Summary
Wnt signaling pathways have been extensively studied in the context of several diseases, including cancer, coronary artery disease, and age-related disorders. β-Catenin plays a central role in the most studied Wnt pathways, the Wnt/β-catenin signaling pathway, commonly referred to as the canonical Wnt signaling pathway. β-catenin is a substrate of glycogen synthase kinase 3β (GSK-3β), and the phosphorylated β-catenin by GSK-3β can be degraded by the proteasome through ubiquitination. Thus, GSK-3β inhibitors have become a widely used chemical biology tool to study the canonical Wnt signaling pathway. Among the varied GSK-3β inhibitors, a compound known as CHIR-99021 is one of the most widely used. Although these inhibitors contribute greatly to our understanding of the canonical Wnt pathway, certain pitfalls associated with such an approach may have been overlooked. In many published studies, micromolar concentrations of CHIR-99021 are used to activate the canonical Wnt pathway. Although CHIR-99021 is a specific GSK-3β inhibitor, it specifically inhibits the kinase at the nanomolar level. Therefore, caution is required when micromolar levels of CHIR-99021 are used for the purpose of activating the canonical Wnt signaling pathway.
Subject areas: Molecular biology, Cell biology
Graphical abstract
Molecular biology; Cell biology
In the current model of the canonical Wnt pathway, secreted Wnt proteins bind the receptor known as Frizzled (Fzd) and lipoprotein-receptor-related protein 5/6 (LRP5/6), thereby activating Disheveled (Dvl) proteins inside the cell (Cadigan and Nusse, 1997; Tran and Zheng, 2017). Dvl then binds to the carboxyl (C)-terminus of Fzd (Gao and Chen, 2009; Wong et al., 2003) and recruits Axin (Song et al., 2014), a protein member of the β-catenin destruction complex, away from the so-called destruction complex and to the cell membrane. Axin also plays an important role in bringing β-catenin and GSK-3β together so that the phosphorylation and subsequent β-catenin can occur in states where there is no Wnt agonist available (Xing et al., 2003). The destruction complex consists of Axin, glycogen synthase kinase-3β (GSK-3β), adenomatous polyposis coli (APC), casein kinase 1α (CK1α), and protein phosphatase 2A (PP2A) (Stamos and Weis, 2013; van Amerongen and Nusse, 2009; Wu and Pan, 2009). Without Wnt stimulation, β-catenin is phosphorylated by the GSK-3β in the destruction complex, and the phosphorylated β-catenin can be degraded through the ubiquitin-proteasome system. When Wnt stimulation occurs and activated Dvl recruits Axin to the membrane, GSK-3β is unable to phosphorylate β-catenin and prevents its degradation. β-catenin then can accumulate in the cell and is transported to the nucleus to initiate transcription of Wnt target genes by binding to T-cell factor/lymphoid enhancer-binding factor (TCF/LEF) transcription reporters, Creb-binding protein, and/or p300 protein (Cadigan and Nusse, 1997).
Therefore, GSK-3β inhibition by small compounds may emulate the removal of Axin with Wnt signaling, likewise leading to accumulation of β-catenin (Song et al., 2014; Wu and Pan, 2009). This in turn activates Wnt signaling. Indeed, in early studies, it was discovered that millimolar concentrations of lithium chloride (LiCl) can specifically inhibit GSK-3β and can also activate the canonical Wnt signaling pathway (Klein and Melton, 1996; Stambolic et al., 1996). Since then, the use of Wnt-β-catenin pathway activators that inhibit GSK-3β has been one of the most studied to date. As an example of the utility of these small molecule inhibitors, lithium chloride has been shown to promote osteoblast differentiation by stimulating Wnt signaling. It has also been shown to improve bone mass in mice through Wnt signaling activation, independent of traditional Wnt second messengers such as LRP5/6 (Clement-Lacroix et al., 2005). The study opened up a new avenue in treatment of degenerative bone disease such as osteoporosis and osteopenia, and may provide lithium chloride with an additional pharmacologic purpose in addition to its usefulness in the treatment of bipolar disorder (Kao and Elinson, 1998).
