Abstract
Aim
The aim of this study was to describe the survival and neurological outcome in patients with OHCA treated with and without mechanical circulatory support (MCS).
Methods
This was a retrospective observational cohort study on patients with OHCA admitted to Aarhus University Hospital, Denmark, between January 2015 and December 2019. Kaplan-Meier estimates were used to evaluate 30-day and 30–180-day survival. Cox regression analysis was used to assess the association between covariates and one-year mortality.
Results
Among 1,015 patients admitted, 698 achieved return of spontaneous circulation (ROSC) before admission, 101 patients with refractory OHCA received mechanical circulatory support (MCS) and the remaining 216 patients with refractory OHCA did not receive MCS treatment. Survival to hospital discharge was 47% (478/1015). Good neurological outcome defined as Cerebral Performance Categories 1–2 were seen among 92% (438/478) of the patients discharged from hospital. Median low-flow was 15 [8–22] minutes in the ROSC group and 105 [94–123] minutes in the MCS group. Mortality rates were high within the first 30 days, however; 30–180-day survival in patients discharged remained constant over time in both patients with ROSC on admission and patients admitted with MCS. Advanced age > 70 years (hazard ratio (HR) 1.98, 95% confidence interval (CI) 1.11–3.49), pulseless electrical activity (HR 2.39, 95% CI 1.25–4.60) and asystole HR 2.70, 95% CI 1.25–5.95) as initial rhythms were associated with one-year mortality in patients with ROSC.
Conclusions
Short-term survival rates were high among patients with ROSC and patients receiving MCS. Among patients who survived to day 30, landmark analyses showed comparable 180-day survival in the two groups despite long low-flow times in the MCS group. Advanced age and initial non-shockable rhythms were independent predictors of one-year mortality in patients with ROSC on admission.
Keywords: Mechanical circulatory support, Out-of-hospital cardiac arrest, Extracorporeal cardiopulmonary resuscitation, Neurological outcome, Impella
Abbreviations: CPC, Cerebral performance category; CPR, Cardiopulmonary resuscitation; ECMO, Extracorporeal membrane oxygenation; ECPR, Extracorporeal cardiopulmonary resuscitation; EMS, Emergency medical service; ICU, Intensive care unit; MCS, Mechanical circulatory support; OHCA, Out-of-hospital cardiac arrest; PCI, Percutaneous coronary intervention; ROSC, Return of spontaneous circulation; V-A, Veno-arterial; VF, Ventricular fibrillation; VT, Ventricular tachycardia
Introduction
Despite significant improvements in the management of out-of-hospital cardiac arrest (OHCA), survival rates remain dismal across various geographical settings.1., 2., 3. In recent years, a growing body of literature has supported centralisation of post-resuscitation care to achieve higher survival rates with good neurological outcomes.4 Furthermore, distance to a cardiac centre was not shown to be associated with survival, indicating the safety of bypassing local hospitals and direct triage to a tertiary centre.5 In Denmark, the concept of direct referral of OHCA to dedicated cardiac arrest centres has emerged as a standard procedure. Currently, in the Central Denmark Region, all OHCAs without obvious non-cardiac aetiology are triaged to the catheterisation laboratory at Aarhus University Hospital, a tertiary centre with access to 24/7 primary percutaneous coronary intervention (PCI), cardiothoracic assistance and treatment for refractory cardiac arrest with mechanical circulatory support (MCS) with either Impella or V-A extracorporeal membrane oxygenation (V-A ECMO). The latter also termed as extracorporeal cardiopulmonary resuscitation (ECPR).
The objective of this study was to describe, at a population-based level, prehospital and in-hospital characteristics, survival and neurological outcomes in three groups of adult patients with OHCA: 1) Patients with ROSC on admission, 2) Patients admitted with refractory OHCA treated with MCS) and 3) Patients admitted with refractory OHCA not treated with MCS. Furthermore, for patients with ROSC the aim was to evaluate the impact of various covariates on one-year mortality.
Methods
This was a retrospective observational cohort study of all adult OHCA patients (≥18 years) admitted directly to the catheterisation laboratory at Aarhus University Hospital from January 2015 to December 2019. The study complies with the principles of the Declaration of Helsinki. The study was approved by the Danish Data Protection Agency (Ref. no. 1-16-02-383-18) and the Danish Patient Safety Authority Services (Ref. no. 1-45-70-34-21). Further ethical approval was exempt due to the retrospective nature of the study. Results are reported in line with the STROBE guidelines.
Study population and data collection
All OHCA patients were identified from local catheterisation laboratory lists and cross-referred with the Danish Cardiac Arrest Registry6 and the emergency medical service (EMS) logistics systems using the unique Danish ten-digit personal identification number in combination with the date and time of the cardiac arrest. The following core data elements from the Utstein template were collected from the prehospital logistics systems and validated with the Danish Cardiac Arrest Registry7: date and time of arrest, witnessed arrest, bystander CPR, initial cardiac rhythm, time of CPR, mechanical CPR and time of return of spontaneous circulation (ROSC). In-hospital data on patient demographics, comorbidities, invasive procedures, level of intensive care and outcomes were obtained from patient records and the Western Denmark Heart Registry. A part of the study population has been published previously.8
Prehospital management
The Central Denmark Region is one of five Danish administrative regions. The region covers 13,000 square kilometres of rural and urban area and has a population of approximately 1.3 million inhabitants. The Central Denmark Region manages nine somatic hospitals among which Aarhus University Hospital functions as the tertiary cardiac care hospital with access to 24/7 cardiac catheterisation service, ECPR, targeted temperature management (TTM) and advanced procedures for neuroprognostication. Currently, Aarhus University Hospital is the only invasive centre in the region.
