Abstract
Background
The human INHA gene encodes the inhibin subunit alpha protein, which is common to both inhibin A and B. The functional importance of inhibins in male sex development, sexual function, and reproduction remain largely unknown.
Objective
We report for the first time two male siblings with homozygous INHAmutations.
Methods
The medical files were examined for clinical, biochemical, and imaging data. Genetic analysis was performed using next-generation and Sanger sequencing methods.
Results
Two brothers complained of gynecomastia, testicular pain, and had a history of hypospadias. Biochemistry revealed low serum testosterone, high gonadotropin and anti-Mullerian hormone, and very low/undetectable inhibin concentrations, where available. Both patients had azoospermia in the spermiogram. We have identified a homozygous 2 bp deletion (c.208_209delAG, R70Gfs*3) variant, which leads to a truncated INHA protein in both patients, and confirmed heterozygosity in the parents. The external genital development, pubertal onset and progression, reproductive functions, serum gonadotropins, and sex hormones of mother and father, who were heterozygous carriers of the identified mutation, were normal.
Conclusion
Homozygosity for INHA mutations causes decreased prenatal and postnatal testosterone production and infertility in males, while the heterozygous female and male carriers of INHA mutations do not have any abnormality in sex development and reproduction.
Introduction
Inhibin A and B are heterodimeric proteins belonging to the transforming growth factor β superfamily, capable of suppressing pituitary follicle-stimulating hormone (FSH) secretion. Inhibin is produced mostly by the granulosa cells in the ovary and Sertoli cells in the testis (1, 2). Inhibins contain either a βA- or βB-subunit and a common α-subunit, which is encoded by INHA (3, 4). Activins are functional counterparts of inhibins, being dimers of the β-subunits with the ability to stimulate FSH secretion. The α-subunit binds competitively to the β-subunits to form inhibin, thereby reducing the formation of activin homodimers; but inhibin is also able to block the binding of the activins to the activin receptors, thereby inhibiting activin signalling (5). Besides the endocrine regulation of FSH biosynthesis, animal and in vitro cell studies have demonstrated that inhibin also has autocrine and/or paracrine actions that regulate gametogenesis and steroidogenesis (6, 7, 8, 9, 10). Knockout of Inha in mice results in the development of gonadal stromal tumours and cachexia-related death in both sexes (6, 7, 11, 12). Human studies have shown that certain variants and polymorphisms in coding sequence or the promoter of INHA are associated with primary ovarian insufficiency (POI) or primary amenorrhea in females (13). There are no reports regarding the effect of inhibins on sex development, steroidogenesis, or reproduction in males.
Here, we report for the first time two male siblings with homozygous INHAmutations and describe their clinical features.
Subjects and methods
Clinical studies
All clinical investigations and genetic analyses were performed according to the guidelines of the Declaration of Helsinki. The Ethical Committee of Marmara University, Istanbul, Turkey approved the study (09.2021.1343). Written informed consent for publication of their clinical details was obtained from the patients and the parents.
Two male siblings from a single family were evaluated for gynecomastia, hypospadias, and primary gonadal insufficiency. Detailed clinical, laboratory, and molecular characteristics of the patients and parents are described.
DNA sequencing
Genomic DNA from peripheral blood was extracted using QIAamp DNA Blood Mini QIAcube Kit (Qiagen), according to the manufacturer’s protocols. All coding exons and exon-intron boundaries of 21 378 and 4493 genes were amplified using Twist HCE (Twist Bioscience HQ, San Francisco, CA, USA) and Clinical Exome Solution kits (SOPHiA Genetics, Boston, USA), for whole-exome sequencing (WES) and clinical exome sequencing (CES) respectively. Libraries were sequenced on the Illumina NextSeq platform (Illumina Inc., San Diego, CA, USA). Detected variation was confirmed via Sanger sequencing on an ABI Prism 3500 Genetic Analyzer (Thermo Fisher Scientific).
