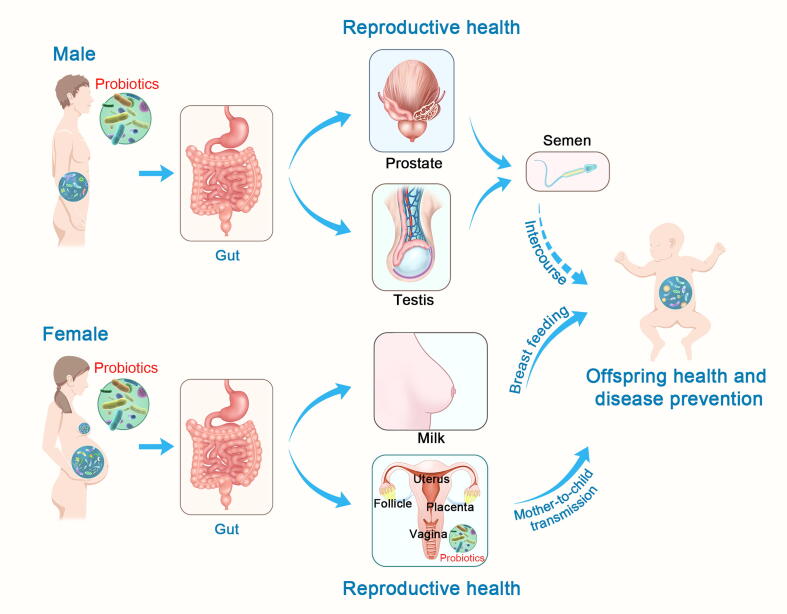
Graphical abstract

Abbreviations: BV, Bacterial vaginosis; BTB, Blood-testis barrier; DEHP, Diethylhexyl phthalate; FMT, Fecal microbiota transplantation; FSH, Follicle-stimulating hormone; GDM, Gestational diabetes mellitus; PCOS, Polycystic ovary syndrome; TLR, Toll-like receptor; VMT, Vaginal microbiota transplantation
Keywords: Reproduction, Reproductive system, Reproductive microbiome, Probiotic, Regulation effect
Abstract
The presence of microbial communities in the reproductive tract has been revealed, and this resident microbiota is involved in the maintenance of health. Intentional modulation via probiotics has been proposed as a possible strategy to enhance reproductive health and reduce the risk of diseases. The male seminal microbiota has been suggested as an important factor that influences a couple’s health, pregnancy outcomes, and offspring health. Probiotics have been reported to play a role in male fertility and to affect the health of mothers and offspring. While the female reproductive microbiota is more complicated and has been identified in both the upper and lower reproductive systems, they together contribute to health maintenance. Probiotics have shown regulatory effects on the female reproductive tract, thereby contributing to homeostasis of the tract and influencing the health of offspring. Further, through transmission of bacteria or through other indirect mechanisms, the parent’s reproductive microbiota and probiotic intervention influence infant gut colonization and immunity development, with potential health consequences. In vitro and in vivo studies have explored the mechanisms underlying the benefits of probiotic administration and intervention, and an array of positive results, such as modulation of microbiota composition, regulation of metabolism, promotion of the epithelial barrier, and improvement of immune function, have been observed. Herein, we review the state of the art in reproductive system microbiota and its role in health and reproduction, as well as the beneficial effects of probiotics on reproductive health and their contributions to the prevention of associated diseases.
1. Introduction
Mounting evidence indicates the existence of an extensive microbiome in the animal system, which commensally contributes to host health and helps sustain normal physiology [1]. Several studies have also manifested the presence of microorganisms in the male and female reproductive tracts. In males, the reproductive microbiota was mainly identified in semen [2], whereas in females, microbiomes were detected in the whole reproductive tract [3], and each region or tissue of the reproductive organs was colonized by a unique microbiome with its own characteristic composition [4], [5]. There is increasing evidence that reproductive microorganisms are key effectors not only in reproductive health but also in associated diseases. Commensal microorganisms help maintain ecological balance in the reproductive tract, thus contributing to host fertility and fitness [3]. Dysbiosis of the reproductive tract microbiome could induce reproductive physiological dysfunction and cause associated diseases and adverse pregnancy outcomes [6]. Furthermore, multiple studies have suggested that gut microbiota play an important regulatory role in maintaining the basal healthy state of the reproductive tract and in the progression of some associated diseases [7].
Considering the evidence that the microbiome plays roles in reproductive health and associated diseases, it is not surprising that probiotic treatment capable of targeting the microbiome is becoming a sensible therapeutic option. In recent years, many studies have demonstrated that dietary supplementation or direct intervention with probiotics can alleviate reproductive dysfunction and have positive therapeutic effects on associated diseases [8], [9], [10]. Probiotics can alter the abundance and activity of microbes, thus exerting their effects by directly regulating the host microbiota composition. In addition, probiotics and the altered microbiota influence host metabolism and health. Probiotics may improve host reproductive function by regulating host metabolism, because metabolic health is correlated with reproductive function [11]. Moreover, probiotics and their produced molecules promote epithelial barrier function, and membrane integrity is essential for successful blastulation and formation of the amnion, chorion, and placenta; thus, it is reasonable that probiotics can influence the different membrane structures involved in reproduction [8]. Furthermore, the immunomodulatory effect of probiotics has been proven by several studies, and specific probiotics have been suggested to have beneficial roles in some reproductive processes and their associated diseases because of their ability to interfere with the inflammatory cascade [12] (see Fig. 1). In general, probiotic treatments have been proposed as a novel strategy to improve reproductive performance and alleviate associated reproductive diseases in both males and females, as well as to improve the health of offspring (see Fig. 2). The effects and modes of action of probiotics on reproductive function have yet to be summarized. Here, we review the current literature on the role of probiotics in improving reproductive function in both males and females and in the health of their offspring.
Fig. 1.
Proposed modes of action of probiotics on reproductive health. Probiotics may exert beneficial effects on reproduction through the modulation of microbiota composition, regulation of metabolism, promotion of the epithelial barrier, and improvement of immune function.
Fig. 2.
Overview of the potential pathway by which probiotics affect reproductive health in both males and females as well as their offspring. 1. Probiotic supplements have positive effects on testicular function, improving semen quality, which may influence couple and offspring health through sexual intercourse. 2. Probiotic supplements can modulate the microbiota composition of the gut and regulate the metabolism of the female, thus impacting microbiome configuration, biofilm integrity, and the immune response of female reproductive organs. The affected female reproductive tract then influences the health of infants through mother-to-child transmission. Additionally, probiotic supplements can influence bacterial transmission from the gut to milk, and then influence infant health through breast feeding. 3. Probiotics may also act through the vagina by changing its microbiota composition directly and balancing its microbial ecology, thus contributing to vaginal reproductive health.
2. Male reproductive microbiome
2.1. Role of the seminal microbiome in reproduction
2.1.1. Microbiomes in semen
Recent studies have shown that a rich microbiome exists in semen and plays an important role in the maintenance of male reproductive health [13], [14]. According to several studies, the composition of serum microflora is correlated with sperm quality. Lactobacilli are typically predominant in semen and preserve sperm motility and viability [15]; therefore, Lactobacillus-predominant semen is of higher quality than semen with other predominant bacteria [16]. Correlations between the existence of Proteobacteria, Anaerococcus, and Bacteroides ureolyticus and depressed sperm quality and poor reproductive outcomes have been identified, suggesting the critical role of semen microbiota composition in male reproductive health [17], [18], [19]. The semen microbiota has been thought to originate from the upper genital tract [20], but a latter common assumption is that it might be derived either from different urogenital tissues or from other tissues, such as the gut, mouth, blood, or vagina [16], [21]. Microbes can interact with sperm by directly adhering to it and affecting spermatozoa functions, or they can impair sperm motility indirectly through their metabolites [14].
2.1.2. Effects of the seminal microbiota on females and offspring
Exchange of microbiota occurs in unprotected sexual intercourse, implying that the transmission of seminal fluid microbiota from males to females affects the health of the couple [18]. Studies have shown that normal sexual intercourse does not alter the consistency of microbial communities, but does increase the diversity of the Gardnerella vaginalis clade in young women with and without bacterial vaginosis (BV), indicating that sexual transmission of commensal and potentially pathogenic clades is possible [22]. Frequent sexual intercourse and multiple sexual partners can cause fluctuations in the vaginal microbiota composition, resulting in episodes of BV [13]. However, other studies have found no correlation between sexual intercourse and BV [23], although BV could be considered a sexually enhanced disease, i.e., one in which the frequency of intercourse is an important factor [24]. Further studies are required to address this discrepancy. Additionally, studies have also shown that the paternal microbiome may affect offspring through the seminal microbiome [25]. Possible mechanisms suggest that the seminal microbiome may affect offspring epigenetics, including the methylome and transcriptome in multiple tissues, which may induce persistent phenotypic changes [26]. Moreover, potential modulation of the immune system has also been observed. One study has shown that long-term exposure of a female to the semen of their partner correlates positively with regulatory T cell development, which limits the maternal anti-fetal immune response [27]. However, the underlying mechanisms remain to be investigated.
2.1.3. Microbiomes in the prostate
The prostate, the largest male accessory gland, plays an important physiological role in male fertility. Prostatic fluid not only influences ejaculation, sperm activation, and capacitation but also enables remodeling of the components of the female reproductive tract, preparing it for conception [28]. It is debatable whether prostate microbiota exists, and few studies have been carried out on prostate microbiomes, partly due to the difficulty in procuring non-disease-state prostates [29]. Recently, Feng et al. [30] discovered the colonized microbiota in both prostate tumors and adjacent benign tissues using integrated metagenomic and metatranscriptomic analyses, and the predominant bacteria included Escherichia, Propionibacterium, Acinetobacter, and Pseudomonas. Enterobacteriaceae, especially Escherichia coli, have been confirmed as the predominant pathogens in acute and chronic bacterial prostatitis [31]. More research is still needed to reveal the relationship between prostate microbiomes and prostate health and disease.
2.2. Effects of probiotics on male reproduction
2.2.1. Testicular function and semen quality
The impacts of probiotics on male fertility have not been thoroughly investigated. However, studies have shown that probiotic strains have beneficial effects on sperm motility and kinematic parameters both in vivo and in vitro [32], [33], as well as in some disease models. Supplementation of Lactobacillus rhamnosus PB01 to diet-induced obese mice resulted in significantly higher percentages of progressively motile sperm and positive effects on weight loss and reproductive hormones [33], while Lactobacillus and Bifidobacterium resulted in improved sperm motility and reduced percentage of sperm DNA fragmentation in asthenozoospermic males [34]. Increased sperm quality and spermatogenesis following fecal microbiota transplantation (FMT) originated from mice fed with alginate oligosaccharide were also found, in which bacteria with probiotic roles in the gut were proliferated by oligosaccharides [35]. Supplementation with probiotics can restore testosterone levels and seminiferous tubule cross-sectional profiles as well as spermatogenesis in aging mice, indicating that probiotics may affect semen quality by influencing testicular function [36]. Studies have demonstrated that the gut microbiome may modulate the permeability of the blood-testis barrier (BTB) and play a regulatory role in testicular function [37]. It is easy to imagine that probiotics may play a role through the BTB, but this requires further investigation. Moreover, by enhancing the quantity and quality of semen, administration of probiotics can increase litter size and litter birth weight of rabbits mated to bucks supplemented with nitrate, which indicates the antisterility effect of probiotics and their positive effect on offspring output [38]. The above evidence, if verified by further investigations, may pave the way to intriguing therapeutic strategies for infertility.