As development of kinase inhibitors is an active frontier of therapeutic development, many potent GSK-3β inhibitors have been discovered and also are commercially available. Most of them are ATP-competitive inhibitors which halt kinase activity through preventing ATP-kinase interactions. The most common GSK-3β inhibitors are listed in Table 1. The power of these GSK-3β inhibitors to stimulate canonical pathway signaling has been leveraged to discover Wnt activation’s numerous effects on the pathogenesis of chronic disease, such as lung and breast cancers and the pathophysiology of heart disease and wound healing.
Table 1.
List of GSK-3β inhibitors and corresponding IC50 and Chemical Abstracts Service Number (CAS No.)
| GSK-3β inhibitors | IC50a | CAS No. |
|---|---|---|
| LiCl | 1 mM (O'Brien et al., 2011) | 7447-41-8 |
| BIO | 8 nM (Wagner et al., 2016) | 667463-62-9 |
| SB-216763 | 12 nM (Wagner et al., 2016) | 280744-09-4 |
| SB-415286 | 78 nM (Coghlan et al., 2000) | 264218-23-7 |
| CHIR-99021 | 4 nM (Wagner et al., 2016) | 252917-06-9 |
| TWS-119 | 30 nM (Wang et al., 2016)] | 601514-19-6 |
| AR-A014418 | 142 nM (Wagner et al., 2016) | 487021-52-3 |
| LY-2090314 | 10 nM (Atkinson et al., 2015) | 603288-22-8 |
| PF-04802367 | 9 nM (Liang et al., 2016) | 1962178-27-3 |
| L807mts | 1 μM (Licht-Murava et al., 2016) | N/A |
| Tideglusib | Irreversible (Dominguez et al., 2012) | 865854-05-3 |
In general, there is no distinction between inhibition of GSK-3α and GSK-3β, as there is much shared overlap of substrates between the two kinases. It has been suggested that the GSKα and β subunits are redundant in nature (McCubrey et al., 2017; Patel and Woodgett, 2017).
For example, 6-bromoindirubin-3′-oxime (BIO) is a GSK-3β inhibitor that has helped investigators discover that hepatocellular carcinoma (HCC) exerts resistance to traditional chemotherapy as a result of Wnt stimulation, and thus renders HCC clinically difficult to eradicate (Yang et al., 2008). BIO has also been observed to maintain stem cells in an undifferentiated state and may prove useful in sustaining a population of stem cells for use in scientific inquiry. SB-216763 is another GSK-3β inhibitor that is particularly used for retinal stem cell proliferation and maintenance of pluripotent stem cell populations and exhibits lower toxicity than other GSK-3β inhibitors in mouse model embryonic stem cells (Inoue et al., 2006). On another note, SB-415286 has been studied as a neuroprotective agent by selectively preserving healthy neural tissue in the context of apoptosis of neuroblastoma cells via hydrogen peroxide (Pizarro et al., 2008). Two more widely used Wnt activators that act via GSK-3β inhibition are TWS-119 and AR-A014418. TWS-119 has been noted in a recent study to mitigate blood-brain barrier disruption that may inadvertently be caused by recombinant tissue plasminogen activator, which is commonly nicknamed as the “clot buster” in the treatment of ischemic stroke (Wang et al., 2016). AR-A014418, interestingly, has been noted to behave similarly to lithium’s effects against the symptoms of bipolar disorder (Gould et al., 2004). Newer GSK-3β inhibitors are being developed in the pipeline and are now available commercially, for example, PF-04802367 (Liang et al., 2016). This compound demonstrates the most selective antagonism of GSK-3β to date and shows promise as a research agent in GSK-3β pharmacodynamics.
So far, two GSK-3β inhibitors have been tested in clinical trials for treating various diseases. One of them is LY-9020314. Because LY-2090314 has antiproliferative and apoptotic effects against in vitro models of neuroblastoma (Kunnimalaiyaan et al., 2018), it was recently applied to acute myeloid leukemia (AML), with a positive safety profile (Gray et al., 2015). Another one is Tideglusib, a non-ATP competitive, irreversible GSK-3β inhibitor (Dominguez et al., 2012). Tideglusib has been tested in clinical trials for diseases such as Alzheimer’s disease (Lovestone et al., 2015) and autism (Martinez-Gonzalez et al., 2021).
