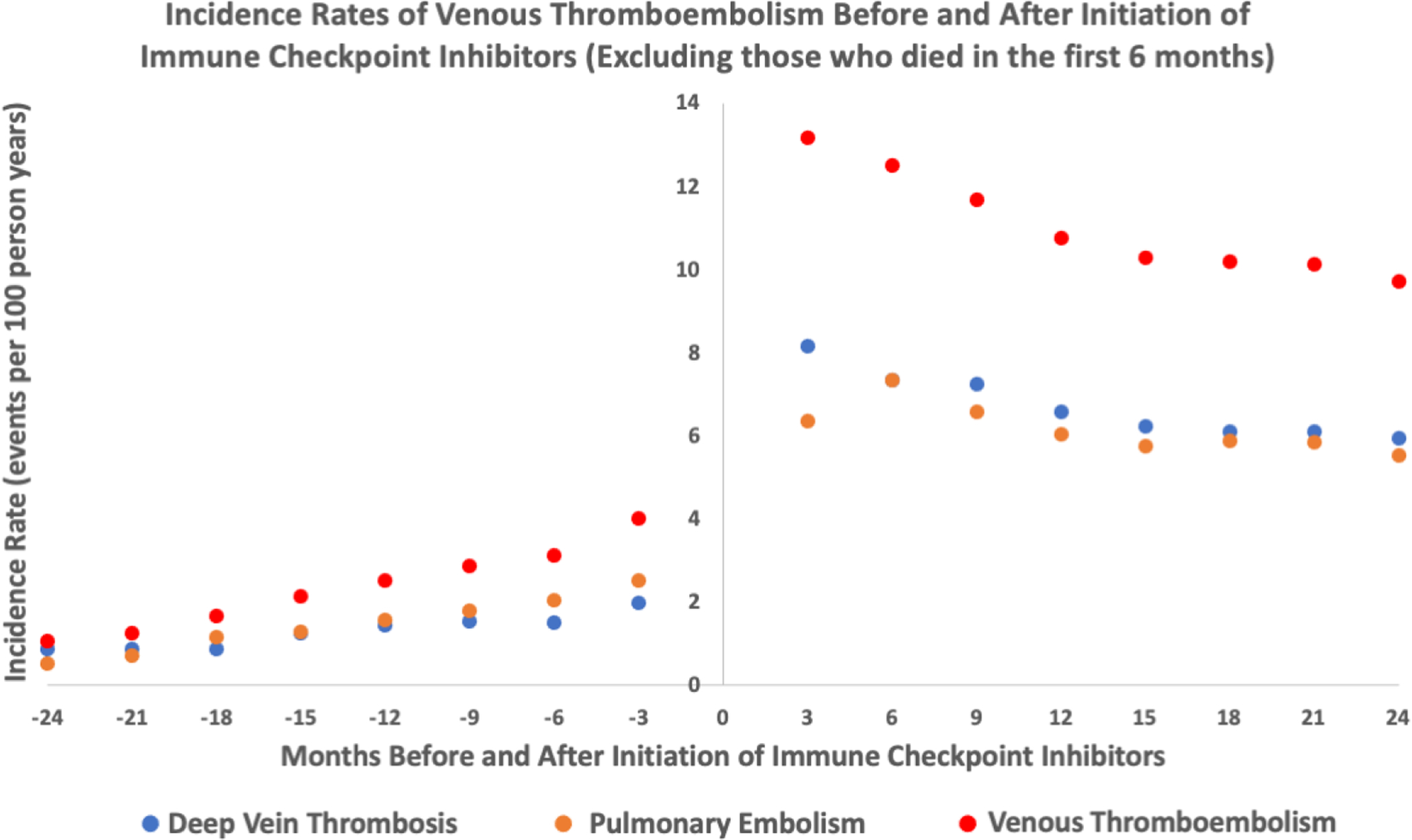
Incidence rates of venous thromboembolism events (deep vein thrombosis, pulmonary embolism, deep vein thrombosis/pulmonary embolism) before and after initiation of immune checkpoint inhibitors- excluding those who died within 6 months of starting ICI (n=2287)
Figure 3:
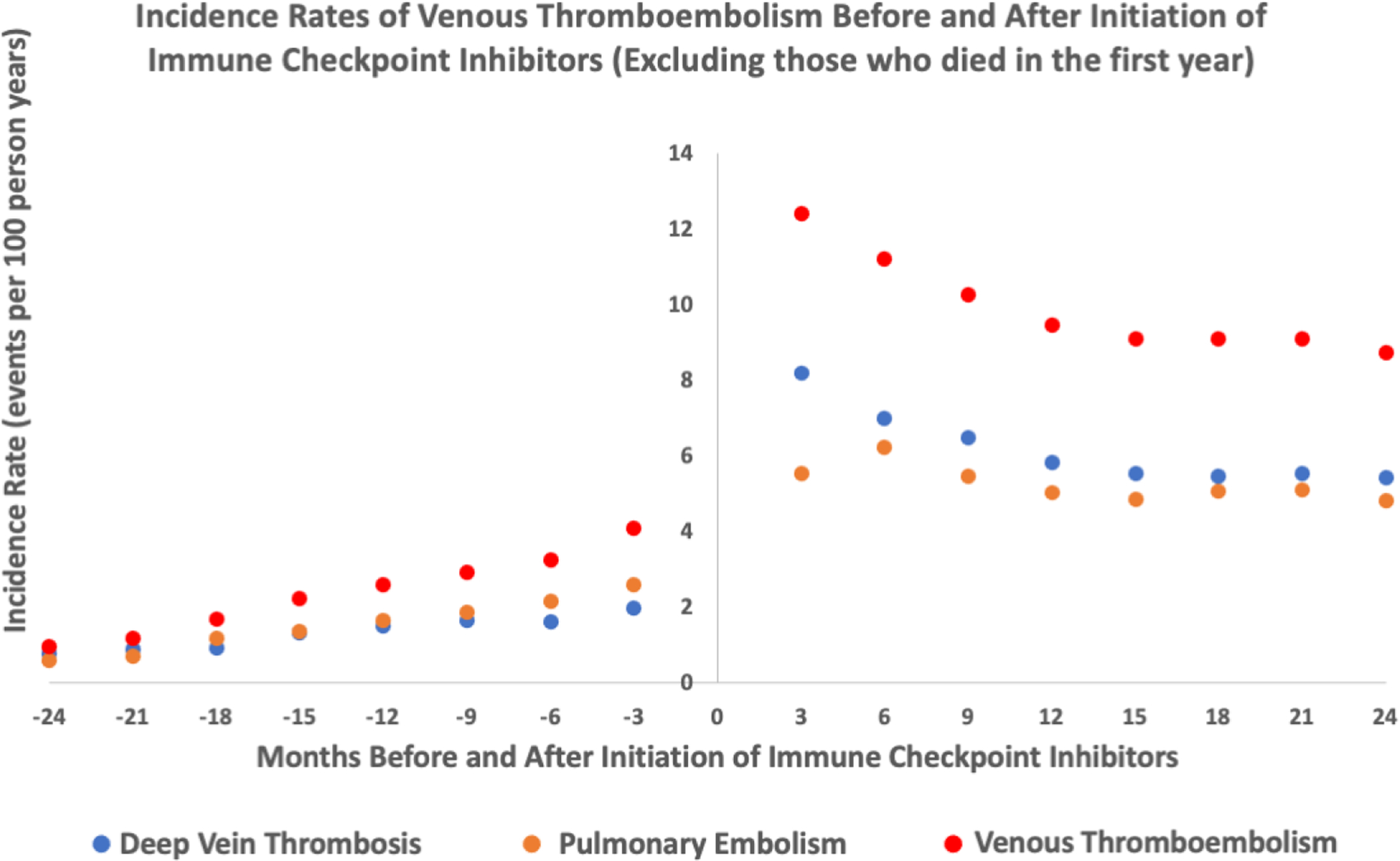
Incidence rates of venous thromboembolism events (deep vein thrombosis, pulmonary embolism, deep vein thrombosis/pulmonary embolism) before and after initiation of immune checkpoint inhibitors- excluding those who died within 1 year of starting ICI (n=2077)
Figure 4:
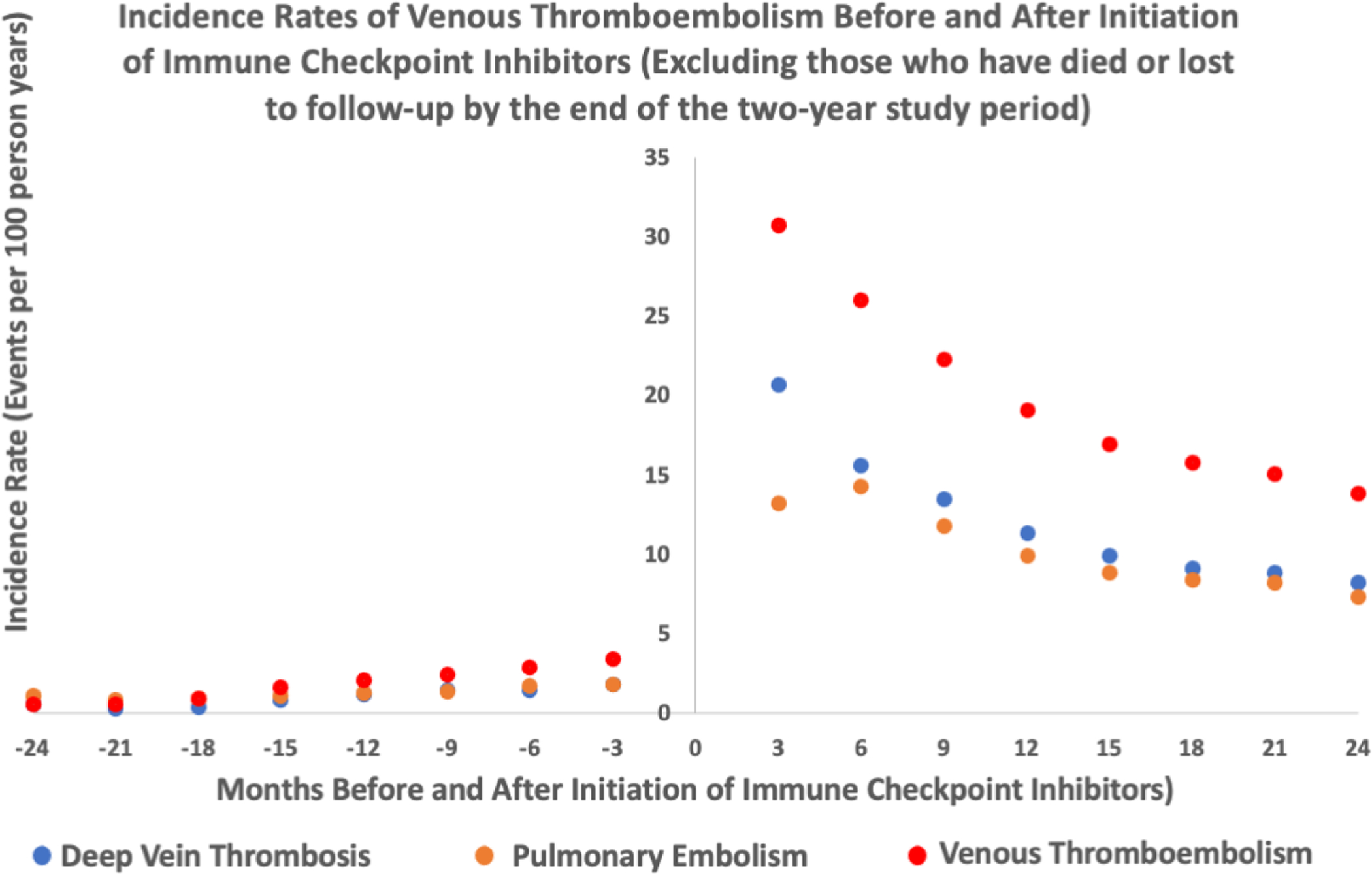
Incidence rates of venous thromboembolism events (deep vein thrombosis, pulmonary embolism, deep vein thrombosis/pulmonary embolism) before and after initiation of immune checkpoint inhibitors- excluding those who died or were lost to follow up within 2 years of starting ICI (n=754)
Risk factors for VTE post ICI
Compared to patients who were VTE free in the two-year period post-ICI, those who developed VTE were modestly younger (63±12 vs. 65±13 years) and were more likely to have prior history of VTE (18.4 vs. 12.5%, Table 1). In the regression analysis, a history of hypertension and age less than 65 predicted VTE in both adjust and unadjusted models (Supplemental Table 4, Table 4). History of prior VTE predicted increased VTE risk in the univariate analysis but not in the multivariate analysis. Patients who had VTE had a higher Khorana Risk Score (p=0.0014, Table 1) and a Khorana score ≥2 predicted a higher risk for VTE post-ICI in both univariate and multivariate models (Table 4, Supplemental Table 4). Patients who had a VTE occur within 2 years of ICI initiation had a higher rate of urothelial cancer (9.4 vs. 6.0%), but a lower rate of melanoma (20.1 vs. 29.0%). Melanoma predicted a lower risk of VTE in both unadjusted and adjusted analyses (Supplemental Table 4, Table 4). To address whether VTE rates with an ICI use differed across cancer types, we