Several hypotheses have been developed to explain the mechanisms by which probiotics augment spermatozoa function. Zhang et al. [39] investigated the suppressive effect of a predominant beneficial genus (Lactobacillus casei) and harmful genus (Pseudomonas aeruginosa) in serum using an in vitro model. They discovered that L. casei significantly depressed the reduction of sperm motility and the damage to mitochondrial activity caused by P. aeruginosa, although L. casei treatment alone did not improve these parameters, revealing that Lactobacillus may promote semen quality by suppressing the negative effect of dominant harmful bacteria on sperm. However, more in vivo studies have revealed that probiotic supplementation may affect testicular function and spermatogenesis by modifying the gut microbiota and acting as an antioxidant. The relationships between key gut microbiota and testicles were investigated using Spearman’s correlation analysis [40]. Bacteroidetes, Deferribacteres, and Firmicutes were significantly connected with the testicular function reduction caused by diethylhexyl phthalate (DEHP). However, Lactobacillus plantarum TW1-1 pretreatment can regulate the abundance of these testicular damage-correlated bacteria and restore testis injury in DEHP-exposed mice, suggesting that probiotic strains may alleviate testicular injury by modulating the intestinal microbiota. Furthermore, it is well known that oxidative stress, which disrupts the integrity of sperm DNA and limits the fertilization potential of sperm, is a major cause of defective sperm function [41]. Studies have shown that administration of L. rhamnosus CECT8361 and Bacteroidetes longum CECT7347 improved sperm motility, reduced DNA fragmentation, and decreased intracellular H2O2 levels in sperm. Thus, these probiotic strains may improve sperm quality by acting as antioxidants [34].
2.2.2. Prostate health
The effects of probiotics on the prostate have been rarely examined, with only a few studies in recent years. Several in vitro experiments have shown that treatment of human prostate cancer cells with certain probiotic strains (L. rhamnosus GG, L. acidophilus La-05, L. casei-01, and Bifidobacterium animalis Bb-12) strongly induced apoptosis [42], [43], indicating the potential of probiotics to suppress prostate cancer. Furthermore, probiotics have been demonstrated to improve prevention of episodes and alleviate symptoms in chronic bacterial prostatitis caused by Enterobacteriaceae [44]. In addition, decreased bacterial load of E. coli and Enterococcus faecalis in urine cultures was observed after probiotic administration in prostatitis [45]. However, no clear etiology exists for prostate disease [46]; thus, the relationship between prostate microbiota and prostatitis and prostate cancer, as well as the potential role of probiotics in their alleviation, is worth further investigation. Effects of microbiomes and probiotics on male reproduction were briefly summarized in Table 1.
Table 1.
Effects of Microbiomes and Probiotics on Male Reproduction.
| Microorganisms | Target(s) | Main effects | Reference |
|---|---|---|---|
| Bacteroides ureolyticus | Sperm | Depressed sperm quality in men | [17] |
| Anaerococcus | Sperm | Considered as a biomarker for low sperm quality in men | [18] |
| Proteobacteria | Sperm | Increased seminal hyperviscosity in men | [19] |
| Lactobacillus brevis, L. salivarius, L. plantarum | Sperm | Preserved sperm motility and viability from ROS in men | [32] |
| L. rhamnosus PB01 | Sperm | Improved sperm motility and kinematic parameters in mice | [33] |
| L. rhamnosus CECT8361, Bifidobacterium longum CECT7347 | Sperm | Improved sperm motility and reduced sperm DNA fragmentation in men | [34] |
| L. casei CGMCC 1.570 | Sperm | Improved sperm motility and mitochondrial activity caused by Pseudomonas aeruginosa in boars | [39] |
| L. rhamnosus CECT8361, Bacteroidetes longum CECT7347 | Sperm | Alleviated sperm oxidative stress in men | [41] |
| Fecal microbiota transplantation | Testis | Improved sperm quality and spermatogenesis in mice | [35] |
| L. reuteri ATCC 6475 | Testis | Increased spermatogenesis and Leydig cell numbers in mice | [36] |
| Clostridium Tyrobutyricum | Testis | Increased BTB permeability in mice | [37] |
| L. acidophilus, Saccharomyces cerevisiae | Testis | Increased testosterone, number and weight of offspring in rabbits | [38] |
| L. plantarum TW1-1 | Testis | Restored testis injury caused by DEHP in mice | [40] |
| Bifiprost® and Serenoa Repens | Prostate | Prevented chronic bacterial prostatitis caused by Enterobacteriaceae in men | [44] |
| PRO-Men Hyperbiotics | Prostate | Reduced inflammatory process of recurrent prostatitis in men | [45] |
| L. acidophilus La-05, L. casei-01, Bifidobacterium animalis Bb-12 | Prostate cancer cells | Induced antiproliferative and apoptotic effects on prostate cancer cells | [42] |
| L. rhamnosus GG | Prostate cancer cells | Decreased cell viability of prostate cancer cells | [43] |
Note: L.: Lactobacillus; ROS: radical oxygen species; BTB: blood-testis barrier; DEHP: diethylhexyl phthalate.
3. Female reproductive microbiome
3.1. Upper and lower tract microbiomes
The female reproductive tract can be divided into two connected parts: the upper and lower reproductive tracts. The former includes the ovaries, fallopian tubes, and uterus, while the latter covers the cervix and vagina. Microorganisms have been identified both in the upper and lower tracts. The upper tract was originally thought to be sterile, but promising research has indicated the existence of microorganisms in the follicle, fallopian tubes, uterus, and placenta [3], [7], [47], [48]. The lower reproductive tract shows high diversity and abundance of microbiomes [7], [49]. The different parts are colonized by their own unique microbiomes with diverse composition and richness [4], [6]. The microbial composition of the reproductive tract is not permanent, but fluctuates with age, physiological conditions, lifestyle, and environmental factors [49].
3.1.1. Follicle
The ovarian follicles can be colonized, and several studies have shown the presence of microorganisms in follicular fluid, not only in humans but also in bovines and swine, among other animals [48], [50]. Follicular fluid plays important roles in ovarian physiology, including in steroidogenesis, follicle growth, ovulation, oocyte maturation, and oviduct transport. Microorganisms colonizing the follicular fluid have been revealed as the normal flora of the vagina (Lactobacillus spp.), gastrointestinal tract (Bifidobacterium spp., enteric bacteria, Streptococcus agalactiae), skin (Staphylococcus spp.), and oral mucosa (Streptococcus spp.) [51]. Lactobacillus spp. are the most predominant bacteria colonizing follicular fluids and are associated with embryo maturation and quality [6]. Lactic acid, produced by Lactobacillus spp., plays a key role in protecting against adverse microbiota during oocyte maturation because of its antimicrobial properties [52]. Microorganisms in the oral mucosa and respiratory tract can spread to the follicular fluid through hematogenous dissemination [53]. Therefore, asymmetrical ovarian vascularization may lead to an uneven distribution of the microbiota in the follicular fluid of different side of the ovaries [51].
3.1.2. Placenta
The placental microbiome is characterized by a low abundance of microbiota but is metabolically rich. There are significant distinctions between the microorganisms harbored in the basal plate and the placental villi [6]. The placental microbiome is hypothesized to be colonized in three ways: ascension from the vagina (vaginal-placental), hematogenous dissemination from oral intake (oral-placental), and transport of intestinal bacteria to the placenta via dendritic cells (gut-placental) [4]. The placental microbiome mainly includes nonpathogenic symbiotic microbiota and is largely similar to that of the oral cavity and the deep endometrium of non-pregnant women when compared to the adjacent vaginal microbiome [54], [55]. McElrath et al. [56] reported that placental microbiota from preterm infants were similar to microbiota resident in the vagina, which may suggest that dysbiosis of placental microorganisms disturbed by the vagina is associated with placental dysfunction and influences pregnancy outcomes. However, some researchers are still convinced that the placenta is sterile and that the identification of microbiota in the placenta is due to contamination, rather than due to the presence of resident microorganisms [57], [58], [59].
3.1.3. Uterus
It was long thought that a healthy uterus should be sterile. Recently, it has been revealed that the uterine cavity is colonized with its own unique microbiome [47], [60], and uterine microbiomes have been characterized in mares, giant pandas, cows, and bitches [4]. Four genera, including Lactobacillus, Bacteroides, Gardnerella, and Provotella, have been identified as highly abundant in the human uterus [4]. Nine genera, including Atopobium, Bifidobacterium, Chryseobacterium, Gardnerella, Haemophilus, Klebsiella, Neisseria, Staphylococcus and Streptococcus in endometrium were associated with clinical miscarriage of infertile patients [60]. Since the cervix serves as a partial filter or barrier against ascending microbiota in the female reproductive tracts, the character of the cervical microbiome shows a transitional phenotype between those of the vagina and uterus, with a significantly lower quantity than that of the vagina [5], [61]. In pregnant women, part of the uterine microbiome originates from cervical bacteria carried upstream with sperm during fertilization [1], while the rest is resident in the uterus.
3.1.4. Vagina
The vaginal microbiome was first reported in 1892 [62]. To date, the vaginal microbiomes of many species, including humans, livestock (cow, ewe, bitch, and mare), wild primates (chimpanzee, baboon, howler, red colobus monkey, lemur, and giant panda), and guinea pigs [4] have been sequenced. It has been widely demonstrated that the human vagina is colonized by commensal bacteria, prevailingly Lactobacilli, and consequently, the vaginal microbiota creates an acidic environment to protect the female reproductive tract against pathogens and to establish normal female reproductive physiology and function [63]. Another role of the dominant Lactobacilli colonizing the vaginal microbiome is to serve as a probiotic, maintaining microbial homeostasis by suppressing overgrowth of other bacterial species in the vagina [64]. Changes in diversity and richness of the vaginal microbiome influence fetal development [5]. Pregnant women with increased vaginal microbiome instability or decreased Lactobacillus dominance have a higher risk of preterm birth [65], [66].
3.2. Effects of probiotics on female reproductive performance
3.2.1. Follicular development
Few studies have described the influence of probiotics on follicular development in women with healthy ovaries. Probiotic supplementation is thought to delay the interruption of ovarian activity and estradiol production in menopausal women to help prevent associated symptoms, including dyslipidemia and obesity, among others [67], [68]. Oral gavage of probiotics originating from feces of healthy women increased estrogen circulation by modifying gut microbiota metabolites in ovariectomized menopausal mice [67], indicating the effects of probiotic treatment on maintaining estradiol levels under ovarian dysfunction. Treatment of perimenopausal women with the probiotic Sanprobi Barrier increases follicle-stimulating hormone (FSH) levels, which helps maintain ovarian activity, and is considered a non-invasive means to impact hormonal homeostasis [68]. Probiotic supplementation has been shown to improve follicle development in birds. Administration of Bacillus significantly increases egg production and egg mass in Hy-Line laying hens [69], [70], while the addition of Enterococcus faecium to AA broiler breeders increases egg weight and eggshell thickness [71]. The above studies show that probiotic treatment can increase levels of reproductive hormones, including FSH, estradiol, and growth hormone, while decreasing adrenal cortical hormone, thus regulating follicle development [70], [71]. In addition, probiotics improve fish follicle maturation. Zebrafish (Danio rerio) fed with L. rhamnosus IMC 501 undergo chemical changes in oocyte composition to promote the oocyte maturation process by increasing the gene expression of neuropeptide hormones (Kiss1 and Kiss2) and metabolic signals (leptin) both on the endocrine system level and at the peripheral level [72]. In livestock, probiotics have significant effects on weaning estrus intervals, especially in sows. Studies have demonstrated that supplementation with either a single or a cocktailed probiotic strain shortened the weaning estrus interval in sows with different parities [73], [74]. It was discovered that weaning estrus interval is correlated with sex hormone secretion and alteration of the gut microbiome in sows after weaning [75], and it can be speculated that the influence of probiotics on weaning estrus intervals may be correlated with regulation of gut microbiota and hormone secretion.
3.2.2. Placental function
The placenta is a temporary tissue that connects the maternal uterus and fetus during gestation. The function of the placenta is to efficiently deliver nutrients and oxygen from mother to fetus to support normal fetal growth [76]. A large number of published papers have reported that orally administered probiotics can affect placental function. Studies have shown that administered probiotics can translocate from the gut to the amniotic fluid of the fetus through the placenta using a genetically labeled E. faecium strain [77], suggesting that probiotic dietary consumption could potentially change the configuration of the placental microbiota, thus influencing placental function [78]. Regulated toll-like receptor (TLR)-related genes and autophagy-related protein expression in the placenta were observed after oral probiotic administration [79], [80]. In an in vitro study, lipopolysaccharide-stimulated TNF-α production in human placental trophoblast cells was inhibited by L. rhamnosus GR-1 [81]. Probiotics also decrease the risk of severe preeclampsia through the reduction of inflammatory responses in the placenta [82]. These results indicate that the effects of probiotics on placental function may be mediated by enhancing the immune response. Furthermore, probiotics combined with prebiotics were found to enhance triacylglycerol concentration in the serum of sows and reduce total cholesterol concentration in umbilical venous serum, implying that the combination may contribute to improved placental lipid metabolism [83]. From the above, it can be suggested that probiotics could improve placental function by modifying microbiota composition and enhancing the immune response, as well as improving metabolic regulation in the placenta during pregnancy.