The EMS of the Central Denmark Region dispatches the appropriate level of care for all incoming 1-1-2 emergency calls from the region. Prehospital care is provided by the prehospital personnel in accordance with current guidelines of the European Resuscitation Council.9 Prehospital critical care physicians facilitate advanced life support, prehospital triage and, and when deemed appropriate, transport of patients with ongoing CPR with mechanical chest compression. Patients with refractory OHCA are considered eligible for MCS if conventional CPR does not result in ROSC after 15 minutes, and meet the following criteria: Age 18-65 years, witnessed arrest, bystander CPR and initial shockable rhythms (in selected cases pulseless electrical activity [PEA]). Contraindications include severe comorbidity, initial presenting rhythm with asystole, no-flow > 10 minutes, end-tidal CO2 < 1.3 kPa, pH < 6.8 and lactate > 15 mmol/L (Supplementary S1). Telemedical communication is effectuated with the tertiary centre including a 12-lead electrocardiogram (ECG). If the cardiac arrest is presumed to be of cardiac or unknown origin, the patients are triaged directly to the catheterisation laboratory. Patients with cardiac arrest of obvious non-cardiac aetiology are transported to local hospitals, and traumatic cardiac arrest patients are referred to the trauma centre at Aarhus University Hospital. In case of treatment futility, physicians terminate resuscitation efforts on scene.
In-hospital management
Patients with refractory OHCA eligible for MCS are evaluated by a team of anaesthesiologists, thoracic surgeons, invasive and non-invasive cardiologists. Upon arrival at the catheterisation laboratory, echocardiography is performed to verify cardiac activity. If intermittent ROSC and/or overall deteriorating haemodynamics or the ECMO team did not find the patient eligible for V-A ECMO (advanced age or severe comorbidity), Impella may be chosen as first treatment option, however in the vast majority of patients V-A ECMO is considered as the principal management for refractory OHCA. In patients receiving V-A ECMO but not achieving optimal unloading, Impella is deployed concurrently or within 24 hours after V-A ECMO commencement. V-A ECMO is inserted percutaneously by Seldinger technique and ultrasound guidance is recommended as well as establishment of distal perfusion to avoid critical limb ischemia. After echocardiography, coronary and pulmonary angiography are performed to confirm or diagnose the underlying cause of cardiac arrest. PCI is done if necessary. Additional imaging with whole-body computed tomography scan is effectuated if required. Post-resuscitation care encompassed TTM during the study period. In comatose survivors, neuroprognostication including protocolised use of neurospecific enolase (NSE), neurological assessment, electroencephalography and magnetic resonance imaging (MRI) are performed routinely in the intensive care unit (ICU). Protocolised weaning is done in patients receiving ECPR.10
Statistical analysis
Patients were classified into three groups (1) ROSC upon arrival at the catheterisation laboratory, 2) refractory OHCA treated with MCS and 3) refractory OHCA not treated with MCS). Categorical variables are presented as absolute values and percentages; continuous variables, as median and interquartile range (IQR) with 25th − 75th percentiles. Kaplan-Meier plots were used to study 30-day and 30-day – 180-day survival (landmark analysis). Predictors of one-year mortality were assessed using univariate and multivariate Cox proportional hazards regression analyses for patients with ROSC on admission. Patients who died within the first five days of hospitalisation were excluded from this analysis in order to be able to distinguish between patients with and without treatment futility upon arrival. We tested the model fit using residual analysis and the proportional hazards assumptions using the log-log survival function. Hazard ratios (HRs) with 95% confidence intervals (CIs) are reported. Selection of risk factors was based on previous literature and clinical relevance. All statistical tests were two-sided, and a p-value below 0.05 was considered statistically significant. All analyses were performed with STATA/BE 17 for Mac, College Station, TX 77845, USA and GraphPad Prism 9.
Outcome measures
The primary outcome was defined as survival at discharge in the tree predefined groups, and neurological outcome at discharge. A good neurological outcome was expressed as Cerebral Performance Categories (CPC) 1 and 2, as previously used in cardiac arrest studies.11 Secondary outcomes included 30- and 30-180-day survival outcomes between patients with ROSC upon arrival and patients with refractory OHCA treated with MCS, and predictors of one-year mortality in the ROSC group.
Results
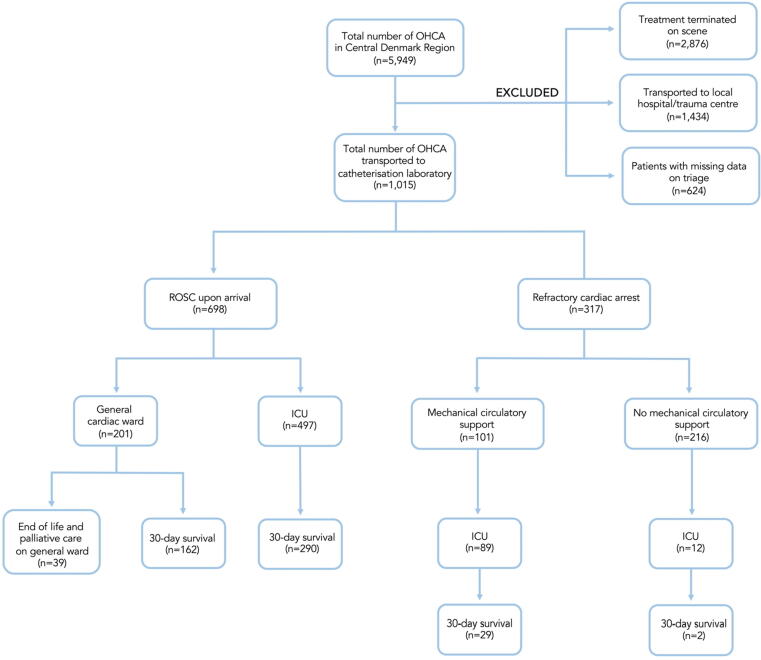
From 1 January 2015 to 31 December 2019, a total of 5,949 patients with OHCA were identified in the Central Denmark Region. Among these, 2,876 were terminated on scene and 1,434 were transported to either local hospitals or referred to the trauma centre (Fig. 1). Direct admission to the catheterisation laboratory at Aarhus University Hospital was seen in 1,015 patients. A total of 698 patients arrived with sustained ROSC. Among 317 patients with refractory OHCA, MCS was commenced in 101 patients. In the remaining 216 patients with refractory OHCA, ROSC was achieved in 12 patients and resuscitation attempts were withdrawn in the catheterisation laboratory in 204 patients. Overall, 47% (478/1,015) survived to hospital discharge. A good neurological outcome with CPC 1-2 was seen among 92% (438/478) of the patients discharged from hospital.