examined incidence rates of VTE pre- and post- ICI across cancer groups (Supplemental Table 6, Supplemental Figure 2). There was an increase in rates of VTE among most cancer types post ICI initiation compared to 2 years prior to ICI use. To interrogate whether the degree of VTE risk potentiation is uniform across cancer types, we used a multi-level categorical variable to describe cancer types to address the mutually exclusive nature of this variable. In comparison to patients with lung cancer, those with melanoma had a lower risk of developing VTE with ICI use (HR 0.42, 95% CI 0.30 – 0.61, p<0.001, Supplemental Table 8), while patients in all other cancer groups had similar increase in risk of VTE with ICI use. No statistically significant difference was observed in the risk of VTE among use of different classes of ICI (Table 1, Supplemental Table 4). There were also no differences in the prior use of potentially cardiotoxic cancer therapy, time from cancer diagnosis to ICI initiation, the occurrence of irAEs, or corticosteroid use. Line-associated DVTs occurred in 14 patients before an ICI (incidence rate 0.39 per 100 person years) and in 13 patients after an ICI (incidence rate 0.62 per 100 person years), an over 3-fold increase in DVTs post ICI initiation (HR 3.25, 95% CI 1.06–9.97, p=0.039). This increase in event rate was comparable to all DVTs within 2 years of starting an ICI (Table 3). A total of 262 patients were on therapeutic anticoagulation at the time of ICI initiation. The median time between start of anticoagulation to the start of ICI was 173 days (IQR 56, 382). Therapeutic anticoagulation at initiation of ICI did not predict VTE risk (Supplemental Table 4, Table 4). Among those who were on therapeutic anticoagulation at the time of ICI, length of time on anticoagulation did not predict risk of VTE with an ICI use (HR 0.96, 95% CI 0.54 – 1.70). However, the increase in VTE risk with ICI use was attenuated by baseline anticoagulation (patients on anticoagulation HR 0.92, 95% CI 0.52 – 1.62; patients not on anticoagulation HR 4.65, 95% CI 3.26 – 6.63, p-value for interaction <0.001, Supplemental Table 5, Supplemental Figure 3).
