Abstract
We are presenting a case of 51‐year‐old female patient, diagnosed with high‐grade NHL‐DLBCL by PET‐CT scan. Immediately, she was started with Rasayana therapy, a specially designed anti‐cancer treatment regimen by our clinic. We observed significant clinical improvement and regression in tumor size in this patient after treatment.
Keywords: Ayurveda, cancer, ECOG, FACT G, lymphoma
Ayurveda‐based Rasayana therapy may serve as a safe and effective alternative treatment option for lymphoma patients who are not eligible for conventional standard of care.

1. INTRODUCTION
Non‐Hodgkin lymphoma (NHL) is a cancer of the lymphatic system. 1 , 2 Diffuse large B‐cell lymphoma (DLBCL) is a type of non‐Hodgkin lymphoma (NHL). 3 , 4 It is the most common form of non‐Hodgkin lymphoma among adults. 4 It develops when the body makes abnormal B lymphocytes. DLBCL can develop in the lymph nodes or in “extra‐nodal sites” (areas outside the lymph nodes) such as the gastrointestinal tract, testes, thyroid, skin, breast, bone, brain, or essentially any organ of the body. 5 It may be localized (in one spot) or generalized (spread throughout the body). Diffuse large B‐cell lymphoma (DLBCL) tends to grow quickly. Despite being an aggressive lymphoma, DLBCL is considered potentially curable. Most often, the treatment is chemotherapy usually with a regimen of four drugs known as CHOP 6 (cyclophosphamide, doxorubicin, vincristine, and prednisone), plus the monoclonal antibody rituximab (Rituxan). 7 This regimen, known as R‐CHOP, is most often given in cycles 3 weeks apart. In spite of yielding promising results, these treatments pose certain side effects. Chemotherapy kills fast‐growing normal cells, including hair cells and mucosal cells of oral cavity, gut, and bone marrow. 8 , 9
Rasayana is one of the specialized branches of Ayurveda. The word Rasayana means the pathway for essence of nutrition (Rasa) toward all body tissue to help attain optimal nourishment for each tissue at cellular level so as to ensure establishment of normal physiological functions in each cell. Ayurveda classics explains that this mode of action of Rasayana ensures longevity, memory, intelligence, health, youthfulness, excellence of luster, complexion and voice, and optimum development of physique and sense organs. 10 , 11 , 12 , 13 In Ayurveda, number of classical Rasayana compounds from herbs, animals, and minerals are described. Many research studies have proved free radical scavenging (anti‐oxidant), immunomodulatory, and anti‐tumor activities of Rasayana drugs and formulations. 14 , 15 , 16 They work similar to adaptogens, nontoxic herbs that work in a nonspecific way to balance the normal physiology of the body, by acting on the Hypothalamic Pituitary Axis and the neuroendocrine system. 17
At Rasayu Cancer Clinic, we have designed a personalized treatment regimen that is based on various clinical aspects such as age, sex, habitat, type of cancer, comorbidities, adverse events of ongoing conventional treatment, anticipating disease progression, and anticipating risk factors. Our treatment protocol is based on the principle of Rasayana therapy which aims toward the establishment of normal physiology and immune response at cellular level of each tissue (called as Dhatu in Ayurveda). We selected Rasayana formulations which will help restore the normal physiology and cell cycle besides modulating the deranged immune response in cancer patients. Our Rasayana protocol not only targets the diseased tissue (Dhatu) but also it ensures optimal functioning and immune response for entire body (all the seven dhatus mentioned in Ayurveda). This ensures the reduction of risk for disease progression or distant metastasis. Besides this, the property of Rasayana formulations to impart longevity in person may also acts on improving overall survival in cancer patients.
The above‐mentioned principles used in designing the treatment protocol are based on the fundamentals of Rasayana therapy mentioned in Ayurveda. However, there lacks experimental evidence establishing the mechanism of action of Rasayana therapy at cellular levels.