Fig. 1.
Flow diagram of study population in the Central Denmark Region 2015–2019. Abbreviations: OHCA out-of-hospital cardiac arrest, ROSC return of spontaneous circulation, ICU intensive care unit, MCS mechanical circulatory support.
Patient demographics
The baseline patient characteristics are presented in Table 1. The overall median age was 66 (IQR, 55–74) years. Patients with ROSC on arrival were older than patients with refractory OHCA receiving MCS (67 (IQR, 56–75) versus 56 (IQR 46–62) years). The proportion of males was similar in all three groups. The ROSC group had a higher rate of witnessed arrest and more often presented with initial shockable rhythm with ventricular tachycardia/fibrillation (VT/VF) than patients with refractory OHCA. Acute myocardial infarction was the most common aetiology of arrest, with a greater proportion in the ROSC group and MCS group than in the refractory OHCA without MCS group (45%, 55% and 26%, respectively).
Table 1.
Baseline characteristics of patients with out-of-hospital cardiac arrest admitted to Aarhus University Hospital.
| Parameter | ROSC on admission (n = 698) | Refractory OHCA with MCS (n = 101) | Refractory OHCA without MCS (n = 216) |
|---|---|---|---|
| Age (years) | 67 [56–75] | 56 [46–62] | 67 [57–74] |
| Age ≤ 65 years | 314 (45) | 85 (84) | 100 (46) |
| Sex (male) | 533 (76) | 81 (80) | 160 (77) |
| Comorbidities | |||
| Ischaemic heart disease | 131 (19) | 14 (14) | 42 (19) |
| Hypertension | 240 (34) | 23 (23) | 62 (29) |
| Diabetes | 90 (13) | 11 (10) | 30 (14) |
| CKD | 23 (3) | 2 (2) | 13 (6) |
| COLD | 86 (12) | 1 (1) | 20 (9) |
| Witnessed arrest | 624 (89) | 84 (83) | 181 (84) |
| Bystander CPR | 645 (92) | 99 (98) | 192 (89) |
| First monitored rhythm | |||
| VF/VT | 501 (72) | 61 (60) | 82 (39) |
| PEA | 103 (15) | 26 (26) | 69 (32) |
| Asystole | 90 (13) | 14 (14) | 63 (29) |
| Pre-hospital defibrillation | 539 (77) | 69 (68) | 105 (49) |
| Signs of life during CPR | 331 (47) | 36 (36) | 28 (13) |
| Time stamps | |||
| No-flow (min) | 0 [0–2] | 0 [0–2] | 0 [0–2] |
| No-flow > 10 min | 39 (6) | 6 (6) | 24 (11) |
| Time from arrest to arrival at catheterisation laboratory | 71 [55–90] | 75 [60–88] | 62 [47–81] |
| Total low-flow (min) | 15 [8–22] | 105 [94–123] | 79 [63–103] |
| Cardiac arrest cause | |||
| AMI | 315 (45) | 56 (55) | 57 (26) |
| Primary arrhythmia | 252 (36) | 18 (18) | 29 (13) |
| Pulmonary embolism | 15 (2) | 10 (10) | 12 (6) |
| Aortic dissection | 5 (1) | 1 (1) | 16 (7) |
| Neurological | 16 (2) | 3 (3) | 2 (1) |
| Respiratory | 49 (7) | 0 (0) | 8 (4) |
| Toxic | 11 (2) | 6 (6) | 1 (1) |
| Other | 7 (1) | 3 (3) | 2 (1) |
| cUnknown | 28 (4) | 4 (4) | 89 (41) |
Data are presented as median and interquartile ranges and absolute number and percentages.
Abbreviations: AMI acute myocardial infarction, CKD chronic kidney disease, COLD chronic obstructive lung disease, CPR cardiopulmonary resuscitation, MCS mechanical circulatory support, PEA pulseless electrical activity, OHCA out-of-hospital cardiac arrest, ROSC return of spontaneous circulation, VF ventricular fibrillation, VT ventricular tachycardia.
In-hospital data
In patients with refractory OHCA, V-A ECMO was commenced as the primary treatment in 93 patients, support with only Impella was seen in four patients, and concomitant treatment was deployed in four patients (Table 2). The most coherent reasons for not deploying MCS in refractory OHCA were severe comorbidities, advanced age, poor initial blood gas analysis upon arrival, low end-tidal CO2 and long no-flow times. Acute coronary angiography was performed in 79% (800/1,015) of the patients; immediate PCI, in 57% (455/800) (Table 2). In a few patients, PCI was not performed despite significant stenoses but scheduled later during admission if the patient recovered from the arrest. The left anterior descending artery (LAD) was most frequently found to be the culprit vessel. A total of 59% (598/1,015) of the patients were admitted to the ICU among whom 75% received TTM. Treatment was terminated in 12 patients receiving MCS before ICU admission: device failure (n = 4), extensive bleeding due to liver or spleen rupture as a complication to mechanical chest compression (n = 4), severe brain injury/stroke (n = 3) and aortic dissection (n = 1). In patients with ROSC on admission, 201 (29%) were admitted to the general cardiac ward, however in 39 patients, further active treatment was deemed futile and these patients received end of life and palliative care.
