Abstract
Purpose
To validate the dose calculation accuracy and dose distribution of GammaTiles for brain tumors, and to suggest a surgically targeted radiation therapy (STaRT) workflow for planning, delivery, radiation safety documentation, and posttreatment validation.
Methods and Materials
Novel surgically targeted radiation therapy, GammaTiles, uses Cs-131 radiation isotopes embedded in collagen-based tiles that can be resorbed after surgery. GammaTile target delineation and dose calculation were performed on MIM Symphony software. Point-based and complex seed distribution calculations in MIM Symphony were verified with hand calculations and BrachyVision calculations. Vendor-provided 2-dimensional dose distribution calculation accuracy was validated using gafchromic EBT3 film measurements at various depths. A workflow was established for safe and effective GammaTile implants.
Results
Good agreement was observed between different calculations. Calculation accuracy of less than 0.5% was achieved for all points except one, which had rounding issues for very low doses and resulted in just below 5% difference. Differences in anisotropy and geometry positioning were noticed in the delineation of Cs-131 IsoRay seeds in the compared systems, resulting in minor discrepancies in the calculated dosimetry distributions. Film measurements showed profiles with relatively good agreement of 0% to 5% in nongradient regions with higher differences between 5% to 10% in the sharp dose fall-off regions.
Conclusions
A comprehensive evaluation of GammaTile geometry, dose distribution, and clinical workflow was conducted. Safe intro-operative implantation of GammaTiles requires extensive preplanning and interdisciplinary collaboration. A STaRT workflow was outlined to provide a guideline for an accurate treatment planning and safe implant process at other institutions.
Introduction
Intracranial neoplasms represent a heterogenous group of tumors with variable clinical behavior.1 Meningioma, glioblastoma, and brain metastasis are the most common malignant brain tumors,2,3 with meningiomas accounting for 30% of central nervous system (CNS) tumors.4,5 These tumors are often treated with a combination of surgery, radiation therapy, and chemotherapy.1 In general, concurrent chemoradiation therapy is used postoperatively for glioblastomas and meningiomas.4,6 Despite best efforts, recurrence is common and requires multidisciplinary effort to provide the best treatment options. GammaTile (GT Medical Technologies Inc, Tempe, AZ) is an emerging treatment modality for operable brain tumors such as meningioma, recurrent glioblastoma, and brain metastasis.7, 8, 9, 10, 11, 12 As a unique form of surgically targeted radiation therapy (STaRT), GammaTiles use Cs-131 seeds to deliver radiation as a permanent implant and are placed directly within the tumor bed at the time of surgery. It can also be a promising treatment option for recurrent meningiomas reirradiation, thin unresectable residual tumors, and recurrent tumors that are too large for conventional external beam radiosurgery.13
Brachytherapy, a radiation therapy technique using radioactive isotopes in close proximity to the target region, has been used for CNS tumors since the 1930s using various forms of radio-isotopes.12 Cs-131 has advantages in its rapid dose falloff from the tumor due to a low energy of around 30keV, via gamma photon emissions.14,15 This minimizes integral dose to normal brain tissue distal to the tumor bed while maintaining prescription doses of 60 to 80 Gy at a curable level. Cs-131 has dosimetric advantages compared with I-125, which was a previously used Brachytherapy seed for recurrent brain lesions.13, 14, 15
Early clinic trials have demonstrated safety and efficacy of using Cs-131 seeds for brain metastasis.7 The use of the collagen based carrier in GammaTile creates separation from normal brain tissue, thus reducing the risk of radionecrosis as a result of direct contact.4 Clinical trials are underway to evaluate treatment outcomes using GammaTiles for resectable metastatic brain tumors in terms of potential risks and survival benefits.16 Yet there is very little data detailing the planning and commissioning aspects of intracranial GammaTiles. Thus, we report a comprehensive overview of the challenges associated with commissioning, dose calculations and verification, and a successfully implemented STaRT workflow for the clinical use of GammaTiles.
Methods and Materials
GammaTile and Cs-131
GammaTile is a 2 cm × 2 cm collagen tile embedded with 4 Cs-131 IsoRay seeds (as shown in Fig. E1). The seeds are oriented as a 2 × 2 array. The center of each seed is 1 cm apart. The tile is 4 mm thick total, with 1 mm above the plane of seeds, and 3 mm underneath. The active length of each seed is 4 mm encased in a Titanium cylindrical capsule with a 4.5 mm total length and a 0.82 mm diameter.17 Cs-131 has a half-life of 9.7 days and average energy of about 30 keV. Owing to the low energy of the emitted photons, the dose falloff is sharp and normal brain distal from the tumor bed can be spared.
