Abstract
Human immunodeficiency virus (HIV) selectively targets and destroys the infection-fighting CD4+ T-lymphocytes of the human immune system, and has a life cycle that encompasses binding to certain cells, fusion to that cell, reverse transcription of its genome, integration of its genome into the host cell DNA, replication of the HIV genome, assembly of the HIV virion, and budding and subsequent release of free HIV virions. Once a host is infected with HIV, the host’s ability to competently orchestrate effective and efficient immune responses against various microorganisms, such as viral infections, is significantly disrupted. Without modern antiretroviral therapy (ART), HIV is likely to gradually destroy the cellular immune system, and thus the initial HIV infection will inexorably evolve into acquired immunodeficiency syndrome (AIDS). Generally, HIV infection in a patient has an acute phase, a chronic phase, and an AIDS phase. During these three clinical stages, patients are found with relatively specific levels of viral RNA, develop rather distinctive immune conditions, and display unique clinical manifestations. Convergent research evidence has shown that hepatitis B virus (HBV) co-infection, a common cause of chronic liver disease, is fairly common in HIV-infected individuals. HBV invasion of the liver can be facilitated by HIV infection at each clinical stage of the infection due to a number of contributing factors, including having identical transmission routes, immunological suppression, gut microbiota dysbiosis, poor vaccination immune response to hepatitis B immunization, and drug hepatotoxicity. However, there remains a paucity of research investigation which critically describes the influence of the different HIV clinical stages and their consequences which tend to favor HBV entrenchment in the liver. Herein, we review advances in the understanding of the mechanisms favoring HBV infection at each clinical stage of HIV infection, thus paving the way toward development of potential strategies to reduce the prevalence of HBV co-infection in the HIV-infected population.
Keywords: HIV, HIV clinical stages, HBV, coinfection, mechanisms
Introduction
Human immunodeficiency virus (HIV) infection has been a major public health issue for the past four decades. Despite extensive global research and study (1–3), a cure for HIV infection has, thus far, proven elusive. Recently, our research group has proposed novel potential therapeutic options for HIV infection (4, 5) which, we believe, could inspire future clinical trials into curative therapeutic options for HIV. Our first proposition concerns the promotion of P-selectin glycoprotein ligand 1 (PSGL-1), an important receptor from innate immunity, which (i) induces the production of membrane defective virions that are unable to attach to or infect new target cells, and (ii) blocks the HIV reverse transcription process. Our second proposition involves the selective elimination of host cells capable of producing HIV virions via the use of a therapeutic cocktail of drugs (latency reversal agents, autophagy inhibitors, apoptosis activators, and antiretroviral drugs).
The World Health Organization (WHO) has proposed that HIV infection may be divided into four clinical stages in adults and adolescents 15 years-of-age and above (6). HIV-positive patients who are asymptomatic or have persistent generalized lymphadenopathy (lymphadenopathy of at least two sites [not including inguinal] for longer than 6 months) are categorized as being in stage 1. Clinical findings included in stage 2 (mildly symptomatic stage) are unexplained weight loss of less than 10 percent of total body weight and recurrent respiratory infections (such as sinusitis, bronchitis, otitis media, and pharyngitis), as well as a range of dermatological conditions including herpes zoster flares, angular cheilitis, recurrent oral ulcerations, papular pruritic eruptions, seborrhoeic dermatitis, and fungal nail infections. Manifestations included in clinical stage 3 (the moderately symptomatic stage) are weight loss of greater than 10 percent of total body weight, prolonged (more than 1 month) unexplained diarrhea, pulmonary tuberculosis, and severe systemic bacterial infections including pneumonia, pyelonephritis, empyema, pyomyositis, meningitis, bone and joint infections, and bacteremia. Stage 4 (the severely symptomatic stage) includes all of the AIDS-defining illnesses, e.g., HIV wasting syndrome, Pneumocystis pneumonia (PCP), recurrent severe or radiological bacterial pneumonia, extrapulmonary tuberculosis, HIV encephalopathy, CNS toxoplasmosis, chronic (more than 1 month) or orolabial herpes simplex infection, esophageal candidiasis, and Kaposi’s sarcoma. WHO HIV clinical staging utilizes standardized clinical parameters to direct medical decision making for patients with HIV/AIDS, and can be used based solely on patient clinical features, thus accommodating treatment facilities that may have limited or no access to sophisticated laboratory testing, such as those in low- and middle-income countries and regions (7). There is, also, the existence of the Fiebig staging system of HIV infection (first published in 2003, and comprising 6 stages), which describes the emergence of virological and immunological markers following infection by HIV. Several discrete clinical phases can thus be recognized for HIV infection; however, it has been generally accepted that HIV infection exhibits an acute phase, a chronic phase, and the acquired immunodeficiency syndrome (AIDS) phase (8).
In 2020, it was estimated that 36.7 million people globally were infected by HIV (9), and thus, the global HIV pandemic continues to pose a material threat to the health of mankind. The large majority of new HIV infections occur in low- and middle-income countries (10). Poverty, stigma associated with HIV disease, cultural and social barriers to appropriate testing and treatment, insufficient and inadequate health care infrastructure to support the large patient pool, poor health literacy, limited provider training, inadequate and inappropriate medical equipment, scarcity of appropriately-trained medical manpower to distribute health care throughout specific regions, and an inadequately low number of accredited medical laboratory facilities are some of the numerous factors that contribute to the almost inexorable global propagation of HIV (11).
At the same time, hepatitis B virus (HBV) is also silently spreading amongst the global population, especially in low- and middle-income countries (12). In 2019, the WHO estimated that 296 million people were living with chronic HBV (with 1.5 million new infections each year). More specifically, the WHO Western Pacific Region and the WHO African Region presents the highest chronic hepatitis B infection rates, with 116 million and 81 million people infected, respectively. Lower proportions occur in (i) the WHO Eastern Mediterranean Region (with 60 million people infected), (ii) the WHO South-East Asia Region (with 18 million people infected), (iii) the WHO European Region (with 14 million people infected), and (iv) the WHO Americas Region (with 5 million people infected) (13). Thus, HBV affects hundreds of millions of people worldwide, and is responsible for progressive liver fibrosis and hepatocellular carcinoma, amongst other chronic health sequelae (14, 15) during the chronic phase of HBV disease. Most cases of HBV infection in adults are arrested early, and are defined as an acute infection that is generally successfully limited by the patient’s own immune system. Only adults with an immunocompromised immune system tend to progress to chronic HBV (16–18). Unfortunately, most cases of HBV infection acquired in infancy or early childhood however, do become chronic (16–18). According to WHO, around one third of the world’s population has been infected by HBV at some point of their lives (16–18). Thus, HIV-HBV coinfection is relatively common (19). Estimations suggest that 10 to 28% of HIV-infected individuals are chronically infected with HBV (20–25). Indeed, the rates of HIV-HBV coinfection vary significantly between regions and risk-based groups. For instance, a study in Vietnam has shown that HIV-HBV coinfection is significantly higher among people who inject drugs (28%) or who are sex workers (15%) (23). Similarly, Xie et al. (26), have reported an estimation of 10% with respect to the existing HIV-HBV coinfection rate in China in general; however, they also state that the prevalence of such HIV-HBV coinfection in China varies between regions from 5% to 15%. In an extensive review on HIV-HBV coinfection, Singh et al. (27), suggested that West Africa and South Africa possess the highest prevalence of HIV-HBV coinfection in the world.
Several past studies have explored the impact of HIV-HBV coinfection on patients’ health, and have found that this comorbid association accelerates HBV progression (higher levels of hepatitis B viremia, higher risk of developing cirrhosis and hepatocellular carcinoma) (28), and materially amplifies the complexities related to treatment (27, 29–31). Among the mechanisms triggered by HIV infection which accelerate the progression of HBV infection, we can list (i) HIV replication in the liver, (ii) HIV-associated microbial translocation and immune activation, and (iii) immune exhaustion and tolerance. Each of these mechanisms mediated by HIV pathogenesis has significant effects on liver disease, as noted by Singh et al. (27). However, to the best of our knowledge, there remains a paucity of published research investigation in the literature which critically describes the influence and consequences of HIV clinical staging that potentially favor HBV establishment in HIV-infected individuals.
We therefore propose, herein, to review the appropriate literature to elucidate the potential mechanisms favoring HBV infection at each clinical stage of HIV infection. In the first part, we discuss the transmission routes of both HIV and HBV, and their subsequent life cycles once they have entered the human body. In the second part, we critically discuss the potential influence of each of the HIV acute, chronic, and AIDS phases that either lead to or may potentially lead to HBV infection.
HIV And HBV: Transmission Routes And Life Cycle
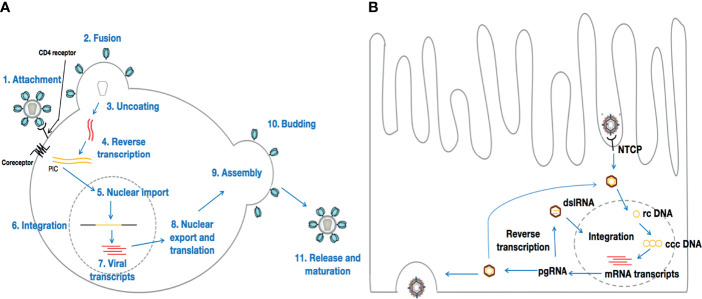
It is well-established that HIV and HBV share the same transmission routes. Indeed, both viruses are known to be transmitted from person to person through sexual intercourse, via contaminated needles used for intravenous drug delivery, from mother to child, and by the therapeutic use of HIV or HBV-infected blood or blood products (32). Thus, individuals who have casual sex in the absence of a condom and those who inject recreational drugs are at a particularly high risk for acquiring not only HIV infection, but also HBV infection (29). Once a person is infected by either HIV or HBV, these viruses exhibit two distinct life cycles within the infected persons body ( Figure 1 ).
Figure 1.
Life cycles of HIV and HBV. (A) represents HIV life cycle once in contact with CD4+ T-cells. Although HIV preferentially infects CD4+ T-cells, HIV tropism is not limited to CD4+ T-cells only. Conversely, HBV [the life cycle of which is depicted in (B)] infects hepatocytes exclusively.
HIV targets immune cells, preferentially CD4+ T-lymphocytes. Then, a viral envelope glycoprotein molecule (gp120) binds to a host cell receptor or co-receptor, such as CCR5 or CXCR4, responsible for HIV entry into lymphocytes and macrophages. The binding of gp120 to these receptors results in a cascade of molecular conformational changes and the exposure of gp41, bringing the HIV virion in much closer proximity to the target cell. Subsequent fusion of the viral envelope with the host cell membrane is essential for the entry of the inner matrix core of the virus into the intracytoplasmic realm of the host cell (33). Within the viral inner core are two strands of viral RNA held together by two small proteins (P6 and P7), and three of the enzymes essential for viral replication, viz., integrase, protease, and reverse transcriptase. Accessory proteins such as Nef, Vpr, and Vif are also found in the core matrix of the virus. Although these accessory proteins are not essential for viral replication, they play crucial roles in counteracting defensive mechanisms activated by the host cell (34, 35). Once within the host cell cytoplasm, the core matrix of the virion disintegrates, releasing the viral capsid as well as the genome of the virus. The viral RNA, together with the essential viral enzymes, is thus exposed to the host cell cytoplasm. The viral RNA then undergoes reverse transcription into viral DNA through a process mediated by the viral reverse transcriptase. Earlier investigations have revealed that the viral DNA generated by the reverse transcription process within the host cytoplasm is part of a broad nucleoprotein complex known as the pre-integration complex (PIC) (36), which also comprises Vpr and the integrase enzyme. Subsequent migration and entry of the PIC into the nucleus is followed by the process termed integration, which is mediated by the integrase enzyme. The preceding view, that conversion of the HIV RNA genome into DNA occurs in the cytoplasm before nuclear entry has, however, been challenged recently. Indeed, Dharan et al., have provided evidence to support the hypothesis that reverse transcription and uncoating can occur in the nucleus of non-dividing cells, such as macrophages or cells treated with the tetracyclic antibiotic, aphidicolin (37). For integration to occur, the integrase within the PIC acts by slicing through the DNA of the host cell, and thus allowing viral DNA to be inserted at a variety of sites on the host DNA, i.e., integrase catalyzes the insertion of viral dsDNA into the host chromosome (38, 39). For HIV, and most viruses that integrate into the host genome (e.g., murine leukemia virus, Herpes simplex virus-1, Ebola virus), observations from past studies (40, 41) reveal evidence of the DNA splicing and joining steps. It is critical to keep in mind that, usually, two nucleotides are removed from 3’ end of the viral DNA. Then, these 3’ ends attack a pair of phosphodiester bonds on opposite strands of the target DNA, across the major groove, leading to a bonding of the covalent 3’ ends of the viral DNA to the target DNA. Finally, the single-strand gaps and the two-nucleotide overhang at the viral DNA’s 5’ ends are repaired by cellular enzymes, in order for integration to be complete. For HIV, the sites are five base pairs apart instead of two, resulting in a five base-pair duplication (42). Once HIV DNA is integrated into the host cell genome in this manner, the host cell is considered to be infected for life. Thus, the integrated viral DNA, referred to as provirus, can be used to generate genomic RNA, which can serve as messenger RNA (mRNA) for the synthesis of viral proteins in the host cytoplasm ( Figure 1A ).