Table 2.
Outcomes measures of patients with out-of-hospital cardiac arrest admitted to Aarhus University Hospital.
| Parameter | ROSC on admission (n = 698) | Refractory OHCA with MCS (n = 101) | Refractory without MCS (n = 216) |
|---|---|---|---|
| V-A ECMO only | 1 (0.14) | 93 (92) | - |
| Impella only | 14 (2) | 4 (4) | - |
| V-A ECMO + Impella | 1 (0.14) | 4 (4) | - |
| Impella for unloading | 1 (100) | 2 (50) | - |
| CAG | 661 (95) | 93 (92) | 46 (21) |
| PCI | 355 (51) | 76 (75) | 24 (11) |
| Culprit | |||
| LM | 20 (6) | 11 (15) | 5 (21) |
| LAD | 169 (48) | 48 (63) | 10 (42) |
| LCX | 81 (23) | 3 (4) | 1 (4) |
| RCA | 84 (24) | 13 (13) | 8 (33) |
| Graft | 1 (0.3) | 1 (1) | 0 (0) |
| Treatment termination before ICU | 23 (3) | 12 (12) | 204 (94) |
| Hospital length of stay (days) | 8 [4–15] | 2 [0–21] | 0 [0-0] |
| No. of patients admitted to general cardiac ward | 201(29) | - | - |
| Hospital length of stay (days) | 6 [3–10] | - | - |
| No. of patients admitted to ICU | 497 (71) | 89 (88) | 12 (6) |
| TTM | 361 (72) | 86 (97) | 1 (50) |
| CRRT | 23 (5) | 29 (26) | 1 (8) |
| ICU length of stay (days) | 2 [1–5] | 11 [6–19] | 6 [2–26] |
| Complications to MCS | |||
| Bleeding at cannulation site | - | 30 (30) | - |
| Gastrointestinal bleeding | - | 10 (10) | - |
| Gastrointestinal ischaemia | - | 6 (6) | - |
| Limb ischaemia | - | 5 (5) | - |
| Ischaemic stroke | - | 2 (2) | - |
| Outcome data | |||
| 30-day survival | 451 (65) | 29 (29) | 2 (1) |
| Hospital discharge | 449 (64) | 27 (27) | 2 (1) |
| Neurological outcome at discharge | |||
| CPC 1 | 291 (42) | 17 (17) | - |
| CPC 2 | 122 (17) | 8 (8) | 1 (0.5) |
| CPC 3 | 27 (4) | 2 (2) | - |
| CPC 4 | 2 (0.3) | 1 (1) | - |
| CPC 5 | 248 (36) | 73 (73) | 215 (99.5) |
| Unknown | 8 (1) | - | - |
| One-year survival | 430 (62) | 24 (24) | 2 (1) |
Data are presented as median and interquartile ranges and absolute number and percentages.
Abbreviations: CAG coronary angiography, CPC cerebral performance category, CRRT continuous renal replacement therapy, ICU intensive care unit, LAD left anterior descending artery, LCX left circumflex artery, LM left main artery, MCS mechanical circulatory support, PCI percutaneous coronary intervention, RCA right coronary artery, ROSC return of spontaneous circulation, TTM targeted temperature management, V-A ECMO veno-arterial extracorporeal membrane oxygenation.
Study outcomes
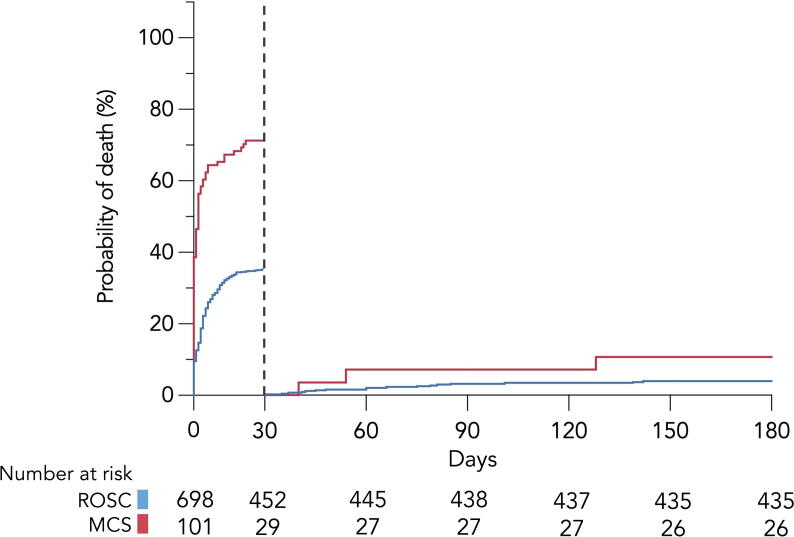
The proportion of patients surviving to hospital discharge was 64% in the ROSC group, 27% in the refractory OHCA with MCS group and 1% in refractory OHCA without MCS group. CPC 1-2 was 92%, 93% and 50% among patients discharged alive, respectively. The 30-day and 30–180-day mortality trends are shown in Fig. 2 for the ROSC group and MCS group. Kaplain-Meier survival curves demonstrated significant difference in survival between groups at day 30 (p < 0.001). The vast majority of patients who were alive on day 30 also survived to the 180-day follow-up in both groups. Survival rates at day 180 were similar for both groups (p = 0.072). Termination of treatment in the ICU was mainly caused by severe brain injury after arrest, multiorgan failure and isolated heart failure.
Fig. 2.
Kaplan-Meier curves showing 30-day mortality and landmark analyses (30–180-day mortality) stratified by group. Abbreviations: ROSC return of spontaneous circulation, MCS mechanical circulatory support.