Data analysis
Sophia DDM software (SOPHiA Genetics, Boston, USA) was used for data analysis. Coverage was 99.11% at a minimum depth of 25 reads for targeted regions. For variant calling, sequencing data were aligned to the human reference genome, hg19. All variants in exons and exon–intron boundaries with a variant fraction over 0.20 and having minor allele frequency (MAF) under 0.01 in GnomAD were evaluated. Genes associated with sexual development and hypogonadism (Online Mendelian Inheritance in Man), were prioritised. The Human Gene Mutation Database Professional (2020) and ClinVar databases were screened for known variants (14, 15). To evaluate the potential effects of these variants, in silico analyses were performed using Mutation Taster, SIFT, Provean, PolyPhen, and Combined Annotation Dependent Depletion (16, 17, 18, 19, 20). Variants were classified according to the American College of Medical Genetics (ACMG) guidelines (21).
Results
Case reports
Patient 1
The patient was referred for evaluation of gynecomastia at the age of 15 years and 9 months. He was born to first-degree cousin parents of Turkish descent (Fig. 1A). His past medical history was unremarkable except for a history of two operations for hypospadias, at 2 and 6 years old. At the presentation, his weight was 75.6 kg (+0.93 SDS) and his height was 163 cm (−1.42 SDS). Physical evaluation showed small, low-set, protruding ears, bilateral gynecomastia of 7 cm with Tanner stage 4 appearance. Testicular volumes were 18 cm3, bilaterally. He had glanular hypospadias. The penile size was normal (9 × 2.2 cm). Pubic hair was at Tanner stage 4. Systemic examinations were normal. Karyotype was 46,XY. Adrenal function tests were normal, but serum gonadotropins were elevated, suggesting hypergonadotropic hypogonadism (Table 1). Measurements of anti- Müllerian hormone (AMH) and serum inhibins in patient 1 (P1) were not possible, due to technical reasons and circumstances beyond our control. Upon follow-up, he was treated with monthly intramuscular testosterone enanthate for 4 years. At the last visit, at 23 years and5 months, his weight was 77.6 kg (+0.5 SDS) and his height was 171.3 cm (−0.84 SDS). Systemic examinations were normal, he had bilateral gynecomastia of 8 cm with Tanner stage 5 appearance. Testicular volumes were 25 cm3 bilaterally. After 5 years of marriage, he presented to a urology clinic due to infertility. Sperm analysis, repeated three times, revealed azoospermia. He was unavailable for further investigation.
Figure 1.
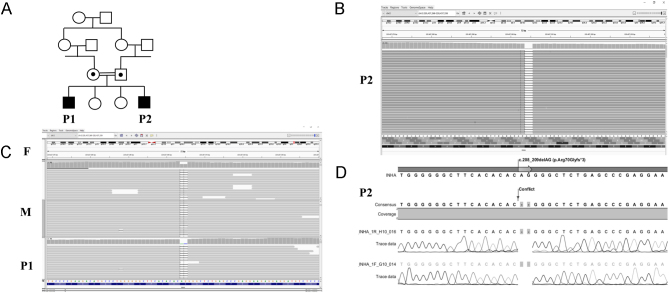
Genetic characteristics of the patients and parents with INHA mutations. (A) The patients (P1 and P2) were born to first-degree cousin parents. (B and C) Integrative genomics viewer images of INHA mutation of patients and parents,with a two nucleotide deletion revealed by next-generation sequencing. P1 and P2 were homozygous, father (F) and mother (M) were heterozygous carriers for the mutation. (D) Electropherogram showing the homozygous INHA variant in P2. A full colour version of this figure is available at https://doi.org/10.1530/EJE-21-1230.
Table 1.
Gonadal and adrenal function test results of patients with INHA mutations at presentation.
