Abstract
Marginal zone lymphoma (MZL) arising from the anterior mediastinum is rare. In the majority of reported cases, the tumor was incidentally discovered, reflecting its indolent clinical features. We present a 38-year-old woman who had no medical history, and presented with a bulky anterior mediastinal tumor complicated by life-threatening compression of the vasculature and bronchi. Biopsy specimens of the neoplasm suggested transformed diffuse large B-cell lymphoma (DLBCL) from MZL. To our best knowledge, this is the first case report of anterior mediastinum MZL associated with an aggressive clinical course and life-threatening complications likely due to transformation to DLBCL.
Keywords: marginal zone lymphoma, diffuse large B-cell lymphoma, anterior mediastinum, cystic lesions
INTRODUCTION
Lymphoma is one of the most common tumors developing in the mediastinum. Primary mediastinal lymphomas include primary mediastinal (thymic) large B-cell lymphoma, Hodgkin lymphoma (HL), T-cell acute lymphoblastic leukemia/lymphoma (T-ALL/LBL), primary thymic marginal zone lymphoma (MZL) of mucosa-associated lymphoid tissue lymphoma (MALT)-type (thymic MZL), primary mediastinal (non-thymic) diffuse large B-cell lymphoma (DLBCL), primary mediastinal small lymphocytic lymphoma, primary mediastinal plasmacytoma, peripheral T-cell lymphoma not otherwise specified, anaplastic large cell lymphoma (including breast implant-associated), and B-cell lymphoma, unclassifiable, with features intermediate between DLBCL and classical HL.1 Among these primary mediastinal lymphomas, thymic MZL is rare.2 Approximately 80% of the reported cases were in Asians and frequently co-occurred with autoimmune diseases, particularly Sjögren syndrome (SjS).3 Most patients were asymptomatic and the tumors were discovered incidentally on imaging tests for other diseases after a few years or decades of the onset of the autoimmune disease.4
This case report describes a patient with anterior mediastinal primary MZL, which was associated with rapid progression and life-threatening complications due to transformation to DLBCL. In addition, we reviewed the literature concerning primary thymic MZL.
CASE REPORT
Physical examination, laboratory data, and imaging findings
A 38-year-old woman presented to a hospital with palpitations, fatigue, and dyspnea on exertion. She was referred to our hospital because chest X-ray and computed tomography (CT) revealed a bulky tumor in the mediastinum. She had no medical history other than asthma. She also had no symptoms suggestive of collagen disease, including SjS. Complete blood counts revealed slight thrombocytosis (40.8×104/μL). Biochemistry tests demonstrated hypoalbuminemia (3.4 g/dL; normal range, 4.1–5.1 g/dL), increased lactate dehydrogenase (451 IU/L; normal range, 124–222 IU/L), and C-reactive protein (6.20 mg/dL; normal range, less than 0.15 mg/dL), but the soluble interleukin-2 receptor level was within the normal range (342.4 U/mL; normal range, 156.6–474.5 U/mL) (Table 1). CT revealed a tumor located in the anterior mediastinum, pericardial effusions, left pleural effusion, and supraclavicular and para-aortic lymphadenopathy (Fig. 1A, B). The anterior mediastinal tumor contained cystic lesions and demonstrated heterogeneous density on contrast CT, suggesting the presence of necrotic changes in the tumor (Fig. 1A, B). The superior vena cava (SVC) and bilateral main bronchi were compressed by the tumor. No abnormal lesion was detected in the lung.