Low-flow times and neurologically intact survival at discharge
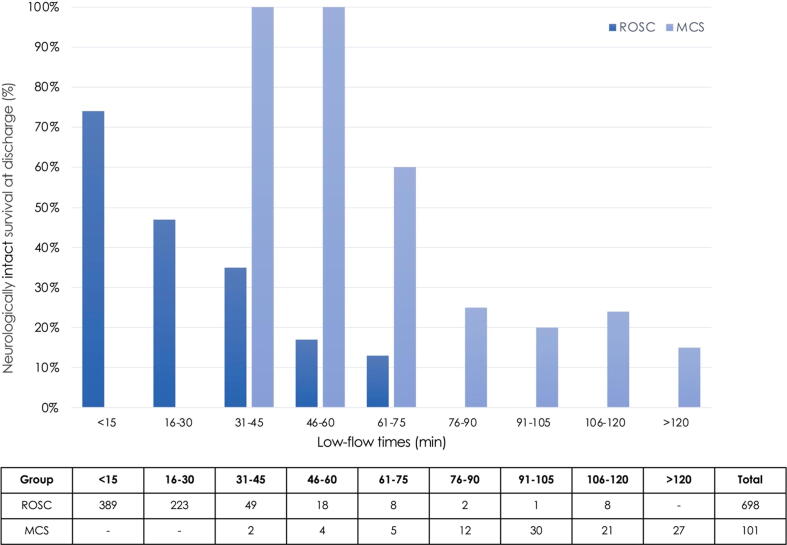
Fig. 3 shows the association between various low-flow times and a good neurological outcome (CPC 1-2) at discharge between the ROSC and the MCS group. No patient survived after low-flow > 75 minutes in the ROSC group. However, > 20% of patients receiving MCS for refractory OHCA still had a good neurological and functional recovery despite low-flow times > 75 min.
Fig. 3.
Association between low-flow times and neurologically intact survival at discharge in patients with return of spontaneous circulation on admission and patients with refractory cardiac arrest treated with mechanical circulatory support. Abbreviations: ROSC return of spontaneous circulation, MCS mechanical circulatory support.
Factors associated with one-year mortality for patients with ROSC on admission
Table 3 presents the univariate and multivariate Cox regression analysis for patients with ROSC upon arrival who had survived to day six. Advanced age > 70 years (HR 1.98, 95% CI 1.11–3.49) and initial non-shockable rhythm (PEA HR 2.39, 95% CI 1.25–4.60, asystole HR 2.70, 95% CI 1.25–5.95) were independently associated with an increased risk of one-year mortality.
Table 3.
Uni- and multivariate Cox regression analysis for one-year mortality for patients with ROSC on admission and at alive after five days of hospitalisation.
| Characteristics | Univariate |
Multivariate |
||
|---|---|---|---|---|
| HR | 95% CI | HR | 95% CI | |
| Age ≥ 70 years | 2.84 | 1.87–4.27 | 1.98 | 1.11–3.49 |
| Witnessed arrest | 0.44 | 0.25–0.76 | 0.94 | 0.44–2.04 |
| Bystander CPR | 0.32 | 0.18–0.59 | 0.49 | 0.22–1.09 |
| No-flow > 10 minutes | 2.61 | 1.26–5.53 | 0.97 | 0.58–1.60 |
| Initial presenting rhythm | ||||
| VT/VF* | Reference | - | Reference | - |
| PEA | 4.44 | 2.74–7.17 | 2.39 | 1.25–4.60 |
| Asystole | 6.20 | 3.63–10.57 | 2.70 | 1.23–5.95 |
| Signs of life during CPR | 0.09 | 0.07–0.13 | 0.16 | 0.11–0.24 |
Data are presented as hazard ratios (HR) and 95% confidence intervals (CI).
*Reference.
Bold font indicates statistical significance (p < 0.05).
Abbreviations: CPR cardiopulmonary resuscitation, PEA pulseless electrical activity, VF ventricular fibrillation, VT ventricular tachycardia.
Discussion
The present study sought to assess the performance of a tertiary cardiac arrest centre regarding survival and neurological outcome in patients admitted with OHCA. The results revealed a hospital discharge rate of 64% in patients admitted with ROSC and 27% in patients admitted with refractory OHCA receiving MCS. Survival at discharge was 1% in patients with refractory OHCA without MCS. A good neurological outcome was found in 92% of patients discharged from hospital in the ROSC group and 93% of patients discharged in the MCS group. Among patients surviving to day 30, a similar 180-day survival was observed for patients admitted with ROSC and patients with refractory OHCA treated with MCS. Advanced age ≥ 70 years and initial non-shockable rhythms were independently associated with one-year mortality in patients admitted with ROSC.
Our data demonstrated a hospital discharge rate of 64% with a good probability of a favourable neurological outcome in patients achieving ROSC in the field. This is remarkably high compared with the literature.12., 13., 14., 15. This may be explained by the very selected patient population referred to our tertiary centre with high rates of known prognostic factors in OHCA: witnessed arrest, bystander CPR and initial shockable rhythms with immediate prehospital defibrillation resulting in short low-flow times. Furthermore, referral to our centre was presumed to be of cardiac origin. Shockable rhythms in OHCA are often associated with ischaemic heart disease and patients with ischaemic heart disease may benefit the most from early access to PCI and intensive cardiac care.16., 17., 18., 19. This is in line with our data, as the majority of patients presenting shockable rhythms did have acute myocardial infarction or severe coronary artery disease in need of PCI. However, we did not distinguish between ST-elevation and non-ST-elevation myocardial infarction in our study, and our data cannot be used to assess the optimal timing of angiography in patients without ST-segment elevation. In the recent TOMAHAWK study, the strategy of performing early angiography in successfully resuscitated OHCA without ST-segment elevation did not benefit patients compared with delayed angiography measured by 30-day mortality (HR 1.25, 95% CI 1.00–1.63, p = 0.06).20 Thus, the high survival rate observed in our analysis most likely reflects the combination of many favourable pre-arrest factors and an assembly of advanced treatment facilities offered to this subpopulation.