The tile is designed with one smooth side and one side with a circular ridge pattern. At the time of surgery, tiles are placed immediately adjacent to one another with the ridge pattern down uniformly covering the entire tumor bed, thus allowing evenly distributed Cs-131 seeds. This type of placement resembles a Quimby system, which is based on a uniform distribution of equal source strength.
Dose calculation system commissioning
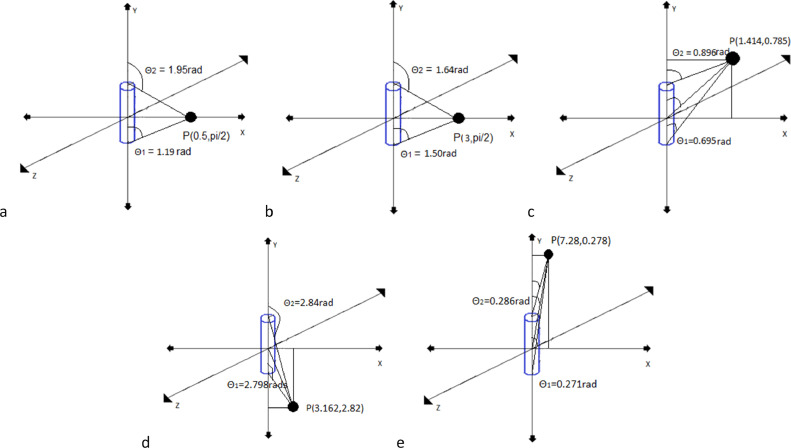
MIM Symphony (MIM Software Inc, Cleveland, OH) provides seed modeling and dose calculation for preplanning and post planning. Dose calculation uses the methodology described in TG-43.18 Accuracy verification of this system involved confirming preconfigured parameters for modeling the 2-dimensional (2D) line source of the IsoRay Medical model CS-1 Rev2 Cs-131 seed as described by TG-43 Supplement 2.17 Point dose calculated in MIM for a single seed was compared and validated using BrachyVision (Varian Medical Systems, Palo Alto, CA), as well as hand calculations following TG-43.18 This simple dose calculation comparison used 5 points at varying distances and anisotropic positions. Figure 1 shows positions of dose calculation points, including point 1: cartesian: (0.5,0,0); polar: (0.5, π/2), point 2: cartesian:(3,0,0); polar: (3, π/2), point 3: cartesian:(1,1,0); polar: (1.414, 0.785), point 4: cartesian:(3,-1,0); polar: (3.162, 2.82), and point 5: cartesian:(0.5,4,0); polar: (7.28, 0.278). Parameters focused in comparison were the active length, the dose rate constant, radial dose function and the anisotropy function.
Fig. 1.
Simple geometry, point dose calculation point relative to a single seed. A, Point 1: cartesian: (0.5, 0, 0); polar: (0.5, π/2); B, point 2: cartesian: (3, 0, 0); polar: (3, π/2); C, point 3: cartesian: (1, 1, 0); polar: (1.414, 0.785); D, point 4: cartesian: (3, −1,0); polar: (3.162, 2.82); E, point 5: cartesian: (0.5,4,0); polar: (7.28, 0.278).
Film measurements
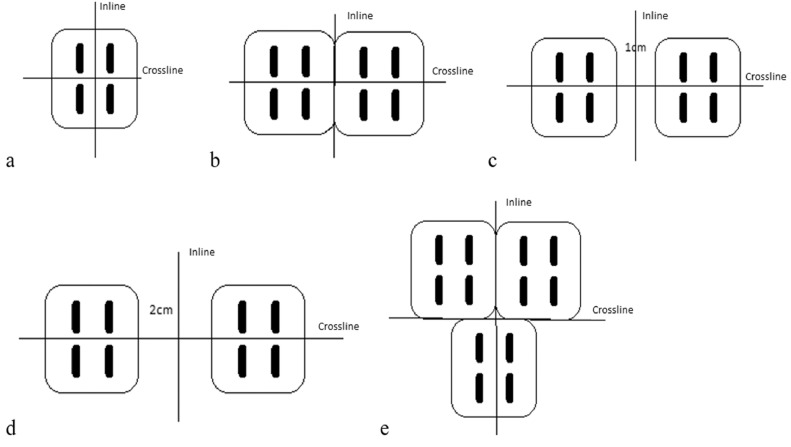
Measured planar dose distributions for specific tile orientations were obtained with gafchromic EBT3 films for validating vendor-provided planar dose distributions at varying depths for different GammaTile positions. Depths of film measurements include 1 mm, 3 mm, 5 mm, and 15 mm in solid water for each tile orientation. A film calibration curve was created to convert the measured intensity to Cs-131 dose. The dose points on the calibration curve were calculated in BrachyVision to depths of 3 mm, 5 mm, 7 mm, 9 mm, 11 mm, and 13 mm for a single GammaTile on water for a 24 hour exposure. Films were placed at these specified depths of solid water slabs and irradiated for 24 hours. A calibration curve was created from all measurements of these films, which were scanned using an Epson flatbed scanner and the FilmQA Pro (Ashland Advanced Materials, Bridgewater, NJ) film analyzer. Given that there can be absorbed dose errors up to 25% if megavoltage calibration curves are used,19,20 it is necessary to create such a calibration curve even though gafchromic films have a low energy dependence. Figure 2 shows 5 tile arrangements and the corresponding 2 orthogonal film placements for obtaining 2 dose profiles of each arrangement. Film-measured dose profiles were compared with the vendor-provided data from the GammaTile Therapy Configuration Dose Atlas (provided by the vendor) at each depth in the inline and crossline directions as shown in Figure 2. For comparison, both data sets were normalized to the same point that represents the maximum dose in the vendor provided Dose Atlas.