HBV targets and replicates solely in the parenchymal cells of the liver (the hepatocytes) (43–46) ( Figure 1B ). Moreover, it has been established that HBV infects only humans, chimpanzees, and to a lesser extent, tree shrews (Tupaia belangeri) (47, 48). Once in contact with the liver, the circulating virion initially attaches to heparan sulfate proteoglycans (HSPGs) (49, 50). Then, the interaction of a specific domain of the HBV L envelope protein with the sodium taurocholate co-transporting polypeptide [NTCP, a hepatocyte-specific transporter of bile acids that is predominantly localized in the basolateral membrane that faces the sinusoidal lumen (51)] on the surface of the hepatocytes contributes to viral entry into the hepatocyte (52). Following entry and uncoating, the nucleocapsid carrying the HBV genome is transported into the nucleus, where it is released as relaxed circular (rc) DNA. There, the rcDNA is converted into an episomal covalently closed circular (ccc) DNA minichromosome by host enzymes (46, 53). Reports suggest that cccDNA is very stable, persisting indefinitely, and is one of the main barriers to cure for hepatitis B disease (46), as it is the template for all HBV RNA transcripts (27, 54) that leave the nucleus unspliced, and produces the viral structural and non-structural proteins (53). Thus, HBV can initiate viral replication with an estimated doubling time of 2-4 days (55, 56). Interestingly, HBV polymerase can encode the pre-genomic RNA (pgRNA) and the reverse transcription of pgRNA can also lead to the formation of double stranded linear HBV DNA (dslDNA). Once in the nucleus, the dslDNA, in a similar manner to HIV, can integrate into the host genome (27). In contrast to HIV, the integrated dslDNA cannot enable viral replication, but it does allow the expression of certain gene products, like the envelope proteins (Env), which are dissimilar to the envelope proteins generated from cccDNA, which coat filamentous and spherical subviral particles (SVPs) (54). In general, acute manifestation of HBV infection occurs within 6 months after a person is exposed to HBV (57). From an acute infection, it can subsequently progress into a chronic infection. Indeed, although most people with healthy immune systems are able to clear the virus at the acute stage, immature immune systems and/or impaired immunity can lead to the establishment of chronic HBV infection in infants and/or adults (58, 59). Once the disease becomes chronic, it becomes a lifelong infection which, in the absence of effective treatment, can cause liver cancer or significant liver damage and scarring, leading to eventual liver failure.
In vitro and in vivo reports suggest that HIV can also infect hepatic stellate cells, sinusoidal endothelial cells, Kupffer cells, and the resident macrophages of the liver [as reported by Chamroonkul and Bansal (60), Housset et al. (61) and Cao et al. (62)]. HIV RNA sequences from the livers of untreated HIV-positive individuals show distinct compartmentalized sequences when compared to RNA sequences from other tissue sites (63). Further studies have demonstrated that HIV can persist in the liver even in patients on antiretroviral therapy (ART), primarily in Kupffer cells (64–66). In this review, therefore, we explore and discuss the influence of HIV infection on the establishment of HBV infection, especially being cognizant of the fact that HIV is known to provoke the fundamentally profound immune system impairment necessary for the onset of chronic HBV. Normally, most people with healthy immune systems are able to clear HBV during the acute phase. Utilizing the combined actions of HBV-specific CD4+ T-cells [essential for the induction and the maintenance of both CD8+ T-cells and antibody responses (67, 68)] and HBV-specific CD8+ T-cells [which kill infected hepatocytes and induce local production of proinflammatory cytokines (69–71)], a healthy person can easily overcome acute HBV infection, and thus avoid the chronic and life-threatening phase of the infection. Subsequently, our discussions will consider HIV as the primary infection, and we reflect further on the immunological consequences of HIV infection that favor HBV infection.
The various mechanisms through which liver injury may occur in patients with HIV infection are numerous; a general breakdown of these mechanisms is presented in Table 1 . A reasonable understanding of these mechanisms is of significant importance to the comprehension of HIV/HBV pathological processes, and any liver injury may further represent an ‘open door’ for HBV to enter hepatocytes and subsequently establish infection. This preceding assertion is speculative at this stage, and further investigation is required to establish precisely how liver injury induced by HIV infection could facilitate HBV invasion of hepatocytes.
Table 1.
Summary of reported mechanisms responsible for liver injury in patients with HIV.
| Mechanism | Contribution | Details | References |
|---|---|---|---|
| Oxidative stress | Moderate | This is a process whereby free reactive oxygen species (ROS) provoke increased activation of Kupffer cells in the liver. In turn, these activated immune cells promote stellate cell activation via nuclear factor kappa-beta (NF-kB) and activator protein 1, leading to increased production of proinflammatory and profibrotic cytokines, resulting in liver damage, fibrosis, and cirrhosis. Nucleoside reverse transcriptase inhibitors (NRTIs) such as didanosine can cause oxidative stress and mitochondrial toxicity. | (21) |
| Mitochondrial injury | Moderate | As the primary source of energy in the hepatocyte, any process that impairs mitochondrial function may lead to hepatic injury. During HIV, mitochondrial injury can occur through increased stress on the endoplasmic reticulum (ER), initiated by activation of the IRE1/TRAF 2 (Inositol Requiring 1/TNF receptor-associated factor 2) pathway. NRTIs and protease inhibitors (PIs) can directly cause mitochondrial toxicity. | (21, 72, 73) |
| Immune-mediated injury | Moderate | HIV can interact with hepatic stellate cells (HSCs) via gp120, producing inappropriate activation and increased HSC production of collagen and monocyte chemoattractant protein (MCP-1) (a macrophage chemoattractant). HIV decreases the number of Kupffer cells in the liver, decreasing the ability of the liver to clear products of microbial translocation. HIV provokes alterations in cytokine profiles resulting from imbalance between CD4+ and CD8+ T-cells |
(21, 74, 75) |
| Cytotoxicity | Mild | HIV triggers apoptosis via the HIV gp120 protein-receptor signaling pathway. | (76) |
| Systematic inflammation | Significant | The systematic inflammation resulting from HIV infection may induce fibrosis via a number of mechanisms, including oxidative stress and mitochondrial dysfunction as a result of ER stress. CD4/CD8 imbalances seen in HIV can lead to underexpression of IFN-gamma (an antifibrotic cytokine), thus favoring induction of apoptosis of activated HSCs, and hepatic progression into a profibrotic state. | (21, 77, 78) |
| Gut microbial translocation | Significant | This leads to hepatic injury primarily via increased hepatic levels of bacterial lipopolysaccharides (LPS), causing hepatic inflammation. More specifically, hepatic inflammation may result from (i) recruitment and activation of inflammatory cells (Kupffer cells and HSCs), (ii) systemic immune responses promoting hepatocyte cell death, or (iii) production of proinflammatory cytokines and acute phase reactants such as transforming growth factor beta 1 (TGFB1), IL-6, and IL-10 | (79–81) |
| Nodular regenerative hyperplasia | Significant | This is a rare condition in which diffuse transformation of the liver parenchyma into micronodules without intervening fibrosis leads to non-cirrhotic portal hypertension in patients with HIV. Pathophysiologically, it is thought that gut bacterial translocation may be responsible for vascular endothelial disruption, vascular and peri-vascular fibrosis and stenosis, and portal hypertension. The epithelial damage observed in the liver isare thought to either be immune-mediated or possibly related to direct viral damage by HIV. | (82–84) |
Acute And Early HIV Infection
Innate Immune Defense Subversion
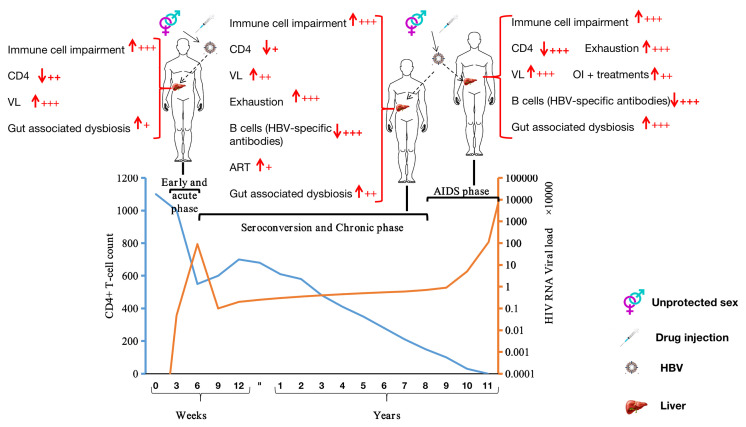
Acute HIV infection (AHI) is the first stage of HIV infection, occurring soon after viral acquisition and before seroconversion. AHI typically lasts 3–4 weeks ( Figure 2 ), and is characterized by the presence of HIV RNA and p24 antigen (Ag) (85) in the circulation. During this short period, HIV concentrations in blood and other body fluids (vaginal secretions and semen) are exceptionally high, increasing the likelihood of HIV transmission (85–92). To reach the high levels of HIV observed, HIV-1 subverts dendritic cell and macrophage activities (preferentially CD4+ T-cells) to increase its replication at mucosal locations (93, 94). This strategy also favors HBV, which does not need to use any specific mechanisms to avoid such immune cells (dendritic cells, macrophages, and T-cells) in an HIV-positive individual. Moreover, HIV adopts a variety of strategies to avoid type 1 interferon (IFN-1) control [repression of HIV restriction factors (95–101) and/or blocking of IFN-1 expression by infected cells (102–105)] via the infected-cells (dendritic cells, macrophages, and T-cells). Indeed, IFN-1 is an innate antiviral defense cytokine, and is known as a pleiotropic cytokine that acts by up-regulating transcription of hundreds of IFN-stimulated genes, including HIV restriction factors (106). To illustrate this point, Gondim et al. (107), for instance, have investigated how IFN-1 can control HIV infection and they have shown that IFN-1 (including IFNα2 and IFNβ) administration can reduce viral replication in CD4+ T-cells and macrophages. Furthermore, three major points depicting the interplay between HIV infection and IFN-I are of particular interest, viz., (i) the sensitivity of HIV-1 isolates to IFN-I inhibition consistently changes over time, (ii) HIV-1 isolates obtained during ART therapy were relatively IFN-I sensitive, and (iii) the viruses that rebounded after treatment interruption displayed the highest degree of IFNα2 and IFNβ resistance. Thus, IFN-1 plays an essential role in inflammation, immunoregulation, tumor cell recognition, and T-cell responses. In the absence of effective expression of IFN-1, or in the absence of response resulting from its expression, the immune system becomes vulnerable to viral infections, such as infections by HBV. In addition to IFN-1, HIV avoids INF-gamma (IFN-γ, a type II interferon) control by (i) destroying CD4+ T-cells [also responsible for IFN-γ secretion (67)] or (ii) repressing, for instance, PSGL-1 (an HIV restriction factor) activities, which has been extensively reviewed by our research group (4). Besides, in order to reduce the protective benefits of innate responses, HIV-1 resists well-demonstrated control by natural killer cell (108–113) [stimulated by innate cytokines including IFN-1, IL-15, IL-18 and receptor-ligand interactions (93)], and may disrupt innate regulation of adaptive responses, as suggested by Borrow (93). By utilizing these mechanisms, HIV infection contributes to HBV evasion of immune cells (particularly effector cells) to establish chronic HBV disease, as described by Lannacone and Guidotti (54).