A poor survival outcome was seen in patients with refractory OHCA not treated with MCS. The majority of these patients presented initial non-shockable rhythms, and non-cardiac causes were more often observed in this subgroup than in other subgroups. Historically, patients presenting with PEA or asystole were reported to have an overall dismal prognosis.21., 22.
In recent years, high-impact literature on the use of ECPR for refractory OHCA has surfaced as two randomized clinical trials and a meta-analysis have been conducted.23., 24., 25. Conversely, evidence for Impella in refractory OHCA is largely limited to case-series and observational single-centre experiences.26., 27., 28. Latest guidelines dot not recommend routine use of V-A ECMO in refractory OHCA; however, ECMO may be considered in selected patients with a potentially reversible cause of arrest.9., 29. Use of Impella for refractory OHCA has not yet been addressed in any guidelines. Nevertheless, considering the growing utilisation of MCS in OHCA with or without refractoriness, it is important to assess the impact of MCS on survival.30 In our cohort, the overall survival at hospital discharge in patients with refractory OHCA treated with MCS was 27%, and a high proportion of these patients had a favourable neurological outcome at discharge. This is consistent with the results of the Extracorporeal Life Support Organization.31 Due to the limited number of Impella cases in our cohort, our data did not allow us to determine which mechanical support device had the greatest impact on outcome. A study from the CARES surveillance group showed that the use of Impella in OHCA was associated with improved survival in an unadjusted analysis (OR = 2.07, 95% CI [1.55–2.77]), however this effect was diminished in the multivariable model (OR = 1.72, 95% CI [0.95–3.06]).32 Although the study used a large dataset from state-wide registries, the results were limited by the low frequency of MCS use in their patient population.
Our setup for refractory OHCA in the Central Denmark Region with a bundle of advanced treatment options, including fast EMS transport to cardiac arrest centre, ECPR and early initiation of invasive strategies, is very similar to the recently conducted Prague OHCA Study.23 This study compared a hyper-invasive OHCA-group (n = 124) with a standard advanced cardiac life support group (ALCS) (n = 132). The authors found no statistical difference in the primary outcome of survival with functional recovery at six months (31% versus 22%, p = 0.09). However, the study was terminated prematurely, which may indicate that the study was possibly underpowered to detect a clinically relevant difference.
An unexplored outcome in patients rescued by MCS is long-term survival and neurological outcome. In the ARREST trial, the cumulative six-month survival was significantly better in the group treated with V-A ECMO than in the ACLS group (HR 0.16, 95% CI 0.006–0.41, p = 0.0001).24 A systematic review of 530 ECPR-treated patients demonstrated that ECPR compared with no ECPR was associated with improved long-term neurologically intact survival after cardiac arrest (relative risk (RR) 3.11, 95% CI 2.06–4.69, p < 0.00001).33 In our cohort, the highest mortality rates among the MCS group were seen in the first 30 days of hospitalisation. Nevertheless, the rates of mortality declined with time parallel with the ROSC group. This finding suggests that overall survival over time in the MCS group is comparable with that seen in the patients who have the highest chance of survival. This is particularly interesting considering the long low-flow times observed in the MCS group. Generally, long low-flow times have been associated with poor survival.34., 35. Our data do not include long-term neurological outcome. Conversely, evidence exists that neurological outcomes evolve over time. The vast majority of patients in this study were discharged with a CPC 1-2. Thus, long-term neurological outcome likely improved over time in survivors in this cohort.
Limitations
The present study was limited by its retrospective observational character. Furthermore, this was a single-centre study with a highly selected patient population, which limits its generalisability. We were unable to provide data from local hospitals or detailed data on patients in whom resuscitation was terminated on scene. Long-term neurologically intact survival was not accessed in this study. This matter is of utter importance to prove the impact of MCS. Survival does not sufficiently characterise success of MCS. Thus, measures of functional status and healthcare related quality-of-life measures should be reported in this group in future trials.
Conclusion
A high survival rate with a favourable neurological outcome at discharge was observed in patients admitted with sustained ROSC and patients with refractory OHCA treated with MCS. Mortality rates were high within the first 30 days of admission. However, among patients who survived to day 30, survival was comparable at day 180 in the two groups despite long low-flow times in the MCS group. The use of MCS in selected patients with refractory OHCA may be associated with good short-term and long-term survival.
Availability of data and materials
The data that support the findings of this study are available from the Danish Data Protection Agency but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are however available from the authors upon reasonable request and with permission of the Danish Data Protection Agency and the Danish Patient Safety Authority Services.
Conflicts of interest
SRM report funding from Aarhus University; Henry og Astrid Møllers Fond; Aase og Ejnar Danielsens Fond; Snedkermester Sophus Jacobsen and hustru Astrid Jacobsens Fond; and speakers honoraria from Getinge. The remaining authors have nothing do declare.
CRediT authorship contribution statement
SRM conceived and designed the study. SRM acquired all data. All authors contributed in the statistical analysis and interpretation of data. CJT supervised the study. SRM drafted the first manuscript. All authors critically revised the manuscript for intellectual content and agree to be accountable for all aspects of the work. All authors approved the final version of the manuscript to be published.