Fig. 2.
Tile arrangements for film measurements and the corresponding locations (2 crossed lines) of the profiles relative to the tile arrangements. A, single tile; B, 2 adjacent tiles; C, 2 tiles spaced by 1 cm; D, 2 tiles spaced by 2 cm; E, 3 adjacent tiles in a T formation.
Treatment planning and delivery workflow
MIM recommended workflow for GammaTile therapy includes preplanning and post planning. Preplanning involves contouring the target and determining the surface area of the projected tumor bed, based upon the tumor itself, which is used to estimate the number of tiles needed for the treatment case, without the need of dose calculation. The number of tiles is calculated based on the following equation.
| (Equation 1) |
The tumor bed is evaluated for sections that should be included in the surface area based on consensus of the radiation oncologist and neurosurgeon. Excluded areas are generally those where tile placement is not feasible (ie, the surgical entry site of the tumor bed). The surface area is then calculated for tile number determination. Postimplantation computed tomography and magnetic resonance imaging are required for seed identification and target dose estimation during the postplanning process. As a secondary dose check, a complex dose comparison for our first GammaTile case was performed using BrachyVision, in which seeds were identified independently. The dose file created from the plan in MIM was exported to BrachyVision for a direct plan and dose comparison, to eliminate any potential discrepancies from dose volume histogram calculation in different systems.
Results
GammaTile and Cs-131
Slight differences in anisotropy factors comparing data used for calculation in MIM and TG-43 supplement were noted (Table S1). The observed differences are primarily in anisotropy factors at angle 0 degrees. The largest relative percent differences from the values specified in TG-43 Supplement 217 are 1.8%, 1.42%, and 1.44% at Θ = 0o r = 0.5 cm, r = 1.0 cm, and r = 10 cm, respectively. All other values are within 0.5% difference, most matching exactly. The dose rate constant in TG-43 is 1.056 while MIM has it at 1.053. Both MIM and TG-43 supplement had identical radial dose functions and active length parameters. Table 1 shows good agreement in point dose calculations for a single seed in hand calculations, BrachyVision, and MIM. All values are less than 0.5% in difference, except the furthest point away from the source, which showed a disagreement of 4.26% as a result of rounding to the nearest cGy in MIM for a very low dose value.
Table 1.