Figure 2.
Stages of HIV infection and factors potentially favoring HBV infection at each clinical stage of HIV infection. ↓: depletion; ↑: augmentation; VL, viral RNA load; OI, opportunistic infection; +: Mild; ++: Moderate; +++: Severe.
High HIV Viral Load and CD4+ T-Cell Depletion
During AHI, the elevated concentration of viral particles in the systemic circulation facilitates infection of the liver by HIV, which in turn promotes multiple pathways that all converge on activated hepatic stellate cells (HSCs), the primary source of collagen synthesis in the injured liver, which encourages hepatic inflammation and fibrosis (60). For instance, it is known that HIV and its envelope gp120 (i) promote direct pro-fibrogenic effects on HSCs, (ii) promote the production of pro-inflammatory cytokines (such as MCP1, IL-8), and (iii) induce apoptosis in hepatocytes (75, 114). Indeed, HIV glycoproteins induce hepatocyte apoptosis via the expression of the TNF-related apoptosis inducing ligand (TRAIL), by stimulation of hepatocytes (115, 116). Furthermore, rapid fibrosis, in addition to causing elevated plasma HIV levels, correlates with reduced CD4+ T-cell counts.
During AHI, there is extensive CD4+ T-cell destruction (HIV-induced CD4+ T-cell depletion) (93). On the one hand, this HIV-induced depletion of CD4+ T-cells relative to CD8+ T-cell recruitment alters the hepatic cytokine profile, establishing a fibrogenic environment. Consequently, an injured liver becomes an ideal target for HBV to establish an acute phase, which progressively metamorphoses into a chronic infection due to the persistence of the systemic inflammation caused by HIV infection. On the other hand, it is recognized that host CD4+ T-cells are essential for the recognition of viral antigens presented by Kupffer cells and the regulation of the activities of (i) CD8+ cytotoxic T-cells, (ii) antibody-producing B-cells, and (iii) cytokine-secreting cells (19, 117–119). When the HIV acute phase leads to drastic depletion of CD4+ T-cells, the immune system is unable to adequately respond to HBV invasion, as HBV antigens presented by Kupffer cells cannot thus be recognized. Moreover, CD8+ T-cells, B-cells, and cytokine-secreting cellular functions are overwhelmed by HIV subvertive activities, which thus facilitates HBV infection establishment.
HIV Seroconversion And The Chronic Phase
During the seroconversion phase, which occurs after the acute phase ( Figure 2 ), the body starts producing detectable levels of HIV-specific antibodies. A seropositive individual may have flu-like symptoms, such as fever and body aches during this phase. The duration for HIV disease progression with clinical symptoms varies widely across individuals, although it usually progresses slowly (120). Most HIV-positive individuals are diagnosed during or after the seroconversion phase [as HIV diagnostic tests generally target HIV-specific antibodies (121)]. During this period, the earlier detection and earlier initiation of appropriate treatment leads to a reduced risk of onward transmission. Due to HIV-specific antibody production, HIV-infection is stabilized at this stage of the infection, meaning that the plasma viral RNA load, despite being high, remains stable, CD4+ T-cells counts increase slightly, and the immune system activation remains persistent. HIV causes several structural, functional, and immunological impairments, resulting from a persisting underlying chronic inflammatory state (122–124). HBV establishment is likely to be favored by HIV infection during the seroconversion and the chronic phases as HIV infection sustains the immunological impairments present during the acute phase, in conjunction with other mechanisms, as described in the following paragraphs.
HIV-Associated Gut Dysbiosis
It has been reported that the gastrointestinal tract (GI) represents the primary site of HIV replication and reservoir persistence (125). Once HIV infection is established, a rapid loss of GI mucosal integrity is noted. Indeed, HIV disrupts the lymphatic system of the gastrointestinal tract, causing a large loss of CD4+ T-lymphocytes in the gut-associated lymphoid tissue (GALT), which disrupts the tight junctions of the intestinal epithelium. Subsequently, this detrimentally alters the integrity of the intestinal mucosal barrier, leading to intestinal microbiomic disorders (126, 127), which manifest as a decrease in gut microbiotic organism diversity, the augmentation of specific species of potentially pathogenic gut microbiomic microorganisms (128), and the promotion of an increased permeability (or “leakiness”) of the intestinal tract. Consequently, harmful bacteria and their products, such as lipopolysaccharide (LPS), via their passage through the portal vein into the liver, may activate the liver’s innate immune system by recognition of Toll-like receptors (TLRs, especially TLR2 and TLR4) (129). Some investigators believe that the levels of translocated microbial products, such as LPS, in the portal vein and/or in the liver (which are both difficult to measure) may be more important than these microbial products being present in the systemic circulation (27, 54). This innate immune response, generated by pathogen-associated molecular patterns (PAMPs) produced by intestinal microbes, may be responsible for hepatocyte damage (130). To further illustrate this point, in a study by Evans et al. (131), using SIV-infected macaques, it was demonstrated that increased microbial load in the liver may also trigger chemokine production and an increased infiltration of CXCR6+ activated NK cells, known for their role in the development of liver fibrosis. An HIV-positive individual displaying an HIV-associated gut dysbiosis profile can, thus, readily develop HBV infection, as HIV-associated microbial translocation favors hepatocyte injury. Our group has recently published an extensive review discussing mechanisms whereby gastrointestinal microbiome dysbiosis and a “leaky” gut in PLWH increases susceptibility to HBV infection (132).
Immune Cell Exhaustion
CD8+ T-cells (levels of which remain elevated in the bloodstream during HIV infection), HIV-associated dysbiosis via microbial translocation (128, 133), and TRAIL [a proapoptotic ligand with an immune effector function promoting the eradication of infected or malignant cells (134)], are some of the identified factors responsible for CD4+ T-cell depletion. CD4+ T-cell depletion is also responsible for liver injury, which facilitates liver invasion by HBV (as described in the preceding section). Since CD4+ T-cells are important for the recruitment of HBV-specific CD8+ T-cells, a sustained CD4+ T-cell depletion restricts the ability of the immune system to adequately and appropriately respond to HBV invasion. Indeed, in such a context, it is difficult for the immune system to locate and recruit HBV-specific CD4+ T-cells (55), which represents an essential facilitator for the induction and maintenance of both CD8+ T-cells and for B-cell antibody responses (68). Researchers have also noted exhaustion signatures in HIV-infected innate immune cells, rendering them less potent at responding not only to HIV, but also to HBV, which is inherently highly efficient at avoiding recognition by the innate immune system, as reported in several studies (135–138). For example, Wang et al., have identified exhausted CD4+ T-cells and CD8+ T-cells, and then, a closer look at the exhausted CD8+ T-cells has indicated that they present less effector function phenotypes than normal CD8+ T-cells (139). Indeed, Wang et al., have identified key upregulated genes [killer cell lectin-like receptor subfamily G member 1 (KLRG1), cluster differentiation (CD160), and T-cell immunoreceptor with Ig and ITIM domains (TIGIT)] that are associated with T-cell exhaustion. Additionally, Nguyen et al. (140), have demonstrated that HIV-specific CD8+ T-cells from the lymph nodes of HIV chronic progressors preferentially express exhaustion signatures [TIGIT, lymphocyte-activation gene 3 (LAG3), CD244 (recognized as inhibitory receptors), KLRG1, and the transcription factor EOMES (Eomesodermin, also known as T-box brain protein 2, Tbr2)] (141–143). Thus, subsequent to HIV infection, remaining CD4+ T-cells and circulating CD8+ T-cells, should they be exhausted, are potentially less potent at assuming essential protective functions compared to normal CD4+ and CD8+ T-cells. A blockade of PD1 (144), CTL-4 (144), KLRG1 (139), for example, may be potentially helpful in effectively restoring the protective functions of exhausted immune cells, which in turn could promptly respond to HBV invasion.
Antiretroviral Treatment (ART)
Since most HIV-positive individuals are diagnosed during or after the seroconversion phase, most HIV-infected patients often initiate ART during or after this phase of the infection. ART efficiently suppresses HIV-1 replication by targeting key mechanisms in its life cycle (145), which in turn (i) reduces HIV viral RNA load to below detectable levels (146, 147), (ii) increases the circulating number of CD4+ T-cells (148, 149), (iii) reduces the incidence of AIDS-related diseases and/or deaths (148, 150), and (iv) effectively prevents the transmission of HIV to the uninfected population (151). Compared to untreated patients, ART reduces rates of hepatic fibrosis in treated patients by effectively increasing CD4+ T-cell numbers. However, active monitoring for ART-induced liver injury should be considered as it has been reported that some ART therapeutic drugs may be toxic to the liver (152, 153). Moreover, it has also been reported that liver-related death is the leading cause of non-AIDS death in patients whose HIV infection is well-controlled by ART (154). Thus, in ART-treated patients, the risk of liver injury does not originate solely from the prevalent HIV RNA viral load or from CD4+ T-cell depletion, but may also result from toxicity associated with ART drugs. This may also represent a potential additional factor facilitating HBV establishment.
HBV Vaccinated Individuals
In people who have received the HBV immunization, the risk for developing HBV remains, as memory B-cells and long-lived plasma cells, recognized as pivotal for maintenance of serological memory to vaccines and infections, have been shown to be reduced in number during HIV-1 infection (155, 156). Interestingly, their numerical decline correlates with reduction of antibody (Ab) titers against childhood vaccinations (157, 158). It is, therefore, reasonable to speculate on the reduction of HBV-specific antibody titers subsequent to memory B-cell reduction, even if it has been demonstrated that ART initiation shortly after HIV infection may restore memory cell numbers to physiological levels in HIV-1-infected children and adults (159, 160). Moreover, exhausted memory B-cells [activated memory B (AM) and tissue-like memory (TLM) B cells)] are expanded in the circulation during HIV-1 infection (161, 162). From the investigations of Wang et al. (139), and Nguyen et al. (140), it is now known that HIV-related exhausted T-cells become less potent at accomplishing their full repertoire of immune functions. Although some clarification remains to be elucidated in this specific subject area, we may relatively confidently assume that due to HIV infection, exhausted B-cells do become dysfunctional as well, and are thus, not as immunologically competent as normal B-cells at producing specific antibodies. Chiodi and Scarlatti (163) have proposed that the B-cell dysfunctional profile (inhibition of both B-cell proliferation and antibody production) due to cellular exhaustion caused by HIV infection, could be explained by a specific pathway engaged via the expression of inhibitory receptors on the surface of TLM B-cells during HIV-1 infection, which includes the inhibitory receptor Fc receptor-like-4 [FCRL4, which is increased in B-cells during HIV-1 (164) infection, and acts by dampening B-cell receptor (BCR) signaling]. Furthermore, presence of IL-6, known to be increased in B-cells during HIV-1 infection, may lead to aberrant B-cell differentiation (164, 165). In such contexts, the liver is vulnerable to HBV invasion, since the expected specific antibody generation resulting from administration of the HBV vaccine would have been somewhat neutralized via B-cell destruction and secondary B-cell functional impairment directly attributable to HIV infection.
