Abstract
Entrainment is a phenomenon in which two oscillators interact with each other, typically through physical or chemical means, to synchronize their oscillations. This phenomenon occurs in biology to coordinate processes from the molecular to organismal scale. Biological oscillators can be entrained within a single cell, between cells or to an external input. Using six illustrative examples of entrainable biological oscillators, we discuss the distinctions between entrainment and synchrony and explore features that contribute to a system's propensity to entrain. Entrainment can either enhance or reduce the heterogeneity of oscillations within a cell population, and we provide examples and mechanisms of each case. Finally, we discuss the known functions of entrainment and discuss potential functions from an evolutionary perspective.
Keywords: entrainment, biological oscillators, synchrony, phase response curve, Arnold tongue
1. Introduction
Oscillating systems can interact with each other in various ways. They can enhance or negate each other's effects (constructive and destructive interference, respectively) or synergize with each other to achieve amplitudes greater than the sum of the two systems (resonance). When two oscillating systems interact, one or both can experience an alteration in frequency to become phase-locked, meaning that the phase difference between the two oscillating systems remains constant in time and is robust to perturbations [1]. This situation is called entrainment.
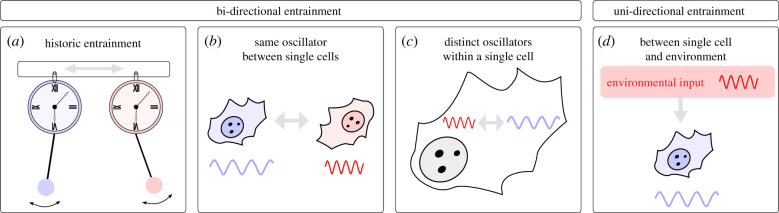
Entrainment was originally described as two pendulum clocks coupled through a wooden structure [2] (figure 1a). Synchronization in this system was achieved via mechanical vibrations through the wooden coupling bar. Oscillations are also found in various biological systems and can operate at the molecular level (e.g. cardiac cell beating) or at the organismal level (e.g. sleep–wake cycles). Entrainment of these oscillations can occur through interactions between single cells, within a single cell or between a cell and its environment (figure 1b,d).
Figure 1.
Entrainment types and their directionality. (a) Entrainment as originally described between two physically connected oscillating pendulums. (b) Entrainment of the same oscillator in two neighbouring single cells. (c) Entrainment of distinct oscillators within a single cell. (d) Entrainment of an oscillator within a single cell by an external periodic input.
Biological oscillators can entrain in a variety of ways. Two biological oscillators in neighbouring cells can interact and influence each other through their extracellular environment (figure 1b). Entrainment between cells often occurs through secreted factors and therefore becomes apparent as cell density increases [3–6]. It allows coordination between cells in a tissue in order to perform a function: for example, cardiac cells synchronize their oscillations in order to provide a strong single voltage that leads to heart contraction [7,8]. Two biological clocks can also entrain within a single cell, as observed between the circadian and cell cycle oscillators (figure 1c). Entrainment of oscillators within a single cell allows for synchronizing the processes controlled by the two individual oscillators. Last, the frequency of a biological clock can entrain to an environmental periodic rhythm (figure 1d) that is itself unaffected by the biological oscillator. The most prevailing example of such unidirectional entrainment is the circadian clock, in which sleep–wake cycles entrain to light–dark cycles [9,10]. Entrainment between a cell and its environment allows organisms to keep their physiology in synchrony with their surrounding rhythms.
2. Key principles of entrainment
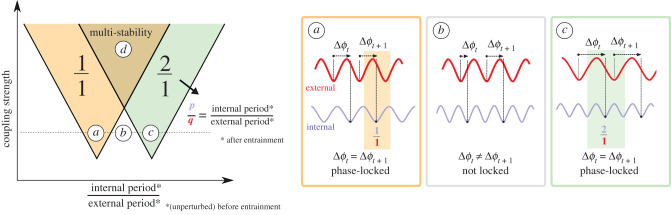
Entrainment depends on two basic conditions: (i) the coupling strength between the oscillator and external input and (ii) the similarity between the intrinsic frequencies of the internal oscillator and the external input in the absence of interaction [1]. Generally, a stronger coupling strength and closer intrinsic frequencies favour entrainment, though the exact requirement varies in different systems. The entrained state (or locked state) is represented by a rational number p/q; after p periods of the internal oscillator and q periods of the external oscillator, the system returns to the same state. As the coupling strength increases, phase locking becomes possible at a wider range of external periods (depicted by a broadening of Arnold tongues, see box 1), and the entrained mode is more robust against random fluctuations. Further increasing coupling strength may result in complex phenomena such as multi-stability, in which multiple entrainment modes coexist, and chaos. These are depicted by the overlap of different ‘tongues’. The transition between a robust locked state and a chaotic one has been observed in a classic example of periodically stimulated cardiac cells, in which a small variation of the period of the electrical stimuli caused a transition between normal and pathological behaviour of cardiac tissue (dysrhythmia) [13].
Box 1. The coupling and uncoupling between the oscillator and external input can be summarized in an ‘Arnold tongue’ plot. The Arnold tongue plot can be interpreted in the following way: with a fixed coupling strength (y-axis), if the intrinsic frequency of one oscillator traverses horizontally across the Arnold tongue plot, the coupled system will either stay not locked (case b) or be locked into distinct frequency modes featuring fixed p/q ratios (cases a and c). Phase locking is defined by measuring ϕ(t) and Δϕ(t), with ϕ(t) being the phase of an oscillator relative to the start of the cycle, expressed as a fraction of the period ϕ(t) ∈[0,2π], and Δϕ(t) being the difference in phase between two periodic signals at a given time Δϕ(t) = ϕoscillator1(t) − ϕoscillator2(t). When the phase difference Δϕ(t) between two signals is constant in time, the two signals are considered to be phase-locked. Traversing vertically over the plot (increasing coupling strength) illustrates how the coupled system becomes more robust against fluctuations (broadening of Arnold tongues) or can lead to multi-stability (case d) or other irregular dynamics such as chaos. ‘Tongues’ associated with high-order entrainment modes (5/4, 3/2, etc.) are usually smaller than that for the equal-frequency model (1/1) and therefore harder to observe experimentally (figure 2, Other entrainment ratios). The amplitude during entrainment remains unaltered as shown both theoretically [11] and experimentally [12].