Relative agreement between MIM and BrachyVision with respect to Hand Calculations for a single seed to specified points (unit: cGy)
| Point (volume) | Hand calculation (cGy) | MIM (cGy) | Percent difference | BrachyVision (cGy) | Percent difference |
|---|---|---|---|---|---|
| Point 1 (0.5,0,0) | 7567.5 | 7549 | −0.24% | 7565.2 | 0.03% |
| Point 2 (3,0,0) | 170.4 | 170 | −0.24% | 170.4 | 0.01% |
| Point 3 (1,1,0) | 910.8 | 909 | −0.20% | 910.6 | 0.02% |
| Point 4 (1,-3,0) | 125.4 | 125 | −0.31% | 125.4 | −0.01% |
| Point 5 (2,7,0) | 9.56 | 9 | −4.26% | 9.6 | −0.41% |
Film measurements validation
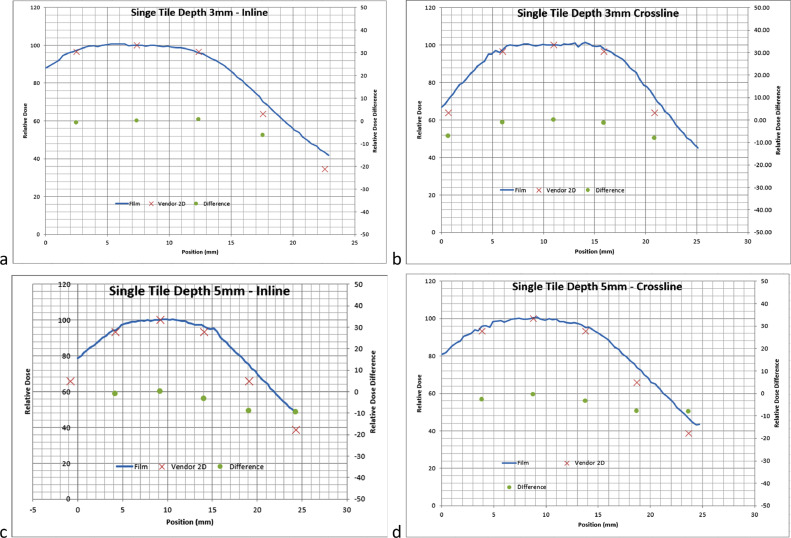
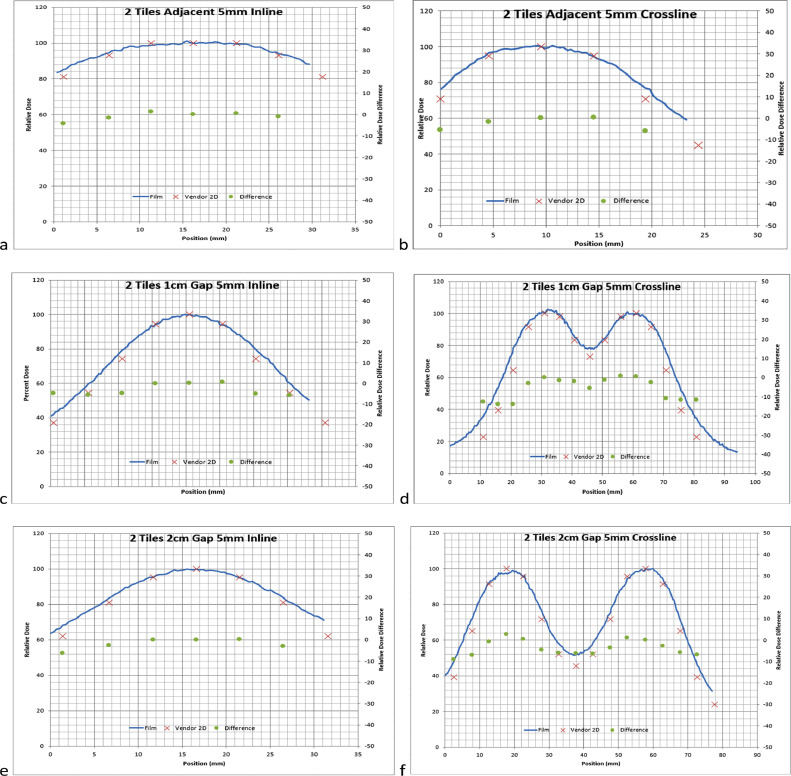
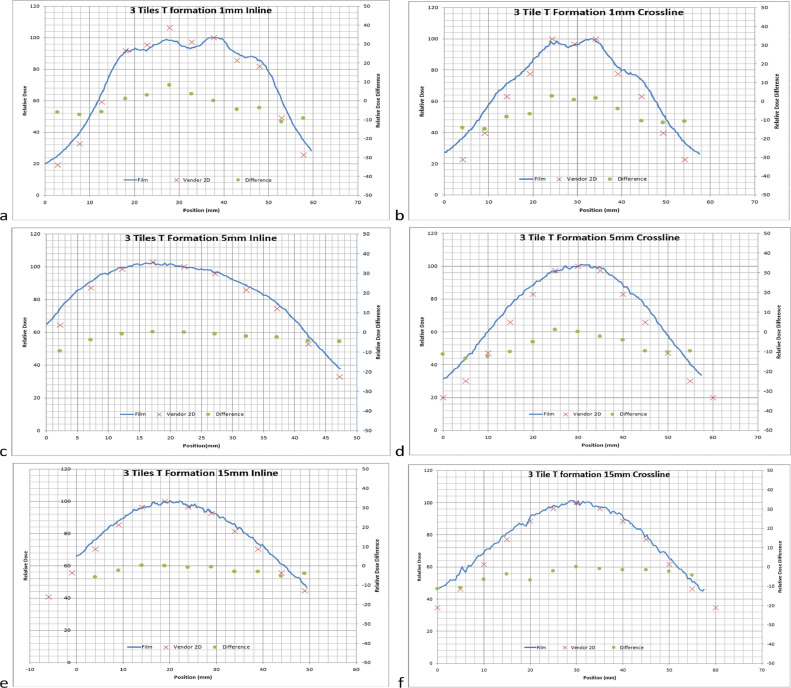
Film measurements generally showed good agreement in the shape of the dose distribution, although there were noticeable differences in the falloff due to course resolution in the vendor provided Dose Atlas. Figures 3, 4, and 5 show varying profile comparisons between vendor-provided data and measured film profiles at varying depths in different tile arrangements. Inline profile comparisons generally had slightly larger disagreement in the sharp dose fall-off regions than that in crossline profiles, varying in between 5% to 15% difference, although both had the same relative shape following vendor-provided data. The profile discrepancies did not show any depth dependency, meaning that each depth showed a similar slight discrepancy. The profiles were normalized to the center of the same point which correlated with the maximum dose in the vendor provided Dose Atlas.
