Abstract
After a long period of endeavor, immunotherapy has become the mainstream of cancer therapies. This success is mostly ascribed to immune checkpoint blockade, chimeric antigen receptor-transduced T cell therapies, and bispecific antibodies. However, these methods have been effective or applicable to only a limited proportion of patients so far. Thus, further development of broadly applicable and effective immunotherapies is eagerly anticipated. Given that innate immunity is key to the induction of robust adaptive immunity and that the immunosuppressive tumor microenvironment is a major hurdle to overcome, intratumoral immunotherapy in which delivery of immunostimulatory microbial agents to the tumor site triggers innate immunity in situ is a rational strategy. There has been a plethora of preclinical and clinical trials conducted involving the delivery of either mimetics of viral nucleic acids or oncolytic viruses intratumorally to trigger innate immunity via various nucleic acid sensors in the tumor site. Many of these have shown significant antitumor effects in mice, particularly in combination with immune checkpoint blockade. Oncolytic herpes simplex virus type 1 has been approved for the treatment of advanced melanoma in the United States and Europe and of glioblastoma in Japan. Whereas direct intratumoral administration has mainly been chosen as a delivery route, several promising compounds amenable to systemic administration have been developed. Intratumoral delivery of immunostimulatory agents will become an important option for cancer immunotherapy as an off-the-shelf, broadly applicable, and rational strategy that exploits the physiology of immunity, namely anti-microbial immunity.
Keywords: intratumoral immunotherapy, innate immunity, STING (stimulator of interferon gene), Toll-like receptor, oncolytic virus
INTRODUCTION
Immunotherapy has recently been revolutionizing the strategy of cancer therapies. Although extensive efforts to develop cancer vaccines have generated only unsatisfactory results, the more-recent development of immune checkpoint blockade, chimeric antigen receptor (CAR)-transduced T cell therapies, and bispecific antibodies has achieved clinically meaningful outcomes. However, immune checkpoint blockade alone is effective for only a proportion of patients. CAR-T cell therapies and bispecific antibodies are applicable to limited B-cell malignancies so far. Thus, it is necessary to develop cancer immunotherapies effective for a broader range of patients.
This review will present the revival of cancer vaccines in the form of intratumoral immunotherapy that exploits antiviral innate immunity in situ at the tumor site. The review will start with a comparison of immunity against cancer with that against microbial pathogens and discussion of the importance of innate immunity in provoking strong adaptive immune responses to a broad range of cancers. The paper will then focus on intratumoral delivery of either mimetics of viral nucleic acids or oncolytic viruses, for which many clinical trials are in progress.
HISTORY OF CANCER IMMUNOTHERAPY
Why is it so difficult to induce effective immune responses to cancer? In principle, the immune system is activated by “danger” and “foreignness”, which are determined by pathogen-associated molecular patterns (PAMPs) or damage-associated molecular patterns that trigger innate immunity through pattern recognition receptors1 and by unexperienced, thereby non-tolerized, antigens that trigger adaptive immunity. Such properties of immunity enable it to react readily to microbial pathogens that have PAMPs and antigens absent in our own cells. In contrast, cancer cells lack PAMPs and share most of the molecules with normal cells, thus resulting in difficulty in inducing antitumor immune responses. Furthermore, the tumor microenvironment (TME) is hostile to antitumor effector cells in most cases due to the presence of immunosuppressive cells (e.g., myeloid-derived suppressor cells and regulatory T cells) and molecules (e.g., interleukin [IL]-10, transforming growth factor-β, and programmed cell death [PD]-1 ligands) in the tumor site.2 To overcome these multiple hurdles to induce effective antitumor immune responses, it will be helpful to convert tumor tissues to “infectious” ones by transferring PAMPs to tumor sites, thereby generating an immunogenic TME. The first example of such attempts is “Coley’s toxins” named after a New York surgeon William Coley (active career 1891–1936).3 Coley’s toxins are a mixture consisting of heat-killed Streptococcus pyogenes and Serratia marcescens. Coley’s toxins injected intratumorally, if accessible, induced remarkable antitumor responses in particular types of malignancies (i.e., sarcomas). Of note, more than 20% of patients with soft-tissue sarcomas were rendered free of clinical evidence of disease for at least 20 years. However, after Coley’s death, clinical interest in the use of his vaccine diminished while more broadly applicable chemotherapy and radiation therapy became prevalent, and Coley’s toxins faded from oncologists’ memory.