3.2.3. Fetal development
Probiotic supplementation during pregnancy has been shown to be beneficial in modulating gut microbiota composition and improving metabolism in pregnant women, which is subsequently beneficial to fetal development [84], [85]. Maternal intervention by specific probiotics, Bifidobacterium lactis and L. rhamnosus, was found to significantly modulate the expression of TLR-related genes in the fetal gut (reflected by meconium), indicating the beneficial effect of probiotics on fetal immune physiology [79]. Rasool et al. [86] reported that probiotics might function as placental therapeutics to prevent preterm birth and poor placental efficiency. Although the effectiveness of maternal probiotic intervention in preventing preterm labor and birth has been studied, insufficient data have been obtained to evaluate the actual effect on preterm birth and its complications [87]. As for animal reproduction and production, fetal development during pregnancy determines birth weight and is highly associated with preweaning mortality and piglet growth after weaning [88]. Studies have demonstrated that probiotic supplementation in late pregnancy can increase birth weight and litter weight both in first and higher parity sows [74], [89]. Increased feed intake, improved immunoglobulin, and regulated gut microbiota were revealed to be the potential pathways by which probiotics influence fetal development [74], [90]. Furthermore, increased growth hormone concentrations in umbilical venous serum and improved placental antioxidant capacity were also interpreted to contribute to fetal development after Bacillus supplementation combined with a prebiotic (isomaltooligosaccharide) [83].
3.3. Effects of probiotics on female reproductive disease prevention
3.3.1. Polycystic ovary syndrome (PCOS)
PCOS is a common endocrine syndrome in women of childbearing age and is accompanied by metabolic disorders mainly characterized by hyperandrogenism, insulin resistance, and disturbances in lipid, carbohydrate, and hormonal metabolism [91], [92]. No single pathological process could result in all cases of PCOS [93], but dysbiosis of the gut microbiota is strongly associated with PCOS progression [94], [95]. The use of probiotics has been recommended to reform gut microbiota composition in PCOS treatment [96]. Dietary probiotic supplementation could promote the growth of short chain fatty acid-producing bacteria and concurrently decrease the number of lipopolysaccharide-producing bacteria in the intestines of patients with PCOS, thus alleviating inflammation [96], [97]. Furthermore, probiotic supplementation could ameliorate insulin resistance and lipid metabolism disturbances in subjects suffering from PCOS, characterized by decreased fasting plasma glucose and fasting insulin as well as decreased total cholesterol and very-low-density lipoprotein cholesterol [98]. Moreover, some lactic acid bacterial strains can also alleviate steroidogenesis dysbiosis in patients with PCOS by modifying the population of sex hormone-related gut microbiota, ultimately regulating FSH, luteinizing hormone, and testosterone levels [99]. Recently, FMT showed considerable effectiveness in PCOS with much lower rates of remission by reestablishing gut eubiosis after gut dysbiosis [100], suggesting the beneficial effect of bacteria with probiotic roles in PCOS.
3.3.2. Bacterial vaginosis (BV)
Bacterial vaginosis is caused by imbalance in the ecology of the vaginal microbiota, and approximately one-third of women worldwide have suffered to BV at different times in their lives [101]. Probiotics are considered an effective treatment to increase the Lactobacillus colonization rate and restore the normal flora in the vagina, thus helping to alleviate vaginal inflammation. Single or multi-strain Lactobacillus spp. can be administered through enteric or intravaginal routes. Enteric probiotic administration (Lactobacillus crispatus strains) can reduce the abundance of vaginal G. vaginalis in patients with BV [102], while vaginal probiotic supplementation (containing L. rhamnosus DSM 14870 and Lactobacillus gasseri DSM 14869) would contribute to the colonization of Lactobacilli in BV patients [103], consequently lowering vaginal pH and increasing production of antimicrobial substances that treat or prevent BV [104]. Another strategy to relieve BV is vaginal microbiota transplantation (VMT), which resets the vaginal microbiome to a healthy state by transplanting vaginal discharge from a healthy individual to a patient with BV [105]. Some patients displayed rapid changes in vaginal bacterial composition as early as one-month post-VMT and closely approached the donor vaginal microbiome configuration. Both probiotics and VMT intervention through the vagina can change the composition of the female vaginal microbiome directly and relieve vaginal inflammation caused by BV. However, studies have shown that oral probiotics administration can positively regulate the vaginal microbial ecosystem [106] and inhibit NF-κB activation and TNF-α expression in the vagina and uterus [107], indicating that the relief from vaginosis symptoms in this manner may be through alteration of the vaginal microbiota or stimulation of the immune system.
3.3.3. Endometrial diseases
The beneficial effects of probiotics on the endometrium have been well documented. An in vitro study showed that the probiotic strain L. rhamnosus BPL005 plays a protective role against endometrial infections, as demonstrated by decreased pH levels and significantly reduced levels of pathogenic bacteria [108]. Another in vitro study demonstrated that probiotic Lactobacilli (Lactobacillus reuteri RC-14 and L. rhamnosus GR-1) treatment can strengthen the barrier function of endometrial epithelial cells destroyed by human immunodeficiency virus-1 [109]. Endometritis is an infectious and inflammatory disorder of the endometrium. The persistent inflammation of the endometrial mucosa is caused by the presence of bacterial pathogens in the uterine cavity [110]. Probiotics have been demonstrated to enhance uterine barrier integrity and inhibit the inflammatory response, thus alleviating Staphylococcus aureus-induced endometritis after intragastric administration of Clostridium tyrobutyricum [111]. Endometriosis is a chronic inflammatory disease characterized by the presence of endometrial-type mucosa outside the uterine cavity, which causes pelvic pain and infertility. Leonardi et al. [112] reviewed that endometriosis was associated with increased abundance of Proteobacteria, Enterobacteriaceae, Streptococcus and E. coli across different microbiome classification. The potential of probiotics to alleviate endometritis is promising. Oral administration of Lactobacillus has been shown to reduce the pain associated with endometriosis [113], [114]. Studies also found that probiotic strain L. gasseri OLL2809 can suppress the development of ectopic endometriotic lesions by enhancing the transcription of IL-12 and natural killer cell activation [115]. The above mentioned studies demonstrated that probiotics alleviate endometritis and endometriosis by changing the endometrial microbiome configuration, inhibiting the inflammatory response, or enhancing the uterine barrier integrity. Furthermore, FMT from mice with endometriosis induced endometriotic lesions, suggesting the influence of gut microbiota on endometriosis [116]. Thus, FMT was proposed as a promising tool for the treatment of female reproductive tract diseases [7], and uterine microbiota transfer has also been offered to be considered for the treatment of endometritis and endometriosis [117].
3.3.4. Gestational diabetes mellitus (GDM)
GDM is defined as any degree of glucose intolerance with onset or first recognition during pregnancy and is associated with significant health risks for both the pregnant woman and the developing fetus. Recent evidence shows that GDM may result from maternal gut microbiome disequilibrium during pregnancy [118]. Less diversity was observed in the intestinal microbial flora of pregnant women with GDM than that of pregnant women without GDM [119]. The preventive effects of specific probiotics on GDM have been widely reviewed [120], [121]. Consumption of probiotic yogurt or a multispecies probiotic mixture prevented increases in serum insulin levels and the development of insulin resistance, thereby improving glucose metabolism and glycemic control [122], [123]. Studies have shown that probiotic supplementation can restore the diversity of the gut microbiota in rats with GDM, and the gut microbial composition was inclined to that of normal pregnant rats [124], indicating that modulation of the gut microbiota via probiotic microorganisms could contribute to GDM prevention [125]. Furthermore, probiotic administration can also alleviate inflammation caused by insulin resistance, decreasing several inflammatory markers (such as IL-6, IL-10, TNF-α, and interferon-gamma, among others) in patients with GDM [121]. It can be speculated that the advantageous outcomes following probiotic intervention in GDM resulted from direct probiotic regulation of the host metabolism and immune response and were mediated through alteration of the gut microbiota composition. However, the exact mechanisms by which probiotics may affect GDM remain unknown. Furthermore, some studies have shown no evidence that probiotics administration decreases the risk of GDM any more than placebos [126], [127], and another study suggests that probiotics (L. rhamnosus and B. animalis subspecies lactis) had limited ability to prevent GDM in overweight and obese pregnant women [128]. Effects of microbiomes and probiotics on female reproduction were briefly summarized in Table 2.
Table 2.
Effects of Microbiomes and Probiotics on Female Reproduction.
| Microorganisms | Target(s) | Main effects | Reference |
|---|---|---|---|
| Sanprobi Barrier | HPO axis | Increased FSH level in perimenopausal women | [68] |
| Bacillus licheniformis | Ovary | Improved follicle development in hens | [69] |
| Bacillus amyloliquefaciens BLCC1-0238 | Ovary | Improved follicle development in hens | [70] |
| Enterococcus faecium | Reproductive system | Improved egg quality and increased FSH in hens | [71] |
| L. rhamnosus IMC 501 | Follicle | Promoted oocyte maturation in fish | [72] |
| Bacillus subtilis C-3102 | Reproductive system | Decreased weaning estrus interval in sows | [73] |
| Bacillus mesentericus strain TO-A, Clostridium butyricum strain TO-A, Enterococcus faecalis strain T-110 | Intestine, reproductive system | Changed gut structure and improved reproductive performance in sows | [74] |
| Enterococcus faecium strain | Placenta | Translocated probiotics from maternal gut to amniotic fluid in women | [77] |
| L. rhamnosus GG, Bifidobacterium lactis | Placenta | Enhanced fetal and placental immune physiology in women | [79] |
| Bifidobacterium, Lactobacillus, Streptococcus | Placenta | Reduced autophagy-related protein expression in women | [80] |
| L. rhamnosus GR-1 | Human placental trophoblast cells | Inhibited lipopolysaccharide-stimulated TNF-α production | [81] |
| L. acidophilus LA-5, B. lactis Bb12, L. rhamnosus GG | Placenta | Decreased the risk of severe preeclampsia in women | [82] |
| Bacillus licheniformis and isomaltooligosaccharide | Placenta | Improved placental lipid metabolism in sows | [83] |
| L. plantarum 30M5 | Intestine | Increased circulating estrogen level and improved SCFAs production | [67] |
| Bifidobacterium lactis probiotic V9 | Intestine | Alleviated inflammation through changing gut microbiome in PCOS patients | [97] |
| L. plantarum HL2, Bifidobacterium longum HB3 | Intestine | Regulated sex hormone related gut microbiota in PCOS patients | [99] |
| Fecal microbiota transplantation | Intestine | Reestablished gut eubiosis in PCOS patients | [100] |
| Gardnerella vaginalis | Vagina | Pathogen of bacterial vaginosis in women | [22] |
| L. crispatus strains | Vagina | Reduced the abundance of vaginal G. vaginalis in BV patients | [102] |
| L. rhamnosus DSM 14870, L. gasseri DSM 14869 | Vagina | Colonized Lactobacilli in BV patients | [103] |
| Vaginal microbiota transplantation | Vagina | Changed in vaginal bacterial composition in BV patients | [105] |
| L. paracasei LPC-S01 | Intestine | Reduced the abundance of Gardnerella spp. in BV patients | [106] |
| L. plantarum NK3, Bifidobacterium longum NK49 | Intestine | Suppressed NF-kB-linked TNF-a expression in the colon in BV patients | [107] |
| L. rhamnosus BPL005 | Primary endometrial epithelial cells | Reduced pathogens in an in vitro endometrial cell model | [108] |
| L. reuteri RC-14, L. rhamnosus GR-1 | Primary endometrial epithelial cells | Enhanced barrier function and reduced proinflammatory cytokines in endometrial cells | [109] |
| Clostridium tyrobutyricum | Intestine | Alleviated Staphylococcus aureus-induced endometritis. | [111] |
| L. gasseri OLL2809 | Intestine | Reduce pain associated with endometriosis | [113] |
| LactoFem® | Intestine | Reduce pain associated with endometriosis | [114] |
| L. gasseri OLL2809 | Intestine | Suppressed the development of ectopic endometriotic lesions | [115] |
| L. acidophilus LA5, Bifidobacterium animalis BB12 | Intestine | Maintained serum insulin levels and prevented developing insulin resistance in pregnant women | [122] |
| Probiotic Mixture (VSL#3) | Intestine | Maintained glycemic control in GDM women | [123] |
| L. rhamnosus, LGG, Bifidobacterium animalis subsp. lactis Bb12 | Intestine | Restored the gut microbiota of GDM rats | [124] |
Note: HPO axis: hypothalamic-pituitary-ovarian axis; FSH: follicle-stimulating hormone; L.: Lactobacillus; SCFAs: short-chain fatty acids; PCOS: polycystic ovary syndrome; BV: bacterial vaginosis; GDM: gestational diabetes mellitus.