The current study reports a case of a patient with high grade NHL‐DLBCL treated with exclusive rasayana regimen.
2. CASE EXAMINATION
A 51‐year‐old female patient reported abdominal pain and epigastric/gastric burning in March 2018. She underwent investigations to find out the cause. The upper GI endoscopy (12/5/2018) revealed severe ulcerations with mucosal thickening in the cardia and body of stomach. Colonoscopy followed by GI scopy showed Grade II internal hemorrhoids. Considering these findings, CT abdomen was also performed on the same day, which showed wall thickening involving cardia fundus, lesser curvature of stomach (2.1 × 5.6 cm in maximum thickness) associated few perigastric lymph nodes (largest 0.98 × 0.9 cm) and hepatomegaly with multiple cysts in liver. Gastric biopsy (31/5/2018) revealed NHL of diffuse large B‐cell phenotype. The diagnosis was further confirmed on PET‐CT Scan (31/5/2018), which reported metabolically active wall thickening involving cardia, fundus, and lesser curvature of stomach (wall thickening 24 mm and SUV‐14.66) consistent with lymphomatous disease involvement. Multiple simple hepatic cysts (largest‐ 16 × 17 mm) without fluorodeoxyglucose (FDG) uptake were noted involving both lobes of liver.
The patient was not willing to opt for conventional anti‐cancer therapies because of her perceptions regarding its toxicity. Hence, the patient approached Rasayu Cancer Clinic for Ayurvedic treatment immediately after the confirmation of the diagnosis (2/6/2018) with symptoms viz. burning sensation in epigastric and gastric region, flatulence, general weakness, constipation, lumbar pain, and cervical pain. The patient started with the treatment on 02/06/2018 with principle aim to achieve remission in the size of tumor, to extend survival with improved quality of life and physical performance as well as to give symptomatic relief.
2.1. Treatment protocol
This patient was prescribed a rasayana regimen that consisted of following Ayurvedic medicines (Table 1):
TABLE 1.
Ayurvedic Rasayana regimen
| Name of the medicine | Generic/P&P | Dose | Frequency | Main ingredients | Anupan (vehicle) |
|---|---|---|---|---|---|
| Pinzar Rasayana | P&P | 1 cap | OD | Hirak Bhasma—40 mg, Suvarna Bhasma—4 mg, Tamra bhasma—5 mg, Roupya Bhasma—5 mg, Abhrak bhasma—40 mg, Jasad bhasma5 mg, Mauktik Bhasma—120 mg, Hartal Bhasma—30 mg, Chapal Bhasma—10 mg, Rasasindoor—30 mg, Suvarnmakshik Bhasma—60 mg | Honey |
| Praval Panchamrit | Generic | 200 mg | BD | Generic | Lukewarm water |
| Kamdudha Rasa | Generic | 200 mg | BD | Generic | Lukewarm water |
| Laghusutshekhar Rasa | Generic | 200 mg | BD | Generic | Lukewarm water |
| Sutshekhar Rasa | Generic | 62 mg | BD | Generic | Lukewarm water |
| Yashtimadhu Churna | Generic | 200 mg | BD | Single drug | Lukewarm water |
| Praval bhasma | Generic | 200 mg | BD | Generic | Lukewarm water |
| Suranvatak | Generic | 200 mg | BD | Generic | Lukewarm water |
| Heeraka Rasayana | P&P | 1 cap | OD | Hirak Bhasma–12.5 mg, Rasayana churna ghana—100 mg | Honey |
| Dasma Rasayana | P&P | 1 cap | OD | Hirak bhasma—5 mg, Somnathi Tamra bhasma—10 mg | Honey |
| Sutendra Rasayana | P&P | 1 cap | OD | Sutshekhar Rasa—115 mg, Suvarna Bhasma—3 mg, Roupya Bhasma—2 mg, Manganese Bhasma—5 mg | Honey |
| Aarewat Kalpa | P&P | 1 cap | OD | Sonamukhi ghana—125 mg, Argwadh ghana—125 mg | Lukewarm water |
All these formulations are Ayurvedic proprietary medicines. The patient is still continued on treatment (~23 months). During this period, she was asked to report the clinic at monthly interval. At every monthly visit, clinical assessment includes weight, systemic examination, response to past complaints, and any new finding. The quality of life and physical performance assessment was also done at monthly interval using Fact G and ECOG scales, respectively. She underwent blood investigations (CBC, LFT, and RFT) after every 2 months, while PET‐CT scan was done after every 6 months.