RESURGENCE OF INTRATUMORAL IMMUNOTHERAPY
Immune responses to microbial pathogens are composed of a series of events: triggering innate immune responses by PAMPs at the infection site, migration of activated dendritic cells (DCs) carrying microbial antigens to the draining lymph nodes, activation of antigen-specific T cells, and their migration to the infection site. For an antitumor immune response to lead to effective killing of tumor cells, similar stepwise events need to happen. Such a series of events in cancer is called “the cancer-immunity cycle” (Figure 1).4 In the first step, tumor cells die and release tumor-associated antigens including neoantigens created by somatic mutations during oncogenesis.5 Intratumoral DCs capture these tumor antigens, carry them to the draining lymph nodes, and activate tumor antigen-specific T cells. The T cells subsequently migrate to the tumor sites and kill tumor cells. For these steps to occur efficiently, the tumor sites need to be enriched with immunostimulatory factors that activate DCs initially and attract tumor-specific T cells finally. In reality, however, the immunosuppressive TME hampers effective antitumor immune responses. Converting such a “cold” tumor into a “hot” one, namely creating an immunogenic TME as observed in an infection site, constitutes a key strategy to generate and maintain the cancer-immunity cycle. In fact, Coley’s toxins exactly represent such a strategy, in which PAMPs injected into tumor sites convert a “cold” tumor into a “hot” one. As the importance of modulating the TME has been recognized, such a strategy as the intratumoral administration of PAMPs has been resurging during the last decade. Such methods are collectively called in situ cancer vaccination6 or intratumoral immunotherapy.7 In particular, a large number of clinical trials have been focusing on various agonists of nucleic acid sensors and oncolytic viruses.
Fig. 1.
The cancer-immunity cycle
The generation of immunity to cancer is a cyclic process that can be self-propagating. (1) Dying tumor cells (dotted circle) release tumor antigens. (2) Intratumoral DCs engulf the tumor antigens and carry them to the draining lymph node. (3) The DCs prime tumor antigen-specific T cells. (4) The tumor-reactive T cells traffic to the tumor site via blood vessels. (5) The T cells recognize and kill tumor cells. How efficiently this cycle is triggered and maintained is key to the success of cancer immunotherapy.
AGONISTS OF NUCLEIC ACID SENSORS
Microbial DNA and RNA represent a major class of PAMPs and are recognized by nucleic acid-sensing pattern recognition receptors located in the endosome (Toll-like receptors [TLRs]) and the cytosol (such as cyclic GMP-AMP synthase [cGAS] and retinoic acid inducible gene I [RIG-I]-like receptors) (Figure 2).8 Viral genomes of DNA viruses trigger DNA sensors such as TLR9 and the cGAS- stimulator of interferon gene (STING) pathway, whereas those of RNA viruses trigger RNA sensors such as TLR3, TLR7, TLR8, and RIG-I-like receptors (RIG-I and melanoma-differentiation-associated gene 5 [MDA5]).8 cGAS recognizes viral DNA and catalyzes the formation of cyclic dinucleotide GMP-AMP (cGAMP), which activates STING, an endoplasmic reticulum-resident transmembrane protein, to induce the production of type I interferon (IFN) and proinflammatory cytokines.9 Notably, dying tumor cell-derived DNA is transferred to host antigen-presenting cells including DCs and stimulates them to produce IFN-β via the cGAS-STING pathway, thus functioning as damage-associated molecular patterns.10 It has also been reported that damaged endogenous DNA generated by genomic instability in tumor cells gain access to cytosolic cGAS in their own cells. cGAMP generated by cGAS in tumor cells is transferred to surrounding cells including DCs.11,12 Then STING in these cells is activated, leading to the induction of spontaneous antitumor immune responses.10 Such mechanisms as the STING-mediated pathway can be exploited for intratumoral immunotherapy, based on a mouse model in which intratumoral injection of a STING agonist induced effective antitumor T-cell responses.13 Such therapy has been combined with immune checkpoint inhibitors in many clinical trials, because these two modalities complement each other.14 Intratumoral immunostimulating agents create an immunogenic TME enabling the recruitment of T cells to a tumor, while immune checkpoint inhibitors release incoming T cells from the constraint of inhibitory signals.
Fig. 2.