4. Impact of maternal microbiomes on offspring
4.1. Potential pathway by which the maternal microbiome affects offspring
It is still unclear whether the newborn is sterile or has prenatal colonization by bacteria [129], [130]. However, it is very clear that the maternal microbiota is extensively transmitted to neonates through bacterial translocation from maternal circulation, direct contact with maternal microbiota during vaginal delivery, and the supply of breastfeeding bacteria during lactation [131], [132], [133]. Fetal microbiomes derived from maternal skin and the vagina seem tentative, while gut microbiomes that migrated and evolved from the mother have proven to be more persistent with better adaptability, following to improve infants’ intestinal microbiota [132]. Maternal microbiomes could have an impact on microbial colonization and microbiota establishment in infants, subsequently affecting infant growth and health [134]. The microbiota composition and homeostasis can be affected by external elements, including diet [135] and infection [136] during gestation, which in turn influence fetal gut microbiomes after farrowing. Probiotic supplementation is a potential strategy to modulate pregnancy and infancy outcomes during the perinatal period and lactation because of its positive role in regulating intestinal and vaginal microflora both in mothers and infants [12], [137]. A pilot study by Schultz et al. [138] showed that after oral intake of probiotic L. rhamnosus strain GG (L. GG) from late pregnancy to farrowing, L. GG could be detected in most fetal fecal samples at 1 and 6 months, and the longest persistence of L. GG was found in infants at 24 months of age. In a study, maternal supplementation with L. acidophilus increased fecal L. acidophilus counts at the onset of weaning in mice, suggesting that maternal transfer occurred [139]. Furthermore, a higher prevalence of Bifidobacterium breve and a lower prevalence of Bifidobacterium adolescentis were observed in infants at 5 days of age whose mothers were treated with L. rhamnosus GG during the perinatal period, and the prevalence of B. adolescentis in the mothers before delivery was also correlated with its presence in infant samples at 1 and 5 months [140]. These results indicate that administered probiotic bacteria can transfer from mother to newborn or that maternal supplementation with probiotics can alter the initial establishment of the microbiome in neonates.
4.2. Impact of maternal microbiome on offspring health
The maternal microbiota is an important determinant of health for infants [141], [142]. Whether the neonate is sterile or not, the maternal microbiome during late pregnancy and early lactation acts as the source of the infant microbiome, while the maternal microbiome during pregnancy is the main cause of adverse pregnancy outcomes and affects neonatal and infant health [141]. Through microbial exposure or action of bacterial metabolites, the maternal gut microbiota influences the composition and establishment of bacteria in the newborn’s gut, thus affecting intestinal function and immune system development [142]. Orally administered probiotic bacteria can be detected in mammary tissue and milk, confirming translocation of bacteria from the gut to milk [143]; thus, the gut-breast milk axis is regarded as a potential pathway through which maternal microbiota drives early offspring health indirectly. Breast-fed infants harbor a characteristic fecal microbiota with a higher abundance of Bifidobacteria than that of formula-fed infants, implying the role of maternal microbiota in infant gut microbiota and immune establishment through the breast [144]. Moreover, vaginal microbes also seem to play a critical role in programming neonatal immunity. Several studies have shown that neonates have different immunity after vaginal and cesarean delivery, that is, infants delivered by cesarean section exhibited fewer Bifidobacteria in their microbiomes early in life and presented stronger humoral immune responses than did infants born vaginally, indicating that vaginal microbiota play a significant role in immune education in neonates [145], [146]. These findings suggest that maternal microorganisms in the gut and reproductive tracts may play a key role in the health of newborns [147].
4.3. Effects of maternal probiotics on offspring
4.3.1. Effects of maternal probiotic supplements on newborn health
Probiotic supplementation during pregnancy has been demonstrated to positively modulate gut microbiota composition and improve metabolism in pregnant women; such interventions have been proposed to transmit benefits to newborns. However, the impacts of probiotic supplementation on pregnant and breastfeeding women on fetal and infant birth weight and growth have only been partially addressed. Several systematic reviews and meta-analyses have indicated that maternal probiotic interventions do not evidently influence newborn birth weight, head circumference, or length in overweight pregnant women or those with GDM [126], [148]. However, some studies have also shown that combined supplementation of the probiotics L. rhamnosus and B. lactis during pregnancy had beneficial effects on infant weight and length gains during the first two years of life, suggesting the potential long-term benefits of maternal supplementation [149], [150]. Several studies have also evaluated the effect of probiotic strain supplementation during pregnancy and lactation on the outcome of gastrointestinal diseases, particularly diarrhea, which is common among infants. Mantaring et al. [150] evaluated the impact of an oral maternal nutritional supplement formulated with the probiotics L. rhamnosus and B. lactis on the incidence of diarrhea in infants from birth to one year of age, but no significant effects were observed.
However, in animal production, many studies have highlighted the effect of maternal probiotic supplementation on fetal and newborn health. Maternal supplementation with probiotics can change the microbiota and metabolism profiles of sows, thereby improving piglet growth and health as demonstrated by higher mean daily gain and body weight at weaning and increased numbers of piglet weaned, as well as reductions in abundance of both Escherichia coli and Clostridium spp. in piglet feces [73]. Microbiota shifts were also observed in livestock animals, and Lan and Kim [151] observed that piglet fecal Lactobacillus and Enterococcus counts linearly increased after E. faecium supplementation in sows, indicating the potential of microbial communities to shift from sows to piglets. This shift can alter the gut microbiota of offspring piglets, thus improving their immune responses and enhancing their production performance and health status. Paciflor was fed to sows 15 days prior to farrowing to weaning, and the piglets from Paciflor-treated mothers were significantly heavier than those from untreated dams, indicating that the use of the probiotic (Paciflor) in sows during late pregnancy and lactation had benefits for piglet growth [152]. Wang et al. [133] supplemented sow diets with probiotic fermentation broth (Lactobacillus spp. combined with yeast) during gestation and lactation and discovered several increased immune indices both in the plasma and small intestine tissue of offspring piglets. They also indicated modulated piglet intestinal microbiota composition and its correlation with the alterations of immunoglobulin and cytokines after maternal probiotic supplementation. Therefore, maternal probiotic supplementation is considered a potential dietary strategy to improve health and production and reduce disease risk in newborn piglets.
4.3.2. Effects of maternal probiotic supplements on newborn disease prevention
During gestation, fetal immune states and metabolic functions are largely up to the mother, and maternal intake of probiotics can regulate immune development in the fetus to decrease the risk of immune abnormalities and improve fetal resistibility [12]. Changes in gut microbes are likely to cause allergies, while the use of probiotics seems to be a practical option for their prevention. Cuello-Garcia et al. [153] and Zuccotti et al. [154] performed a systematic review and meta-analysis to assess the effects of probiotic supplementation during pregnancy and early infancy and concluded that probiotics consumed by pregnant women or breastfeeding mothers and/or given to infants reduced the risk of eczema and prevented atopic diseases in infants. This suggests that maternal or infantile probiotic intervention is a feasible, effective, relatively short-term method to decrease the risk of allergy-related diseases in infants [12], [155]. Asthma is another type of allergic disorder. However, another systematic review and meta-analysis of randomized controlled trials evaluating the correlation of probiotic administration during pregnancy or infancy with asthma and wheeze in childhood concluded that there is little evidence to verify the protective association between perinatal use of probiotics and allergic disease [156]. Further investigation is warranted to define the role of probiotics in the prevention of childhood autoimmune diseases. Therefore, clinical use of probiotics for these purposes is still far away, and the probiotic strains used, as well as their preparation methods, matrix, and delivery vehicles, need to be considered. For example, Szajewska and Horvath [157] concluded that L. GG supplementation (regardless of the timing of administration) does not reduce the risk of eczema, suggesting that specific strains would be indicated when recommending the use of probiotics to prevent these diseases. Effects of microbiomes and probiotics on offspring were briefly summarized in Table 3.
Table 3.
Effects of Microbiomes and Probiotics on Offspring.
| Microorganisms | Target(s) | Main effects | Reference |
|---|---|---|---|
| Bacillus subtilis C-3102 | Fetus | Supplemented to mother and improved piglet growth and health | [73] |
| Enterococcus faecium DSM 7134 | Fetus | Increased feed intake and weight performance in piglets | [89] |
| L. plantarum B90, Saccharomyces cerevisiae P11 | Fetus | Supplemented to mother and increased intestinal immunity in piglets | [133] |
| L. rhamnosus GG | Fetus | Transferred from maternal intestine to fetal intestine in human | [138] |
| L. acidophilus | Fetus | Transferred from maternal intestine to fetal intestine in mice | [139] |
| L. rhamnosus GG | Fetus | Transferred and established fecal bifidobacterial microbiota in neonates | [140] |
| Lactococcus lactis MG1614, L. salivarius PS2 | Milk | Transferred from intestine to milk in mice | [143] |
| L. rhamnosus GG, B. lactis Bb12 | Fetus | Diminished the risk of larger birth size and weight in GDM mother | [149] |
| L. rhamnosus CGMCC 1.3724, Bifidobacterium lactis CNCC I-3446 | Fetus | Supplemented to mother and increased infant weight and length gains at 12-month old | [150] |
| Enterococcus faecium | Fetus | Supplemented to mother and increased fecal Lactobacillus and Enterococcus counts in piglets | [151] |
| Paciflor | Fetus | Supplemented to mother and increased piglet growth | [152] |
Note: L.: Lactobacillus; GDM: gestational diabetes mellitus.
5. Summary and outlook
Modern omics techniques have helped prove the existence of microorganisms in almost the entire reproductive tract, and their vital roles in maintaining the reproductive health of males and females was also discovered. Exploration of reproductive-related microorganisms offers a potential opportunity to develop specific treatments aimed at their modification, including probiotics. This review investigated the currently available evidence for the impact of probiotic intervention on the reproductive health of males and females, as well as on their offspring. The suggested mechanism by which probiotics benefit reproduction may include modulation of microbiota composition, regulation of metabolism, promotion of the epithelial barrier, and improvement of immune function. Probiotic intervention may be a tolerable, relatively affordable, and effective way to improve reproductive health in males, females, and their offspring, but we still cannot draw any firm conclusions about using probiotics in reproductive health improvement. A larger number of randomized trials have been suggested to illuminate the potential role of certain probiotics in improving reproductive health. Moreover, further studies are required to elucidate the underlying mechanisms by which probiotics may influence host reproductive physiology and thus to explain and rationally exploit their modes of action.
CRediT authorship contribution statement
Tao Feng: Conceptualization, Methodology, Writing – review & editing, Project administration, Funding acquisition. Yan Liu: Supervision, Writing – review & editing, Funding acquisition.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgments
We would like to thank Dr. Jing Wang, an animal nutritionist of the Institute of Animal Husbandry and Veterinary Medicine, Beijing Academy of Agriculture and Forestry Sciences, for her contributions to the structure and editing of this manuscript.
Funding sources
This research was supported in part by the National Natural Science Foundation of China (31972575), Beijing Natural Science Foundation (6202009), the Science Foundation of Beijing Academy of Agriculture and Forestry Sciences (CZZJ202205), the Science Foundation of the Institute of Animal Husbandry and Veterinary Medicine, Beijing Academy of Agriculture and Forestry Sciences (XMS2022-01).