Table 1. Patient’s laboratory data on admission.
| <Complete blood count> | BUN | 7.4 | mg/dL | <Coagulation> | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| WBC | 6120 | /μL | Cr | 0.57 | mg/dL | PT | 11.4 | sec | ||
| RBC | 440 | ×104/μL | UA | 2.5 | mg/dL | APTT | 35.8 | sec | ||
| Hb | 12.3 | g/dL | Na | 140 | mmol/L | Fib | 696 | mg/dL | ||
| Ht | 38.4 | % | K | 4.4 | mmol/L | FDP | 10.1 | μg/mL | ||
| MCV | 86.6 | fL | Cl | 102 | mmol/L | D-dimer | 6.5 | μg/mL | ||
| MCH | 27.7 | pg | Ca | 9.3 | mg/dL | AT3 | 90 | % | ||
| MCHC | 32.1 | g/dL | CRP | 6.2 | mg/dL | |||||
| Plt | 40.8 | ×104/μL | Ferritin | 242.0 | ng/mL | <Tumor markers> | ||||
| IgG | 961.1 | mg/dL | β-HCG | 0.2 | IU/mL | |||||
| <Biochemistry> | IgA | 246.1 | mg/dL | CYFRA | 0.7 | ng/dL | ||||
| TP | 6.9 | g/dL | IgM | 99.6 | mg/dL | CEA | 0.44 | ng/mL | ||
| Alb | 3.4 | g/dL | IFE | Not detectable | AFP | 1.3 | ng/mL | |||
| T-Bil | 0.48 | mg/dL | RF | 0.30 | U/mL | NSE | 31.1 | ng/mL | ||
| AST | 20 | IU/L | ANA | Not detectable | ProGRP | 26.9 | mmol/L | |||
| ALT | 23 | IU/L | Anti SS-A Ab | 0.50 | IU/mL | SCC | 0.3 | mg/dL | ||
| ALP | 190 | IU/L | Anti SS-B Ab | 0.50 | IU/mL | SIL-2R | 342.4 | IU/mL | ||
| γ-GTP | 45 | IU/L | BNP | 8.5 | pg/mL | |||||
| LDH | 451 | IU/L | ||||||||
Abbreviations: WBC, white blood cells; RBC, red blood cells; Hb, hemoglobin; Ht, hematocrit; MCV, mean corpuscular volume; MCH, mean corpuscular hemoglobin; MCHC, mean corpuscular hemoglobin concentration; Plt, platelets; TP, total protein; Alb, albumin; T-Bil, total bilirubin; AST, aspartate aminotransferase; ALT, alanine aminotransferase; ALP, alkaline phosphatase; γ-GTP, gamma glutamyltranspeptidase; LDH, lactate dehydrogenase; BUN, blood urea nitrogen; Cr, creatinine; UA, uric acid; Na, natrium; Cl, chloride; K, potassium; Ca, calcium; CRP, C-reactive protein; IgG, immunoglobulin G; IgA, immunoglobulin A; IgM, immunoglobulin M; IFE, immunofixation electrophoresis; RF, rheumatoid factor; ANA, anti-nuclear antibody; Ab, antibody; BNP, brain natriuretic peptide; PT, prothrombin time; APTT, activated partial thromboplastin time; Fib, fibrinogen; FDP, fibrin/fibrinogen degradation products; AT3, antithrombin 3; β-HCG, β-human chorionic gonadotropin; CYFRA, cytokeratin 19 fragment; CEA, carcinoembryonic antigen; AFP, α-fetoprotein; NSE, neuron-specific enolase; ProGRP, pro-gastrin-releasing peptide; SCC, squamous cell carcinoma; SIL-2R, soluble interleukin-2 receptor
Fig. 1.
Radiological findings of the anterior mediastinal tumor
Chest-abdominal computed tomography shows a bulky anterior mediastinal tumor with cystic and necrotic lesions (A, B, arrows), complicated by pericardial (A, arrowheads) and left pulmonary effusions. The tumor is compressing the superior vena cava and bilateral main bronchi (B, arrowheads). Chest X-ray performed 4 months before admission shows an abnormal shadow around the left pulmonary artery trunk in the front view (C, arrowheads) and the anterior mediastinum in the side view (D, area circled by the red broken line); however, these findings were not observed one and a half years ago (E, F).