Acknowledgements
This study was funded by Aarhus University; Henry og Astrid Møllers Fond; Aase og Ejnar Danielsens Fond; Snedkermester Sophus Jackobsen og hustru Astrid Jacobsens Fond to Dr. SRM. None of the mentioned sources participated in the study design, collection, analysis or interpretation of data, writing of the manuscript or final decision to submit the manuscript for publication.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.resplu.2022.100230.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- 1.Rea T.D., Eisenberg M.S., Becker L.J., Murray J.A., Hearne T. Temporal trends in sudden cardiac arrest: a 25-year emergency medical services perspective. Circulation. 2003;107:2780–2785. doi: 10.1161/01.CIR.0000070950.17208.2A. [DOI] [PubMed] [Google Scholar]
- 2.Yan S., Gan Y., Jiang N., et al. The global survival rate among adult out-of-hospital cardiac arrest patients who received cardiopulmonary resuscitation: a systematic review and meta-analysis. Crit Care. 2020;24:61. doi: 10.1186/s13054-020-2773-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berdowski J., Berg R.A., Tijssen J.G., Koster R.W. Global incidences of out-of-hospital cardiac arrest and survival rates: Systematic review of 67 prospective studies. Resuscitation. 2010;81:1479–1487. doi: 10.1016/j.resuscitation.2010.08.006. [DOI] [PubMed] [Google Scholar]
- 4.Kajino K., Iwami T., Daya M., et al. Impact of transport to critical care medical centers on outcomes after out-of-hospital cardiac arrest. Resuscitation. 2010;81:549–554. doi: 10.1016/j.resuscitation.2010.02.008. [DOI] [PubMed] [Google Scholar]
- 5.Tranberg T., Lippert F.K., Christensen E.F., et al. Distance to invasive heart centre, performance of acute coronary angiography, and angioplasty and associated outcome in out-of-hospital cardiac arrest: a nationwide study. Eur Heart J. 2017;38:1645–1652. doi: 10.1093/eurheartj/ehx104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ringgren KB, Christensen HC, Schønau L, et al. Danish Cardiac Arrest Registry 2001-2018. (2018); 22 Mar 2021: https://hjertestopregister.dk/wp-content/uploads/2019/11/Dansk-Hjertestopregister-2018-2.pdf.
- 7.Perkins G.D., Jacobs I.G., Nadkarni V.M., et al. Cardiac arrest and cardiopulmonary resuscitation outcome reports: update of the Utstein Resuscitation Registry Templates for Out-of-Hospital Cardiac Arrest: a statement for healthcare professionals from a task force of the International Liaison Committee on Resuscitation (American Heart Association, European Resuscitation Council, Australian and New Zealand Council on Resuscitation, Heart and Stroke Foundation of Canada, InterAmerican Heart Foundation, Resuscitation Council of Southern Africa, Resuscitation Council of Asia); and the American Heart Association Emergency Cardiovascular Care Committee and the Council on Cardiopulmonary, Critical Care, Perioperative and Resuscitation. Circulation. 2015;132:1286–1300. doi: 10.1161/CIR.0000000000000144. [DOI] [PubMed] [Google Scholar]
- 8.Mork S.R., Stengaard C., Linde L., et al. Mechanical circulatory support for refractory out-of-hospital cardiac arrest: a Danish nationwide multicenter study. Crit Care. 2021;25:174. doi: 10.1186/s13054-021-03606-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Soar J., Bottiger B.W., Carli P., et al. European Resuscitation Council Guidelines 2021: Adult advanced life support. Resuscitation. 2021;161:115–151. doi: 10.1016/j.resuscitation.2021.02.010. [DOI] [PubMed] [Google Scholar]
- 10.Mork S.R., Frederiksen C.A., Nielsen R.R., et al. A systematic approach to weaning from extracorporeal membrane oxygenation in patients with refractory cardiac failure. Acta Anaesthesiol Scand. 2021;65:936–943. doi: 10.1111/aas.13814. [DOI] [PubMed] [Google Scholar]
- 11.Jennett B., Bond M. Assessment of outcome after severe brain damage. Lancet. 1975;1:480–484. doi: 10.1016/s0140-6736(75)92830-5. [DOI] [PubMed] [Google Scholar]
- 12.Wampler D.A., Collett L., Manifold C.A., Velasquez C., McMullan J.T. Cardiac arrest survival is rare without prehospital return of spontaneous circulation. Prehosp Emerg Care. 2012;16:451–455. doi: 10.3109/10903127.2012.695435. [DOI] [PubMed] [Google Scholar]
- 13.Komatsu T., Kinoshita K., Sakurai A., et al. Shorter time until return of spontaneous circulation is the only independent factor for a good neurological outcome in patients with postcardiac arrest syndrome. Emerg Med J. 2014;31:549–555. doi: 10.1136/emermed-2013-202457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barnard E.B.G., Sandbach D.D., Nicholls T.L., Wilson A.W., Ercole A. Prehospital determinants of successful resuscitation after traumatic and non-traumatic out-of-hospital cardiac arrest. Emerg Med J. 2019;36:333–339. doi: 10.1136/emermed-2018-208165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Petrie J., Easton S., Naik V., Lockie C., Brett S.J., Stumpfle R. Hospital costs of out-of-hospital cardiac arrest patients treated in intensive care; a single centre evaluation using the national tariff-based system. BMJ Open. 2015;5 doi: 10.1136/bmjopen-2014-005797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Risgaard B., Lynge T.H., Wissenberg M., et al. Risk factors and causes of sudden noncardiac death: A nationwide cohort study in Denmark. Heart Rhythm. 2015;12:968–974. doi: 10.1016/j.hrthm.2015.01.024. [DOI] [PubMed] [Google Scholar]
- 17.Granfeldt A., Adelborg K., Wissenberg M., et al. Severity of ischemic heart disease and presenting rhythm in patients with out-of-hospital cardiac arrest. Resuscitation. 2018;130:174–181. doi: 10.1016/j.resuscitation.2018.07.018. [DOI] [PubMed] [Google Scholar]