2.2. Outcome
After starting Ayurvedic treatment, the patient showed improvement in quality of life as evident by baseline Fact G score 85 to post‐treatment score of 104. (Figure 1). Her physical performance as rated on ECOG also showed improvement from 1 to 0. (Figure 2). She also showed increase in weight from 52.6 to 56.9 kg (Figure 3). Her baseline prognostic nutritional index was 28.87 (which indicates malnutrition) which improved to 49.35.
FIGURE 1.
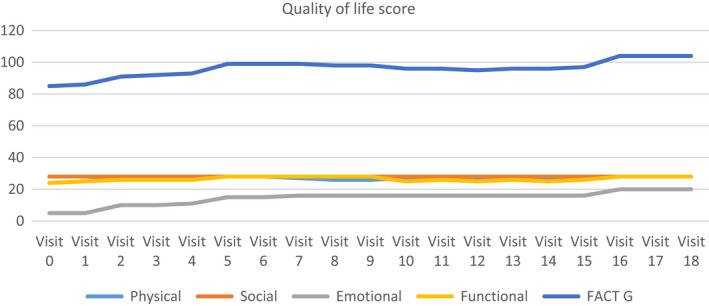
Health related quality of life score measured by Fact G scale
FIGURE 2.
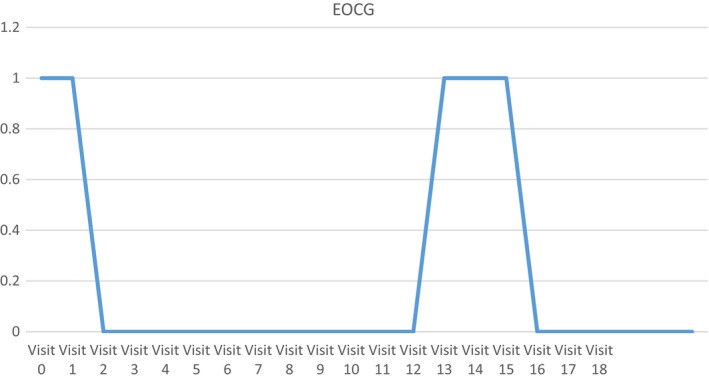
Physical performance status measured by ECOG Scale
FIGURE 3.
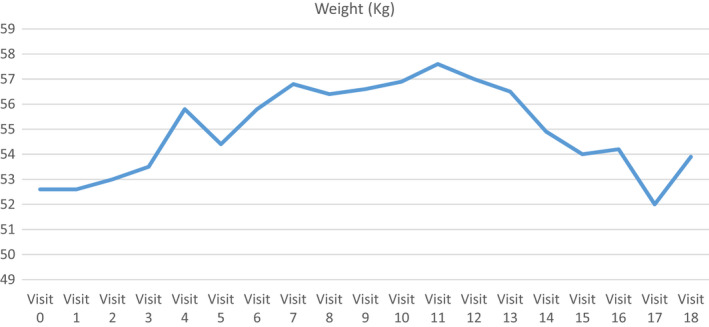
Weight Chart
The patient underwent PET‐CT scan for total 3 times since the start of treatment. These scans showed gradual reduction in residual wall thickening and improvement in SUV. The findings are summarized below. (Table 2). Her biochemical parameters did not show any clinical significant fluctuation (Table 3).
