Abstract
Pneumococci can enter and survive inside human lung alveolar carcinoma cells. We examined the activity of azithromycin, gentamicin, levofloxacin, moxifloxacin, penicillin G, rifampin, telithromycin, and trovafloxacin against pneumococci inside and outside cells. We found that moxifloxacin, trovafloxacin, and telithromycin were the most active, but only telithromycin killed all intracellular organisms.
In 1916 Rouss and Jones showed that living, but not dead, phagocytes could protect microbes from killing by antiserum and potassium cyanide (11). Though the pneumococcus is usually considered to be an extracellular pathogen, studies have indicated that pneumococci can enter and survive inside human lung alveolar carcinoma cells (type II pneumocytes, A549 cells) (14). The clinical relevance of this observation is not clear. Furthermore, pneumococci may persist in tissue sites despite in vitro sensitivities to antibiotics and adequate antimicrobial levels. A recent clinical study documented the persistence of Streptococcus pneumoniae in the middle-ear fluid of patients treated with several different antibiotics (2). The role of intracellular persistence in this phenomenon is unknown.
We studied several antimicrobial agents with good activity against the pneumococcus to determine their ability to kill the organism inside A549 cells. The agents studied included penicillin G and gentamicin (7, 10, 12), which remain largely extracellular, and azithromycin, levofloxacin, rifampin, trovafloxacin, telithromycin (3, 5, 8, 15), and moxifloxacin (9), all of which have been reported to penetrate cells well. The minimal bacteristatic and bactericidal concentrations of each agent for S. pneumoniae 14.8 were determined in RPMI 1640 (the A549 culture medium; Biowhittaker, Walkersville, Md.).
Purified polymorphonuclear leukocytes were obtained from heparinized (10 U/ml; Lymphomed Fujisawa USA Inc., Deerfield, Ill.) human venous blood by a Ficoll-Hypaque separation procedure adapted from the work of Ferrante and Thong (4). Cells were resuspended in Hanks balanced salt solution (HBSS), counted using a hemocytometer, and found to be 95% pure polymorphonuclear leukocytes. Micrococcus luteus ATCC 9341 (American Type Culture Collection, Rockville, Md.) and Staphylococcus aureus ATCC 27217 were cultured in tryptic soy broth (Difco, Detroit, Mich.) or on 5% sheep blood agar (Becton Dickinson, Cockeysville, Md.). S. pneumoniae 14.8 was supplied by Daniel Musher, Infectious Disease Section, Veterans Administration Medical Center, Houston, Tex. A549, a human lung alveolar carcinoma (type II pneumocyte) cell line, was obtained from ATCC. Cells were grown in RPMI 1640 (Biowhittaker) with 10% fetal calf serum (Biowhittaker), 25 mM HEPES (Sigma Chemical Company, St. Louis, Mo.), and 100 U of penicillin G per ml with 100 μg of streptomycin (Biowhittaker) per ml. A549 monolayers were grown to confluence in 24-well tissue culture plates (Nalge Nunc International, Roskilde, Denmark) at 37°C with 5% CO2.
Penicillin G and gentamicin sulfate were purchased from Sigma Chemical Company. Levofloxacin was provided by the R. W. Johnson Pharmaceutical Research Institute, Spring House, Pa. Stock solutions of penicillin G, gentamicin sulfate, and levofloxacin were made up in HBSS. Rifampin was purchased from Sigma Chemical Company, and a stock solution was made fresh daily by dissolving rifampin in methanol and diluting this further with HBSS. Trovafloxacin was obtained from Pfizer Pharmaceuticals, New York, N.Y. Telithromycin was supplied by Hoechst-Marion-Roussel, Romainville, France. Stock solutions of trovafloxacin and telithromycin were made by resuspending powder in 1% HCl and sterile water. Moxifloxacin was obtained from Bayer Corporation, West Haven, Conn., and stock solutions were made by resuspending the drug in sterile water. Azithromycin was provided by Pfizer Pharmaceuticals and a stock solution was made by initially dissolving azithromycin in ethanol and then diluting this with HBSS.
In order to make meaningful comparisons of antimicrobial agent activity, we utilized in vitro antibiotic concentrations (except with gentamicin and rifampin) similar to peak concentrations in serum, as reported in the literature (1, 13, 16). In some cases lower concentrations were also used.
The MICs and minimum bactericidal concentrations (MBCs) for S. pneumoniae were determined by a broth dilution method using a concentration of 5 × 105 CFU/ml in RPMI 1640 (6). Incubation was carried out at 37°C for 24 h, and growth of any viable organisms was checked by using a 10-μl loop to inoculate the organisms onto blood agar.