Nucleic acid-sensing innate immune signaling
The nucleic acid sensing system plays a fundamental role in anti-viral immunity. A cytosolic DNA sensor cGAS recognizes DNA from DNA viruses and generate the second messenger cGAMP, which activates STING located on the endoplasmic reticulum. TLR9 in the endosome also recognizes virus-derived DNA and transmits the downstream signal through the adaptor MyD88. Notably, DNA released from dying tumor cells is also incorporated into intratumoral DCs and activates them through STING and TLR9. TLR7 and TLR8 in the endosome recognize single-stranded RNA (ssRNA) from RNA virus and transmits the downstream signal through the adaptor MyD88, whereas TLR3 in the endosome recognizes double-stranded RNA (dsRNA) from RNA viruses and transmits the downstream signal through a different adaptor, TRIF/TICAM-1. Cytosolic RNA sensors, RIG-I-like receptors (RIG-I and MDA5), also recognize dsRNA and transmit the downstream signal though the adaptor IPS-1/MAVS. Engagement of all these nucleic acid sensors leads to the final common pathways: the TBK1-IRF3/7 pathway to the production of type I IFN (IFN-α/β) and the IKK-NF-κB pathway to the production of inflammatory cytokines such as TNF and IL-6. Whereas the cytosolic nucleic acid sensors are expressed broadly in numerous tissue types of both immune and non-immune origin, expression of the endosomal TLRs is largely restricted to antigen-presenting cells including monocytes, macrophages, DCs, and B cells. MyD88, myeloid differentiation primary response 88; TRIF, Toll-interleukin-1 receptor domain-containing adapter inducing interferon-β; TICAM-1, Toll-like receptor adaptor molecule 1; IPS-1, interferon-β promoter stimulator 1; MAVS, mitochondrial antiviral signaling; TBK1, TNAK-binding kinase 1; IRF, interferon regulatory factor; IKK, IκB kinase; NF-κB, nuclear factor-κB.
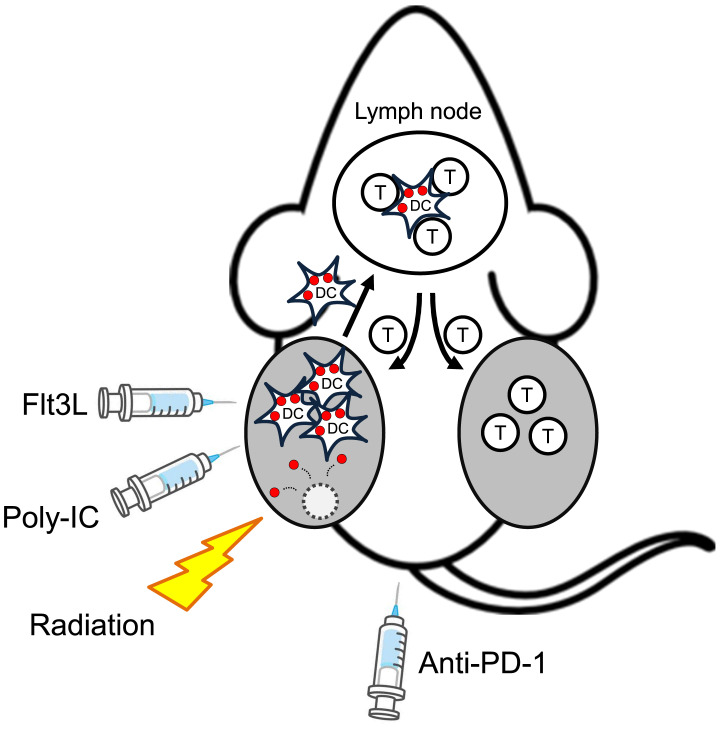
There have also been many clinical trials of intratumoral administration of agonists for nucleic acid-sensing TLRs.14 Among them, a promising strategy using a TLR3 agonist for indolent lymphoma has been reported.15 TLR3 recognizes double-stranded RNA (dsRNA) and transmits a signal to induce the production of type I IFN. Polyinosinic: polycytidylic acid (poly-IC) is a synthetic TLR3 agonist due to its structural similarity to dsRNA.16 Notably, mouse conventional type 1 DCs (cDC1) and their human equivalent, CD141+ DCs, express a high level of TLR3, secrete type I IFN in response to poly-IC, and efficiently cross-present endocytosed antigens to CD8+ T cells.17 Thus, activation of this DC subset in the tumor site is expected to induce effective antitumor immune responses. A combination of four components, intratumoral injection of fms-like tyrosine kinase 3 ligand (Flt3L) and poly-IC, local radiotherapy, and systemic administration of anti-PD-1 antibody exhibited a maximal antitumor effect on subcutaneous A20 lymphoma in mice (Figure 3).15 In this combination, Flt3L mobilizes DC precursors and expands conventional and plasmacytoid DCs at the tumor site. Local radiotherapy induces immunogenic tumor cell death,18 which contributes to the activation of DCs. Intratumorally injected poly-IC further activates the cross-presenting TLR3+ cDC1. The combined effect leads to engulfment of tumor antigens by activated cross-presenting DCs abundant at the tumor site. In this situation, expression levels of PD-L1 and PD-1 become upregulated on tumor cells and T cells, respectively. Accordingly, anti-PD-1 further enhances the antitumor effect of the triple combination of Flt3L, poly-IC, and radiotherapy. Of note, the combination therapy was effective not only for the Flt3L/poly-IC-injected tumor but also for the contralateral non-injected tumor. Such a systemic effect of a local therapy on remote tumors is called the abscopal effect (Figure 3).19 It originally referred to the fact that localized radiotherapy can induce an antitumor effect on distant tumors, and such an effect is also essential for intratumoral immunotherapy to be systemically effective. The combination therapy of Flt3L, poly-IC, and radiotherapy has been translated into a phase I/II trial for advanced indolent lymphoma that has at least one site of disease accessible for intratumoral injection percutaneously (NCT01976585).15 Eight of 11 patients had partial or complete regression of the treated tumor. At distant tumors, 3 patients showed significant regressions and 6 had stable disease or minor regressions. Adding PD-1 blockade is expected to improve the outcome, as observed in the preclinical model.