3. Examples of biological oscillators exhibiting entrainment
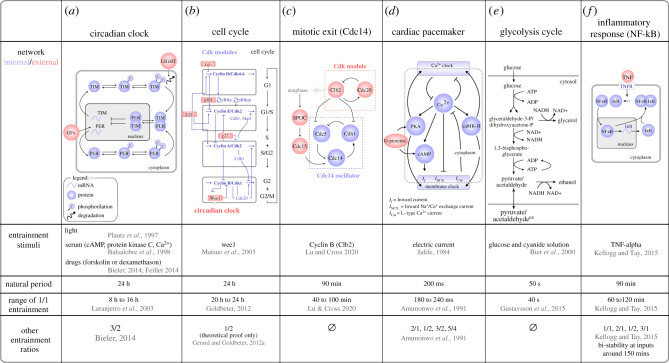
We will explore entrainment focusing on six biological examples of autonomous oscillators, which have been shown to entrain experimentally (figure 2). For each example, we present the simplest model that accounts for oscillatory behaviour, along with the node(s) receiving the stimuli for entrainment. Each example follows the basic principle of biochemical oscillators but differs in terms of its network architecture, the nature of the oscillations, their time-scale and the number of entrainment modes. All examples have a negative feedback loop within their core network (see blue networks in figure 2) with additional positive feedback loops providing robustness [24]. Other details of the networks vary with regard to the number of nodes, number of positive and negative interactions and number of points of coupling to external oscillators. In addition, the oscillating factors differ between the various systems. For example, in the circadian clock example, mRNA and protein levels oscillate [25], nuclear factor kappa B (NF-κB) and Cdc14 oscillate in their nuclear–cytoplasmic localization [23,26] and the glycolysis network oscillates in the products of enzymatic reactions [27]. The time-scale of oscillations also varies between these systems, with transcriptionally regulated systems exhibiting longer time-scales (hours for NF-κB and the circadian clock) and oscillators relying on enzymatic reactions operating on shorter time-scales (less than a minute for glycolytic oscillations) (figure 2, Natural period). In this review, we will not focus on systems that show irregular oscillations, such as bursting dynamics of calcium ions [28], nuclear translocation of Msn2 [29], insulin secretion by B-cells [30] or neuron spiking [31].
Figure 2.
Six biological cases of entrainment. For each oscillator, the internal minimal network (blue) and external nodes (red) are portrayed, along with the stimuli used for entrainment and the observed entrainment ratios. (a) The fly circadian clock is regulated at the levels of transcription, protein stability and post-translational modifications [14]. It responds to light and GFs, but it can oscillate freely in the dark [9]. (b) The mammalian cell cycle network contains four coupled modules each centred around one cycle/Cdk complex which promotes progression or transition into the ordered succession of the cell cycle phases G1, S, G2 and M. The cell cycle components Wee1, p21 and cyclin E are transcriptionally regulated by the circadian clock [15]. (c) The Cdc14 network module is a negative feedback loop controlling cycles of nucleolar sequestration and release of Cdc14, which is essential for mitotic exit in budding yeast [16]. Each component of this loop (Cdc14, Cdc5, Cdh1) is coupled to the cell cycle. (d) Cardiomyocytes of the sinoatrial node (SAN) autonomously oscillate through action potentials that result from the opening and closing of sodium, calcium and potassium channels in their membrane, creating depolarization and repolarization oscillations [17,18]. (e) Glycolysis consists of the step-by-step breakdown of glucose and storage of the released Gibbs energy in the form of ATP. Oscillations correspond to changes in the concentration of glycolytic metabolites nicotinamide adenine dinucleotide plus hydrogen (NADH) and ATP. The molecular mechanism for oscillations is based on the speed of enzymatic reactions [12]. Sustained glycolytic oscillations require both glucose and cyanide to be present in the medium [19,20]. (f) The transcription factor NF-κB oscillates between the cytoplasm and nucleus in response to the inflammatory signal TNF-alpha [21,22]. TNF-alpha signalling induces the dissociation of the IκB::NF-κB complex in the cytoplasm, allowing NF-κB to enter the nucleus and activate transcription of its inhibitor IκB, which sequesters NF-κB in the cytoplasm [23].
The six biological examples covered here have been extensively modelled using ordinary differential equations (ODEs) to describe their regulatory networks [21,32–37]. Dynamical systems tools, such as ODEs, phase portraits and bifurcation diagrams, are keys to understand how various systems differ in their requirements for initiating and sustaining oscillations [38]. For example, a model of the cell cycle [39] shows self-sustained oscillations only in the presence of growth factor (GF), thus defining GFs as a trigger between quiescence (non-oscillatory state) and proliferation (oscillatory state). Glycolytic oscillations require both glucose and cyanide to be sustained [19,20], with glucose alone leading to dampened oscillations but the addition of cyanide leading to sustained oscillations. GF and cyanide are thus Hopf parameters that are responsible for a Hopf bifurcation [20,36,39], meaning that they lead the system to transition from steady state (non-oscillatory) to a limit cycle (self-sustained oscillations). In most cases, oscillation triggers (Hopf parameters) also serve as entrainment stimuli. For example, entrainment of glycolytic oscillations by cyanide [20], or entrainment of NF-κB by tumour necrosis factor (TNF) [40], but that is not always the case, for example GFs only initiate but cannot entrain the cell cycle [39].
4. Distinguishing between entrainment and other mechanisms leading to synchrony
Synchrony is the empirical observation of two systems oscillating in phase, which can result from either entrainment or other mechanisms such as gating [41,42]. During entrainment, all phases of the follower oscillator must be affected by the leading oscillator—in other words, the oscillatory curve of the follower must be proportionately stretched out or compressed through all phases. By contrast, during gating, the leading oscillator defines windows of time in which different phases of the follower oscillator can occur. As opposed to entrainment, a gating mechanism follows these three principles: (i) arresting the lead oscillator at any constant level will arrest the follower oscillator; (ii) only 1 : 1 ratios will be observed; and (iii) the leading oscillator impacts only specific phases of the follower oscillator.