Acquired Immunodeficiency Syndrome (Aids) Phase
The global success of ART in treating HIV infection and AIDS has led some to some doubt whether a curative solution to AIDS is necessary. Only patients not on ART or those who are infected with HIV strains resistant to ART can progress to the AIDS phase of HIV infection (166). In general, in untreated people or inadequately treated people, it takes several years to gradually progress from primary HIV infection to the AIDS phase, which is characterized by the onset of symptoms and signs of severe HIV illnesses and profound immunosuppression. The immunological and other issues encountered during the acute and the chronic phases of HIV infection are significantly exacerbated in the AIDS phase. A patient at this stage of the infection may have a substantially high viral load, which may, in addition to a very low CD4+ T-cell count ( Figure 2 ), lead to further liver injury, thus favoring HBV infection. The overtly symptomatic stage of HIV illness denotes the late stage of HIV disease (AIDS) in which patients (i) have a CD4+ T-cell count of less than 200 cells/mm3 and (ii) are vulnerable to additional opportunistic infections (OIs) (167) (such as infections by Mycobacterium avium complex, Mycobacterium tuberculosis, Pneumocystis jirovecii, Cytomegalovirus, Toxoplasma gondii, and Candida species) or the occurrence of aggressive forms of Kaposi’s sarcoma or B-cell lymphoma (32). Unfortunately, numerous OIs are known to be associated with liver injury, which is a vital facilitator for HBV invasion of the liver (168–173). The liver is frequently affected by opportunistic infections, most commonly in infections by mycobacteria and Cytomegalovirus (174). Compared with non-TB HIV-infected patients, TB-HIV co-infected patients present with more significantly aberrant liver function profiles, with higher serum total bilirubin, alanine transaminase (ALT) and alkaline phosphatase (ALK-P) levels (175). Dey et al., showed that Mycobacterium tuberculosis can be an etiological factor for liver abscesses in HIV-infected patients (168). Infection by Toxoplasma gondii has also been reported to promote chronic liver disease in HIV-infected individuals (169). Hepatitis C virus infection is also known to act as an opportunistic disease in AIDS patients, directly causing progressive liver damage, which may also result in liver cirrhosis and hepatocellular carcinoma (176, 177).
Moreover, the medications associated with the drug treatment of opportunistic diseases are further contributing factors to persisting liver damage. The current first-line drug treatment for TB is a regimen of four drugs, i.e., isoniazid (INH), rifampicin (RIF), ethambutol (EMB), and pyrazinamide (PZA) (178). However, hepatotoxicity has been frequently observed as a serious adverse reaction following the use of these anti-TB drugs, especially with use of PZA, INH, and RIF, with a 2–28% incidence rate (179–183). Among PLWH, a higher incidence of hepatotoxicity has been seen, and Araújo-Mariz et al., have reported a 30.6% cumulative incidence rate of hepatotoxicity in PLWH following the use of recommended drugs for TB treatment (184). Sulfonamides, including trimethoprim/sulfamethoxazole (TMP/SMZ) and sulfadiazine, are other drugs which have been widely used in AIDS patients, and have been recommended as drugs of first choice for infections by Pneumocystis jirovecii and Toxoplasma gondii in HIV-infected patients (185). These drugs have also been frequently reported to induce hepatotoxicity (186–189).
Other contributing factors that may occur during the AIDS stage, such as paradoxical and unmasking immune reconstitution inflammatory syndrome (IRIS) and drug-drug interactions, may also result in liver disease or toxicity (190, 191). However, further studies of the baseline liver status of patients (uninfected by HBV) during the AIDS stage and studies of liver enzyme profiles in these patients during the AIDS stage are warranted to further assess other potential influencing factors for HBV establishment in patients with AIDS.
Conclusion
It is known that HIV infection induces an immunodeficiency syndrome, rendering the patient vulnerable to infections, including HBV infection. The present review is the first to critically discuss the specific mechanisms leading to HBV establishment in a patient who is already HIV-positive. We report that the acute phase is responsible for a sudden immune system defense subversion, a CD4+ T-cell depletion, and a high viral RNA load, all contributing to increasing the vulnerability of the liver, which subsequently inexorably develops a permissiveness to HBV. During the chronic phase of HIV infection, gut-associated dysbiosis and immune cell exhaustion, compounded by the hepatotoxic phenomena encountered during the acute phase, are two major consequences of HIV infection which are likely to enhance the probability of subsequent HBV invasion of the liver. The other possible facilitatory causes for HBV invasion of the liver in HIV-infected patients that we have discussed herein are the use of modern ART, and HIV-associated B-cell depletion. Finally, the AIDS phase of HIV infection is often defined by particularly low CD4+ T-cell counts, OIs (and OI-related drug treatments), and extraordinarily high viral RNA loads, all of which, as we have described herein, conspire to inflict further sustained injury to the liver, which also favors HBV establishment.
Author Contributions
SZ and JO wrote the first draft of the manuscript. YLC, YJ, and HW provided critical revision of the manuscript. YKC conceived and designed the study. All authors read and approved the manuscript and its submission for publication.
Funding
This work was supported by the Chongqing Talent Cultivation Program (cstc2021ycjh-bgzxm0275), the Joint Medical Research Project (2020GDRC010) of Chongqing Science & Technology Bureau and Chongqing Health Commission, the Chinese Federation of Public Health foundation (GWLM202024), and the Chongqing Science & Technology Bureau project (cstc2020jscx-cylh0004).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- 1. Xia H, Wang Y, Sun HL, Gao LY, Cao Y, Zaongo SD, et al. Safety and Efficacy of Allogeneic Natural Killer Cell Immunotherapy on Human Immunodeficiency Virus Type 1 Immunological Non-Responders: A Brief Report. Chin Med J (2020) 133(23):2803–7. doi: 10.1097/cm9.0000000000001189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zaongo SD, Xia H, Ma P. HIV Gene Therapy Strategies and Safety: What Do We Know From the Recent Publications? AIDS Rev (2020) 23(3):195–202. doi: 10.24875/AIDSRev.20000008 [DOI] [PubMed] [Google Scholar]
- 3. Falkenhagen A, Joshi S. Genetic Strategies for HIV Treatment and Prevention. Mol Ther Nucleic Acids (2018) 13:514–33. doi: 10.1016/j.omtn.2018.09.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zaongo SD, Liu Y, Harypursat V, Song F, Xia H, Ma P, et al. P-Selectin Glycoprotein Ligand 1: A Potential HIV-1 Therapeutic Target. Front Immunol (2021) 12:710121. doi: 10.3389/fimmu.2021.710121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zaongo SD, Wang Y, Ma P, Song FZ, Chen YK. Selective Elimination of Host Cells Harboring Replication-Competent Human Immunodeficiency Virus Reservoirs: A Promising Therapeutic Strategy for HIV Cure. Chin Med J (2021) 134(23):2776–87. doi: 10.1097/cm9.0000000000001797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. World Health Organization . Interim WHO Clinical Staging of HIV/AIDS and HIV/AIDS Case Definitions for Surveillance. Geneva, Switzerland: World Health Organisation; (2005). Available at: https://apps.who.int/iris/bitstream/handle/10665/69058/WHO_HIV_2005.02.pdf. [Google Scholar]
- 7. Weinberg JL, Kovarik CL. The WHO Clinical Staging System for HIV/AIDS. Virtual Mentor (2010) 12(3):202–6. doi: 10.1001/virtualmentor.2010.12.3.cprl1-1003 [DOI] [PubMed] [Google Scholar]
- 8. Naif HM. Pathogenesis of HIV Infection. Infect Dis Rep (2013) 5(Suppl 1):e6. doi: 10.4081/idr.2013.s1.e6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. UNAIDS . AIDSinfo (2020). Available at: https://aidsinfo.unaids.org/ (Accessed January 12, 2022).
- 10. Bendavid E, Young SD, Katzenstein DA, Bayoumi AM, Sanders GD, Owens DK. Cost-Effectiveness of HIV Monitoring Strategies in Resource-Limited Settings: A Southern African Analysis. Arch Intern Med (2008) 168(17):1910–8. doi: 10.1001/archinternmed.2008.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Charles M, Boyle BA. Excess and Access: The Continuing Controversy Regarding HIV and Health Care in Africa. AIDS Read (2002) 12(7):288–92. [PubMed] [Google Scholar]
- 12. Zampino R, Boemio A, Sagnelli C, Alessio L, Adinolfi LE, Sagnelli E, et al. Hepatitis B Virus Burden in Developing Countries. World J Gastroenterol (2015) 21(42):11941–53. doi: 10.3748/wjg.v21.i42.11941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. World Health Organization . Hepatitis B (2021). Available at: https://www.who.int/news-room/fact-sheets/detail/hepatitis-b (Accessed February 23, 2022).
- 14. Potthoff A, Manns MP, Wedemeyer H. Treatment of HBV/HCV Coinfection. Expert Opin Pharmacother (2010) 11(6):919–28. doi: 10.1517/14656561003637659 [DOI] [PubMed] [Google Scholar]
- 15. Mavilia MG, Wu GY. HBV-HCV Coinfection: Viral Interactions, Management, and Viral Reactivation. J Clin Transl Hepatol (2018) 6(3):296–305. doi: 10.14218/jcth.2018.00016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Locarnini S, Hatzakis A, Chen DS, Lok A. Strategies to Control Hepatitis B: Public Policy, Epidemiology, Vaccine and Drugs. J Hepatol (2015) 62(1 Suppl):S76–86. doi: 10.1016/j.jhep.2015.01.018 [DOI] [PubMed] [Google Scholar]
- 17. Yuen MF, Chen DS, Dusheiko GM, Janssen HLA, Lau DTY, Locarnini SA, et al. Hepatitis B Virus Infection. Nat Rev Dis Primers (2018) 4:18035. doi: 10.1038/nrdp.2018.35 [DOI] [PubMed] [Google Scholar]
- 18. Revill PA, Chisari FV, Block JM, Dandri M, Gehring AJ, Guo H, et al. A Global Scientific Strategy to Cure Hepatitis B. Lancet Gastroenterol Hepatol (2019) 4(7):545–58. doi: 10.1016/s2468-1253(19)30119-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Shire NJ, Sherman KE. Management of HBV/HIV-Coinfected Patients. Semin Liver Dis (2005) 25(Suppl 1):48–57. doi: 10.1055/s-2005-915646 [DOI] [PubMed] [Google Scholar]
- 20. Utsumi T, Lusida MI. Viral Hepatitis and Human Immunodeficiency Virus Co-Infections in Asia. World J Virol (2015) 4(2):96–104. doi: 10.5501/wjv.v4.i2.96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kaspar MB, Sterling RK. Mechanisms of Liver Disease in Patients Infected With HIV. BMJ Open Gastroenterol (2017) 4(1):e000166. doi: 10.1136/bmjgast-2017-000166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kourtis AP, Bulterys M, Hu DJ, Jamieson DJ. HIV-HBV Coinfection–a Global Challenge. N Engl J Med (2012) 366(19):1749–52. doi: 10.1056/NEJMp1201796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Dunford L, Carr MJ, Dean J, Nguyen LT, Ta Thi TH, Nguyen BT, et al. A Multicentre Molecular Analysis of Hepatitis B and Blood-Borne Virus Coinfections in Viet Nam. PloS One (2012) 7(6):e39027. doi: 10.1371/journal.pone.0039027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Templeton DJ, Wright ST, McManus H, Lawrence C, Russell DB, Law MG, et al. Antiretroviral Treatment Use, Co-Morbidities and Clinical Outcomes Among Aboriginal Participants in the Australian HIV Observational Database (AHOD). BMC Infect Dis (2015) 15:326. doi: 10.1186/s12879-015-1051-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bell TG, Makondo E, Martinson NA, Kramvis A. Hepatitis B Virus Infection in Human Immunodeficiency Virus Infected Southern African Adults: Occult or Overt–That Is the Question. PloS One (2012) 7(10):e45750. doi: 10.1371/journal.pone.0045750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Xie J, Han Y, Qiu Z, Li Y, Li Y, Song X, et al. Prevalence of Hepatitis B and C Viruses in HIV-Positive Patients in China: A Cross-Sectional Study. J Int AIDS Soc (2016) 19(1):20659. doi: 10.7448/ias.19.1.20659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Singh KP, Crane M, Audsley J, Avihingsanon A, Sasadeusz J, Lewin SR. HIV-Hepatitis B Virus Coinfection: Epidemiology, Pathogenesis, and Treatment. AIDS (2017) 31(15):2035–52. doi: 10.1097/qad.0000000000001574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hoffmann CJ, Thio CL. Clinical Implications of HIV and Hepatitis B Co-Infection in Asia and Africa. Lancet Infect Dis (2007) 7(6):402–9. doi: 10.1016/s1473-3099(07)70135-4 [DOI] [PubMed] [Google Scholar]