Fig. 3.
The abscopal effect induced by intratumoral administration of immunostimulatory agents
Hammerich et al.15 reported that intratumoral administration of DC-recruiting Flt3L, DC-activating poly-IC, together with tumor-killing radiation, to subcutaneous A20 mouse lymphoma induced tumor cell death (dotted circle) that released tumor antigens (red circles) and engulfment of them by locally accumulated and activated cross-presenting cDC1. The activated DCs migrate to the draining lymph nodes and prime tumor-reactive T cells. The activated T cells migrate not only to the injected tumor but also to non-injected tumors, thus exhibiting the abscopal effect. As the treatment upregulates the expression of PD-L1 and PD-1 on tumor cells and immune cells in the tumor, anti-PD-1 antibody augments the antitumor immune responses.
mRNA-based cancer vaccines are also promising. Although vaccines using in vitro transcribed mRNA have been investigated, mRNA instability, high innate immunogenicity, and inefficient in vivo delivery hampered the development of mRNA vaccines.20 However, major technological innovations have enabled mRNA to become a promising tool for vaccination against cancer and infection. The latter has culminated in the great success of mRNA vaccines against SARS-CoV-2.21 mRNA vaccines have several important advantages such as safety due to virtually no risk of insertional mutagenesis and the ease of synthesis and scalability of GMP-compliant mRNA production. Development of an appropriate packaging system to protect mRNA and enhance delivery contributed to the success of mRNA vaccines. In addition, mRNA itself functions as an adjuvant by stimulating cells through RNA-recognizing TLRs (TLR3, TLR7, and TLR8) and cytosolic RNA sensors (e.g., RIG-I and MDA5). There are many preclinical and clinical trials of mRNA-based cancer vaccines that transduce immunostimulatory molecules into cells in the tumor sites.20 A promising example is intratumoral delivery in mice of a combination of OX40L-, CD80-, and CD86-encoding mRNA combined with a novel biodegradable carrier called charge-altering releasable transporters.22 The intratumorally injected mRNA-charge-altering releasable transporter complexes transfect various cells in the tumor site such as DCs, macrophages, T cells, and tumor cells, and expression of OX40L, CD80, and CD86 on these cells lead to strong stimulation of T cells. By delivering mRNAs encoding various combinations of immunostimulatory molecules to the tumor site, it may be possible to induce optimal antitumor immune responses.
ONCOLYTIC VIRUSES
Another group of promising intratumoral therapeutics is oncolytic viruses. These are genetically engineered or naturally occurring viruses that can selectively replicate in and kill tumor cells without harming normal cells (Figure 4).23 Originally, oncolytic viruses had been invented based on many anecdotal reports that hematological malignancies such as leukemia and lymphoma resolved after viral infection.24-26 Although oncolytic viruses were thought to have an antitumor effect mainly through direct killing activity, namely oncolysis, it is now recognized that indirect immune-mediated mechanisms play a dominant role in the antitumor effect.23 A variety of viruses have been used for oncolytic virus therapy, including DNA (adenovirus, herpes simplex virus [HSV], vaccinia virus) and RNA (reovirus, coxsackievirus, measles virus, Newcastle disease virus, vesicular stomatitis virus) viruses.27
Fig. 4.