Cell cultures infected with intracellular pneumococci were prepared as follows. A549 monolayers grown to confluence were washed to remove penicillin G and streptomycin from the growth media. S. pneumoniae 14.8 was cultured for 16 to 18 h in Todd-Hewitt broth (Difco) supplemented with 0.5% yeast extract (Difco) and 0.25% choline bitartrate salt (Sigma) and was prepared by centrifugation and washing of the pellet in normal saline. The pellet was then resuspended in HBSS, and the optical density at a wavelength of 580 nm was adjusted to 1.10, which was indicative of 1 × 109 CFU/ml. Samples of 9 × 107 CFU of S. pneumoniae 14.8 were incubated for 2 h at 37°C with A549 monolayers in 1 ml of A549 growth medium (omitting the penicillin G and streptomycin) per well. Monolayers containing intracellular pneumococci were then washed with HBSS. A549 growth medium containing gentamicin (50 μg/ml) was added to each well and incubated for 2 h at 37°C to kill extracellular pneumococci. Cultures showed 12,572 ± 2,609 (standard error of the mean, n = 8) CFU after the gentamicin incubation. The monolayers were then washed with HBSS. A549 growth medium with or without antibiotic was added and incubated with the A549 cells containing pneumococci for 18 h. Trypan blue staining of A549 cells showed that in the absence of antibiotics all cells were nonviable and that with all antibiotics tested more than 98% of the A549 cells were viable. After incubation, the supernatants were serially diluted and plated onto chocolate agar, and the monolayers were washed with HBSS to remove the antibiotics. One hundred microliters of trypsin-EDTA (Sigma Chemical) was added per well and incubated for 10 min at 37°C to lift the monolayers, which were then lysed by the addition of 900 μl of sterile water to each well for 10 min at 37°C. Viable organisms were enumerated by serial dilutions and counts of CFU on chocolate agar.
Those monolayers incubated without antibiotics showed “too numerous to count” growth of pneumococci, indicating that the penicillin G and streptomycin present in the original A549 growth medium had no significant effect on bacterial growth.
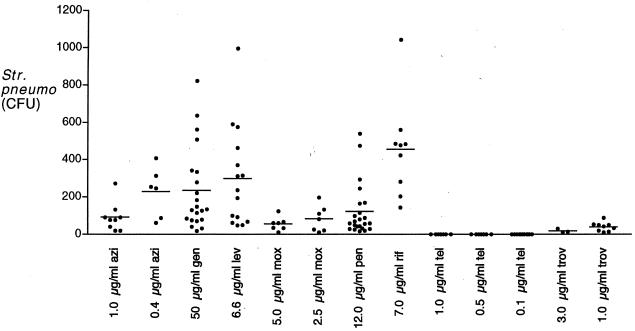
Table 1 shows the MICs and MBCs of the antibiotics used against S. pneumoniae 14.8. Results of the assays to determine the ability of antibiotics to kill intracellular pneumococci are shown in Fig. 1. In all experiments, supernatant cultures showed no growth. Despite concentrations of gentamicin, penicillin G, and rifampin far exceeding the MBCs in A549 growth medium, significant numbers of pneumococci survived inside the cells after 18 h of incubation. Data were analyzed using a Mann-Whitney rank sum nonparametric test. All of the antimicrobial agents tested were more active than penicillin G in killing intracellular organisms (P < 0.05). Telithromycin was most active (P < 0.01) and was unique in that it consistently sterilized the cultures, even at low extracellular concentrations. In more than 100 experiments, the only agent that sterilized the cells was telithromycin. Experiments were performed with 2- and 8-h incubation times (data not shown), and pneumococcal survival decreased in a linear fashion with no difference in the rank order of effectiveness.
TABLE 1.
MICs and MBCs of various antibiotics against S. pneumoniae 14.8 in RPMI
| Antibiotics | MIC (μg/ml) | MBC (μg/ml) |
|---|---|---|
| Azithromycin | 0.0006 | 0.02 |
| Gentamicin | 1.56 | 6.25 |
| Levofloxacin | 0.6 | 2.5 |
| Moxifloxacin | 0.08 | 0.31 |
| Penicillin G | 0.01 | 0.01 |
| Rifampin | 0.05 | 1.56 |
| Telithromycin | 0.0002 | 0.001 |
| Trovafloxacin | 0.125 | 0.5 |
FIG. 1.
Growth of pneumococci in the presence of antibiotics. Details of the experiment are given in the text. Azi, azithromycin; gen, gentamicin; lev, levofloxacin; mox, moxifloxacin; pen, penicillin G; rif, rifampin; tel, telithromycin; trov, trovafloxacin.
In order for an antimicrobial agent to kill intracellular organisms, it must enter the cell and the compartment where the microbe resides at concentrations above the MBC for that milieu. Ideally, an antibiotic should eradicate pathogens from tissue sites, including intracellular locations. This may be more important for some organisms than others. We know, for example, that beta-lactam antibiotics failed to cure patients with Legionnaires' disease, despite the causative organism's susceptibility to these agents in vitro. The presumed explanation is that the intracellular location of the organism protected it from the action of the antibiotic. Agents that do get into cells, such as macrolides and fluoroquinolones, are effective against this disease.