| Normal range | P1 | P2 | Mother | Father | |
|---|---|---|---|---|---|
| Age (years) | 15 9/12 | 12 7/12 | 50 | 55 | |
| Karyotype | 46, XY | 46, XY | |||
| FSH (mIU/mL) | 1.27–19.26 | 41.16 | 74 | 2.98 | 1.18 |
| LH (mIU/mL) | 1.7–8.6 | 13.74 | 26.7 | 1.63 | 5.57 |
| Testosterone (ng/mL) | 2.4–9.5 | 0.68 | 1.48 | – | 2.98 |
| Oestradiol (pg/mL) | < 25 | 23.7 | <20 | 192 | – |
| Cortisol (µg/dL) | 5–21 | 18.73 | 17.1 | ||
| ACTH (pg/mL) | 5–46 | 67.2 | 37.1 | ||
| DHEAS (µg/dL) | 80–560 | 267 | 129 | ||
| Androstenedione (ng/mL) | 0.6–3.1 | 2.02 | 1.31 | ||
| Prolactin (ng/mL) | 3.52–16.3 | 26 | 13.9 | ||
| Beta HCG (mIU/mL) | <5 | NA | <1.2 | ||
| CEA (ng/mL) | <5 | NA | 1.01 | ||
| AFP (ng/mL) | <9 | NA | <1 |
Patient 2
This patient is the affected sibling of P1. The patient was referred for evaluation at the age of 12 years 7 months, due to gynecomastia and scrotal pain. His past medical history was unremarkable. At the presentation, his weight was 49.4 kg (+0.18 SDS) and his height was 154.8 cm (+0.01 SDS). Physical evaluation showed small, low-set, protruding ears, and bilateral gynecomastia of 5 cm with Tanner stage 4 appearance. Testicular volumes were 15 cm3 bilaterally and testes were painful at palpation. He had glanular hypospadias. Penile size was normal (9 × 2.5 cm). Pubic hair was at Tanner stage 3. Systemic examinations were normal. Karyotype was 46,XY. Adrenal function tests were normal, but serum gonadotropins were elevated suggesting hypergonadotropic hypogonadism; anti-Mullerian hormone concentration was high for his age and sex, serum inhibin A and B were undetectable, and tumour markers were negative (Table 1 and Supplementary Table 1, see section on supplementary materials given at the end of this article). Upon follow-up, he was treated with monthly intramuscular testosterone enanthate (50 mg/i.m.) for 4 months. His gonadotropins remained elevated and testosterone concentrations were below normal (Supplementary Table 1). He had two hospital admissions for bilateral scrotal pain with suspicion of testicular torsion. He was operated on due to varicocele on the left testis at 16 years and 2 months. At the last visit, at 17 years and 1 month, his weight was 69.3 kg (-0.01 SDS) and his height was 168.4 cm (−1.04 SDS). Systemic examinations were normal; he had bilateral gynecomastia of 5 cm with Tanner stage 4 appearance. Testicular volumes were 25 cm3 (right) and 30 cm3 (left) and testes were painful at palpation. Testicular ultrasound revealed some parenchymal heterogeneities, calcifications, varicocele, and hydrocele on repeated occasions (Supplementary Table 2). Sperm analysis, repeated three times at 18 years and 10 months, revealed azoospermia.
The father of the patient was 55 years old and had typical male external genitalia following normal pubertal onset and normal pubertal course. His gonadal function tests are seen in Table 1. Serum inhibin A and inhibin B concentrations were 306 and <1 pg/mL (N: 75–475 pg/mL and <2 pg/mL, respectively). Testicular ultrasound was normal (testis volumes: right, 38.6 cm3; left, 40 cm3).
The mother of the patient was 50 years old, with normal pubertal development and menarche at 12 years old. She still has regular menstrual bleeding and her gonadal function tests are seen in Table 1.
Two healthy sisters of the patients had regular menstrual cycles, which started at 12 and 13 years of age. One sister is 27 years of age, married and with two children.
Molecular characterisations
WES (Patient 2 (P2)) and CES (P1) were performed. Identified variants were then excluded from consideration unless they met the following criteria: (i) variants with a MAF less than 0.01; (ii) variants in the coding sequence, splice variants, indels or duplications, and nonsynonymous changes; (iii) variants with a coverage of at least 20 reads; (iv) variants not recorded as benign in ClinVar database; (v) selecting pathogenic or disease-causing variants by all base conservation scores and functional prediction tools. Only one variant met all the above criteria: a homozygous variant (NM_002191.3:chr2:220437304_220437305delAG, c.208_209delAG, R70Gfs*3) in INHA (LOF Z-Score = 1.54, n < 0.7). This variant was investigated for segregation with the disease in the parents by CES and the homozygous variant was also tested in P1 and P2 by Sanger sequencing (Fig. 1B, C and D). The variant was homozygous in patients and heterozygous in parents (Fig. 1B, C and D). We evaluated WES and CES data of P1 and P2 for all individual and shared homozygous, heterozygous and hemizygous variants of known genes causing testicular dysgenesis, impaired testosterone biosynthesis, disorders of sex development and primary gonadal failure, as well as pathogenic or likely pathogenic variants in the other genes. None of the individual or shared variants were associated with pathogenicity. Moreover, we have also evaluated all other INHA variants in CES and WES data of P1 and P2 for any other potentially pathogenic variants and found no other variants that can be associated with the phenotype. All sequence data are available on request.