Table 4:
Multivariate Cox regression analysis for association between risk factors and a VTE event post ICI initiation comparing patients who developed VTE while on an ICI (n=259) to those who did not have a VTE event (n=2610)
| Hazard Ratio (95% confidence interval) | P value | |
|---|---|---|
| Age >65 | 0.75 (0.58, 0.98) | 0.03 |
| History of hypertension | 1.37 (1.06, 1.77) | 0.015 |
| History of smoking | 1.24 (0.89, 1.73) | 0.21 |
| VTE prior to ICI | 1.42 (0.99, 2.06) | 0.06 |
| Melanoma | 0.59 (0.41, 0.85) | 0.004 |
| Non-small cell lung cancer | 1.10 (0.81, 1.51) | 0.54 |
| Renal and genitourinary | 1.47 (0.92, 2.35) | 0.10 |
| Therapeutic anticoagulation at initiation of ICI | 1.04 (0.67, 1.63) | 0.86 |
| Khorana Risk Score ≥2 | 1.54 (1.14, 2.09) | 0.005 |
DISCUSSION
In this study, we characterized the risk for VTE among 2854 patients treated with an ICI. The risk for a VTE at 6 months and 1 year after starting an ICI was high at 7.4% and 13.8%, respectively. In comparison to the period prior to ICI, the risk of developing VTE was more than 4-fold higher after starting an ICI. The higher rate for VTE events was observed for both PE and DVT. A history of hypertension and a higher Khorana risk score predicted a higher risk of VTE, while older age and a history of melanoma were associated with lower risk of VTE. This increase in VTE risk with ICI use was lowered with the use of anticoagulation.
There have been individual case reports describing VTE events in patients with cancer undergoing ICI treatment22–25. Beyond case reports, several studies have evaluated the combined outcome of arterial and venous thromboembolic events with ICI use with conflicting results; most of these studies have not performed a control comparison limiting the conclusions. For example, in a report of 122 patients on an ICI, incidence of combined arterial and venous