Distinguishing between gating and entrainment mechanisms has met with varying degrees of success. Strong evidence in favour of entrainment was obtained for the coordination between the cell cycle and Cdc14 nucleolar sequestration and release [43]. Blocking the cell cycle by maintaining cyclin B at constant physiological levels did not block Cdc14 oscillations, ruling out a gating mechanism. The mechanisms governing other synchronized systems, such as the synchronization between the cell cycle and circadian rhythm, have not reached consensus. Among the studies in favour of entrainment [15,33,44], Feillet et al. [44] reset the circadian clock using a glucocorticoid agonist and observed a variety of coupled states between the clock and the cell cycle (1 : 1, 1 : 2, 3 : 2), supporting an entrainment mechanism and aligned with computational studies [15]. Among the studies suggesting a gating mechanism [45–47], Laranjeiro et al. [45] manipulated light–dark cycles in zebrafish cells to vary the period of the circadian clock and observed an exclusive effect on the length of G1 with S/G2/M phases remaining relatively constant. As articulated above, impact over specific phases of the follower oscillator is characteristic of a gating mechanism.
Most studies in favour of entrainment between the circadian and cell cycle oscillators consider unidirectional entrainment with the circadian clock unidirectionally entraining the cell cycle (figure 1d). Circadian rhythms persisted in cells whose division was inhibited, initially suggesting unidirectional entrainment [48]. However, the possibility of bi-directional entrainment has not been ruled out [15]. It is plausible that altered cell cycle dependent changes in transcription or reduced protein concentrations after cell division may affect the circadian phase [49–51]. Future work using synthetic biology approaches to study isolated or minimally coupled oscillators could help elucidate both the mechanisms leading to synchrony in other systems (entrainment versus gating) and the directionality of entrainment (uni- versus bi-directionality).
5. Different biological oscillators vary in their propensity for entrainment
The study of entrainment can be greatly simplified by studying the response of an oscillator to a single pulse instead of to a periodic input. Such single perturbation is often shorter than the period of the oscillating system and can cause a shift in the original phase, either advancing or retarding the oscillations depending on its start time relative to the phase of the natural oscillator. A common way to capture this dependency is through phase response curves (PRCs) [52,53]. The features of a PRC, such as its magnitude (amplitude in the y-axis), zero points (intercept of the x-axis) and discontinuities (i.e. phase singularities), impact the propensity for entrainment [1] (box 2).
Box 2. The inclination of a system to be entrained depends on its sensitivity to the perturbation and can be interpreted from the shape and properties of the phase response curves (PRCs). A PRC describes the magnitude of phase changes (also called phase shift) by plotting how much the oscillation is shifted in time (i.e. new phase ϕnew minus unperturbed old phase ϕold on the y-axis) as a function of the phase at which it is received (x-axis).
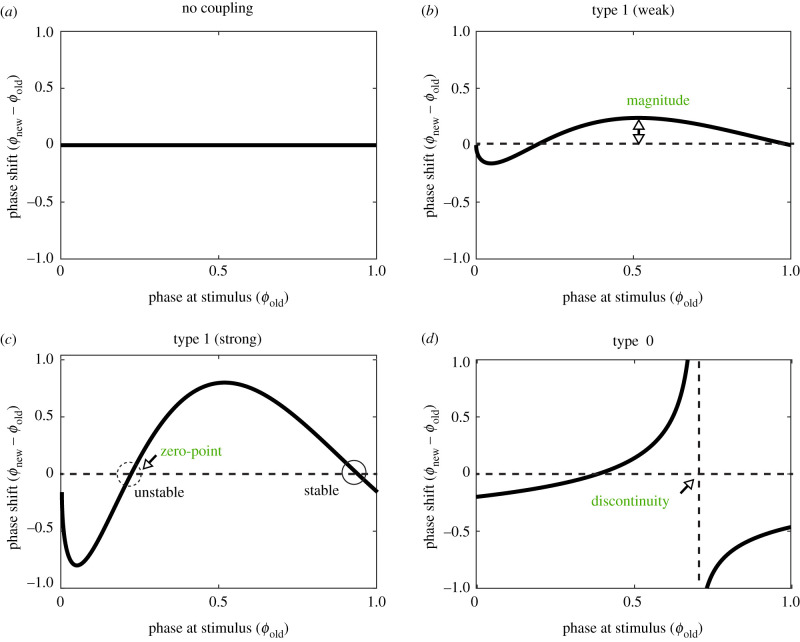
A system's PRCs can change by varying the amplitude or duration of the external pulse [54]. Stable entrainment of NF-κB oscillators (figure 2f) requires a minimal duration and minimal concentration of the synchronizing TNF pulse [55]. The sensitivity of fly circadian clock has been tested by varying the duration of light pulses, which mainly affect the degradation of the clock gene TIM and can entrain the system in all tissues (figure 2a) (both neuronal and non-neuronal tissues in drosophila are photoreceptive) [9,10]. Short light pulses lead to a PRC with a small magnitude and a continuous transition between phase advance and phase delay (called ‘type 1’ resetting) [56] (box 2b). As the duration of the light pulse increases, the PRC's magnitude increases (box 2c) and may show discontinuity between phase advance and phase delay regions (called ‘type 0’ resetting) (box 2d). Around this discontinuity, the new phase after perturbation is highly sensitive to the old phase and may lead to complex behaviours of the system, such as chaos [57,58]. Similarly, the PRC of the circadian clock of cyanobacteria is continuous, i.e. lacks phase singularities, under a short temperature pulse [59]. The phase shift increases with the increase of pulse duration, while the transition between phase advance and delay becomes sharper. Consequently, the PRC exhibits a singularity point above a certain pulse duration. This phase singularity may cause population-level arrhythmicity when certain perturbations cause stochastic phases of oscillations in individual cells. In the case of cardiac pacemaker, discontinuity of PRC has been suggested to lead to cardiac arrhythmias [1,60,61].
Absence of phase shift, i.e. flat curve (box 2a), indicates no possibility for entrainment. PRCs with low (box 2b) or high (box 2c) magnitude on phase shift indicate lower or higher propensity for entrainment. A PRC may have multiple zero points, with well-known examples in the circadian system [48,57,59,62], meaning that a perturbation administrated when the system's phase is at these points will not cause phase change [1]. A PRC may have multiple zeros corresponding to distinct entrainment modes. The slope at a zero point of a PRC dictates the stability of this entrainment state: a negative or positive slope predicts stable or unstable (i.e. further from or closer to uncoupling regions) entrainment, respectively. Last, PRCs can exhibit phase singularities (marked by a vertical line in box 2d), at which the phase resetting is very sensitive to the phase at stimulus.