-
PET‐CT scan‐post 6 months of treatment‐04/11/2018
Significant regression in wall thickening and FDG uptake noted involving cardia, fundus, and lesser curvature of stomach. Residual wall thickening—16 mm (SUV −9).
Multiple simple hepatic cysts without FDG uptake noted involving both lobes of liver. Largest—16 × 17 mm.
-
PET‐CT scan‐post 12 months‐ 08/06/2019
Further mild regression in metabolic activity and wall thickening involving cardia, fundus, and lesser curvature of stomach. Residual wall thickening—12 mm (SUV max‐8.2).
Multiple simple hepatic cysts without any FDG avid uptake in both lobes of liver. Larger—18 × 17 mm.
-
PET‐CT scan‐post 18 months‐ 06/12/2019
Further marked regression in the metabolic activity and wall thickening involving cardia, fundus, and lesser curvature of the stomach. Residual wall thickening 8 mm (SUV max—1.3).
Multiple simple hepatic cysts without any FDG avid uptake in both lobes of liver. Larger—20 × 16 mm.
TABLE 2.
Tumor response (Evaluated by PET‐CT scan)
| 31st May 2018 (Baseline) | 4th Nov 2018 Post 6 months | 8th Jun 2019 Post 12 months | 6th Dec 2019 Post 18 months | |
|---|---|---|---|---|
| Thickness of gastric wall | 24 mm | 16 mm | 12 mm | 8 mm |
| SUV | 14.66 | 9 | 8.2 | 1.3 |
| Multiple simple hepatic cysts. Size of largest one | 16 × 17 mm | 16 × 17 mm | 18 × 17 mm | 20 × 16 mm |
| SUV | No FDG uptake | No FDG uptake | No FDG uptake | No FDG uptake |
TABLE 3.
Blood investigations
| Parameters | 02‐Jun‐18 | 14‐Aug‐18 | 26‐Oct‐18 | 07‐Jan‐19 | 10‐Mar‐19 | 06‐May‐19 | 18‐Jul‐19 | 05‐Dec‐19 |
|---|---|---|---|---|---|---|---|---|
| Hb (gm/dl) | 10.6 | 11 | 11.8 | 10.4 | 9.5 | 11.2 | 10.8 | 10.8 |
| WBC cells/cu.mm | 5100 | 3600 | 5700 | 4900 | 6000 | 4900 | 8500 | 3900 |
| Platelet Cells/μl | 289,000 | 254,000 | 233,000 | 214,000 | 208,000 | 245,000 | 199,000 | 456,000 |
| T. Bilirubin mg/dl | 0.22 | 0.52 | 0.4 | 0.58 | 0.7 | 0.62 | 1 | 1 |
| Direct Bilirubin mg/dl | 0.1 | 0.21 | 0.2 | 0.21 | 0.23 | 0.17 | 0.34 | |
| Indirect Bilirubin mg/dl | 0.12 | 0.19 | 0.38 | 0.49 | 0.39 | 0.83 | 0.66 | |
| SGPT U/L | 28 | 19.2 | 15.5 | 30.12 | 38 | 31.12 | 28.3 | 16.2 |
| SGOT U/L | 21 | 15.3 | 24.03 | 24.19 | 14 | 19 | 22.9 | 14.5 |
| ALP U/L | 83 | 258 | 186.3 | 197.6 | 190 | 171.13 | 196.5 | 158.2 |
| T. Protein g/dl | 6.92 | 6.22 | 6.2 | 6.03 | 6.7 | 6.27 | 6.85 | 6.8 |
| Albumin g/dl | 3.37 | 4.16 | 4.02 | 4.19 | 4.2 | 4.03 | 4.55 | 4.5 |
| Globulin g/dl | 3.55 | 2.06 | 2.08 | 1.84 | 2.5 | 2.24 | 2.3 | 2.3 |
| S. Creatinine mg/dl | 0.75 | 0.66 | 0.62 | 0.58 | 0.54 | 1.26 | 0.92 | 0.87 |
| Urea mg/dl | 22 | 19.3 | 15.17 | 18.3 | 16.2 | 14.6 | 12.6 | 35 |
| eGFR (CKD EPI creatinine equation) ml/min/1.73m2 | 92 | 102 | 104 | 106 | 108 | 49 | 72 | 77 |
The upper GI endoscopy was repeated on 25/12/2019, which revealed small area of ulceration with erythema in cardia region. Finally, biopsy was carried out on 01/01/2020, in which no evidence of lymphoma was noted.