- 29. Cheng Z, Lin P, Cheng N. HBV/HIV Coinfection: Impact on the Development and Clinical Treatment of Liver Diseases. Front Med (2021) 8:713981. doi: 10.3389/fmed.2021.713981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ferrante ND, Lo Re V. Epidemiology, Natural History, and Treatment of Hepatitis Delta Virus Infection in HIV/Hepatitis B Virus Coinfection. Curr HIV/AIDS Rep (2020) 17(4):405–14. doi: 10.1007/s11904-020-00508-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ionita G, Malviya A, Rajbhandari R, Schluter WW, Sharma G, Kakchapati S, et al. Seroprevalence of Hepatitis B Virus and Hepatitis C Virus Co-Infection Among People Living With HIV/AIDS Visiting Antiretroviral Therapy Centres in Nepal: A First Nationally Representative Study. Int J Infect Dis (2017) 60:64–9. doi: 10.1016/j.ijid.2017.04.011 [DOI] [PubMed] [Google Scholar]
- 32. Janeway CA, Jr, Travers P, Walport M, Shlomchik MJ. Immunobiology: The Immune System in Health and Disease. 5th Edition. New York: Garland Science; (2001). [Google Scholar]
- 33. Wilen CB, Tilton JC, Doms RW. HIV: Cell Binding and Entry. Cold Spring Harb Perspect Med (2012) 2(8):a006866. doi: 10.1101/cshperspect.a006866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Simon V, Bloch N, Landau NR. Intrinsic Host Restrictions to HIV-1 and Mechanisms of Viral Escape. Nat Immunol (2015) 16(6):546–53. doi: 10.1038/ni.3156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sugden SM, Bego MG, Pham TN, Cohen ÉA. Remodeling of the Host Cell Plasma Membrane by HIV-1 Nef and Vpu: A Strategy to Ensure Viral Fitness and Persistence. Viruses (2016) 8(3):67. doi: 10.3390/v8030067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bowerman B, Brown PO, Bishop JM, Varmus HE. A Nucleoprotein Complex Mediates the Integration of Retroviral DNA. Genes Dev (1989) 3(4):469–78. doi: 10.1101/gad.3.4.469 [DOI] [PubMed] [Google Scholar]
- 37. Dharan A, Bachmann N, Talley S, Zwikelmaier V, Campbell EM. Nuclear Pore Blockade Reveals That HIV-1 Completes Reverse Transcription and Uncoating in the Nucleus. Nat Microbiol (2020) 5(9):1088–95. doi: 10.1038/s41564-020-0735-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Krishnan L, Engelman A. Retroviral Integrase Proteins and HIV-1 DNA Integration. J Biol Chem (2012) 287(49):40858–66. doi: 10.1074/jbc.R112.397760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ambrose Z, Aiken C. HIV-1 Uncoating: Connection to Nuclear Entry and Regulation by Host Proteins. Virology (2014) 454-455:371–9. doi: 10.1016/j.virol.2014.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Fujiwara T, Mizuuchi K. Retroviral DNA Integration: Structure of an Integration Intermediate. Cell (1988) 54(4):497–504. doi: 10.1016/0092-8674(88)90071-2 [DOI] [PubMed] [Google Scholar]
- 41. Brown PO, Bowerman B, Varmus HE, Bishop JM. Retroviral Integration: Structure of the Initial Covalent Product and Its Precursor, and a Role for the Viral IN Protein. Proc Natl Acad Sci USA (1989) 86(8):2525–9. doi: 10.1073/pnas.86.8.2525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Craigie R, Bushman FD. HIV DNA Integration. Cold Spring Harb Perspect Med (2012) 2(7):a006890. doi: 10.1101/cshperspect.a006890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Gish RG, Given BD, Lai CL, Locarnini SA, Lau JY, Lewis DL, et al. Chronic Hepatitis B: Virology, Natural History, Current Management and a Glimpse at Future Opportunities. Antivir Res (2015) 121:47–58. doi: 10.1016/j.antiviral.2015.06.008 [DOI] [PubMed] [Google Scholar]
- 44. Tong S, Revill P. Overview of Hepatitis B Viral Replication and Genetic Variability. J Hepatol (2016) 64(1 Suppl):S4–s16. doi: 10.1016/j.jhep.2016.01.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Revill PA, Locarnini SA. New Perspectives on the Hepatitis B Virus Life Cycle in the Human Liver. J Clin Invest (2016) 126(3):833–6. doi: 10.1172/jci86650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Nassal M. HBV cccDNA: Viral Persistence Reservoir and Key Obstacle for a Cure of Chronic Hepatitis B. Gut (2015) 64(12):1972–84. doi: 10.1136/gutjnl-2015-309809 [DOI] [PubMed] [Google Scholar]
- 47. Hillis WD. Viral Hepatitis Associated With Sub-Human Primates. Transfusion (1963) 3:445–54. doi: 10.1111/j.1537-2995.1963.tb04673.x [DOI] [PubMed] [Google Scholar]
- 48. Walter E, Keist R, Niederöst B, Pult I, Blum HE. Hepatitis B Virus Infection of Tupaia Hepatocytes. Vitro Vivo Hepatol (1996) 24(1):1–5. doi: 10.1002/hep.510240101 [DOI] [PubMed] [Google Scholar]
- 49. Schulze A, Gripon P, Urban S. Hepatitis B Virus Infection Initiates With a Large Surface Protein-Dependent Binding to Heparan Sulfate Proteoglycans. Hepatology (2007) 46(6):1759–68. doi: 10.1002/hep.21896 [DOI] [PubMed] [Google Scholar]
- 50. Sureau C, Salisse J. A Conformational Heparan Sulfate Binding Site Essential to Infectivity Overlaps With the Conserved Hepatitis B Virus a-Determinant. Hepatology (2013) 57(3):985–94. doi: 10.1002/hep.26125 [DOI] [PubMed] [Google Scholar]
- 51. Döring B, Lütteke T, Geyer J, Petzinger E. The SLC10 Carrier Family: Transport Functions and Molecular Structure. Curr Top Membr (2012) 70:105–68. doi: 10.1016/b978-0-12-394316-3.00004-1 [DOI] [PubMed] [Google Scholar]
- 52. Yan H, Zhong G, Xu G, He W, Jing Z, Gao Z, et al. Sodium Taurocholate Cotransporting Polypeptide Is a Functional Receptor for Human Hepatitis B and D Virus. eLife (2012) 1:e00049. doi: 10.7554/eLife.00049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Seeger C, Mason WS. Molecular Biology of Hepatitis B Virus Infection. Virology (2015) 479-480:672–86. doi: 10.1016/j.virol.2015.02.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Iannacone M, Guidotti LG. Immunobiology and Pathogenesis of Hepatitis B Virus Infection. Nat Rev Immunol (2022) 22(1):19–32. doi: 10.1038/s41577-021-00549-4 [DOI] [PubMed] [Google Scholar]
- 55. Asabe S, Wieland SF, Chattopadhyay PK, Roederer M, Engle RE, Purcell RH, et al. The Size of the Viral Inoculum Contributes to the Outcome of Hepatitis B Virus Infection. J Virol (2009) 83(19):9652–62. doi: 10.1128/jvi.00867-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Whalley SA, Murray JM, Brown D, Webster GJ, Emery VC, Dusheiko GM, et al. Kinetics of Acute Hepatitis B Virus Infection in Humans. J Exp Med (2001) 193(7):847–54. doi: 10.1084/jem.193.7.847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. National Institutes of Health . HIV and Hepatitis B (2021). Available at: https://hivinfo.nih.gov/understanding-hiv/fact-sheets/hiv-and-hepatitis-b (Accessed January 12, 2022).
- 58. Prendergast AJ, Klenerman P, Goulder PJ. The Impact of Differential Antiviral Immunity in Children and Adults. Nat Rev Immunol (2012) 12(9):636–48. doi: 10.1038/nri3277 [DOI] [PubMed] [Google Scholar]
- 59. Tsai KN, Kuo CF, Ou JJ. Mechanisms of Hepatitis B Virus Persistence. Trends Microbiol (2018) 26(1):33–42. doi: 10.1016/j.tim.2017.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Chamroonkul N, Bansal MB. HIV and the Liver. Nat Rev Gastroenterol Hepatol (2019) 16(1):1–2. doi: 10.1038/s41575-018-0085-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Housset C, Lamas E, Courgnaud V, Boucher O, Girard PM, Marche C, et al. Presence of HIV-1 in Human Parenchymal and non-Parenchymal Liver Cells. Vivo J Hepatol (1993) 19(2):252–8. doi: 10.1016/s0168-8278(05)80579-3 [DOI] [PubMed] [Google Scholar]
- 62. Cao YZ, Dieterich D, Thomas PA, Huang YX, Mirabile M, Ho DD. Identification and Quantitation of HIV-1 in the Liver of Patients With AIDS. AIDS (1992) 6(1):65–70. doi: 10.1097/00002030-199201000-00008 [DOI] [PubMed] [Google Scholar]
- 63. Penton PK, Blackard JT. Analysis of HIV Quasispecies Suggests Compartmentalization in the Liver. AIDS Res Hum Retroviruses (2014) 30(4):394–402. doi: 10.1089/aid.2013.0146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Denton PW, Long JM, Wietgrefe SW, Sykes C, Spagnuolo RA, Snyder OD, et al. Targeted Cytotoxic Therapy Kills Persisting HIV Infected Cells During ART. PloS Pathog (2014) 10(1):e1003872. doi: 10.1371/journal.ppat.1003872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. North TW, Higgins J, Deere JD, Hayes TL, Villalobos A, Adamson L, et al. Viral Sanctuaries During Highly Active Antiretroviral Therapy in a Nonhuman Primate Model for AIDS. J Virol (2010) 84(6):2913–22. doi: 10.1128/jvi.02356-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Kandathil AJ, Durand CM, Quinn J, Cameron A, Thomas DL, Balagopal A. Liver Macrophages and HIV-1 Persistence. Seattle: CROI; (2015). [Google Scholar]
- 67. Xiao J, Wan X, Wang H, Deng G. Analysis of HBV-Specific CD4 T-Cell Responses and Identification of HLA-DR-Restricted CD4 T-Cell Epitopes Based on a Peptide Matrix. J Vis Exp (2021), e62387. doi: 10.3791/62387 [DOI] [PubMed] [Google Scholar]
- 68. Guidotti LG, Chisari FV. Immunobiology and Pathogenesis of Viral Hepatitis. Annu Rev Pathol (2006) 1:23–61. doi: 10.1146/annurev.pathol.1.110304.100230 [DOI] [PubMed] [Google Scholar]
- 69. Thimme R, Wieland S, Steiger C, Ghrayeb J, Reimann KA, Purcell RH, et al. CD8(+) T Cells Mediate Viral Clearance and Disease Pathogenesis During Acute Hepatitis B Virus Infection. J Virol (2003) 77(1):68–76. doi: 10.1128/jvi.77.1.68-76.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Phillips S, Chokshi S, Riva A, Evans A, Williams R, Naoumov NV. CD8(+) T Cell Control of Hepatitis B Virus Replication: Direct Comparison Between Cytolytic and Noncytolytic Functions. J Immunol (2010) 184(1):287–95. doi: 10.4049/jimmunol.0902761 [DOI] [PubMed] [Google Scholar]
- 71. Morvan MG, Teque FC, Locher CP, Levy JA. The CD8(+) T Cell Noncytotoxic Antiviral Responses. Microbiol Mol Biol Rev (2021) 85(2):e00155–20. doi: 10.1128/mmbr.00155-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Kao E, Shinohara M, Feng M, Lau MY, Ji C. Human Immunodeficiency Virus Protease Inhibitors Modulate Ca2+ Homeostasis and Potentiate Alcoholic Stress and Injury in Mice and Primary Mouse and Human Hepatocytes. Hepatology (2012) 56(2):594–604. doi: 10.1002/hep.25702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Williams K, Rao YP, Natarajan R, Pandak WM, Hylemon PB. Indinavir Alters Sterol and Fatty Acid Homeostatic Mechanisms in Primary Rat Hepatocytes by Increasing Levels of Activated Sterol Regulatory Element-Binding Proteins and Decreasing Cholesterol 7alpha-Hydroxylase mRNA Levels. Biochem Pharmacol (2004) 67(2):255–67. doi: 10.1016/j.bcp.2003.08.044 [DOI] [PubMed] [Google Scholar]
- 74. Del Cornò M, Cappon A, Donninelli G, Varano B, Marra F, Gessani S. HIV-1 Gp120 Signaling Through TLR4 Modulates Innate Immune Activation in Human Macrophages and the Biology of Hepatic Stellate Cells. J Leukoc Biol (2016) 100(3):599–606. doi: 10.1189/jlb.4A1215-534R [DOI] [PubMed] [Google Scholar]