6. The impact of entrainment on heterogeneity between individual cells
During entrainment, each single-cell oscillator locks to the external input (figure 1d). If the population of cells is initially heterogeneous in its oscillations, phase locking results in a loss of heterogeneity. For example, the glycolytic oscillations of isolated yeast cells (figure 2e) display a broad distribution of frequencies around half a minute [63]. Periodic cyanide input can entrain this heterogeneous population through phase shifting (see section above). All cells’ oscillations become synchronized after the first cyanide pulse [12] reducing population heterogeneity. Furthermore, both robust and weak (or non-) oscillating cells entrain to the periodic input, further reducing population heterogeneity.
Entrainment through intercellular communication (figure 1c) can also decrease cell-to-cell variability. During glycolysis in yeast cultures, acetaldehyde secreted by cells induces synchronization of metabolic oscillations (even converting non-oscillating cells to an oscillatory state) [64]; this effect occurs only above a minimal cell density [3,19,63,65]. Similarly, dissociated cells of many organs show high heterogeneity of their oscillations. Isolated individual sinoatrial node cardiac pacemaker cells have varying periods [66–69], but at high density, they exhibit the stereotypical 80 beats per minute [61,70]. Dispersed cultures of suprachiasmatic nucleus (SCN) neurons behave as non-synchronous single-cell oscillators and fire with widely varying circadian periods distinct from 24 h [71,72]. When neurons are maintained at high density, either in explants or dispersals, their periods synchronize [5,73] to achieve tissue-level synchrony, in which all cells oscillate at the stereotypical 24 h period. The secreted factor synchronizing circadian oscillations of SCN neurons is less clear than that for glycolysis. Separation of the dorsal and ventral SCN resulted in a loss of synchrony of the neural rhythms of the dorsal (but not ventral) SCN, suggesting that a neurotransmitter released by the ventral SCN maintains synchrony throughout the SCN [74]. Indeed, some of the candidate synchronizing factors (neurotransmitters γ-aminobutyric acid, vasoactive intestinal peptide and gastrin-releasing peptide) changed the firing rate of dorsal SCN neurons [73,75,76].
When an initially heterogeneous cell population entrains through intercellular communication to become more homogeneous, it is not clear which cells will dominate the final behaviour of the population. When two cell suspensions of yeast oscillating out of phase were mixed, synchronization was dominated by the culture whose NADH levels were decreasing [77,78]. A different mechanism operated when non-synchronized oscillating cardiomyocytes were placed into physical contact through a connected agarose microchamber [79,80] to synchronize their beating. The cells synchronized to the one showing smaller fluctuations in beating. Thus, it appears that different mechanisms can be employed to determine which of two functionally equivalent oscillators dominates during entrainment.
In some cases, entrainment can increase population heterogeneity, for example when it involves bi-stable responses. This phenomenon has been observed following periodic stimulation of the NF-κB pathway by the cytokine TNF-alpha (figure 2f) [40]. A single pulse of TNF-alpha leads to NF-κB oscillations with a period of 90 min. When the TNF-alpha signal was provided in an oscillatory manner, cells entrained at multiple ratios for a given TNF periodic input. The multi-stability in entrainment ratios depended on the input frequency. When the stimulation period corresponded to the original unaltered period, 90 min, the population entrained nearly homogeneously with a 90 min phase-locked oscillation (1 : 1 ratio). By contrast, during a 150 min stimulation period cells showed a mixture of cellular responses including 150 min oscillation (1 : 1), 75 min oscillation (1 : 2), or without phase locking. Multi-stability rose from extrinsic noise (variation in signalling parameters between cells) that caused a significant broadening of the entrainment Arnold tongues regions (see box 1), revealing an important function of noise in allowing for a heterogeneous response to a periodic stimulus [40].
7. Plausible functions of entrainment
Entrainment is a ubiquitous phenomenon in biology, found across species and in diverse systems. In some cases, the function of entertainment is clear. For example, systems in which a population of cells synchronizes to achieve a specific coordinated task, such as the synchronization of SCN neurons to light–dark cycles provides further synchronization in downstream organs [81]. In the cardiac rhythms, synchronization of pacemaker cells provides blood circulation [61]. In systems in which cellular information is encoded in frequency, such as the frequency modulation of the transcription factor Crz1 by extracellular calcium concentration ensuring appropriate downstream expression [82] or frequency of motor protein-based oscillations in neurons is a read-out for axonal length [83]; a potential function for entrainment is to strengthen such a modality of signalling. However, in many other systems, the biological function for entrainment remains unclear. For example, despite its ubiquity, the physiological function of glycolytic oscillations and their entrainment are still uncertain [63]. In addition, while entrainment of NF-κB was shown to coordinate the transcriptional response downstream of NF-κB [40], entrainment in this system was achieved artificially through period stimulation by TNF-alpha, which is not known to oscillate in vivo.
Entrainment of biological clocks may also play an important role during evolution. As one example, the oscillation of cyclin-dependent kinase (CDK) activity drives other periodic events, such as DNA replication and chromosome separation, during the cell cycle. Interestingly, CDKs seem to have appeared late during evolution [84], raising the question as to how cells synchronize the series of events required for proliferation prior to CDK emergence. Recent studies in yeast identified several processes that show periodic behaviours even in the absence of CDK oscillator. These CDK-independent oscillators include budding, DNA replication, centrosome duplication, transcription and Cdc14 release [85–89]. Intriguingly, their intrinsic periods are close to the normal cell cycle duration. It has been speculated that cell cycle processes may be intrinsically oscillatory before the emergence of CDK, and these oscillators entrain each other to create an aggregate rhythm [43]. The master CDK oscillator may have evolved to regulate other oscillators in order to yield a stable entrainment structure. This satisfies the evolutionary requirement of utility of intermediate forms [90]. Entrainment of autonomous oscillators could have been important in early cell cycle evolution, raising the possibility that it plays a role in promoting a stable cell cycle rhythm in modern eukaryotes.
8. Future perspectives
Many aspects of entrainment remain unexplored mainly due to the complex network interactions controlling and connecting oscillations in biology. One approach that can be useful in disentangling interconnected oscillatory systems is synthetic biology. Synthetic biology allows precise control of entrainment networks and has been used to study extremely complex systems such as a built-in circadian clock [81,91] or quorum sensing [4]. In the future, building synthetic oscillators that are heavily intertwined in nature (such as the cell cycle or the Cdc14 oscillators) could elucidate the mechanisms behind their coupling and avoid the use of genetic manipulation in their original natural systems. Finally, the potential of an oscillatory system to be entrained has not been explored in many networks, even in well studied oscillatory systems such as p53 or Msn2 [29,92,93], both having the potential to be entrained using distinct combinations of drugs.