Flow cytometry analysis and histological examination
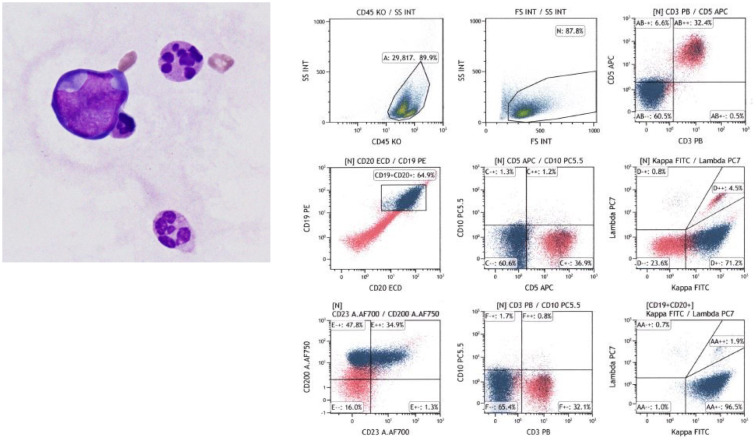
As the patient presented with signs of cardiac tamponade, including hypotension, tachycardia, and low voltage on electrocardiography, emergency pericardial drainage was performed. Flow cytometry analysis of the pericardial fluid revealed abnormal cells that were CD3−, CD5−, CD10−, CD19+, and CD20+, with immunoglobulin light chain restriction (kappa) (Fig. 2), strongly suggesting a mature B-cell neoplasm. CT-guided needle biopsy of the mediastinal tumor along with biopsy of the right supraclavicular lymph node were performed. Histological examination of these biopsy specimens using hematoxylin and eosin staining demonstrated proliferation of predominantly small to medium-sized abnormal lymphoid cells with monocytoid features (Fig. 3A), but large lymphoid cells were partially observed in some areas (Fig. 3B). The border between the two components was vague as the cells of different sizes were intermingled. Both medium and large lymphoid cells were CD3−, CD5−, CD10−, CD20+, CD23+, MUM1+, BCL2+, BCL6+, c-myc−, cyclin D1−, and SOX11− on immunohistochemistry, and Epstein–Barr virus-encoded small RNA in situ hybridization was negative. The Ki-67 labeling index was 10% in the medium cell area and 40% in the large cell area (Fig. 3C, D). Hassall’s corpuscles, which are found in the thymus, were not observed in the specimen of the anterior mediastinum tumor. Although CD23 and BCL6 were generally negative in MZL, there have been reports of MZL expressing these markers.5-8 Therefore, based on the cell morphology and other immunohistochemistry studies, we diagnosed the medium cell regions with a low Ki-67 labeling index as MZL. On the other hand, we diagnosed the large cell regions with a high Ki-67 labeling index as DLBCL.
Fig. 2.
Cytological examination of the pericardial fluid and flow cytometry analysis
May-Giemsa staining shows abnormal cells with an irregularly shaped nucleus (left, ×1000). Flow cytometry analysis revealed that the lymphoid cells were positive for CD19, CD20, and immunoglobulin kappa, and negative for CD3, CD5, and CD10.
Fig. 3.
Histological findings of the tissue biopsy specimens
Hematoxylin and eosin staining shows predominantly atypical small- to medium-sized lymphoid cells with monocytoid features in most parts of the tumor (A, ×200; inset, ×1000), although large lymphoid cells are also observed in some areas (B, ×200; inset, ×1000). The Ki-67 labeling index is low in the medium cell area (C, ×400) and high in the large cell area (D, ×400).
Molecular analyses of the lymphoma
To investigate whether the MZL and DLBCL in this patient had the same origin, we performed immunoglobulin heavy chain (IgH) gene rearrangement analyses of the medium and large cell components using polymerase chain reaction (PCR) of laser micro-dissected samples from a formalin-fixed paraffin-embedded section. However, monoclonal peaks were not found at any of the sites (data not shown). Although we were unable to exclude the possibility of a composite lymphoma (MZL+DLBCL), the similarity of features on immunohistochemistry studies between the two types of lymphoma cells suggested that the origin of MZL and DLCBL in this patient was closely associated. Thus, she was diagnosed with transformed DLBCL (Hans criteria:9 non-germinal center B-cell type) from MZL in the anterior mediastinum (The Lugano Classification:10 Stage IV).