Among all the GSK-3β inhibitors, perhaps, CHIR-99021 is the most commonly used GSK-3β inhibitor and is considered the standard small-molecule Wnt agonist. It has, for example, been used to mimic Wnt signaling in preadipocytes and inhibit adipogenesis (Bennett et al., 2002), and this small molecule maintains an integral role in the study of certain Wnt-related disease states such as coronary artery disease, Type II diabetes mellitus, and cancer (Ring et al., 2003). Because Wnt signaling plays a key role in regulating stem cells, this aforementioned compound is a member of the 2i (two inhibitors) and 3i (three inhibitors) cocktails that have been widely used to stimulate stem cells (Li et al., 2008; Ying et al., 2008). This compound has been so commonly used to activate Wnt signaling that it has become the “gold standard” of Wnt activator.
Indeed, compared with other GSK-3β inhibitors, CHIR-99021 is a potent inhibitor with high selectivity. A kinomescan showed that CHIR-99021 and AR-A014418 have similar degree of high kinase selectivity as opposed to the more promiscuous kinase binding as exhibited by BIO and SB-216763, of which BIO demonstrated the lowest kinase selectivity against most of the 359 kinases assayed in their study (Wagner et al., 2016). The kinase profile of CHIR-99021 indicates strong inhibition, as expected, against GSK-3α and GSK3β and also moderate inhibition of BRAF, CDK2/CycE1, and moderate-strong inhibition of DYKR1B and CDK2/CycA2, among others (An et al., 2010; Wagner et al., 2016).
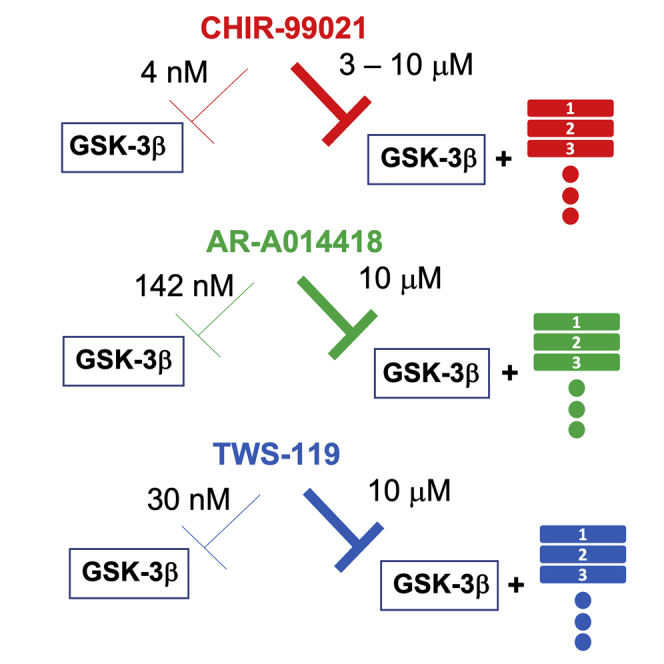
However, a pressing issue exists regarding this compound and its usage in the studies of Wnt signaling pathway. Because CHIR-99021 is a potent inhibitor, and it inhibits GSK-3β in the nanomolar range (Bennett et al., 2002; Ring et al., 2003), many of the specificity studies were carried out at the nanomolar level. On the other hand, for the purpose of Wnt activation, CHIR-99021 is generally (and only) used in the 3 to 10 μM range. It even has been reported that CHIR-99021 does not activate Wnt signaling at low concentrations (below 3 μM) (Lee and Evans, 2019). Although CHIR-99021 exhibits high kinase selectivity, at the micromolar concentration, it may collaterally bind to and inhibit other enzymes. Indeed, even the most state-of-the-art GSK-3β inhibitors still inhibit a few other kinases at higher concentrations (Bernard-Gauthier et al., 2019). Therefore, the view that this compound activates Wnt signaling by merely inhibiting GSK-3β might oversimplify this complex situation. Indeed, in general, this is one of the common concerns in the field of chemical biology. For this reason, in several published commentaries and reviews that tried to lay down the requirements (or rules) for chemical biology studies, one unanimously agreed requirement is that an appropriate chemical study should include at least two structurally distinct chemical probes as a control, frequently referred as the Blagg-Workman guideline (Blagg and Workman, 2017). Unfortunately, using CHIR-99021 at the micromolar range simply as a chemical probe to activate canonical Wnt signaling has been so widely used, and it is viewed as a scientific presumption. Because of this quasi-standard use dosage, more and more additional studies employ this approach, and the support from the scientific community for using the micromolar range becomes even stronger. Therefore, we feel that the research community should take caution against the consequences of not including control in their studies. Given that there are many GSK-3β inhibitors, most of which are widely available (Table 1), thus including the use of another