Advances in technologies such as microfluidic devices, microscopy and optical traps allow precise spatial and temporal control of a cell's environment and facilitate single-cell measurements of oscillatory behaviours. Synthetic biology approaches along with technological advances will be essential to explore fundamental questions of entrainment such as the molecular determinants of the entrainment capability of a system and the functional consequences of entrainment.
Acknowledgements
We would like to thank Bill Jia and Adrian Granada for discussions.
Data accessibility
This article has no additional data.
Authors' contributions
A.Ji.: conceptualization, writing—original draft, writing—review and editing; Y.L.: conceptualization, writing—original draft, writing—review and editing; A.Ja.: conceptualization, supervision, writing—original draft, writing—review and editing; G.L.: conceptualization, supervision, writing—original draft, writing—review and editing.
All authors gave final approval for publication and agreed to be held accountable for the work performed therein.
Competing interests
We declare we have no competing interests.
Funding
Research in the Lahav Lab is supported by National Institutes of Health grant no. NIH R35 GM139572 and by the Ludwig Center at Harvard. Research in the Lu Lab is supported by National Institutes of Health grant no. NIH R01 GM134064-01.
References
- 1.Winfree A. 2001. The geometry of the biological time. New York, NY: Springer. [Google Scholar]
- 2.Huygens C. 1665. Correspondence 1664–1665. In Oeuvres complètes de Christiaan Huygens vol. V (La societe hollandaise des sciences, 1893). The Hague, The Netherlands: M. Nijhoff. [Google Scholar]
- 3.De Monte S, D'Ovidio F, Danø S, Sørensen PG. 2007. Dynamical quorum sensing: population density encoded in cellular dynamics. Proc. Natl Acad. Sci. USA 104, 18 377-18 381. ( 10.1073/pnas.0706089104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Garcia-Ojalvo J, Elowitz MB, Strogatz SH. 2004. Modeling a synthetic multicellular clock: repressilators coupled by quorum sensing. Proc. Natl Acad. Sci. USA 101, 10 955-10 960. ( 10.1073/pnas.0307095101) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nakamura W, Honma S, Shirakawa T, Honma KI. 2001. Regional pacemakers composed of multiple oscillator neurons in the rat suprachiasmatic nucleus. Eur. J. Neurosci. 14, 666-674. ( 10.1046/j.0953-816x.2001.01684.x) [DOI] [PubMed] [Google Scholar]
- 6.Ypey DL, Clapham DE, DeHaan RL. 1979. Development of electrical coupling and action potential synchrony between paired aggregates of embryonic heart cells. J. Membr. Biol. 51, 75-96. ( 10.1007/BF01869344) [DOI] [PubMed] [Google Scholar]
- 7.Jalife J. 1984. Mutual entrainment and electrical coupling as mechanisms for synchronous firing of rabbit sino-atrial pace-maker cells. J. Physiol. 356, 221-243. ( 10.1113/jphysiol.1984.sp015461) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Michaels DC, Matyas EP, Jalife J. 1986. Dynamic interactions and mutual synchronization of sinoatrial node pacemaker cells: a mathematical model. Circ. Res. 58, 706-720. ( 10.1161/01.RES.58.5.706) [DOI] [PubMed] [Google Scholar]
- 9.Plautz JD, Kaneko M, Hall JC, Kay SA. 1997. Independent photoreceptive circadian clocks throughout Drosophila. Science 278, 1632-1635. ( 10.1126/science.278.5343.1632) [DOI] [PubMed] [Google Scholar]
- 10.Zeng H, Qian Z, Myers MP, Rosbash M. 1996. A light-entrainment mechanism for the Drosophila circadian clock. Nature 380, 129-135. ( 10.1038/380129a0) [DOI] [PubMed] [Google Scholar]
- 11.Strogatz SH. 2000. From Kuramoto to Crawford: exploring the onset of synchronization in populations of coupled oscillators. Physica D 143, 1-20. ( 10.1016/S0167-2789(00)00094-4) [DOI] [Google Scholar]
- 12.Gustavsson AK, Adiels CB, Mehlig B, Goksör M. 2015. Entrainment of heterogeneous glycolytic oscillations in single cells. Sci. Rep. 5, 9404. ( 10.1038/srep09404) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Almendral J, Caulier-Cisterna R, Rojo-Álvarez JL. 2013. Resetting and entrainment of reentrant arrhythmias: part i: concepts, recognition, and protocol for evaluation: surface ECG versus intracardiac recordings. Pacing Clin. Electrophysiol. 36, 508-532. ( 10.1111/pace.12064) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rosato E, Tauber E, Kyriacou CP. 2006. Molecular genetics of the fruit-fly circadian clock. Eur. J. Hum. Genet. 14, 729-738. ( 10.1038/sj.ejhg.5201547) [DOI] [PubMed] [Google Scholar]
- 15.Yan J, Goldbeter A. 2019. Robust synchronization of the cell cycle and the circadian clock through bidirectional coupling. J. R. Soc. Interface 16, 20190376. ( 10.1098/rsif.2019.0376) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Azzam R, Chen SL, Shou W, Mah AS, Alexandru G, Nasmyth K, Annan RS, Carr SA, Deshaies RJ. 2004. Phosphorylation by cyclin B-Cdk underlies release of mitotic exit activator Cdc14 from the nucleolus. Science 305, 516-519. ( 10.1126/science.1099402) [DOI] [PubMed] [Google Scholar]
- 17.Lakatta EG, Yaniv Y, Maltsev VA. 2014. Cardiac impulse is initiated by a coupled system of membrane ion channels and Ca2+ cycling proteins. In Cardiac electrophysiology: from cell to bedside, 6th edn (eds Zipes DP, Jalife J), pp. 243-252. Philadelphia, PA: Saunders/Elsevier. [Google Scholar]