The INHA variant identified in our patients was not found in 200 ethnically matched in-house Turkish exomes, the Turkish whole-exome database, nor in 100 in-house exomes from other Turkish 46,XY disorder/differences of sex developmentor hypogonadism patients. This variant was not seen in either GnomAD, ExAC, 1000 Genomes or 6500ESP.
Discussion
This is the first report of male patients presenting with hypospadias, primary testicular failure, and infertility due to homozygous INHA mutations. Together, these data suggest significant roles for the inhibins in human sex development, steroidogenesis, and reproduction in 46,XY males.
The human INHA gene encodes a 366-amino acid inhibin subunit alpha protein, which is common to both inhibin A and B. Therefore, the homozygous 2-bp deletion mutation (c.208_209delAG, R70Gfs*3) identified in INHA,resulting in a frameshift in the coding sequence and a severely truncated inhibin subunit alpha protein, is predicted to cause significantly low serum inhibin A and B, as shown biochemically in P2. Although we were unable to measure serum activin levels, deficiency of inhibin subunit alpha protein could facilitate the formation of activin homodimers and enhance activin signalling in these patients. Hence, pituitary FSH secretion may increase the subsequent loss of negative feedback by inhibin and also potentially due to increased activin stimulation. High FSH induces constant stimulation of Sertoli cells, which could explain excessive AMH production, normal/high testicular volumes, and testicular pain observed in the patients. This contrasts with the general presentation of primary testicular failure, characterised by high FSH but low AMH and small testes. Male Inha knockout mice similarly exhibit testicular enlargement and grossly visible foci of haemorrhage by 5 weeks of age (6). However, these mice also develop testicular stromal tumours, which can contribute to increased testis size. Zebrafish lacking the inhibin a-subunit (inha−/−) also develop gonadal tumours in both sexes, similar to mice (9). Our patients did not develop tumours during their long follow-up period in adolescence and early adulthood. Nevertheless, the testicular USG of our patient revealed parenchymal heterogeneity and calcifications, which indicate close monitoring for the development of testicular tumours, considering the evidence from animal studies.
Testosterone deficiency was a major clinical feature of our patients. Knockdown of Inha expression impairs androgen biosynthesis by significant decreases in the expression of Cyp17a1, Cyp11a1, and Nr5a1, in mouse TM3 Leydig cell line (22). Similarly, knockdown of Inha expression in bovine ovarian theca cells resulted in suppression of androgen production subsequent to decreased Cyp17a1 (10). It has been hypothesised that testosterone deficiency develops postnatally as a result of extensive somatic cell tumours causing Leydig cell regression and damage (6, 7, 8). However, both patients with INHA mutations had hypospadias and did not have testicular tumours, suggesting that androgen deficiency exists prenatally and impairs the hormone-dependent stage of external genitalia development in males. Postnatally, low testosterone promotes LH secretion, which could explain high LH concentrations in our patients, as an indirect effect of inhibin deficiency. This is in contrast to mice, in which inhibin has no effect on LH secretion (8). Additionally, adult rat Leydig cells and the MA-10 Leydig cell line provides support for the hypothesis that not only the lack of inhibin action but also unopposed activin signalling contribute to the decline in testosterone production when hypospermatogenesis is present (23). During human chorionic gonadotrophin (hCG) stimulation, activin A directly suppresses testosterone secretion but enhances progesterone secretion from rat Leydig cell primary cultures. Likewise, treatment of MA-10 cells with activin-A enhances cAMP-stimulated progesterone secretion and STAR expression (23).