Apoptosis inhibitor 2 gene (API2)-MALT lymphoma-associated translocation 1 (MALT1) and IgH-MALT1 fusion transcripts were not detectable by fluorescence in situ hybridization analysis. Cytogenetic analysis of the right supraclavicular lymph node demonstrated a complex karyotype: 50, XX, +X, +X, +9, +12 [14/20 cells] / 50, idem, t(4;17)(q27;p13) [6/20 cells]. There was no evidence of lymphoma in the bone marrow or gastrointestinal tract.
Treatment and clinical course
Treatment was initiated with R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisolone). After one cycle of R-CHOP, the anterior mediastinal tumor and lymphadenopathy both decreased in size, along with disappearance of the pericardial effusions and alleviation of compression of the SVC and bronchi. Positron emission tomography-computed tomography (PET-CT) after four cycles of R-CHOP demonstrated complete metabolic response (CMR); therefore, she subsequently received two cycles of R-CHOP and involved-site radiation therapy as consolidation therapy.
We retrospectively reviewed the medical records to estimate the onset of the lymphoma. Chest X-ray performed at an annual medical checkup four months before admission revealed an abnormal shadow around the left pulmonary artery trunk (Fig. 1C), along with a decrease in X-ray penetrance around the anterior mediastinum in the lateral view (Fig. 1D), suggesting the development of an indolent tumor. These findings were not observed on chest X-ray taken one and a half years ago (Fig. 1E, F), indicating the emergence of the mediastinal tumor during this time.
DISCUSSION
Primary thymic MZL was first described by Isaacson and colleagues,11 and less than 100 cases have been reported in the English literature thus far (Supplementary Table 1). Most cases have been reported from east Asian countries with a female predominance (male:female ratio of 1:3–4). A close association between thymic MZL and autoimmune diseases, especially SjS, has been reported.4 Although some reported thymic MZL cases had anti-SSA antibody without clinical symptoms of SjS (Supplementary Table 1), interpretation of the results of anti-SSA antibody requires caution because anti-SSA antibody is detected even in healthy individuals.12 According to previous reports, around 70% of thymic MZL patients were asymptomatic, with the tumor being accidentally detected on imaging tests performed for investigating the symptoms of other diseases. As for the characteristic radiological findings, thymic MZL often presents as an anterior mediastinal mass with cystic lesions on CT (up to 70%); however, these findings are not specific to thymic MZL, as they are also observed in thymoma, nodular-sclerosis CHL, DLBCL, and LBL.13,14 As the necrotic lesions in lymphoma tissues reflect the aggressive proliferation of tumor cells15,16 and it is one of the predictors of poor prognosis in DLBCL,17-19 we initially assumed that the lymphoma in this patient was aggressive. Typical histological findings of thymic MZL include replacement of the normal thymic structure by lymphoepithelial lesions accompanied by infiltration of the lymphoma cells into Hassall’s corpuscles and multilobular cysts of different sizes in the tumor.3,4 We did not observe these findings in the biopsy specimen obtained from the mediastinal tumor. This was possibly because the specimen was not sufficient size to include the thymic tissue. Regarding cytogenetical alterations, chromosomal translocations specific to or associated with MALT lymphoma resulting in API2-MALT1 fusion or IgH gene rearrangement were rarely observed,4 whereas trisomy 3 was frequently detected (50%) in thymic MZL.20
Although previous reports suggested a good prognosis for thymic MZL,3,4 whether the prognosis of concurrent DLBCL and indolent non-Hodgkin lymphoma (NHL) at diagnosis is better than that of DLBCL alone is controversial. Wang et al. reported that the prognosis of concurrent DLBCL and indolent NHL including MZL was similar to that of DLBCL alone.21 Conversely, another study revealed that concurrent DLBCL with indolent lymphoma other than follicular lymphoma has a significantly poor prognosis.22 Indeed, one MZL case in the mediastinum that transformed to DLBCL was refractory to R-CHOP therapy.23 Therefore, although CMR with chemoradiotherapy was achieved in our case, careful follow-up is necessary in the future.