thrombosis events was 8.2% with VTE in 5 (4.1%) patients26. In a study evaluating 672 patients treated with an ICI there was a cumulative incidence of 12.9% for VTE15. In contrast, two prior studies of patients with non-small cell lung cancer who received ICIs (vs. traditional chemotherapy) identified no difference in acute vascular events (both arterial and venous) after initiation of therapy16,17. Further, randomized trials testing the effect of ICIs have not reported a high incidence of VTEs27–29. In a meta-analysis of 20,273 patients from 68 retrospective and prospective studies, the authors found the incidence rate of VTE to be similar in both ICI and non-ICI arms30. The reason for the difference in findings is not clear. Several negative studies had a relatively smaller sample size and, here, we provide data on a population of almost 3,000 patients. We also compared the rates of VTE with a control period and found that the rate of VTE increased 4-fold after ICI treatment and reported a similar rate to prior studies of VTE on an ICI at 12 months14, 15. Additionally, we included all cancer types expanding the external relevance of the study findings. It is also possible that meta-analysis of clinical trials may underestimate the occurrence of a VTE or may reflect the different population included. Specifically, the meta-analysis reported a 2.7% rate of VTE at a follow-up that varied from a few months up to three years30. In comparison, multiple studies, including ours, report a VTE rate that ranges from approximately 13 to 16% at one year14, 15. Finally, prior studies that evaluated the efficacy of combination therapy of ICI with anti-angiogenic therapies, which are known to be thrombogenic, did not report VTE as a potential side effect. This finding might be due to the fact that these studies confined adverse effects to those that occurred in at least 10% of the patients31–34. Our results suggest use of ICI as a risk factor for VTE and further studies to corroborate such findings should be pursued.