Diseases caused by extracellular pathogens such as the pneumococcus clearly respond to therapy with agents, such as penicillin G, which penetrate cells poorly. Only a small proportion (about 8%) of the pneumococci incubated with A549 cells were found to be viable after incubation for 18 h with the extracellular antibiotic penicillin G. However, it may be advantageous to destroy organisms in intracellular sites to reduce relapse, recolonization, and possibly the development of resistance. Clinical studies to prove this hypothesis will be difficult to perform because of the variables associated with antimicrobial agents of different classes.
Acknowledgments
This work was supported by grants from Hoechst-Marion-Roussel, Ortho-McNeil Pharmaceutical, Raritan, N.J., and Bayer Corporation.
REFERENCES
- 1.Amsden G W, Schentag J. Tables of antimicrobial agent pharmacology. In: Mandell G L, Bennett J, Dolin R, editors. Principles and practice of infectious disease. 5th ed. New York, N.Y: Churchill Livingstone; 2000. pp. 566–589. [Google Scholar]
- 2.Brook I, Gober A E. Microbiologic characteristics of persistent otitis media. Arch Otolaryngol Head Neck Surg. 1998;124:1350–1352. doi: 10.1001/archotol.124.12.1350. [DOI] [PubMed] [Google Scholar]
- 3.Doern G V. In vitro activity of selected fluoroquinolones versus Streptococcus pneumoniae. Infect Dis Clin Practice. 1999;8(Suppl):S3–S6. [Google Scholar]
- 4.Ferrante A, Thong Y H. Optimal conditions for simultaneous purification of mononuclear and polymorphonuclear leukocytes from human blood by the Hypaque-Ficoll method. J Immunol. 1980;36:109–117. doi: 10.1016/0022-1759(80)90036-8. [DOI] [PubMed] [Google Scholar]
- 5.Hamilton-Miller J M T, Shah S. Comparative in-vitro activity of ketolide (HMR 3647) and four macrolides against gram-positive cocci of known erythromycin susceptibility status. J Antimicrob Chemother. 1998;41:649–653. doi: 10.1093/jac/41.6.649. [DOI] [PubMed] [Google Scholar]
- 6.Jones R N, Barry A L, Gavan T L, Washington J A., II . Susceptibility tests: microdilution and macrodilution broth procedures. In: Lennette E H, Balows A, Hausler W J Jr, Shadomy H J, editors. Manual of clinical microbiology. 4th ed. Washington, D.C.: American Society for Microbiology; 1985. pp. 972–977. [Google Scholar]
- 7.Mandell G L. Interaction of intraleukocytic bacteria and antibiotics. J Clin Investig. 1973;52:1673–1679. doi: 10.1172/JCI107348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miossec-Bartoli C, Pilatre L, Peyron P, N'Diaye E-N, Collart-Dutilleul V, Maridonneau-Parini I, Diu-Hercend A. The new ketolide HMR3647 accumulates in the azurophil granules of human polymorphonuclear cells. Antimicrob Agents Chemother. 1999;43:2457–2462. doi: 10.1128/aac.43.10.2457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pascual A, García I, Ballesta S, Perea E J. Uptake and intracellular activity of moxifloxacin in human neutrophils and tissue-cultured epithelial cells. Antimicrob Agents Chemother. 1999;43:12–15. doi: 10.1128/aac.43.1.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Prokesch R C, Hand W L. Antibiotic entry into human polymorphonuclear leukocytes. Antimicrob Agents Chemother. 1982;21:373–380. doi: 10.1128/aac.21.3.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rous P, Jones F S. The protection of pathogenic microorganisms by living tissue cells. J Exp Med. 1916;23:601. doi: 10.1084/jem.23.5.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schwab J C, Mandell G L. The importance of penetration of antimicrobial agents into cells. Infect Dis Clin N Am. 1989;3:461–467. [PubMed] [Google Scholar]
- 13.Stass H, Kubitza D. Pharmacokinetics and elimination of moxifloxacin after oral and intravenous administration in man. J Antimicrob Chemother. 1999;43(Suppl. B):83–90. doi: 10.1093/jac/43.suppl_2.83. [DOI] [PubMed] [Google Scholar]
- 14.Talbot U M, Paton A W, Paton J C. Uptake of Streptococcus pneumoniae by respiratory epithelial cells. Infect Immun. 1996;64:3772–3777. doi: 10.1128/iai.64.9.3772-3777.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vazifeh D, Preira A, Bryskier A, Labro M T. Interactions between HMR 3647, a new ketolide, and human polymorphonuclear neutrophils. Antimicrob Agents Chemother. 1998;42:1944–1951. doi: 10.1128/aac.42.8.1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wise R, Honeybourne D. Pharmacokinetics and pharmacodynamics of fluoroquinolones in the respiratory tract. Eur Respir J. 1999;14:221–229. doi: 10.1034/j.1399-3003.1999.14a38.x. [DOI] [PubMed] [Google Scholar]