The concept of oncolytic virus therapy
Genetically engineered oncolytic viruses exploit biochemical differences in survival or antiviral signaling between tumor and normal cells so that the viruses replicate in tumor cells and induce immunogenic tumor cell death but do not do so in normal cells. The replicating viruses are released from dying tumor cells (dotted circle) and further infect neighboring tumor cells. Intratumoral DCs activated by viruses and immunogenically killed tumor cells engulf tumor antigens (red circles) released from dying tumor cells (dotted circle) and prime tumor-reactive T cells in the draining lymph node, thus generating the cancer-immunity cycle.
Oncolytic viruses are mainly injected intratumorally. They provoke innate immune responses in the tumor site by engaging nucleic acid sensors and by inducing immunogenic cell death of tumor cells, thus converting the immunosuppressive TME to an immunogenic one. This leads to generation of the cancer-immunity cycle (Figure 4).4 Thus, oncolytic virus therapy represents a rational cancer immunotherapy.
Among oncolytic viruses, HSV-1 has been leading the field.28 Oncolytic HSV-1 had been originally developed to treat glioma.29 A recent phase I trial of genetically engineered oncolytic HSV-1 for pediatric high-grade glioma showed a good safety profile and robust infiltration of T cells into the tumor site.30 Oncolytic HSV-1, G47Δ (teserpaturev), carrying three mutations that enhance viral replication and tumor immunogenicity31 improved the overall survival rate at 1 year compared with the historical control in a phase II trial for residual or recurrent glioblastoma (UMIN000015995) and has recently been approved in Japan.
Oncolytic HSV-1 encoding granulocyte-macrophage colony-stimulating factor (talimogene laherparepvec: T-VEC) has also been approved for the treatment of unresectable stage IIIB, IIIC or IVM1a melanoma in Europe and up to IVM1c melanoma in the USA.32,33 Intratumoral injection of T-VEC induced significantly longer durable responses than the control arm (subcutaneous granulocyte-macrophage colony-stimulating factor). T-VEC improved overall survival in patients without visceral metastases. The combination of T-VEC and anti-CTLA-4 antibody ipilimumab induced significantly better objective responses including visceral lesions without additional safety concerns, compared with ipilimumab alone in patients with advanced melanoma.34 Furthermore, the combination of T-VEC and anti-PD-1 antibody pembrolizumab for stage IIIB-IV melanoma showed promising results in a phase Ib trial, with a 62% overall response rate and 33% complete response rate.35 T-VEC is currently being tested in phase I trials together with an anti-PD-1 or anti-PD-L1 antibody for its safety and efficacy in primary and metastatic liver cancer (NCT02509507) and triple negative breast cancer (NCT03802604).
Oncolytic HSV-1 may also be effective for hematological malignancies. HSV-1 killed various lineages of hematological tumor cells.36,37 Notably, intratumoral injection of T-VEC into primary cutaneous B-cell lymphoma in a phase I trial induced 6 complete responses and 5 partial responses out of 13 patients in injected lesions.38 Two out of 3 patients with non-injected lesions showed an abscopal effect on these lesions. Single-cell sequences of fine-needle aspirates demonstrated that remodeling of the TME can happen in both injected and non-injected lesions.
SYSTEMIC DELIVERY OF AGONISTS
Compared with systemic administration, intratumoral administration avoids systemic off-target toxicities, reduces the amounts of drug required, and achieves high local concentrations. However, deep-seated lesions may not be easily accessible for intratumoral injection. In addition, intrinsic differences, such as the clonal heterogeneity of tumor cells and variations in antigen repertoire, have been described between primary tumors and growing metastases within the same individual.39 Therefore, differences in distal lesions rendering them untargetable by immune responses induced in the treated lesion may limit the efficacy of intratumoral approaches for inducing abscopal effects. To overcome these limitations, systemic administration of nucleic acid sensor agonists and oncolytic viruses has been attempted.
The natural cyclic dinucleotide ligands for STING are not suitable for systemic administration due to their metabolic instability. Thus, nucleic acid sensor agonists that are metabolically stable and preferentially targeted to tumor sites need to be developed. High-throughput screening identified a non-nucleotide STING agonist that is more than 400-fold more potent than cGAMP in inducing IFN-β production by human blood mononuclear cells in vitro.40 Intravenous administration of the compound also induced potent antitumor responses in tumor-bearing mice in vivo. Two other non-nucleotide STING agonists available for systemic administration have been reported.41,42 Notably, one of them is amenable to oral administration and becomes an active dimer in acidic conditions.41 Thus, this compound may engage STING in the acidified TME preferentially, thereby mitigating off-tumor toxicity in normal tissue. Intravenous STING agonists are being evaluated in clinical trials (NCT03843359, NCT04420884, and NCT04096638).