GSK-3β inhibitor as control when CHIR-99021 is used as a chemical probe in Wnt activation is a straightforward approach. In addition, besides those ATP-competitive inhibitors, there is a myristoylated compound known as L807mts that has recently been developed. It acts as an in situ substrate-inhibitor converter, i.e., converts enzymatic substrates into inhibitors of their parent enzyme and has greater specificity for GSK3 kinases in in vitro studies (Licht-Murava et al., 2016). Of note, because lithium chloride displays relatively specific GSK-3β inhibition at the millimolar level (O'Brien et al., 2011), and many studies already use millimolar concentrations of probes as their upper limit, we think lithium may also be a good choice as a probe control, especially for cellular studies.
To further demonstrate the importance of using at least one different probe as a positive control when CHIR-99021 is used at the micromolar level to study the effect of activated Wnt signaling in a biological system, we obtained information regarding CHIR-99021 and two other GSK-3β inhibitors, TWS-119 and AR-A014418, from the L1000 database (Keenan et al., 2018). The LINCS L1000 kinome database contains information on how these three compounds at three different concentrations affect the gene expressions of two cell lines: PC3, a well-studied prostate cancer cell line, and HA1E, an SV40+TERT-immortalized kidney cell line. All the obtained data are listed in Tables S1 and S2. Although the two cell lines are very different, more than half of the affected genes shared between the two cell lines when treated with any of the three compounds exhibit overlap, suggesting that the three compounds can robustly affect cellular function. However, there are minimal overlaps in genes (four genes) that are affected by all three compounds regardless of the cell line (Table 2). Compounds CHIR-99021 and TWS-119 downregulate ADO and RAB30 in both PC3 and HA1E cells. However, the connection between the two genes and Wnt signaling has not been studied. Nevertheless, there are some potential connections between Wnt signaling and other two genes, DFFB (DNA fragmentation factor subunit β) and S100A8. DFFB is one of those unique genes; it encodes a DNAse that promotes cell differentiation and degrades DNA during apoptosis (Liu et al., 1997). DFFB is downregulated in the CHIR-99021 or TWS-119-treated HA1E. Although we have not found a direct connection between DFFB and Wnt signaling in the literature, it is a known marker for various cancers. Another gene is S100A8 (also known as MRP8); S100A8 is upregulated in HA1E and treated by AR-A014418 or TWS-119. Protein S100A8 functions as a heterodimeric calcium and zinc-binding zipper moiety in the initiation of the neutrophil-mediated inflammatory response, along with leukocyte recruitment and cytokine release (Wang et al., 2018). It has been reported that S100A8 is a potential Wnt-targeted gene in different systems (Lee et al., 2019; van den Bosch et al., 2016). Therefore, it is likely that the alterations of expression of both DFFB and S100A8 are because of the Wnt activations by the compounds. Nevertheless, the data clearly demonstrates that at the micromolar level, while these GSK-3β inhibitors can activate Wnt signaling, they also trigger many other cellular effects, and the effects of those compounds are, for the most part, completely different. Therefore, when CHIR-99021 is used to activate Wnt signaling, a positive control such as another GSK-3β inhibitor is critically needed to ensure that the observed phenotype(s) induced by CHIR-99021 is because of activated Wnt signaling in the studied system.
Table 2.