References
- 1.Dominguez-Bello M.G., Godoy-Vitorino F., Knight R., Blaser M.J. Role of the microbiome in human development. Gut. 2019;68(6):1108–1114. doi: 10.1136/gutjnl-2018-317503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lundy S.D., Sangwan N., Parekh N.V., Selvam M.K.P., Gupta S., McCaffrey P., et al. Functional and taxonomic dysbiosis of the gut, urine, and semen microbiomes in male infertility. Eur Urol. 2021;79(6):826–836. doi: 10.1016/j.eururo.2021.01.014. [DOI] [PubMed] [Google Scholar]
- 3.Rowe M., Veerus L., Trosvik P., Buckling A., Pizzari T. The Reproductive microbiome: an emerging driver of sexual selection, sexual conflict, mating systems, and reproductive isolation. Trends Ecol Evol. 2020;35(3):220–234. doi: 10.1016/j.tree.2019.11.004. [DOI] [PubMed] [Google Scholar]
- 4.Heil B.A., Paccamonti D.L., Sones J.L. Role for the mammalian female reproductive tract microbiome in pregnancy outcomes. Physiol Genomics. 2019;51(8):390–399. doi: 10.1152/physiolgenomics.00045.2019. [DOI] [PubMed] [Google Scholar]
- 5.Koedooder R., Mackens S., Budding A., Fares D., Blockeel C., Laven J., et al. Identification and evaluation of the microbiome in the female and male reproductive tracts. Hum Reprod Update. 2019;25(3):298–325. doi: 10.1093/humupd/dmy048. [DOI] [PubMed] [Google Scholar]
- 6.Schoenmakers S., Steegers-Theunissen R., Faas M. The matter of the reproductive microbiome. Obstet Med. 2019;12(3):107–115. doi: 10.1177/1753495X18775899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Quaranta G., Sanguinetti M., Masucci L. Fecal microbiota transplantation: a potential tool for treatment of human female reproductive tract diseases. Front Immunol. 2019;10:2653. doi: 10.3389/fimmu.2019.02653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reid J.N.S., Bisanz J.E., Monachese M., Burton J.P., Reid G. The rationale for probiotics improving reproductive health and pregnancy outcome. Am J Reprod Immunol. 2013;69:558–566. doi: 10.1111/aji.12086. [DOI] [PubMed] [Google Scholar]
- 9.Helli B., Kavianpour M., Ghaedi E., Dadfar M., Haghighian H.K. Probiotic effects on sperm parameters, oxidative stress index, inflammatory factors and sex hormones in infertile men. Hum Fertil. 2020:1–9. doi: 10.1080/14647273.2020.1824080. [DOI] [PubMed] [Google Scholar]
- 10.Abbasi A., Aghebati-Maleki A., Yousefi M., Aghebati-Maleki L. Probiotic intervention as a potential therapeutic for managing gestational disorders and improving pregnancy outcomes. J Reprod Immunol. 2021;143 doi: 10.1016/j.jri.2020.103244. [DOI] [PubMed] [Google Scholar]
- 11.Palmer N.O., Bakos H.W., Owens J.A., Setchell B.P., Lane M. Diet and exercise in an obese mouse fed a high fat diet improves metabolic health and reverses perturbed sperm function. Am J Physiol Endocrinol Metab. 2012;302:E768–E780. doi: 10.1152/ajpendo.00401.2011. [DOI] [PubMed] [Google Scholar]
- 12.Sanz Y. Gut microbiota and probiotics in maternal and infant health. Am J Clin Nutr. 2011;94(6 suppl):2000S–2005S. doi: 10.3945/ajcn.110.001172. [DOI] [PubMed] [Google Scholar]
- 13.Altmäe S., Franasiak J.M., Mändar R. The seminal microbiome in health and disease. Nat Rev Urol. 2019;16:703–721. doi: 10.1038/s41585-019-0250-y. [DOI] [PubMed] [Google Scholar]
- 14.Tomaiuolo R., Veneruso I., Cariati F., D'Argenio V. Microbiota and human reproduction: the case of male infertility. High-Throughput. 2020;9(2):10. doi: 10.3390/ht9020010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weng S.L., Chiu C.M., Lin F.M., Huang W.C., Liang C., Yang T., et al. Bacterial communities in semen from men of infertile couples: metagenomic sequencing reveals relationships of seminal microbiota to semen quality. PLoS ONE. 2014;9(10) doi: 10.1371/journal.pone.0110152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mändar R., Punab M., Borovkova N., Lapp E., Kiiker R., Korrovits P., et al. Complementary seminovaginal microbiome in couples. Res Microbiol. 2015;166:440–447. doi: 10.1016/j.resmic.2015.03.009. [DOI] [PubMed] [Google Scholar]
- 17.Balmelli T., Stamm J., Dolina-Giudici M., Peduzzi R., Piffaretti-Yanez A., Balerna M. Bacteroides ureolyticus in men consulting for infertility. Andrologia. 1994;26:35–38. doi: 10.1111/j.1439-0272.1994.tb00751.x. [DOI] [PubMed] [Google Scholar]
- 18.Hou D., Zhou X., Zhong X., Settles M.L., Herring J., Wang L., et al. Microbiota of the seminal fluid from healthy and infertile men. Fertil Steril. 2013;100:1261–1269. doi: 10.1016/j.fertnstert.2013.07.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Monteiro C., Marques P.I., Cavadas B., Damião I., Almeida V., Barros N., et al. Characterization of microbiota in male infertility cases uncovers differences in seminal hyperviscosity and oligoasthenoteratozoospermia possibly correlated with increased prevalence of infectious bacteria. Am J Reprod Immunol. 2018;79 doi: 10.1111/aji.12838. [DOI] [PubMed] [Google Scholar]
- 20.Kermes K., Punab M., Lõivukene K., Mändar R. Anaerobic seminal fluid micro-flora in chronic prostatitis/chronic pelvic pain syndrome patients. Anaerobe. 2003;9:117–123. doi: 10.1016/S1075-9964(03)00085-4. [DOI] [PubMed] [Google Scholar]
- 21.Jeon S.J., Cunha F., Vieira-Neto A., Bicalho R.C., Lima S., Bicalho M.L., et al. Blood as a route of transmission of uterine pathogens from the gut to the uterus in cows. Microbiome. 2017;5(1):109. doi: 10.1186/s40168-017-0328-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vodstrcil L.A., Twin J., Garland S.M., Fairley C.K., Hocking J.S., Law M.G., et al. The influence of sexual activity on the vaginal microbiota and Gardnerella vaginalis clade diversity in young women. PLoS ONE. 2017;12(2) doi: 10.1371/journal.pone.0171856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Morison L., Ekpo G., West B., Demba E., Mayaud P., Coleman R., et al. Bacterial vaginosis in relation to menstrual cycle, menstrual protection method, and sexual intercourse in rural Gambian women. Sex Transm Infect. 2005;81(3):242–247. doi: 10.1136/sti.2004.011684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Verstraelen H., Verhelst R., Vaneechoutte M., Temmerman M. The epidemiology of bacterial vaginosis in relation to sexual behaviour. BMC Infect Dis. 2010;10:81. doi: 10.1186/1471-2334-10-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rando O.J., Simmons R.A. I’m eating for two: Parental dietary effects on offspring metabolism. Cell. 2015;161(1):93–105. doi: 10.1016/j.cell.2015.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pan W.H., Sommer F., Falk-Paulsen M., Ulas T., Best P., Fazio A., et al. Exposure to the gut microbiota drives distinct methylome and transcriptome changes in intestinal epithelial cells during postnatal development. Genome Med. 2018;10(1):27. doi: 10.1186/s13073-018-0534-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sisti G., Kanninen T.T., Witkin S.S. Maternal immunity and pregnancy outcome: Focus on preconception and autophagy. Genes Immun. 2016;17(1):1–7. doi: 10.1038/gene.2015.57. [DOI] [PubMed] [Google Scholar]
- 28.Motrich R.D., Salazar F.C., Breser M.L., Mackern-Oberti J.P., Godoy G.J., Olivera C., et al. Implications of prostate inflammation on male fertility. Andrologia. 2018;50(11) doi: 10.1111/and.13093. [DOI] [PubMed] [Google Scholar]
- 29.Porter C.M., Shrestha E., Peiffer L.B., Sfanos K.S. The microbiome in prostate inflammation and prostate cancer. Prostate Cancer Prostatic Dis. 2018;21(3):345–354. doi: 10.1038/s41391-018-0041-1. [DOI] [PubMed] [Google Scholar]
- 30.Feng Y., Ramnarine V.R., Bell R., Volik S., Davicioni E., Hayes V.M., et al. Metagenomic and metatranscriptomic analysis of human prostate microbiota from patients with prostate cancer. BMC Genomics. 2019;20(1):146. doi: 10.1186/s12864-019-5457-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nickel J.C. Is chronic prostatitis/chronic pelvic pain syndrome an infectious disease of the prostate? Investig Clin Urol. 2017;58:149–151. doi: 10.4111/icu.2017.58.3.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Barbonetti A., Cinque B., Vassallo M.R., Mineo S., Francavilla S., Cifone M.G., et al. Effect of vaginal probiotic lactobacilli on in vitro-induced sperm lipid peroxidation and its impact on sperm motility and viability. Fertil Steril. 2011;95(8):2485–2488. doi: 10.1016/j.fertnstert.2011.03.066. [DOI] [PubMed] [Google Scholar]
- 33.Dardmeh F., Alipour H., Gazerani P., van der Horst G., Brandsborg E., Nielsen H.I. Lactobacillus rhamnosus PB01 (DSM 14870) supplementation affects markers of sperm kinematic parameters in a diet-induced obesity mice model. PLoS ONE. 2017;12(10) doi: 10.1371/journal.pone.0185964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Valcarce D.G., Genovés S., Riesco M.F., Martorell P., Herráez M.P., Ramón D., et al. Probiotic administration improves sperm quality in asthenozoospermic human donors. Benef Microb. 2017;8(2):193–206. doi: 10.3920/BM2016.0122. [DOI] [PubMed] [Google Scholar]
- 35.Zhang P., Feng Y., Li L., Ge W., Yu S., Hao Y., et al. Improvement in sperm quality and spermatogenesis following faecal microbiota transplantation from alginate oligosaccharide dosed mice. Gut. 2021;70(1):222–225. doi: 10.1136/gutjnl-2020-320992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Poutahidis T., Springer A., Levkovich T., Qi P., Varian B.J., Lakritz J.R., et al. Probiotic microbes sustain youthful serum testosterone levels and testicular size in aging mice. PLoS ONE. 2014;9(1) doi: 10.1371/journal.pone.0084877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Al-Asmakh M., Stukenborg J.B., Reda A., Anuar F., Strand M.L., Hedin L., et al. The gut microbiota and developmental programming of the testis in mice. PLoS ONE. 2014;9(8) doi: 10.1371/journal.pone.0103809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Attia Y.A., Abd El Hamid E.A., Ismaiel A.M., El-Nagar A. The detoxication of nitrate by two antioxidants or a probiotic, and the effects on blood and seminal plasma profiles and reproductive function of New Zealand White rabbit bucks. Animal. 2013;7(4):591–601. doi: 10.1017/S1751731112002054. [DOI] [PubMed] [Google Scholar]
- 39.Zhang J., Liu H., Yang Q., Li P., Wen Y., Han X., et al. Genomic sequencing reveals the diversity of seminal bacteria and relationships to reproductive potential in boar sperm. Front Microbiol. 2020;11:1873. doi: 10.3389/fmicb.2020.01873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tian X., Yu Z., Feng P., Ye Z., Li R., Liu J., et al. Lactobacillus plantarum TW1-1 alleviates diethylhexylphthalate-induced testicular damage in mice by modulating gut microbiota and decreasing inflammation. Front Cell Infect Microbiol. 2019;9:221. doi: 10.3389/fcimb.2019.00221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Aitken R.J., Smith T.B., Jobling M.S., Baker M.A., De Iuliis G.N. Oxidative stress and male reproductive health. Asian J Androl. 2014;16(1):31–38. doi: 10.4103/1008-682X.122203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rosa L.S., Santos M.L., Abreu J.P., Balthazar C.F., Rocha R.S., Silva H.L.A., et al. Antiproliferative and apoptotic effects of probiotic whey dairy beverages in human prostate cell lines. Food Res Int. 2020;137 doi: 10.1016/j.foodres.2020.109450. [DOI] [PubMed] [Google Scholar]