The risk factors for VTE with ICI are also not completely understood. A recent single center retrospective cohort study evaluated 228 patients with melanoma who received ICIs, where the use of combination therapy was high, and identified that VTEs developed in 37 (16.2%) patients over median follow-up of 27.3 months. The study identified combination therapy, a Khorana Score ≥1, a history of coronary disease, and anticoagulation at time of ICI as risk factors for developing VTE while on an ICI. Our findings are complementary but also significantly additive. We identified that a history of hypertension, a younger age, and a Khorana Risk Score ≥2 were predictive of the occurrent of VTE after an ICI whereas melanoma was associated with a lower risk.
Immunomodulatory therapy in other cancers has been associated with an increase in VTE. Patients with multiple myeloma (MM) have an increased incidence of VTE due to both the malignancy itself and the therapy. This risk is heightened in those treated with combination therapy35, 36. For example, the combination of thalidomide with dexamethasone increased the incidence of VTE from baseline rate of 3 – 4% to 14 – 26%35. Additional studies found that prophylactic anticoagulation in patients with MM on immunomodulatory drugs can reduce VTE risk37. These findings have resulted in a scoring system and established guidelines for the prophylactic anticoagulation in patients with MM treated with combination therapy including thalidomide/lenalidomide35, 38, 39. We identified that while therapeutic anticoagulation did not predict VTE, it mediated the increase in VTE seen with ICI use. Our results suggest use of ICI may be a risk factor for VTE. Further studies to corroborate such findings should be pursued and, if confirmed, the subsequently performance of clinical trials to evaluate the efficacy of prophylactic anticoagulation in patients without contraindication undergoing ICI treatment would be indicated.
Study Limitations:
This was a retrospective self-controlled study. The self-controlled nature of the analysis limits our ability to control for time-dependent factors but is effective in eliminating the effect of time-invariant confounders. Our study lacked the data to differentiate those who received ICI only therapy as compared to those who received combination of chemotherapy with ICI, limiting our ability to interrogate the potential effect of combination therapy on risk of VTE. Furthermore, we did not have another cohort of patients to validate our findings in, limiting the strength of the findings. We interrogated the effect of the cancer progression on VTE risks by eliminating those who died within 6 months and one year of starting ICI; thus, we could demonstrate a persistently elevated VTE risk associated with ICI initiation. We did not observe any difference in time from cancer diagnosis to ICI initiation between patients who did and did not develop VTE. Both findings suggest but not confirm that the higher VTE risk we observed is not likely solely a function of more advanced cancer but may also be an effect of exposure to an ICI. While the length of observation was limited by the high death and lost to follow up rate during the post-ICI period, we performed sensitivity analysis by eliminating patients who were either dead or lost to follow up by the end of 2-year study period and demonstrated similar findings. Our study may also be prone to ascertainment bias where more VTE events would have been detected if patients received more surveillance scans with active treatment. In total, almost half of the PE diagnoses (43%) were made via staging scans (Supplemental Table 9). However, more PEs were diagnosed incidentally on staging scans in the pre-ICI period as compared to post-ICI period (51% vs 34%). Thus, it is unlikely that increased rates of PEs observed in the risk period is a reflection of increased incidental diagnosis via an increased number of surveillance scans. In understanding the effect of anticoagulation at the time of ICI on the risk VTE with ICI use, the study design was limited by its inability to control for baseline VTE, and thus how anticoagulation associates with VTE. This would need to be addressed using a non-ICI control cohort to compare risks of VTE between the two groups, while adjusting for anticoagulation history and also history of VTE in a multivariate model.
In conclusion, this study demonstrated an increased risk of VTE in cancer patients in the first 2 years after initiating ICI. Younger age, a history of hypertension, and a higher Khorana score predicted higher risk of VTE, whereas patients with melanoma had a lower risk of developing VTE. Anticoagulation at the time of ICI initiation significantly mediated this risk. Our study highlights the need for increased clinical suspicion for VTE in patients with cancer receiving ICIs. Upon confirmation of the findings via additional studies, randomized trials testing the effect of prophylactic anticoagulation with ICI initiation would be reasonable.