The numbers of the genes and the commonly affected genes by three GSK-3β inhibitors, CHIR-99021, AR-A014418, and TWS-119, in two cell lines, PC3 and HA1E, documented in the L1000 database
| CHIR-99021 | AR-A014418 | TWS-119 | ||
|---|---|---|---|---|
| PC3 | Upregulated | 11 genes | 10 genes | 5 genes |
| Downregulated |
4 genes including ADO (10) RAB30 (10) |
10 genes |
21 genes including ADO (10) DFFB (3.3 & 10) |
|
| HA1E | Upregulated | 12 genes | 15 genes including S100A8 (10) | 11 genes including S100A8 (10) |
| Downregulated | 11 genes including DFFB (10) |
12 genes | 32 genes Including ADO (10) DFFB (10) RAB30 (3.3) |
|
Treated concentrations (in μM) are listed in parentheses next to the selected gene.
In conclusion, in the studies where CHIR-99021 was used as merely a chemical probe to activate canonical Wnt signaling, especially when the concentration of CHIR-99021 used is high, the numerous consequent biological effect(s) may not be simply because of Wnt activation. Though undoubtedly, with these micromolar concentrations, the inhibition of GSK-3β and Wnt activation occurred, CHIR-99021 may affect many other biological events, which in turn contributed to the phenotypes that were observed and reported. Therefore, when inhibiting GSK-3β, it would be prudent to use at least two chemically distinct GSK-3β inhibitors. If a control compound fails to generate a similar biological effect, the inhibition pattern of CHIR-99021 may shed light on the relation between Wnt signaling and other cell signaling pathways that CHIR-99021 can affect and especially those that play a role in stem cell regulation. In this case, further investigation into the kinome properties of CHIR-99021 may lead to a greater understanding of the Wnt signaling cascade and how to use the Wnt signaling pathway in developing additional high potency, low toxicity small-molecule probes for scientific inquiry and therapeutic applications.
Acknowledgments
Supported in part by the National Eye Institute (R01EY0028557 and 5P30EY000331) and an unrestricted grant from Research to Prevent Blindness. We thank Dr. Simon K. Law for many helpful discussions.
Author contributions
Conceptualization, J.J.Z.; Methodology, J.J.Z. and S.M.L.; Investigation, S.M.L.; Writing – Original Draft, S.M.L.; Writing – Review & Editing, S.M.L. and J.J.Z..
Declaration of interests
The authors declare no competing interests.
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.isci.2022.104159.
Supplemental information
References
- An W.F., Germain A.R., Bishop J.A., Nag P.P., Metkar S., Ketterman J., Walk M., Weiwer M., Liu X., Patnaik D., et al. Probe Reports from the NIH Molecular Libraries Program. National Center for Biotechnology Information (US); 2010. Discovery of potent and highly selective inhibitors of GSK3b.https://www.ncbi.nlm.nih.gov/books/NBK133436/ [PubMed] [Google Scholar]
- Atkinson J.M., Rank K.B., Zeng Y., Capen A., Yadav V., Manro J.R., Engler T.A., Chedid M. Activating the Wnt/beta-catenin pathway for the treatment of melanoma--application of LY2090314, a novel selective inhibitor of glycogen synthase kinase-3. PLoS One. 2015;10:e0125028. doi: 10.1371/journal.pone.0125028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett C.N., Ross S.E., Longo K.A., Bajnok L., Hemati N., Johnson K.W., Harrison S.D., MacDougald O.A. Regulation of Wnt signaling during adipogenesis. J. Biol. Chem. 2002;277:30998–31004. doi: 10.1074/jbc.M204527200. [DOI] [PubMed] [Google Scholar]