- 43.Celebioglu H.U. Effects of potential synbiotic interaction between Lactobacillus rhamnosus GG and salicylic acid on human colon and prostate cancer cells. Arch Microbiol. 2021;203(3):1221–1229. doi: 10.1007/s00203-021-02200-1. [DOI] [PubMed] [Google Scholar]
- 44.Chiancone F., Carrino M., Meccariello C., Pucci L., Fedelini M., Fedelini P. The use of a combination of Vaccinium Macracarpon, Lycium barbarum L. and Probiotics (Bifiprost®) for the prevention of chronic bacterial prostatitis: a double-blind randomized study. Urol Int. 2019;103(4):423–426. doi: 10.1159/000502765. [DOI] [PubMed] [Google Scholar]
- 45.Pacifici L., Santacroce L., Dipalma G., Haxhirexha K., Topi S., Cantore S., et al. Gender medicine: the impact of probiotics on male patients. Clin Ter. 2021;171(1):e8–e15. doi: 10.7417/CT.2021.2274. [DOI] [PubMed] [Google Scholar]
- 46.Neill M.G., Appu S., Zlotta A.R. Strategies to preserve prostate health. Drugs Today (Barc) 2009;45(1):63–80. doi: 10.1358/dot.2009.45.1.1315920. [DOI] [PubMed] [Google Scholar]
- 47.Baker J.M., Chase D.M., Herbst-Kralovetz M.M. Uterine microbiota: residents, tourists, or invaders? Front Immunol. 2018;9:208. doi: 10.3389/fimmu.2018.00208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Salary A., Kafi M., Derakhshandeh A., Moezzi M.S. Detection of bacteria in bovine ovarian follicular fluid. Lett Appl Microbiol. 2020;70(3):137–142. doi: 10.1111/lam.13254. [DOI] [PubMed] [Google Scholar]
- 49.Tomaiuolo R., Veneruso I., Cariati F., D'Argenio V. Microbiota and human reproduction: the case of female infertility. High-Throughput. 2020;9(2):12. doi: 10.3390/ht9020012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pelzer E.S., Allan J.A., Cunningham K., Mengersen K., Allan J.M., Launchbury T., et al. Microbial colonization of follicular fluid: alterations in cytokine expression and adverse assisted reproduction technology outcomes. Hum Reprod. 2011;26(7):1799–1812. doi: 10.1093/humrep/der108. [DOI] [PubMed] [Google Scholar]
- 51.Pelzer E.S., Allan J.A., Waterhouse M.A., Ross T., Beagley K.W., Knox C.L. Microorganisms within human follicular fluid: effects on IVF. PLoS ONE. 2013;8(3) doi: 10.1371/journal.pone.0059062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tachedjian G., Aldunate M., Bradshaw C.S., Cone R.A. The role of lactic acid production by probiotic Lactobacillus species in vaginal health. Res Microbiol. 2017;168:782–792. doi: 10.1016/j.resmic.2017.04.001. [DOI] [PubMed] [Google Scholar]
- 53.Goulet M., Dular R., Tully J., Billowes G.S. Isolation of Mycoplasma pneumoniae from the human urogenital tract. J Clin Microbiol. 1995;33(11):2823–2825. doi: 10.1128/jcm.33.11.2823-2825.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Aagaard K., Ma J., Antony K.M., Ganu R., Petrosino J., Versalovic J. The placenta harbors a unique microbiome. Sci Transl Med. 2014;6(237):237ra65 doi: 10.1126/scitranslmed.3008599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Verstraelen H., Vilchez-Vargas R., Desimpel F., Jauregui R., Vankeirsbilck N., Weyers S., et al. Characterisation of the human uterine microbiome in non-pregnant women through deep sequencing of the V1–2 region of the 16S rRNA gene. PeerJ. 2016;4 doi: 10.7717/peerj.1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.McElrath T.F., Hecht J.L., Dammann O., Boggess K., Onderdonk A., Markenson G., et al. Pregnancy disorders that lead to delivery before the 28th week of gestation: an epidemiologic approach to classification. Am J Epidemiol. 2008;168(9):980–989. doi: 10.1093/aje/kwn202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kuperman A.A., Zimmerman A., Hamadia S., Ziv O., Gurevich V., Fichtman B., et al. Deep microbial analysis of multiple placentas shows no evidence for a placental microbiome. BJOG. 2020;127(2):159–169. doi: 10.1111/1471-0528.15896. [DOI] [PubMed] [Google Scholar]
- 58.Theis K.R., Romero R., Greenberg J.M., Winters A.D., Garcia-Flores V., Motomura K., et al. No consistent evidence for microbiota in murine placental and fetal tissues. mSphere. 2020;5(1):e00933–e1019. doi: 10.1128/mSphere.00933-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Theis K.R., Romero R., Winters A.D., Jobe A.H., Gomez-Lopez N. Lack of evidence for microbiota in the placental and fetal tissues of rhesus macaques. mSphere. 2020;5(3):e00210–e220. doi: 10.1128/mSphere.00210-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Moreno I., Garcia-Grau I., Perez-Villaroya D., Gonzalez-Monfort M., Bahçeci M., Barrionuevo M.J., et al. Endometrial microbiota composition is associated with reproductive outcome in infertile patients. Microbiome. 2022;10(1):1. doi: 10.1186/s40168-021-01184-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mitchell CM, Haick A, Nkwopara E, Garcia R, Rendi M, Agnew K, et al. Colonization of the upper genital tract by vaginal bacterial species in nonpregnant women. Am J Obstet Gynecol 2015; 212(5):611.e1–9. [DOI] [PMC free article] [PubMed]
- 62.Stavropoulou E., Bezirtzoglou E. Probiotics in medicine: a long debate. Front Immunol. 2020;11:2192. doi: 10.3389/fimmu.2020.02192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wee B.A., Thomas M., Sweeney E.L., Frentiu F.D., Samios M., Ravel J., et al. A retrospective pilot study to determine whether the reproductive tract microbiota differs between women with a history of infertility and fertile women. Aust N Z J Obstet Gynaecol. 2017;58(3):341–348. doi: 10.1111/ajo.12754. [DOI] [PubMed] [Google Scholar]
- 64.Franasiak J.M., Scott R.T., Jr. Reproductive tract microbiome in assisted reproductive technologies. Fertil Steril. 2015;104:1364–1371. doi: 10.1016/j.fertnstert.2015.10.012. [DOI] [PubMed] [Google Scholar]
- 65.DiGiulio D.B., Callahan B.J., McMurdie P.J., Costello E.K., Lyell D.J., Robaczewska A., et al. Temporal and spatial variation of the human microbiota during pregnancy. Proc Natl Acad Sci U S A. 2015;112(35):11060–11065. doi: 10.1073/pnas.1502875112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Stout M.J., Zhou Y., Wylie K.M., Tarr P.I., Macones G.A., Tuuli M.G. Early pregnancy vaginal microbiome trends and preterm birth. Am J Obstet Gynecol. 2017;217(3):356:e1–e18. doi: 10.1016/j.ajog.2017.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chen Q., Wang B., Wang S., Qian X., Li X., Zhao J., et al. Modulation of the gut microbiota structure with probiotics and isoflavone alleviates metabolic disorder in ovariectomized mice. Nutrients. 2021;13(6):1793. doi: 10.3390/nu13061793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Szydłowska I., Marciniak A., Brodowska A., Loj B., Ciećwież S., Skonieczna-Żydecka K., et al. Effects of probiotics supplementation on the hormone and body mass index in perimenopausal and postmenopausal women using the standardized diet. A 5-week double-blind, placebo-controlled, and randomized clinical study. Eur Rev Med Pharmacol Sci. 2021;25(10):3859–3867. doi: 10.26355/eurrev_202105_25953. [DOI] [PubMed] [Google Scholar]
- 69.Lei K., Li Y.L., Yu D.Y., Rajput I.R., Li W.F. Influence of dietary inclusion of Bacillus licheniformis on laying performance, egg quality, antioxidant enzyme activities, and intestinal barrier function of laying hens. Poult Sci. 2013;92(9):2389–2395. doi: 10.3382/ps.2012-02686. [DOI] [PubMed] [Google Scholar]
- 70.Zhou Y., Li S., Pang Q., Miao Z. Bacillus amyloliquefaciens BLCC1-0238 can effectively improve laying performance and egg quality via enhancing immunity and regulating reproductive hormones of laying hens. Probiotics Antimicrob Proteins. 2020;12(1):246–252. doi: 10.1007/s12602-019-9524-1. [DOI] [PubMed] [Google Scholar]
- 71.Zhao S., Zhang K., Ding X., Celi P., Yan L., Bai S., et al. The impact of dietary supplementation of different feed additives on performances of broiler breeders characterized by different egg-laying rate. Poult Sci. 2019;98(11):6091–6099. doi: 10.3382/ps/pez316. [DOI] [PubMed] [Google Scholar]
- 72.Gioacchini G., Maradonna F., Lombardo F., Bizzaro D., Olivotto I., Carnevali O. Increase of fecundity by probiotic administration in zebrafish (Danio rerio) Reproduction. 2010;140(6):953–959. doi: 10.1530/REP-10-0145. [DOI] [PubMed] [Google Scholar]
- 73.Kritas S.K., Marubashi T., Filioussis G., Petridou E., Christodoulopoulos G., Burriel A.R., et al. Reproductive performance of sows was improved by administration of a sporing bacillary probiotic (Bacillus subtilis C-3102) J Anim Sci. 2015;93(1):405–413. doi: 10.2527/jas.2014-7651. [DOI] [PubMed] [Google Scholar]
- 74.Hayakawa T., Masuda T., Kurosawa D., Tsukahara T. Dietary administration of probiotics to sows and/or their neonates improves the reproductive performance, incidence of post-weaning diarrhea and histopathological parameters in the intestine of weaned piglets. Anim Sci J. 2016;87(12):1501–1510. doi: 10.1111/asj.12565. [DOI] [PubMed] [Google Scholar]
- 75.Xu K., Bai M., Liu H., Duan Y., Zhou X., Wu X., et al. Gut microbiota and blood metabolomics in weaning multiparous sows: Associations with oestrous. J Anim Physiol Anim Nutr (Berl) 2020;104(4):1155–1168. doi: 10.1111/jpn.13296. [DOI] [PubMed] [Google Scholar]
- 76.Sun C., Groom K.M., Oyston C., Chamley L.W., Clark A.R., James J.L. The placenta in fetal growth restriction: What is going wrong? Placenta. 2020;96:10–18. doi: 10.1016/j.placenta.2020.05.003. [DOI] [PubMed] [Google Scholar]
- 77.Jiménez E., Fernández L., Marín M.L., Martín R., Odriozola J.M., Nueno-Palop C., et al. Isolation of commensal bacteria from umbilical cord blood of healthy neonates born by cesarean section. Curr Microbiol. 2005;51(4):270–274. doi: 10.1007/s00284-005-0020-3. [DOI] [PubMed] [Google Scholar]
- 78.Voreades N., Kozil A., Weir T.L. Diet and the development of the human intestinal microbiome. Front Microbiol. 2014;5:494. doi: 10.3389/fmicb.2014.00494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rautava S., Collado M.C., Salminen S., Isolauri E. Probiotics modulate host-microbe interaction in the placenta and fetal gut: a randomized, double-blind, placebo-controlled trial. Neonatology. 2012;102(3):178–184. doi: 10.1159/000339182. [DOI] [PubMed] [Google Scholar]
- 80.Yang P., Li Z., Tye K.D., Chen Y., Lu T., He Z., et al. Effects of an orally supplemented probiotic on the autophagy protein LC3 and Beclin1 in placentas undergoing spontaneous delivery during normal pregnancy. BMC Pregnancy Childbirth. 2020;20(1):216. doi: 10.1186/s12884-020-02905-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yeganegi M, Watson CS, Martins A, Kim SO, Reid G, Challis JR, et al. Effect of Lactobacillus rhamnosus GR-1 supernatant and fetal sex on lipopolysaccharide-induced cytokine and prostaglandin-regulating enzymes in human placental trophoblast cells: implications for treatment of bacterial vaginosis and prevention of preterm labor. Am J Obstet Gynecol 2009; 200(5):532.e1–8. [DOI] [PubMed]