To target an immunostimulatory agent to the tumor site after systemic administration, immune-stimulating antibody conjugates (ISACs) comprising a TLR7/8 dual agonist conjugated to tumor-targeting antibodies have been developed.43 Systemically administered human epidermal growth factor receptor 2 (HER2)-targeted ISACs localize to the HER2+ tumor site, where TLR7/8+ myeloid antigen-presenting cells phagocytose the ISACs-bound tumor cells through Fcγ receptors, become activated, and induce tumor-specific T cell responses in the draining lymph nodes. Anti-HER2-TLR7/8 agonist ISAC therapy induced a durable antitumor immune response in a HER2+ tumor-bearing mouse model, and it is being investigated as a single agent or in combination with anti-PD-1 antibody in a phase I/II trial (NCT04278144).
Because of the shortcomings of intratumoral administration described here, systemic intravenous administration of oncolytic viruses has been attempted.44 For example, recombinant oncolytic vaccinia virus JX-594 was administered intravenously to patients with treatment-refractory solid tumors in a phase I trial.45 As vaccinia virus has evolved mechanisms for intravenous stability and spread to distant tissues, JX-594 was successfully delivered to metastatic tumor sites and replicated there in a dose-dependent manner. Dose-related antitumor activity was also demonstrated. Whereas this study showed a promising result, limited success has been reported with intravenous delivery of oncolytic viruses in other trials so far. This is because of the requirement for a large amount of infectious viral particles owing to dilution in the blood volume, rapid clearance by neutralizing antibodies, sequestration in non-target organs, and inability of the virus to extravasate through the tumor vasculature efficiently. To overcome these hurdles, delivery system such as cell carriers and lipid nanoparticles have been tried to shield oncolytic virus from neutralizing antibodies and to increase a tropism for tumor tissues.44 As oncolytic viruses are genetically engineered so that they cannot actively replicate in normal cells, virus-infected normal carrier cells can convey oncolytic viruses to tumor sites without being killed.
CONCLUSION
Delivery of nucleic acid sensor agonists or oncolytic viruses to the tumor site is gaining traction because of various advantages including its nature as an “off-the-shelf” vaccine, conversion of the immunosuppressive TME to an immunogenic one, and no necessity for prior identification of tumor antigens. Direct intratumoral administration has additional advantages such as the high achievable local concentrations with lower doses and reduced off-target toxicities. Recent development of novel compounds amenable to intravenous or even oral administration40-42 will greatly broaden the applicability of the intratumoral delivery to anatomically inaccessible tumors. As intratumoral immunotherapy and immune checkpoint blockade is a rational combination, numerous clinical trials of such combination immunotherapy are ongoing.14
Although a lot of research has presented a promising outlook for intratumoral immunotherapy, there are still multiple outstanding questions. Which immunotherapeutic agent (e.g., agonists for STING, TLR3, TLR7/8 or TLR9 and oncolytic virus) is more potent than others? Are any of the agents more potent than others, depending on the cancer type or specific patient? In the case of direct intratumoral administration, how can we enhance the abscopal effect by combining with other therapeutic modalities (e.g., immune checkpoint blockade, chemotherapy, or targeted therapy)? Will systemic delivery of immunotherapeutic agents cause unacceptable off-target toxicities? Further basic and clinical research will give answers and expand the possibility of intratumoral cancer immunotherapy, given that this strategy is rational in that it is based on the physiology of immunity, namely anti-microbial immunity, which Coley’s toxins successfully exploited to combat cancer 100 years ago.
ACKNOWLEDGMENTS
The author thanks Haruyuki Fujita and Jun-ichiro Kida (Kagawa University, Japan) for reviewing the manuscript.
Footnotes
CONFLICT OF INTEREST
The author declares no conflict of interest.