- Bernard-Gauthier V., Mossine A.V., Knight A., Patnaik D., Zhao W.N., Cheng C., Krishnan H.S., Xuan L.L., Chindavong P.S., Reis S.A., et al. Structural basis for achieving GSK-3beta inhibition with high potency, selectivity, and brain exposure for positron emission tomography imaging and drug discovery. J. Med. Chem. 2019;62:9600–9617. doi: 10.1021/acs.jmedchem.9b01030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blagg J., Workman P. Choose and use your chemical probe Wisely to explore cancer biology. Cancer Cell. 2017;32:9–25. doi: 10.1016/j.ccell.2017.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadigan K.M., Nusse R. Wnt signaling: a common theme in animal development. Genes Dev. 1997;11:3286–3305. doi: 10.1101/gad.11.24.3286. [DOI] [PubMed] [Google Scholar]
- Clement-Lacroix P., Ai M., Morvan F., Roman-Roman S., Vayssiere B., Belleville C., Estrera K., Warman M.L., Baron R., Rawadi G. Lrp5-independent activation of Wnt signaling by lithium chloride increases bone formation and bone mass in mice. Proc. Natl. Acad. Sci. U S A. 2005;102:17406–17411. doi: 10.1073/pnas.0505259102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coghlan M.P., Culbert A.A., Cross D.A., Corcoran S.L., Yates J.W., Pearce N.J., Rausch O.L., Murphy G.J., Carter P.S., Roxbee Cox L., et al. Selective small molecule inhibitors of glycogen synthase kinase-3 modulate glycogen metabolism and gene transcription. Chem. Biol. 2000;7:793–803. doi: 10.1016/s1074-5521(00)00025-9. [DOI] [PubMed] [Google Scholar]
- Dominguez J.M., Fuertes A., Orozco L., del Monte-Millan M., Delgado E., Medina M. Evidence for irreversible inhibition of glycogen synthase kinase-3beta by tideglusib. J. Biol. Chem. 2012;287:893–904. doi: 10.1074/jbc.M111.306472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao C., Chen Y.G. Dishevelled: the hub of Wnt signaling. Cell Signal. 2009;22:717–727. doi: 10.1016/j.cellsig.2009.11.021. S0898-6568(09)00368-4 [pii] [DOI] [PubMed] [Google Scholar]
- Gould T.D., Einat H., Bhat R., Manji H.K. AR-A014418, a selective GSK-3 inhibitor, produces antidepressant-like effects in the forced swim test. Int. J. Neuropsychopharmacol. 2004;7:387–390. doi: 10.1017/S1461145704004535. [DOI] [PubMed] [Google Scholar]
- Gray J.E., Infante J.R., Brail L.H., Simon G.R., Cooksey J.F., Jones S.F., Farrington D.L., Yeo A., Jackson K.A., Chow K.H., et al. A first-in-human phase I dose-escalation, pharmacokinetic, and pharmacodynamic evaluation of intravenous LY2090314, a glycogen synthase kinase 3 inhibitor, administered in combination with pemetrexed and carboplatin. Invest. New Drugs. 2015;33:1187–1196. doi: 10.1007/s10637-015-0278-7. [DOI] [PubMed] [Google Scholar]
- Inoue T., Kagawa T., Fukushima M., Shimizu T., Yoshinaga Y., Takada S., Tanihara H., Taga T. Activation of canonical Wnt pathway promotes proliferation of retinal stem cells derived from adult mouse ciliary margin. Stem Cells. 2006;24:95–104. doi: 10.1634/stemcells.2005-0124. [DOI] [PubMed] [Google Scholar]
- Kao K.R., Elinson R.P. The legacy of lithium effects on development. Biol. Cell. 1998;90:585–589. [PubMed] [Google Scholar]
- Keenan A.B., Jenkins S.L., Jagodnik K.M., Koplev S., He E., Torre D., Wang Z., Dohlman A.B., Silverstein M.C., Lachmann A., et al. The library of integrated network-based cellular signatures NIH program: system-level cataloging of human cells response to perturbations. Cell Syst. 2018;6:13–24. doi: 10.1016/j.cels.2017.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein P.S., Melton D.A. A molecular mechanism for the effect of lithium on development. Proc. Natl. Acad. Sci. U S A. 1996;93:8455–8459. doi: 10.1073/pnas.93.16.8455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunnimalaiyaan S., Schwartz V.K., Jackson I.A., Clark Gamblin T., Kunnimalaiyaan M. Antiproliferative and apoptotic effect of LY2090314, a GSK-3 inhibitor, in neuroblastoma in vitro. BMC Cancer. 2018;18:560. doi: 10.1186/s12885-018-4474-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H., Evans T. TMEM88 inhibits Wnt signaling by promoting Wnt signalosome localization to multivesicular bodies. iScience. 2019;19:267–280. doi: 10.1016/j.isci.2019.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J.H., List A., Sallman D.A. Molecular pathogenesis of myelodysplastic syndromes with deletion 5q. Eur. J. Haematol. 2019;102:203–209. doi: 10.1111/ejh.13207. [DOI] [PubMed] [Google Scholar]