Fig. 3.
A, 1 Tile profiles at 3 mm in the inline direction. B, 1 Tile profiles at 3 mm in the crossline direction. C, 1Tile profiles at 5 mm in the inline direction. D, 1 Tile profiles at 5 mm in the crossline direction. Solid lines: film profiles, and red Xs: vendor provided profiles (x-axis: position in mm; left y-axis: normalized to the maximum of vendor provided profiles). Green dots: percent difference between film and vendor data, normalized to maximum of vendor provided profiles (right y-axis: percent difference).
Fig. 4.
A, Shows 2 tiles adjacent profiles at 5 mm in the inline direction. B, Shows 2 tiles profiles adjacent at 5 mm in the crossline direction. C, Shows 2 tiles separated by 1-cm profiles at 5 mm in the inline direction. D, Shows 2 tiles profiles separated by 1 cm at 5 mm in the crossline direction. E, Shows 2 tiles separated by 2-cm profiles at 5 mm in the inline direction. F, Shows 2 Tiles profiles separated by 2 cm at 5 mm in the crossline direction. Solid lines: film profiles, and red Xs: vendor provided profiles (x-axis: position in mm; Left y-axis: normalized to the maximum of vendor provided profiles). Green dots: percent difference between film and vendor data, normalized to maximum of vendor provided profiles (right y-axis: percent difference).
Fig. 5.
A, Shows 3 tiles in a T formation profiles at 1 mm in the inline direction. B, Shows 3 tiles in a T formation profiles at 1 mm in the crossline direction. C, 3 Tiles in a T formation profiles at 5 mm in the inline direction. D, 3 Tiles in a T formation profiles at 5 mm in the crossline direction. E, 3 tiles in a T formation profiles at 15 mm in the inline direction. F, 3 tiles in a T formation profiles at 15 mm in the crossline direction. Solid lines: film profiles, and red Xs: vendor provided profiles (x-axis: position in mm; left y-axis: normalized to the maximum of vendor provided profiles). Green dots: percent difference between film and vendor data, normalized to maximum of vendor provided profiles (right y-axis: percent difference).
Clinical case treatment and workflow
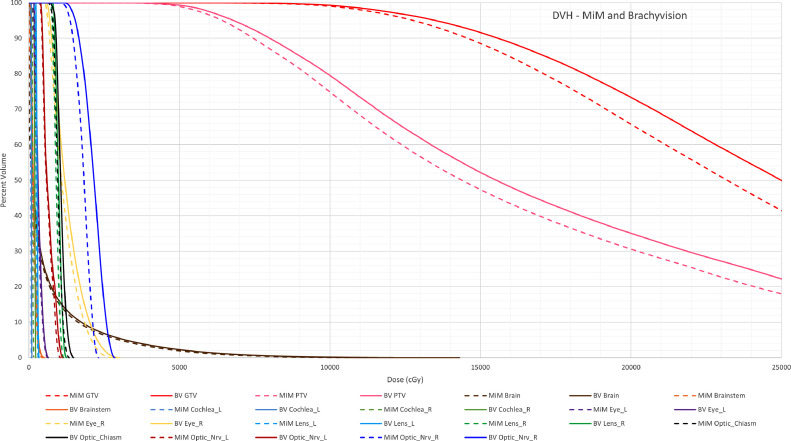
A patient that underwent GammaTile treatment at our institution is herein shown as an example. Total dose prescription for this patient was 60 Gy. Based on the surface contour using Equation 1, a total of 15 tiles were ordered. The surface contour was created slightly larger to ensure there would not be a shortage of seeds. At the time of treatment, 12 tiles (48 seeds) were implanted for the patient. Dose distributions in the axial, sagittal and coronal views obtained in postplanning on the patient CT image are shown in Figure E2. Dose distributions show isodose lines that have the same shape and falloff that is very similar. Mean and maximum dose comparisons in organs at risk (OARs) were listed in Table 2 between MIM and BrachyVision systems. DVHs of OARS and targets for each plan were generated in BrachyVision and overlayed together in Figure 6. Both plans showed similar doses to OARs and tumor bed coverage with some discrepancies for some OARs due to anisotropy differences.