- 82.Brantsaeter A.L., Myhre R., Haugen M., Myking S., Sengpiel V., Magnus P., et al. Intake of probiotic food and risk of preeclampsia in primiparous women: the Norwegian mother and child cohort study. Am J Epidemiol. 2011;174(7):807–815. doi: 10.1093/aje/kwr168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gu X.L., Li H., Song Z.H., Ding Y.N., He X., Fan Z.Y. Effects of isomaltooligosaccharide and Bacillus supplementation on sow performance, serum metabolites, and serum and placental oxidative status. Anim Reprod Sci. 2019;207:52–60. doi: 10.1016/j.anireprosci.2019.05.015. [DOI] [PubMed] [Google Scholar]
- 84.Jarrett P., Meczner A., Costeloe K., Fleming P. Historical aspects of probiotic use to prevent necrotising enterocolitis in preterm babies. Early Hum Dev. 2019;135:51–57. doi: 10.1016/j.earlhumdev.2019.05.015. [DOI] [PubMed] [Google Scholar]
- 85.Mirpuri J., Neu J. Maternal microbial factors that affect the fetus and subsequent offspring. Semin Perinatol. 2021;151449 doi: 10.1016/j.semperi.2021.151449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Rasool A., Alvarado-Flores F., O'Tierney-Ginn P. Placental impact of dietary supplements: more than micronutrients. Clin Ther. 2021;43(2):226–245. doi: 10.1016/j.clinthera.2020.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Othman M, Neilson JP, Alfirevic Z. Probiotics for preventing preterm labour. Cochrane Database Syst Rev 2007; (1):CD005941. [DOI] [PMC free article] [PubMed]
- 88.Rootwelt V., Reksen O., Farstad W., Framstad T. Postpartum deaths: piglet, placental, and umbilical characteristics. J Anim Sci. 2013;91(6):2647–2656. doi: 10.2527/jas.2012-5531. [DOI] [PubMed] [Google Scholar]
- 89.Böhmer B.M., Kramer W., Roth-Maier D.A. Dietary probiotic supplementation and resulting effects on performance, health status, and microbial characteristics of primiparous sows. J Anim Physiol Anim Nutr (Berl) 2006;90(7–8):309–315. doi: 10.1111/j.1439-0396.2005.00601.x. [DOI] [PubMed] [Google Scholar]
- 90.Romano-Keeler J., Weitkamp J.H. Maternal influences on fetal microbial colonization and immune development. Pediatr Res. 2015;77(1–2):189–195. doi: 10.1038/pr.2014.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Szczuko M., Kikut J., Szczuko U., Szydłowska I., Nawrocka-Rutkowska J., Ziętek M., et al. Nutrition strategy and life style in polycystic ovary syndrome-narrative review. Nutrients. 2021;13(7):2425. doi: 10.3390/nu13072452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Yu Y., Tan P., Zhuang Z., Wang Z., Zhu L., Qiu R., et al. Untargeted metabolomic approach to study the serum metabolites in women with polycystic ovary syndrome. BMC Med Genomics. 2021;14(1):206. doi: 10.1186/s12920-021-01058-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Patel S. Polycystic ovary syndrome (PCOS), an inflammatory, systemic, lifestyle endocrinopathy. J Steroid Biochem Mol Biol. 2018;182:27–36. doi: 10.1016/j.jsbmb.2018.04.008. [DOI] [PubMed] [Google Scholar]
- 94.Jiao N, Baker SS, Nugent CA, Tsompana M, Cai L, Wang Y, et al. 2018. Gut microbiome may contribute to insulin resistance and systemic inflammation in obese rodents: A meta-analysis. Physiol Genomics 2018; 50(4):244–54. [DOI] [PubMed]
- 95.Qi X., Yun C., Sun L., Xia J., Wu Q., Wang Y., et al. Gut microbiota-bile acid-interleukin-22 axis orchestrates polycystic ovary syndrome. Nat Med. 2019;25(8):1225–1233. doi: 10.1038/s41591-019-0509-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Shirvani-Rad S., Tabatabaei-Malazy O., Mohseni S., Hasani-Ranjbar S., Soroush A.R., Hoseini-Tavassol Z., et al. Probiotics as a complementary therapy for management of obesity: a systematic review. Evid Based Complement Alternat Med. 2021;2021:6688450. doi: 10.1155/2021/6688450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zhang J., Sun Z., Jiang S., Bai X., Ma C., Peng Q., et al. Bifidobacterium lactis probiotic V9 regulates the secretion of sex hormones in polycystic ovary syndrome patients through the gut-brain axis. mSystems. 2019;4(2):e00017–e19. doi: 10.1128/mSystems.00017-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Li Y., Tan Y., Xia G., Shuai J. Effects of probiotics, prebiotics, and synbiotics on polycystic ovary syndrome: a systematic review and meta-analysis. Crit Rev Food Sci Nutr. 2021:1–17. doi: 10.1080/10408398.2021.1951155. [DOI] [PubMed] [Google Scholar]
- 99.He Y., Wang Q., Li X., Wang G., Zhao J., Zhang H., et al. Lactic acid bacteria alleviate polycystic ovarian syndrome by regulating sex hormone related gut microbiota. Food Funct. 2020;11(6):5192–5204. doi: 10.1039/c9fo02554e. [DOI] [PubMed] [Google Scholar]
- 100.Corrie L., Gulati M., Vishwas S., Kapoor B., Singh S.K., Awasthi A., et al. Combination therapy of curcumin and fecal microbiota transplant: Potential treatment of polycystic ovarian syndrome. Med Hypotheses. 2021;154 doi: 10.1016/j.mehy.2021.110644. [DOI] [PubMed] [Google Scholar]
- 101.Joseph R.J., Ser H.L., Kuai Y.H., Tan L.T., Arasoo V.J.T., Letchumanan V., et al. Finding a balance in the vaginal microbiome: how do we treat and prevent the occurrence of bacterial vaginosis? Antibiotics (Basel) 2021;10(6):719. doi: 10.3390/antibiotics10060719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Rostok M., Hütt P., Rööp T., Smidt I., Štšepetova J., Salumets A., et al. Potential vaginal probiotics: Safety, tolerability and preliminary effectiveness. Benef Microbes. 2019;10(4):385–393. doi: 10.3920/BM2016.0123. [DOI] [PubMed] [Google Scholar]
- 103.Marcotte H., Larsson P.G., Andersen K.K., Zuo F., Mikkelsen L.S., Brandsborg E., et al. An exploratory pilot study evaluating the supplementation of standard antibiotic therapy with probiotic lactobacilli in south African women with bacterial vaginosis. BMC Infect Dis. 2019;19(1):824. doi: 10.1186/s12879-019-4425-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Homayouni A., Bastani P., Ziyadi S., Mohammad-Alizadeh-Charandabi S., Ghalibaf M., Mortazavian A.M., et al. Effects of probiotics on the recurrence of bacterial vaginosis: a review. J Low Genit Tract Dis. 2014;18(1):79–86. doi: 10.1097/LGT.0b013e31829156ec. [DOI] [PubMed] [Google Scholar]
- 105.Lev-Sagie A., Goldman-Wohl D., Cohen Y., Dori-Bachash M., Leshem A., Mor U., et al. Vaginal microbiome transplantation in women with intractable bacterial vaginosis. Nat Med. 2019;25(10):1500–1504. doi: 10.1038/s41591-019-0600-6. [DOI] [PubMed] [Google Scholar]
- 106.Koirala R., Gargari G., Arioli S., Taverniti V., Fiore W., Grossi E., et al. Effect of oral consumption of capsules containing Lactobacillus paracasei LPC-S01 on the vaginal microbiota of healthy adult women: a randomized, placebo-controlled, double-blind crossover study. FEMS Microbiol Ecol. 2020;96(6):fiaa084 doi: 10.1093/femsec/fiaa084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kim D.E., Kim J.K., Han S.K., Jang S.E., Han M.J., Kim D.H. Lactobacillus plantarum NK3 and Bifidobacterium longum NK49 alleviate bacterial vaginosis and osteoporosis in mice by suppressing NF-kB-linked TNF-a expression. J Med Food. 2019;22:1022–1031. doi: 10.1089/jmf.2019.4419. [DOI] [PubMed] [Google Scholar]
- 108.Chenoll E., Moreno I., Sánchez M., Garcia-Grau I., Silva Á., González-Monfort M., et al. Selection of new probiotics for endometrial health. Front Cell Infect Microbiol. 2019;9:114. doi: 10.3389/fcimb.2019.00114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Dizzell S., Nazli A., Reid G., Kaushic C. Protective effect of probiotic bacteria and estrogen in preventing HIV-1-mediated impairment of epithelial barrier integrity in female genital tract. Cells. 2019;8(10):1120. doi: 10.3390/cells8101120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Cicinelli E., De Ziegler D., Nicoletti R., Colafiglio G., Saliani N., Resta L., et al. Chronic endometritis: Correlation among hysteroscopic, histologic, and bacteriologic findings in a prospective trial with 2190 consecutive office hysteroscopies. Fertil Steril. 2008;89(3):677–684. doi: 10.1016/j.fertnstert.2007.03.074. [DOI] [PubMed] [Google Scholar]
- 111.Hu X., Guo J., Xu M., Jiang P., Yuan X., Zhao C., et al. Clostridium tyrobutyricum alleviates Staphylococcus aureus-induced endometritis in mice by inhibiting endometrial barrier disruption and inflammatory response. Food Funct. 2019;10(10):6699–6710. doi: 10.1039/c9fo00654k. [DOI] [PubMed] [Google Scholar]
- 112.Leonardi M., Hicks C., El-Assaad F., El-Omar E., Condous G. Endometriosis and the microbiome: a systematic review. BJOG. 2020;127(2):239–249. doi: 10.1111/1471-0528.15916. [DOI] [PubMed] [Google Scholar]
- 113.Itoh H., Uchida M., Sashihara T., Ji Z., Li J., Tang Q., et al. Lactobacillus gasseri OLL2809 is effective especially on the menstrual pain and dysmenorrhea in endometriosis patients: Randomized, double-blind, placebo-controlled study. Cytotechnology. 2011;63(2):153–161. doi: 10.1007/s10616-010-9326-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Khodaverdi S., Mohammadbeigi R., Khaledi M., Mesdaghinia L., Sharifzadeh F., Nasiripour S., et al. Beneficial effects of oral lactobacillus on pain severity in women suffering from endometriosis: A pilot placebo-controlled randomized clinical trial. Int J Fertil Steril. 2019;13(3):178–183. doi: 10.22074/ijfs.2019.5584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Itoh H., Sashihara T., Hosono A., Kaminogawa S., Uchida M. Lactobacillus gasseri OLL2809 inhibits development of ectopic endometrial cell in peritoneal cavity via activation of NK cells in a murine endometriosis model. Cytotechnology. 2011;63:205–210. doi: 10.1007/s10616-011-9343-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Chadchan S.B., Cheng M., Parnell L.A., Yin Y., Schriefer A., Mysorekar I.U., et al. Antibiotic therapy with metronidazole reduces endometriosis disease progression in mice: A potential role for gut microbiota. Hum Reprod. 2019;34(6):1106–1116. doi: 10.1093/humrep/dez041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Molina N.M., Sola-Leyva A., Saez-Lara M.J., Plaza-Diaz J., Tubić-Pavlović A., Romero B., et al. New opportunities for endometrial health by modifying uterine microbial composition: Present or future? Biomolecules. 2020;10(4):593. doi: 10.3390/biom10040593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Mullins T.P., Tomsett K.I., Gallo L.A., Callaway L.K., McIntyre H.D., Dekker Nitert M., et al. Maternal gut microbiota displays minor changes in overweight and obese women with GDM. Nutr Metab Cardiovasc Dis. 2021;31(7):2131–2139. doi: 10.1016/j.numecd.2021.03.029. [DOI] [PubMed] [Google Scholar]
- 119.Cortez R.V., Taddei C.R., Sparvoli L.G., Ângelo A.G.S., Padilha M., Mattar R., et al. Microbiome and its relation to gestational diabetes. Endocrine. 2019;64(2):254–264. doi: 10.1007/s12020-018-1813-z. [DOI] [PubMed] [Google Scholar]