- 75. Tuyama AC, Hong F, Saiman Y, Wang C, Ozkok D, Mosoian A, et al. Human Immunodeficiency Virus (HIV)-1 Infects Human Hepatic Stellate Cells and Promotes Collagen I and Monocyte Chemoattractant Protein-1 Expression: Implications for the Pathogenesis of HIV/hepatitis C Virus-Induced Liver Fibrosis. Hepatology (2010) 52(2):612–22. doi: 10.1002/hep.23679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Blackard JT, Sherman KE. HCV/ HIV Co-Infection: Time to Re-Evaluate the Role of HIV in the Liver? J Viral Hepat (2008) 15(5):323–30. doi: 10.1111/j.1365-2893.2008.00970.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Mastroianni CM, Lichtner M, Mascia C, Zuccalà P, Vullo V. Molecular Mechanisms of Liver Fibrosis in HIV/HCV Coinfection. Int J Mol Sci (2014) 15(6):9184–208. doi: 10.3390/ijms15069184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Suhail M, Abdel-Hafiz H, Ali A, Fatima K, Damanhouri GA, Azhar E, et al. Potential Mechanisms of Hepatitis B Virus Induced Liver Injury. World J Gastroenterol (2014) 20(35):12462–72. doi: 10.3748/wjg.v20.i35.12462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Sacchi P, Cima S, Corbella M, Comolli G, Chiesa A, Baldanti F, et al. Liver Fibrosis, Microbial Translocation and Immune Activation Markers in HIV and HCV Infections and in HIV/HCV Co-Infection. Dig Liver Dis (2015) 47(3):218–25. doi: 10.1016/j.dld.2014.11.012 [DOI] [PubMed] [Google Scholar]
- 80. Page EE, Nelson M, Kelleher P. HIV and Hepatitis C Coinfection: Pathogenesis and Microbial Translocation. Curr Opin HIV AIDS (2011) 6(6):4–7. doi: 10.1097/COH.0b013e32834bbc71 [DOI] [PubMed] [Google Scholar]
- 81. Wright SD, Ramos RA, Tobias PS, Ulevitch RJ, Mathison JC. CD14, a Receptor for Complexes of Lipopolysaccharide (LPS) and LPS Binding Protein. Science (1990) 249(4975):1431–3. doi: 10.1126/science.1698311 [DOI] [PubMed] [Google Scholar]
- 82. Chang PE, Miquel R, Blanco JL, Laguno M, Bruguera M, Abraldes JG, et al. Idiopathic Portal Hypertension in Patients With HIV Infection Treated With Highly Active Antiretroviral Therapy. Am J Gastroenterol (2009) 104(7):1707–14. doi: 10.1038/ajg.2009.165 [DOI] [PubMed] [Google Scholar]
- 83. Wanless IR. Micronodular Transformation (Nodular Regenerative Hyperplasia) of the Liver: A Report of 64 Cases Among 2,500 Autopsies and a New Classification of Benign Hepatocellular Nodules. Hepatology (1990) 11(5):787–97. doi: 10.1002/hep.1840110512 [DOI] [PubMed] [Google Scholar]
- 84. Sood A, Castrejón M, Saab S. Human Immunodeficiency Virus and Nodular Regenerative Hyperplasia of Liver: A Systematic Review. World J Hepatol (2014) 6(1):55–63. doi: 10.4254/wjh.v6.i1.55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Pilcher CD, Eron JJ, Jr., Galvin S, Gay C, Cohen MS. Acute HIV Revisited: New Opportunities for Treatment and Prevention. J Clin Invest (2004) 113(7):937–45. doi: 10.1172/jci21540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Fiebig EW, Wright DJ, Rawal BD, Garrett PE, Schumacher RT, Peddada L, et al. Dynamics of HIV Viremia and Antibody Seroconversion in Plasma Donors: Implications for Diagnosis and Staging of Primary HIV Infection. AIDS (2003) 17(13):1871–9. doi: 10.1097/00002030-200309050-00005 [DOI] [PubMed] [Google Scholar]
- 87. Quinn TC, Brookmeyer R, Kline R, Shepherd M, Paranjape R, Mehendale S, et al. Feasibility of Pooling Sera for HIV-1 Viral RNA to Diagnose Acute Primary HIV-1 Infection and Estimate HIV Incidence. AIDS (2000) 14(17):2751–7. doi: 10.1097/00002030-200012010-00015 [DOI] [PubMed] [Google Scholar]
- 88. Zetola NM, Pilcher CD. Diagnosis and Management of Acute HIV Infection. Infect Dis Clin North Am (2007) 21(1):19–48, vii. doi: 10.1016/j.idc.2007.01.008 [DOI] [PubMed] [Google Scholar]
- 89. Pilcher CD, Shugars DC, Fiscus SA, Miller WC, Menezes P, Giner J, et al. HIV in Body Fluids During Primary HIV Infection: Implications for Pathogenesis, Treatment and Public Health. AIDS (2001) 15(7):837–45. doi: 10.1097/00002030-200105040-00004 [DOI] [PubMed] [Google Scholar]
- 90. Pilcher CD, Joaki G, Hoffman IF, Martinson FE, Mapanje C, Stewart PW, et al. Amplified Transmission of HIV-1: Comparison of HIV-1 Concentrations in Semen and Blood During Acute and Chronic Infection. AIDS (2007) 21(13):1723–30. doi: 10.1097/QAD.0b013e3281532c82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Pilcher CD, Tien HC, Eron JJ, Jr, Vernazza PL, Leu SY, Stewart PW, et al. Brief But Efficient: Acute HIV Infection and the Sexual Transmission of HIV. J Infect Dis (2004) 189(10):1785–92. doi: 10.1086/386333 [DOI] [PubMed] [Google Scholar]
- 92. Morrison CS, Demers K, Kwok C, Bulime S, Rinaldi A, Munjoma M, et al. Plasma and Cervical Viral Loads Among Ugandan and Zimbabwean Women During Acute and Early HIV-1 Infection. AIDS (2010) 24(4):573–82. doi: 10.1097/QAD.0b013e32833433df [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Borrow P. Innate Immunity in Acute HIV-1 Infection. Curr Opin HIV AIDS (2011) 6(5):353–63. doi: 10.1097/COH.0b013e3283495996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Weinberg A, Tugizov S, Pandiyan P, Jin G, Rakshit S, Vyakarnam A, et al. Innate Immune Mechanisms to Oral Pathogens in Oral Mucosa of HIV-Infected Individuals. Oral Dis (2020) 26(Suppl 1):69–79. doi: 10.1111/odi.13470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Stremlau M, Owens CM, Perron MJ, Kiessling M, Autissier P, Sodroski J. The Cytoplasmic Body Component TRIM5alpha Restricts HIV-1 Infection in Old World Monkeys. Nature (2004) 427(6977):848–53. doi: 10.1038/nature02343 [DOI] [PubMed] [Google Scholar]
- 96. Sheehy AM, Gaddis NC, Choi JD, Malim MH. Isolation of a Human Gene That Inhibits HIV-1 Infection and Is Suppressed by the Viral Vif Protein. Nature (2002) 418(6898):646–50. doi: 10.1038/nature00939 [DOI] [PubMed] [Google Scholar]
- 97. Neil SJ, Zang T, Bieniasz PD. Tetherin Inhibits Retrovirus Release and Is Antagonized by HIV-1 Vpu. Nature (2008) 451(7177):425–30. doi: 10.1038/nature06553 [DOI] [PubMed] [Google Scholar]
- 98. Hrecka K, Hao C, Gierszewska M, Swanson SK, Kesik-Brodacka M, Srivastava S, et al. Vpx Relieves Inhibition of HIV-1 Infection of Macrophages Mediated by the SAMHD1 Protein. Nature (2011) 474(7353):658–61. doi: 10.1038/nature10195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Laguette N, Sobhian B, Casartelli N, Ringeard M, Chable-Bessia C, Ségéral E, et al. SAMHD1 Is the Dendritic- and Myeloid-Cell-Specific HIV-1 Restriction Factor Counteracted by Vpx. Nature (2011) 474(7353):654–7. doi: 10.1038/nature10117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Rosa A, Chande A, Ziglio S, De Sanctis V, Bertorelli R, Goh SL, et al. HIV-1 Nef Promotes Infection by Excluding SERINC5 From Virion Incorporation. Nature (2015) 526(7572):212–7. doi: 10.1038/nature15399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Usami Y, Wu Y, Göttlinger HG. SERINC3 and SERINC5 Restrict HIV-1 Infectivity and Are Counteracted by Nef. Nature (2015) 526(7572):218–23. doi: 10.1038/nature15400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Solis M, Nakhaei P, Jalalirad M, Lacoste J, Douville R, Arguello M, et al. RIG-I-Mediated Antiviral Signaling Is Inhibited in HIV-1 Infection by a Protease-Mediated Sequestration of RIG-I. J Virol (2011) 85(3):1224–36. doi: 10.1128/jvi.01635-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Yan N, Regalado-Magdos AD, Stiggelbout B, Lee-Kirsch MA, Lieberman J. The Cytosolic Exonuclease TREX1 Inhibits the Innate Immune Response to Human Immunodeficiency Virus Type 1. Nat Immunol (2010) 11(11):1005–13. doi: 10.1038/ni.1941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Doehle BP, Hladik F, McNevin JP, McElrath MJ, Gale M., Jr. Human Immunodeficiency Virus Type 1 Mediates Global Disruption of Innate Antiviral Signaling and Immune Defenses Within Infected Cells. J Virol (2009) 83(20):10395–405. doi: 10.1128/jvi.00849-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Harman AN, Lai J, Turville S, Samarajiwa S, Gray L, Marsden V, et al. HIV Infection of Dendritic Cells Subverts the IFN Induction Pathway via IRF-1 and Inhibits Type 1 IFN Production. Blood (2011) 118(2):298–308. doi: 10.1182/blood-2010-07-297721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Schoggins JW, Wilson SJ, Panis M, Murphy MY, Jones CT, Bieniasz P, et al. A Diverse Range of Gene Products Are Effectors of the Type I Interferon Antiviral Response. Nature (2011) 472(7344):481–5. doi: 10.1038/nature09907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Gondim MVP, Sherrill-Mix S, Bibollet-Ruche F, Russell RM, Trimboli S, Smith AG, et al. Heightened Resistance to Host Type 1 Interferons Characterizes HIV-1 at Transmission and After Antiretroviral Therapy Interruption. Sci Transl Med (2021) 13(576):eabd8179. doi: 10.1126/scitranslmed.abd8179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Alter G, Martin MP, Teigen N, Carr WH, Suscovich TJ, Schneidewind A, et al. Differential Natural Killer Cell-Mediated Inhibition of HIV-1 Replication Based on Distinct KIR/HLA Subtypes. J Exp Med (2007) 204(12):3027–36. doi: 10.1084/jem.20070695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Ward J, Bonaparte M, Sacks J, Guterman J, Fogli M, Mavilio D, et al. HIV Modulates the Expression of Ligands Important in Triggering Natural Killer Cell Cytotoxic Responses on Infected Primary T-Cell Blasts. Blood (2007) 110(4):1207–14. doi: 10.1182/blood-2006-06-028175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Kielczewska A, Pyzik M, Sun T, Krmpotic A, Lodoen MB, Munks MW, et al. Ly49P Recognition of Cytomegalovirus-Infected Cells Expressing H2-Dk and CMV-Encoded M04 Correlates With the NK Cell Antiviral Response. J Exp Med (2009) 206(3):515–23. doi: 10.1084/jem.20080954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Richard J, Sindhu S, Pham TN, Belzile JP, Cohen EA. HIV-1 Vpr Up-Regulates Expression of Ligands for the Activating NKG2D Receptor and Promotes NK Cell-Mediated Killing. Blood (2010) 115(7):1354–63. doi: 10.1182/blood-2009-08-237370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Ward J, Davis Z, DeHart J, Zimmerman E, Bosque A, Brunetta E, et al. HIV-1 Vpr Triggers Natural Killer Cell-Mediated Lysis of Infected Cells Through Activation of the ATR-Mediated DNA Damage Response. PloS Pathog (2009) 5(10):e1000613. doi: 10.1371/journal.ppat.1000613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Fausther-Bovendo H, Vieillard V, Sagan S, Bismuth G, Debré P. HIV Gp41 Engages Gc1qr on CD4+ T Cells to Induce the Expression of an NK Ligand Through the PIP3/H2O2 Pathway. PloS Pathog (2010) 6(7):e1000975. doi: 10.1371/journal.ppat.1000975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Crane M, Iser D, Lewin SR. Human Immunodeficiency Virus Infection and the Liver. World J Hepatol (2012) 4(3):91–8. doi: 10.4254/wjh.v4.i3.91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Babu CK, Suwansrinon K, Bren GD, Badley AD, Rizza SA. HIV Induces TRAIL Sensitivity in Hepatocytes. PloS One (2009) 4(2):e4623. doi: 10.1371/journal.pone.0004623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Herbeuval JP, Boasso A, Grivel JC, Hardy AW, Anderson SA, Dolan MJ, et al. TNF-Related Apoptosis-Inducing Ligand (TRAIL) in HIV-1-Infected Patients and Its Vitro Prod by Antigen-Presenting Cells. Blood (2005) 105(6):2458–64. doi: 10.1182/blood-2004-08-3058 [DOI] [PubMed] [Google Scholar]
- 117. Koziel MJ. The Immunopathogenesis of HBV Infection. Antivir Ther (1998) 3(Suppl 3):13–24. [PubMed] [Google Scholar]
- 118. Park JS, Saraf N, Dieterich DT. HBV Plus HCV, HCV Plus HIV, HBV Plus HIV. Curr Gastroenterol Rep (2006) 8(1):67–74. doi: 10.1007/s11894-006-0066-9 [DOI] [PubMed] [Google Scholar]
- 119. Joshi D, O'Grady J, Dieterich D, Gazzard B, Agarwal K. Increasing Burden of Liver Disease in Patients With HIV Infection. Lancet (2011) 377(9772):1198–209. doi: 10.1016/s0140-6736(10)62001-6 [DOI] [PubMed] [Google Scholar]
- 120. Cunningham AL, Li S, Juarez J, Lynch G, Alali M, Naif H. The Level of HIV Infection of Macrophages Is Determined by Interaction of Viral and Host Cell Genotypes. J Leukoc Biol (2000) 68(3):311–7. [PubMed] [Google Scholar]
- 121. University of Californian San Francisco . HIV Diagnosis (2022). Available at: https://www.ucsfhealth.org/conditions/hiv/diagnosis (Accessed January 12, 2022).