Table 2.
Relative agreement to OARs between BrachyVision with respect to MIM normalized to the Prescription of 60 Gy
| OAR | Max MIM (%) | Max BrachyVision (%) | Normalized relative percent difference | Mean MIM (%) | Mean BrachyVision (%) | Normalized relative percent difference |
|---|---|---|---|---|---|---|
| Brain | 243.95 | 238.37 | −5.58 | 9.65 | 10.62 | 0.97 |
| Brain stem | 8.67 | 8.85 | 0.18 | 2.25 | 2.77 | 0.52 |
| Optic nerve Lt | 17.47 | 18.73 | 1.27 | 10.25 | 10.93 | 0.68 |
| Optic nerve Rt | 41.85 | 47.55 | 5.70 | 30.22 | 35.62 | 5.40 |
| Optic chiasm | 21.57 | 24.72 | 3.15 | 15.07 | 17.77 | 2.70 |
| Cochlea Lt | 1.33 | 1.65 | 0.32 | 1.10 | 1.48 | 0.38 |
| Cochlea Rt | 2.67 | 3.77 | 1.10 | 2.15 | 3.25 | 1.10 |
| Lens Lt | 5.57 | 5.62 | 0.05 | 4.25 | 4.60 | 0.35 |
| Lens Rt | 19.33 | 20.90 | 1.57 | 15.00 | 16.47 | 1.47 |
| Eye Lt | 11.00 | 10.80 | −0.20 | 5.00 | 5.42 | 0.42 |
| Eye Rt | 37.67 | 50.17 | 12.50 | 16.93 | 21.65 | 4.72 |
Abbreviations: Lt = left; max = maximum; OAR = organs at risk; Rt = right.
Fig. 6.
Dose volume histogram of plans created in MIM and BrachyVision. The dose file and structure set from the MIM plan was imported into BrachyVision to overlay and export to txt file together. Dashed Lines are Structures from the plan generated in MIM, Solid Lines are Structures from the plan generated in BrachyVision. x-axis: dose in cGy; y-axis is percent volume.
GammaTile implants require interdisciplinary collaboration from the radiation oncologist, neurosurgeon, medical physicist, radiation safety officer, Post Anesthesia Care Unit (PACU) staff, transportation staff, and nursing units responsible for inpatient care once the patient is released from the PACU. Physics roles include preplanning, tile ordering, seed assay, tile transportation to operative room, and postplanning. The radiation safety officer has an important role in initial education and coordination among different care takers including PACU and other inpatient nursing staff. Numerous collaborative meetings with representatives from each department were held, including radiation safety training for nonradiation workers. A workflow was created in addition to documentation for post implant surveys, which is visible in Figure E3. The workflow requires the physicist to be the primary point of contact to move the process forward to work with all the departments for preplanning, planning, and postplanning stages. In preplanning, it is important that the neurosurgeon and radiation oncologist work together to define the tumor bed. Accurate determination of the surface area allows accurate estimation of the needed number of tiles, which specifically requires accurately determining exclusion areas. Too few or too many tiles would likely be ordered if the exclusion areas are not appropriately determined.
Discussion
Preliminary calculations showed good agreement between all types of calculation methods and planning systems. Dose differences were generally less than 0.5%, with one exception of 4.26% point dose difference, translating to a very low dose of 9 cGy. This difference is largely attributed to the rounding error in MIM. Overall, MIM calculation was fully validated with hand calculation and BrachyVision calculation. Dose profile agreement was observed for inline and crossline directions at various depths in various tile arrangements.
Slight differences in the values measured for different OARs were observed. Possible reasons for these differences are multifold. The major source of these subtle differences is likely a calculation limitation in MIM. All seeds are oriented in the superior-inferior direction. This means that if a seed is rotated or tilted after implantation, the corresponding dose distribution in MIM is modeled as a seed positioned vertically along the y axis. Figure E4 shows the difference between actual seed placements (upper figure) and seed modeling (lower figure) in 3-dimensional rendering. This can cause differences in dose anisotropy and result in dose underestimation for structures superior and inferior of seed locations. This is shown in Figure 6 in organs that are immediately inferior to the tile placement, such as Optic_Nrv_R and, to a lesser extent, Eye_R. A second reason for differences might be manual identification of those implanted seeds in each TPS. MIM does have an algorithm for automatic seed identification and delineation, although some seed locations needed to be modified, especially those adjacent to skull. Slight differences in seed placement can have an effect on overall dose distribution. A third reason for differences in dose statistics could be differences in structure contour or dose matrix resolutions in the 2 systems. Structures have subtle differences in shape and owing to how a voxel is defined in each TPS, OARs may have different borders. Cs-131 has a low energy, thus results in rapid dose fall-off, which therefore, corresponds to high sensitivity in maximum dose to structures closest to seeds that was rendered slightly differently.