Supplementary Material
Highlights.
Rate of venous thromboembolism (VTE) increases after starting an ICI
Patients with a higher Khorana score are more likely to develop VTE with an ICI
Compared to lung cancer, patients with melanoma have lower risk of VTE on an ICI
Anticoagulation can attenuate the increase in risk of VTE with an ICI use.
Clinical suspicion for VTE in patients receiving ICIs should be increased.
Acknowledgments:
We gratefully acknowledge the Cardiovascular Imaging Research Center (CIRC) research team for providing feedback on the study design and interpretation; the CIRC is a combined effort from the Division of Cardiology and the Department of Radiology at Massachusetts General Hospital.
Role of the funder:
The funders had no role in the design of the study; the collection, analysis, and interpretation of the data; the writing of the manuscript; and the decision to submit the manuscript for publication.
FUNDING
Dr. Neilan is supported by a gift from A. Curt Greer and Pamela Kohlberg, and grants from the National Institutes of Health/National Heart, Lung, and Blood Institute grants R01HL130539, R01HL137562, K24HL150238, and National Institutes of Health/Harvard Center for AIDS Research grant P30 AI060354; Dr. Alvi, Dr. Zafar and Dr. Raghu are supported by U.S. National Institutes of Health/National Heart, Lung, and Blood Institute grant T32HL076136. Dr. Leyre Zubiri is supported by a gift from Mr. Gordan Pugh and Dr. Christine Olsen.
Declaration of competing interest
Dr. Neilan has been a consultant to and received fees from Parexel Imaging, Intrinsic Imaging, H3-Biomedicine, Amgen, Genentech, and AbbVie, outside of the current work. Dr. Neilan also reports consultant fees from Bristol Myers Squibb for a Scientific Advisory Board focused on myocarditis related to immune checkpoint inhibitors and grant funding from Astra Zeneca for a study focused on atherosclerosis with immune checkpoints. Dr. Sullivan has been a consultant to Asana, Bristol Myers Squibb, Merck, Replimune; and received research funding from Amgen and Merck, all outside of the current work. Dr. Reynolds has received research funding from Project Datasphere. Other authors have no conflicts of interest or financial disclosures.
Abbreviation List:
- CI
Confidence Interval
- DVT
Deep Vein Thrombosis
- HR
Hazard Ratio
- ICI
Immune Checkpoint Inhibitors
- irAE
immune-related Adverse Event
- MM
Multiple myeloma
- PE
Pulmonary Embolism
- RPDR
Research Patient Data Registry
- VTE
Venous Thromboembolism
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Prior presentations: The findings were presented in abstract format at the Scientific Sessions of the American Heart Association, Friday, November 13th, 2020.
Declaration of interests
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:
Dr. Neilan has been a consultant to and received fees from Parexel Imaging, Intrinsic Imaging, H3-Biomedicine, and AbbVie, outside of the current work. Dr. Neilan also reports consultant fees from Bristol Myers Squibb for a Scientific Advisory Board focused on myocarditis related to immune checkpoint inhibitors and grant funding from Astra Zeneca for a study focused on atherosclerosis with immune checkpoints. Dr. Sullivan has been a consultant to Asana, Bristol Myers Squibb, Merck, Replimune; and received research funding from Amgen and Merck, all outside of the current work. Dr. Reynolds has received research funding from Project Datasphere. Other authors have no conflicts of interest or financial disclosures.