REFERENCES
- 1.Amarante-Mendes GP, Adjemian S, Branco LM, et al. Pattern Recognition Receptors and the Host Cell Death Molecular Machinery. Front Immunol. 2018; 9: 2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Binnewies M, Roberts EW, Kersten K, et al. Understanding the tumor immune microenvironment (TIME) for effective therapy. Nat Med. 2018; 24: 541-550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Starnes CO. Coley’s toxins in perspective. Nature. 1992; 357: 11-12. [DOI] [PubMed] [Google Scholar]
- 4.Chen DS, Mellman I. Oncology meets immunology: the cancer-immunity cycle. Immunity. 2013; 39: 1-10. [DOI] [PubMed] [Google Scholar]
- 5.Schumacher TN, Scheper W, Kvistborg P. Cancer Neoantigens. Annu Rev Immunol. 2019; 37: 173-200. [DOI] [PubMed] [Google Scholar]
- 6.Hammerich L, Binder A, Brody JD. In situ vaccination: cancer immunotherapy both personalized and off-the-shelf. Mol Oncol. 2015; 9: 1966-1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marabelle A, Tselikas L, de Baere T, Houot R. Intratumoral immunotherapy: using the tumor as the remedy. Ann Oncol. 2017; 28: xii33-xii43. [DOI] [PubMed] [Google Scholar]
- 8.McWhirter SM, Jefferies CA. Nucleic Acid Sensors as Therapeutic Targets for Human Disease. Immunity. 2020; 53: 78-97. [DOI] [PubMed] [Google Scholar]
- 9.Burdette DL, Vance RE. STING and the innate immune response to nucleic acids in the cytosol. Nat Immunol. 2013; 14: 19-26. [DOI] [PubMed] [Google Scholar]
- 10.Woo SR, Fuertes MB, Corrales L, et al. STING-dependent cytosolic DNA sensing mediates innate immune recognition of immunogenic tumors. Immunity. 2014; 41: 830-842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ablasser A, Schmid-Burgk JL, Hemmerling I, et al. Cell intrinsic immunity spreads to bystander cells via the intercellular transfer of cGAMP. Nature. 2013; 503: 530-534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marcus A, Mao AJ, Lensink-Vasan M, et al. Tumor-Derived cGAMP Triggers a STING-Mediated Interferon Response in Non-tumor Cells to Activate the NK Cell Response. Immunity. 2018; 49: 754-763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Corrales L, Glickman LH, McWhirter SM, et al. Direct Activation of STING in the Tumor Microenvironment Leads to Potent and Systemic Tumor Regression and Immunity. Cell Rep. 2015; 11: 1018-1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen M, Hu S, Li Y, et al. Targeting nuclear acid-mediated immunity in cancer immune checkpoint inhibitor therapies. Signal Transduct Target Ther. 2020; 5: 270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hammerich L, Marron TU, Upadhyay R, et al. Systemic clinical tumor regressions and potentiation of PD1 blockade with in situ vaccination. Nat Med. 2019; 25: 814-824. [DOI] [PubMed] [Google Scholar]
- 16.Matsumoto M, Seya T. TLR3: Interferon induction by double-stranded RNA including poly(I:C). Adv Drug Deliv Rev. 2008; 60: 805-812. [DOI] [PubMed] [Google Scholar]
- 17.Villadangos JA, Shortman K. Found in translation: the human equivalent of mouse CD8+ dendritic cells. J Exp Med. 2010; 207: 1131-1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kroemer G, Galluzzi L, Kepp O, Zitvogel L. Immunogenic cell death in cancer therapy. Annu Rev Immunol. 2013; 31: 51-72. [DOI] [PubMed] [Google Scholar]
- 19.Ngwa W, Irabor OC, Schoenfeld JD, et al. Using immunotherapy to boost the abscopal effect. Nat Rev Cancer. 2018; 18: 313-322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pardi N, Hogan MJ, Porter FW, Weissman D. mRNA vaccines— a new era in vaccinology. Nat Rev Drug Discov. 2018; 17: 261-279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Polack FP, Thomas SJ, Kitchin N, et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N Engl J Med. 2020; 383: 2603-2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Haabeth OAW, Blake TR, McKinlay CJ, et al. Local Delivery of Ox40l, Cd80, and Cd86 mRNA Kindles Global Anticancer Immunity. Cancer Res. 2019; 79: 1624-1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bommareddy PK, Shettigar M, Kaufman HL. Integrating oncolytic viruses in combination cancer immunotherapy. Nat Rev Immunol. 2018; 18: 498-513. [DOI] [PubMed] [Google Scholar]
- 24.Bierman HR, Crile DM, Dod KS, et al. Remissions in leukemia of childhood following acute infectious disease: staphylococcus and streptococcus, varicella, and feline panleukopenias. Cancer. 1953; 6: 591-605. [DOI] [PubMed] [Google Scholar]