- 122. Buzón MJ, Massanella M, Llibre JM, Esteve A, Dahl V, Puertas MC, et al. HIV-1 Replication and Immune Dynamics Are Affected by Raltegravir Intensification of HAART-Suppressed Subjects. Nat Med (2010) 16(4):460–5. doi: 10.1038/nm.2111 [DOI] [PubMed] [Google Scholar]
- 123. Mutlu EA, Keshavarzian A, Losurdo J, Swanson G, Siewe B, Forsyth C, et al. A Compositional Look at the Human Gastrointestinal Microbiome and Immune Activation Parameters in HIV Infected Subjects. PloS Pathog (2014) 10(2):e1003829. doi: 10.1371/journal.ppat.1003829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. McGrath N, Eaton JW, Newell ML, Hosegood V. Migration, Sexual Behaviour, and HIV Risk: A General Population Cohort in Rural South Africa. Lancet HIV (2015) 2(6):e252–9. doi: 10.1016/s2352-3018(15)00045-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Yoder AC, Guo K, Dillon SM, Phang T, Lee EJ, Harper MS, et al. The Transcriptome of HIV-1 Infected Intestinal CD4+ T Cells Exposed to Enteric Bacteria. PloS Pathog (2017) 13(2):e1006226. doi: 10.1371/journal.ppat.1006226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Brenchley JM, Schacker TW, Ruff LE, Price DA, Taylor JH, Beilman GJ, et al. CD4+ T Cell Depletion During All Stages of HIV Disease Occurs Predominantly in the Gastrointestinal Tract. J Exp Med (2004) 200(6):749–59. doi: 10.1084/jem.20040874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Li Q, Duan L, Estes JD, Ma ZM, Rourke T, Wang Y, et al. Peak SIV Replication in Resting Memory CD4+ T Cells Depletes Gut Lamina Propria CD4+ T Cells. Nature (2005) 434(7037):1148–52. doi: 10.1038/nature03513 [DOI] [PubMed] [Google Scholar]
- 128. Ponte R, Mehraj V, Ghali P, Couëdel-Courteille A, Cheynier R, Routy JP. Reversing Gut Damage in HIV Infection: Using Non-Human Primate Models to Instruct Clinical Research. EBioMedicine (2016) 4:40–9. doi: 10.1016/j.ebiom.2016.01.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Sehgal R, Bedi O, Trehanpati N. Role of Microbiota in Pathogenesis and Management of Viral Hepatitis. Front Cell Infect Microbiol (2020) 10:341. doi: 10.3389/fcimb.2020.00341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Yang R, Xu Y, Dai Z, Lin X, Wang H. The Immunologic Role of Gut Microbiota in Patients With Chronic HBV Infection. J Immunol Res (2018) 2018:2361963. doi: 10.1155/2018/2361963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Evans TI, Li H, Schafer JL, Klatt NR, Hao XP, Traslavina RP, et al. SIV-Induced Translocation of Bacterial Products in the Liver Mobilizes Myeloid Dendritic and Natural Killer Cells Associated With Liver Damage. J Infect Dis (2016) 213(3):361–9. doi: 10.1093/infdis/jiv404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Ouyang J, Zaongo SD, Zhang X, Qi M, Hu A, Wu H, et al. Microbiota-Meditated Immunity Abnormalities Facilitate Hepatitis B Virus Co-Infection in People Living With HIV: A Review. Front Immunol (2022) 12:755890(5783). doi: 10.3389/fimmu.2021.755890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Brenchley JM, Price DA, Schacker TW, Asher TE, Silvestri G, Rao S, et al. Microbial Translocation Is a Cause of Systemic Immune Activation in Chronic HIV Infection. Nat Med (2006) 12(12):1365–71. doi: 10.1038/nm1511 [DOI] [PubMed] [Google Scholar]
- 134. Cummins N, Badley A. The TRAIL to Viral Pathogenesis: The Good, the Bad and the Ugly. Curr Mol Med (2009) 9(4):495–505. doi: 10.2174/156652409788167078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Guidotti LG, Rochford R, Chung J, Shapiro M, Purcell R, Chisari FV. Viral Clearance Without Destruction of Infected Cells During Acute HBV Infection. Science (1999) 284(5415):825–9. doi: 10.1126/science.284.5415.825 [DOI] [PubMed] [Google Scholar]
- 136. Wieland SF, Spangenberg HC, Thimme R, Purcell RH, Chisari FV. Expansion and Contraction of the Hepatitis B Virus Transcriptional Template in Infected Chimpanzees. Proc Natl Acad Sci USA (2004) 101(7):2129–34. doi: 10.1073/pnas.0308478100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Wieland S, Thimme R, Purcell RH, Chisari FV. Genomic Analysis of the Host Response to Hepatitis B Virus Infection. Proc Natl Acad Sci USA (2004) 101(17):6669–74. doi: 10.1073/pnas.0401771101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Suslov A, Boldanova T, Wang X, Wieland S, Heim MH. Hepatitis B Virus Does Not Interfere With Innate Immune Responses in the Human Liver. Gastroenterology (2018) 154(6):1778–90. doi: 10.1053/j.gastro.2018.01.034 [DOI] [PubMed] [Google Scholar]
- 139. Wang S, Zhang Q, Hui H, Agrawal K, Karris MAY, Rana TM. An Atlas of Immune Cell Exhaustion in HIV-Infected Individuals Revealed by Single-Cell Transcriptomics. Emerg Microbes Infect (2020) 9(1):2333–47. doi: 10.1080/22221751.2020.1826361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Nguyen S, Deleage C, Darko S, Ransier A, Truong DP, Agarwal D, et al. Elite Control of HIV Is Associated With Distinct Functional and Transcriptional Signatures in Lymphoid Tissue CD8(+) T Cells. Sci Transl Med (2019) 11(523):eaax4077. doi: 10.1126/scitranslmed.aax4077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Buggert M, Tauriainen J, Yamamoto T, Frederiksen J, Ivarsson MA, Michaëlsson J, et al. T-Bet and Eomes Are Differentially Linked to the Exhausted Phenotype of CD8+ T Cells in HIV Infection. PloS Pathog (2014) 10(7):e1004251. doi: 10.1371/journal.ppat.1004251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Wherry EJ, Kurachi M. Molecular and Cellular Insights Into T Cell Exhaustion. Nat Rev Immunol (2015) 15(8):486–99. doi: 10.1038/nri3862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Tauriainen J, Scharf L, Frederiksen J, Naji A, Ljunggren HG, Sönnerborg A, et al. Perturbed CD8(+) T Cell TIGIT/CD226/PVR Axis Despite Early Initiation of Antiretroviral Treatment in HIV Infected Individuals. Sci Rep (2017) 7:40354. doi: 10.1038/srep40354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Gubin MM, Zhang X, Schuster H, Caron E, Ward JP, Noguchi T, et al. Checkpoint Blockade Cancer Immunotherapy Targets Tumour-Specific Mutant Antigens. Nature (2014) 515(7528):577–81. doi: 10.1038/nature13988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Spach DH. Antiretroviral Medications and Initial Therapy (2021). Available at: https://www.hiv.uw.edu/go/antiretroviral-therapy/general-information/core-concept/all (Accessed January 12, 2022).