- Li P., Tong C., Mehrian-Shai R., Jia L., Wu N., Yan Y., Maxson R.E., Schulze E.N., Song H., Hsieh C.L., et al. Germline competent embryonic stem cells derived from rat blastocysts. Cell. 2008;135:1299–1310. doi: 10.1016/j.cell.2008.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang S.H., Chen J.M., Normandin M.D., Chang J.S., Chang G.C., Taylor C.K., Trapa P., Plummer M.S., Para K.S., Conn E.L., et al. Discovery of a highly selective glycogen synthase kinase-3 inhibitor (PF-04802367) that modulates tau phosphorylation in the brain: translation for PET neuroimaging. Angew. Chem. Int. Ed. Engl. 2016;55:9601–9605. doi: 10.1002/anie.201603797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Licht-Murava A., Paz R., Vaks L., Avrahami L., Plotkin B., Eisenstein M., Eldar-Finkelman H. A unique type of GSK-3 inhibitor brings new opportunities to the clinic. Sci. Signal. 2016;9:ra110. doi: 10.1126/scisignal.aah7102. [DOI] [PubMed] [Google Scholar]
- Liu X., Zou H., Slaughter C., Wang X. DFF, a heterodimeric protein that functions downstream of caspase-3 to trigger DNA fragmentation during apoptosis. Cell. 1997;89:175–184. doi: 10.1016/s0092-8674(00)80197-x. [DOI] [PubMed] [Google Scholar]
- Lovestone S., Boada M., Dubois B., Hull M., Rinne J.O., Huppertz H.J., Calero M., Andres M.V., Gomez-Carrillo B., Leon T., et al. A phase II trial of tideglusib in Alzheimer's disease. J. Alzheim. Dis. 2015;45:75–88. doi: 10.3233/JAD-141959. [DOI] [PubMed] [Google Scholar]
- Martinez-Gonzalez L., Gonzalo-Consuegra C., Gomez-Almeria M., Porras G., de Lago E., Martin-Requero A., Martinez A. Tideglusib, a non-ATP competitive inhibitor of GSK-3beta as a drug candidate for the treatment of amyotrophic lateral sclerosis. Int. J. Mol. Sci. 2021;22:8957. doi: 10.3390/ijms22168975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCubrey J.A., Fitzgerald T.L., Yang L.V., Lertpiriyapong K., Steelman L.S., Abrams S.L., Montalto G., Cervello M., Neri L.M., Cocco L., et al. Roles of GSK-3 and microRNAs on epithelial mesenchymal transition and cancer stem cells. Oncotarget. 2017;8:14221–14250. doi: 10.18632/oncotarget.13991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien W.T., Huang J., Buccafusca R., Garskof J., Valvezan A.J., Berry G.T., Klein P.S. Glycogen synthase kinase-3 is essential for beta-arrestin-2 complex formation and lithium-sensitive behaviors in mice. J. Clin. Invest. 2011;121:3756–3762. doi: 10.1172/JCI45194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel P., Woodgett J.R. Glycogen synthase kinase 3: a kinase for all pathways? Curr. Top Dev. Biol. 2017;123:277–302. doi: 10.1016/bs.ctdb.2016.11.011. [DOI] [PubMed] [Google Scholar]
- Pizarro J.G., Yeste-Velasco M., Rimbau V., Casadesus G., Smith M.A., Pallas M., Folch J., Camins A. Neuroprotective effects of SB-415286 on hydrogen peroxide-induced cell death in B65 rat neuroblastoma cells and neurons. Int. J. Dev. Neurosci. 2008;26:269–276. doi: 10.1016/j.ijdevneu.2008.02.002. [DOI] [PubMed] [Google Scholar]
- Ring D.B., Johnson K.W., Henriksen E.J., Nuss J.M., Goff D., Kinnick T.R., Ma S.T., Reeder J.W., Samuels I., Slabiak T., et al. Selective glycogen synthase kinase 3 inhibitors potentiate insulin activation of glucose transport and utilization in vitro and in vivo. Diabetes. 2003;52:588–595. doi: 10.2337/diabetes.52.3.588. [DOI] [PubMed] [Google Scholar]