3. DISCUSSION
The present case report showcases the management of high‐grade NHL‐DLBCL with Ayurvedic rasayana regimen. The patient did not receive any conventional treatment throughout the treatment duration. We observed complete regression of tumor with Ayurvedic treatment. Our findings give hope to the patients who are unable to tolerate or are not eligible or willing conventional anti‐cancer therapy. Moreover, Ayurveda therapy can be of potential benefits in patents who are resistant to chemotherapeutic agents and in whom no conventional therapies are seen to be effective. In such patients, Ayurvedic treatment can prove as an effective alternative.
The rasayana regimen consists of many bhasmas (incinerated ash of metals) and herbo‐mineral formulations. These drugs are often under microscope due to their alleged safety. However, in our experience, we have not observed signs of toxicity in any of the patients treated at our clinic so far (data being published separately). Even in the present case, the hematological and biochemical values of our patient remained in normal range after treatment for 23 months. Further, no adverse effects were seen throughout the treatment duration. This finding indicates safety of the regimen and indirectly of the metallic formulations.
Interestingly, the after‐treatment biopsy showed no evidence of lymphoma, which is a direct indicator of treatment success. There was an improvement in the quality of life and physical performance of the patient. The weight of patient improved during the treatment course. These are additional benefits of Ayurvedic treatment, which are generally not associated with the conventional treatment. The rasayana treatment regimen has additional benefits such as it can be administered orally. It does not require hospitalization and can be taken by the patient even staying at home. The cost of rasayana treatment is lower than the cost of conventional cancer treatment. All the above‐mentioned points make the rasayana treatment cost‐effective as well.
Further, it will be worth exploring if integrating Rasayana therapy with conventional standard of care will improve therapeutic outcomes in patients of high‐grade NHL‐DLBCL. This anecdotal evidence warrants further clinical studies for conforming these results.
4. CONCLUSION
The present case demonstrated successful management of high‐grade NHL‐DLBCL with exclusive Ayurvedic rasayana regimen.
CONFLICT OF INTEREST
None.
AUTHOR CONTRIBUTIONS
YB, AK, AP, and PB were involved in conceptualization of study, data analysis, and interpretation of results. AP and PB were involved in data collection, clinical examination, and follow‐up of study participants. YB, AK, AP, and PB drafted the manuscript. All the authors reviewed, edited, and approved the final manuscript.
ETHICAL APPROVAL
Personal data have been respected.
CONSENT
Written informed consent was obtained from the patient for publication of this case report and any accompanying images. A copy of the written consent is available for review by the Editor‐in‐Chief of this journal upon request.
ACKNOWLEDGEMENTS
We would like to record my deep sense of gratitude and profound thanks to Dr. Supriya Bhalerao, Assistant Professor, IRSHA, Bharati Vidyapeeth (Deemed to be) University for her keen interest and guidance in manuscript preparation.
Narayan Bendale Y, Kadam A, Patil A, Kantilal Birari‐Gawande P. Complete tumor regression with exclusive Ayurvedic rasayana regimen in high‐grade diffuse large B‐cell lymphoma: A case report. Clin Case Rep. 2022;10:e05696. doi: 10.1002/ccr3.5696
Funding information
None
DATA AVAILABILITY STATEMENT
All data generated or analyzed during this study are included in this published paper.