- 146. Volberding PA, Deeks SG. Antiretroviral Therapy and Management of HIV Infection. Lancet (2010) 376(9734):49–62. doi: 10.1016/s0140-6736(10)60676-9 [DOI] [PubMed] [Google Scholar]
- 147. Kalichman SC, Cherry C, Amaral CM, Swetzes C, Eaton L, Macy R, et al. Adherence to Antiretroviral Therapy and HIV Transmission Risks: Implications for Test-and-Treat Approaches to HIV Prevention. AIDS patient Care STDs (2010) 24(5):271–7. doi: 10.1089/apc.2009.0309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Wilson EM, Sereti I. Immune Restoration After Antiretroviral Therapy: The Pitfalls of Hasty or Incomplete Repairs. Immunol Rev (2013) 254(1):343–54. doi: 10.1111/imr.12064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Autran B, Carcelain G, Li TS, Blanc C, Mathez D, Tubiana R, et al. Positive Effects of Combined Antiretroviral Therapy on CD4+ T Cell Homeostasis and Function in Advanced HIV Disease. Science (1997) 277(5322):112–6. doi: 10.1126/science.277.5322.112 [DOI] [PubMed] [Google Scholar]
- 150. Michaels SH, Clark R, Kissinger P. Declining Morbidity and Mortality Among Patients With Advanced Human Immunodeficiency Virus Infection. N Engl J Med (1998) 339(6):405–6. doi: 10.1056/nejm199808063390612 [DOI] [PubMed] [Google Scholar]
- 151. Le Guillou A, Buchbinder S, Scott H, Liu A, Havlir D, Scheer S, et al. Population Impact and Efficiency of Improvements to HIV PrEP Under Conditions of High ART Coverage Among San Francisco Men Who Have Sex With Men. J Acquir Immune Defic Syndr (2021) 88(4):340–7. doi: 10.1097/qai.0000000000002781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Taramasso L, Lorenzini P, Di Biagio A, Lichtner M, Marchetti G, Rossotti R, et al. Incidence and Risk Factors for Liver Enzyme Elevation Among Naive HIV-1-Infected Patients Receiving ART in the ICONA Cohort. J Antimicrob Chemother (2019) 74(11):3295–304. doi: 10.1093/jac/dkz353 [DOI] [PubMed] [Google Scholar]
- 153. Debes JD, Bohjanen PR, Boonstra A. Mechanisms of Accelerated Liver Fibrosis Progression During HIV Infection. J Clin Transl Hepatol (2016) 4(4):328–35. doi: 10.14218/jcth.2016.00034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Weber R, Sabin CA, Friis-Møller N, Reiss P, El-Sadr WM, Kirk O, et al. Liver-Related Deaths in Persons Infected With the Human Immunodeficiency Virus: The D:A:D Study. Arch Intern Med (2006) 166(15):1632–41. doi: 10.1001/archinte.166.15.1632 [DOI] [PubMed] [Google Scholar]
- 155. Catherine FX, Piroth L. Hepatitis B Virus Vaccination in HIV-Infected People: A Review. Hum Vaccin Immunother (2017) 13(6):1–10. doi: 10.1080/21645515.2016.1277844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Jose-Abrego A. Some Considerations About HBV Vaccination Among HIV Patients From Latin America and the Caribbean. Ann Hepatol (2019) 18(5):656–7. doi: 10.1016/j.aohep.2019.06.005 [DOI] [PubMed] [Google Scholar]
- 157. Cagigi A, Nilsson A, Pensieroso S, Chiodi F. Dysfunctional B-Cell Responses During HIV-1 Infection: Implication for Influenza Vaccination and Highly Active Antiretroviral Therapy. Lancet Infect Dis (2010) 10(7):499–503. doi: 10.1016/s1473-3099(10)70117-1 [DOI] [PubMed] [Google Scholar]
- 158. Amu S, Ruffin N, Rethi B, Chiodi F. Impairment of B-Cell Functions During HIV-1 Infection. AIDS (2013) 27(15):2323–34. doi: 10.1097/QAD.0b013e328361a427 [DOI] [PubMed] [Google Scholar]
- 159. Pensieroso S, Cagigi A, Palma P, Nilsson A, Capponi C, Freda E, et al. Timing of HAART Defines the Integrity of Memory B Cells and the Longevity of Humoral Responses in HIV-1 Vertically-Infected Children. Proc Natl Acad Sci USA (2009) 106(19):7939–44. doi: 10.1073/pnas.0901702106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Pogliaghi M, Ripa M, Pensieroso S, Tolazzi M, Chiappetta S, Nozza S, et al. Beneficial Effects of cART Initiated During Primary and Chronic HIV-1 Infection on Immunoglobulin-Expression of Memory B-Cell Subsets. PloS One (2015) 10(10):e0140435. doi: 10.1371/journal.pone.0140435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Moir S, Ho J, Malaspina A, Wang W, DiPoto AC, O'Shea MA, et al. Evidence for HIV-Associated B Cell Exhaustion in a Dysfunctional Memory B Cell Compartment in HIV-Infected Viremic Individuals. J Exp Med (2008) 205(8):1797–805. doi: 10.1084/jem.20072683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Pensieroso S, Galli L, Nozza S, Ruffin N, Castagna A, Tambussi G, et al. B-Cell Subset Alterations and Correlated Factors in HIV-1 Infection. AIDS (2013) 27(8):1209–17. doi: 10.1097/QAD.0b013e32835edc47 [DOI] [PubMed] [Google Scholar]
- 163. Chiodi F, Scarlatti G. Editorial: HIV-Induced Damage of B Cells and Production of HIV Neutralizing Antibodies. Front Immunol (2018) 9:297. doi: 10.3389/fimmu.2018.00297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Siewe B, Nipper AJ, Sohn H, Stapleton JT, Landay A. FcRL4 Expression Identifies a Pro-Inflammatory B Cell Subset in Viremic HIV-Infected Subjects. Front Immunol (2017) 8:1339. doi: 10.3389/fimmu.2017.01339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Rieckmann P, D'Alessandro F, Nordan RP, Fauci AS, Kehrl JH. IL-6 and Tumor Necrosis Factor-Alpha. Autocrine and Paracrine Cytokines Involved in B Cell Function. J Immunol (1991) 146(10):3462–8. [PubMed] [Google Scholar]
- 166. Deeks SG, Lewin SR, Havlir DV. The End of AIDS: HIV Infection as a Chronic Disease. Lancet (2013) 382(9903):1525–33. doi: 10.1016/s0140-6736(13)61809-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. El-Atrouni W, Berbari E, Temesgen Z. HIV-Associated Opportunistic Infections. Bacterial Infections. J Med Liban (2006) 54(2):80–3. [PubMed] [Google Scholar]
- 168. Dey J, Gautam H, Venugopal S, Porwal C, Mirdha BR, Gupta N, et al. Tuberculosis as an Etiological Factor in Liver Abscess in Adults. Tuberc Res Treat (2016) 2016:8479456. doi: 10.1155/2016/8479456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Hryzhak IH. Infection With Toxoplasma Gondii Can Promote Chronic Liver Diseases in HIV-Infected Individuals. Folia Parasitol (2020) 67:034. doi: 10.14411/fp.2020.034 [DOI] [PubMed] [Google Scholar]
- 170. Manolaki N, Vaos G, Zavras N, Sbokou D, Michael C, Syriopoulou V. Inflammatory Myofibroblastic Tumor of the Liver Due to Mycobacterium Tuberculosis in an Immunocompetent Girl. Pediatr Surg Int (2009) 25(5):451–4. doi: 10.1007/s00383-009-2361-7 [DOI] [PubMed] [Google Scholar]
- 171. Jeena PM, Coovadia HM, Chrystal V. Pneumocystis Carinii and Cytomegalovirus Infections in Severely Ill, HIV-Infected African Infants. Ann Trop Paediatr (1996) 16(4):361–8. doi: 10.1080/02724936.1996.11747852 [DOI] [PubMed] [Google Scholar]
- 172. Radin DR, Baker EL, Klatt EC, Balthazar EJ, Jeffrey RB, Jr., Megibow AJ, et al. Visceral and Nodal Calcification in Patients With AIDS-Related Pneumocystis Carinii Infection. AJR Am J Roentgenol (1990) 154(1):27–31. doi: 10.2214/ajr.154.1.2104720 [DOI] [PubMed] [Google Scholar]
- 173. BBunchorntavakul C, Reddy KR. Epstein-Barr Virus and Cytomegalovirus Infections of the Liver. Gastroenterol Clin North Am (2020) 49(2):331–46. doi: 10.1016/j.gtc.2020.01.008 [DOI] [PubMed] [Google Scholar]
- 174. Bonacini M. Hepatobiliary Complications in Patients With Human Immunodeficiency Virus Infection. Am J Med (1992) 92(4):404–11. doi: 10.1016/0002-9343(92)90271-c [DOI] [PubMed] [Google Scholar]
- 175. Dey SK, Ghosh I, Bhattacharjee D, A P, Jha S, Dasgupta A, et al. Liver Function Profile Anomalies in HIV Seropositive Tuberculosis. J Clin Diagn Res (2013) 7(6):1068–72. doi: 10.7860/jcdr/2013/5156.3047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Manns MP, Buti M, Gane E, Pawlotsky JM, Razavi H, Terrault N, et al. Hepatitis C Virus Infection. Nat Rev Dis Primers (2017) 3:17006. doi: 10.1038/nrdp.2017.6 [DOI] [PubMed] [Google Scholar]
- 177. Sulkowski MS, Mast EE, Seeff LB, Thomas DL. Hepatitis C Virus Infection as an Opportunistic Disease in Persons Infected With Human Immunodeficiency Virus. Clin Infect Dis (2000) 30 Suppl 1:S77–84. doi: 10.1086/313842 [DOI] [PubMed] [Google Scholar]
- 178. World Health Organization . Global Tuberculosis Report (2021). Available at: https://www.who.int/teams/global-tuberculosis-programme/tb-reports/global-tuberculosis-report-2021 (Accessed January 12, 2022).
- 179. Hussain Z, Zhu J, Ma X. Metabolism and Hepatotoxicity of Pyrazinamide, an Antituberculosis Drug. Drug Metab Dispos (2021) 49(8):679–82. doi: 10.1124/dmd.121.000389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Sharma R, Kaur R, Mukesh M, Sharma VL. Assessment of Hepatotoxicity of First-Line Anti-Tuberculosis Drugs on Wistar Rats. Naunyn Schmiedebergs Arch Pharmacol (2018) 391(1):83–93. doi: 10.1007/s00210-017-1434-8 [DOI] [PubMed] [Google Scholar]
- 181. Cybulski DJ, White BK. Fatal Isoniazid Hepatotoxicity in the Deployed Environment. Mil Med (2021) 186(5-6):619–22. doi: 10.1093/milmed/usaa414 [DOI] [PubMed] [Google Scholar]
- 182. Kim JH, Nam WS, Kim SJ, Kwon OK, Seung EJ, Jo JJ, et al. Mechanism Investigation of Rifampicin-Induced Liver Injury Using Comparative Toxicoproteomics in Mice. Int J Mol Sci (2017) 18(7):1417. doi: 10.3390/ijms18071417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Tostmann A, Boeree MJ, Aarnoutse RE, de Lange WC, van der Ven AJ, Dekhuijzen R. Antituberculosis Drug-Induced Hepatotoxicity: Concise Up-to-Date Review. J Gastroenterol Hepatol (2008) 23(2):192–202. doi: 10.1111/j.1440-1746.2007.05207.x [DOI] [PubMed] [Google Scholar]
- 184. Araújo-Mariz C, Lopes EP, Acioli-Santos B, Maruza M, Montarroyos UR, Ximenes RA, et al. Hepatotoxicity During Treatment for Tuberculosis in People Living With HIV/AIDS. PloS One (2016) 11(6):e0157725. doi: 10.1371/journal.pone.0157725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. AIDS and Hepatitis C Professional Group. Society of Infectious Diseases. Chinese Medical Association . Chinese Center for Disease Control and Prevention. Chinese Guidelines for Diagnosis and Treatment of HIV/AIDS (2018). Zhonghua nei ke za zhi (2018) 57(12):867–84. doi: 10.3760/cma.j.issn.0578-1426.2018.12.002 [DOI] [PubMed] [Google Scholar]
- 186. Khalili H, Soudbakhsh A, Talasaz AH. Severe Hepatotoxicity and Probable Hepatorenal Syndrome Associated With Sulfadiazine. Am J Health Syst Pharm (2011) 68(10):888–92. doi: 10.2146/ajhp100516 [DOI] [PubMed] [Google Scholar]
- 187. Bell TL, Foster JN, Townsend ML. Trimethoprim-Sulfamethoxazole-Induced Hepatotoxicity in a Pediatric Patient. Pharmacotherapy (2010) 30(5):539. doi: 10.1592/phco.30.5.539 [DOI] [PubMed] [Google Scholar]
- 188. Zhao H, Wang Y, Mu M, Guo M, Yu H, Xing M. Lycopene Alleviates Sulfamethoxazole-Induced Hepatotoxicity in Grass Carp (Ctenopharyngodon Idellus) via Suppression of Oxidative Stress, Inflammation and Apoptosis. Food Funct (2020) 11(10):8547–59. doi: 10.1039/d0fo01638a [DOI] [PubMed] [Google Scholar]
- 189. Yang JJ, Huang CH, Liu CE, Tang HJ, Yang CJ, Lee YC, et al. Multicenter Study of Trimethoprim/Sulfamethoxazole-Related Hepatotoxicity: Incidence and Associated Factors Among HIV-Infected Patients Treated for Pneumocystis Jirovecii Pneumonia. PloS One (2014) 9(9):e106141. doi: 10.1371/journal.pone.0106141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Van J, Reau N. Disseminated AIDS-Related Kaposi Sarcoma Immune Reconstitution Inflammatory Syndrome With Infiltrative Liver Disease. ACG Case Rep J (2021) 8(9):e00660. doi: 10.14309/crj.0000000000000660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Núñez M, González-Requena D, González-Lahoz J, Soriano V. Short Communication: Interactions Between Nevirapine Plasma Levels, Chronic Hepatitis C, and the Development of Liver Toxicity in HIV-Infected Patients. AIDS Res Hum Retroviruses (2003) 19(3):187–8. doi: 10.1089/088922203763315687 [DOI] [PubMed] [Google Scholar]