- Song X., Wang S., Li L. New insights into the regulation of Axin function in canonical Wnt signaling pathway. Protein Cell. 2014;5:186–193. doi: 10.1007/s13238-014-0019-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stambolic V., Ruel L., Woodgett J.R. Lithium inhibits glycogen synthase kinase-3 activity and mimics wingless signalling in intact cells. Curr. Biol. 1996;6:1664–1668. doi: 10.1016/s0960-9822(02)70790-2. [DOI] [PubMed] [Google Scholar]
- Stamos J.L., Weis W.I. The beta-catenin destruction complex. Cold Spring Harbor Perspect. Biol. 2013;5:a007898. doi: 10.1101/cshperspect.a007898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran F.H., Zheng J.J. Modulating the Wnt signaling pathway with small molecules. Protein Sci. 2017;26:650–661. doi: 10.1002/pro.3122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Amerongen R., Nusse R. Towards an integrated view of Wnt signaling in development. Development. 2009;136:3205–3214. doi: 10.1242/dev.033910. 136/19/3205 [pii] [DOI] [PubMed] [Google Scholar]
- van den Bosch M.H., Blom A.B., Schelbergen R.F., Vogl T., Roth J.P., Sloetjes A.W., van den Berg W.B., van der Kraan P.M., van Lent P.L. Induction of canonical Wnt signaling by the alarmins S100a8/A9 in murine knee joints: implications for osteoarthritis. Arthritis Rheumatol. 2016;68:152–163. doi: 10.1002/art.39420. [DOI] [PubMed] [Google Scholar]
- Wagner F.F., Bishop J.A., Gale J.P., Shi X., Walk M., Ketterman J., Patnaik D., Barker D., Walpita D., Campbell A.J., et al. Inhibitors of glycogen synthase kinase 3 with exquisite kinome-Wide selectivity and their functional effects. ACS Chem. Biol. 2016;11:1952–1963. doi: 10.1021/acschembio.6b00306. [DOI] [PubMed] [Google Scholar]
- Wang S., Song R., Wang Z., Jing Z., Wang S., Ma J. S100A8/A9 in inflammation. Front. Immunol. 2018;9:1298. doi: 10.3389/fimmu.2018.01298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W., Li M., Wang Y., Li Q., Deng G., Wan J., Yang Q., Chen Q., Wang J. GSK-3beta inhibitor TWS119 attenuates rtPA-induced hemorrhagic transformation and activates the Wnt/beta-catenin signaling pathway after acute ischemic stroke in rats. Mol. Neurobiol. 2016;53:7028–7036. doi: 10.1007/s12035-015-9607-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong H.C., Bourdelas A., Krauss A., Lee H.J., Shao Y.M., Wu D., Mlodzik M., Shi D.L., Zheng J. Direct binding of the PDZ domain of Dishevelled to a conserved internal sequence in the C-terminal region of Frizzled. Mol. Cell. 2003;12:1251–1260. doi: 10.1016/s1097-2765(03)00427-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu D., Pan W. GSK3: a multifaceted kinase in Wnt signaling. Trends Biochem. Sci. 2009;35:161–168. doi: 10.1016/j.tibs.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing Y., Clements W.K., Kimelman D., Xu W. Crystal structure of a beta-catenin/axin complex suggests a mechanism for the beta-catenin destruction complex. Genes Dev. 2003;17:2753–2764. doi: 10.1101/gad.1142603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang W., Yan H.X., Chen L., Liu Q., He Y.Q., Yu L.X., Zhang S.H., Huang D.D., Tang L., Kong X.N., et al. Wnt/beta-catenin signaling contributes to activation of normal and tumorigenic liver progenitor cells. Cancer Res. 2008;68:4287–4295. doi: 10.1158/0008-5472.CAN-07-6691. [DOI] [PubMed] [Google Scholar]
- Ying Q.L., Wray J., Nichols J., Batlle-Morera L., Doble B., Woodgett J., Cohen P., Smith A. The ground state of embryonic stem cell self-renewal. Nature. 2008;453:519–523. doi: 10.1038/nature06968. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