Film measurements of dose profiles generally showed good agreement, with moderate discrepancies in lower dose regions. One reason for the discrepancy was from dose distribution resolution (a pixel size of 5 mm x 5 mm) in the vendor provided 2D dose distribution in the GammaTile Therapy Configuration Dose Atlas used for comparison. Dose on sharp gradients can get averaged out or values at peaks or troughs could be averaged higher or lower by the partial volume effect. Another issue is that vendor-provided dose distributions are displayed symmetrical in all directions, meaning that a profile perpendicular to the seed orientation had the same distribution as one in the parallel direction. The symmetry in the geometrically cylindrical Cs-131 seed with a 4 mm active length and a 0.25 mm diameter was represented as a square. These factors can contribute to dose discrepancies between the measured profiles and system-calculated profiles. The relative shape of the dose distributions agreed with measurements with relative values agreeing within 0% to 5% in more homogenous dose regions and varying between 5% to 15% in the sharp dose fall-off regions. There was one point in the highest dose region that exhibits 10% difference in Figure 5A and contradicts the vendor provided data. This point is a measurement taken at 1 mm depth for 3 Tiles. At this depth sharp gradients occur in the measurement due to the proximity of the seed. At shallow depths the seeds are visible in the film and small positioning errors can lead to high dose discrepancy, which may have led to the discrepancy at this point. Therefore, it is recommended that users should validate the vendor provided data against measurements as part of their commissioning processes.
GammaTile treatment workflow at this institution requires multiple departments to collaborate to ensure safe planning, implantation and postimplant care of the patient. In preplanning, the collaboration between the neurosurgeon and radiation oncologist in target delineation is crucial to ensure the appropriate number of tiles are ordered.
Patient transition between departments requires strong communication from a radiation safety standpoint. It is important to ensure that the radiation safety information, signage, and instantaneous dosimeter for staff is available with multiple staff and location changes. A written log denoting the exposure received by staff as well as written instructions for staff about location of highest and lowest exposure rates should be readily available and present in caretaking instructions. Documentation of release instructions given to the patient are necessary to minimize exposure to the general public. Important steps and tasks that are most applicable to any facility must be determined before scheduling of the first fraction.
Conclusions
A comprehensive evaluation of GammaTile has been performed and the characterization of the dose distribution of GammaTiles in numerous TPS matches and is accurate in the quantification of dose to critical structures. To a large degree, measurements and calculation comparisons agreed well with the exception of a few differences in the falloff region of film measurements. This is attributed to resolution and partial volume averaging issues. Establishing a well-thought out workflow greatly streamlined the treatment process and ensured safety. Planning and multidisciplinary communication are vital to ensure safe implementation.
Footnotes
Sources of support: This work had no specific funding.
Disclosures: none.
Research data are stored in an institutional server and will be shard upon request to the corresponding author.
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.adro.2022.100910.