- 18.Yaniv Y, Lakatta EG, Maltsev VA. 2015. From two competing oscillators to one coupled-clock pacemaker cell system. Front. Physiol. 6, 28. ( 10.3389/fphys.2015.00028) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bier M, Bakker BM, Westerhoff HV. 2000. How yeast cells synchronize their glycolytic oscillations: a perturbation analytic treatment. Biophys. J. 78, 1087-1093. ( 10.1016/S0006-3495(00)76667-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gustavsson AK, Van Niekerk DD, Adiels CB, Kooi B, Goksör M, Snoep JL. 2014. Allosteric regulation of phosphofructokinase controls the emergence of glycolytic oscillations in isolated yeast cells. FEBS J. 281, 2784-2793. ( 10.1111/febs.12820) [DOI] [PubMed] [Google Scholar]
- 21.Tay S, Hughey JJ, Lee TK, Lipniacki T, Quake SR, Covert MW. 2010. Single-cell NF-B dynamics reveal digital activation and analogue information processing. Nature 466, 267-271. ( 10.1038/nature09145) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hoffmann A, Levchenko A, Scott ML, Baltimore D. 2002. The IκB-NF-κB signaling module: temporal control and selective gene activation. Science 298, 1241-1245. ( 10.1126/science.1071914) [DOI] [PubMed] [Google Scholar]
- 23.Nelson DE, et al. 2004. Oscillations in NF-κB signaling control the dynamics of gene expression. Science 306, 704-708. ( 10.1126/science.1099962) [DOI] [PubMed] [Google Scholar]
- 24.Tsai TYC, Yoon SC, Ma W, Pomerening JR, Tang C, Ferrell JE. 2008. Robust, tunable biological oscillations from interlinked positive and negative feedback loops. Science 321, 126-139. ( 10.1126/science.1156951) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hardin PE, Hall JC, Rosbash M. 1990. Feedback of the Drosophila period gene product on circadian cycling of its messenger RNA levels. Nature 343, 536-540. ( 10.1038/343536a0) [DOI] [PubMed] [Google Scholar]
- 26.Visintin R, Hwang ES, Amon A. 1999. Cfi1 prevents premature exit from mitosis by anchoring Cdc14 phosphatase in the nucleolus. Nature 398, 818-823. ( 10.1038/19775) [DOI] [PubMed] [Google Scholar]
- 27.Duysens LN, Amesz J. 1957. Fluorescence spectrophotometry of reduced phosphopyridine nucleotide in intact cells in the near-ultraviolet and visible region. Biochim. Biophys. Acta 24, 19-26. ( 10.1016/0006-3002(57)90141-5) [DOI] [PubMed] [Google Scholar]
- 28.Toescu EC. 1995. Temporal and spatial heterogeneities of Ca2+ signaling: mechanisms and physiological roles. Am. J. Physiol. 269, G173-G185. ( 10.1152/ajpgi.1995.269.2.g173) [DOI] [PubMed] [Google Scholar]
- 29.Hao N, O'Shea EK. 2012. Signal-dependent dynamics of transcription factor translocation controls gene expression. Nat. Struct. Mol. Biol. 19, 31-40. ( 10.1038/nsmb.2192) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nunemaker CS, Satin LS. 2014. Episodic hormone secretion: a comparison of the basis of pulsatile secretion of insulin and GnRH. Endocrine 47, 49-63. ( 10.1007/s12020-014-0212-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wright T, Gillespie LN, O'Leary SJ, Needham K. 2016. Firing frequency and entrainment maintained in primary auditory neurons in the presence of combined BDNF and NT3. Sci. Rep. 6, 1. ( 10.1038/s41598-016-0001-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Borghans JAM, Dupont G, Goldbeter A. 1997. Complex intracellular calcium oscillations. a theoretical exploration of possible mechanisms. Biophys. Chem. 66, 25-41. ( 10.1016/S0301-4622(97)00010-0) [DOI] [PubMed] [Google Scholar]
- 33.Gérard C, Goldbeter A. 2012. Entrainment of the mammalian cell cycle by the circadian clock: modeling two coupled cellular rhythms. PLoS Comput. Biol. 8, e1002516. ( 10.1371/journal.pcbi.1002516) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Guevara MR, Glass L, Shrier A. 2011. Phase locking, period-doubling bifurcations, and irregular dynamics in periodically stimulated cardiac cells. Science 214, 1350-1353. ( 10.1126/science.7313693) [DOI] [PubMed] [Google Scholar]
- 35.Hancioglu B, Tyson JJ. 2012. A mathematical model of mitotic exit in budding yeast: the role of Polo kinase. PLoS ONE 7, e30810. ( 10.1371/journal.pone.0030810) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Leloup JC, Goldbeter A. 2003. Toward a detailed computational model for the mammalian circadian clock. Proc. Natl Acad. Sci. USA 100, 7051-7056. ( 10.1073/pnas.1132112100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Panda S, Hogenesch JB, Kay SA. 2002. Circadian rhythms from flies to human. Nature 417, 329-335. ( 10.1038/417329a) [DOI] [PubMed] [Google Scholar]
- 38.Ballesta A, Innominato PF, Dallmann R, Rand DA, Lévi FA. 2017. Systems chronotherapeutics. Pharmacol. Rev. 69, 161-199. ( 10.1124/pr.116.013441) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gérard C, Goldbeter A. 2009. Temporal self-organization of the cyclin/Cdk network driving the mammalian cell cycle. Proc. Natl Acad. Sci. USA 106, 21 643-21 648. ( 10.1073/pnas.0903827106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kellogg RA, Tay S. 2015. Noise facilitates transcriptional control under dynamic inputs. Cell 160, 381-392. ( 10.1016/j.cell.2015.01.013) [DOI] [PubMed] [Google Scholar]
- 41.Edmunds LN. 1988. Cellular and molecular bases of biological clocks: models and mechanisms for circadian timekeeping. New York, NY: Springer. [Google Scholar]
- 42.Wille JJ, Ehret CF. 1968. Light synchronization of an endogenous circadian rhythm of cell division in tetrahymena. J. Protozool. 15, 785-789. ( 10.1111/j.1550-7408.1968.tb02214.x) [DOI] [PubMed] [Google Scholar]