References:
- 1.Ribas A, Wolchok JD. Cancer immunotherapy using checkpoint blockade. Science. Mar 23 2018;359(6382):1350–1355. doi: 10.1126/science.aar4060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Robert C, Thomas L, Bondarenko I, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med. Jun 2011;364(26):2517–26. doi: 10.1056/NEJMoa1104621 [DOI] [PubMed] [Google Scholar]
- 3.Gong J, Chehrazi-Raffle A, Reddi S, Salgia R. Development of PD-1 and PD-L1 inhibitors as a form of cancer immunotherapy: a comprehensive review of registration trials and future considerations. J Immunother Cancer. January 2018;6(1):8. doi: 10.1186/s40425-018-0316-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Topalian SL, Taube JM, Pardoll DM. Neoadjuvant checkpoint blockade for cancer immunotherapy. Science. January 2020;367(6477)doi: 10.1126/science.aax0182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mahmood SS, Fradley MG, Cohen JV, et al. Myocarditis in Patients Treated With Immune Checkpoint Inhibitors. J Am Coll Cardiol. April 2018;71(16):1755–1764. doi: 10.1016/j.jacc.2018.02.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Awadalla M, Mahmood SS, Groarke JD, et al. Global Longitudinal Strain and Cardiac Events in Patients With Immune Checkpoint Inhibitor-Related Myocarditis. J Am Coll Cardiol. Feb November 2020;75(5):467–478. doi: 10.1016/j.jacc.2019.11.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang L, Awadalla M, Mahmood SS, et al. Cardiovascular magnetic resonance in immune checkpoint inhibitor-associated myocarditis. Eur Heart J. May 7 2020;41(18):1733–1743. doi: 10.1093/eurheartj/ehaa051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang L, Zlotoff DA, Awadalla M, et al. Major Adverse Cardiovascular Events and the Timing and Dose of Corticosteroids in Immune Checkpoint Inhibitor-Associated Myocarditis. Circulation. Jun 2020;141(24):2031–2034. doi: 10.1161/CIRCULATIONAHA.119.044703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Awadalla M, Golden DLA, Mahmood SS, et al. Influenza vaccination and myocarditis among patients receiving immune checkpoint inhibitors. J Immunother Cancer. February 2019;7(1):53. doi: 10.1186/s40425-019-0535-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.DA Z, MZ H, A Z, et al. Electrocardiographic features of immune checkpoint inhibitor associated myocarditis. 2021;9(e002007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Anderson FA, Spencer FA. Risk factors for venous thromboembolism. Circulation. Jun 2003;107(23 Suppl 1):I9–16. doi: 10.1161/01.CIR.0000078469.07362.E6 [DOI] [PubMed] [Google Scholar]
- 12.Levi M, van der Poll T, Büller HR. Bidirectional relation between inflammation and coagulation. Circulation. Jun 2004;109(22):2698–704. doi: 10.1161/01.CIR.0000131660.51520.9A [DOI] [PubMed] [Google Scholar]
- 13.Drobni ZD, Alvi RM, Taron J, et al. Association Between Immune Checkpoint Inhibitors With Cardiovascular Events and Atherosclerotic Plaque. Circulation. Dec 2020;142(24):2299–2311. doi: 10.1161/CIRCULATIONAHA.120.049981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sussman TA, Li H, Hobbs B, Funchain P, McCrae KR, Khorana AA. Incidence of thromboembolism in patients with melanoma on immune checkpoint inhibitor therapy and its adverse association with survival. J Immunother Cancer. Jan 2021;9(1)doi: 10.1136/jitc-2020001719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moik F, Chan WE, Wiedemann S, et al. Incidence, risk factors and outcomes of venous and arterial thromboembolism in immune checkpoint inhibitor therapy. Blood. Oct 2020;doi: 10.1182/blood.2020007878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bar J, Markel G, Gottfried T, et al. Acute vascular events as a possibly related adverse event of immunotherapy: a single-institute retrospective study. Eur J Cancer. Oct 2019;120:122–131. doi: 10.1016/j.ejca.2019.06.021 [DOI] [PubMed] [Google Scholar]
- 17.Nichetti F, Ligorio F, Zattarin E, et al. Is There an Interplay between Immune Checkpoint Inhibitors, Thromboprophylactic Treatments and Thromboembolic Events? Mechanisms and Impact in Non-Small Cell Lung Cancer Patients. Cancers (Basel). Dec 2019;12(1)doi: 10.3390/cancers12010067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li R, Stewart B, Weintraub E. Evaluating efficiency and statistical power of self-controlled case series and self-controlled risk interval designs in vaccine safety. J Biopharm Stat. 2016;26(4):686–93. doi: 10.1080/10543406.2015.1052819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Khorana AA, Kuderer NM, Culakova E, Lyman GH, Francis CW. Development and validation of a predictive model for chemotherapy-associated thrombosis. Blood. May 2008;111(10):4902–7. doi: 10.1182/blood-2007-10-116327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lin D, Wei L. The Robust Inference for the Cox Proportional Hazards Model. Journal of the American Statistical Association. 1989;84(408):1074–1078. [Google Scholar]