Both patients with INHA mutations exhibited significant gynecomastia. Expression of ovarian aromatase (cyp19a1a) increases dramatically in the ovary of inha−/−zebrafish. Juvenile female mutants show signs of early follicle activation or precocious puberty onset (9). It is known that the testicular tumours in Inha−/−mice also produce excessive quantities of oestradiol (6, 7, 12). Although oestrogens were not high in these patients, gynecomastia could have developed as a result of disrupted testosterone/oestrogen balance due to low testosterone/oestrogen ratio or increased local aromatase activity.
Although the effects of inhibin on fertility have been reported in different species, sometimes with discrepancies, its functional importance in male reproduction remains largely unknown in humans. Knockout of the Inha gene in mice resulted in infertility in both females and males, mostly due to the formation of gonadal tumours and cachexia-related death (6). Deletion of inha in female zebrafish disrupted follicle development and maturation; however, in contrast to females, inha null male zebrafish showed normal spermatogenesis and fertility (9). In males, inhibin inhibits spermatogonial DNA synthesis (24) and reduces spermatogonia number (25), whereas activin stimulates spermatogonial proliferation in vitro (26). Inhibin synthesis by Sertoli cells fluctuates during the stages of spermatogenesis (27, 28). The binding of inhibin to different populations of germ cells (29) also changes during the various stages of spermatogenesis. These data support the critical intragonadal paracrine and/or autocrine role of inhibins, which mediate the interaction between Sertoli and germ cells. We have observed azoospermia and infertility in our patients with homozygous INHA mutations. Our results, therefore, emerge as the first human evidence of the physiological significance of inhibins in male reproduction. Some heterozygous missense variants in INHA including Ala257Thr, Ser92Asn, His175Gln, and Ala182Asp have been associated with POI, primary and secondary amenorrhoea in women (13, 30). Male and female mice heterozygous for the Inha deletion are normal and fertile (6). We identified no females homozygous for the INHA mutation in this family. However, the heterozygous mother and father of our patients had normal sex development, normal pubertal development and normal reproductive functions, and normal gonadotropin, AMH, and inhibin concentrations.
As testicular descent was normal in our patients, neither testosterone deficiency nor azoospermia could be explained by cryptorchidism.
As a potential limitation of our study, we could not perform activity studies of the INHA variant identified in our patients. Thus, it remains possible, although unlikely, that despite the severe truncation predicted due to the c.208_209delAG mutation of INHA, some biologic activity of the INHA protein is retained. Furthermore, inhibin and AMH concentrations could only be measured in P2. Nevertheless, available biochemical, clinical, and molecular findings support INHA deficiency in these two siblings – similar to the knockout animal models.
In conclusion, the clinical and molecular characteristics of these two patients suggest that homozygous INHA mutations cause decreased prenatal and postnatal testosterone production and infertility in males. Our results highlight the essential role of INHA and inhibins in human male sex development, testicular function, and reproduction.
Supplementary Material
Declaration of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of this article.
Funding
This work has been supported by the Medical Research Council of Marmara University (Project Grant SAG-A-120418-0152). Research in AG’s laboratory was funded by the UK Medical Research Council through core funding (MC_U142684167) at the Mammalian Genetics Unit, MRC Harwell Institute.
Author contribution statement
E A A, M E, A G and T G designed the study. B G T, T S M, B S, S T and A B recruited and clinically characterised the patients. T G and M E conducted and analysed biochemical measurements. E A A performed and analysed the sequencing data. T G, A G, E A A and A B prepared the draft manuscript. All authors contributed to the discussion of results, and edited and approved the final manuscript.
Acknowledgements
The authors are deeply grateful to the patients and family without whom this study could not have been performed.