Appendix. Supplementary materials
References
- 1.DeAngelis LM. Brain tumors. N Engl J Med. 2001;344:114–123. doi: 10.1056/NEJM200101113440207. [DOI] [PubMed] [Google Scholar]
- 2.Ohgaki H, Kleihues P. The definition of primary and secondary glioblastoma. Clin Cancer Res. 2013;19:764–772. doi: 10.1158/1078-0432.CCR-12-3002. [DOI] [PubMed] [Google Scholar]
- 3.Ostrom QT, Gittleman H, Xu J, et al. CBTRUS statistical report: Primary brain and other central nervous system tumors diagnosed in the United States in 2009-2013. Neuro-Oncol. 2016;18(Suppl 5):v1–v75. doi: 10.1093/neuonc/now207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ali-Reza F, Ulrich R. Meningioma. Curr Neurol Neurosci Rep. 2013;13:337. doi: 10.1007/s11910-013-0337-4. [DOI] [PubMed] [Google Scholar]
- 5.Wen PY, Black PM, Loeffler JS. In: Cancer: Principles and Practice of Oncology. DeVita V, Hellman S, Rosenberg SA, editors. Lippincott, Williams, & Wilkins; Philadelphia, PA: 2001. Metastatic brain cancer; pp. 2655–2670. [Google Scholar]
- 6.Barani IJ, Larson DA. In: Raizer J, Parsa A, editors. Vol. 163. Springer; Champaign: 2015. Radiation therapy of glioblastoma; pp. 49–73. (Current Understanding and Treatment of Gliomas. Cancer Treatment and Research). [DOI] [PubMed] [Google Scholar]
- 7.Brachman DG, Youssef E, Dardis CJ, et al. Resection and permanent intracranial brachytherapy using modular, biocompatible cesium-131 implants: Results in 20 recurrent, previously irradiated meningiomas. J Neurosurg. 2019;131:1819–1828. doi: 10.3171/2018.7.JNS18656. [DOI] [PubMed] [Google Scholar]
- 8.Stupp R, Wong ET, Kanner AA, et al. NovoTTF-100A versus physician's choice chemotherapy in recurrent glioblastoma: A randomised phase III trial of a novel treatment modality. Euro J Cancer. 2012;48:2192–2202. doi: 10.1016/j.ejca.2012.04.011. [DOI] [PubMed] [Google Scholar]
- 9.Brachman D, Youssef E, Dardis C, Smith K, Pinnaduwage D, Nakaji P. Surgically targeted radiation therapy: Safety profile of collagen tile brachytherapy in 79 recurrent, previously irradiated intracranial neoplasms on a prospective clinical trial. Brachytherapy. 2019;18:S35–S36. [Google Scholar]
- 10.Brachman D, Rogers C, et al. Resection and surgically targeted radiation therapy for initial or salvage treatment of aggressive meningioma: Results from a prospective trial. Cureus. 2020;12:e11570. doi: 10.7759/cureus.11570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brachman D, Nakaji P, et al. A prospective trial of resection plus surgically targeted radiation therapy for brain metastasis. Neurooncol Adv. 2020;2(suppl 2):ii6. [Google Scholar]
- 12.Gessler DJ, Ferreira C, Dusenbery K, Chen CC. GammaTile: Surgically targeted radiation therapy for glioblastomas. Future Oncol. 2020;16:2445–2455. doi: 10.2217/fon-2020-0558. [DOI] [PubMed] [Google Scholar]
- 13.Magill ST, Schwartz TH, Theodosopoulos PV, McDermott MW. Brachytherapy for Meningiomas. Handb Clin Neurol. 2020;170:303–307. doi: 10.1016/B978-0-12-822198-3.00049-5. [DOI] [PubMed] [Google Scholar]
- 14.Han DY, Ma L, Braunstein S, Raleigh D, Sneed PK, McDermott M. Resection cavity contraction effects in the use of radioactive sources (1-25 versus Cs-131) for intra-operative brain implants. Cureus. 2018;10:e2079. doi: 10.7759/cureus.2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chiu-Tsao S-T, Napoli JJ, Davis SD, Hanley J, Rivard MJ. Dosimetry for 131Cs and 125I seeds in solid water phantom using radiochromic EBT film. Appl Radiat Isotopes. 2014;92:102–114. doi: 10.1016/j.apradiso.2014.06.014. [DOI] [PubMed] [Google Scholar]
- 16.Weinberg J, McAleer MF, Tawbi H, Lang F. A randomized, multicenter phase iii trial of surgery plus stereotactic radiosurgery (SRS) compared with surgery plus permanently implanted collagen tile brachytherapy (CTBT) for resectable metastatic brain tumors-protocol in progress. Neurooncol Adv. 2020;2(suppl 2):ii11. [Google Scholar]
- 17.Rivard MJ, Ballester F, Butler WM, et al. Supplement 2 for the 2004 update of the AAPM Task Group No. 43 Report: Joint recommendations by the AAPM and GEC-ESTRO. Med Phys. 2017;44:297–337. doi: 10.1002/mp.12430. [DOI] [PubMed] [Google Scholar]
- 18.Rivard MJ, Coursey BM, DeWerd LA, et al. Update of AAPM TaskGroup No. 43 Report: A revised AAPM protocol for brachytherapy dose calculations. Med Phys. 2004;31:633–667. doi: 10.1118/1.1646040. [DOI] [PubMed] [Google Scholar]
- 19.Devic S, LiHeng L, Tomic N, Bekerat H, et al. Dose measurements nearby low energy electronic brachytherapy sources using radiochromic film. Phys Med. 2019;64:40–44. doi: 10.1016/j.ejmp.2019.05.017. [DOI] [PubMed] [Google Scholar]
- 20.Campojola L, Casolaro P, Di Capua F. Absolute dose calibration of EBT3 Gafchromic films. J Instrum. 2017;12:8–15. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.