In conclusion, we reported a case of anterior mediastinal MZL that transformed into DLBCL and exhibited rapid progression, which is commonly observed in aggressive lymphomas of the mediastinum. The clinical course of thymic MZL was reported to be indolent even in the presence of a DLBCL component,23,24 and most cases were accidentally detected. Thus, to the best of our knowledge, this is the first case report of MZL developing in the anterior mediastinum that transformed into DLBCL and presented with an aggressive clinical course. It is necessary to suspect transformed DLBCL from MZL if an anterior mediastinal tumor with cystic lesions and aggressive clinical features is discovered.
Supplemental Materials and Methods
ACKNOWLEDGMENTS
We thank Asami Nishikori for performing the IgH gene rearrangement analyses. The manuscript was edited and proofread by Editage (https://www.editage.jp/).
Footnotes
CONFLICT OF INTEREST
The authors declare that they have no conflicts of interest.
REFERENCES
- 1.Piña-Oviedo S, Moran CA. Primary mediastinal nodal and extranodal non-Hodgkin lymphomas: current concepts, historical evolution, and useful diagnostic approach: Part 2. Adv Anat Pathol. 2019; 26: 371-389. [DOI] [PubMed] [Google Scholar]
- 2.Maeshima AM, Taniguchi H, Suzuki T, et al. Distribution of malignant lymphomas in the anterior mediastinum: a single-institution study of 76 cases in Japan, 1997–2016. Int J Hematol. 2017; 106: 675-680. [DOI] [PubMed] [Google Scholar]
- 3.Shimizu K, Yoshida J, Kakegawa S, et al. Primary thymic mucosa-associated lymphoid tissue lymphoma: diagnostic tips. J Thorac Oncol. 2010; 5: 117-121. [DOI] [PubMed] [Google Scholar]
- 4.Inagaki H, Chan JKC, Ng JWM, et al. Primary thymic extranodal marginal-zone B-cell lymphoma of mucosa-associated lymphoid tissue type exhibits distinctive clinicopathological and molecular features. Am J Pathol. 2002; 160: 1435-1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boveri E, Arcaini L, Merli M, et al. Bone marrow histology in marginal zone B-cell lymphomas: correlation with clinical parameters and flow cytometry in 120 patients. Ann Oncol. 2009; 20: 129-136. [DOI] [PubMed] [Google Scholar]
- 6.Shi Y, Li X, Lai Y, Jia L. CD21 and CD23 expression differences in small B-cell lymphomas: comparative analysis in follicular dendritic cells and tumor cells. Int J Clin Exp Pathol. 2016; 9: 8395-8405. [Google Scholar]
- 7.Traverse-Glehen A, Felman P, Callet-Bauchu E, et al. A clinicopathological study of nodal marginal zone B-cell lymphoma. A report on 21 cases. Histopathology. 2006; 48: 162-173. [DOI] [PubMed] [Google Scholar]
- 8.Poveda J, Cassidy DP, Zhou Y, et al. Expression of germinal center cell markers by extranodal marginal zone lymphomas of MALT type within colonized follicles, a diagnostic pitfall with follicular lymphoma. Leuk Lymphoma. 2021; 62: 1116-1122. [DOI] [PubMed] [Google Scholar]
- 9.Hans CP, Weisenburger DD, Greiner TC, et al. Confirmation of the molecular classification of diffuse large B-cell lymphoma by immunohistochemistry using a tissue microarray. Blood. 2004; 103: 275-282. [DOI] [PubMed] [Google Scholar]