References
- 1.Woodruff TK, Meunier H, Jones PB, Hsueh AJ, Mayo KE. Rat inhibin: molecular cloning of alpha- and beta-subunit complementary deoxyribonucleic acids and expression in the ovary. Molecular Endocrinology 19871561–568. ( 10.1210/mend-1-8-561) [DOI] [PubMed] [Google Scholar]
- 2.Cuevas P, Ying SY, Ling N, Ueno N, Esch F, Guillemin R, Healy D, Ta S. Immunohistochemical detection of inhibin in the gonad. Biochemical and Biophysical Research Communications 198714223–30. ( 10.1016/0006-291x(8790446-3) [DOI] [PubMed] [Google Scholar]
- 3.Robertson DM, Foulds LM, Leversha L, Morgan FJ, Hearn MT, Burger HG, Wettenhall RE, de Kretser DM. Isolation of inhibin from bovine follicular fluid. Biochemical and Biophysical Research Communications 1985126220–226. ( 10.1016/0006-291x(8590594-7) [DOI] [PubMed] [Google Scholar]
- 4.Ling N, Ying SY, Ueno N, Esch F, Denoroy L, Guillemin R. Isolation and partial characterization of a Mr 32,000 protein with inhibin activity from porcine follicular fluid. PNAS 1985827217–7221. ( 10.1073/pnas.82.21.7217) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Martens JW, de Winter JP, Timmerman MA, McLuskey A, van Schaik RH, Themmen AP, de Jong FH. Inhibin interferes with activin signaling at the level of the activin receptor complex in Chinese hamster ovary cells. Endocrinology 19971382928–2936. ( 10.1210/endo.138.7.5250) [DOI] [PubMed] [Google Scholar]
- 6.Matzuk MM, Finegold MJ, Su JG, Hsueh AJ, Bradley A. Alpha-inhibin is a tumour-suppressor gene with gonadal specificity in mice. Nature 1992360313–319. ( 10.1038/360313a0) [DOI] [PubMed] [Google Scholar]
- 7.Matzuk MM, Kumar TR, Shou W, Coerver KA, Lau AL, Behringer RR, Finegold MJ. Transgenic models to study the roles of inhibins and activins in reproduction, oncogenesis, and development. Recent Progress in Hormone Research 199651123–154; discussion 55–57. [PubMed] [Google Scholar]
- 8.Wijayarathna R, de Kretser DM, Meinhardt A, Middendorff R, Ludlow H, Groome NP, Loveland KA, Hedger MP. Activin over-expression in the testis of mice lacking the inhibin α-subunit gene is associated with androgen deficiency and regression of the male reproductive tract. Molecular and Cellular Endocrinology 2018470188–198. ( 10.1016/j.mce.2017.10.013) [DOI] [PubMed] [Google Scholar]
- 9.Lu H, Zhao C, Zhu B, Zhang Z, Ge W. Loss of inhibin advances follicle activation and female puberty onset but blocks oocyte maturation in zebrafish. Endocrinology 2020161 bqaa184. ( 10.1210/endocr/bqaa184) [DOI] [PubMed] [Google Scholar]
- 10.Laird M, Glister C, Cheewasopit W, Satchell LS, Bicknell AB, Knight PG. ‘Free’ inhibin α subunit is expressed by bovine ovarian theca cells and its knockdown suppresses androgen production. Scientific Reports 20199 19793. (https://doi.org/) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cipriano SC, Chen L, Kumar TR, Matzuk MM. Follistatin is a modulator of gonadal tumor progression and the activin-induced wasting syndrome in inhibin-deficient mice. Endocrinology 20001412319–2327. ( 10.1210/endo.141.7.7535) [DOI] [PubMed] [Google Scholar]
- 12.Nagaraja AK, Agno JE, Kumar TR, Matzuk MM. Luteinizing hormone promotes gonadal tumorigenesis in inhibin-deficient mice. Molecular and Cellular Endocrinology 200829419–28. ( 10.1016/j.mce.2008.06.019) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dixit H, Deendayal M, Singh L. Mutational analysis of the mature peptide region of inhibin genes in Indian women with ovarian failure. Human Reproduction 2004191760–1764. ( 10.1093/humrep/deh342) [DOI] [PubMed] [Google Scholar]