- 21.JP F, RJ G. A proportional hazards model for the Subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- 22.Cortellini A, Parisi A, Fargnoli MC, et al. Safe Administration of Ipilimumab, Pembrolizumab, and Nivolumab in a Patient with Metastatic Melanoma, Psoriasis, and a Previous Guillain-Barré Syndrome. Case Rep Oncol Med. 2018;2018:2783917. doi: 10.1155/2018/2783917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kunimasa K, Nishino K, Kimura M, et al. Pembrolizumab-induced acute thrombosis: A case report. Medicine (Baltimore). May 2018;97(20):e10772. doi: 10.1097/MD.0000000000010772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tsukamoto J, Monteiro M, Vale S, et al. Thromboembolic Events Related to Treatment with Checkpoint Inhibitors: Report of Two Cases. Case Rep Oncol. 2018 Sep–Dec 2018;11(3):648–653. doi: 10.1159/000492463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Horio Y, Takamatsu K, Tamanoi D, et al. Trousseau’s syndrome triggered by an immune checkpoint blockade in a non-small cell lung cancer patient. Eur J Immunol. October 2018;48(10):1764–1767. doi: 10.1002/eji.201847645 [DOI] [PubMed] [Google Scholar]
- 26.Ando Y, Hayashi T, Sugimoto R, et al. Risk factors for cancer-associated thrombosis in patients undergoing treatment with immune checkpoint inhibitors. Invest New Drugs. August 2020;38(4):1200–1206. doi: 10.1007/s10637-019-00881-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Antonia SJ, Villegas A, Daniel D, et al. Durvalumab after Chemoradiotherapy in Stage III Non-Small-Cell Lung Cancer. N Engl J Med. Nov 16 2017;377(20):1919–1929. doi: 10.1056/NEJMoa1709937 [DOI] [PubMed] [Google Scholar]
- 28.Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. N Engl J Med. Oct 2015;373(17):1627–39. doi: 10.1056/NEJMoa1507643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kaufman HL, Russell J, Hamid O, et al. Avelumab in patients with chemotherapyrefractory metastatic Merkel cell carcinoma: a multicentre, single-group, open-label, phase 2 trial. Lancet Oncol. Oct 2016;17(10):1374–1385. doi: 10.1016/S1470-2045(16)30364-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Solinas C, Saba L, Sganzerla P, Petrelli F. Venous and arterial thromboembolic events with immune checkpoint inhibitors: A systematic review. Thromb Res. December 2020;196:444–453. doi: 10.1016/j.thromres.2020.09.038 [DOI] [PubMed] [Google Scholar]
- 31.Finn RS, Qin S, Ikeda M, et al. Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N Engl J Med. May 2020;382(20):1894–1905. doi: 10.1056/NEJMoa1915745 [DOI] [PubMed] [Google Scholar]
- 32.Socinski MA, Jotte RM, Cappuzzo F, et al. Atezolizumab for First-Line Treatment of Metastatic Nonsquamous NSCLC. N Engl J Med. Jun 2018;378(24):2288–2301. doi: 10.1056/NEJMoa1716948 [DOI] [PubMed] [Google Scholar]
- 33.Rini BI, Plimack ER, Stus V, et al. Pembrolizumab plus Axitinib versus Sunitinib for Advanced Renal-Cell Carcinoma. N Engl J Med. March 2019;380(12):1116–1127. doi: 10.1056/NEJMoa1816714 [DOI] [PubMed] [Google Scholar]
- 34.Makker V, Rasco D, Vogelzang NJ, et al. Lenvatinib plus pembrolizumab in patients with advanced endometrial cancer: an interim analysis of a multicentre, open-label, single-arm, phase 2 trial. Lancet Oncol. May 2019;20(5):711–718. doi: 10.1016/S1470-2045(19)30020-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Palumbo A, Rajkumar SV, Dimopoulos MA, et al. Prevention of thalidomide- and lenalidomide-associated thrombosis in myeloma. Leukemia. Feb 2008;22(2):414–23. doi: 10.1038/sj.leu.2405062 [DOI] [PubMed] [Google Scholar]
- 36.Rajkumar SV, Jacobus S, Callander NS, et al. Lenalidomide plus high-dose dexamethasone versus lenalidomide plus low-dose dexamethasone as initial therapy for newly diagnosed multiple myeloma: an open-label randomised controlled trial. Lancet Oncol. Jan 2010;11(1):29–37. doi: 10.1016/S1470-2045(09)70284-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Palumbo A, Bringhen S, Caravita T, et al. Oral melphalan and prednisone chemotherapy plus thalidomide compared with melphalan and prednisone alone in elderly patients with multiple myeloma: randomised controlled trial. Lancet. Mar 2006;367(9513):825–31. doi: 10.1016/S0140-6736(06)68338-4 [DOI] [PubMed] [Google Scholar]
- 38.Wang M, Weber DM, Delasalle K, Alexanian R. Thalidomide-dexamethasone as primary therapy for advanced multiple myeloma. Am J Hematol. Jul 2005;79(3):194–7. doi: 10.1002/ajh.20382 [DOI] [PubMed] [Google Scholar]
- 39.Palumbo A, Falco P, Corradini P, et al. Melphalan, prednisone, and lenalidomide treatment for newly diagnosed myeloma: a report from the GIMEMA--Italian Multiple Myeloma Network. J Clin Oncol. Oct 2007;25(28):4459–65. doi: 10.1200/JCO.2007.12.3463 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