- 10.Cheson BD, Fisher RI, Barrington SF, et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J Clin Oncol. 2014; 32: 3059-3067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Isaacson PG, Chan JKC, Tang C, Addis BJ. Low-grade B-cell lymphoma of mucosa-associated lymphoid tissue arising in the thymus. A thymic lymphoma mimicking myoepithelial sialadenitis. Am J Surg Pathol. 1990; 14: 342-351. [DOI] [PubMed] [Google Scholar]
- 12.Zimmermann C, Fabini G, Höfler E, Smolen JS, Steiner G. Analysis of anti-Ro52 antibodies in sera of healthy subjects. Arthritis Res Ther. 2001; 3: 118. [Google Scholar]
- 13.Yamada S, Tadasu K, Mingyon M, Yoshiaki A, Noriko M. Mucosa-associated lymphoid tissue lymphoma of the thymus resected using combined thoracoscopic and transcervical approaches. Ann Thorac Surg. 2003; 76: 293-295. [DOI] [PubMed] [Google Scholar]
- 14.Kuroki S, Nasu K, Murakami K, et al. Thymic MALT lymphoma: MR imaging findings and their correlation with histopathological findings on four cases. Clin Imaging. 2004; 28: 274-277. [DOI] [PubMed] [Google Scholar]
- 15.Saito A, Takashima S, Takayama F, et al. Spontaneous extensive necrosis in non-Hodgkin lymphoma: prevalence and clinical significance. J Comput Assist Tomogr. 2001; 25: 482-486. [DOI] [PubMed] [Google Scholar]
- 16.Wislez M, Cadranel J, Antoine M, et al. Lymphoma of pulmonary mucosa-associated lymphoid tissue: CT scan findings and pathological correlations. Eur Respir J. 1999; 14: 423-429. [DOI] [PubMed] [Google Scholar]
- 17.Song MK, Chung JS, Shin DY, et al. Tumor necrosis could reflect advanced disease status in patients with diffuse large B cell lymphoma treated with R-CHOP therapy. Ann Hematol. 2017; 96: 17-23. [DOI] [PubMed] [Google Scholar]
- 18.Adams HJA, de Klerk JMH, Fijnheer R, et al. Prognostic value of tumor necrosis at CT in diffuse large B-cell lymphoma. Eur J Radiol. 2015; 84: 372-377. [DOI] [PubMed] [Google Scholar]
- 19.Adams HJA, de Klerk JMH, Fijnheer R, et al. Tumor necrosis at FDG-PET is an independent predictor of outcome in diffuse large B-cell lymphoma. Eur J Radiol. 2016; 85: 304-309. [DOI] [PubMed] [Google Scholar]
- 20.Kominato S, Nakayama T, Sato F, et al. Characterization of chromosomal aberrations in thymic MALT lymphoma. Pathol Int. 2012; 62: 93-98. [DOI] [PubMed] [Google Scholar]
- 21.Wang Y, Link BK, Witzig TE, et al. Impact of concurrent indolent lymphoma on the clinical outcome of newly diagnosed diffuse large B-cell lymphoma. Blood. 2019; 134: 1289-1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Witte H, Biersack H, Kopelke S, et al. Indolent lymphoma with composite histology and simultaneous transformation at initial diagnosis exhibit clinical features similar to de novo diffuse large B-cell lymphoma. Oncotarget. 2018; 9: 19613-19622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rymkiewicz G, Ptaszyński K, Walewski J, et al. Unusual cyclin D1 positive marginal zone lymphoma of mediastinum. Med Oncol. 2006; 23: 423-428. [DOI] [PubMed] [Google Scholar]
- 24.Lorsbach RB, Pinkus GS, Shahsafaei A, Dorfman DM. Primary marginal zone lymphoma of the thymus. Am J Clin Pathol. 2000; 113: 784-791. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.