- 120.Isolauri E., Rautava S., Collado M.C., Salminen S. Role of probiotics in reducing the risk of gestational diabetes. Diabetes Obes Metab. 2015;17(8):713–719. doi: 10.1111/dom.12475. [DOI] [PubMed] [Google Scholar]
- 121.Dallanora S., Medeiros de Souza Y., Deon R.G., Tracey C.A., Freitas-Vilela A.A., Wurdig Roesch L.F., et al. Do probiotics effectively ameliorate glycemic control during gestational diabetes? A systematic review. Arch Gynecol Obstet. 2018;298(3):477–485. doi: 10.1007/s00404-018-4809-2. [DOI] [PubMed] [Google Scholar]
- 122.Asemi Z., Samimi M., Tabassi Z., Naghibi Rad M., Rahimi Foroushani A., Khorammian H., et al. Effect of daily consumption of probiotic yoghurt on insulin resistance in pregnant women: a randomized controlled trial. Eur J Clin Nutr. 2013;67(1):71–74. doi: 10.1038/ejcn.2012.189. [DOI] [PubMed] [Google Scholar]
- 123.Jafarnejad S., Saremi S., Jafarnejad F., Arab A. Effects of a multispecies probiotic mixture on glycemic control and inflammatory status in women with gestational diabetes: a randomized controlled clinical trial. J Nutr Metab. 2016;2016:5190846. doi: 10.1155/2016/5190846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Zheng Q.X., Jiang X.M., Wang H.W., Ge L., Lai Y.T., Jiang X.Y., et al. Probiotic supplements alleviate gestational diabetes mellitus by restoring the diversity of gut microbiota: a study based on 16S rRNA sequencing. J Microbiol. 2021;59(9):827–839. doi: 10.1007/s12275-021-1094-8. [DOI] [PubMed] [Google Scholar]
- 125.Homayouni A., Bagheri N., Mohammad-Alizadeh-Charandabi S., Kashani N., Mobaraki-Asl N., Mirghafurvand M., et al. Prevention of gestational diabetes mellitus (GDM) and probiotics: mechanism of action: a review. Curr Diabetes Rev. 2020;6(6):538–545. doi: 10.2174/1573399815666190712193828. [DOI] [PubMed] [Google Scholar]
- 126.Okesene-Gafa K.A., Moore A.E., Jordan V., McCowan L., Crowther C.A. Probiotic treatment for women with gestational diabetes to improve maternal and infant health and well-being. Cochrane Database Syst Rev. 2020;6(6):CD012970 doi: 10.1002/14651858.CD012970.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Davidson S.J., Barrett H.L., Price S.A., Callaway L.K., Dekker N.M. Probiotics for preventing gestational diabetes. Cochrane Database Syst Rev. 2021;4(4):CD009951 doi: 10.1002/14651858.CD009951.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Callaway L.K., McIntyre H.D., Barrett H.L., Foxcroft K., Tremellen A., Lingwood B.E., et al. Probiotics for the prevention of gestational diabetes mellitus in overweight and obese women: findings from the SPRING double-blind randomized controlled trial. Diabetes Care. 2019;42(3):364–371. doi: 10.2337/dc18-2248. [DOI] [PubMed] [Google Scholar]
- 129.Perez-Muñoz M.E., Arrieta M.-C., Ramer-Tait A.E., Walter J. A critical assessment of the “sterile womb” and “in utero colonization” hypotheses: implications for research on the pioneer infant microbiome. Microbiome. 2017;5(1):48. doi: 10.1186/s40168-017-0268-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Walker R.W., Clemente J.C., Peter I., Loos R.J.F. The prenatal gut microbiome: are we colonized with bacteria in utero? Pediatr Obes. 2017;12(Suppl 1):3–17. doi: 10.1111/ijpo.12217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Stinson L.F., Payne M.S., Keelan J.A. Planting the seed: Origins, composition, and postnatal health significance of the fetal gastrointestinal microbiota. Crit Rev Microbiol. 2017;43(3):352–369. doi: 10.1080/1040841X.2016.1211088. [DOI] [PubMed] [Google Scholar]
- 132.Ferretti P, Pasolli E, Tett A, Asnicar F, Gorfer V, Fedi S, et al. Mother-to-infant microbial transmission from different body sites shapes the developing infant gut microbiome. Cell Host Microbe 2018; 24(1):133–45.e5. [DOI] [PMC free article] [PubMed]
- 133.Wang K., Hu C., Tang W., Azad M.A.K., Zhu Q., He Q., et al. The enhancement of intestinal immunity in offspring piglets by maternal probiotic or synbiotic supplementation is associated with the alteration of gut microbiota. Front Nutr. 2021;8 doi: 10.3389/fnut.2021.686053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Hashemi A., Villa C.R., Comelli E.M. Probiotics in early life: a preventative and treatment approach. Food Funct. 2016;7(4):1752–1768. doi: 10.1039/c5fo01148e. [DOI] [PubMed] [Google Scholar]
- 135.Liu X., Li X., Xia B., Jin X., Zou Q., Zeng Z., et al. High-fiber diet mitigates maternal obesity-induced cognitive and social dysfunction in the offspring via gut-brain axis. Cell Metab. 2021;33(5):923–938. doi: 10.1016/j.cmet.2021.02.002. [DOI] [PubMed] [Google Scholar]
- 136.Kim S., Kim H., Yim Y.S., Ha S., Atarashi K., Tan T.G., et al. Maternal gut bacteria promote neurodevelopmental abnormalities in mouse offspring. Nature. 2017;549(7673):528–532. doi: 10.1038/nature23910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Gomez Arango L.F., Barrett H.L., Callaway L.K., Nitert M.D. Probiotics and pregnancy. Curr Diab Rep. 2015;15(1):567. doi: 10.1007/s11892-014-0567-0. [DOI] [PubMed] [Google Scholar]
- 138.Schultz M., Gottl C., Young R.J., Iwen P., Vanderhoof J.A. Administration of oral probiotic bacteria to pregnant women causes temporary infantile colonization. J Pediatr Gastroenterol Nutr. 2004;38(3):293–297. doi: 10.1097/00005176-200403000-00012. [DOI] [PubMed] [Google Scholar]
- 139.Fak F., Ahrne S., Molin G., Jeppsson B., Westrom B. Maternal consumption of Lactobacillus plantarum 299v affects gastrointestinal growth and function in the suckling rat. Br J Nutr. 2008;100(2):332–338. doi: 10.1017/S0007114507883036. [DOI] [PubMed] [Google Scholar]
- 140.Gueimonde M., Sakata S., Kalliomaki M., Isolauri E., Benno Y., Salminen S. Effect of maternal consumption of Lactobacillus GG on transfer and establishment of fecal bifidobacterial microbiota in neonates. J Pediatr Gastroenterol Nutr. 2006;42(2):166–170. doi: 10.1097/01.mpg.0000189346.25172.fd. [DOI] [PubMed] [Google Scholar]
- 141.Dunlop A.L., Mulle J.G., Ferranti E.P., Edwards S., Dunn A.B., Corwin E.J. Maternal microbiome and pregnancy outcomes that impact infant health: a review. Adv Neonatal Care. 2015;15(6):377–385. doi: 10.1097/ANC.0000000000000218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Nyangahu D.D., Jaspan H.B. Influence of maternal microbiota during pregnancy on infant immunity. Clin Exp Immunol. 2019;198:47–56. doi: 10.1111/cei.13331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.de Andrés J., Jiménez E., Chico-Calero I., Fresno M., Fernández L., Rodríguez J.M. Physiological translocation of lactic acid bacteria during pregnancy contributes to the composition of the milk microbiota in mice. Nutrients. 2017;10(1):14. doi: 10.3390/nu10010014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Bezirtzoglou E., Tsiotsias A., Welling G.W. Microbiota profile in feces of breast- and formula-fed newborns by using fluorescence in situ hybridization (FISH) Anaerobe. 2011;17:478–482. doi: 10.1016/j.anaerobe.2011.03.009. [DOI] [PubMed] [Google Scholar]
- 145.Huurre A., Kalliomaki M., Rautava S., Rinne M., Salminen S., Isolauri E. Mode of delivery-effects on gut microbiota and humoral immunity. Neonatology. 2008;93:236–240. doi: 10.1159/000111102. [DOI] [PubMed] [Google Scholar]
- 146.Schlinzig T., Johansson S., Stephansson O., Hammarström L., Zetterström R.H., von Döbeln U., et al. Surge of immune cell formation at birth differs by mode of delivery and infant characteristics – a population-based cohort study. PLoS ONE. 2017;12:1–14. doi: 10.1371/journal.pone.0184748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Gomez de Aguero M., Ganal-Vonarburg S.C., Fuhrer T., Rupp S., Uchimura Y., Li H., et al. The maternal microbiota drives early postnatal innate immune development. Science. 2016;351:1296–1302. doi: 10.1126/science.aad2571. [DOI] [PubMed] [Google Scholar]
- 148.Wang C.C., Tung Y.T., Chang H.C., Lin C.H., Chen Y.C. Effect of probiotic supplementation on newborn birth weight for mother with gestational diabetes mellitus or overweight/obesity: a systematic review and meta-analysis. Nutrients. 2010;12(11):3477. doi: 10.3390/nu12113477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Luoto R., Laitinen K., Nermes M., Isolauri E. Impact of maternal probiotic-supplemented dietary counselling on pregnancy outcome and prenatal and postnatal growth: a double-blind, placebo-controlled study. Br J Nutr. 2010;103(12):1792–1799. doi: 10.1017/S0007114509993898. [DOI] [PubMed] [Google Scholar]
- 150.Mantaring J., Benyacoub J., Destura R., Pecquet S., Vidal K., Volger S., et al. Effect of maternal supplement beverage with and without probiotics during pregnancy and lactation on maternal and infant health: a randomized controlled trial in the Philippines. BMC Pregnancy Childbirth. 2018;18(1):193. doi: 10.1186/s12884-018-1828-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Lan R., Kim I. Enterococcus faecium supplementation in sows during gestation and lactation improves the performance of sucking piglets. Vet Med Sci. 2020;6(1):92–99. doi: 10.1002/vms3.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Alexopoulos C., Karagiannidis A., Kritas S.K., Boscos C., Georgoulakis I.E., Kyriakis S.C. Field evaluation of a bioregulator containing live Bacillus cereus spores on health status and performance of sows and their litters. J Vet Med A Physiol Pathol Clin Med. 2001;48(3):137–145. doi: 10.1046/j.1439-0442.2001.00342.x. [DOI] [PubMed] [Google Scholar]
- 153.Cuello-Garcia C.A., Brożek J.L., Fiocchi A., Pawankar R., Yepes-Nuñez J.J., Terracciano L., et al. Probiotics for the prevention of allergy: A systematic review and meta-analysis of randomized controlled trials. J Allergy Clin Immunol. 2015;36(4):952–961. doi: 10.1016/j.jaci.2015.04.031. [DOI] [PubMed] [Google Scholar]
- 154.Zuccotti G., Meneghin F., Aceti A., Barone G., Callegari M.L., Di Mauro A., et al. Probiotics for prevention of atopic diseases in infants: systematic review and meta-analysis. Allergy. 2015;70(11):1356–1371. doi: 10.1111/all.12700. [DOI] [PubMed] [Google Scholar]
- 155.Rautava S., Kainonen E., Salminen S., Isolauri E. Maternal probiotic supplementation during pregnancy and breast-feeding reduces the risk of eczema in the infant. J Allergy Clin Immunol. 2012;130(6):1355–1360. doi: 10.1016/j.jaci.2012.09.003. [DOI] [PubMed] [Google Scholar]
- 156.Azad M.B., Coneys J.G., Kozyrskyj A.L., Field C.J., Ramsey C.D., Becker A.B., et al. Probiotic supplementation during pregnancy or infancy for the prevention of asthma and wheeze: systematic review and meta-analysis. BMJ. 2013;347 doi: 10.1136/bmj.f6471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Szajewska H., Horvath A. Lactobacillus rhamnosus GG in the primary prevention of eczema in children: a systematic review and meta-analysis. Nutrients. 2018;10(9):1319. doi: 10.3390/nu10091319. [DOI] [PMC free article] [PubMed] [Google Scholar]