- 25.Bluming AZ, Ziegler JL. Regression of Burkitt’s lymphoma in association with measles infection. Lancet. 1971; 298: 105-106. [DOI] [PubMed] [Google Scholar]
- 26.Taqi AM, Abdurrahman MB, Yakubu AM, Fleming AF. Regression of Hodgkin’s disease after measles. Lancet. 1981; 317: 1112. [DOI] [PubMed] [Google Scholar]
- 27.Kaufman HL, Kohlhapp FJ, Zloza A. Oncolytic viruses: a new class of immunotherapy drugs. Nat Rev Drug Discov. 2015; 14: 642-662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bommareddy PK, Peters C, Saha D, Rabkin SD, Kaufman HL. Oncolytic Herpes Simplex Viruses as a Paradigm for the Treatment of Cancer. Annu Rev Cancer Biol. 2018; 2: 155-173. [Google Scholar]
- 29.Martuza RL, Malick A, Markert JM, Ruffner KL, Coen DM. Experimental therapy of human glioma by means of a genetically engineered virus mutant. Science. 1991; 252: 854-856. [DOI] [PubMed] [Google Scholar]
- 30.Friedman GK, Johnston JM, Bag AK, et al. Oncolytic HSV-1 G207 Immunovirotherapy for Pediatric High-Grade Gliomas. N Engl J Med. 2021; 384: 1613-1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Todo T, Martuza RL, Rabkin SD, Johnson PA. Oncolytic herpes simplex virus vector with enhanced MHC class I presentation and tumor cell killing. Proc Natl Acad Sci USA. 2001; 98: 6396-6401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Andtbacka RHI, Kaufman HL, Collichio F, et al. Talimogene Laherparepvec Improves Durable Response Rate in Patients With Advanced Melanoma. J Clin Oncol. 2015; 33: 2780-2788. [DOI] [PubMed] [Google Scholar]
- 33.Ressler JM, Karasek M, Koch L, et al. Real-life use of talimogene laherparepvec (T-VEC) in melanoma patients in centers in Austria, Switzerland and Germany. J Immunother Cancer. 2021; 9: e001701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chesney J, Puzanov I, Collichio F, et al. Randomized, Open-Label Phase II Study Evaluating the Efficacy and Safety of Talimogene Laherparepvec in Combination With Ipilimumab Versus Ipilimumab Alone in Patients With Advanced, Unresectable Melanoma. J Clin Oncol. 2018; 36: 1658-1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ribas A, Dummer R, Puzanov I, et al. Oncolytic Virotherapy Promotes Intratumoral T Cell Infiltration and Improves Anti-PD-1 Immunotherapy. Cell. 2017; 170: 1109-1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ishino R, Kawase Y, Kitawaki T, et al. Oncolytic Virus Therapy with HSV-1 for Hematological Malignancies. Mol Ther. 2021; 29: 762-774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Oku M, Ishino R, Uchida S, et al. Oncolytic herpes simplex virus type 1 (HSV-1) in combination with lenalidomide for plasma cell neoplasms. Br J Haematol. 2021; 192: 343-353. [DOI] [PubMed] [Google Scholar]
- 38.Ramelyte E, Tastanova A, Balázs Z, et al. Oncolytic virotherapy-mediated anti-tumor response: a single-cell perspective. Cancer Cell. 2021; 39: 394-406. [DOI] [PubMed] [Google Scholar]
- 39.Jiménez-Sánchez A, Memon D, Pourpe S, et al. Heterogeneous Tumor-Immune Microenvironments among Differentially Growing Metastases in an Ovarian Cancer Patient. Cell. 2017; 170: 927-938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ramanjulu JM, Pesiridis GS, Yang J, et al. Design of amidobenzimidazole STING receptor agonists with systemic activity. Nature. 2018; 564: 439-443. [DOI] [PubMed] [Google Scholar]
- 41.Pan BS, Perera SA, Piesvaux JA, et al. An orally available non-nucleotide STING agonist with antitumor activity. Science. 2020; 369: eaba6098. [DOI] [PubMed] [Google Scholar]
- 42.Chin EN, Yu C, Vartabedian VF, et al. Antitumor activity of a systemic STING-activating non-nucleotide cGAMP mimetic. Science. 2020; 369: 993-999. [DOI] [PubMed] [Google Scholar]
- 43.Ackerman SE, Pearson CI, Gregorio JD, et al. Immune-stimulating antibody conjugates elicit robust myeloid activation and durable antitumor immunity. Nat Can. 2021; 2: 18-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Harrington K, Freeman DJ, Kelly B, Harper J, Soria JC. Optimizing oncolytic virotherapy in cancer treatment. Nat Rev Drug Discov. 2019; 18: 689-706. [DOI] [PubMed] [Google Scholar]
- 45.Breitbach CJ, Burke J, Jonker D, et al. Intravenous delivery of a multi-mechanistic cancer-targeted oncolytic poxvirus in humans. Nature. 2011; 477: 99-102. [DOI] [PubMed] [Google Scholar]