- 43.Lu Y, Cross FR. 2010. Periodic cyclin-cdk activity entrains an autonomous cdc14 release oscillator. Cell 141, 268-279. ( 10.1016/j.cell.2010.03.021) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Feillet C, et al. 2014. Phase locking and multiple oscillating attractors for the coupled mammalian clock and cell cycle. Proc. Natl Acad. Sci. USA 111, 9828-9833. ( 10.1073/pnas.1320474111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Laranjeiro R, Tamai TK, Letton W, Hamilton N, Whitmore D. 2018. Circadian clock synchronization of the cell cycle in zebrafish occurs through a gating mechanism rather than a period-phase locking process. J. Biol. Rhythms 33, 137-150. ( 10.1177/0748730418755583) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mori T, Binder B, Johnson CH. 1996. Circadian gating of cell division in cyanobacteria growing with average doubling times of less than 24 hours. Proc. Natl Acad. Sci. USA 93, 10 183-10 188. ( 10.1073/pnas.93.19.10183) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yang Q, Pando BF, Dong G, Golden SS, Van Oudenaarden A. 2010. Circadian gating of the cell cycle revealed in single cyanobacterial cells. Science 327, 1522-1526. ( 10.1126/science.1181759) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Matsuo T, Yamaguchi S, Mitsui S, Emi A, Shimoda F, Okamura H. 2003. Control mechanism of the circadian clock for timing of cell division in vivo. Science 302, 255-259. ( 10.1126/science.1086271) [DOI] [PubMed] [Google Scholar]
- 49.Bieler J, Cannavo R, Gustafson K, Gobet C, Gatfield D, Naef F. 2014. Robust synchronization of coupled circadian and cell cycle oscillators in single mammalian cells. Mol. Syst. Biol. 10, 739. ( 10.15252/msb.20145218) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nagoshi E, Saini C, Bauer C, Laroche T, Naef F, Schibler U. 2004. Circadian gene expression in individual fibroblasts: cell-autonomous and self-sustained oscillators pass time to daughter cells. Cell 119, 693-705. ( 10.1016/j.cell.2004.11.015) [DOI] [PubMed] [Google Scholar]
- 51.Zopf CJ, Quinn K, Zeidman J, Maheshri N. 2013. Cell-cycle dependence of transcription dominates noise in gene expression. PLoS Comput. Biol. 9, e1003161. ( 10.1371/journal.pcbi.1003161) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fei C, Cao Y, Ouyang Q, Tu Y. 2018. Design principles for enhancing phase sensitivity and suppressing phase fluctuations simultaneously in biochemical oscillatory systems. Nat. Commun. 9, 1434. ( 10.1038/s41467-018-03826-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Granada A, Hennig RM, Ronacher B, Kramer A, Herzel H. 2009. Phase response curves: elucidating the dynamics of coupled oscillators. Methods Enzymol. 454, 1-27. ( 10.1016/S0076-6879(08)03801-9) [DOI] [PubMed] [Google Scholar]
- 54.Masuda K, Tokuda IT, Nakamichi N, Fukuda H. 2021. The singularity response reveals entrainment properties of the plant circadian clock. Nat. Commun. 12, 864. ( 10.1038/s41467-021-21167-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zambrano S, De Toma I, Piffer A, Bianchi ME, Agresti A. 2016. NF-κB oscillations translate into functionally related patterns of gene expression. eLife 5, e09100. ( 10.7554/eLife.09100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Johnson CH. 1999. Forty years of PRCs: what have we learned? Chronobiol. Int. 16, 711-743. ( 10.3109/07420529909016940) [DOI] [PubMed] [Google Scholar]
- 57.Granada A, Herzel H. 2009. How to achieve fast entrainment? The timescale to synchronization. PLoS ONE 4, e7057. ( 10.1371/journal.pone.0007057) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Heltberg ML, Krishna S, Kadanoff LP, Jensen MH. 2021. A tale of two rhythms: locked clocks and chaos in biology. Cell Syst. 12, 291-303. ( 10.1016/j.cels.2021.03.003) [DOI] [PubMed] [Google Scholar]
- 59.Gan S, O'Shea EK. 2017. An unstable singularity underlies stochastic phasing of the circadian clock in individual cyanobacterial cells. Mol. Cell 67, 659-672. ( 10.1016/j.molcel.2017.07.015) [DOI] [PubMed] [Google Scholar]
- 60.Abramovich-Sivan S, Akselrod S. 1998. A single pacemaker cell model based on the phase response curve. Biol. Cybern. 79, 67-76. ( 10.1007/s004220050459) [DOI] [PubMed] [Google Scholar]
- 61.Fenske S, et al. 2020. cAMP-dependent regulation of HCN4 controls the tonic entrainment process in sinoatrial node pacemaker cells. Nat. Commun. 11, 5555. ( 10.1038/s41467-020-19304-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Diekman CO, Bose A. 2016. Entrainment maps: a new tool for understanding properties of circadian oscillator models. J. Biol. Rhythms 31, 598-616. ( 10.1177/0748730416662965) [DOI] [PubMed] [Google Scholar]
- 63.Weber A, Zuschratter W, Hauser MJB. 2020. Partial synchronisation of glycolytic oscillations in yeast cell populations. Sci. Rep. 10, 1. ( 10.1038/s41598-019-56847-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Richard P, Bakker BM, Teusink B, Van Dam K, Westerhoff HV. 1996. Acetaldehyde mediates the synchronization of sustained glycolytic oscillations in populations of yeast cells. Eur. J. Biochem. 235, 238-241. ( 10.1111/j.1432-1033.1996.00238.x) [DOI] [PubMed] [Google Scholar]
- 65.Aldridge J, Pye EK. 1976. Cell density dependence of oscillatory metabolism. Nature 259, 670-671. ( 10.1038/259670a0) [DOI] [PubMed] [Google Scholar]
- 66.Kim MS, et al. 2018. Heterogeneity of calcium clock functions in dormant, dysrhythmically and rhythmically firing single pacemaker cells isolated from SA node. Cell Calcium 74, 168-179. ( 10.1016/j.ceca.2018.07.002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lyashkov AE, Juhaszova M, Dobrzynski H, Vinogradova TM, Maltsev VA, Juhasz O, Spurgeon HA, Sollott SJ, Lakatta EG. 2007. Calcium cycling protein density and functional importance to automaticity of isolated sinoatrial nodal cells are independent of cell size. Circ. Res. 100, 1723-1731. ( 10.1161/CIRCRESAHA.107.153676) [DOI] [PubMed] [Google Scholar]