REFERENCES
- 1. https://www.cancer.org/cancer/non‐hodgkin‐lymphoma/about/what‐is‐non‐hodgkin‐lymphoma.html. Accessed January 12, 2021.
- 2. https://www.nhs.uk/conditions/non‐hodgkin‐lymphoma/n. Accessed January 12, 2021.
- 3. Padala SA, Kallam A. Diffuse large B cell lymphoma. [Updated 2020 May 14]. In StatPearls [Internet]. Stat Pearls Publishing; 2020. https://www.ncbi.nlm.nih.gov/books/NBK557796/ [PubMed] [Google Scholar]
- 4. Liu Y, Barta SK. Diffuse large B‐cell lymphoma: 2019 update on diagnosis, risk stratification, and treatment. Am J Hematol. 2019;94:604‐616. [DOI] [PubMed] [Google Scholar]
- 5. Shen H, Wei Z, Zhou D, et al. Primary extra‐nodal diffuse large B‐cell lymphoma: a prognostic analysis of 141 patients. Oncol Lett. 2018;16:1602‐1614. doi: 10.3892/ol.2018.8803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fisher RI, Gaynor ER, Dahlberg S, et al. Comparison of a standard regimen (CHOP) with three intensive chemotherapy regimens for advanced non‐Hodgkin's lymphoma. N Engl J Med. 1993;328:1002‐1006. [DOI] [PubMed] [Google Scholar]
- 7. Kwak JY. Treatment of diffuse large B cell lymphoma. Korean J Intern Med. 2012;27:369‐377. doi: 10.3904/kjim.2012.27.4.369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Coiffier B, Sarkozy C. Diffuse large B‐cell lymphoma: R‐CHOP failure‐what to do? Hematology Am Soc Hematol Educ Program. 2016;2016:366‐378. doi: 10.1182/asheducation-2016.1.366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Meya U, Hitzb F, Lohric A, et al. Diagnosis and treatment of diffuse large B‐cell lymphoma. Swiss Med Wkly. 2012;142:w13511. [DOI] [PubMed] [Google Scholar]
- 10. Singh AK, Gupta AK, Singh PK. Rasayan therapy: a magic contribution of Ayurveda for healthy long life. Int J Res Ayurveda Pharm. 2014;5:41‐47. [Google Scholar]
- 11. Sharma R, Amin H. Rasayana therapy: Ayurvedic contribution to improve quality of life. World J Pharmacol Res Tech. 2015;4:23‐33. [Google Scholar]
- 12. Balasubramani SP, Venkatasubramanian P, Kukkupuni SK, Patwardhan B. Plant‐based rasayana drugs from Ayurveda. Chin J Integr Med. 2011;17:88‐94. [DOI] [PubMed] [Google Scholar]
- 13. Shastri K, Chaturvedi G, editors. Charak Samhita, Vidyodini Hindi commentary (Chikitsasthan 1st pada, Chp 1, verse 7‐8), Vol II; 16th edn. Chaukhamba Bharati Academy; 1989: 5. [Google Scholar]
- 14. Rekha PS, Kuttan G, Kuttan R. Antioxidant activity of brahma rasayana. Indian J Exp Biol. 2001;39:447‐452. [PubMed] [Google Scholar]
- 15. Mukherjee S, Pawar N, Kulkarni O, et al. Evaluation of free‐radical quenching properties of standard Ayurvedic formulation Vayasthapana rasayana. BMC Complement Altern Med. 2011;11:1‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Baliga MS, Sharake M, Kumar L, Rao S, Palatty PL. Rasayana drugs from the Ayurvedic system of medicine as possible radioprotective agents in cancer treatment. Integr Cancer Ther. 2013;12:455‐463. [DOI] [PubMed] [Google Scholar]
- 17. Chauhan N, Saraf D, Vinod D. Effect of vajikaran rasayana herbs on pituitary–gonadal axis. Eur J Integr Med. 2010;2:89‐91. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published paper.