- 14.Stenson PD, Mort M, Ball EV, Howells K, Phillips AD, Thomas NS, Cooper DN. The Human Gene Mutation Database: 2008 update. Genome Medicine 20091 13. ( 10.1186/gm13) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Landrum MJ, Lee JM, Benson M, Brown GR, Chao C, Chitipiralla S, Gu B, Hart J, Hoffman D, Jang W. ClinVar: improving access to variant interpretations and supporting evidence. Nucleic Acids Research 201846 D1062–D1067. ( 10.1093/nar/gkx1153) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sim NL, Kumar P, Hu J, Henikoff S, Schneider G, Ng PC. SIFT web server: predicting effects of amino acid substitutions on proteins. Nucleic Acids Research 201240W452–W457. ( 10.1093/nar/gks539) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Adzhubei I, Jordan DM, Sunyaev SR. Predicting functional effect of human missense mutations using PolyPhen-2. Current Protocols in Human Genetics 2013Chapter 7Unit7.20. ( 10.1002/0471142905.hg0720s76) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Choi Y, Chan AP. PROVEAN web server: a tool to predict the functional effect of amino acid substitutions and indels. Bioinformatics 2015312745–2747. ( 10.1093/bioinformatics/btv195) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schwarz JM, Cooper DN, Schuelke M, Seelow D. MutationTaster2: mutation prediction for the deep-sequencing age. Nature Methods 201411361–362. ( 10.1038/nmeth.2890) [DOI] [PubMed] [Google Scholar]
- 20.Kircher M, Witten DM, Jain P, O’Roak BJ, Cooper GM, Shendure J. A general framework for estimating the relative pathogenicity of human genetic variants. Nature Genetics 201446310–315. ( 10.1038/ng.2892) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, Grody WW, Hegde M, Lyon E, Spector Eet al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genetics in Medicine 201517405–424. ( 10.1038/gim.2015.30) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang Y, Bilandzic M, Ooi GT, Findlay JK, Stenvers KL. Endogenous inhibins regulate steroidogenesis in mouse TM3 Leydig cells by altering SMAD2 signalling. Molecular and Cellular Endocrinology 201643668–77. ( 10.1016/j.mce.2016.07.026) [DOI] [PubMed] [Google Scholar]
- 23.Winters SJ, Moore JP, Jr, Clark BJ. Leydig cell insufficiency in hypospermatogenesis: a paracrine effect of activin-inhibin signaling? Andrology 20186262–271. ( 10.1111/andr.12459) [DOI] [PubMed] [Google Scholar]
- 24.Franchimont P, Croze F, Demoulin A, Bologne R, Hustin J. Effect of inhibin on rat testicular desoxyribonucleic acid (DNA) synthesis in vivo and in vitro. Acta Endocrinologica 198198312–320. ( 10.1530/acta.0.0980312) [DOI] [PubMed] [Google Scholar]
- 25.van Dissel-Emiliani FM, Grootenhuis AJ, de Jong FH, de Rooij DG. Inhibin reduces spermatogonial numbers in testes of adult mice and Chinese hamsters. Endocrinology 19891251899–1903. ( 10.1210/endo-125-4-1898) [DOI] [PubMed] [Google Scholar]
- 26.Mather JP, Attie KM, Woodruff TK, Rice GC, Phillips DM. Activin stimulates spermatogonial proliferation in germ-Sertoli cell cocultures from immature rat testis. Endocrinology 19901273206–3214. ( 10.1210/endo-127-6-3206) [DOI] [PubMed] [Google Scholar]
- 27.Bhasin S, Krummen LA, Swerdloff RS, Morelos BS, Kim WH, diZerega GS, Ling N, Esch F, Shimasaki S, Toppari J. Stage dependent expression of inhibin alpha and beta-B subunits during the cycle of the rat seminiferous epithelium. Endocrinology 1989124987–991. ( 10.1210/endo-124-2-987) [DOI] [PubMed] [Google Scholar]
- 28.Cai K, Hua G, Ahmad S, Liang A, Han L, Wu C, Yang F, Yang L. Action mechanism of inhibin α-subunit on the development of Sertoli cells and first wave of spermatogenesis in mice. PLoS ONE 20116 e25585. ( 10.1371/journal.pone.0025585) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Woodruff TK, Borree J, Attie KM, Cox ET, Rice GC, Mather JP. Stage-specific binding of inhibin and activin to subpopulations of rat germ cells. Endocrinology 1992130871–881. ( 10.1210/endo.130.2.1310280) [DOI] [PubMed] [Google Scholar]
- 30.Dixit H, Rao KL, Padmalatha V, Kanakavalli M, Deenadayal M, Gupta N, Chakravarty BN, Singh L. Expansion of the germline analysis for the Inha gene in Indian women with ovarian failure. Human Reproduction 2006211643–1644. ( 10.1093/humrep/del129) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



 This work is licensed under a
This work is licensed under a