- 18.Garcia S., Drexel T., Bekwelem W., et al. Early Access to the Cardiac Catheterization Laboratory for Patients Resuscitated From Cardiac Arrest Due to a Shockable Rhythm: The Minnesota Resuscitation Consortium Twin Cities Unified Protocol. J Am Heart Assoc. 2016;5 doi: 10.1161/JAHA.115.002670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yeo J.W., Ng Z.H.C., Goh A.X.C., et al. Impact of Cardiac Arrest Centers on the Survival of Patients With Nontraumatic Out-of-Hospital Cardiac Arrest: A Systematic Review and Meta-Analysis. J Am Heart Assoc. 2021:e023806. doi: 10.1161/JAHA.121.023806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Desch S., Freund A., Akin I., et al. Angiography after Out-of-Hospital Cardiac Arrest without ST-Segment Elevation. N Engl J Med. 2021;385:2544–2553. doi: 10.1056/NEJMoa2101909. [DOI] [PubMed] [Google Scholar]
- 21.Grunau B., Reynolds J.C., Scheuermeyer F.X., et al. Comparing the prognosis of those with initial shockable and non-shockable rhythms with increasing durations of CPR: Informing minimum durations of resuscitation. Resuscitation. 2016;101:50–56. doi: 10.1016/j.resuscitation.2016.01.021. [DOI] [PubMed] [Google Scholar]
- 22.Herlitz J., Svensson L., Engdahl J., Silfverstolpe J. Characteristics and outcome in out-of-hospital cardiac arrest when patients are found in a non-shockable rhythm. Resuscitation. 2008;76:31–36. doi: 10.1016/j.resuscitation.2007.06.027. [DOI] [PubMed] [Google Scholar]
- 23.Belohlavek J., Smalcova J., Rob D., et al. Effect of Intra-arrest Transport, Extracorporeal Cardiopulmonary Resuscitation, and Immediate Invasive Assessment and Treatment on Functional Neurologic Outcome in Refractory Out-of-Hospital Cardiac Arrest: A Randomized Clinical Trial. JAMA. 2022;327:737–747. doi: 10.1001/jama.2022.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yannopoulos D., Bartos J., Raveendran G., et al. Advanced reperfusion strategies for patients with out-of-hospital cardiac arrest and refractory ventricular fibrillation (ARREST): a phase 2, single centre, open-label, randomised controlled trial. Lancet. 2020;396:1807–1816. doi: 10.1016/S0140-6736(20)32338-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Scquizzato T., Bonaccorso A., Consonni M., et al. Extracorporeal cardiopulmonary resuscitation for out-of-hospital cardiac arrest: A systematic review and meta-analysis of randomized and propensity score-matched studies. Artif Organs. 2022 doi: 10.1111/aor.14205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vase H., Christensen S., Christiansen A., et al. The Impella CP device for acute mechanical circulatory support in refractory cardiac arrest. Resuscitation. 2017;112:70–74. doi: 10.1016/j.resuscitation.2016.10.003. [DOI] [PubMed] [Google Scholar]
- 27.Stottrup N.B., Jakobsen L., Krusell L.R., Terkelsen C.J. Utility of Impella(R) left ventricular assist device during cardiac arrest: A case report. Int J Cardiol. 2016;225:111–112. doi: 10.1016/j.ijcard.2016.09.074. [DOI] [PubMed] [Google Scholar]
- 28.Davidsen C., Packer E.J.S., Loland K.H., et al. Impella use in acute myocardial infarction complicated by cardiogenic shock and cardiac arrest: Analysis of 10 years registry data. Resuscitation. 2019;140:178–184. doi: 10.1016/j.resuscitation.2019.04.022. [DOI] [PubMed] [Google Scholar]
- 29.Panchal A.R., Bartos J.A., Cabanas J.G., et al. Part 3: Adult Basic and Advanced Life Support: 2020 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2020;142:S366–S468. doi: 10.1161/CIR.0000000000000916. [DOI] [PubMed] [Google Scholar]
- 30.Patel N.J., Patel N., Bhardwaj B., et al. Trends in utilization of mechanical circulatory support in patients hospitalized after out-of-hospital cardiac arrest. Resuscitation. 2018;127:105–113. doi: 10.1016/j.resuscitation.2018.04.007. [DOI] [PubMed] [Google Scholar]
- 31.Extracoporeal Life Support Organization Registry Report & International Summary of Statistics. 2021: https://www.elso.org/Registry/InternationalSummary.aspx.
- 32.Tram J., Pressman A., Chen N.W., et al. Percutaneous mechanical circulatory support and survival in patients resuscitated from Out of Hospital cardiac arrest: A study from the CARES surveillance group. Resuscitation. 2021;158:122–129. doi: 10.1016/j.resuscitation.2020.10.046. [DOI] [PubMed] [Google Scholar]
- 33.Miraglia D., Miguel L.A., Alonso W. Long-term neurologically intact survival after extracorporeal cardiopulmonary resuscitation for in-hospital or out-of-hospital cardiac arrest: A systematic review and meta-analysis. Resusc Plus. 2020;4:100045. doi: 10.1016/j.resplu.2020.100045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wengenmayer T., Rombach S., Ramshorn F., et al. Influence of low-flow time on survival after extracorporeal cardiopulmonary resuscitation (eCPR) Crit Care. 2017;21:157. doi: 10.1186/s13054-017-1744-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Debaty G., Babaz V., Durand M., et al. Prognostic factors for extracorporeal cardiopulmonary resuscitation recipients following out-of-hospital refractory cardiac arrest. A systematic review and meta-analysis. Resuscitation. 2017;112:1–10. doi: 10.1016/j.resuscitation.2016.12.011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the Danish Data Protection Agency but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are however available from the authors upon reasonable request and with permission of the Danish Data Protection Agency and the Danish Patient Safety Authority Services.