- 68.Monfredi O, Tsutsui K, Ziman B, Stern MD, Lakatta EG, Maltsev VA. 2018. Electrophysiological heterogeneity of pacemaker cells in the rabbit intercaval region, including the SA node: insights from recording multiple ion currents in each cell. Am. J. Physiol. Heart Circ. Physiol. 314, H403-H414. ( 10.1152/ajpheart.00253.2016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wilders R, Jongsma HJ. 1993. Beating irregularity of single pacemaker cells isolated from the rabbit sinoatrial node. Biophys. J. 65, 2601-2613. ( 10.1016/S0006-3495(93)81289-X) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Easterling M, Rossi S, Mazzella AJ, Bressan M. 2021. Assembly of the cardiac pacemaking complex: electrogenic principles of sinoatrial node morphogenesis. J. Cardiovasc. Dev. Dis. 8, 40. ( 10.3390/jcdd8040040) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Herzog ED, Takahashi JS, Block GD. 1998. Clock controls circadian period in isolated suprachiasmatic nucleus neurons. Nat. Neurosci. 1, 708-713. ( 10.1038/3708) [DOI] [PubMed] [Google Scholar]
- 72.Welsh DK, Logothetis DE, Meister M, Reppert SM. 1995. Individual neurons dissociated from rat suprachiasmatic nucleus express independently phased circadian firing rhythms. Neuron 14, 697-706. ( 10.1016/0896-6273(95)90214-7) [DOI] [PubMed] [Google Scholar]
- 73.Aton SJ, Herzog ED. 2005. Come together, right…now: synchronization of rhythms in a mammalian circadian clock. Neuron 48, 531-534. ( 10.1016/j.neuron.2005.11.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yamaguchi S, Isejima H, Matsuo T, Okura R, Yagita K, Kobayashi M, Okamura H. 2003. Synchronization of cellular clocks in the suprachiasmatic nucleus. Science 302, 1408-1412. ( 10.1126/science.1089287) [DOI] [PubMed] [Google Scholar]
- 75.Liu C, Reppert SM. 2000. GABA synchronizes clock cells within the suprachiasmatic circadian clock. Neuron 25, 123-128. ( 10.1016/S0896-6273(00)80876-4) [DOI] [PubMed] [Google Scholar]
- 76.Piggins HD, Antle MC, Rusak B. 1995. Neuropeptides phase shift the mammalian circadian pacemaker. J. Neurosci. 15, 5612-5622. ( 10.1523/JNEUROSCI.15-08-05612.1995) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Danø S, Hynne F, De Monte S, d'Ovidio F, Sørensen PG, Westerhoff H. 2001. Synchronization of glycolytic oscillations in a yeast cell population. Faraday Discuss. 120, 261-276. ( 10.1039/b103238k) [DOI] [PubMed] [Google Scholar]
- 78.Pye E. 1969. Biochemical mechanisms underlying the metabolic oscillations in yeast. Can. J. Bot. 2, 271-285. ( 10.1139/b69-040) [DOI] [Google Scholar]
- 79.Kojima K, Kaneko T, Yasuda K. 2006. Role of the community effect of cardiomyocyte in the entrainment and reestablishment of stable beating rhythms. Biochem. Biophys. Res. Commun. 351, 209-215. ( 10.1016/j.bbrc.2006.10.037) [DOI] [PubMed] [Google Scholar]
- 80.Yasuda K. 2020. Dominant rule of community effect in synchronized beating behavior of cardiomyocyte networks. Biophys. Rev. 12, 481-501. ( 10.1007/s12551-020-00688-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Reppert SM, Weaver DR. 2002. Coordination of circadian timing in mammals. Nature 418, 935-941. ( 10.1038/nature00965) [DOI] [PubMed] [Google Scholar]
- 82.Cai L, Dalal CK, Elowitz MB. 2008. Frequency-modulated nuclear localization bursts coordinate gene regulation. Nature 455, 485-490. ( 10.1038/nature07292) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bressloff PC, Karamched BR. 2015. A frequency-dependent decoding mechanism for axonal length sensing. Front. Cell. Neurosci. 9, 281. ( 10.3389/fncel.2015.00281) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Krylov DM, Nasmyth K, Koonin EV. 2003. Evolution of eukaryotic cell cycle regulation: stepwise addition of regulatory kinases and late advent of the CDKs. Curr. Biol. 13, 173-177. ( 10.1016/S0960-9822(03)00008-3) [DOI] [PubMed] [Google Scholar]
- 85.Gard DL, Hafezi S, Zhang T, Doxsey SJ. 1990. Centrosome duplication continues in cycloheximide-treated Xenopus blastulae in the absence of a detectable cell cycle. J. Cell Biol. 110, 2033-2042. ( 10.1083/jcb.110.6.2033) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Haase SB, Winey M, Reed SI. 2001. Multi-step control of spindle pole body duplication by cyclin-dependent kinase. Nat. Cell Biol. 3, 38-42. ( 10.1038/35050543) [DOI] [PubMed] [Google Scholar]
- 87.Haase SB, Reed SI. 1999. Evidence that a free-running oscillator drives G1 events in the budding yeast cell cycle. Nature 401, 394-397. ( 10.1038/43927) [DOI] [PubMed] [Google Scholar]
- 88.McCleland ML, O'Farrell PH. 2008. RNAi of mitotic cyclins in Drosophila uncouples the nuclear and centrosome cycle. Curr. Biol. 18, 245-254. ( 10.1016/j.cub.2008.01.041) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sluder G, Miller FJ, Cole R, Rieder CL. 1990. Protein synthesis and the cell cycle: centrosome reproduction in sea urchin eggs is not under translational control. J. Cell Biol. 110, 2025-2032. ( 10.1083/jcb.110.6.2025) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Darwin C. 1859. On the origin of species by means of natural selection. London, UK: John Murray. [Google Scholar]
- 91.Mondragón-Palomino O, Danino T, Selimkhanov J, Tsimring L, Hasty J. 2011. Entrainment of a population of synthetic genetic oscillators. Science 333, 1315-1319. ( 10.1126/science.1205369) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Harton MD, Koh WS, Bunker AD, Singh A, Batchelor E. 2019. P53 pulse modulation differentially regulates target gene promoters to regulate cell fate decisions. Mol. Syst. Biol. 15, e8685. ( 10.15252/msb.20188685) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lahav G, Rosenfeld N, Sigal A, Geva-Zatorsky N, Levine AJ, Elowitz MB, Alon U. 2004. Dynamics of the p53-Mdm2 feedback loop in individual cells. Nat. Genet. 36, 147-150. ( 10.1038/ng1293) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This article has no additional data.